Abstract
A better understanding of the maturational correlates of inflammatory activity during adolescence is needed to more appropriately study both normal and abnormal development. Inflammation is the immune system’s first response to infection, injury, or psychological stress, and it has been shown to be elevated in individuals with both physical and psychological conditions. This study examined unique associations between (1) pubertal status and inflammatory biomarkers, and (2) age and inflammatory biomarkers, and whether these relationships differed by sex in a diverse sample of 155 adolescents (54.2% female, 45.8% male; Mage = 16.22) from a northeastern city in the US. A more advanced pubertal status was uniquely associated with lower levels of tumor necrosis factor alpha (TNF-α) and interleukin-8 (IL-8). Chronological age was uniquely associated with lower IL-8 levels. The association between pubertal status and C-reactive protein (CRP) levels differed by sex: more mature females had higher CRP, whereas pubertal status and CRP were not significantly associated in males. These findings highlight an important relation between pubertal development and inflammatory activity during adolescence.
Keywords: adolescence, puberty, age, sex, inflammation
Introduction
Adolescence is broadly defined as the developmental period from the ages of 11 to 25 during which youth must meet a host of developmental milestones, including physical, cognitive, social, and emotional milestones (Curtis, 2015). Although many youth successfully achieve these milestones, adolescence is also a vulnerable period for the emergence of physical and psychological conditions that negatively impact normative development. Specifically, during adolescence, rates of depression (e.g., Kessler, Avenevoli, & Merikangas, 2001), bipolar disorder (Lewinsohn, Seeley, Buckley, & Klein, 2002), anxiety disorders (Merikangas et al., 2010), eating disorders (e.g., Lewinsohn, Striegel-Moore, & Seeley, 2000), substance use (e.g., Merikangas et al., 2010), and schizophrenia (e.g., Van Nimwegen, de Haan, van Beveren, van den Brink, & Linszen, 2005) all increase. Rates of certain autoimmune disorders (Beeson, 1994) and diabetes also increase in prevalence during this vulnerable developmental period (Maahs, West, Lawrence, & Mayer-Davis, 2010). Understanding the causes and correlates of this increase in physical and psychological conditions is essential to identify youth at increased risk for these negative outcomes and offer prevention and intervention efforts to promote normal development.
Developmental research highlights the importance of understanding normal development in order to inform our understanding of abnormal development and underscores the importance of conceptualizing these outcomes as the result of an interaction between an individual and the environmental context (Drabick & Kendall, 2010). This work has focused on a number of biopsychosocial factors, including genetics, temperament, race, gender, early childhood experiences, family structure and dynamics, socioeconomic status, cognitive abilities, and peer relationships (Holmbeck, 2002). A critical developmental milestone is the pubertal transition to adolescence (Holmbeck, 2002). During puberty, youth undergo significant neuroendocrine changes that initiate sex-specific somatic maturation as well as a deepening of gender-related differences in psychological development. Consequently, puberty results in dramatic physical, cognitive, emotional, and social changes and has long been identified as an important transition stage in the etiology of a variety of physical and psychological conditions (Curtis, 2015). In order to adequately investigate the way that pubertally-induced changes interact with environmental factors to confer risk or resilience for negative outcomes, the field needs a better understanding of the way pubertal processes influence other biological systems and, in turn, the individual. The current study aimed to elucidate the relation between pubertal development and inflammatory physiology during adolescence.
Pubertal Status and Physical and Psychological Conditions
There is evidence that pubertal status, or the degree of physical maturation at the time of observation, predicts psychological and physical health outcomes. Pubertal status differentially predicted the incidence of depression in boys and girls beyond the effect of age (Angold, Costello, & Worthman, 1998). In another study, advanced pubertal status was associated with more depressive symptoms, although only among females (Hayward, Gotlib, Schraedley, & Litt, 1999), and there is some evidence that this association can be explained by increases in reproductive hormones (Angold, Costello, Erkanli, & Worthman, 1999). Similarly, pubertal status predicted social anxiety symptoms among girls (Deardorff, Hayward, Wilson, Bryson, Hammer, & Agras, 2007) and anxiety symptoms increased significantly after the onset of menarche in girls (Patton et al., 1996). Increases in incidence of both eating disorders (Killen et al 1992) and substance use also are associated with a more advanced pubertal stage (Patton, McMorris, Toumbourou, Hemphill, Donath, & Catalano, 2004).
It also has been hypothesized that the maturational changes in certain immune responses after puberty may precipitate the first onset of schizophrenia symptoms (e.g., Walker & Bollini, 2002; Kinney et al., 2010). In addition, the prevalence of several autoimmune conditions, including systemic lupus erythematosus, autoimmune thyroid conditions, rheumatoid arthritis, and type 1 diabetes, all increase during the pubertal transition to adolescence (Beeson, 1994). In sum, a change in immune functioning appears to be associated with many physical and psychological conditions that increase in incidence during adolescence.
Inflammation as a Potential Mechanism
Although there is empirical support for pubertal development as a potential cause of the increase in psychological and physical conditions during adolescence, the mechanisms of this association still are not clearly understood. One understudied potential mechanism is the role of proinflammatory biomarkers, like interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α), and acute phase reactants like C-reactive protein (CRP). Proinflammatory biomarkers are signaling proteins released by many different types of cells and tissues to stimulate cellular functions as part of the immune response. The levels of these proteins increase during infection or after trauma and also can communicate with the brain about the health of the individual (Janeway, 1989). When the levels increase markedly, individuals evince “sickness behaviors,” like fatigue, loss of appetite, and a reduced interest in rewarding activities (Dantzer & Kelley, 2007). It is thought that these symptoms serve to help the individual conserve energy and enable a more effective response to the infectious pathogen or injured tissue (Irwin & Cole, 2011). However, the levels of proinflammatory biomarkers also can change in response to emotional challenges and social stress (Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). Whereas chronic stressors often suppress immune functioning, acute provocations can result in increased levels of several proinflammatory biomarkers (Segerstrom & Miller, 2004).
Four biomarkers commonly studied in relation to psychological factors are CRP and IL-6, IL-8, and TNF-α. Each biomarker has different tissue origins and functions. For instance, IL-6 is a cytokine produced by white blood cells, as well as by fat and liver cells. IL-6 is considered a pleiotropic cytokine, which means that it affects the activity of multiple cellular systems and organs and has diverse actions throughout the body, including on the heart, lungs, central nervous system, liver, gut, kidneys, and skeletal system. IL-6 also is involved in the body’s acute phase response to a new infection or trauma, and the release of IL-6 can be further stimulated by increases in cortisol after psychological stress (Van Snick, 1990). Finally, in addition to its role in acute inflammatory responses, and stimulating the release of CRP by the liver, IL-6 also plays an important role in the transition from acute to chronic inflammation (Gabay, 2006). Another potent inflammatory cytokine is TNF-α. It is often released quickly, and then because of its potency, the levels return to baseline within a few hours. TNF-α can initiate the release of other cytokines, such as IL-6 and IL-8, and can influence whether an immune response is more dominated by the production of antibody or a cellular response (Streiter, Kunkel, & Bone, 1993). It also has an important a role mediating homeostatic functions, like sleep, circadian rhythms, and appetite. Importantly, both IL-6 and TNF-α can influence the neuroendocrine system, including both the hypothalamic-pituitary-adrenal (HPA) and hypothalamic-pituitary-gonadal (HPG) axes (Turnbull & Rivier, 1999). There are many other cytokines to potentially consider when studying inflammatory physiology, but IL-8 frequently is selected because it is derived from different cellular sources, including skin cells and other types of white blood cells, such as monocytes/macrophages (types of white blood cells). Its biological activity is more associated with a different leukocyte, the stimulation of the more primitive phagocytic neutrophils (Remick, 2005).
When studying inflammatory cytokines, most studies also include an assessment of CRP, a protein that is released from the liver as part of the acute-phase response to infection or trauma. Acute increases in CRP levels often are used by physicians in clinical practice to monitor for a recent bacterial or viral infection. CRP levels are commonly correlated with the levels of IL-6 in the blood stream, especially in older, overweight individuals. Many studies have found that CRP levels also are elevated in a number of psychiatric disorders, including both depression and psychosis (Pepys & Hirschfield, 2003).
In light of evidence demonstrating increased inflammatory activity in response to psychosocial stress, other research has focused on whether proinflammatory biomarkers can be used as predictors of psychological outcomes and physical health problems (Kendall-Tackett, 2009). Indeed, some individuals with mood and anxiety disorders (Goldsmith, Rapaport, & Miller, 2016; Munkholm, Vinberg, & Kessing, 2013), schizophrenia (Goldsmith et al., 2016), eating (Solmi et al., 2015) and substance use disorders (Cook, 1998; Fox et al., 2012) have elevated levels of a number of these proinflammatory biomarkers in peripheral blood. These proteins also are involved in the pathophysiology of disease, including diabetes and autoimmune conditions, but with levels that are not typically as high as found in patients (Dandona, Aljada, & Bandyopadhyay, 2004; Moudgil & Choubey, 2011).
Puberty and Inflammation
Research on the effects of hormones on immune functioning also conveys that there is a link between pubertal development and inflammatory physiology (Klein & Flanagan, 2016). Reproductive hormones, including sex-typical ones such as estrogen and testosterone, increase substantially during puberty (Shirtcliff, Dahl, & Pollack, 2009), and both are known to influence immune responses and inflammatory pathways. Estrogen often is characterized as being proinflammatory, whereas progesterone and testosterone are considered to be anti-inflammatory (Cutolo & Wilder, 2000). Changes in these hormones during the peripubertal transition also may influence the HPA-axis. Pubertal development results in sex-specific changes to the HPA-axis, such that a more advanced pubertal status is associated with an increase in cortisol reactivity and cortisol levels, particularly among females (Stroud, Papandonatos, Williamson, & Dahl, 2011). Further, for both males and females, chronic stress and increased secretion of cortisol can lead to higher levels of peripheral proinflammatory cytokines (Hansel, Hong, Cámara, & von Kanel, 2010). Generally, it is hypothesized that larger changes in inflammation will be evident in females than males, but there is still a need for empirical research such as the current study’s analysis to test potential sex differences. The importance of hormones on inflammatory physiology and immunity becomes especially evident during pregnancy, when the endocrine changes lead to many immune alterations that are required to ensure that the mother’s immune system does not reject the fetus and that increases in inflammatory physiology do not lead to a premature birth (Elenkov & Chrousos, 2002). However, there has been limited research on pubertal status and levels of proinflammatory biomarkers, and this body of work primarily has investigated how the relation between inflammation and depression differs across pubertal development (Mills, Scott, Wray, Cohen-Woods, & Baune, 2013). Therefore, the present analyses address an important gap in our knowledge with regard to normative maturational associations with inflammatory physiology in a community sample of healthy adolescents.
Sex Differences
It is important to note that many (but not all) of the physical and psychological conditions that increase in prevalence during adolescence seem to occur more commonly in females (e.g., depression, anxiety, and eating disorders and several autoimmune conditions; Bulik, 2002; Gater et al., 1998; Jacobson, Gange, Rose, & Graham, 1997; Lewinsohn, Gotlib, Lewinsohn, Seeley, & Allen, 1998; Merikangas et al, 2010; Zahn-Waxler, Shirtcliff, & Marceau, 2008). For example, females are 2–3 times as likely to meet criteria for Major Depressive Disorder than males after age 13 (Hankin, Abramson, Moffitt, Silva, McGee, & Angell, 1998), and females are estimated to be at 2.7 times greater risk for developing an autoimmune disease than males (Jacobson et al., 1997). There also is evidence for a sexual dimorphism in certain immune responses, with some studies finding that females mount larger responses to infection than males (Verthelyi, 2001; Whitacre 2001). Conversely, studies have found the cells of males are more reactive when stimulated with cell stimulants in in vitro cultures (Casimir et al., 2010). However, both types of findings led us to hypothesize that there would be sex differences in the maturational correlates of inflammatory physiology across adolescence. More systematic knowledge is needed in order to better understand why individuals or subgroups of individuals (i.e., females and males) have a differential risk for negative outcomes.
The Confounding Effect of Age
It also is important to consider the likely effect of chronological age on inflammation. Although pubertal staging is highly correlated with age, there are likely to be intrinsic age-associated changes within the immune system, which are not directly mediated by the reproductive hormones. For example, it is known that the size and structure of a key regulatory gland within the immune system, the thymus, changes markedly from childhood to adolescence, and then, continues to undergo additional modifications in older adulthood (Linton & Dorshkind, 2004). These changes contribute to age-related differences in immune markers among adolescents (Rudy, Wilson, Durako, Moscicki, Muenz, & Douglas, 2002). Further, the levels of several commonly studied inflammatory biomarkers in the blood are related to age. Cytokines such as IL-6 are usually higher in older than younger adults (e.g., Cohen, Pieper, Harris, Rao, & Currie, 1997; Riancho, Zarrabeitia, Amado, Olmos, & González-Macias, 1994). Therefore, our analysis was designed to delineate the independent influences of the participant’s age and pubertal stage on the levels of the four biomarkers in circulation.
The Current Study
More systematic information is needed to better understand how inflammatory activity is affected by pubertal maturation in adolescence. This knowledge can inform our understanding about physiological processes that may contribute to the emergence of physical and psychological problems during this critical developmental period. The primary aim of this study was to determine whether pubertal status was significantly associated with the levels of several proinflammatory biomarkers, above and beyond the association with age. It was hypothesized that more advanced pubertal status would be associated with higher levels, and that the associations would be stronger in female than male adolescents.
Methods
Participants
Participants were drawn from the Adolescent Cognition and Emotion (ACE) project at Temple University, a large, public university located in an urban setting in the United States. A community sample of 639 adolescents (aged 12–13 years at baseline) and their mothers or primary female caregivers were recruited from the Philadelphia area. Recruitment involved both mailings and follow-up calls to families with children attending Philadelphia area public and private middle schools (68% of the total sample) and advertisement in local newspapers (32% of the sample). Inclusion criteria included sufficient competence with the English language to complete the assessments. Additionally, adolescents had to identify as either Caucasian/White, African American/Black, or biracial. Individuals who identified as members of other racial or ethnic groups were excluded, as the investigation of differences in the etiology of depression comparing Caucasian/White and African American/Black youth was one of the aims of Project ACE. All demographic information was self-reported during the first visit of the study. Exclusion criteria also included a history of severe psychiatric illness or developmental disorders (see Alloy et al., 2012 for further information). Informed written consent was obtained from mothers and written assent from adolescents at the first study visit. About four years after the start of data collection, a supplementary grant allowed for the annual collection of blood samples to assay proinflammatory biomarkers; 315 participants from the parent study completed at least one blood draw.
The current sample consisted of a subsample of 155 adolescents (mean age at blood draw = 16.22; SD = 1.52 years, range = 12.11–20.01 years) of the 315 who completed at least one blood draw. The data used were drawn from their first annual blood draw (or only blood draw if they only completed one). The final sample was 54.2% female, 42.6% Caucasian, 57.4% African American (see Tables 1a–c for descriptive statistics and Table 2 for a correlation matrix of study variables). There were no differences between the analytic sample and the rest of the parent study sample on sex (t(639) = .19, p = .85), or SES (t(610) = 1.31, p = .19), but the analytic sample was significantly more likely to be African American (t(634) = −2.04, p = .04). There were no differences between the analytic sample and the rest of the sample that completed at least one blood draw on sex (t(313) = −.52, p = .60), race (t(313) = .57, p = .56), or SES (t(301) = .21, p = .84).
Table 1a.
Descriptive statistics of primary study variables overall and by sex.
| Overall Sample | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | |
| CRP | 1.72 | 0.59 | 0.00–2.97 | 1.64 | 0.56 | 0.00–2.93 | 1.79 | 0.64 | 0.00–2.97 |
| IL-8 | 2.52 | 0.24 | 2.04–3.14 | 2.52 | 0.26 | 2.05–3.14 | 2.51 | 0.23 | 2.04–3.12 |
| IL-6 | 1.53 | 0.29 | 0.85–2.43 | 1 44*** | 0.25 | 0.85–2.01 | 1.60*** | 0.30 | 1.04–2.43 |
| TNF-α | 2.15 | 0.12 | 1.82–2.44 | 2.17* | 0.12 | 1.82–2.44 | 2.13* | 0.12 | 1.83–2.40 |
| Age | 16.22 | 1.47 | 12.11–20.01 | 16.20 | 1.41 | 12.96–19.39 | 16.23 | 1.61 | 12.11–20.01 |
| PDS | 3.31 | 0.56 | 1.80–4.00 | 2 99*** | 0.52 | 1.80–4.00 | 3.58*** | 0.40 | 2.20–4.00 |
Note: CRP = C-reactive protein; IL-8 = interleukin-8; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; PDS = Pubertal Development Scale; SD = standard deviation.
p<.10,
p<.001.
Table 1c.
A breakdown of PDS means and ranges by age group in the sample.
| Overall Sample PDS | Males PDS | Females PDS | ||||
|---|---|---|---|---|---|---|
| Age Range | Mean | Range | Mean | Range | Mean | Range |
| 12.00–13.99 | 2.85 | 1.80–3.80 | 2.15 | 1.80–2.80 | 3.26 | 2.20–3.80 |
| 14.00–15.99 | 3.19 | 1.80–4.00 | 2.91 | 1.80–4.00 | 3.55 | 2.40–4.00 |
| 16.00–17.99 | 3.32 | 2.20–4.00 | 3.18 | 2.20–4.00 | 3.60 | 2.40–4.00 |
| 18.00–20.01 | 3.57 | 2.60–4.00 | 3.23 | 2.60–3.80 | 3.80 | 3.20–4.00 |
Note: PDS = Pubertal Development Scale.
Table 2.
Bivariate correlations among primary study variables.
| CRP | IL-8 | IL-6 | TNF-α | Age | PDS | Sex | BMI | |
|---|---|---|---|---|---|---|---|---|
| CRP | - | |||||||
| IL-8 | −0.16 | - | ||||||
| IL-6 | 0.43** | −.019* | - | |||||
| TNF-α | −0.01 | 0.08 | 0.13 | - | ||||
| Age | 0.18* | −0.25** | 0.19* | −0.07 | - | |||
| PDS | 0.14 | −0.21** | 0.20* | −0.27** | 0.34** | - | ||
| Sex | 0.13 | −0.01 | 0.29** | −0.16* | 0.01 | 0.54** | - | |
| BMI | 0.54** | −0.14 | 0.38** | −0.08 | 0.12 | 0.18** | 0.10 | - |
Note: CRP = C-reactive protein; IL-8 = interleukin-8; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; PDS = Pubertal Development Scale; BMI = Body Mass Index.
p<.05,
p<.01;
p<.001.
Measures
Pubertal Status.
The Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988) is a self-report questionnaire designed to assess pubertal development. Both mothers and adolescents completed the five-item questionnaire, but only adolescent-report was used in analyses (correlation between mother and adolescent report: r = .84, p < .001). The questions ask about growth in height, body hair, skin change, breast (females) or voice (males) change, and facial hair (males) or menstruation (females). All questions aside from menstruation are rated on a 4-point scale (1 = no development, 2 = development has barely begun, 3 = development is definitely underway, 4 = development is complete). Menstruation is scored as 1 = “I have not yet begun to menstruate” or 4 = “I have begun to menstruate”. Item scores are averaged, and the scale yields a final score ranging from 1–4 (less to more pubertally developed). The PDS has acceptable psychometric properties (average α of .77 for five items) and good convergent validity (r’s of .61–.67 with physician ratings) (Petersen et al, 1988). The PDS has been shown to adequately capture variability in basal hormones (Shirtcliff, Dahl, & Pollack, 2009). The PDS has been used in samples of older adolescents (Kong et al., 2013) and demonstrated a correlation of r = .70 with observational measures of pubertal development in a sample of high school students (Leon, Fulkerson, Perry, & Early-Zald, 1995). Descriptive statistics for the PDS and a more detailed breakdown of the mean and range of PDS score by age group in the sample is presented in Tables 1a–c. Internal consistency in this sample was α = .58 for girls and α = .79 for boys.
Although pubertal timing was not a focus of the current study, it is also included as an independent variable in alternate models reported in this manuscript. Consistent with past research assessing pubertal timing (Alloy, Hamilton, Hamlat, & Abramson, 2016; Dorn, Dahl, Woodward, & Biro, 2006), timing scores were obtained by regressing PDS total score on age. Timing scores were computed separately for males and females. The residual was used as a continuous measure of pubertal timing.
Inflammatory Biomarkers.
Three proinflammatory cytokines were quantified by multi-cytokine array (IL-6, IL-8, and TNF-α), and high-sensitivity CRP was determined in a singleplex assay, using an electrochemiluminescence platform and a QuickPlex SQ 120 imager for analyte detection (Meso Scale Discovery, Gaithersburg, MD). Each specimen was assayed in duplicate. The intra-assay coefficients of variation were 1.94 – 4.38%. Values were calculated with respect to a standard curve generated from 7 calibrators with known concentrations. The lower limit of detection (LLOD) for the cytokines was 0.1 pg/mL, with a large dynamic range up to 2000 pg/mL. CRP is present in blood at higher concentrations, and thus, plasma was diluted to correspond to the standard curve. The LLOD for CRP was 0.1 mg/L. Values below the LLOD were set at the LLOD. Values were converted to mg/L units to be consistent with the clinical literature (Breen et al., 2011; Dabitao, Margolick, Lopez, & Bream, 2011). These biomarkers were selected because they are some of the most commonly studied inflammatory biomarkers in relation to adolescence and psychological disorders (and many medical disorders).
Demographic Information.
Sex, race, birth date (for calculating age), and eligibility for subsidized school lunch (a proxy for SES that accounts for number of individuals in the household) were collected via self-report at Time 1 of the parent study. Immunomodulating medication status, and diagnosis of an autoimmune disease, and diagnosis of any other medication condition that could influence inflammation (e.g., diabetes, asthma, pregnancy, bone fracture, asthma, or blood-clotting disorder) also were collected via self-report on the day of the blood draw. One variable was computed for whether the person had any pertinent medical condition that could affect inflammation (e.g., diabetes, autoimmune condition, asthma).
Procedure
The ongoing longitudinal Project ACE attempted to interview participants every six months. After the start of the supplementary grant to collect blood samples, participants were approached annually with the opportunity to complete an optional blood draw. The data used in the current study are from participants’ first blood draw. If participants consented to participate in the blood draw, blood was obtained via antecubital venipuncture by a certified phlebotomist into a 10 mL vacutainer designed for freezing plasma separated from the cells within the vial (BD Hemogard with K2 EDTA). Vacutainers were stored in an ultracold freezer at −80 °C, and later thawed on the day of assay. Collection time for the blood draw and participants’ body mass index (BMI) based on direct measurement of height and weight were recorded.
Because the PDS was not given at every session in the study, the PDS closest to the date of the blood draw was used. Only those PDS assessments that were completed within 70 days of the time of the blood draw were used (M = 16.44 days, SD = 17.85 days). Ninety-four cases were dropped due to missing data on the PDS. The PDS was completed on the same date as the blood draw for 45.1% of cases. The date that the PDS was completed was within one month of the date of the blood draw for 87.7% of participants and within 70 days of the date of the blood draw for all participants.
Results
Preliminary Analyses
All analyses were run in Mplus (Muthén & Muthén, 2015). First, the distribution of each proinflammatory biomarker was examined. Consistent with literature indicating that a CRP value > 10 indicates a possible acute infection (Bell et al., 2017; De Ferranti, Gauvreau, Ludwih, Newburger, & Rifai, 2006), all participants with a CRP value > 10 were removed. All participants with IL-6, IL-8 and TNF-α values more than 3 standard deviations from the mean also were removed; 66 total cases were removed due to extreme biomarker values. After removing cases who didn’t have a PDS within 70 days of the blood draw and cases who had extreme biomarker values, there were a total of 155 cases used in the present analyses. Despite removing outliers, CRP, IL-6, IL-8, and TNF-α were skewed (IL-8: skewness = 1.75, kurtosis = 3.10; TNF-α: skewness = .51, kurtosis = .52; IL-6: skewness = 3.17, kurtosis = 14.26; CRP: skewness = 2.51, kurtosis = 6.80). Thus, a log transformation was applied to the raw values (log(100*value)), resulting in skewness statistics that did not violate the assumptions of normality (IL-8: skewness = .49, kurtosis = −.27; TNF-α: skewness = −.36, kurtosis = .31; IL-6: skewness = .42, kurtosis = .28; CRP: skewness = −.10, kurtosis = −.37). Although the values for TNF-α did not violate established cutoffs for skewness or kurtosis, it was log-transformed to be comparable to the other biomarkers, as well as the extant literature. PDS values were normally distributed (skewness = −.71 and kurtosis = .08).
Correlations between each inflammatory biomarker and several non-demographic potential covariates previously reported to be associated with proinflammatory biomarkers were also examined. The relationship between each biomarker and time of blood draw, diagnosis of pertinent medical condition, use of immunomodulating medications1, race, and BMI were tested. Variables that were correlated with a specific inflammatory biomarker were included as covariates in the model predicting that biomarker. CRP was associated with BMI (r = .54, p < .001), IL-6 was associated with BMI (r = .38, p < .001), IL-8 was associated with child race (r = −.14, p = .08, BMI (r = −.14, p = .07), and pertinent medical condition diagnosis (r = .17, p = .05), and TNF-α was associated with time of blood draw (r = −.16, p = .05) and race (r = −.15, p =.07).
Differences in the levels of each inflammatory biomarker by sex, race, and SES were investigated using independent samples t-tests. The levels of IL-6 were higher in females than males (IL-6: t(153) = −3.72, p < .001); the levels of TNF-α were higher in males than females (t(153) = 2.03, p = .045). However, there were no significant differences between males and females in mean levels of CRP (t(153) = −1.60, p = .11) or IL-8 (t(153) = .15, p = .88). There were no significant differences between individuals who identified as Caucasian versus African American in mean CRP (t(153) = −.49, p = .66) or IL-6 levels (t(153) = −.94, p = .35), but African American participants tended to have lower levels of TNF-α (t(153) = 1.84, p = .07) and IL-8 (t(153) = 1.77 p = .08). SES was not associated with significant differences for any of the four inflammatory biomarkers (CRP: (t(145) = 1.39, p = .11); IL-8: (t(145) = .70, p = .48); IL-6: (t(145) = −.25, p = .81); TNF-α (t(145) = .71, p = .48)).
Age was positively correlated with IL-6 (r = .19, p = .02) and CRP (r = .18, p = .02) and negatively correlated with IL-8 levels (r = −.25, p = .002). A more advanced pubertal status was significantly positively correlated with IL-6 (r = .20, p = .01) and age (r = .34, p < .001), and significantly negatively correlated with IL-8 (r = −.21, p = .01) and TNF-α (r = −.27, p = .001). BMI was significantly positively associated with CRP (r = .54, p < .001), IL-6 (r = .38, p < .001), and PDS (r = .18, p = .03). Descriptive statistics and bivariate correlations among primary study variables are presented in Tables 1a–c and 2, respectively.
Associations of Pubertal Status and Age with Proinflammatory Biomarkers
Linear regression models were run to examine associations of age and pubertal status with each proinflammatory biomarker. Every model included days between the pubertal status assessment and the blood draw as a covariate. In addition, each model controlled for variables that were significantly associated with the dependent variable (inflammatory biomarker). Finally, because the aim of the study was to examine the unique associations between pubertal status and inflammatory biomarkers controlling for age, and age and inflammatory biomarkers controlling for pubertal status, just one model predicting each biomarker was conducted with pubertal status and age both entered as predictors, as well as other relevant covariates. In addition, because sex was tested as a moderator of each association, sex was not included as a covariate in the linear regression models. The linear regression models testing pubertal status and age as predictors of each proinflammatory marker are presented in Table 3.
Table 3.
Linear regression analyses testing pubertal status and age as predictors of each pro-inflammatory biomarker. Pubertal status and age are entered into the same model to evaluate their unique contribution to variance in each pro-inflammatory biomarker.
| CRP | |||
|---|---|---|---|
| b | B | SE | |
| Days Difference | −.002 | −0.07 | 0.002 |
| BMI | 0.05 | 0.53*** | 0.01 |
| Pubertal Status | 0.004 | 0.004 | 0.08 |
| Age | 0.05 | 0.12+ | 0.03 |
| R2=.31*** | |||
| IL-6 | |||
| b | B | SE | |
| Days Difference | 0.000 | 0.08 | 0.001 |
| BMI | 0.02 | 0.36*** | 0.003 |
| Pubertal Status | 0.05 | 0.10 | 0.04 |
| Age | 0.02 | 0.11 | 0.02 |
| R2=.18*** | |||
| TNF-α | |||
| b | B | SE | |
| Days Difference | −0.001 | −0.10 | 0.001 |
| Time of BD | −0.01 | −0.18* | 0.004 |
| Race | −0.05 | −0.21** | 0.02 |
| Pubertal Status | −0.06 | −0.27** | 0.02 |
| Age | 0.001 | 0.01 | 0.006 |
| R2=.15** | |||
| IL-8 | |||
| b | B | SE | |
| Days Difference | −0.001 | −0.04 | 0.001 |
| Race | −0.09 | −0.17* | 0.04 |
| BMI | −0.003 | −0.07 | 0.003 |
| Pertinent Medical Condition | −0.07 | −0,10 | 0.05 |
| Pubertal Status | −0.07 | −0.15+ | 0.04 |
| Age | −0.03 | −0.20* | 0.01 |
| R2=.14** |
Note: CRP = C-reactive protein; IL-8 = interleukin-8; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; BMI = body mass index; BD = blood draw; b= unstandardized beta; B=standardized beta; SE = standard error.
p<.10;
p<.05;
p<.01;
p<.001.
After controlling for age and other variables associated with each biomarker, pubertal status was negatively associated with TNF-α (B = −.27, SE = .02, p = .001) and marginally negatively associated with IL-8 (B = −.15, SE = .04, p = .08). Pubertal status was not significantly associated with CRP (B = .004, SE = .08, p = .96) or with IL-6 (B = .10, SE = .04, p = .18).
There was a significant effect of age, such that age was negatively associated with IL-8 and marginally positively associated with CRP (IL-8: B = −.20, SE = .01, p = .02; CRP: B = .12, SE = .03, p = .098), but not IL-6 or TNF-α (IL-6: B = .11, SE = .02 p = .15; TNF-α: B = .01, SE = .006, p = .86).
Pubertal Status x Sex Interactions
To evaluate whether these associations differed by sex, moderation analyses were conducted to assess the Age X Sex interaction and Pubertal Status x Sex interaction for predicting each biomarker. In each moderation analysis, covariates, main effects, and the interaction terms were entered into the model. All predictors (pubertal status, age, and sex) were mean-centered. To probe significant interactions, the independent variable’s relationship with the dependent variable was plotted for males and females separately (Aiken & West, 1991).
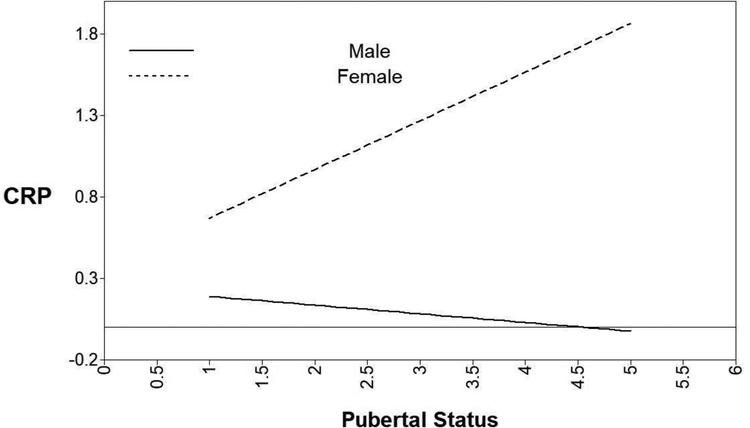
The pubertal status x sex interaction was significant for CRP (Table 4; Figure 1; B = .13, SE = .17, p = .04). Decomposition of the interaction revealed that the simple slope for boys was not significant (b = −.05, SE = .09, p = .53); however, the association between pubertal status and CRP was marginally significant for girls (b = .30, SE = .16, p = .07). There was not a significant pubertal status x sex interaction predicting IL-6, TNF-α, or IL-8 levels (B = .11 SE = .09, p = .13; B = .000, SE = .04, p = .998; B = −.02, SE = .08, p = .84, respectively).
Table 4.
Pubertal status x sex interaction predicting CRP.
| b | B | SE | |
|---|---|---|---|
| Days Difference | −0.002 | −0.06 | 0.002 |
| Age | 0.06 | 0.15* | 0.03 |
| BMI | 0.05 | 0.54*** | 0.01 |
| Pubertal Status | −0.05 | −0.05 | 0.09 |
| Sex | 0.13 | 0.11 | 0.09 |
| Pubertal Status x Sex | 0.35 | 0.13* | 0.17 |
| R2= .34*** |
Note: CRP = C-reactive protein; BMI = body mass index; b= unstandardized beta; B=standardized beta; SE = standard error.
p<.05;
p<.01;
p<.001.
Figure 1.
Decomposition of the interaction between pubertal status and sex predicting CRP (C-Reactive Protein (log mg/DL)).
The age x sex interaction did not predict any of the four inflammatory biomarkers significantly (CRP: B = .11, SE = .06, p = .10; IL-6: B = −.08, SE = .03, p = .29; TNF-α: B = −.12, SE = .01, p = .12; IL-8: B = .05, SE = .03, p = .54).
Alternate Model Analyses
Models testing whether pubertal timing, rather than pubertal status, predicted any of the four biomarkers and whether these relations differed by sex also were run. Since the pubertal timing variable was computed based on age at the time of the assessment, age was not entered as a covariate in these models. Pubertal timing did not predict CRP (B = .04, SE = .03, p = .55), IL-6 (B = −.001, SE = .02, p = .99), TNF-α (B = −.03, SE = .01, p = .71), or IL-8 (B = −.13, SE = .02, p = .11). In addition, the pubertal timing x sex interaction did not significantly predict any of the four biomarkers (CRP: B = −.05, SE = .07, p = .50; IL-6: B = −.004, SE = .03, p = .96; TNF-α: B = .03, SE = .02, p = .67; IL-8: B = .01, SE = .03, p = .91).
Additionally, models were run including all participants, regardless of when their PDS assessment was completed (N=183). The mean number of days between the blood draw and the PDS in this sample was −27.04 (SD = 101.36). The pattern of results was largely the same as when these participants were excluded. However, when these participants were included, pubertal status significantly predicted IL-6 (B = .15, SE = .04, p = .04).
Discussion
A comprehensive understanding of pubertal development is integral to the study of adolescence and is relevant to identifying the processes that underlie the increase in negative physical and psychological health outcomes in adolescents. Changes in inflammatory physiology during the pubertal period may play a role, especially when considered in the context of the emotional and stress-related volatility of adolescence. However, to date, previous analyses have not attempted to delineate the specific contributions of pubertal staging from chronological age on the levels of proinflammatory biomarkers in adolescents.
After controlling for age and several other variables that could influence each proinflammatory biomarker, a more advanced pubertal status was uniquely associated with lower levels of TNF-α and marginally associated with lower levels of IL-8 among both females and males. The association of pubertal status with CRP was more selective and sex-specific, with a more advanced pubertal status significantly associated with higher CRP levels among females only. However, when controlling for pubertal status and other variables that have been associated with each biomarker, older age marginally was associated with higher CRP among both male and female adolescents. Participant age also was significantly correlated with lower IL-8 levels among both male and female adolescents.
These results are consistent with prior work suggesting that sex hormones, which increase during puberty, can have an immunomodulatory effect on the immune system and inflammatory physiology (Corcoran. Meydani, Liechtenstein, Schaefer, Dillard, & Lamon-Fava, 2010). Further, increases in the levels of CRP in the blood stream have been associated with a number of psychological conditions, including depression that also increase in prevalence after puberty (Beeson, 1994; Deardorff et al., 2007; Hayward et al., 1999; Kinney et al., 2010; Patton et al., 2004). Therefore, the current findings are suggestive of a shared mechanism or parallel processes that may contribute to the more common occurrence of depression in adolescent females. However, the cross-sectional nature of our study, and the lack of a diagnostic verification of depression precludes a more definitive statement, and this hypothesis should be addressed with a prospective, longitudinal design.
Some of the findings differed from the generalization from prior literature that females typically would have higher levels of inflammatory proteins in their blood and that this sex difference would become more divergent with age during the pubertal period. The prior literature indicates that sex-typical hormones, including estrogen, progesterone and testosterone, have immunomodulatory effects. Specifically, estrogen often has been reported to stimulate inflammatory responses, whereas progesterone and testosterone have been described as largely anti-inflammatory (Cutolo & Wilder, 2000; DaSilva, 1995; Malkin et al., 2004; Verthelyi, 2001). Therefore, we hypothesized that a more advanced pubertal status would be associated with higher levels of proinflammatory biomarkers in females. However, a more advanced pubertal status actually was associated with lower levels of two important cytokines, TNF-α and IL-8. Although counter to expectations, the findings suggest a need for a more nuanced understanding of the relationship among sex-typical development and inflammatory physiology. For example, one other study of the cellular immune responses of younger girls and boys found that the white blood cells of males released higher levels of cytokines compared to females (Casimir et al., 2010). A study of older adults found that testosterone was associated with lower TNF-α, but not IL-6 or CRP. There also have been several studies that have failed to find an effect of estrogen on TNF-α or IL-6, but CRP levels were affected (dependent on other factors, such lipids; Corcoran et al., 2010). Finally, one study found that IL-8 levels were higher in estrogen-deficient patients (Payne, Reinhardt, Masada, DuBois, & Allison, 1993). Collectively, these findings indicate that more research needs to be done to better understand the extent of the sex-related differences in inflammatory physiology and immunity, especially during adolescence. Many of the previous reviews may have over-generalized their conclusions from the immune changes associated with pregnancy, when reproductive hormones are much higher than in women during normal cycles. In addition, many of the experimental findings have been generated from animal models that may show larger sex-specific differences than found in humans.
Additionally, pubertal status did not predict levels of IL-6. However, IL-6 concentrations were significantly higher in females in this sample. It is possible that the nature of the sample contributed to the lack of an effect. Specifically, the earliest stages of pubertal development were not captured in this sample, and by virtue of girls starting puberty earlier than boys (Abbassi, 1998), males in this sample were significantly less pubertally developed than females. Perhaps this effect would be evident in the earlier stages of pubertal development. It is critical to replicate the current findings using a younger, less developed sample.
In addition, African Americans have been shown to have higher levels of IL-6 and CRP than Caucasian individuals, and this effect is particularly strong for women (Carroll et al., 2009; Khera et al., 2005). However, in the current study, levels of IL-6 and CRP did not differ between Caucasian and African American youth, and African American youth had marginally lower levels of TNF-α and IL-8. It would be interesting to investigate whether the pattern of results in the present study differs by race (and sex). However, we did not have adequate power in the present study sample to test a three-way interaction or to split the sample by race to appropriately interrogate this hypothesis.
Similarly, pubertal timing, rather than pubertal status, is a well-documented independent predictor of many psychological disorders (e.g., mood and anxiety disorders; Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997) and physical health disorders (Day, Elks, Murray, Ong, & Perry, 2015). Therefore, we tested whether pubertal timing was associated with the levels of proinflammatory biomarkers independent of pubertal status. We found that pubertal timing did not predict any of the four biomarkers. However, at the time of the first blood draw in this study, youth were 16 years old on average, which is well beyond the average age that children now enter puberty. Consequently, there was reduced variation in our measure of pubertal timing at the time of the blood draw, which could explain these null findings.
It is important to interpret these findings in light of the limitations of the current study. First, the lowest score on the PDS in the current sample was 1.80, meaning that the current sample had already begun puberty, and we could not capture youth at the very early stages of pubertal development. Although there was variation in pubertal stage at each age group in the sample (Table 1c), this sample could not capture the full range of development, and it is possible that youth of younger or older ages may show different patterns of associations between pubertal development and proinflammatory biomarker concentrations. However, this study is an important first step in elucidating pubertally-related associations with proinflammatory concentrations in adolescents. Future work should aim to replicate these findings in a sample that includes youth in earlier stages of development.
Second, this study was cross-sectional, so causality cannot be inferred from these findings. However, these results demonstrate an association between pubertal status and proinflammatory biomarkers independent of age that future work should investigate longitudinally. Investigating within-person changes in pubertal development over time and proinflammatory biomarkers could allow us to verify whether the observed variations in inflammatory biomarkers in this study are, in fact, due to more advanced pubertal development.
We also used a self-report measure of pubertal status, as opposed to a biological measure such as hormone levels, so the mechanisms of the association cannot be inferred from the current analyses. It is important to note that there is evidence that pubertal stage as assessed by the PDS is correlated with hormone levels (Shirtcliff et al., 2009). However, this was not tested directly. It is possible that increases in sex hormones in male and female adolescents as they advance through puberty are responsible for changes in proinflammatory biomarkers, especially in light of evidence that sex hormones have immunomodulatory effects (Verthelyi, 2001). Alternatively, pubertal development may induce changes in the HPA-axis and cortisol levels (Stroud et al., 2011), which, in turn, may alter immune functioning and levels of inflammation (Hansel et al., 2010). Future work should empirically test these hypotheses.
Relatedly, some of the PDS assessments used in the analyses were collected on a different day than the day of the blood draw. About half of the sample completed their PDS on the day of the blood draw, but some youth completed the measure as much as 70 days after the blood draw. Consequently, youth may have actually been less pubertally developed on the day of the blood draw. We accounted for this discrepancy by controlling for the number of days between the PDS assessment and the blood draw. However, there is variability in the amount of time it takes for youth to progress through puberty (Mendle, Harden, Brooks-Gunn, & Graber, 2010). This is referred to as pubertal tempo, and pubertal tempo is linked to mood-related disorders and symptoms (Mendle, 2014). Thus, it is possible that this variability may have affected the results.
However, the current study also has a number of important strengths. It is the first study to attempt to disentangle the independent associations of age and pubertal status with concentrations of proinflammatory biomarkers among adolescents. Currently, there is a dearth of literature on normative developmental factors associated with proinflammatory markers among adolescents. The current study adds to our understanding of the pattern of proinflammatory biomarkers among adolescents and whether this pattern varies as a function of age or pubertal development. This knowledge is critical for the field, as it highlights: 1) basic, much needed information about important correlates of peripheral inflammation in adolescence; and 2) processes that may contribute to the increase in physical and psychological disorders during adolescence. This knowledge also has important methodological implications, as it suggests that future work investigating proinflammatory biomarker levels in adolescents should account for variation due to pubertal status. Although there are no direct implications for society from these findings, they could lead to new research on adolescent development, which, in turn, could inform improved prevention and intervention efforts to promote normal development during a vulnerable developmental period. In addition, the study was conducted with a community sample that was diverse in race and SES and included females and males across a wide age range, allowing these results to be generalizable to many youth in the population.
Conclusion
The current study aimed to disentangle the independent associations of age and pubertal status with inflammatory biomarker concentrations in youth and improve knowledge about normative developmental correlates of inflammatory activity during adolescence. These findings demonstrated that pubertal staging is uniquely associated with inflammatory physiology above and beyond the effects of chronological age. The results indicate the need to consider both participant age and pubertal maturation when investigating inflammatory physiology in adolescents. Further, the sex-specific effect of pubertal stage on CRP levels suggests that biomarker concentrations either may be a contributory factor or reflective of the emergence of certain psychological conditions that also differ in expression during adolescence. In addition, our findings on inflammatory biomarkers are consistent with clinical research that has documented that there are significant sex differences in the prevalence of some inflammatory diseases that change before and after puberty. Further work is needed to integrate the findings from the psychological and clinical literatures and to elucidate the underlying biological mediators.
Table 1b.
Total N and percent of study sample in each pubertal stage and age range.
| Overall Sample | Males | Females | ||||
|---|---|---|---|---|---|---|
| Pubertal Stage | N | % | N | % | N | % |
| 1 | 3 | 1.90% | 3 | 4.20% | 0 | - |
| 2 | 29 | 18.70% | 24 | 33.80% | 5 | 6.00% |
| 3 | 101 | 65.20% | 41 | 57.70% | 60 | 71.40% |
| 4 | 22 | 14.20% | 3 | 4.20% | 19 | 22.60% |
| Age Range | N | % | N | % | N | % |
| 12.00–13.99 | 11 | 7.10% | 4 | 5.60% | 7 | 8.30% |
| 14.00–15.99 | 62 | 40.00% | 35 | 49.30% | 27 | 32.10% |
| 16.00–17.99 | 62 | 40.00% | 24 | 33.80% | 38 | 45.20% |
| 18.00–20.01 | 20 | 12.90% | 8 | 11.30% | 12 | 14.30% |
Acknowledgments
Funding
This research was supported by National Institute of Mental Health Grants MH101168 and MH079369 to Lauren B. Alloy.
Biography
Allison Stumper is a doctoral student in Lauren B. Alloy’s Mood and Cognition Lab at Temple University. Her major research interests include how biological and environmental factors interact to confer risk for adolescent depression, with an emphasis on the role of biological changes as a result of the pubertal transition.
Daniel P. Moriarity is a doctoral student in Lauren B. Alloy’s Mood and Cognition Lab at Temple University. His major research interests include how cognitive vulnerabilities and inflammation interact to confer risk for mood disorders.
Christopher L. Coe is the W.B. Cannon Professor of Biopsychology at the University of Wisconsin-Madison. He received his doctorate in the behavioral neurosciences at Downstate Medical Center, SUNY. His research interests include the relationship between biomarkers and health, with a focus on resilience and vulnerability at the beginning and end of the life course, specifically the prenatal period and old age.
Lauren M. Ellman is an Associate Professor of Psychology at Temple University. She received her doctorate in Clinical Psychology at the University of California-Los Angeles. Her major research interests include neurodevelopmental risk factors, including inflammation, for psychotic disorders (e.g. schizophrenia) and depression.
Lyn Y. Abramson is the Sigmund Freud Professor of Psychology at the University of Wisconsin-Madison. She received her doctorate in Clinical Psychology from the University of Pennsylvania. Her major research interests include the developmental, cognitive, motivational, and cultural determinants of information processing about the self and the effects of early psychological, physical, and sexual maltreatment on the development of cognitive styles and vulnerability to depression in adulthood.
Lauren B. Alloy is Laura H. Carnell Professor and Joseph Wolpe Distinguished Faculty in Psychology at Temple University. She received her doctorate in Experimental and Clinical Psychology from the University of Pennsylvania. Her major research interests include cognitive, psychosocial, developmental, and, neurobiological processes in the onset and course of depression and bipolar disorder.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data Sharing Declaration
The datasets generated and/or analyzed during the current study are not publicly available but may be available from the corresponding author on reasonable request.
Conflicts of Interest
The authors report no conflict of interests.
Ethical approval
The Temple University Institutional Review Board approved the protocol (IRB protocol #6844).
Informed Consent
Written informed consent was collected from all study participants after explaining their role in the study and before starting data collection.
Immunomodulatory medication use was not significantly associated with any dependent variables, and therefore, was not included in the models. However, when it was included, the pattern of results remained the same. Similarly, only IL-8 was associated with the diagnosis of a medical condition that could affect inflammation, so only models predicting IL-8 included this variable as a covariate. However, when these cases were excluded, the pattern of results remained the same.
References
- Abbassi V (1998). Growth and normal puberty. Pediatrics, 102(Supplement 3), 507–511. [PubMed] [Google Scholar]
- Aiken LS, West SG, & Reno RR (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Alloy LB, Black SK, Young ME, Goldstein KE, Shapero BG, Stange JP, … Abramson LY (2012). Cognitive vulnerabilities and depression versus other psychopathology symptoms and diagnoses in early adolescence. Journal of Clinical Child and Adolescent Psychology, 41(5), 539–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Hamilton JL, Hamlat EJ, & Abramson LY (2016). Pubertal development, emotion regulatory styles, and the emergence of sex differences in internalizing disorders and symptoms in adolescence. Clinical Psychological Science, 4, 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, & Worthman CM (1999). Pubertal changes in hormone levels and depression in girls. Psychological Medicine, 29(5), 1043–1053. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, & Worthman CM (1998). Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine, 28(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med 1994; 96: 457–62. [DOI] [PubMed] [Google Scholar]
- Bell JA, Kivimäki M, Bullmore ET, Steptoe A, Bullmore E, Vértes PE, … & Hume D (2017). Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Translational Psychiatry, 7(8), e1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, … Norris PJ (2011). Multisite comparison of high-sensitivity multiplex cytokine assays. Clinical and Vaccine Immunology, 18(8), 1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM (2002). Eating disorders in adolescents and young adults. Child and Adolescent Psychiatric Clinics of North America, 11(2), 201–218. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Fulda KG, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, … & Cardarelli R (2009). Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity, 17(7), 1420–1427. [DOI] [PubMed] [Google Scholar]
- Casimir GJ, Heldenbergh F, Hanssens L, Mulier S, Heinrichs C, Lefevre N, … & Duchateau J (2010). Gender differences and inflammation: an in vitro model of blood cells stimulation in prepubescent children. Journal of inflammation, 7(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, & Chrousos GP (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences, 966(1), 290–303. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Pieper CF, Harris T, Rao KMK, & Currie MS (1997). The association of plasma IL-6 levels with functional disability in community-dwelling elderly. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 52(4), M201–M208. [DOI] [PubMed] [Google Scholar]
- Cook RT (1998). Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcoholism: Clinical and Experimental Research, 22(9), 1927–1942. [PubMed] [Google Scholar]
- Curtis AC (2015). Defining adolescence. Journal of Adolescent and Family Health, 7(2), 2. [Google Scholar]
- Cutolo M, & Wilder RL (2000). Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheumatic Disease Clinics of North America, 26(4), 825–839. [DOI] [PubMed] [Google Scholar]
- Dabitao D, Margolick JB, Lopez J, & Bream JH (2011). Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. Journal of immunological methods, 372(1–2), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandona P, Aljada A, & Bandyopadhyay A (2004). Inflammation: the link between insulin resistance, obesity and diabetes. Trends in Immunology, 25(1), 4–7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, & Kelley KW (2007). Twenty years of research on cytokine-induced sickness behavior. Brain, behavior, and immunity, 21(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Elks CE, Murray A, Ong KK, & Perry JR (2015). Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Scientific Reports, 5, 11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, & Rifai N (2006). Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clinical Chemistry, 52(7), 1325–1330. [DOI] [PubMed] [Google Scholar]
- Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, & Lamon-Fava S (2010). Sex hormone modulation of proinflammatory cytokine and CRP expression in macrophages from older men and postmenopausal women. The Journal of endocrinology, 206(2), 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. [Google Scholar]
- Drabick DA, & Kendall PC (2010). Developmental psychopathology and the diagnosis of mental health problems among youth. Clinical Psychology: Science and Practice, 17(4), 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, D’sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, & Sinha R (2012). Immune system inflammation in cocaine dependent individuals: implications for medications development. Human Psychopharmacology: Clinical and Experimental, 27(2), 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C (2006). Interleukin-6 and chronic inflammation. Arthritis research & therapy, 8(2), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, & Olatawura MO (1998). Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Archives of General Psychiatry, 55(5), 405–413. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, & Brooks-Gunn J (1997). Is psychopathology associated with the timing of pubertal development?. Journal of the American Academy of Child & Adolescent Psychiatry, 36(12), 1768–1776. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, & Miller BJ (2016). A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Molecular psychiatry, 21(12), 1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of abnormal psychology, 107(1), 128. [DOI] [PubMed] [Google Scholar]
- Hänsel A, Hong S, Camara RJ, & Von Kaenel R (2010). Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neuroscience & Biobehavioral Reviews, 35(1), 115–121. [DOI] [PubMed] [Google Scholar]
- Hayward C, Gotlib IH, Schraedley PK, & Litt IF (1999). Ethnic differences in the association between pubertal status and symptoms of depression in adolescent girls. Journal of Adolescent Health, 25(2), 143–149. [DOI] [PubMed] [Google Scholar]
- Irwin MR, & Cole SW (2011). Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology, 11(9), 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA (1989, January). Approaching the asymptote? Evolution and revolution in immunology In Cold Spring Harbor symposia on quantitative biology (Vol. 54, pp. 1–13). Cold Spring Harbor Laboratory Press. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K (2009). Psychological trauma and physical health: A psychoneuroimmunology approach to etiology of negative health effects and possible interventions. Psychological Trauma: theory, research, practice, and policy, 1(1), 35. [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, … & de Lemos JA (2005). Race and gender differences in C-reactive protein levels. Journal of the American College of Cardiology, 46(3), 464–469. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, & Merikangas KR (2001). Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry, 49(12), 1002–1014. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, & Glaser R (2002). Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annual review of psychology, 53(1), 83–107. [DOI] [PubMed] [Google Scholar]
- Killen JD, Hayward C, Litt I, Hammer LD, Wilson DM, Miner B, … & Shisslak C (1992). Is puberty a risk factor for eating disorders?. American Journal of Diseases of Children, 146(3), 323–325. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Hintz K, Shearer EM, Barch DH, Riffin C, Whitley K, & Butler R (2010). A unifying hypothesis of schizophrenia: abnormal immune system development may help explain roles of prenatal hazards, post-pubertal onset, stress, genes, climate, infections, and brain dysfunction. Medical Hypotheses, 74(3), 555–563. [DOI] [PubMed] [Google Scholar]
- Klein SL, & Flanagan KL (2016). Sex differences in immune responses. Nature Reviews Immunology, 16(10), 626. [DOI] [PubMed] [Google Scholar]
- Holmbeck GN (2002). A developmental perspective on adolescent health and illness: An introduction to the special issues. Journal of pediatric psychology, 27(5), 409–416. [DOI] [PubMed] [Google Scholar]
- Leon GR, Fulkerson JA, Perry CL, & Early-Zald MB (1995). Prospective analysis of personality and behavioral vulnerabilities and gender influences in the later development of disordered eating. Journal of abnormal psychology, 104(1), 140. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, & Allen NB (1998). Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology, 107(1), 109. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Buckley ME, & Klein DN (2002). Bipolar disorder in adolescence and young adulthood. Child and Adolescent Psychiatric Clinics of North America, 11(3), 461–75. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Striegel-Moore RH, & Seeley JR (2000). Epidemiology and natural course of eating disorders in young women from adolescence to young adulthood. Journal of the American Academy of Child & Adolescent Psychiatry, 39(10), 1284–1292. [DOI] [PubMed] [Google Scholar]
- Linton PJ, & Dorshkind K (2004). Age-related changes in lymphocyte development and function. Nature immunology, 5(2), 133. [DOI] [PubMed] [Google Scholar]
- Maahs DM, West NA, Lawrence JM, & Mayer-Davis EJ (2010). Epidemiology of type 1 diabetes. Endocrinology and Metabolism Clinics of North America, 39(3), 481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, & Jones TH (2004). The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. The Journal of Clinical Endocrinology & Metabolism, 89(7), 3313–3318. [DOI] [PubMed] [Google Scholar]
- Mendle J (2014). Beyond pubertal timing: New directions for studying individual differences in development. Current Directions in Psychological Science, 23(3), 215–219. [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, & Graber JA (2010). Development’s tortoise and hare: pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Developmental Psychology, 46(5), 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, … & Swendsen J (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen‐Woods S, & Baune BT (2013). Research review: the role of cytokines in depression in adolescents: a systematic review. Journal of Child Psychology and Psychiatry, 54(8), 816–835. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Vinberg M, & Kessing LV (2013). Cytokines in bipolar disorder: a systematic review and meta-analysis. Journal of Affective Disorders, 144(1–2), 16–27. [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén B (2015). Mplus. The comprehensive modelling program for applied researchers: user’s guide, 5. [Google Scholar]
- Moudgil KD, & Choubey D (2011). Cytokines in autoimmunity: role in induction, regulation, and treatment. Journal of Interferon & Cytokine Research, 31(10), 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mahony L, Holland J, Jackson J, Feighery C, Hennessy TPJ, & Mealy K (1998). Quantitative intracellular cytokine measurement: age-related changes in proinflammatory cytokine production. Clinical and Experimental Immunology, 113(2), 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, & Catalano RF (2004). Puberty and the onset of substance use and abuse. Pediatrics, 114(3), e300–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JB, Reinhardt RA, Masada MP, DuBois LM, & Allison AC (1993). Gingival crevicular fluid IL‐8: correlation with local IL‐1β levels and patient estrogen status. Journal of periodontal research, 28(6), 451–453. [PubMed] [Google Scholar]
- Pepys MB, & Hirschfield GM (2003). C-reactive protein: a critical update. The Journal of clinical investigation, 111(12), 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Remick DG (2005). Interleukin-8. Critical care medicine, 33(12), S466–S467. [DOI] [PubMed] [Google Scholar]
- Riancho A, Zarrabeitia MT, Amado A, Olmos M, & González-Macías J (1994). Age-related differences in cytokine secretion. Gerontology, 40(1), 8–12. [DOI] [PubMed] [Google Scholar]
- Rudy BJ, Wilson CM, Durako S, Moscicki AB, Muenz L, & Douglas SD (2002). Peripheral blood lymphocyte subsets in adolescents: a longitudinal analysis from the REACH project. Clin. Diagn. Lab. Immunol, 9(5), 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological bulletin, 130(4), 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal development: correspondence between hormonal and physical development. Child development, 80(2), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solmi M, Veronese N, Favaro A, Santonastaso P, Manzato E, Sergi G, & Correll CU (2015). Inflammatory cytokines and anorexia nervosa: A meta-analysis of cross-sectional and longitudinal studies. Psychoneuroendocrinology, 51, 237–252. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kunkel SL, & Bone RC (1993). Role of tumor necrosis factor-alpha in disease states and inflammation. Critical care medicine, 21(10 Suppl), S447–63. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, & Dahl RE (2011). Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology, 36(8), 1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, & Rivier CL (1999). Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiological reviews, 79(1), 1–71. [DOI] [PubMed] [Google Scholar]
- Van Nimwegen L, De Haan L, Van Beveren N, Van Den Brink W, & Linszen D (2005). Adolescence, schizophrenia and drug abuse: a window of vulnerability. Acta Psychiatrica Scandinavica, 111, 35–42. [DOI] [PubMed] [Google Scholar]
- Van Snick J (1990). Interleukin-6: an overview. Annual review of immunology, 8(1), 253–278. [DOI] [PubMed] [Google Scholar]
- Verthelyi D (2001). Sex hormones as immunomodulators in health and disease. International Immunopharmacology, 1(6), 983–993. [DOI] [PubMed] [Google Scholar]
- Walker E, & Bollini AM (2002). Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophrenia research, 54(1–2), 17–23. [DOI] [PubMed] [Google Scholar]
- Whitacre CC (2001). Sex differences in autoimmune disease. Nature Immunology, 2(9), 777. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, & Marceau K (2008). Disorders of childhood and adolescence: Gender and psychopathology. Annu. Rev. Clin. Psychol, 4, 275–303. [DOI] [PubMed] [Google Scholar]