Abstract
Objective:
Frascati international research criteria for HIV-associated neurocognitive disorders (HAND) are controversial; some investigators have argued that Frascati criteria are too liberal, resulting in a high false positive rate. Meyer et al. (2013) recommended more conservative revisions to HAND criteria, including exploring other commonly used methodologies for neurocognitive impairment in HIV including the global deficit score (GDS). This study compares neurocognitive impairment classifications by Frascati, Meyer, and GDS methods, in relation to neuroimaging markers of brain integrity in HIV.
Method:
241 persons living with HIV (PLWH) without current substance use disorder or severe (confounding) comorbid conditions underwent comprehensive neurocognitive testing and brain structural MRI and MR spectroscopy. Participants were classified using Frascati criteria vs. Meyer criteria: concordant unimpaired (Frascati(Un)/Meyer(Un)), concordant impaired (Frascati(Imp)/Meyer(Imp)), or discordant (Frascati(Imp)/Meyer(Un)) which were impaired via Frascati criteria but unimpaired via Meyer criteria. To investigate the GDS vs. Meyer criteria, the same groupings were utilized using GDS criteria instead of Frascati criteria.
Results:
When examining Frascati vs. Meyer criteria, discordant Frascati(Imp)/Meyer(Un) individuals had less cortical gray matter, greater sulcal CSF volume, and greater evidence of neuroinflammation (i.e., choline) than concordant Frascati(Un)/Meyer(Un) individuals. GDS vs. Meyer comparisons indicated that discordant GDS(Imp)/Meyer(Un) individuals had less cortical gray matter and lower levels of energy metabolism (i.e., creatine) than concordant GDS(Un)/Meyer(Un) individuals. In both sets of analyses, the discordant group did not differ from the concordant impaired group on any neuroimaging measure.
Conclusions:
The Meyer criteria failed to capture a substantial portion of PLWH with brain abnormalities. These findings support continued use of Frascati or GDS criteria to detect HIV-associated CNS dysfunction.
Keywords: MRI, MRS, cognition, infectious disease, HIV-associated neurocognitive disorders, Frascati Criteria
Introduction
Since the introduction of combination antiretroviral therapy (cART), life expectancies among people living with HIV (PLWH) have increased while rates of medical morbidity and mortality have decreased. However, HIV-associated Neurocognitive Disorders (HAND), especially milder forms of neurocognitive impairment (NCI), have persisted in the cART era (Heaton et al., 2011). Frascati criteria, the current international research nosology for HAND, were developed to meet the need for updated, operationalized classification methods, especially as literature emerged showing that even mild NCI in HIV was associated with a range of unfavorable functional outcomes (Antinori et al., 2007). Using Frascati criteria, HAND prevalence ranges from 30-50%, depending upon comorbidities and HIV disease treatment histories (Heaton et al., 2010; Saloner & Cysique, 2017). The majority of those with HAND are classified with mild-to-moderate impairment (>1 SD below the mean in 2 cognitive domains), which is further divided into Asymptomatic Neurocognitive Impairment (ANI; meeting criteria for cognitive, but not functional decline) and Mild Neurocognitive Disorder (MND; meeting criteria for both cognitive and functional decline; Antinori et al. 2007).
There remains controversy regarding Frascati criteria’s clinical applicability. Some investigators have proposed that the Frascati criteria for milder HAND are too liberal, resulting in high false positive classifications, overestimation of HAND, and undue distress for patients (Gisslén, Price, & Nilsson, 2011; Meyer, Boscardin, Kwasa, & Price, 2013; Torti, Focà, Cesana, & Lescure, 2011). Consequently, Meyer et al. (2013) advocate for stricter cognitive impairment cut-offs (1.5 SD below the mean) and a smaller test battery (i.e., 3-5 domains versus 7 domains). Although false positive misclassifications may result in adverse consequences, detecting HIV-associated neurological dysfunction at its earliest point may be important for early intervention. For example, detection of early CNS dysfunction may allow for cognitive neurorehabilitation interventions and mitigate risk for NCI-related adverse functional outcomes (Iudicello, Hussain, Watson, Morgan, & Heaton, in press). Additionally, constraining cutoffs to 1.5 SD below the mean may result in a loss of sensitivity for detecting brain disorders (Heaton, Miller, Taylor, & Grant, 2004; Heaton et al., 2004) and the probability that PLWH with clinically significant neurocognitive difficulties may be overlooked.
An alternate, slightly more conservative approach relative to Frascati for detecting NCI in PLWH is the Global Deficit Score (GDS; Carey et al., 2004) method. The GDS, an objective, algorithmic approach to classifying NCI, is often used in research settings and has demonstrated reliability in detecting NCI in HIV (Cysique et al., 2010; Heaton et al., 1995; Kabuba, Menon, Franklin, Heaton, & Hestad, 2017; Kanmogne et al., 2010) and is related to functional- and health-related outcomes (Andrade et al., 2013; Hinkin et al., 2004). The GDS is a weighted average of neuropsychological test deficit scores ranging from 0 (no impairment) to 5 (severe impairment); therefore, it considers both number and severity of deficits. Blackstone et al. (2012) illustrated that GDS-determined NCI nearly guarantees meeting Frascati criteria for mild NCI, though the opposite is not necessarily true. Additionally, those impaired using only Frascati criteria had less neurocognitive and functional deficits than those impaired using the GDS methodology, further suggesting the GDS approach is more conservative than Frascati criteria.
In addition to neurocognitive assessment, non-invasive magnetic resonance imaging (MRI) techniques have enhanced understanding of HIV-related brain abnormalities and may have potential in diagnosing HAND, as there is no reliable biomarker correlate of HAND. Structural MRI studies report cortical and subcortical gray matter atrophy and white matter atrophy as well as increased abnormal white matter volume, and these findings have persisted into the cART era (Becker et al., 2012; Kallianpur et al., 2013; Nichols et al., 2019). With respect to cognitive functioning, greater brain atrophy correlates with widespread neurocognitive deficits (Alakkas et al., 2018; Bonnet et al., 2013; Cohen et al., 2010; Patel et al., 2002; Thames et al., 2012). Magnetic resonance spectroscopy (MRS) studies demonstrate cerebral metabolic abnormalities indicative of neuroinflammation (e.g., elevated choline and myo-inositol) and neuronal loss (e.g., reduced N-acetyl-aspartate and glutamate) across cortical and subcortical tissues (Chang et al., 2004; Cysique et al., 2013; Ernst, Jiang, Nakama, Buchthal, & Chang, 2010; Harezlak et al., 2011; Paul et al., 2008). PLWH with the greatest evidence of neuroinflammation and neuronal loss are at higher risk for HAND, with decrements executive function, working memory, learning, recall, and complex motor skills (Ernst et al., 2010; Mohamed et al., 2010; Mohamed, Barker, Skolasky, & Sacktor, 2018; Paul et al., 2008). Given these findings, some authors have advocated for the inclusion of neuroimaging biomarkers in HAND diagnostic decision-making (Ances & Hammoud, 2014; Masters & Ances, 2014; Saloner & Cysique, 2017).
Those who have criticized current HAND nosology suggest Frascati criteria overdiagnose HAND, potentially resulting in a decrease in power to detect associations between HAND and imaging markers (Meyer et al., 2013; Torti et al., 2011). However, analyses of neuroimaging data have not been reported. Therefore, the current study aims to examine structural and metabolite brain imaging markers in PLWH who are impaired via Frascati criteria but unimpaired via Meyer criteria compared to those that are concordantly unimpaired and concordantly impaired. Additionally, Meyer et al. (2013) recommended exploring other commonly-used classifications, including the GDS; therefore, we also examined those that are impaired via GDS but unimpaired via Meyer criteria compared to those that are concordanly unimpaired or concordantly impaired. These findings may help determine if discrepantly classified PLWH exhibit neuroanatomical profiles that more closely resemble the concordantly impaired or the concordanly unimpared groups.
Methods
Participants
The present cross-sectional study included 241 PLWH in the NIH-funded CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) study who completed comprehensive neuropsychological testing, neuromedical assessment, and high-resolution multi-channel structural MRI and MRS between 2004 and 2007 (Jernigan et al., 2011). Participants were excluded from analysis if there was evidence of severe confounding comorbid conditions as described in Antinori et al. (2007) and Heaton et al. (2010), current substance use disorder, or insufficient neuropsychological data. Additionally, one participant was excluded for meeting criteria for Meyer impairment but being classified as unimpaired via Frascati criteria (see Supplemental Figure 1).
Participants were recruited and examined at one of five sites: Johns Hopkins University (Baltimore, MD, n = 38); Icahn School of Medicine at Mount Sinai (New York, NY, n = 56); University of California, San Diego (San Diego, CA, n = 68); University of Texas Medical Branch (Galveston, TX, n = 53); and University of Washington (Seattle, WA, n = 26). The procedures were approved by the Institutional Review Board at each university, and all participants provided written informed consent.
Neuropsychological Assessment
Participants completed comprehensive neuropsychological testing, which assesses seven cognitive domains commonly affected by HIV (verbal fluency, working memory, speed of information processing, executive functioning, learning, recall, and motor). Specific tests are described in detail in Heaton et al., 2010. Raw test scores were converted to T-scores corrected for the effects of age, education, sex, and race (Heaton et al., 2004; Heaton, Taylor, & Manly, 2003; Norman et al., 2011).
Clinical Ratings (Frascati Criteria).
As referenced in the Antinori et al. (2007) presentation of Frascati criteria, clinical ratings (CR) are described in detail by Woods et al. (2004). Demographically corrected T-scores were not averaged per domain to obtain a domain T-score, which would allow a good score on one test to obscure or offset a very poor score on another test. Instead, more weight is given to the most impaired scores per domain: if there are two domain scores at that level, this defines the level of the domain, whereas if there is only one score at that level the domain rating is one rating score less (Woods et al., 2004). Tests and domains were then assigned a CR, using a 9-point scale ranging from 1 (i.e., above average functioning) to 9 (i.e., severe impairment). Performance in a domain is considered to be impaired if the domain CR is greater than or equal to 5 (CR of 5 is defined as “mild impairment” and is equivalent to a T-score of 35-39). A participant was classified with HAND if at least two domains of the seven domains assessed were in the impaired range (i.e., CR≥5). However, if learning and memory were the only impaired domains, the participant had to demonstrate impaired recall retention (i.e., forgetting or impaired retrieval) as well as impaired learning, in order to avoid double-penalization for learning deficits. The CR methodology operationalizes and is consistent with current Frascati criteria for classifying HAND (Antinori et al., 2007).
The 13-item Lawton-Brody ADL questionnaire (Lawton & Brody, 1969) was used to assess functional impairment (versus best level of prior functioning) in performing instrumental activities of daily living. The 33-item Patient’s Assessment of Own Functioning (PAOFI; Chelune, Heaton, & Lehman, 1986) was used to assess current cognitive difficulties in everyday life. Functional decline was defined as at least two types of evidence of decreased everyday functioning. Those who were classified as impaired using Frascati CR guidelines were classified with ANI, MND, or HIV-associated dementia (HAD). In line with Frascati criteria described in Antinori et at. (2007), those demonstrating mild NCI (i.e., at least 1 SD below the mean in at least 2 cognitive domains) but not endorsing functional impairment were classified as ANI, those demonstrating mild NCI and also endorsing decline in functional abilities were classified as MND, and those with major NCI (i.e., at least 2 SD below the mean in at least 2 cognitive domains) and more severe functional decline were classified as HAD.
Meyer Criteria.
Meyer et al. (2013) recommend analyses be limited to three to five cognitive domains, the threshold for impairment be lowered to less than or equal to −1.5 standard deviations below the demographically-corrected mean, and the average domain score be used to define an “abnormal” domain. Meyer et al. (2013) do not recommend which domains be removed from consideration; we selected verbal fluency and speed of information processing as these domains show the lowest prevalence of impairment in HIV (Heaton et al 2011), leaving 5 cognitive domains (learning, delayed recall, working memory, complex motor, and executive function). Individual test T-scores were averaged in each domain to create domain T-scores, and a participant was classified as impaired via Meyer criteria if at least two domains had an average T-score of less than or equal to 35 (equivalent to Meyer’s recommendations of ≤ −1.5 standard deviations below the average domain score). Participants were categorized into three groups for Frascati CR vs. Meyer analyses: 1) a dually unimpaired group (Frascati(Un)/Meyer(Un)): unimpaired by Frascati CR and Meyer criteria; 2) Frascati CR-only impairment (Frascati(Imp)/Meyer(Un)): impaired by Frascati CR but unimpaired by Meyer criteria; and 3) a dually impaired group (Frascati(Imp)/Meyer(Imp)): impaired by Frascati CR and Meyer criteria.
Global Deficit Score (GDS).
To generate the GDS, individual test T-scores were first converted to deficit scores, which range from 0 (T-score ≥ 40; unimpaired) to 5 (T < 20; severely impaired). Deficit scores were averaged across all tests in the seven domains assessed to obtain a global deficit score (GDS) and global impairment was defined by a GDS≥0.5. To compare patterns of impairment by domain, tests in each domain were averaged to obtain a domain deficit score (DDS); an impaired domain was defined as a DDS>0.5. These cut-offs are commonly utilized and have previously been shown to yield the best sensitivity to specificity ratio (Carey et al., 2004; Heaton et al., 2004). Participants were categorized into three groups for GDS vs. Meyer analyses: 1) a dually unimpaired group (GDS(Un)/Meyer(Un)): unimpaired by GDS and Meyer criteria; 2) GDS-only impairment (GDS(Imp)/Meyer(Un)): impaired by GDS but unimpaired by Meyer criteria; and 3) a dually impaired group (GDS(Imp)/Meyer(Imp)): impaired using both Meyer and GDS criteria.
Cognitive Reserve Variables.
Participants also were administered the Reading Subtest from the Wide Range Achievement Test-3 (WRAT; Wilkinson, 1993). The WRAT is a reliable estimator of premorbid IQ, despite cognitive decline associated with HIV-infection (Casaletto et al., 2014; Olsen et al., 2015). Additionally, the Hollingshead Index of Social Status (Hollingshead, 1975) was calculated using a weighted average of educational and occupational attainment.
Neuromedical and Psychiatric Assessment
Medical history and HIV disease history were gathered via standardized neuromedical evaluation. Common medical comorbidities listed in Table 1 and Table 2 were determined by self-report or taking medication for the condition. Psychiatric comorbidities were assessed using the Composite International Diagnostic Interview (World Health Organization, 1997), which is a computer-based, fully-structured interview to assess lifetime and current affective and substance use disorders in line with the DSM-IV. A senior clinician (R.K.H.) reviewed all medical, educational, and psychiatric histories to classify each participant into comorbidity subgroups as defined by Antinori et al. (2007) and Heaton et al. (2010). Participants with severe comorbidities were classified as “confounded” and were excluded from this study. Participants with mild-to-moderate comorbidities were classified as having “contributing” comorbidities and were included in this study. These mild-to-moderate comorbidities could have contributed to NCI, but the timing or severity of the NCI suggests an HIV component. The remainder had no more than minimal comorbidities that were considered “incidental” to a HAND diagnosis.
Table 1.
Participant Characteristics by Clinical Rating (Frascati) vs. Meyer Impairment Grouping
| Frascati(Un)/Meyer(Un) (U/U; n=144) |
Frascati(Imp)/Meyer(Un) (I/U; n=64) |
Frascati(Imp)/Meyer(Imp) (I/I; n=33) |
p | Pair-wise comparisons1 |
|
|---|---|---|---|---|---|
|
Median [IQR], Mean
(SD), or n (%) |
|||||
| Demographics | |||||
| Age (years) | 46.0 (8.3) | 43.1 (7.6) | 44.6 (7.4) | 0.10 | |
| Gender (male) | 120 (83.3%) | 49 (76.6%) | 25 (75.8%) | 0.40 | |
| Ethnicity | -- | -- | -- | 0.06 | |
| Non-Hispanic White | 60 (41.7%) | 25 (39.1%) | 16 (48.5%) | -- | |
| African American | 72 (50.0%) | 28 (43.8%) | 9 (27.3%) | -- | |
| Hispanic | 10 (6.9%) | 10 (15.6%) | 8 (24.2%) | -- | |
| Other | 2 (1.4%) | 1 (1.6%) | 0 (0.0%) | -- | |
| Education (years) | 12.9 (2.3) | 12.9 (2.6) | 14.2 (2.3) | 0.02 | I/I > I/U, U/U |
| WRAT-3 Reading | 97.0 (13.2) | 93.0 (13.2) | 93.2 (14.0) | 0.10 | |
| Hollingshead2 | 40.4 (10.7) | 40.3 (11.5) | 38.4 (13.4) | 0.63 | |
| Employed | 50 (34.7%) | 17 (26.6%) | 13 (39.4%) | 0.37 | |
| HIV Disease Characteristics | |||||
| History of AIDS | 97 (67.4%) | 43 (67.2%) | 24 (72.7%) | 0.82 | |
| Detectable viral load3 | 73 (50.7%) | 30 (47.6%) | 13 (40.6%) | 0.58 | |
| Current CD4 count | 442 [266 – 611] | 463 [329 – 630] | 516 [268 – 667] | 0.55 | |
| Nadir CD4 count | 150 [30 – 300] | 172 [19 – 277] | 109 [28 – 224] | 0.50 | |
| Estimated duration of infection (years) | 11.0 [5.8 – 15.6] | 13.3 [4.8 – 17.0] | 12.7 [6.2 – 15.3] | 0.47 | |
| On ART | 107 (74.3%) | 50 (78.1%) | 31 (93.9%) | 0.05 | |
| Failing ART (% detectable on ART) | 38 (35.5%) | 18 (36.0%) | 11 (36.7%) | 0.99 | |
| Medical Comorbidities and Characteristics | |||||
| Hypertension4 | 20 (14.6%) | 13 (21.0%) | 9 (28.1%) | 0.16 | |
| Hyperlipidemia4 | 12 (8.8%) | 9 (14.5%) | 5 (15.6%) | 0.25 | |
| Hepatitis C4 | 41 (29.9%) | 18 (29.0%) | 9(28.1%) | 0.98 | |
| Diabetes4 | 14 (10.2%) | 9 (14.5%) | 4 (12.5%) | 0.67 | |
| Psychiatric Diagnoses | |||||
| LT MDD | 80 (55.6%) | 37 (57.8%) | 19 (57.6%) | 0.95 | |
| Current MDD5 | 14 (9.7%) | 8 (12.7%) | 1 (3.0%) | 0.31 | |
| LT SUD | 111 (77.1%) | 46 (71.9%) | 19 (57.6%) | 0.07 | |
| LT alcohol | 83 (57.6%) | 32 (50.0%) | 14 (42.4%) | 0.23 | |
| LT cannabis | 42 (29.2%) | 17 (26.6%) | 7 (21.2%) | 0.64 | |
| LT methamphetamine | 25 (17.4%) | 11 (17.2%) | 5 (15.2%) | 0.95 | |
| Contributing Diagnoses | 35 (24.3%) | 20 (31.3%) | 18 (54.6%) | <0.01 | I/I > U/U |
Note. Frascati(Un)/Meyer(Un)=Unimpaired by both criteria; Frascati(Imp)/Meyer(Un)= Impaired by Frascati CR criteria and Unimpaired by Meyer; Frascati(Imp)/Meyer(Imp)=Impaired by both criteria. WRAT=Wide Range Achievement Test, 3rd edition; ART = antiretroviral therapy; BMI= body mass index; LT = lifetime; MDD = major depressive disorder; SUD = substance use disorder;
Pair-wise comparisons were examined using Tukey’s H.S.D. (α = 0.05) for continuous outcomes and Bonferroni-adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes
n=236;
n=239;
n=231;
n=240
Table 2.
Participant Characteristics by GDS vs. Meyer Grouping
| GDS(Un)/Meyer(Un) (U/U; n=172) |
GDS(Imp)/Meyer(Un) (I/U; n=36) |
GDS(Imp)/Meyer(Imp) (I/I; n=33) |
p | Pair-wise comparisons1 |
|
|---|---|---|---|---|---|
| Median [IQR], Mean (SD), or n (%) | |||||
| Demographics | |||||
| Age (years) | 43.4 (7.8) | 44.4 (6.1) | 44.6 (7.4) | 0.17 | |
| Gender (male) | 141 (82.0%) | 28 (77.8%) | 25 (75.8%) | 0.64 | |
| Ethnicity | -- | -- | -- | 0.09 | |
| Non-Hispanic White | 69 (40.1%) | 16 (44.4%) | 16 (48.5%) | -- | |
| African American | 85 (49.4%) | 15 (41.7%) | 9 (27.3%) | -- | |
| Hispanic | 15 (8.7%) | 5 (13.9%) | 8 (24.2%) | -- | |
| Other | 3 (1.7%) | 0 (0.0%) | 0 (0.0%) | -- | |
| Education (years) | 12.8 (2.4) | 13.3 (2.5) | 14.2 (2.3) | <0.01 | I/I > U/U |
| WRAT | 96.6 (13.0) | 91.7 (16.4) | 93.2 (14.0) | 0.09 | |
| Hollingshead2 | 40.5 (10.7) | 39.9 (12.2) | 38.4 (13.4) | 0.60 | |
| Employed | 56 (32.6%) | 11 (30.6%) | 13 (39.3%) | 0.70 | |
| HIV Disease Characteristics | |||||
| History of AIDS | 115 (66.9%) | 25 (69.4%) | 24 (72.7%) | 0.79 | |
| Detectable viral load3 | 86 (50.3%) | 17 (47.2%) | 13 (40.6%) | 0.60 | |
| Current CD4 count | 450 [267 – 621] | 477 [359 – 630] | 516 [268 – 667] | 0.35 | |
| Nadir CD4 count | 150 [36 – 300] | 155 [14 – 277] | 109 [28 – 224] | 0.36 | |
| Estimated duration of infection (years) | 11.2 [5.7 – 15.6] | 14.3 [5.0 – 17.9] | 12.7 [6.2 – 15.3] | 0.40 | |
| On ART | 127 (73.8%) | 30 (83.3%) | 31 (93.9%) | 0.03 | I/I > U/U |
| Failing ART (% detectable on ART) | 45 (35.4%) | 11 (36.7%) | 11 (36.7%) | 0.99 | |
| Medical Comorbidities and Characteristics | |||||
| Hypertension4 | 24 (14.6%) | 9 (25.7%) | 9 (28.1%) | 0.09 | |
| Hyperlipidemia4 | 16 (9.8%) | 5 (14.3%) | 5 (15.6%) | 0.52 | |
| Hepatitis C4 | 50 (30.5%) | 9 (25.7%) | 9 (28.1%) | 0.84 | |
| Diabetes4 | 18 (11.0%) | 5 (14.3%) | 4 (12.5%) | 0.85 | |
| Psychiatric Diagnoses | |||||
| LT MDD | 95 (55.2%) | 22 (61.1%) | 19 (57.6%) | 0.80 | |
| Current MDD5 | 15 (8.7%) | 7 (20.0%) | 1 (3.0%) | 0.05 | |
| LT SUD | 131 (76.2%) | 26 (72.2%) | 19 (57.6%) | 0.09 | |
| LT alcohol | 98 (57.0%) | 17 (47.2%) | 14 (42.4%) | 0.22 | |
| LT cannabis | 51 (29.7%) | 8 (22.2%) | 7(21.2%) | 0.46 | |
| LT methamphetamine | 30 (17.4%) | 6 (16.7%) | 5 (15.2%) | 0.95 | |
| Contributing Diagnoses | 43 (25.0%) | 12(33.3%) | 18 (54.6%) | <0.01 | I/I > U/U |
Note. GDS(Un)/Meyer(Un)=Unimpaired by both Meyer and GDS criteria; GDS(Imp)/Meyer(Un)= Impaired by GDS criteria and Unimpaired by Meyer; GDS(Imp)/Meyer(Imp)=impaired by both criteria. WRAT=Wide Range Achievement Test, 3rd edition; ART = antiretroviral therapy; BMI= body mass index; LT = lifetime; MDD = major depressive disorder; SUD = substance use disorder;
Pair-wise comparisons were examined using Tukey’s H.S.D. (α = 0.05) for continuous outcomes and Bonferroni-adjustments (α = 0.05/3 = 0.0167) for dichotomous outcomes
n=236;
n=239;
n=231;
n=240
Nadir CD4+ T-cell count was estimated via a combination of self-report and medical records. Current CD4+ T-cell count was measured with flow cytometry, and HIV RNA level was measured by ultra-sensitive PCR (Amplicor, Roche Diagnostic System, Indianapolis IN; lower limit of detection <50 copies/ml) in a CLIA-certified clinical laboratory.
Neuroimaging Assessments
MRI data were acquired on six General Electric 1.5-Tesla scanners across five sites, annually reviewed for quality; because scanner differences (e.g., hardware upgrades) can influence neuroimaging measurements, we included a “scanner” variable in statistical analyses to account for these effects between sites (Fennema-Notestine et al., 2007) as in our prior work (Fennema-Notestine et al., 2013; Jernigan et al., 2011). Four series were acquired for structural morphometric analysis: coronal two-dimensional T2- and proton-density (PD)-weighted fast spin echo sequences (section thickness=2.0 mm), and three-dimensional sagittal T1- and PD-weighted spoiled gradient recalled acquisitions (section thickness=1.3 mm; as in Fennema-Notestine et al., 2013; Jernigan et al., 2011). MRS was performed using a standardized point-resolved spectroscopy protocol (echo time=35ms, repetition time=3000ms; as in Anderson et al., 2015).
Multi-channel Structural MRI.
As described previously (Fennema-Notestine et al., 2013; Jernigan et al., 2011), we used the multi-channel structural images in a semi-automated workflow to measure cortical and subcortical gray matter; total cerebral and abnormal (e.g., hyperintense regions on T2-weighted images) white matter; and ventricular and cerebral sulcal CSF, as well as supra-tentorial cranial vault volume to account for individual differences in head size. The workflow includes inspection for motion and other artifacts, re-slicing to a standard space, intra-subject mutual information registration, bias-correction with nonparametric nonuniformity normalization, removal of non-brain tissue, three-tissue segmentation (gray matter, white matter, and CSF), abnormal white matter designation, and anatomical labeling performed by trained anatomists. This approach includes the identification of cerebral white matter regions with abnormal MRI signal characteristics that are segmented as gray matter but anatomically located within the white matter.
Single-Voxel MRS.
As described previously (Anderson et al., 2015), three voxels were acquired: frontal gray matter (20×20×20mm and 64 acquisitions), frontal white matter (20×20×20mm and 64 acquisitions), and basal ganglia (20×20×15mm and 96 acquisitions). MRS concentrations of N-acetyl-aspartate, choline, myo-inositol, and creatine were quantified using LCModel with water suppression (Provencher, 2001). Water suppression allows for examination of absolute metabolite levels; although ratios to creatine have been commonly reported in the past for standardization across sites and studies, that approach is limited by evidence that HIV infection independently affects creatine levels directly, confounding the interpretation of ratio values and existing findings (as in Anderson et al., 2015; Jansen, Backes, Nicolay, & Kooi, 2006). Only metabolite estimates for adequate spectra (standard deviation<21) were used; therefore, sample size varied by MRS metabolite. Structural segmentation was used to estimate the proportion of relevant tissue volume within each MRS voxel (e.g., amount of gray matter in frontal gray matter voxel) to control for variability in individual sampling.
Statistical Analyses
First, ANOVAs or Kruskal-Wallis tests for continuous variables and Chi-square tests for categorical variables were used in order to compare both Frascati CR vs. Meyer and GDS vs. Meyer group differences on demographics, HIV disease characteristics, medical comorbidities, and psychiatric diagnoses. To control for multiple comparisons, Tukey’s Honest Significant Difference test was applied to ANOVA tests and Bonferroni correction was applied for Kruskal-Wallis and Chi-square tests.
Multivariable linear regression analyses were performed to examine the associations between group and log-transformed MRI structural volumes and MRS metabolites. Both age and scanner were included as covariates in every model. Additionally, supratentorial cranial vault volume was included in models predicting MRI structural volume analyses to control for differences in head size, and proportion of relevant tissue in each voxel was included in MRS analyses. Standardized beta coefficients are reported in text and tables. For significant findings, an effect size that is analogous to Cohen’s d was estimated by dividing the regression coefficient by the residual standard deviation. These analyses were run twice: once to compare groups using Frascati CR and Meyer criteria, and again to compare GDS and Meyer criteria. In all multivariable linear regression analyses, the Impaired/Unimpaired group was specified as the reference group to compare differences between the concordant impaired and concordant unimpaired groups. JMP version 13.0.0 was used for all analyses.
Results
Sample Characteristics
Most participants were non-Hispanic white men with an average age of 43.9 years and 13.1 years of education. 78% of participants were being prescribed antiretroviral therapy (ART) and 51% were virally suppressed (i.e., <50 copies/ml plasma).
Frascati CR vs. Meyer Criteria.
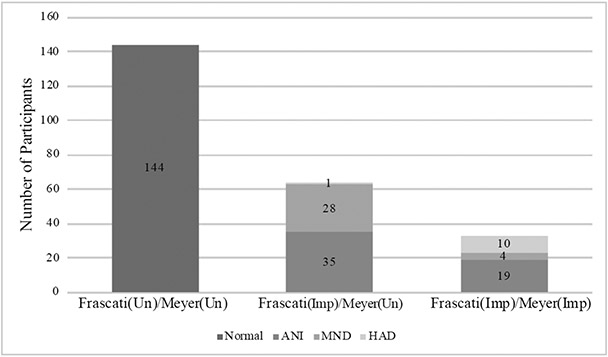
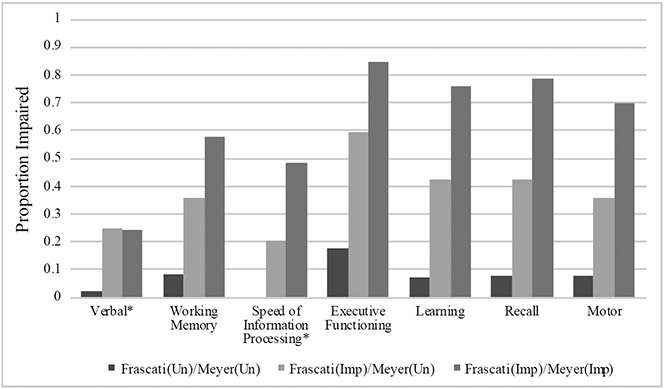
Participants’ characteristics by Frascati CR vs. Meyer Impairment Grouping are displayed in Table 1. The groups had similar demographics, HIV disease characteristics, medical comorbidities, and psychiatric diagnoses. However, after correcting for multiple comparisons, the Frascati(Imp)/Meyer(Imp) group had higher average education than both the Frascati(Un)/Meyer(Un) and Frascati(Imp)/Meyer(Un) groups, and the Frascati(Imp)/Meyer(Imp) group had a greater proportion classified with contributing comorbidities than the Frascati(Un)/Meyer(Un) group. Of note, the proportion of those demonstrating evidence of functional impairment and classified as MND or HAD (Figure 1) were similar in the Frascati(Imp)/Meyer(Un) group (45.3%) and Frascati(Imp)/Meyer(Imp) group (42.4%); put another way, 29.9% of the functionally impaired participants in the Frascati(Imp) group were classified as normal by the Meyer criteria. Proportion impaired, as defined by a CR of ≥5, in each cognitive domain by group are displayed in Figure 3. A stair-step pattern (Frascati(Un)/Meyer(Un) < Frascati(Imp)/Meyer(Un) < Frascati(Imp)/Meyer(Imp)) was observed for all domains except for verbal fluency in which the Frascati(Imp)/Meyer(Un) and Frascati(Imp)/Meyer(Imp) groups had similar rates of impairment.
Figure 1.
Distribution of subjects per group using Frascati Clinical Rating vs. Meyer Criteria.
Note. Frascati(Un)/Meyer(Un)=Unimpaired by both criteria; Frascati(Imp)/Meyer(Un)= Impaired by Frascati criteria and Unimpaired by Meyer; Frascati(Imp)/Meyer(Imp)=Impaired by both criteria. HAND subtypes defined using Frascati criteria: ANI = asymptomatic neurocognitive impairment; MND = mild neurocognitive disorder; HAD = HIV-associated dementia.
Figure 3.
Neuropsychological impairment rates by Clinical Rating (CR; Frascati) vs. Meyer group.
Note. Frascati(Un)/Meyer(Un)=Unimpaired by both criteria; Frascati(Imp)/Meyer(Un)= Impaired by Frascati CR criteria and Unimpaired by Meyer; Frascati(Imp)/Meyer(Imp)=Impaired by both criteria. Impairment defined as a CR ≥ 5.
*Domain not included in Meyer criteria
GDS vs. Meyer Criteria.
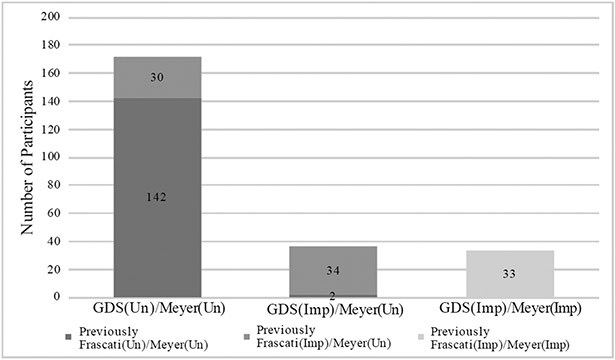
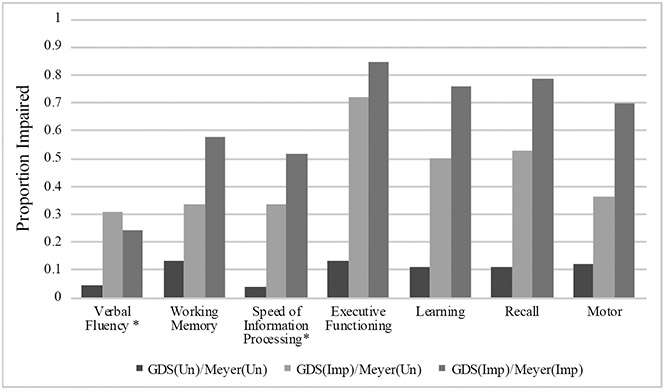
Participants’ characteristics by GDS vs. Meyer Impairment grouping are displayed in Table 2. The groups were similar on most characteristics; however, the GDS(Imp)/Meyer(Imp) group had more education, a greater proportion prescribed antiretroviral therapy, and a greater proportion of those classified as having contributing comorbidities than the GDS(Un)/Meyer(Un) group. Thirty of 64 participants (46.9%) who were categorized as Frascati(Imp)/Meyer(Un) using Frascati CR criteria were classified as GDS(Un)/Meyer(Un) using GDS methodology, and 2 of 144 (1.4%) participants who were previously classified as Frascati(Un)/Meyer(Un) using Frascati CR criteria were classified as GDS(Imp)/Meyer(Un) using GDS methodology (Figure 2). Proportion impaired, as defined by DDS>0.5, in each cognitive domain by group is displayed in Figure 4. A similar stair-step pattern of proportion impaired (GDS(Un)/Meyer(Un) < GDS(Imp)/Meyer(Un) < GDS(Imp)/Meyer(Imp)) was observed for all cognitive domains except for verbal fluency.
Figure 2.
Distribution of subjects per group using GDS vs. Meyer Criteria.
Note. Depicts number of participants that were previously Frascati(Imp)/Meyer(Un) via Frascati vs. Meyer Criteria and are GDS(Un)/Meyer(Un) via GDS vs. Meyer Criteria (n=30) and those that were Frascati(Un)/Meyer(Un) via Frascati vs. Meyer Criteria and GDS(Un)/Meyer(Un) via GDS vs. Meyer criteria (n=2).
Figure 4.
Neuropsychological impairment rates by GDS vs. Meyer group. GDS(Un)/Meyer(Un)=Unimpaired by both Meyer and GDS criteria; GDS(Imp)/Meyer(Un)= Impaired by GDS criteria and Unimpaired by Meyer; GDS(Imp)/Meyer(Imp)=impaired by both criteria.
Note. Impairment defined as a domain deficit score (DDS) > 0.5
*Domain not included in Meyer criteria
Frascati CR vs. Meyer Criteria Imaging Measures
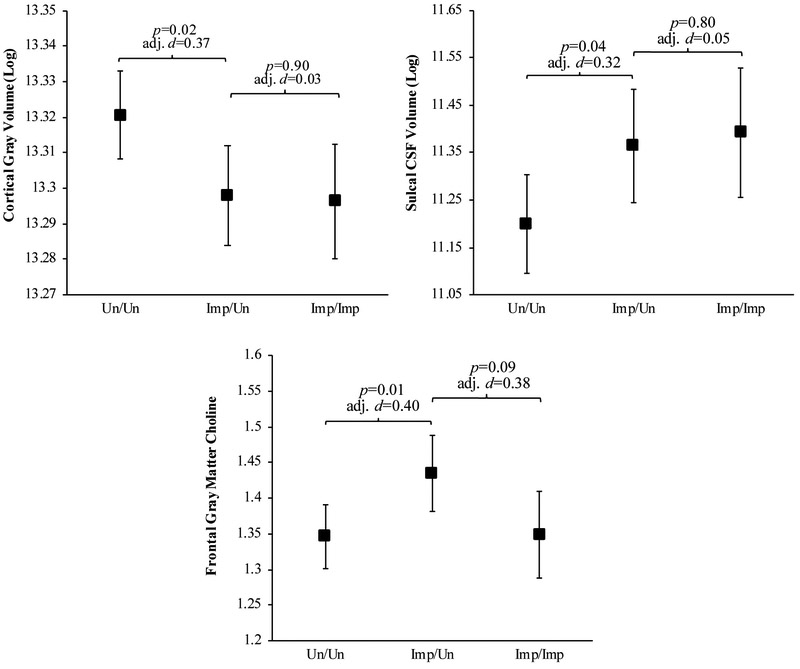
Standardized beta coefficients (β) from the linear regression models predicting structural MRI and MRS variables that compare Frascati CR vs. Meyer Frascati(Un)/Meyer(Un) and Frascati(Imp)/Meyer(Imp) groups to the Frascati(Un)/Meyer(Imp) group are displayed in Table 3, and predicted means and effect sizes from the significant finding are displayed in Figure 5.
Table 3.
Standardized beta coefficients for structural MRI and MRS measures comparing Frascati Clinical Ratings vs. Meyer criteria
| Cortical Gray |
Subcortical Gray |
Abnormal White Matter |
Total White Matter |
Ventricular CSF |
Sulcal CSF |
|
|---|---|---|---|---|---|---|
| Structural | ||||||
| Frascati(Un)/Meyer(Un) | 0.082* | 0.013 | −0.046 | 0.024 | −0.102 | −0.135* |
| Frascati(Imp)/Meyer(Imp) | −0.004 | −0.053 | 0.080 | −0.039 | 0.051 | 0.016 |
|
N-acetyl aspartate |
Choline | Creatine | Myo-inositol | Glutamate | ||
| MRS Frontal White Matter | ||||||
| Frascati(Un)/Meyer(Un) | 0.033 | −0.051 | 0.097 | 0.016 | −0.089 | |
| Frascati(Imp)/Meyer(Imp) | −0.053 | −0.012 | 0.033 | −0.025 | −0.073 | |
| MRS Frontal Gray Matter | ||||||
| Frascati(Un)/Meyer(Un) | −0.009 | −0.173* | −0.010 | 0.016 | −0.021 | |
| Frascati(Imp)/Meyer(Imp) | −0.066 | −0.119 | −0.027 | 0.005 | −0.052 | |
| MRS Basal Ganglia | ||||||
| Frascati(Un)/Meyer(Un) | 0.118 | −0.067 | 0.030 | −0.125 | 0.050 | |
| Frascati(Imp)/Meyer(Imp) | −0.039 | −0.064 | −0.006 | −0.066 | −0.013 | |
Note. Frascati(Un)/Meyer(Un)=Unimpaired by both criteria; Frascati(Imp)/Meyer(Un)=Impaired by Frascati CR criteria and Unimpaired by Meyer; Frascati(Imp)/Meyer(Imp)=Impaired by both criteria. CSF = cerebrospinal fluid.
All analyses were in comparison to the Frascati(Imp)/Meyer(Un) group derived from Frascati CR vs. Meyer criteria. Predicted means for significant relationships are presented in Figure 5.
p<0.05
Figure 5.
Predicted means with error bars denoting standard error for cortical gray matter volume (log), sulcal CSF volume (log), and frontal gray matter choline for the Frascati CR vs. Meyer groups.
Note. Un/Un=Unimpaired by both criteria; Imp/Un=Impaired by Frascati CR criteria and Unimpaired by Meyer; Imp/Imp=Impaired by both criteria; adj. d= adjusted Cohen’s d. The Frascati(Imp)/Meyer(Un) group had less cortical gray matter, greater sulcal CSF volume, and greater FGM choline than the Frascati(Un)/Meyer(Un) group. The Frascati(Imp)/Meyer(Un) group did not significantly differ from the Frascati(Imp)/Meyer(Imp) group on these measures.
Structural MRI.
After adjusting for scanner, age, and cerebral vault volume, the Frascati(Imp)/Meyer(Un) group had less cortical gray matter volume (β=0.08, p=0.02) and more sulcal CSF volume (β=−0.14, p=0.04) than the Frascati(Un)/Meyer(Un) group. The Frascati(Imp)/Meyer(Un) group did not significantly differ from the Frascati(Imp)/Meyer(Imp) on any MRI structural variables.
MRS.
After accounting for relevant covariates, choline in the frontal gray matter was significantly greater in the Frascati(Imp)/Meyer(Un) group compared to the Frascati(Un)/Meyer(Un) group (β=−0.17, p=0.01). The Frascati(Imp)/Meyer(Un) group also tended to have higher choline in frontal gray matter than the Frascati(Imp)/Meyer(Imp) group, but this was not significant (β=−0.12, p=0.08) nor did any other Frascati(Imp)/Meyer(Un) vs. Frascati(Imp)/Meyer(Imp) MRS metabolite comparisons differ significantly.
GDS vs. Meyer Criteria Imaging Measures
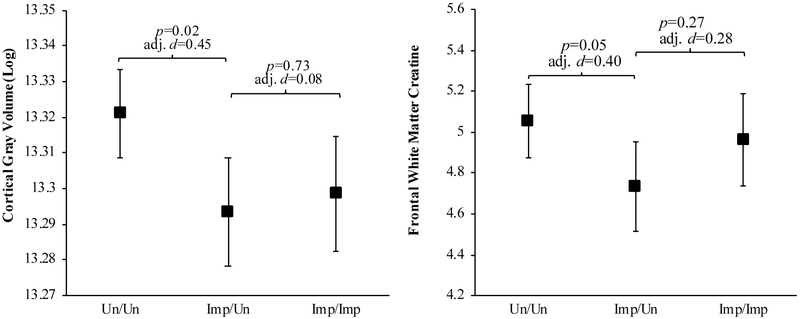
Standardized beta coefficients (β) from the linear regression models predicting structural MRI and MRS variables that compare GDS vs. Meyer GDS(Un)/Meyer(Un) and GDS(Imp)/Meyer(Imp) groups to the GDS(Un)/Meyer(Imp) group are displayed in Table 4, and predicted means and effect sizes from the significant finding are displayed in Figure 6.
Table 4.
Standardized beta coefficients for structural MRI and MRS measures comparing GDS vs. Meyer criteria
| Cortical Gray |
Subcortical Gray |
Abnormal White Matter |
Total White Matter |
Ventricular CSF |
Sulcal CSF |
|
|---|---|---|---|---|---|---|
| Structural | ||||||
| GDS(Un)/Meyer(Un) | 0.092* | 0.108 | −0.050 | −0.034 | −0.051 | −0.074 |
| GDS(Imp)/Meyer(Imp) | 0.013 | 0.008 | 0.071 | −0.071 | 0.069 | 0.035 |
|
N-acetyl aspartate |
Choline | Creatine | Myo-inositol | Glutamate | ||
| MRS Frontal White Matter | ||||||
| GDS(Un)/Meyer(Un) | 0.000 | −0.057 | 0.176* | −0.019 | −0.027 | |
| GDS(Imp)/Meyer(Imp) | −0.069 | −0.024 | 0.098 | −0.046 | −0.048 | |
| MRS Frontal Gray Matter | ||||||
| GDS(Un)/Meyer(Un) | 0.061 | −0.016 | 0.106 | 0.052 | 0.065 | |
| GDS(Imp)/Meyer(Imp) | −0.026 | −0.051 | 0.081 | 0.030 | −0.001 | |
| MRS Basal Ganglia | ||||||
| GDS(Un)/Meyer(Un) | 0.087 | −0.085 | 0.100 | −0.136 | 0.044 | |
| GDS(Imp)/Meyer(Imp) | −0.041 | −0.084 | 0.038 | −0.090 | −0.009 | |
Note. GDS(Un)/Meyer(Un)=Unimpaired by both Meyer and GDS criteria; GDS(Imp)/Meyer(Un)=Impaired by GDS criteria and Unimpaired by Meyer; GDS(Imp)/Meyer(Imp)=impaired by both criteria. CSF = cerebrospinal fluid.
All analyses were in comparison to the GDS(Imp)/Meyer(Un) group derived from GDS vs. Meyer criteria. Predicted means for significant relationships are presented in Figure 6.
p<0.05
Figure 6.
Predicted means with error bars denoting standard error for cortical gray matter volume (log) and frontal white matter creatine for the GDS vs. Meyer groups.
Note. Un/Un=Unimpaired by both Meyer and GDS criteria; Imp/Un=Impaired by GDS criteria and Unimpaired by Meyer; Imp/Imp=impaired by both criteria; adj. d= adjusted Cohen’s d. The GDS(Imp)/Meyer(Un) group had less cortical gray matter and less FGM choline than the GDS(Un)/Meyer(Un) group. The GDS(Imp)/Meyer(Un) group did not significantly differ from the GDS(Imp)/Meyer(Imp) group on these measures.
Structural MRI.
When analyzing the structural MRI variables using the GDS vs. Meyer criteria, the GDS(Imp)/Meyer(Un) group had significantly less cortical gray matter volume than the GDS(Un)/Meyer(Un) group (β=0.9, p=0.02), after adjusting for covariates. As seen in the Frascati CR vs. Meyer criteria analyses, the GDS(Imp)/Meyer(Un) group did not statistically differ from the GDS(Imp)/Meyer(Imp) group on any MRI structural variables.
MRS.
When comparing the groups derived from the GDS vs. Meyer criteria, the GDS(Imp)/Meyer(Un) group had significantly less creatine in the frontal white matter than the GDS(Un)/Meyer(Un) group (β=0.18, p=0.05). The GDS(Imp)/Meyer(Un) and the GDS(Imp)/Meyer(Imp) groups did not differ significantly on any MRS metabolites.
Discussion
Optimal classification of neurocognitive impairment within the context of HIV enables evaluation of potential mechanisms of CNS injury and prediction of functional outcomes. It is also clinically relevant, as false positive and false negative errors are both important: false positive errors that are communicated to patients could cause undue stress, and false negative diagnoses can result in missed opportunities to more closely monitor or intervene. This study utilized neuroimaging to examine different NCI criteria and help determine the optimal criteria for detecting NCI within the context of HIV with regards to brain integrity. Our findings indicate that those classified as impaired using Frascati criteria or GDS criteria, but unimpaired by the Meyer criteria, differ neuroanatomically from those with unimpaired cognition. Based on these findings, the Meyer criteria fail to capture a sizable group of PLWH with cortical atrophy. The group that was not captured by the Meyer criteria is not small; over a quarter (26%) of the sample was in the discordant Frascati(Imp)/Meyer(Un) group when classifying using Frascati CR, and 15% of the sample was in the discordant GDS(Imp)/Meyer(Un) group when classifying using GDS. While Meyer et al. (2013) are correct that false positives will negatively affect power to detect associations between cognitive impairment and brain integrity, it is important to also note that false negatives reduce power as well, which should be considered when examining optimal neurocognitive impairment criteria within the context of HIV.
Consistent across the Frascati CR and GDS criteria, the discordant group displayed similarly small cortical gray matter volumes compared to concordant impaired group, and significantly smaller volumes of cortical gray matter compared to the concordant unimpaired group. While it is difficult to compare this study which splits HAND into two groups (i.e., Impaired/Unimpaired and Impaired/Impaired) to other imaging studies examining HAND as one group, our findings were somewhat consistent with other studies. Recent cART era studies comparing neuroanatomical profiles of PLWH to seronegative controls have identified cortical thinning, particularly in frontal and temporal structures, as a prevalent neuroimaging correlate within the context of HIV (Bonnet et al., 2013; Cohen, Seider, & Navia, 2015; Thompson et al., 2005). Additionally, these findings are consistent with a recent study by the CHARTER group in an overlapping population that found those classified as MND and HAD had smaller cortical gray matter volumes relative to those without NCI (Alakkas et al., 2018). Furthermore, reductions in frontal gray matter have been detected in neurocognitively intact PLWH in the absence of subcortical and white matter changes (Towgood et al., 2012). Thus, while the Meyer criteria classify individuals in the discordant Impaired/Unimpaired group as neurocognitively unimpaired, the subtler neurocognitive deficits defined by Frascati CR and GDS criteria may include early HIV-related CNS injury. Although discordant Impaired/Unimpaired individuals also displayed some evidence of subcortical gray and white matter tissue injury compared to concordant unimpaired individuals, these differences were not statistically significant and are therefore consistent with observations that PLWH in the cART era may be less vulnerable to subcortical and white matter damage than the pre-cART era (O'Connor, Zeffiro, & Zeffiro, 2018). Given that our sample was characterized by high rates of impairment in executive functioning, learning, and recall, it is not surprising that discordant Impaired/Unimpaired and concordant unimpaired individuals were best differentiated by our estimate of cortical gray matter volume, which encompasses frontal and temporal structures that support memory and higher-order thinking.
Discordant Impaired/Unimpaired individuals also displayed evidence of neurochemical abnormalities in cortical gray and white matter tissues. For the Frascati CR vs. Meyer comparisons, the Frascati(Imp)/Meyer(Un) had higher levels of choline, a marker of cellular membrane turnover that is elevated with glial proliferation and neuroinflammatory responses, in frontal gray matter than the concordant unimpaired group. Previous research has shown that PLWH display elevated levels of choline in the context of both chronic-infection (Harezlak et al., 2011; Mohamed et al., 2010) and early infection (Lentz et al., 2011; Valcour et al., 2012) compared to seronegative controls. Research has also shown higher choline is associated with worse neurocognitive functioning (Chang et al., 2004; Paul et al., 2008; Alakkas et al. 2018). The neurocognitive ramifications associated with choline may in part be mediated by monocyte activation, a known driver of neural injury that has been linked to choline in PLWH (Anderson et al., 2015; Kamat et al., 2012; Lentz et al., 2011). However, while myo-inositol, another marker of neuroinflammation, was greatest in the Frascati(Imp)/Meyer(Un) group, this difference was not statistically significant. Myo-inositol is more variable than choline when measured using a 1.5 Tesla scanner, so this variability may be affecting our power to detect a significant difference, or the neuroinflammatory differences between groups may be more specific to choline. For the GDS vs. Meyer comparisons, the GDS(Imp)/Meyer(Un) group had lower levels of creatine, a marker of brain energy metabolism, in frontal white matter than the GDS(Un)/Meyer(Un) group. Although creatine has been employed as a reference marker in MRS studies to generate metabolite/creatine ratios (Jansen et al., 2006), we have reported correlations between frontal white matter creatine levels and HIV RNA in plasma and CSF, nadir CD4 count, and CSF biomarkers of monocyte and macrophage activation (Anderson et al., 2015). Thus, the altered frontal white matter brain energy metabolism in GDS(Imp)/Meyer(Un), compared to GDS(Un)/Meyer(Un) individuals, may reflect neural dysfunction related to HIV-associated neurotoxicity and neuroinflammation.
Analyses comparing impairment groupings’ demographics suggest that these three groups were fairly comparable with the exception of formal educational attainment. The concordant impaired group had the highest level of education for both analyses. This is surprising given the ample evidence showing that greater education is protective against cognitive impairment in PLWH (Foley et al., 2012; Maki et al., 2015; Tozzi et al., 2007). Education, which is often used as a proxy variable for cognitive reserve, is a protective factor against HAND (Morgan et al., 2012; Stern, Silva, Chaisson, & Evans, 1996). However, cognitive reserve as estimated from pre-morbid IQ (i.e., WRAT) and the Hollingshead Index, did not differ among the groups. In fact, the concordant unimpaired group had the highest pre-morbid IQ but also had the lowest educational attainment. The concordant impaired group had the most years (quantity) of education, but the quality of their education and/or occupational attainment may have been lower. All neuropsychological tests were adjusted for the effects of education; therefore, the educational normative adjustments with T-scores (in the potential case of lower education quality) may be negatively impacting the concordant impaired group resulting in greater impairment rates.
The three groups were fairly similar on most HIV disease characteristics. The concordant impaired group was more likely to be on ART, but this was only significant in the GDS vs. Meyer comparisons and may result from earlier policies that ART not be initiated until severe immunosuppression was detected. Of note, the proportion of those on ART that were not virally suppressed did not differ across groups. While the specific medical and psychiatric comorbidities did not differ between the three groups, the concordant impaired group had a greater proportion with mild-to-moderate (i.e., “contributing” versus “incidental”) comorbidities compared to both the concordant unimpaired and discordant group. However, this comparison was only significantly different with regard to the concordant unimpaired group. We chose to exclude those with severe (i.e., “confounded”) comorbidities, as confounding conditions preclude diagnosis of HAND whereas contributing conditions were included because they may exacerbate HIV-associated NCI or brain abnormalities and NC impairment, but are unlikely to fully account for them. This decision is further supported by a recent neuroimaging study by the CHARTER group which found that those with “contributing” comorbidities were fairly comparable to those with “incidental” comorbidities (Saloner et al., 2019). However, greater comorbidity burden has been shown to increase risk for impairment (Heaton et al., 2010; Patel et al., 2013), and some groups have found comorbid conditions and sociodemographic risk factors are associated with a greater risk of NCI than HIV status (Maki et al., 2015). Additionally, comorbidity burden may also contribute to volumetric abnormalities in PLWH (Masters & Ances, 2014; Lake et al., 2017). Therefore, the concordant impaired group would be expected to have more widespread cognitive impairments and a higher prevalence of comorbidities and brain atrophy. The aim of this study was to use neuroimaging to help assess neurocognitive criteria for identifying CNS differences. This study and the Meyer criteria do not address the relative contribution of HIV versus comorbidities to the CNS difference. Therefore, future studies that include an HIV-uninfected comparison groups are needed to parse apart the effect of HIV-infection compared to comorbidities with respect to imaging measures and cognition.
Several limitations of our study should be considered. First, this study was cross-sectional in nature; therefore, we cannot make inferences with regard to causality or timing of NCI in relation to structural or metabolic brain abnormalities. Longitudinal studies examining long-term neuropsychological outcomes of these groups in conjunction with imaging measures are warranted. Next, because we did not study HIV-uninfected controls, we cannot assess if the observed associations are totally or primarily due to HIV infection; therefore, these results should be interpreted within the context of HIV rather than fully due to HIV. Additionally, while the overall sample was fairly large, some groups, particularly the concordant impaired group, had a smaller sample size. Standardized betas were utilized in order to compare magnitude of the associations; however, this study may be underpowered to observe some differences between the discordant group and the concordant impaired group. Also, generalizability of the current findings to other populations (e.g., to women) should be considered. For example, this sample was predominantly men. Additionally, while the rate of viral suppression was similar to Center for Disease Control (CDC) estimates for viral suppression in the United States, viral suppression was somewhat lower than CDC estimates for viral suppression of those engaged in medical care (80% at <200 copies/mL; Center for Disease Control and Prevention, 2016). Lastly, this study analyzed global measures of brain structure acquired on 1.5 Tesla systems. Future studies should utilize scanners with greater field strength (e.g., 3 Tesla) as well as examine specific brain structures and other imaging modalities such as functional MRI and diffusion tensor imaging that have been shown to be sensitive to cognitive functioning in HIV (Ances et al., 2009; Cysique et al., 2017; du Plessis et al., 2017; Masters & Ances, 2014).
In a large group of demographically and geographically diverse PLWH, we found that both Frascati and GDS criteria captured a group of PLWH with worse brain integrity as compared to those without neurocognitive impairment. While Frascati and GDS criteria may be improved by further research, these findings suggest that the cutoff for mild impairment in HIV research studies should remain at 1 SD below the mean (i.e., the cutoff used in Frascati and GDS criteria). Future neuroimaging studies that track and attempt to predict longitudinal progression of HIV-related NCI are needed in order to assess the possible clinical utility of neuroimaging in helping to identify those at risk for HIV-related NCI.
Supplementary Material
Supplemental Figure 1. Flowchart to illustrate inclusion/exclusion criteria and neuropsychological criteria groupings.
Acknowledgements
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) N01 MH2205 and HHSN271201000036C, to I. Grant; R01 MH107345, to R.K. Heaton and S.L. Letendre; and P30 MH062512, to R.K. Heaton.
Funding
This research was supported by National Institute of Health awards N01 MH22005, HHSN271201000036C, and HHSN271201000030C. Ms. Campbell and Ms. Hussain were supported by T32 DA031098 from the National Institute on Drug Abuse. Mr. Saloner was supported by T32AA013525 from the National Institute on Alcohol Abuse and Alcoholism. The study was more broadly supported by the HIV Neurobehavioral Research Center (HNRC) Award P30 MH062512. The authors declare no conflict of interest.
Footnotes
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: Scott L. Letendre, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Davey M. Smith, M.D. (P.I.); Imaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Michael J. Taylor, Ph.D., Rebecca Theilmann, Ph.D.; Data Management Component: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman,; Statistics Component: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Vincent Rogalski; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Will Toperoff, N.P..; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P., Sheryl Storey, PA-C; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, … Fennema-Notestine C (2018). White matter damage, neuroinflammation, and neuronal integrity in HAND. Journal of NeuroVirology. doi: 10.1007/s13365-018-0682-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances B, Sisti D, Vaida F, Liang C, Leontiev O, Perthen J, … Little S (2009). Resting cerebral blood flow a potential biomarker of the effects of HIV in the brain. Neurology,73, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, & Hammoud DA (2014). Neuroimaging of HIV associated neurocognitive disorders (HAND). Current Opinion in HIV and AIDS, 9, 545–551. doi: 10.1097/coh.0000000000000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, … Letendre SL (2015). CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. Journal of NeuroVirology. doi: 10.1007/s13365-015-0359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade AS, Deutsch R, S AC, Duarte NA, Marcotte TD, Umlauf A, … Collier AC (2013). Relationships among neurocognitive status, medication adherence measured by pharmacy refill records, and virologic suppression in HIV-infected persons. Journal of Acquired Immune Deficiency Syndromes, 62, 282–292. doi: 10.1097/QAI.0b013e31827ed678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, … Wojna VE (2007). Updated research nosology for HIV-associated neurocognitive disorders. Neurology, 69, 1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, … Selnes O (2012). Factors affecting brain structure in men with HIV disease in the post-HAART era. Neuroradiology, 54, 113–121. doi: 10.1007/s00234-011-0854-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, … Heaton RK (2012). Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. The Clinical Neuropsychologist, 26, 894–908. doi: 10.1080/13854046.2012.694479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy FA, … Chene G (2013). Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS, 27, 391–400. doi: 10.1097/QAD.0b013e32835b1019 [DOI] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, & Heaton RK (2004). Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology, 26, 307–319. [DOI] [PubMed] [Google Scholar]
- Casaletto KB, Cattie J, Franklin DR, Moore DJ, Woods SP, Grant I, … Group H (2014). The Wide Range Achievement Test-4 Reading subtest “holds” in HIV-infected individuals. Journal of Clinical and Experimental Neuropsychology, 36, 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (2016). HIV in the United States and Dependent Areas. Retrieved from https://www.cdc.gov/hiv/statistics/overview/ataglance.html
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, … Navia BA (2004). A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage, 23, 1336–1347. doi: 10.1016/j.neuroimage.2004.07.067 [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Heaton RK, & Lehman RA (1986). Neuropsychological and personality correlates of patients’ complaints of disability Advances in Clinical Neuropsychology (pp. 95–126). Springer, Boston, MA. [Google Scholar]
- Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, … Navia B (2010). Effects of nadir CD4 count and duration of HIV infection on brain volumes in the HAART era. Journal of NeuroVirology, 16, 25–32. doi: 10.3109/13550280903552420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Seider TR, & Navia B (2015). HIV effects on age-associated neurocognitive dysfunction: Premature cognitive aging or neurodegenerative disease? Alzheimer's Research & Therapy, 7, 37. doi: 10.1186/s13195-015-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, … Grant I (2010). Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS, 24, 983–990. doi: 10.1097/QAD.0b013e32833336c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, … Rae C (2013). HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a (1)H MRS study. PLoS One, 8, e61738. doi: 10.1371/journal.pone.0061738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Soares JR, Geng G, Scarpetta M, Moffat K, Green M, … Rae C (2017). White matter measures are near normal in controlled HIV infection except in those with cognitive impairment and longer HIV duration. Journal of NeuroVirology, 23, 539–547. [DOI] [PubMed] [Google Scholar]
- du Plessis L, Paul RH, Hoare J, Stein DJ, Taylor PA, Meintjes EM, & Joska JA (2017). Resting-state functional magnetic resonance imaging in clade C HIV: Within-group association with neurocognitive function. Journal of NeuroVirology, 23, 875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Jiang CS, Nakama H, Buchthal S, & Chang L (2010). Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder. Journal of Magnetic Resonance Imaging, 32, 1045–1053. doi: 10.1002/jmri.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, … Grant, I. (2013). Increases in brain white matter abnormalities and subcortical gray matter are linked to CD4 recovery in HIV infection. Journal of NeuroVirology, 19, 393–401. doi: 10.1007/s13365-013-0185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, … Gollub RL (2007). Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics, 5, 235–245. doi: 10.1007/s12021-007-9003-9 [DOI] [PubMed] [Google Scholar]
- Foley JM, Ettenhofer ML, Kim MS, Behdin N, Castellon SA, & Hinkin CH (2012). Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Applied Neuropsychology: Adult, 19, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén M, Price RW, & Nilsson S (2011). The definition of HIV-associated neurocognitive disorders: are we overestimating the real prevalence? BMC infectious diseases, 11, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, … Navia B (2011). Persistence of HIV-associated cognitive impairment, inflammation and neuronal injury in era of highly active antiretroviral treatment. AIDS, 25, 625–633. doi: 10.1097/QAD.0b013e3283427da7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, … Grant I (2010). HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 75, 2087–2096. doi: 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, … Grant I (2011). HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of NeuroVirology, 17, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, … Abramson I (1995). The HNRC 500--neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society, 1, 231–251. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, … Grant I (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society,, 10, 317–331. doi: 10.1017/S1355617704102130 [DOI] [PubMed] [Google Scholar]
- Heaton RK, Taylor M, & Manly J (2003). Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. Clinical interpretation of the WAIS-III and WMS-III, 181. [Google Scholar]
- Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, & Stefaniak M (2004). Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS, 18 Suppl 1, S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status. Unpublished manuscript, Department of Sociology, Yale University, New Haven, CT. [Google Scholar]
- Iudicello JE, Hussain MA, Watson CWM, Morgan EE, & Heaton RK (in press). HIV-associated neurocognitive disorders In Stern RA, and Alosco ML (Eds.) Oxford Handbook of Adult Cognitive Disorders. New York: Oxford University Press. [Google Scholar]
- Jansen JF, Backes WH, Nicolay K, & Kooi ME (2006). 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology, 240, 318–332. doi: 10.1148/radiol.2402050314 [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, … Grant, I. (2011). Clinical factors related to brain structure in HIV: The CHARTER study. Journal of NeuroVirology, 17, 248–257. doi: 10.1007/s13365-011-0032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuba N, Anitha Menon J, Franklin DR Jr., Heaton RK, & Hestad KA (2017). Use of western neuropsychological test battery in detecting HIV-associated neurocognitive disorders (HAND) in Zambia. AIDS and Behavior, 21, 1717–1727. doi: 10.1007/s10461-016-1443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallianpur KJ, Shikuma C, Kirk GR, Shiramizu B, Valcour V, Chow D, … Sailasuta N(2013). Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology, 80, 1792–1799. doi: 10.1212/WNL.0b013e318291903f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, & Gabuzda D (2012). Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of Acquired Immune Deficiency Syndromes, 60, 234–243. doi: 10.1097/QAI.0b013e318256f3bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Kuate CT, Cysique LA, Fonsah JY, Eta S, Doh R, … Njamnshi AK (2010). HIV-associated neurocognitive disorders in sub-Saharan Africa: A pilot study in Cameroon. BMC Neurology, 10, 60. doi: 10.1186/1471-2377-10-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JE, Popov M, Post WS, Palella FJ, Sacktor N, Miller EN, … & Becker JT (2017). Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. Journal of neurovirology, 23, 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, & Brody EM (1969). Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist, 9, 179–186. [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, … Gonzàlez RG (2011). Alterations in brain metabolism during the first year of HIV infection. Journal of NeuroVirology, 17, 220–229. doi: 10.1007/s13365-011-0030-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, … Alden C (2015). Cognitive function in women with HIV Findings from the Women's Interagency HIV Study. Neurology, 84, 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters MC, & Ances BM (2014). Role of neuroimaging in HIV associated neurocognitive disorders (HAND). Seminars in Neurology, 34, 89–102. doi: 10.1055/s-0034-1372346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AC, Boscardin WJ, Kwasa JK, & Price RW (2013). Is it time to rethink how neuropsychological tests are used to diagnose mild forms of HIV-associated neurocognitive disorders? Impact of false-positive rates on prevalence and power. Neuroepidemiology, 41, 208–216. doi: 10.1159/000354629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M, Barker P, Skolasky R, Selnes OA, Moxley R, Pomper M, & Sacktor N (2010). Brain metabolism and cognitive impairment in HIV infection: A 3 Tesla magnetic resonance spectroscopy Study. Magnetic Resonance Imaging, 28, 1251–1257. doi: 10.1016/j.mri.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M, Barker PB, Skolasky RL, & Sacktor N (2018). 7T Brain MRS in HIV infection: Correlation with cognitive impairment and performance on neuropsychological tests. American Journal of Neuroradiology, 39, 704–712. doi: 10.3174/ajnr.A5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA, Lentz MR, Lee V, Halpern EF, Sacktor N, Selnes O, … Pomper MG (2010). Factor analysis of proton MR spectroscopic imaging data in HIV infection: Metabolite-derived factors help identify infection and dementia. Radiology, 254, 577–586. doi: 10.1148/radiol.09081867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Smith C, Weber E, Scott JC, & Grant I (2012). Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS and Behavior, 16, 2279–2285. doi: 10.1007/s10461-012-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols MJ, Gates TM, Soares JR, Moffat KJ, Rae CD, Brew BJ, & Cysique LA (2019). Atrophic brain signatures of mild forms of neurocognitive impairment in virally suppressed HIV infection. AIDS, 33, 55–66. [DOI] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, Franklin D Jr., Cysique L, Ake C, … Group H (2011). Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. Journal of Clinical and Experimental Neuropsycholgy, 33, 793–804. doi: 10.1080/13803395.2011.559157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor EE, Zeffiro TA, & Zeffiro TA (2018). Brain structural changes following HIV infection: Meta-analysis. American Journal of Neuroradiology, 39, 54–62. doi: 10.3174/ajnr.A5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JP, Fellows RP, Rivera-Mindt M, Morgello S, Byrd DA, & Bank MHB(2015). Reading ability as an estimator of premorbid intelligence: does it remain stable among ethnically diverse HIV+ adults? The Clinical Neuropsychologist, 29, 1034–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, … Grossman RI.(2002). Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. American Journal of Neuroradiology, 23, 543–549. [PMC free article] [PubMed] [Google Scholar]
- Patel SM, Thames AD, Arbid N, Panos SE, Castellon S, & Hinkin CH (2013). The aggregate effects of multiple comorbid risk factors on cognition among HIV-infected individuals. Journal of Clinical Experimental Neuropsycholgy, 35, 421–434. doi: 10.1080/13803395.2013.783000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Ernst T, Brickman AM, Yiannoutsos CT, Tate DF, Cohen RA, & Navia BA (2008). Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. Journal of the International Neuropsychological Society, 14, 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 14, 260–264. [DOI] [PubMed] [Google Scholar]
- Saloner R, & Cysique LA (2017). HIV-associated neurocognitive disorders: A global perspective. Journal of the International Neuropsychological Society, 23, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner R, Heaton RK, Campbell LM, Chen A, Franklin D Jr., Ellis RJ, … Fennema-Notestine C (2019). Effects of comorbidity burden and Age on brain integrity in HIV. AIDS. Advance online publication. doi: 10.1097/QAD.0000000000002192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Silva SG, Chaisson N, & Evans DL (1996). Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology, 53, 148–153. [DOI] [PubMed] [Google Scholar]
- Thames AD, Foley JM, Wright MJ, Panos SE, Ettenhofer M, Ramezani A, …Hinkin CH (2012). Basal ganglia structures differentially contribute to verbal fluency: evidence from Human Immunodeficiency Virus (HIV)-infected adults. Neuropsychologia, 50, 390–395. doi: 10.1016/j.neuropsychologia.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, & Becker JT (2005). Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America, 102, 15647–15652. doi: 10.1073/pnas.0502548102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti C, Focà E, Cesana BM, & Lescure FX (2011). Asymptomatic neurocognitive disorders in patients infected by HIV: Fact or fiction? BMC Medicine, 9, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towgood KJ, Pitkanen M, Kulasegaram R, Fradera A, Kumar A, Soni S, … Kopelman MD (2012). Mapping the brain in younger and older asymptomatic HIV-1 men: Frontal volume changes in the absence of other cortical or diffusion tensor abnormalities. Cortex, 48, 230–241. doi: 10.1016/j.cortex.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, … Antinori A (2007). Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. Journal of Acquired Immune Deficiency Syndromes, 45, 174–182. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, … Ananworanich J (2012). Central nervous system viral invasion and inflammation during acute HIV infection. Journal of Infectious Diseases, 206, 275–282. doi: 10.1093/infdis/jis326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G (1993). Wide Range Achievement Test 3—Administration Manual. Wilmington, DE: Jastak Associates. In: Inc. [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, … Heaton RK (2004). Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsycholgy, 26, 759–778. doi: 10.1080/13803390490509565 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1997). Composite International Diagnostic Interview (CIDI, version 2.1). Geneva, Switzerland: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flowchart to illustrate inclusion/exclusion criteria and neuropsychological criteria groupings.