Abstract
Background
Mechanisms of postoperative delirium remain poorly understood, limiting development of effective treatments. We tested the hypothesis that intraoperative oxidative damage is associated with delirium and neuronal injury and that disruption of the blood-brain barrier modifies these associations.
Methods
In a prespecified cohort study of four hundred cardiac surgery patients enrolled in a clinical trial of atorvastatin to reduce kidney injury and delirium, we measured plasma concentrations of F2-isoprostanes and isofurans using gas chromatography-mass spectrometry to quantify oxidative damage, ubiquitin carboxyl-terminal hydrolase-1 to quantify neuronal injury, and S100 calcium-binding protein B using enzyme-linked immunosorbent assays to quantify blood-brain barrier disruption prior to, during, and following surgery. We performed the Confusion Assessment Method for the Intensive Care Unit twice daily to diagnose delirium. We measured the independent associations between intraoperative F2-isoprostanes and isofurans and delirium (primary outcome) and postoperative ubiquitin C-terminal hydrolase-1 (secondary outcome), and we assessed if S100 calcium-binding protein B modified these associations.
Results
Delirium occurred in 109 of 400 (27.3%) patients for a median (10th, 90th percentile) of 1.0 (0.5, 3.0) day. In the total cohort, plasma ubiquitin C-terminal hydrolase-1 concentration was 6.3 ng/ml (2.7, 14.9) at baseline and 12.4 ng/ml (7.9, 31.2) on postoperative day 1. F2-isoprostanes and isofurans increased throughout surgery, and the log-transformed sum of intraoperative F2-isoprostanes and isofurans was independently associated with increased odds of postoperative delirium (OR: 3.70 [95% CI: 1.41 to 9.70]; P=0.008) and with increased postoperative ubiquitin C-terminal hydrolase-1 (ratio of geometric means: 1.42 [1.11 to 1.81]; P=0.005). The association between increased intraoperative F2-isoprostanes and isofurans and increased postoperative ubiquitin C-terminal hydrolase-1 was amplified in patients with elevated S100 calcium-binding protein B (P=0.049).
Conclusions
Intraoperative oxidative damage was associated with increased postoperative delirium and neuronal injury, and the association between oxidative damage and neuronal injury was stronger among patients with increased blood-brain barrier disruption.
INTRODUCTION
Postoperative delirium is a state of acute cerebral dysfunction following anesthesia and surgery.1 It occurs in 25 to 52% of patients undergoing cardiac surgery and is associated with increased mortality, increased duration of hospitalization, and long-term cognitive decline.2,3 The underlying pathophysiologic mechanisms of cardiac surgery-induced delirium are unclear but may include acute inflammation, effects of anesthetics, exposure to artificial circulation or altered perfusion, and microemboli.4 Alterations in systemic and regional perfusion and oxygenation, cardiopulmonary bypass (CPB), and rapid changes in plasma pH and cellular metabolism during cardiac surgery induce an oxidative stress that could contribute to postoperative delirium.5,6 In fact, hydrogen peroxide, a reactive oxygen species generated during tissue ischemia and reperfusion, suppresses neuron function,7 and delirium-susceptible mice have increased systemic oxidative stress and are protected by treatments that reduce oxidative stress.8 Oxidative stress leads to oxidative damage, but a functional blood-brain barrier might reduce the injurious effects of circulating oxidants on neurons, unless oxidative damage disrupts the blood-brain barrier.9
F2-isoprostanes and isofurans are oxidative damage end-products of arachidonic acid peroxidation that increase in proportion to oxidative stress, are specific to free radical-initiated oxidation, are stable in plasma, and have been independently associated with other organ injuries following cardiac surgery.10–13 Data on the relationships among oxidative damage, blood-brain barrier disruption, neuronal injury, and risk of delirium are limited. We conducted this study to test the hypothesis that increased intraoperative oxidative damage is associated with increased postoperative delirium and neuronal injury and that disruption of the blood-brain barrier modifies these associations.
METHODS
Patients
This observational study was prospectively planned as part of a clinical trial of statin therapy to reduce kidney injury and delirium following cardiac surgery (Clinicaltrials.gov identifier ).14 Adults receiving elective coronary artery bypass grafting (with or without CPB), heart valve surgery, or surgery on the ascending aorta at Vanderbilt University Medical Center (Nashville, TN) were eligible to participate. Patients unable to participate in preoperative cognitive testing or with acute coronary syndrome, hepatitis, chronic liver dysfunction, statin intolerance, cytochrome P450 3A4 inhibitors or cyclosporine use, pregnancy, on dialysis, a history of kidney transplantation or insufficient blood availability were excluded. The Vanderbilt Institutional Review Board approved the study, and all participants provided written informed consent prior to surgery.
Baseline assessments
Research nurses measured baseline preoperative cognitive function with the Mini Mental State Exam, estimated severity of illness with the Charlson comorbidity index, and documented past medical history, vital signs, and baseline laboratory data.
Standardized patient management
Perioperative anesthetic, surgical, and postoperative patient management was conducted according to institutional protocol (Supplemental Digital Content, Supplemental Methods).
Postoperative delirium was assessed beginning on the night of surgery and continuing until patients were transferred out of the intensive care unit by research nurses and coordinators who were blinded to treatment and biomarker data. Delirium assessment training is described in the Supplemental Digital Content. Briefly, trained research personnel assessed patients for level of arousal and delirium twice daily using the Richmond Agitation Scale Score and the Confusion Assessment Method for the Intensive Care Unit tool, respectively. The Confusion Assessment Method for the Intensive Care Unit tool is validated in ventilated and non-ventilated patients and is used to assess acute changes or a fluctuating course of mental status, inattention, an altered level of consciousness, and disorganized thinking to diagnose delirium.15–18 This tool has been frequently utilized to assess for delirium after cardiac surgery.3,19–21 Participants with a noncomatose state (Richmond Agitation Scale Score −3 or greater) and a positive Confusion Assessment Method for the Intensive Care Unit assessment were considered to have delirium.
Quantification of oxidative damage, blood-brain barrier disruption, and neuronal injury
We collected arterial blood at the induction of general anesthesia (baseline), 30 minutes after initiation of cardiopulmonary bypass (CPB) or off-pump coronary artery bypass (CABG) grafting, after termination of CPB or completion of off-pump CABG grafting, at intensive care unit admission, and at 09:00 on postoperative day 1. Blood was collected in 0.105M sodium citrate- and EDTA-coated tubes, immediately placed on ice, centrifuged for 15 minutes at 1000G, and plasma separated and frozen at −80°C until thawed for batched analysis. Lab technicians were blinded to the results of delirium assessments and all clinical data. To measure oxidative damage, we quantified the non-esterified free plasma concentrations of F2-isoprostanes and isofurans using gas chromatography-mass spectrometry. F2-isoprostanes and isofurans are products of arachidonic acid peroxidation that represent the gold-standard for the assessment of oxidative damage in vivo because they increase in direct proportion to oxidative stress and are stable in biologic specimens.10 F2-isoprostanes and isofurans are differentially expressed from a common lipid radical intermediate in response to local oxygen tension.22 When oxygen administration and tissue oxygen tensions are heterogenous both temporally and regionally, such as during cardiac surgery, the combined metric of F2-isoprostanes and isofurans best reflects systemic lipid peroxidation.
To quantify neuronal injury, we measured plasma concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1 in duplicate at baseline, intensive care unit admission, and on postoperative day 1, using an enzyme-linked immunosorbent assay (Abnova, Taiwan). Ubiquitin carboxyl-terminal hydrolase isozyme L1 is a neuron-specific enzyme that tags proteins with ubiquitin for proteasome degradation and assists with recycling of ubiquitin among other neuron specific functions.23 Plasma concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1 reflect neuronal damage in conjunction with blood-brain barrier disruption, active or passive transport of ubiquitin carboxyl-terminal hydrolase isozyme L1 across the blood-brain barrier, diffusion of ubiquitin carboxyl-terminal hydrolase isozyme L1 across the blood-brain barrier, or glymphatic passage.24,25 Plasma concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1 are increased in patients with traumatic brain injury and following controlled hypoxic-ischemic brain injury in preclinical studies.26,27
To measure disruption or injury to the blood-brain barrier, we measured S100 calcium binding protein B concentrations in plasma in duplicate at baseline, intensive care unit admission, and on postoperative day 1 using ELISA (Millipore Sigma, USA). S100 calcium binding protein B is released from astrocytes after injury or ischemia and has previously been validated as a marker of blood-brain barrier disruption by comparing cerebrospinal fluid-serum albumin quotients and magnetic resonance images after blood-brain barrier injury.28–30 S100 calcium binding protein B concentrations may also reflect neuronal injury independent of blood-brain barrier disruption via glymphatic passage,24 and there are some extracranial sources of S100 calcium binding protein B,31 although these are considered minor contributors to circulating concentrations.32,33
Statistical Analyses
The association between intraoperative oxidative damage and the development of postoperative delirium was estimated using logistic regression, adjusting for potential confounders including age, baseline Mini Mental State Exam score, Charlson comorbidity index, history of diabetes (yes/no), current smoking status (yes/no), history of stroke (yes/no), use of CPB during surgery (yes/no), perioperative atorvastatin treatment (yes/no), and baseline F2-isoprostanes and isofurans measurements (natural log-transformed). Intraoperative oxidative damage was quantified as the sum of the natural log-transformed intraoperative F2-isoprostane and isofuran measurements, averaged among the 30 minutes into CPB or off-pump CABG grafting, the post CPB or off-pump CABG grafting, and the intensive care unit admission time points. This measure is equivalent to the natural logarithm of the geometric mean of F2-isoprostanes and isofurans measurements at these three time points. Thus, by exponentiation, the overall measure can be summarized in the original units of the F2-isoprostane and isofuran measurements. Quantitative factors were modeled using a four-knot natural cubic spline to allow for possible nonlinear associations. These terms were omitted in the absence of significant statistical evidence of nonlinear effects (P>0.05), assessed using a multiple-degree-of-freedom chi-square test. The effect of intraoperative oxidative stress on the incidence of postoperative delirium was summarized using an odds ratio and 95% CI and tested for statistical significance using the associated Wald-test. P-values less than 0.05 were considered statistically significant.
The effects of intraoperative oxidative damage on neuronal injury, measured as natural log-transformed plasma concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1 on postoperative day 1, were quantified using linear regression adjusting for the same set of factors listed previously.
To examine whether the extent of blood-brain barrier disruption, measured by the natural log-transformed plasma concentration of S100 calcium binding protein B immediately after surgery, modifies the association between intraoperative oxidative damage and the incidence of delirium or the extent of neuronal injury, we added the intensive care unit admission S100 calcium binding protein B concentration and a cross-product interaction term between intraoperative oxidative damage and intensive care unit admission S100 calcium binding protein B (F2-isoprostanes & isofurans × S100 calcium binding protein B) to the delirium logistic regression and the ubiquitin carboxyl-terminal hydrolase isozyme L1 linear regression models. We then examined the association between these interaction terms and delirium and ubiquitin carboxyl-terminal hydrolase isozyme L1, respectively.
Finally, as a sensitivity analysis, we measured the association between neuronal injury and the odds of delirium using logistic regression methods, adjusting for perioperative oxidative damage and the other potential confounders listed previously. In addition, we performed a subgroup analysis on patients who received on-pump surgery.
Due to evidence of nonlinear associations among factors, all associations are summarized by comparing the odds/geometric means corresponding to the 90th versus 10th percentile of the independent variable, and effect estimates are presented graphically with 95% confidence bands. Multiple-degree-of-freedom chi-square tests were used to evaluate statistical significance.
We selected a sample size for this study based on prior studies of perioperative oxidative damage and established rates of postoperative delirium. To detect a 5 ± 15 pg/ml difference in F2-isoprostanes and isofurans concentration between patients who did and who did not develop delirium, a concentration of F2-isoprostanes and isofurans previously demonstrated to be independently associated with kidney injury following surgery,11 we studied 400 patients and assumed a 25% incidence of delirium in the cohort and a type I error rate of 5%. This number of patients provides 83% power to reject the null hypothesis. In addition, a cohort of 400 patients and 109 events provided the opportunity to reliably fit a model with up to 11 degrees of freedom for logistic regression modeling and 27 degrees of freedom for linear regression modeling.34 We used R version 3.4.1 for all statistical analyses.
RESULTS
Patient characteristics and delirium
Four hundred patients comprised the cohort. The median (10th percentile, 90th percentile) age of patients was 67 (50, 81) years, 32.8% were female, 30.8% were diabetic, and 70.8% had CPB during surgery (Table 1). One hundred nine patients (27.3%) developed postoperative delirium for a median of 1.0 (0.5, 3.0) day. The median onset of delirium was on the morning of postoperative day one (0.5 [0.0, 2.0] days after intensive care unit admission). Patients who developed delirium experienced increased rates of postoperative atrial fibrillation (47.7% vs. 30.2%) and acute kidney injury (30.3% vs. 20.6%) and remained in the intensive care unit and hospital 2.0 days longer than patients who did not develop delirium. Similar to other trials of short-term statins, study drug administration (atorvastatin vs. placebo) did not affect delirium.14,35,36
Table 1. Baseline and intraoperative patient characteristics.
| Patient characteristic | No delirium (n=291) | Delirium (n=109) | Total (n = 400) |
|---|---|---|---|
| Age, years | 64 (47, 81) | 72 (56, 82) | 66 (50, 81) |
| Female sex | 78 (26.8%) | 53 (48.6%) | 131 (32.8%) |
| African American ancestry | 11 (3.8%) | 8 (7.3%) | 19 (4.8%) |
| Height, cm | 175 (157, 183) | 167 (157, 183) | 173 (157, 183) |
| Weight, kg | 85 (63, 101) | 77 (61, 102) | 82 (62, 109) |
| Body Mass Index | 27.2 (22.6, 36.1) | 27.5 (22.0, 36.9) | 27.4 (22.5, 36.5) |
| Medical history | |||
| ASA physical status class | 4 (3, 4) | 4, (3,4) | 4, (3,4) |
| Congestive heart failure | 109 (37.5%) | 54 (49.5%) | 163 (40.8%) |
| Left ventricular ejection fraction, % | 60 (40, 60) | 57 (30, 60) | 60 (35, 60) |
| Hypertension | 249 (85.6%) | 99 (90.8%) | 348 (87.0%) |
| Chronic obstructive pulmonary disease | 24 (8.2%) | 23 (21.1%) | 47 (11.8%) |
| Current smoking | 49 (16.8%) | 18 (16.5%) | 67 (16.8%) |
| Obstructive sleep apnea | 44 (15.1%) | 13 (11.9%) | 57 (14.2%) |
| Charlson comorbidity index | 2 (0, 5) | 3 (0, 5) | 2 (0, 5) |
| Diabetes | 88 (30.2%) | 35 (32.1%) | 123 (30.8%) |
| Peripheral vascular disease | 79 (27.1%) | 34 (31.2%) | 113 (28.2%) |
| Stroke | 12 (4.5%) | 9 (8.3%) | 22 (5.5%) |
| Education, years* | 12 (10, 16) | 12 (9,16) | 12 (10, 16) |
| Mini Mental State Exam score | 29 (27, 30) | 28 (24, 30) | 29 (26, 30) |
| Trails B score, seconds | 105 (70, 195) | 134 (75, 222) | 110 (70, 207) |
| Medication use | |||
| Baseline angiotensin converting enzyme inhibitor use | 92 (31.6%) | 33 (30.3) | 125 (31.3%) |
| Baseline statin use | 184 (63.2%) | 66 (60.6%) | 250 (62.5%) |
| Perioperative statin treatment | 151 (51.9%) | 53 (48.6%) | 204 (51.0%) |
| Baseline laboratory and hemodynamic data | |||
| Heart rate, beats/minute | 63 (49, 83) | 66 (49, 85) | 64 (49, 83) |
| Mean arterial Pressure, mmHg | 78 (62, 96) | 81 (61, 98) | 78 (62, 96) |
| Central venous pressure, mmHg | 13 (7, 21) | 14 (7, 22) | 13 (7, 21) |
| Cardiac index, liters/min/m2 | 2.2 (1.6, 3.0) | 2.1 (1.4, 3.2) | 2.2 (1.5, 3.0) |
| Blood glucose, mg/dl | 107 (88, 162) | 109 (90, 162) | 108 (89, 162) |
| Hematocrit, % | 36.0 (29.0, 43.0) | 34.0 (28.0, 41.0) | 35.0 (29.0, 42.1) |
| Platelet count, x103/μL | 213 (142, 306) | 208 (135, 339) | 141 (212, 313) |
| Leukocyte count, x103/L | 7.7 (5.0, 10.8) | 7.7 (4.7, 11.9) | 7.3 (5.0, 10.9) |
| Arterial pH | 7.39 (7.33, 7.47) | 7.41 (7.34, 7.47) | 7.40 (7.33, 7.47) |
| PaCO2, mmHg | 42 (35, 49) | 42 (35, 50) | 42 (35, 49) |
| Arterial lactate, mg/dl | 0.7 (0.4, 1.4) | 0.7 (0.4, 1.4) | 0.7 (0.4, 1.4) |
| Procedure characteristics | |||
| Duration of surgery, minutes | 298 (216, 463) | 336 (226, 510) | 301 (218, 474) |
| Coronary artery bypass surgery | 144 (49.5%) | 50 (45.9%) | 194 (48.5%) |
| Valve surgery | 182 (62.5%) | 78 (71.6%) | 260 (65.0%) |
| On-pump surgery | 196 (67.4%) | 87 (79.8%) | 283 (70.8%) |
| Off-pump surgery | 95 (32.6%) | 22 (20.2%) | 117 (29.2%) |
| Cardiopulmonary bypass time, minutes† | 131 (89, 233) | 146 (90, 249) | 135 (89, 238) |
| Aorta cross-clamp use | 135 (46.4%) | 57 (52.3%) | 192 (48.0%) |
| Circulatory arrest use | 9 (3.1%) | 2 (1.8%) | 11 (2.8%) |
Binary characteristics are reported as n (%) and continuous characteristics as median (10th percentile, 90 percentile).
Education years start at 1st grade. For example, 16 years of education is 12 grades and 4 years of post-high school education (college or equivalent).
Among on-pump surgery patients. Medical history diagnoses were obtained from the medical record and from direct patient questioning at the time of enrollment.
Oxidative damage and postoperative delirium
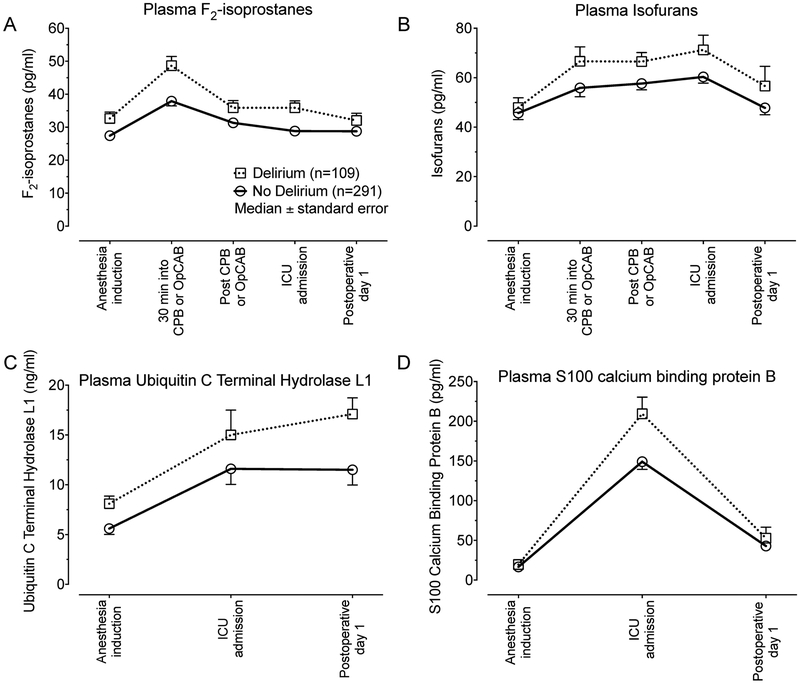
The median (10th percentile, 90th percentile) plasma concentration of F2-isoprostanes was 28.9 pg/ml (14.8, 58.4) at baseline, increased to a peak of 40.8 pg/ml (20.6, 79.6) 30-minutes into CPB or off-pump CABG grafting, and decreased towards baseline by postoperative day 1 (Figure 1, Panel A). The median plasma concentration of isofurans was 46.1 pg/ml (22.3, 110.2) at baseline, increased throughout surgery to a peak of 64.0 pg/ml (32.6, 132.3) at intensive care unit admission, and decreased towards baseline on postoperative day 1 (Figure 1, Panel B).
Figure 1. Perioperative plasma expression of F2-isoprostanes, isofurans, ubiquitin carboxyl-terminal hydrolase isozyme L1, and S100 calcium binding protein B.
Plasma concentrations of markers of oxidative damage (F2-isoprostanes, Panel A and isofurans, Panel B), neuronal injury (ubiquitin carboxyl-terminal hydrolase isozyme L1, Panel C), and blood-brain barrier disruption (S100 calcium binding protein B, Panel D) during the perioperative period in patients who developed delirium and those who did not develop delirium.
Increased intraoperative oxidative damage was independently associated with increased odds of developing postoperative delirium. Quantitatively, patients at the 90th percentile of intraoperative oxidative damage (combined intraoperative F2-isoprostane and isofuran concentrations) were, on average, 3.7 times more likely to develop postoperative delirium than patients at the 10th percentile of intraoperative F2-isoprostanes and isofurans (odds ratio: 3.70 [95% CI: 1.41 to 9.70]; P=0.008; Table 2, Model Delirium, independent variable F2-isoPs & isoFs). This analysis was adjusted for the effects of potential confounders between oxidative damage and delirium, risk factors for delirium, and baseline concentrations of F2-isoprostanes and isofurans. Concentrations of F2-isoprostane and isofurans at baseline were not associated with delirium (P=0.200). Results were qualitatively similar when individual concentrations of F2-isoprostanes or isofurans were used in the model, as compared to the combined F2-isoprostanes and isofurans metric of oxidative damage. Baseline Mini Mental State Exam and F2-isoprostanes and isofurans were missing for 12 and 33 patients, respectively. S100 calcium binding protein B and ubiquitin carboxyl-terminal hydrolase isozyme L1 measurements were missing for 22 and 9 participants, respectively. No other patient data were missing. Records with missing data were excluded from regression analyses.
Table 2. Effects of oxidative damage and blood-brain barrier disruption on postoperative delirium and neuronal injury.
The Delirium model summarizes the independent association between cumulative intraoperative plasma concentrations of F2-isoprostanes and isofurans and the odds of postoperative delirium. The DeliriumS100B model summarizes S100 calcium binding protein B’s modification of the association between F2-isoprostanes and isofurans and odds of delirium, as well as the independent association between S100 calcium binding protein B at intensive care unit admission and odds of delirium. The UCHL1 model summarizes the association between F2-isoprostanes and isofurans and the geometric mean of postoperative ubiquitin carboxyl-terminal hydrolase L1. The UCHL1S100B model summarizes S100 calcium binding protein B’s modification of the association between F2-isoprostanes and isofurans and the geometric mean of ubiquitin carboxy-terminal hydrolase L1, as well as the association between plasma concentrations of S100 calcium binding protein B and the geometric mean of ubiquitin carboxyl-terminal hydrolase L1. The effect of S100 calcium binding protein B is adjusted to the median F2-isoprostane and isofuran concentration. Due to nonlinearity, all associations are summarized by comparing the odds/geometric means corresponding to the 90th versus 10th percentile of the independent variable. All models are adjusted for age, Charlson comorbidity index, baseline Mini Mental State Exam score, current smoking, history of stroke, history of diabetes mellitus, use of cardiopulmonary bypass, study drug, and baseline F2-isoprostane and isofuran concentrations.
| Model | Independent variable | Delirium odds ratio (95% CI) |
P-value |
|---|---|---|---|
| Delirium | F2-isoprostanes & isofurans | 3.70 (1.41 to 9.7) | 0.008 |
| DeliriumS100B | F2-isoprostanes & isofurans at S100 calcium binding protein B 10th percentile | 3.03 (0.71 to 13.0) | 0.135 |
| F2-isoprostanes & isofurans at S100 calcium binding protein B 90th percentile | 4.4 (1.26 to 15.3) | 0.020 | |
| S100 calcium binding protein B | 2.50 (1.13 to 5.5) | 0.024 | |
| F2-isoprostanes & isofurans × S100 calcium binding protein B interaction | 0.665 | ||
| UCHL1 ratio of geometric means (95% CI) | |||
| UCHL1 | F2-isoprostanes & isofurans | 1.42 (1.11 to 1.81) | 0.005 |
| UCHL1S100B | F2-isoprostanes & isofurans at S100 calcium binding protein B 10th percentile | 1.02 (0.75 to 1.41) | 0.881 |
| F2-isoprostanes & isofurans at S100 calcium binding protein B 90th percentile | 1.54 (1.09 to 2.18) | 0.014 | |
| S100 calcium binding protein B | 1.30 (1.04 to 1.63) | 0.024 | |
| F2-isoprostanes & isofurans × S100 calcium binding protein B interaction | 0.049 |
Oxidative damage and postoperative neuronal injury
The median (10th percentile, 90th percentile) plasma concentration of ubiquitin carboxyl-terminal hydrolase isozyme L1 at baseline was 6.3 ng/ml (2.7, 14.9), increased during surgery to 12.2 ng/ml (5.0, 42.7) at intensive care unit admission, and peaked at 12.4 ng/ml (7.9, 31.2) on postoperative day 1 (Figure 1, Panel C). The mean coefficient of variation for ubiquitin carboxyl-terminal hydrolase isozyme L1 duplicate measurements was 5.23%. Increased intraoperative oxidative damage was independently associated with increased postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1. For example, patients with intraoperative F2-isoprostane and isofuran concentrations at the 90th percentile had, on average, a postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 concentration 41.8% greater (ratio of geometric means: 1.42 [95% CI: 1.11 to 1.81]; P=0.005) than patients who had intraoperative F2-isoprostane and isofuran concentrations at the 10th percentile, adjusted for potential confounders, risk factors for delirium, and baseline F2-isoprostane and isofuran concentrations (Table 2, Model UCHL1, independent variable F2-isoPs & isoFs).
Impact of blood-brain barrier disruption on the associations between intraoperative oxidative damage and delirium and between intraoperative oxidative damage and neuronal injury
The median plasma concentration of S100 calcium binding protein B at baseline was 17.2 pg/ml (10th percentile, 90th percentile: 9.0, 32.3), peaked at 163.4 pg/ml (70.1, 414.0) at intensive care unit admission, and decreased towards baseline by postoperative day 1 (Figure 1, Panel D). The mean coefficient of variation for S100 calcium binding protein B duplicate measurements was 5.99%. Increased S100 calcium binding protein B plasma concentrations at intensive care unit admission were independently associated with increased development of delirium and with postoperative neuronal injury. Patients with S100 calcium binding protein B concentrations at the 90th percentile had, on average, 2.5-fold greater odds of developing postoperative delirium (odds ratio: 2.50 [95% CI: 1.13 to 5.51]; P=0.024) than those with concentrations at the 10th percentile, adjusted for potential confounders and delirium risk factors (Table 2, Model DeliriumS100B, independent variable S100B). Patients with S100 calcium binding protein B concentrations at the 90th percentile at intensive care unit admission also had, on average, 30% greater plasma ubiquitin carboxyl-terminal hydrolase isozyme L1 concentrations on postoperative day 1 (ratio of geometric means: 1.30 [95% CI: 1.04 to 1.63]; P=0.024) than patients with S100 calcium binding protein B concentration at the 10th percentile (Table 2, Model UCHL1S100B, independent variable S100 calcium binding protein B).
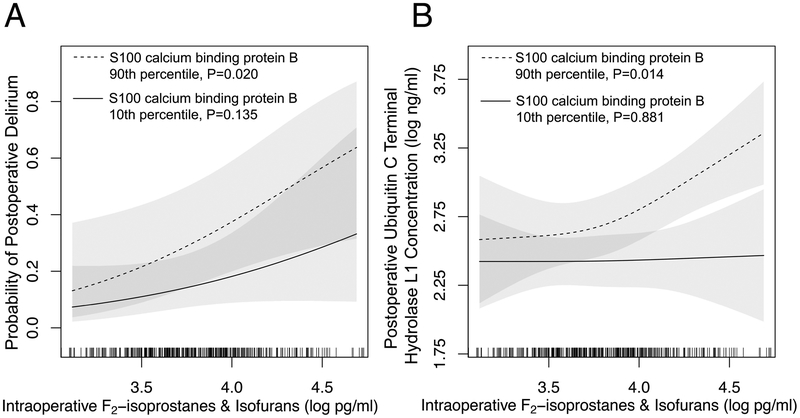
The extent of blood-brain barrier disruption did not modify the association between increased oxidative damage and increased odds of developing delirium (P=0.665; Table 2, Model DeliriumS100B, F2-isoPs & isoFs × S100B interaction term; Figure 2, Panel A) but did modify the association between increased oxidative damage and increased neuronal injury (P=0.049; Table 2, Model UCHL1S100B, F2-isoPs & isoFs × S100B interaction term). For example, at the 10th percentile of S100 calcium binding protein B there was no association between increased oxidative damage and ubiquitin carboxyl-terminal hydrolase isozyme L1 (ratio of geometric means: 1.02 [95% CI: 0.75 to 1.41]; P=0.881), while at the 90th percentile of S100 calcium binding protein B, patients with intraoperative F2-isoprostane and isofuran concentrations at the 90th percentile experienced a 54% increase in postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 concentrations compared to ubiquitin carboxyl-terminal hydrolase isozyme L1 concentrations among patients at the 10th percentile of F2-isoprostanes and isofurans (ratio of geometric means: 1.54 [95% CI: 1.09 to 2.18]; P=0.014). The S100B effect modification on the association between intraoperative oxidative damage and neuronal injury is illustrated in Figure 2, Panel B.
Figure 2. Intraoperative oxidative damage versus postoperative delirium and neuronal injury.
Intraoperative oxidative damage, quantified as the plasma concentration of F2-isoprostanes and isofurans, was independently associated with increased probability of postoperative delirium (Panel A) and plasma concentration of ubiquitin carboxyl-terminal hydrolase isozyme L1 on postoperative day 1 (Panel B) after adjusting for potential confounders. This association with ubiquitin carboxyl-terminal hydrolase isozyme L1 concentration was differentially modified by the extent of blood-brain barrier disruption (measured as plasma S100 calcium binding protein B concentration at the time of intensive care unit admission). The association with delirium was not modified by S100 calcium binding protein B. Shaded areas represent the 95% CI of the probability of delirium (Panel A) and ubiquitin carboxyl-terminal hydrolase isozyme L1 concentration (Panel B). Tick marks at the bottom of the figure indicate the observed values among the study cohort.
A post hoc subgroup analysis involving on-pump surgery was added during peer review. The results were similar between the subgroup of patients who received on-pump surgery and the total cohort (Supplemental Table 1).
Neuronal injury and postoperative delirium
In the sensitivity analysis, postoperative neuronal injury was also associated with delirium. Specifically, after adjusting for the effects of oxidative stress and potential confounders between ubiquitin carboxyl-terminal hydrolase isozyme L1 and delirium, the odds of developing postoperative delirium were, on average, 2.16-fold greater (odds ratio: 2.16 [95% CI: 1.05 to 4.43]; P=0.043) in patients with postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 concentrations in the 90th percentile compared to in patients with postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 concentrations in the 10th percentile.
DISCUSSION
In this cohort of cardiac surgery patients, increased intraoperative oxidative damage was independently associated with the development of postoperative delirium and with postoperative neuronal injury. Moreover, blood-brain barrier disruption modified the relationship between oxidative damage on postoperative neuronal injury – the association between increased intraoperative oxidative damage and postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 was stronger in patients with increased S100 calcium binding protein B than in patients with decreased S100 calcium binding protein B. Thus, these findings indicate that intraoperative systemic oxidative damage may promote postoperative brain dysfunction and injury and that blood-brain barrier disruption and neuronal injury may contribute to acute brain dysfunction after surgery.
Clinicians diagnose delirium in patients using neurocognitive instruments that assess inattention, alterations in behavior, and confusion. These clinical manifestations of delirium are hypothesized to be caused by impaired neuronal function and neuronal networks, persistent GABAa receptor current in the hippocampus, alterations of neurotransmitter availability, residual effects of anesthetics, altered microvascular blood flow, and neuroinflammation.7,37–40 Evidence that postoperative delirium may be a consequence of oxidative damage and direct neuronal injury, conversely, is limited. For example, many previous studies have focused on the role of anesthetics and sedatives in the development of postoperative delirium.41 This study demonstrates that intraoperative oxidative damage, common during surgery and previously noted to be associated with acute kidney and myocardial injury,11,42 is independently associated with postoperative brain dysfunction and injury.
Intraoperative oxidative damage is a consequence of excess production or decreased elimination of reactive oxygen species, reactive nitrogen species, and lipid radicals. These compounds are generated during surgery secondary to ischemia and reperfusion, hyperoxygenation, mitochondrial dysfunction, and hemolysis.43,44 The tissue specific source of intraoperative free radicals and end-products of oxidative stress including F2-isoprostanes and isofurans is unknown, however, as is the impact of systemic oxidants on the brain and the role of the blood-brain barrier on the movement of F2-isoprostanes and isofurans between the systemic circulation and the cerebrum. F2-isoprostanes and isofurans do possess vascular biologic activities that could affect the brain,45 and a prior study of geriatric patients receiving hip surgery noted increased postoperative F2-isoprostane concentrations in patients with acute brain dysfunction.46 In a previous study that also used a subgroup of patients who participated in the Statin AKI Cardiac Surgery RCT we noted that intraoperative hyperoxic cerebral reperfusion, measured by cerebral oximetry, was associated with increased postoperative oxidative damage and delirium and that postoperative oxidative damage partially mediated this association and was independently associated with delirium.6 The current study expands upon that research to focus on intraoperative oxidative damage and markers of neuron injury and blood brain barrier disruption, in order to better characterize the time and nature of the cerebral insult from surgery.
Intracellular proteins that are released into the plasma when neurons or the blood-brain barrier are damaged provide a feasible measurement of neurologic injury in humans.26,27 Increased concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1 have been associated with increased severity of brain injury and poor neurologic outcomes in patients with traumatic head injuries and with cognitive impairment after critical illness,47,48 and increased plasma concentrations of S100B have been associated with septic encephalopathy and delirium during critical illness.9,49 The temporal relationships among these markers and delirium and the effect modification of S100B on the association between F2-isoprostanes and isofurans and ubiquitin carboxyl-terminal hydrolase isozyme L1 in the current study provide evidence that intraoperative systemic oxidative stress may increase neuronal injury and lead to delirium. Delirium, therefore, may be a behavioral manifestation of oxidative damage-induced neuronal injury, and treatments that decrease oxidative damage may be useful to decrease the development of delirium in patients. This warrants further study. To that end, we are currently conducting a clinical trial to further study this mechanism by randomly assigning cardiac surgery patients to intraoperative normoxia or hyperoxia and assessing for oxidative damage and organ injury.50
This study has noteworthy strengths and limitations. Specifically, trained research staff prospectively collected delirium outcomes in the setting of a clinical trial; we measured markers of oxidative stress, neuronal injury, and blood-brain barrier disruption at numerous perioperative time points representing nearly 4000 samples; and the large cohort included multiple types of cardiac surgery, increasing the generalizability of the results. The observational design of the study, however, prevents determinations of causality and is subject to unknown confounding, although we were able to adjust for several important potential baseline and hospital course confounders. In addition, we did not directly measure cellular events in the brain but relied upon well-validated surrogate measurements of the injury pathways. We chose these specific plasma markers because previous studies have shown that these markers reflect the processes – oxidative damage, neuronal injury, and blood-brain barrier disruption – we sought to quantify. These markers, however, are not without limitation. Plasma concentrations of ubiquitin carboxyl-terminal hydrolase isozyme L1, for example, reflect neuronal injury but require translocation of ubiquitin carboxyl-terminal hydrolase isozyme L1 from the brain to the systemic circulation via passive and active processes. Plasma concentrations of S100 calcium binding protein B may also be affected by similar efflux mechanisms,24 also increase when there is brain injury,51 and may be affected by extracranial sources.31,33 Each of these characteristics is a potential limitation to examining S100 calcium binding protein B as a marker of blood-brain barrier disruption. The increased magnitude of the association between intraoperative oxidative damage and postoperative ubiquitin carboxyl-terminal hydrolase isozyme L1 that was observed in patients with elevated S100 calcium binding protein B at the end of surgery (i.e., effect modification), however, supports the conclusions that blood-brain barrier disruption is associated with an increased correlation between oxidative stress and neuronal injury and that these plasma biomarkers are not measuring the same process. These observations are temporally valid and biologically plausible. Studies using cerebrospinal fluid, although difficult in most surgical populations, or a panel approach to assess neuron-enriched proteins in plasma could provide additional valuable data.
In conclusion, intraoperative oxidative damage was independently associated with the development of postoperative delirium and with postoperative neuronal injury. Increased blood-brain barrier disruption modified the association between increased oxidative damage and increased neuronal injury and also was associated with the development of delirium. These findings support the idea that postoperative delirium may, in part, be a result of neuronal injury and that intraoperative oxidative damage may affect this process. Studies that target intraoperative oxidative damage, blood-brain barrier disruption, and neuronal injury are needed to further evaluate the relationships between these mechanisms of delirium and to determine if reducing intraoperative oxidative damage decreases postoperative brain dysfunction and injury.
Supplementary Material
Acknowledgments:
We acknowledge Patricia Hendricks, RN, from the VUMC Dept. of Anesthesiology (Nashville, TN, USA) for nursing support.
Funding Statement: MGL received funding from the Foundation for Anesthesia Education and Research (Schaumberg, IL, USA) and the U.S. NIH (Bethesda, MD, USA) grant K23GM129662. FTB received funding from NIH grants K23GM102676 and R01GM112871.We acknowledge support from the Vanderbilt Institute for Clinical and Translational Research which is supported by NIH UL1TR000445.
Footnotes
Prior presentations: A portion of these data were presented as an abstract at the Society of Critical Care Medicine 2017 Congress (Honolulu, HI, USA).
Conflicts of interest: The authors report no conflicts of interest.
REFERENCES
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG, Nomenclature Consensus Working Group: Recommendations for the Nomenclature of Cognitive Change Associated with Anaesthesia and Surgery-2018. Anesthesiology 2018; 129: 872–879 [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr., Inouye SK, Bernard GR, Dittus RS Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004; 291: 1753–62 [DOI] [PubMed] [Google Scholar]
- 3.Brown CHt, Probert J, Healy R, Parish M, Nomura Y, Yamaguchi A, Tian J, Zehr K, Mandal K, Kamath V, Neufeld KJ, Hogue CW: Cognitive Decline after Delirium in Patients Undergoing Cardiac Surgery. Anesthesiology 2018; 129: 406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown CH: Delirium in the cardiac surgical ICU. Curr Opin Anaesthesiol 2014; 27: 117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver CN, Starke-Reed PE, Stadtman ER, Liu GJ, Carney JM, Floyd RA: Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A 1990; 87: 5144–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez MG, Pandharipande P, Morse J, Shotwell MS, Milne GL, Pretorius M, Shaw AD, Roberts LJ, 2nd, Billings FTt: Intraoperative cerebral oxygenation, oxidative injury, and delirium following cardiac surgery. Free Radic Biol Med 2017; 103: 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penna A, Wang DS, Yu J, Lecker I, Brown PM, Bowie D, Orser BA: Hydrogen peroxide increases GABAA receptor-mediated tonic current in hippocampal neurons. J Neurosci 2014; 34: 10624–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Gao J, Guo G, Li S, Zhan G, Xie Z, Yang C, Luo A: Anesthesia and surgery induce delirium-like behavior in susceptible mice: the role of oxidative stress. Am J Transl Res 2018; 10: 2435–2444 [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes CG, Pandharipande PP, Thompson JL, Chandrasekhar R, Ware LB, Ely EW, Girard TD: Endothelial Activation and Blood-Brain Barrier Injury as Risk Factors for Delirium in Critically Ill Patients. Crit Care Med 2016; 44: e809–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadiiska MB, Gladen BC, Baird DD, Germolec D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, Brot N, Fitzgerald GA, Floyd RA, George M, Heinecke JW, Hatch GE, Hensley K, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ 2nd, Rokach J, Shigenaga MK, Sohal RS, Sun J, Tice RR, Van Thiel DH, Wellner D, Walter PB, Tomer KB, Mason RP, Barrett JC: Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med 2005; 38: 698–710 [DOI] [PubMed] [Google Scholar]
- 11.Billings IV FT, Pretorius M, Schildcrout JS, Mercaldo ND, Byrne JG, Ikizler TA, Brown NJ: Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol 2012; 23: 1221–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts LJ, Morrow JD: Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 2000; 28: 505–13 [DOI] [PubMed] [Google Scholar]
- 13.Milne GL, Sanchez SC, Musiek ES, Morrow JD: Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc 2007; 2: 221–6 [DOI] [PubMed] [Google Scholar]
- 14.Billings IV FT, Hendricks PA, Schildcrout JS, Shi Y, Petracek MR, Byrne JG, Brown NJ: High-Dose Perioperative Atorvastatin and Acute Kidney Injury Following Cardiac Surgery: A Randomized Clinical Trial. JAMA 2016; 315: 877–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R: Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286: 2703–10 [DOI] [PubMed] [Google Scholar]
- 16.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK: The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–44 [DOI] [PubMed] [Google Scholar]
- 17.van Eijk MM, van Marum RJ, Klijn IA, de Wit N, Kesecioglu J, Slooter AJ: Comparison of delirium assessment tools in a mixed intensive care unit. Crit Care Med 2009; 37: 1881–5 [DOI] [PubMed] [Google Scholar]
- 18.Neufeld KJ, Leoutsakos JS, Sieber FE, Joshi D, Wanamaker BL, Rios-Robles J, Needham DM: Evaluation of two delirium screening tools for detecting post-operative delirium in the elderly. Br J Anaesth 2013; 111: 612–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avidan MS, Maybrier HR, Abdallah AB, Jacobsohn E, Vlisides PE, Pryor KO, Veselis RA, Grocott HP, Emmert DA, Rogers EM, Downey RJ, Yulico H, Noh GJ, Lee YH, Waszynski CM, Arya VK, Pagel PS, Hudetz JA, Muench MR, Fritz BA, Waberski W, Inouye SK, Mashour GA, Group PR: Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017; 390: 267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, Katznelson R: Dexmedetomidine versus Propofol Sedation Reduces Delirium after Cardiac Surgery: A Randomized Controlled Trial. Anesthesiology 2016; 124: 362–8 [DOI] [PubMed] [Google Scholar]
- 21.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN: Cognitive trajectories after postoperative delirium. N Engl J Med 2012; 367: 30–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ, 2nd: Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc Natl Acad Sci U S A 2002; 99: 16713–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graham SH, Liu H: Life and death in the trash heap: The ubiquitin proteasome pathway and UCHL1 in brain aging, neurodegenerative disease and cerebral Ischemia. Ageing Res Rev 2017; 34: 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M: Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci 2015; 35: 518–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiber H, Peter JB: Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci 2001; 184: 101–22 [DOI] [PubMed] [Google Scholar]
- 26.Papa L, Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, Zheng W, Robinson G, Robicsek SA, Gabrielli A, Heaton SC, Hannay HJ, Demery JA, Brophy GM, Layon J, Robertson CS, Hayes RL, Wang KK: Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit Care Med 2010; 38: 138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnaoutakis GJ, George TJ, Wang KK, Wilson MA, Allen JG, Robinson CW, Haggerty KA, Weiss ES, Blue ME, Talbot CC, Jr., Troncoso JC, Johnston MV, Baumgartner WA: Serum levels of neuron-specific ubiquitin carboxyl-terminal esterase-L1 predict brain injury in a canine model of hypothermic circulatory arrest. J Thorac Cardiovasc Surg 2011; 142: 902–910 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A, Stocklein V, Bazarian JJ: Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma 2009; 26: 1497–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, Stevens GH, Masaryk T, Aumayr B, Vogelbaum MA, Barnett GH, Janigro D: Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer 2003; 97: 2806–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchi N, Rasmussen P, Kapural M, Fazio V, Kight K, Mayberg MR, Kanner A, Ayumar B, Albensi B, Cavaglia M, Janigro D: Peripheral markers of brain damage and blood-brain barrier dysfunction. Restor Neurol Neurosci 2003; 21: 109–21 [PMC free article] [PubMed] [Google Scholar]
- 31.Unden J, Bellner J, Eneroth M, Alling C, Ingebrigtsen T, Romner B: Raised serum S100B levels after acute bone fractures without cerebral injury. J Trauma 2005; 58: 59–61 [DOI] [PubMed] [Google Scholar]
- 32.Cata JP, Abdelmalak B, Farag E: Neurological biomarkers in the perioperative period. Br J Anaesth 2011; 107: 844–58 [DOI] [PubMed] [Google Scholar]
- 33.Pham N, Fazio V, Cucullo L, Teng Q, Biberthaler P, Bazarian JJ, Janigro D: Extracranial sources of S100B do not affect serum levels. PLoS One 2010; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrell FE: Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York, Springer, 2001 [Google Scholar]
- 35.Needham DM, Colantuoni E, Dinglas VD, Hough CL, Wozniak AW, Jackson JC, Morris PE, Mendez-Tellez PA, Ely EW, Hopkins RO: Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med 2016; 4: 203–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page VJ, Casarin A, Ely EW, Zhao XB, McDowell C, Murphy L, McAuley DF: Evaluation of early administration of simvastatin in the prevention and treatment of delirium in critically ill patients undergoing mechanical ventilation (MoDUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2017; 5: 727–737 [DOI] [PubMed] [Google Scholar]
- 37.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB: The neuroinflammatory hypothesis of delirium. Acta Neuropathol 2010; 119: 737–54 [DOI] [PubMed] [Google Scholar]
- 38.Hala M: Pathophysiology of postoperative delirium: systemic inflammation as a response to surgical trauma causes diffuse microcirculatory impairment. Med Hypotheses 2007; 68: 194–6 [DOI] [PubMed] [Google Scholar]
- 39.Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC: Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc 2010; 85: 18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP: Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology 2018; 129: 829–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW: Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298: 2644–53 [DOI] [PubMed] [Google Scholar]
- 42.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A: Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 2001; 88: 529–35 [DOI] [PubMed] [Google Scholar]
- 43.Caputo M, Mokhtari A, Rogers CA, Panayiotou N, Chen Q, Ghorbel MT, Angelini GD, Parry AJ: The effects of normoxic versus hyperoxic cardiopulmonary bypass on oxidative stress and inflammatory response in cyanotic pediatric patients undergoing open cardiac surgery: a randomized controlled trial. J Thorac Cardiovasc Surg 2009; 138: 206–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT: The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signal 2004; 6: 954–66 [DOI] [PubMed] [Google Scholar]
- 45.Fukunaga M, Makita N, Roberts LJ, 2nd, Morrow JD, Takahashi K, Badr KF: Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am J Physiol 1993; 264: C1619–24 [DOI] [PubMed] [Google Scholar]
- 46.Zheng YB, Ruan GM, Fu JX, Su ZL, Cheng P, Lu JZ: Postoperative plasma 8-iso-prostaglandin F2alpha levels are associated with delirium and cognitive dysfunction in elderly patients after hip fracture surgery. Clin Chim Acta 2016; 455: 149–53 [DOI] [PubMed] [Google Scholar]
- 47.Hughes CG, Patel MB, Brummel NE, Thompson JL, McNeil JB, Pandharipande PP, Jackson JC, Chandrasekhar R, Ware LB, Ely EW, Girard TD: Relationships between markers of neurologic and endothelial injury during critical illness and long-term cognitive impairment and disability. Intensive Care Med 2018; 44: 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welch RD, Ayaz SI, Lewis LM, Unden J, Chen JY, Mika VH, Saville B, Tyndall JA, Nash M, Buki A, Barzo P, Hack D, Tortella FC, Schmid K, Hayes RL, Vossough A, Sweriduk ST, Bazarian JJ: Ability of Serum Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase-L1, and S100B To Differentiate Normal and Abnormal Head Computed Tomography Findings in Patients with Suspected Mild or Moderate Traumatic Brain Injury. J Neurotrauma 2016; 33: 203–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Munster BC, Korevaar JC, Korse CM, Bonfrer JM, Zwinderman AH, de Rooij SE: Serum S100B in elderly patients with and without delirium. Int J Geriatr Psychiatry 2010; 25: 234–9 [DOI] [PubMed] [Google Scholar]
- 50.Lopez MG, Pretorius M, Shotwell MS, Deegan R, Eagle SS, Bennett JM, Sileshi B, Liang Y, Gelfand BJ, Kingeter AJ, Siegrist KK, Lombard FW, Richburg TM, Fornero DA, Shaw AD, Hernandez A, Billings FT: The Risk of Oxygen during Cardiac Surgery (ROCS) trial: study protocol for a randomized clinical trial. Trials 2017; 18: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thelin EP, Nelson DW, Bellander BM: A review of the clinical utility of serum S100B protein levels in the assessment of traumatic brain injury. Acta Neurochir (Wien) 2017; 159: 209–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.