Abstract
Oxytocin (OXT) regulates adult social behavior and has been implicated in its development. Because mammalian milk contains OXT and we have recently identified OXT receptors (OXTR) in the face and oronasal cavity of pre-weaning mice, we hypothesize that orally applied OXT may impact brain activity and acute behavior in developing mice. Oral OXT may have effects in the absence of sensory stimulation or perhaps by modulating sensory input, such as whisker stimulation. The present study investigates the acute c-Fos response in the paraventricular nucleus of the hypothalamus (PVN) and along whisker sensory processing brain regions (trigeminothalamocortical circuit) to orally applied OXT, compared to saline, with and without whisker stimulation in postnatal day (P) 14 and P21 male and female mice. Acute behavioral responses were also quantified after oral OXT with whisker stimulation in a non-social context. Oral OXT with and without whisker stimulation increased c-Fos activity in the PVN of males and decreased c-Fos in the ventroposterior medial thalamus in both males and females compared to saline. Additionally, oral OXT with whisker stimulation decreased c-Fos activity across whisker sensory processing brain regions in males and females and decreased c-Fos activity in the trigeminal motor nucleus of females. Lastly, oral OXT with whisker stimulation increased males’ locomotor behavior and decreased females’ oromotor behavior compared to saline-treated controls. These data indicate that orally applied OXT has acute brain and behavioral effects on developing mice. OXT-modulated sensory signals may bias brain and behavior development toward the social world.
Keywords: oxytocin, oxytocin receptor, pre-weaning, oromotor behavior, oronasal cavity, c-Fos, oxtr-egfp, whisker stimulation, trigeminal ganglion
Introduction:
It is well documented that oxytocin (OXT) acts through the OXT receptor (OXTR) and vasopressin receptors to affect homeostatic processes and social behaviors in mammals1. OXT action in the central nervous system regulates social behaviors such as maternal-offspring interactions in mammals, pair bonding in prairie voles, and interacts with the dopaminergic system to regulate social reward, among other effects2-5. OXT action in the peripheral nervous system is well-known to facilitate labor and delivery as well as the milk let down reflex for nursing. In addition, accumulating evidence suggests that OXT interacts with the environment to regulate species typical development. For example, early life maternal-offspring experience alters the trajectory of adult OXT function in the brain with consequences for maternal behavior6,7. Similarly, altering the OXT system early in life alters behaviors during development and in adulthood, such as huddling behavior in rat pups and pair bonds in adult prairie voles8-11. Significantly, OXT also interacts with the environment to regulate cross-modal sensory plasticity in the cortex of developing mice12 during a putative sensitive period in which there is an increase in cortical OXTR13. At postnatal day (P) 14 in mice, there is a transient peak in cortical OXTR expression which decreases by P2113 and sensory experience increases while sensory deprivation decreases OXT synthesis in the paraventricular nucleus of the hypothalamus (PVN)12, a major neural site for OXT production. Pre-weaning sensory experience also regulates individual differences in OXT levels released from the PVN into the neocortex at P14, which directly impact multisensory cortical plasticity during this time12. Altogether, these data implicate a role for OXT in multi-sensory integration during a sensitive time of development that corresponds with peak cortical OXTR expression in mice. These mechanisms during development may train the mouse brain to modulate the OXT system for its adult role in social signal processing and behavior14-16. However, the mechanisms by which OXT interacts with the environment throughout development to organize neural circuits that regulate adult behavior are unclear.
OXT exchange between conspecifics detected by peripheral OXTRs offers a provocative hypothesis for a mechanism to regulate brain and behavior throughout development in an experience-dependent manner. OXTRs in the periphery have been characterized in the oronasal cavity, anogenital region, and whisker pads of developing mice, raising the intriguing possibility that socially-acquired OXT may modulate sensory development beginning at the sensory apparatus itself13,17. OXT exchanged between conspecifics may act as an external signal via peripheral OXTR to affect sensory processing, endogenous OXT activity, and the integration of sensory signals14. Both mammalian milk and saliva contain OXT which may be exchanged exclusively during social encounters, especially between parents and offspring early in life. Indeed, maternal interactions are the first and most conserved source of sensory experiences during mammalian development ensuring life-sustaining nutrition, protection, and warmth and greatly influences the socioemotional development of the offspring. Since milk contains OXT, nursing ensures an exogenous source of OXT to potentially act on oronasal OXTR and is contingent with social sensory experience to influence offspring’s neural development. It may be that this mechanism has unique and differential significance during specific time points of development, such as during the third postnatal week in mice in which there is a transient peak of OXTR expression in the neocortex, pups’ full sensory capabilities are evident in the neocortex, nursing continues, exploratory behavior increases as pups leave the nest for the first time, and experience-dependent OXT regulates cross modal cortical plasticity12. Social experience may modulate the OXT system via peripheral OXTR at this time14.
In the following experiments, we address a straightforward exploration of this hypothesis by asking if it is anatomically possible for orofacial afferent signals to be modulated by OXT. We quantified the behavioral and c-Fos response to orally applied OXT or saline in P14 and P21 male and female mice. These ages were chosen to reflect the onset of multisensory integration and the established peak of OXTR in multisensory layers II/III of primary somatosensory cortex at a time when pups are still nursing (e.g. P14- when all senses are capable of activating neurons in their primary neocortical representations) and when mice are routinely weaned and no longer exposed to maternal sources of OXT (e.g. P21 in laboratory models). First, we determined if OXTR is present in trigeminal sensory neurons innervating the face in P14 and P21 mice. This was achieved by observing EGFP expression under the control of the Oxtr promoter in the trigeminal ganglion of OXTR:EGFP reporter mice. Then, to test if oral OXT alters neural activity in brain regions that receive trigeminal sensory input during a putative sensitive period of sensory dependent OXT signaling, we quantified a neuronal marker of activation, c-Fos, after oral application of OXT or saline to the mouths of P14 and P21 males and females in two separate experiments. The first experiment (study 1) examined the baseline effects of oral OXT compared to saline on c-Fos. In the second experiment (study 2), the c-Fos response to oral OXT versus saline with sensory stimulation of the face (whisker brushing) was quantified. In study 2, we also video-recorded behavior of the mice in the holding cage for the first 10 minutes after oral application of OXT and whisker brushing. Whisker brushing was chosen as the mode of sensory stimulation because it was reproducible by the experimenter and likely plays a role in suckling. Whisker stimulation is also known to modulate the development of the PVN and is a potent activator of a known circuitry (the trigeminothalamocortical circuit), making it a tractable system. To address our hypothesis, we restricted our focus to the PVN, trigeminal brainstem nuclei, and the trigeminothalamocortical circuit. We measured c-Fos in the PVN because it has consistently been shown to be responsive to or associated with peripheral sensory stimulation including suckling12,18,19and there are traceable projections from the caudal trigeminal brainstem nuclei to the hypothalamus20-23. We had a main a priori hypothesis that oral OXT would induce c-Fos in the PVN of the hypothalamus. We also hypothesized that oral OXT would modulate c-Fos activity in trigeminothalamocortical neural circuits receiving sensory-dependent input from the whiskers. Finally, we performed exploratory analyses of the effects of oral OXT with whisker brushing on the behavior of preweaning mice in a socially isolated environment.
Mice
All procedures were conducted after approval by the Institutional Animal Care and Use Committee of Florida State University. All mice in this study were on a mixed FVB/N x Swiss-Webster background. Mice were all wild-type for Oxtr, but some carry an additional transgene under the control of the OXTR promoter24. Due to experimental plans to perform experiments in genetically manipulated animals, subjects in study 1 were the offspring of wild-type (FVB/N x Swiss-Webster mixed background strain) and OXTR:EGFP (GO185Gsat/Mmucd)25 and/or OXTR:Cre (ON66Gsat/Mmucd)26 transgenic breeder pairs. Subjects in study 2 included the offspring of wild-type and OXTR:EGFP transgenic breeder pairs. Supplementary Table 1 lists the genotypes of breeder pairs as well as subjects and the number of subjects per group. All animals were maintained on a 12:12 light cycle and had continuous access to standard chow (LabDiet 5001, PMI Nutrition International, LLC, Brentwood, MO) and water. Pre-weaning male and female subjects were left in the home cage undisturbed until experimental manipulation on P14 or P21, with the day of birth designated as P0, determined by daily observation of breeder cages.
Experimental Procedures
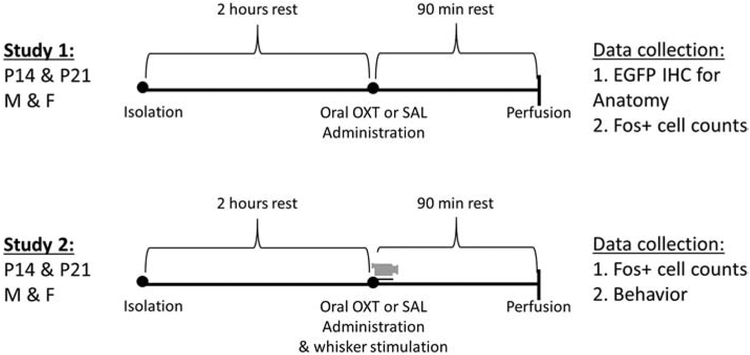
Figure 1 illustrates experimental details and timelines for studies 1 and 2. All experimental procedures were performed during the light phase from 9am to 5pm under standard room lighting and were identical for studies 1 and 2 except for unilateral whisker stimulation and behavioral recording which took place only in study 2. Previous studies indicate that dams nurse their offspring more in the light phase compared to the dark phase with consequences for maternal care and offspring brain and behavioral development27-30. Thus, oral OXT delivery during the light phase in nocturnal mice may achieve greater ethological relevance for pup feeding. When subjects reached P14 or P21, the parents were removed from the home cage and subjects’ body weight and sex were recorded. Subjects were then individually isolated in testing cages for a minimum of two hours before drug administration to control for potential individual differences in recent home cage nursing activity. The testing cage (identical to cages used in housing) consisted of a single clean polycarbonate cage lined with wood chip bedding and covered with an empty wire hopper lid. During isolation and testing, the subjects were not supplied with food and water or nesting material, in order to constrain the available behaviors to be displayed. Further, the subjects were tested alone, so the recorded behaviors were all in a non-social context.
Figure 1. Timeline and experimental details for studies 1 and 2:
Postnatal day (P) 14 and P21 males and females were administered oxytocin (OXT) or saline 2 hours after social isolation. Subjects in study 2 then received unilateral whisker brushing and their acute behavioral responses in a non-social environment were video recorded (video camera icon). Abbreviations: OXT = oxytocin; EGFP = enhanced green fluorescent protein; Fos+ = c-Fos positive neurons; IHC = immunohistochemistry
OXT Administration
OXT (oxytocin; H-2510.0005, Bachem) delivery was identical in studies 1 and 2; subjects were removed from the testing cage, gently scruffed, and administered orally 2μl of either a low or high dose of OXT dissolved in saline or saline only via a micropipette. While subjects were lightly scruffed, the tip of a 10μl micropipette tip was gently inserted in the lower mouth and 2μl of solution was dispensed between the tip of the tongue and bottom incisor teeth area, and visually confirmed to fully enter the oral cavity. The low dose of OXT (1,56pg/μl) dissolved in saline represents a physiological concentration of OXT previously reported in mammalian milk31,32 and is hereafter referred to as the “Milk” dose. The high dose of OXT (1.25ng/μl) dissolved in saline represents a clinical dose calculated to reflect OXT dosing in international units per kg prevalent in human clinical trials of intranasal drug delivery33-35 and is hereafter referred to as the “Clinical” dose. Drug administration was counterbalanced across treatment group, sex, and litter. After drug administration, subjects in study 1 were placed immediately back into the test cage, while subjects in study 2 underwent whisker stimulation (see next), then were returned to the test cage.
Unilateral Whisker Stimulation
In study 2, unilateral whisker brushing for approximately 60 seconds immediately followed drug administration while the animal was still gently scruffed. A small dry paint brush (Liquitex Basics, flat, Level 1) was used to gently stroke the entire right or left whisker pad in a clockwise and counterclockwise rotation as well as up and down directions. Unilateral whisker brushing (left or right) was counterbalanced across treatment group, sex, and litter. Subjects were immediately placed back into the testing cage after whisker brushing and left undisturbed for 90 minutes until they were collected for perfusion.
Behavioral Scoring
Behaviors for all subjects in study 2 were video recorded using 1080p HD video recorders (Lorex) placed on the side of the testing cages. Behaviors were later scored by a single researcher blind to the experimental condition of the subjects using JWatcher36 software. Behaviors scored include resting, locomotion, rearing, wall leaning, climbing cage top, quivering (shivering), jumping, chewing-like jaw movement, sniffing, and autogrooming. The duration and frequency of each behavior was quantified for the first ten minutes after the subject was placed back into the testing cage after drug administration and unilateral whisker brushing.
Body Weights
All subjects were weighed upon separation from their home cage just before habituation in the testing apparatus. Subjects were weighed again immediately prior to OXT or saline delivery and lastly directly prior to perfusion. Because study subjects did not have access to food or water during the experimental session, weight changes between timepoints were used to calculate “weight loss”, which could be influenced by experimentally-induced changes in thermogenesis, respiration, and/or urination/defecation.
Perfusion and Immunohistochemistry
Following 90 minutes after drug administration with or without unilateral whisker brushing, subjects were injected with a lethal dose of sodium pentobarbital (Fatal Plus). After final body weights were recorded and tail snips collected for genotyping, subjects were transcardially perfused with 1X phosphate buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (pH 7.4). Heads were post-fixed in 4% paraformaldehyde for approximately 24 hours at 4°C followed by cryoprotection in 10%, 20%, and then 30% sucrose in PBS, each for approximately 24 hours. Brains were dissected and stored at 4°C in 30% sucrose in PBS.
Brains were sectioned in the sagittal plane at 40μm in six series on a microtome for each subject and stored at −20°C in cryoprotectant until immunohistochemistry. In study 1, sections were collected in a successive series of six microcentrifuge tubes. In study 2, right and left hemisphere sections were collected separately in two separate sets of six series for hemisphere-specific examination.
To accommodate all subjects, multiple batches of IHC were performed. One subject per group was included in each IHC staining batch to control for potential stain intensity differences. Free floating brain sections were stained in net wells on a platform shaker for all washes and in 2mL microcentrifuge tubes on a rotator wheel during antibody and avidin-biotin incubations. Cryoprotectant was washed from tissue slices in 1x PBS multiple times followed by a quench in 0.5% hydrogen peroxide in PBS for 5 minutes to inactivate endogenous peroxidases. Next, tissue sections were rinsed in PBS and then washed in 0.1M Tris – Glycine (pH 7.4) 3 times for 10 minutes each wash to compete off extra aldehydes and reduce background. After additional PBS rinses, tissue was washed 4 times for 10 minutes in a blocking solution consisting of 4% non-fat dry milk in PBS and 0.2% TritonX – 100. Sections were then incubated in blocking solution and 1:20,000 primary c-Fos anti-rabbit antibody (Millipore, Ab-5 PC-38) for 48 hours at 4°C. After 48 hours, the primary antibody was rinsed away in blocking solution and sections were incubated in blocking solution with 1:1,000 biotinylated donkey anti-rabbit secondary antibody (Jackson, 711-165-152) for 1 hour at room temperature. After PBS rinsing, sections were incubated for 1 hour at room temperature with Vectastain ABC Elite (Vector Labs, PK-6100). Tissue sections for c-Fos were then rinsed in PBS and stained in a nickel enhanced 3,3′-diaminobenzidine tetrahydrochloride (Ni-DAB) reaction consisting of 10mg/mL cobalt chloride hexahydrate, 10mg/mL nickel sulfate hexahydrate, 1mg/mL 3,3′-diaminobenzidine tetrahydrochloride, and 0.0045% hydrogen peroxide in PBS for 10 minutes. Sections were then rinsed thoroughly in PBS and mounted with 0.5% gelatin on charged microscope slides. After drying, slides were dehydrated in ethanol followed by defatting in Citrisolv (Fisher, 22-143975) and coverslipped using DPX mounting medium (Electron Microscopy Sciences, 13512).
The same procedure was used to detect EGFP in the brain, substituting the primary (chicken anti-EGFP 1:10,000 Abcam 13970-50) and secondary (donkey anti-chicken 1:1,000 Jackson, 703-065-155) antibodies, and without using nickel enhanced staining.
Genotyping
Tail samples were collected directly prior to perfusion, after subjects were anesthetized. Tail samples were processed for EGFP and Cre genotypes via polymerase chain reaction (PCR). All PCRs contained appropriate positive, negative, and no template controls. To determine EGFP and Cre gene status, the forward Oxtr primer (5’ – GCCACACTTTAAAGAGCCTCAA -3’) was used, while the reverse primer for EGFP (5’ - TAGCGGCTGAAGCACTGCA - 3’) or Cre (5’ - CGGCAAACGGACAGAAGCATT - 3’) were used under the following thermal cycling conditions: 94°C for 5 minutes, followed by 10 cycles of 94°C at 15 seconds, 65 - 55°C for 30 seconds with a decrease of 1°C per cycle, and 72°C for 40 seconds, 30 cycles of 94°C at 15 seconds, 55°C for 30 seconds, and 72°C for 40 seconds, and finally 5 minutes at 72°C.
Additionally, the sex of subjects with developmentally ambiguous external genitalia were confirmed by PCR with the forward primer (5’ – CCGCTGCCAAATTCTTTGG - 3’) and the reverse primer (5’ – TGAAGCTTTTGGCTTTGAG - 3’) to detect the Smcy gene on the Y chromosome and the Smcx gene on the X chromosome under the following thermal cycling conditions: 95°C for 7 minutes followed by 35 cycles of 93°C, 58°C, and 72°C for 30 seconds each, and finally 10 minutes at 72°C.
PCR products were visualized by gel electrophoresis followed by ethidium bromide assisted imaging. The PCR products generated consisted of a 320bp amplicon for the EGFP transgene, a 250bp amplicon for the Cre transgene, a 330bp amplicon for the Smcx gene, and a 290bp amplicon for the Smcy gene.
EGFP Fluorescence Microscopy
Sections containing the trigeminal ganglion from P14 and P21 OXTR:EGFP+ and OXTR:EGFP- males and females were rinsed in 1X PBS and then incubated in DAPI in 1X PBS for 5 minutes. Sections were then rinsed in 1X PBS, mounted onto Superfrost plus slides (VWR 48311-703) and coverslipped with Vectashield mounting medium (Vector Labs, H1000). A Keyence microscope (BZ-X710) and software were used to image the trigeminal ganglion at 10X and 20X magnification. EGFP and DAPI images were taken separately and digitally overlayed (Keyence image analyzer software).
c-Fos Quantification
Brain regions of interest were projected on to a computer via an Amscope camera connected to a light microscope (Olympus) at 4X, 5X, or 10X magnification. Images were taken using Amscope imaging software. All images for one brain region of interest for each age group were taken on the same microscope with the same microscope and software settings. All brain regions were identified with the assistance of a mouse atlas (Paxinos & Franklin). Brain regions of interest were isolated and c-Fos cell density was analyzed using ImageJ37 in the following brain regions: trigeminal principal sensory nucleus (PrV), trigeminal spinal nucleus (SpV), trigeminal motor nucleus (MV), paraventricular nucleus of the hypothalamus (PVN), ventroposterior medial thalamus (VPM), and whisker barrel cortex (S1). For each brain area, a single researcher blind to the experimental condition of the subjects identified cells positive for c-Fos (c-Fos+) as a black nuclear stain and cell counts were generated via manual thresholding in ImageJ. Cell counts were divided by the counting area to obtain cell densities in cells/mm2. An average of 2-6 sections for each subject were analyzed for c-Fos depending on the brain region.
Data Analysis
To accommodate immunohistochemistry (IHC) of subjects across treatments, ages, and sexes, brain sections spanning brainstem to cortex were stained in 28 batches for both experiments. Bilateral c-Fos average cell densities for each brain region were analyzed for both studies by fitting data to a linear mixed effects model with IHC batch as a covariate. When possible in study 2, ipsilateral and contralateral c-Fos counts were also analyzed separately to determine if there was a stronger effect related to the stimulated side (ipsilateral to whisker brushing in the brainstem nuclei and PVN and contralateral to whisker brushing in the VPM thalamus and somatosensory cortex). Behavioral data from study 2 was also analyzed with a linear effects model to account for unequal variances across groups. Quivering and jumping behaviors were not analyzed due to extremely low frequency. To determine the effects of oral OXT on c-Fos cell densities across a network of brain regions and on multiple behaviors, a principle component analysis (PCA) was performed on c-Fos cell densities in both studies as well as behaviors from study 2. For the PCA, to control for potential staining differences between IHC batches, total cell density for each subject and brain region was standardized by converting to z-scores within an IHC batch. Z-scores representing bilateral c-Fos cell density average per brain region within an IHC batch were used in PCA to generate factor scores based on correlated brain regions. Effects of oral OXT on PCA factor scores were probed with a linear mixed effects model with treatment, age, and sex fitted as fixed effects. Significant interactions were probed with a priori post-hoc comparisons with Bonferroni adjusted critical values to correct for multiple comparisons. Effect sizes for main effects and interactions are reported as partial eta squared for ANOVA results in the tables, and effect sizes for post-hoc contrasts on group means are reported as Cohen’s d within the text and correlations are reported as Pearson’s r. All data analyses were conducted in SPSS software (IBM).
Results:
EGFP positive cells identified in P14 and P21 trigeminal ganglion and P14 whisker barrel cortex
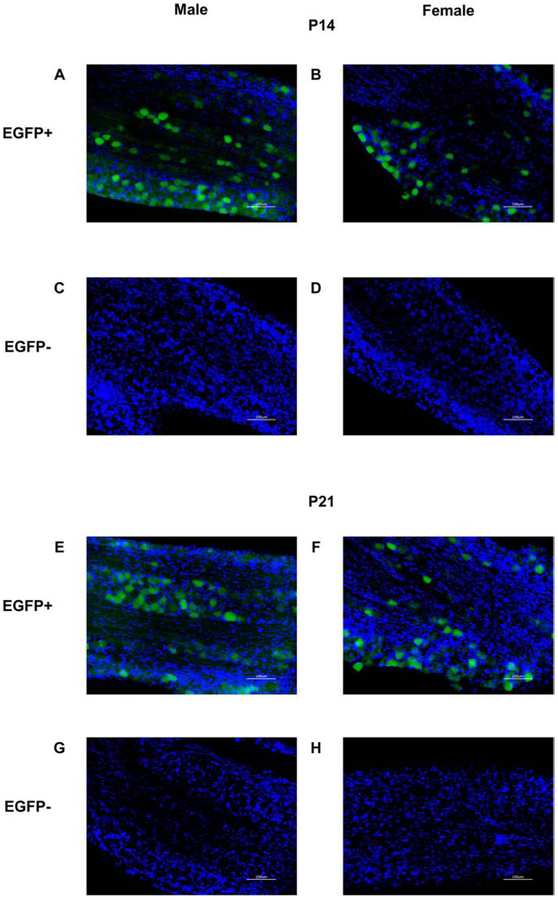
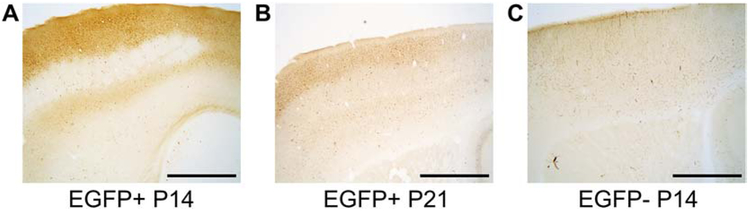
Endogenous EGFP fluorescence signal was observed in the trigeminal ganglion of P14 and P21 OXTR:EGFP+ males and females (figure 2A, B, E, F), but not in EGFP- mice (figure 2 C, D, G, H). EGFP signal was also seen in the trigeminal ganglion with the anti-EGFP antibody (not shown). Additionally, EGFP+ cells were observed in neocortex by endogenous fluorescence at P14 (not shown) and immunohistochemistry revealed EGFP immunoreactive cells in OXTR:EGFP+ neocortex at P14 (figure 3A) and to a lesser extent at P21 (figure 3B), but not in OXTR:EGFP- mice (figure 3C). EGFP immunoreactive cells and neuropil surround the whisker barrels in the primary whisker barrel cortex at P14.
Figure 2. Enhanced green fluorescent protein (EGFP) in the trigeminal ganglion of postnatal day (P) 14 and P21 male and female OXTR:EGFP transgenic mice:
Fluorescent microscopy for EGFP (green) and DAPI (blue) in OXTR:EGFP+ (A, B, E, F,) and OXTR:EGFP−(C, D, G, H) trigeminal ganglion at P14 (A-D) and P21 (E-H) males (A, C, E, G) and females (B, D, F, H). EGFP is present in the trigeminal ganglion of OXTR:EGFP+, but not OXTR:EGFP−, P14 and P21 males and females. Images taken at 20X magnification. Scale bar = 100μm.
Figure 3. Immunohistochemistry for EGFP in the primary whisker barrel cortex of postnatal day (P) 14 and P21 mice:
Dense EGFP immunoreactivity with DAB staining is present surrounding whisker barrels in the primary whisker barrel cortex at P14 (A), and to a lesser extent at P21 (B), in OXTR:EGFP+ but not OXTR:EGFP- mice (C). Images taken at 4X magnification. Scale bar = 1mm.
Analysis of c-Fos mean densities
Based off of EGFP fluorescence and IHC results showing EGFP+ cells in the trigeminal nerve of OXTR:EGFP mice and previous reports of OXTR binding in orofacial tissues innervated by the trigeminal ganglion13,17 and intranasal OXT altering trigeminal ganglion activity38, we quantified c-Fos after oral OXT or saline treatment in several brain regions of interest receiving direct or indirect information from the trigeminal nerve. These brain regions include the PVN, trigeminal spinal nucleus, trigeminal sensory nucleus, trigeminal motor nucleus, ventroposterior medial thalamus (VPM thalamus), and whisker barrel cortex. Due to non-homogeneity of variance and correlated brain regions, c-Fos cell densities were analyzed by linear mixed effects modeling with treatment, sex and age as fixed effects and IHC batch as a covariate. All statistical tests (ANOVA) results and justified post-hoc tests are presented in Tables 1 through 4. Significant main effects, interactions, and post-hoc results are presented below.
Table 1: Study 1 results for c-Fos cell density in six brain regions after a high (Clinical) or low (Milk) dose of oral oxytocin (OXT) versus saline in postnatal day (P) 14 and P21 male and female OXTR:EGFP mice.
Average c-Fos cell density is expressed as mean ± standard error of the mean (SEM). Main and interaction effects p – values are from multivariate mixed model analyses on mean c – Fos densities and effect sizes are expressed in parentheses as partial eta squared values. Bold font emphasizes statistical significance. Post-hoc results are Bonferroni corrected for multiple comparisons. T = treatment; S = sex; A = age; PVN = paraventricular nucleus of the hypothalamus
| Study 1 c- Fos |
Mean ± SEM | p-value (ηp2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treat ment |
Sex | Age | T × S | T × A | A × S |
T × S × A |
post hoc | |||
| P14 | P21 | P14 | P21 | |||||||||
| PVN | ||||||||||||
| Saline | 476 ± 60 | 591 ± 137 | 385 ± 83 | 731 ± 185 | 0.68 (0.01) |
0.01 (0.07) |
0.01 (0.07) |
0.04 (0.06) |
0.16 (0.04) |
0.60 (0.00) |
0.06 (0.06) |
saline ♂<Clinical ♂ P14 < P21 ♂ > ♀ |
| Milk | 428 ± 83 | 950 ± 136 | 482 ± 63 | 534 ± 78 | ||||||||
| Clinical | 796 ± 104 | 782 ± 147 | 411 ± 83 | 440 ± 104 | ||||||||
| Trigeminal sensory | ||||||||||||
| Saline | 86 ± 12 | 65 ± 18 | 104 ± 8 | 76 ± 16 | 0.46 (0.02) |
0.81 (0.00) |
0.00 (0.90) |
0.44 (0.02) |
0.91 (0.00) |
0.93 (0.00) |
0.61 (0.01) |
P14 > P21 |
| Milk | 98 ± 23 | 54 ± 7 | 76 ± 14 | 54 ± 8 | ||||||||
| Clinical | 80 ± 23 | 63 ± 5 | 93 ± 18 | 55 ± 12 | ||||||||
| Trigeminal spinal | ||||||||||||
| Saline | 113 ± 19 | 66 ± 19 | 127 ± 23 | 80 ± 14 | 0.84 (0.00) |
0.53 (0.00) |
0.00 (0.11) |
0.64 (0.01) |
0.56 (0.01) |
0.52 (0.00) |
0.52 (0.01) |
P14 > P21 |
| Milk | 119 ± 26 | 71 ± 9 | 109 ± 15 | 678 ± 12 | ||||||||
| Clinical | 92 ± 10 | 94 ± 13 | 129 ± 16 | 83 ± 19 | ||||||||
| Trigeminal motor | ||||||||||||
| Saline | 84 ± 26 | 127 ± 42 | 49 ± 10 | 135 ± 30 | 0.81 (0.00) |
0.81 (0.00) |
0.03 (0.05) |
0.88 (0.00) |
0.23 (0.03) |
0.02 (0.05) |
0.67 (0.01) |
P14 ♂ > P14♀ P14 < P21 |
| Milk | 108 ± 28 | 111 ± 25 | 73 ± 24 | 139 ± 30 | ||||||||
| Clinical | 135 ± 25 | 80 ± 3 | 86 ± 11 | 140 ± 36 | ||||||||
| Ventroposterior medial thalamus | ||||||||||||
| Saline | 83 ± 43 | 144 ± 81 | 96 ± 46 | 19 ± 4 |
0.05 (0.06) |
0.20 (0.02) |
0.89 (0.00) |
0.28 (0.03) |
0.89 (0.00) |
0.25 (0.01) |
0.28 (0.03) |
saline > Milk |
| Milk | 18 ± 9 | 12 ± 2 | 43 ± 20 | 25 ± 8 | ||||||||
| Clinical | 65 ± 29 | 72 ± 54 | 18 ± 8 | 33 ± 6 | ||||||||
| Barrel cortex | ||||||||||||
| Saline | 156 ± 38 | 72 ± 31 | 250 ± 56 | 77 ± 27 | 0.86 (0.00) |
0.50 (0.00) |
0.00 (0.15) |
0.17 (0.03) |
0.37 (0.01) |
0.73 (0.00) |
0.68 (0.01) |
P14 > P21 |
| Milk | 209 ± 85 | 59 ± 20 | 185 ± 43 | 34 ± 11 | ||||||||
| Clinical | 216 ± 64 | 112 ± 32 | 117 ± 44 | 43 ± 13 | ||||||||
Table 4: Study 2 results for c-Fos principal component analysis (PCA) factor scores after a high (Clinical) or low (Milk) dose of oral oxytocin (OXT) versus saline with whisker brushing in postnatal day (P) 14 and P21 male and female OXTR:EGFP mice.
Average c-Fos cell densities were z-scored per IHC batch and z-scores were entered into a PCA. Factor scores are expressed as mean ± standard error of the mean (SEM). Main and interaction effects p – values are from multivariate mixed model analyses on factor scores and effect sizes are expressed in parentheses as partial eta squared values. Bold font emphasizes statistical significance. Post-hoc results are Bonferroni corrected for multiple comparisons. * = significance did not survive Bonferroni corrected post-hoc test. T = treatment; S = sex; A = age; PVN = paraventricular nucleus of the hypothalamus
| Study 2 PCA c-Fos |
Mean ± SEM | p-value (ηp2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treat ment |
Sex | Age | T × S | T × A | A × S |
T × S × A |
post hoc | |||
| P14 | P21 | P14 | P21 | |||||||||
| Factor 1 sensory | ||||||||||||
| Saline | 0.40 ± 0.52 | 0.47 ± 0.31 | 0.49 ± 0.25 | 0.25 ± 0.42 |
0.02 (0.09) |
0.94 (0.00) |
0.93 (0.00) |
0.96 (0.00) |
0.57 (0.02) |
0.04* (0.06) |
0.37 (0.03) |
saline > Clinical |
| Milk | −0.27 ± 0.52 | 0.32 ± 0.35 | 0.64 ± 0.29 | −0.44 ± 0.14 | ||||||||
| Clinical | −0.63 ± 0.37 | −0.02 ± 0.30 | −0.26 ± 0.38 | −0.31 ± 0.35 | ||||||||
| Factor 2 PVN | ||||||||||||
| Saline | −0.51 ± 0.24 | 0.37 ± 0.22 | −0.51 ± 0.34 | −0.60 ± 0.29 |
0.00 (0.18) |
0.00 (0.14) |
0.24 (0.02) |
0.00 (0.16) |
0.67 (0.01) |
0.00 (0.14) |
0.26 (0.04) |
♂saline < ♂Clinical ♂P14 < ♂P21 |
| Milk | −0.54 ± 0.08 | −0.20 ± 0.37 | −0.12 ± 0.31 | −0.35 ± 0.32 | ||||||||
| Clinical | 0.55 ± 0.43 | 1.63 ± 0.21 | 0.13 ± 0.29 | −0.68 ± 0.30 | ||||||||
| Factor 3 motor | ||||||||||||
| Saline | −0.39 ± 0.25 | 0.16 ± 0.30 | 1.22 ± 0.29 | 0.03 ± 0.43 | 0.23 (0.04) |
0.95 (0.00) |
0.71 (0.00) |
0.05 (0.08) |
0.41 (0.02) |
0.11 (0.03) |
0.16 (0.05) |
♀saline > ♀Milk & Clinical |
| Milk | −0.04 ± 0.40 | 0.09 ± 0.44 | −0.59 ± 0.09 | −0.21 ± 0.31 | ||||||||
| Clinical | −0.12 ± 0.47 | 0.44 ± 0.53 | −0.22 ± 0.19 | −0.18 ± 0.50 | ||||||||
PVN c-Fos density increases with oral OXT in males and with age
Study 1 Oral OXT
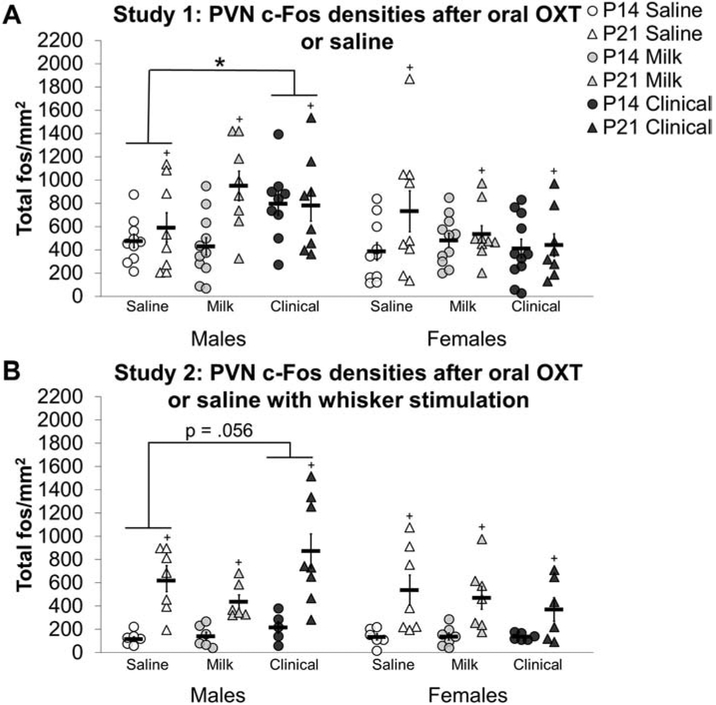
A sex by treatment interaction (F2, 100 = 3.235, p<0.05; figure 4A) indicated that the PVN of males, but not females was impacted by oral OXT treatment. Specifically, Clinical treated males had higher PVN c-Fos cell densities compared to saline treated males (d = 0.73). A main effect of age (F1,100 = 8.032, p<0.01; d = 0.47) also revealed higher PVN c-Fos densities in P21 subjects compared to P14 subjects.
Figure 4: Oral oxytocin (OXT) increases c-Fos in the paraventricular nucleus of the hypothalamus (PVN) of males:
A high dose (Clinical; black shapes) of oral OXT increased c-Fos cell densities in the PVN of postnatal day (P) 14 (circles) and P21 (triangles) males (right side of the graphs), compared to saline (white shapes) in study 1 (A) and tended to increase PVN c-Fos densities in study 2 (B) after whisker stimulation. Black shapes represent the Clinical dose of oral OXT, gray shapes reflect the Milk dose, and white shapes reflect oral saline treatment. Individual data points are graphed as well as mean ± SEM. * = p < 0.05, + = main effect of age p < 0.05.
Study 2 Oral OXT with whisker stimulation
With whisker stimulation, Clinical treated males had higher PVN bilateral c-Fos cell densities compared to saline treated males in study 2 (treatment main effect F2, 78 = 3.464, p < 0.05; figure 4B), but this effect did not survive Bonferroni adjusted significance in post-hoc tests (Clinical versus saline post-hoc adjusted p = 0.056; d = 0.618). Bilateral analysis was followed with ipsilateral and contralateral analyses. In the ipsilateral PVN, a treatment main effect (F2, 64 = 4.166, p < 0.05) also revealed that Clinical treated subjects had higher c-Fos cell densities compared to saline (d = 0.38) and Milk treated subjects (d = 0.51). In the PVN contralateral to whisker brushing, P21 Clinical treated females had decreased c-Fos cell densities (age by sex by treatment interaction F2, 60 = 9.822, p < 0.001) compared to saline treated P21 females (d = 1.97) while P21 Clinical treated males had increased c-Fos cell densities compared to P21 Milk treated males (d = 1.99).
Like study 1, P21 males and females had higher bilateral c-Fos cell densities in the PVN compared to P14 subjects (total PVN F1,78 = 64.511, p < 0.001; d = 1.178). This age effect was observed in both hemispheres either ipsilateral or contralateral to the stimulated side (ipsilateral PVN F1, 64 53.66, p < 0.001; d = 1.5; contralateral PVN F1, 60 = 40.813, p < 0.001; d = 1.3). Furthermore, a main effect of sex revealed males had higher c-Fos cell densities bilaterally in the PVN compared to females (F1, 78 = 4.166, p < 0.05; d =0.47). In the ipsilateral PVN, a sex by age interaction (F2, 64 = 8.935, p < 0.01) revealed P21 females had lower c-Fos cell densities in the PVN ipsilateral to whisker brushing compared to P21 males (d = 1.16).
Lastly, c-Fos cell densities in the PVN contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral PVN (r(64) = 0.79, p <0.001, supplementary figure 1A).
Trigeminal brainstem nuclei c-Fos levels decrease with age and oral OXT with stimulation
Study 1 Oral OXT
There were no main effects or interaction effects of oral OXT treatment on c-Fos cell densities in brainstem trigeminal nuclei in study 1 (supplementary figure 2). There was a significant main effect of age on c-Fos cell density within the trigeminal sensory nucleus (F1, 116 = 11.390, p<0.01) and the trigeminal spinal nucleus (F1, 117 = 14.582, p<0.01). C-Fos cell densities were higher in P14 trigeminal sensory and spinal nucleus compared to P21 subjects (sensory: d = 0.65; spinal: d = 0.73; supplementary figure 2A and 2B). There was also a significant interaction between age and sex for c-Fos cell densities in the trigeminal motor nucleus (F1,114 = 5.465, p<0.05). Follow up analyses revealed that P14 females had lower c-Fos densities in the trigeminal motor nucleus compared to P21 females (F1, 60 = 13.295, p < 0.01; d = 0.88; supplementary figure 2C) and compared to P14 males (F1, 57 = 4.807, p<0.05; d =0.58).
Study 2 Oral OXT with whisker stimulation
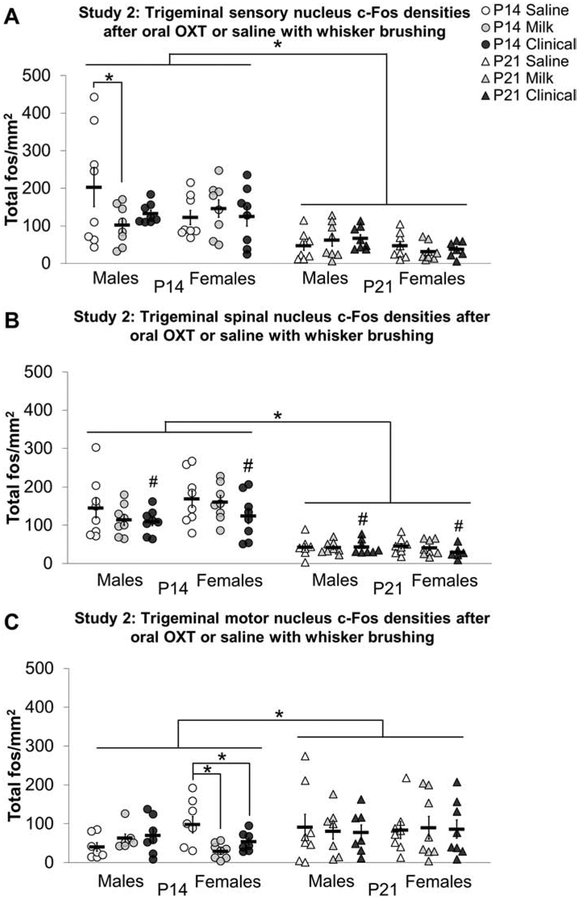
Trigeminal sensory nucleus:
In study 2, an age by sex by treatment interaction (F2, 95 = 3.510, p < 0.05) revealed that Milk treated P14 males had decreased c-Fos density in the trigeminal sensory nucleus compared to P14 saline treated males (d = 0.88, figure 5A). Analysis by hemisphere did not reveal any treatment main effects or interactions in the ipsilateral or contralateral trigeminal sensory nucleus.
Figure 5: Oral oxytocin (OXT) with whisker brushing decreases c-Fos cell densities in trigeminal brainstem nuclei:
Oral OXT with whisker brushing decreased c-Fos cell densities in trigeminal nuclei of pre-weaning mice compared to saline in study 2. The Milk dose of oral OXT (gray shapes) with whisker brushing decreased c-Fos densities in the trigeminal sensory nucleus of postnatal day (P) 14 (circles) males compared to saline (white circles; A). The Clinical dose of oral OXT (black shapes) with whisker brushing tended to decrease c-Fos in the trigeminal spinal nucleus of P14 and P21 (triangles) males and females compared to saline (B). The Milk and Clinical dose of oral OXT with whisker brushing decreased c-Fos densities in the trigeminal motor nucleus of P14 females compared to saline (C). Individual data points are graphed as well as mean ± SEM. *p = < 0.05; # = Main effect of treatment (p < 0.05) followed by Bonferroni corrected post-hoc Clinical vs saline p = 0.063
As in study 1 without whisker stimulation, study 2 revealed a main effect of age on c-Fos cell density in the trigeminal sensory nucleus (F1, 95 = 54.306, p <0.001; d = 1.40), with higher c-Fos densities in P14 subjects. Additionally, a main effect of age revealed that P14 subjects had higher c-Fos cell densities in the ipsilateral (F1, 92 = 34.773, p < 0.001 d = 1.13) and contralateral (F1, 93 = 49.815, p < 0.001; d = 1.42) trigeminal sensory nucleus. C-Fos cell densities in the trigeminal sensory nucleus contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral trigeminal sensory nucleus (r(92) = 0.67, p <0.001, supplementary figure 1B).
Trigeminal spinal nucleus:
In the trigeminal spinal nucleus, there was a non-significant trend for a main effect of treatment (Figure 5B). To evaluate if laterality would clarify this trend, analyses were conducted on c-Fos densities ipsi- and contralateral to the stimulated side. In the trigeminal spinal nucleus ipsilateral to whisker brushing, Clinical treated P14 subjects had lower c-Fos cell densities compared to P14 saline (d = 1.22) and Milk (d = 1.25) treated subjects (treatment by age interaction F2, 91 = 5.458, p < 0.01). In the contralateral trigeminal spinal nucleus, there were no effects of treatment.
Compared to P14 subjects, P21 subjects had lower c-Fos cell densities in the trigeminal spinal nucleus bilaterally (F1, 93 = 133.230, p < 0.001; d = 0.97) as well as ipsilateral (F1, 91 = 108.335, p < 0.001; d = 2) and contralateral (F1, 91 = 104.029, p < 0.001; d = 1.91) to whisker brushing. Additionally, a sex by age interaction (F1, 91 = 9.216, p < 0.01) revealed that P14 females had higher c-Fos cell densities in the contralateral trigeminal spinal nucleus compared to P14 males (d = 0.97).
Lastly, c-Fos cell densities in the trigeminal spinal nucleus contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral trigeminal spinal nucleus (r(91) = 0.71, p <0.001, supplementary figure 1C).
Trigeminal motor nucleus:
P14 Milk (d = 1.71) and Clinical (d = 1.16) females had lower c-Fos cell densities in the trigeminal motor nucleus compared to P14 saline treated females (sex by treatment interaction F2, 36 = 6.617, p < 0.01; figure 5C). In the ipsilateral trigeminal motor nucleus, an age by sex by treatment three-way interaction (F2, 82 = 3.156, p < 0.05) revealed Milk treated P14 females had decreased c-Fos cell densities compared to P14 saline treated females (d = 1.87). P14 Clinical treated females tended to have lower c-Fos cell densities in the ipsilateral motor nucleus compared to P14 saline treated females as well but this did not survive Bonferroni corrections (adjusted p = 0.076; d = 1.04). Follow up analyses revealed a main effect of treatment in P14 females in the ipsilateral trigeminal motor nucleus (F2, 20 = 8.082, p < 0.01). There were no effects of treatment in the trigeminal motor nucleus contralateral to whisker brushing.
P14 subjects had lower c-Fos cell densities, compared to P21 subjects, in the trigeminal motor nucleus bilaterally (F1, 88 = 4.180, p < 0.05; d = 0.45) as well as contralateral (F1, 87 = 4.790, p < 0.05; d = .46) to whisker brushing. Further, c-Fos cell densities in the trigeminal motor nucleus contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral trigeminal motor nucleus (r(78) = 0.57, p <0.001, supplementary figure 1D).
VPM thalamus c-Fos levels decrease with oral OXT
Study 1 Oral OXT
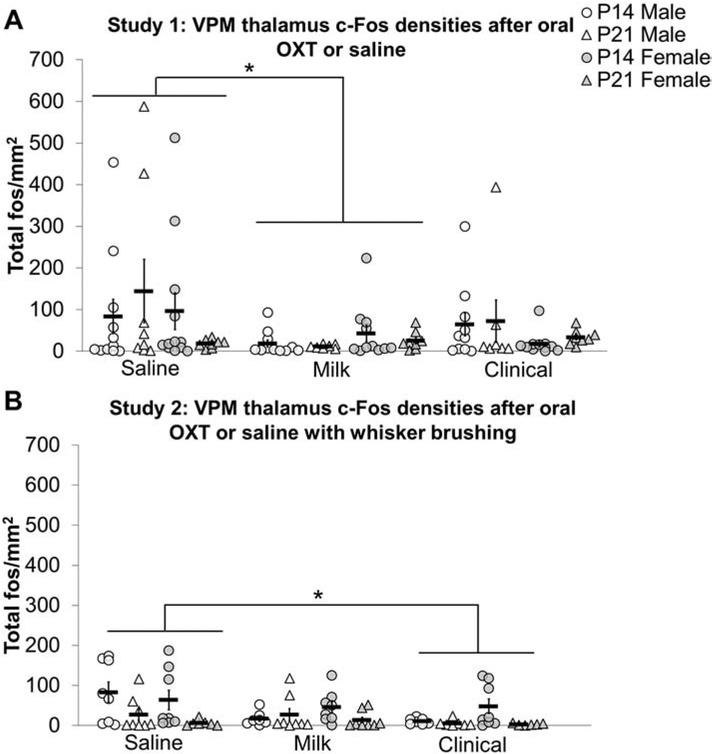
Milk treated subjects had decreased c-Fos densities in the VPM thalamus compared to saline treated subjects in study 1 (F2,100 = 3.143, p<0.05; d = 0.31, figure 6A).
Figure 6: Oral oxytocin (OXT) decreases c-Fos densities in the ventroposteriomedial (VPM) thalamus:
In study 1, c-Fos cell densities were decreased in the VPM thalamus of postnatal day (P) 14 (circles) and P21 (triangles) Milk treated males (white shapes) and females (gray shapes) compared to saline treated subjects (A). In study 2, whisker brushing with Clinical treatment decreased c-Fos densities in the VPM thalamus in P14 and P21 males and females compared to whisker brushed and saline treated subjects. Individual data points are graphed as well as mean ± SEM. *p = < 0.05; + = main effect of age p < 0.05
Study 2 Oral OXT with whisker stimulation
In study 2, Clinical treated subjects had decreased c-Fos cell density in the VPM thalamus compared to saline treated subjects (F2, 78 = 3.412, p < 0.05; d = 0.55; figure 6B). In the ipsilateral VPM thalamus, P14 Clinical (d = 1.12) and Milk (d = 1.12) treated males had decreased c-Fos densities compared to P14 saline males (age by sex by treatment three-way interaction F2, 76 = 4.044, p < 0.05). In the contralateral VPM thalamus, a significant age by treatment interaction (F2, 87 = 3.686, p < 0.05) revealed that Milk (d = 0.85) and Clinical (d = 0.99) treated P14 subjects had lower c-Fos cell densities in the contralateral VPM thalamus compared to saline treated P14 subjects.
Compared to P21 males and females, P14 subjects had higher c-Fos cell densities in the VPM thalamus bilaterally (F1, 78 = 11.872, p < 0.01; d = 0.72) as well as ipsilateral (F1, 76 = 13.114, p < 0.01; d = 0.75) and contralateral (F1, 75 = 9.712, p < 0.01; d = 0.66) to whisker brushing. Additionally, c-Fos cell densities in the VPM thalamus contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral VPM thalamus (r(82) = 0.46, p <0.001, supplementary figure 1E).
Barrel cortex c-Fos levels decrease with age
Study 1 and Study 2
There were no main effects or interaction effects of oral OXT treatment on c-Fos cell densities within the barrel cortex in study 1 (supplementary figure 3A) or study 2 (supplementary figure 3B). Additionally, there were no effects of treatment on c-Fos cell densities within the whisker barrel cortex of either the ipsilateral or contralateral side to whisker brushing in study 2. However, a significant main effect of age on c-Fos cell density within the whisker barrel cortex in study 1 (F1, 115 = 19.931, p<0.001; d = 0.73) and study 2 (F1, 89 = 105.508, p < 0.001; d = 0.72) revealed higher c-Fos densities in P14 barrel cortex compared to P21 subjects in both studies. C-Fos cell densities in the whisker barrel cortex contralateral to whisker brushing were positively correlated with c-Fos cell densities in the ipsilateral whisker barrel cortex in study 2 (r(85) = 0.85, p <0.001, supplementary figure 1F).
Network analysis suggests OXT increases c-Fos in PVN and decreases sensory dependent c-Fos in trigeminal sensory and motor circuits sex specifically
Because brain regions of interest in this study exist within functional circuits, we sought to identify c-Fos patterns of covariation across brain regions using a principal component analysis (PCA) conducted on a correlation matrix, with pairwise deletions, and an oblique transformation. Bilateral average c-Fos cell density z-scores across six brain regions from all subjects were assigned as variables in the PCA. Regression factor scores were generated from PCA results that predict the location of each subject on each factor component across age, sex, and treatment.
Study 1 Oral OXT
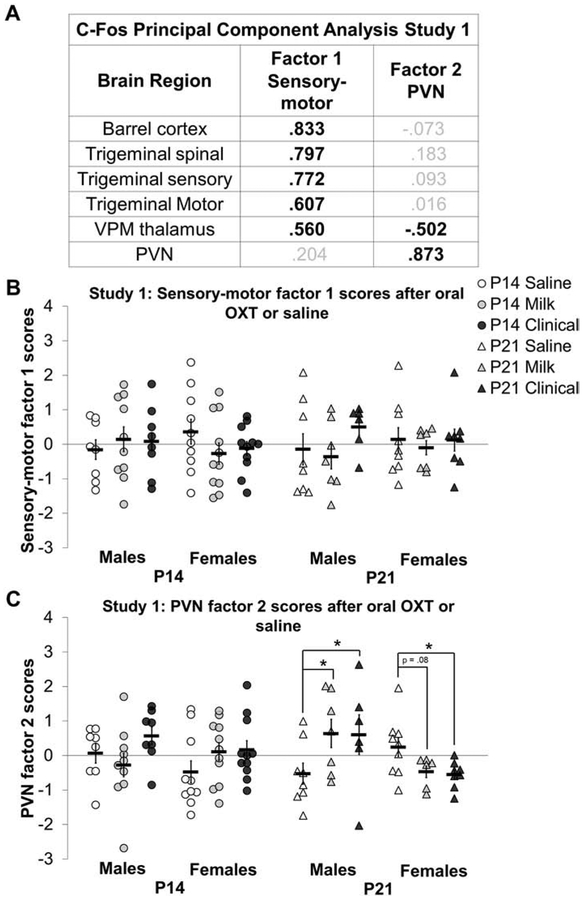
In study 1, PCA on c-Fos density z-scores extracted two main factors with an eigenvalue above 1 and accounting for 62% of the total variance. Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was 0.757 and Bartlett’s test of sphericity was significant (X2 (15) = 146.701, p<0.001). Figure 7A reflects extracted factors and factor loadings. Factor 1 consists of high loadings from the trigeminal sensory, trigeminal spinal, and whisker barrel cortex, with more moderate but appreciable, loadings from the trigeminal motor nucleus and VPM thalamus. the Factor 2 consists of high PVN loading, with the VPM thalamus moderately loading in the opposite direction.
Figure 7: Oral oxytocin (OXT) increases c-Fos paraventricular nucleus of the hypothalamus (PVN) factor 2 scores in P21 males compared to saline:
C-Fos principal component analysis factor scores for study 1 after oral OXT (a low Milk dose or high Clinical dose) or saline treatment in study 1 in postnatal day (P) 14 (white circles) and P21 (white triangles) males and P14 (gray circles) and P21 (gray triangles) females. C-Fos cell density z scores within an IHC batch extracted two main factors (A) reflecting sensory-motor (factor 1) and PVN (factor 2) components. Bold black font emphasizes high loadings. Factor loadings were used to calculate c-Fos factor regression scores for sensory-motor factor 1 (B) and PVN factor 2 (C) for P14 (circles) and P21 (triangles) males (white markers) and females (gray markers). There were no treatment differences in sensory-motor factor 1 scores in study 1 (B). P21 Milk and Clinical treated males had higher factor 2 scores compared to saline treated males while P21 Clinical treated females had lower factor 2 scores compared to saline treated females (C). Individual data points are graphed as well as mean ± SEM. *p = < 0.05.
There were no significant differences in factor 1 (sensory) scores across sex, group or treatment in study 1 (figure 7B). In contrast, factor 2 (PVN) scores in study 1 showed a significant age by sex by treatment three-way interaction (F2,90 = 4.954, p < 0.05); P21 Milk (d = 1.11) and Clinical (d = 0.89) treated males had higher factor 2 scores compared to saline treated P21 males (figure 7C). Females seemed to show the opposite effect, but this effect did not survive Bonferroni correction (adjusted p = 0.08; d = 1.04).
Study 2 Oral OXT with whisker stimulation
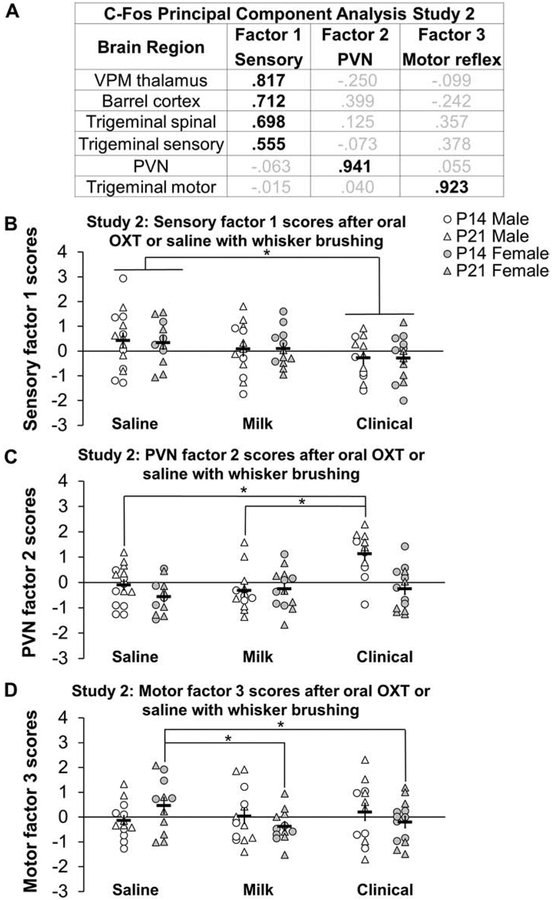
Right and left hemisphere c-Fos densities were averaged in the PCA for study 2 because doing so increased the number of subjects put into the model and increased KMO’s measure of sampling adequacy which is a measure of model fit. Additionally, including all the data accounted for more of the variance than just using data from the side receiving whisker stimulation. In study 2, a PCA on bilateral c-Fos density z scores generated three eigenvalues close to or above 1. Based off residuals for two factor versus three factor models, a three factor model was judged to represent the best fit. Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was 0.67 and Bartlett’s test of sphericity was significant (X2 (15) = 74.923, p<0.001). Three factors accounted for 73% of the total variance. Factor loadings for each factor component are shown in figure 8A. All measured sensory processing brain regions loaded highly on factor 1. Factor 2 consisted primarily of the PVN and factor 3 consisted primarily of the trigeminal motor nucleus.
Figure 8: Oral oxytocin (OXT) with whisker brushing decreases c-Fos sensory factor 1 scores in males and females, increases PVN factor 2 scores in males, and decreases motor factor 3 scores in females:
C-Fos principal component analysis factor scores after a low dose (Milk) or high dose (Clinical) of oral OXT or saline and whisker brushing in study 2 in P14 (circles) and P21 (triangles) males (white shapes) and females (gray shapes). C-Fos cell density z scores within an IHC batch extracted three main components (A) reflecting sensory brain regions (factor 1), the paraventricular nucleus of the hypothalamus (PVN) (factor 2), and a trigeminal motor component (factor 3). Bold black font emphasizes high loadings. Factor loadings were used to calculate c-Fos factor regression scores for sensory factor 1 (B), PVN factor 2 (C), and trigeminal motor factor 3 (D) scores. Clinical treated postnatal day (P)14 and P21 males and females had decreased c-Fos sensory factor 1 scores compared to saline treatment (B). Clinical treated P14 and P21 males had higher c-Fos PVN factor 2 scores compared to saline treated males (C). Milk and Clinical treated P14 and P21 females had lower c-Fos motor factor 3 scores compared to saline treated females (D). Data collapsed across ages. Individual data points are graphed as well as mean ± SEM. *p = < 0.05.
A treatment main effect for factor 1 (sensory) scores in study 2 (F2, 75 = 3.337, p<0.05) revealed that Clinical treated subjects had lower factor 1 scores compared to saline treated subjects (d = 0.71, figure 8B). For factor 2 (PVN) scores, Clinical treated males had higher factor 2 PVN scores compared to Saline treated males (sex by treatment interaction F2, 75 = 5.816, p<0.01; d = 1.48, figure 8C). Additionally, P21 males had higher factor 2 PVN scores compared to P14 males (age by sex interaction F1, 75 = 12.348, p < 0.01; d = 0.76). Lastly, Milk (d = 0.92) and Clinical (d = 0.67) treated females had lower factor 3 trigeminal motor scores compared to saline treated females (sex by treatment interaction F2, 74 = 3.223, p<0.05; figure 8D).
Exploratory behavior analysis
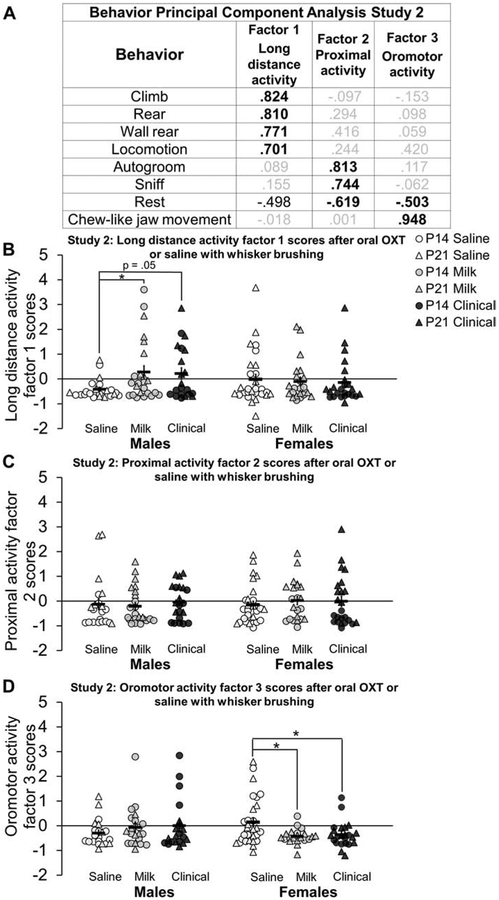
Study 2 Behavior PCA
The duration of behaviors for 10 minutes after oral OXT or saline treatment and whisker brushing were quantified and entered as variables into a PCA which included data for all ages, sexes, and treatment groups in study 2. KMO measure of sampling adequacy was 0.68 and Bartlett’s test of sphericity was significant (X2 (28) = 505.455, p<0.001). Three factors were extracted (figure 9A) which accounted for 75% of the total variance. Factor 1 was composed of exploratory locomotor behaviors including climbing, rearing, wall rearing, and locomotion. Factor 2 consisted of proximal activity behavior including autogrooming, sniffing in place, and resting. Factor 3 was primarily composed of chew-like jaw movements. ANOVA and post-hoc results on behavioral PCA scores are summarized in Table 5. For long distance activity factor 1 behavioral scores, a sex by treatment interaction (F2, 141 = 3.662, p<0.05) revealed that Milk treated males had higher behavioral factor 1 scores compared to saline treated males (d = 0.75, figure 9B). Clinical treated males tended to also have higher factor 1 (locomotor) scores compared to saline treated males, but this effect did not survive Bonferroni adjustments (adjusted p = 0.052; d = 0.82). Additionally, Milk (d = 0.80) and Clinical (d = 0.65) treated females had lower oromotor factor 3 behavioral scores compared to saline treated females (sex by treatment interaction F2, 136 = 3.603, p<0.05; figure 9D). There were no group differences for proximal activity factor 2 behavioral scores (figure 9C). A significant main effect of age on locomotor activity factor 1 behavioral scores (F1, 141 = 13.763, p < 0.001; d = 0.63) and proximal activity factor 2 behavioral scores (F1, 139 = 83.282, p < 0.001; d = 1.54) revealed higher activity in P21 compared to P14 subjects.
Figure 9: Oral oxytocin (OXT) with whisker brushing increases long distance activity factor 1 scores in males and decreases oromotor behavior factor 3 scores in females compared to saline:
Behavior principal component analysis and factor scores after a low dose (Milk; gray shapes) or high dose (Clinical; black shapes) of oral OXT or saline (white shapes) and whisker brushing in study 2 in postnatal day (P)14 (circles) and P21 (triangles) males and females in study 2. Behavior durations (in seconds) for ten minutes directly after oral OXT or saline administration with whisker brushing extracted three factors (A) reflecting long distance activity (factor 1), proximal activity (factor 2) and oromotor activity (factor 3). Bold black font emphasizes high loadings. Factor loadings were used to calculate behavioral factor regressions scores for long distance activity factor 1 (B), proximal activity factor 2 (C) and oromotor activity factor 3 (D). Clinical and Milk treated P14 and P21 males had higher long distance activity factor 1 scores compared to saline treated males, reflecting increased locomotor behavior (B). There were no differences between proximal activity factor 2 behavioral scores (C). Milk and Clinical treated P14 and P21 females had lower oromotor factor 3 scores compared to saline treated females, largely reflecting decreased chewing-like behavior (D). Individual data points are graphed ± SEM. *p = < 0.05.
Table 5: Study 2 results for behavioral principal component analysis (PCA) factor scores after a high (Clinical) or low (Milk) dose of oral oxytocin (OXT) versus saline with whisker brushing in postnatal day (P) 14 and P21 male and female OXTR:EGFP mice.
Average behavioral durations were entered into a PCA. Factor scores are expressed as mean ± standard error of the mean (SEM). Main and interaction effects p – values are from multivariate mixed model analyses on factor scores and effect sizes are expressed in parentheses as partial eta squared values. Bold font emphasizes statistical significance. Post-hoc results are Bonferroni corrected for multiple comparisons. T = treatment; S = sex; A = age; PVN = paraventricular nucleus of the hypothalamus
| Study 2 PCA behavior |
Mean ± SEM | p-value (ηp2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treat ment |
Sex | Age | T × S | T × A | A × S |
T × S × A |
post hoc | |||
| P14 | P21 | P14 | P21 | |||||||||
| Factor 1 long distance activity | ||||||||||||
| Saline | −0.47 ± 0.09 | −0.33 ± 0.18 | −0.18 ± 0.18 | 0.15 ± 0.37 | 0.28 (0.02) |
0.23 (0.01) |
0.00 (0.09) |
0.04 (0.05) |
0.28 (0.02) |
0.89 (0.00) |
0.95 (0.00) |
♂saline < ♂Milk P14 < P21 |
| Milk | 0.04 ± 0.35 | 0.69 ± 0.35 | −0.50 ± 0.09 | 0.13 ± 0.26 | ||||||||
| Clinical | −0.19 ± 0.26 | 0.67 ± 0.36 | −0.60 ± 0.05 | 0.24 ± 0.30 | ||||||||
| Factor 2 proximal activity | ||||||||||||
| Saline | −0.59 ± 0.10 | 0.63 ± 0.58 | −0.54 ± 0.09 | 0.30 ± 0.26 | 0.98 (0.00) |
0.64 (0.00) |
0.00 (0.38) |
0.85 (0.00) |
0.93 (0.00) |
0.94 (0.00) |
0.27 (0.02) |
P14 < P21 |
| Milk | −0.64 ± 0.07 | 0.46 ± 0.25 | −0.51 ± 0.14 | 0.39 ± 0.23 | ||||||||
| Clinical | −0.44 ± 0.17 | 0.41 ± 0.19 | −0.76 ± 0.06 | 0.62 ± 0.29 | ||||||||
| Factor 3 oromotor activity | ||||||||||||
| Saline | −0.44 ± 0.08 | −0.05 ± 0.27 | 0.18 ± 0.23 | 0.11 ± 0.28 | 0.39 (0.01) |
0.58 (0.00) |
0.13 (0.02) |
0.03 (0.05) |
0.11 (0.03) |
0.78 (0.00) |
0.21 (0.02) |
♀saline > ♀Milk & Clinical |
| Milk | 0.05 ± 0.25 | −0.27 ± 0.15 | −0.27 ± 0.12 | −0.52 ± 0.07 | ||||||||
| Clinical | 0.35 ± 0.38 | −0.41 ± 0.11 | −0.27 ± 0.19 | −0.43 ± 0.11 | ||||||||
Body Weights
A repeated measures ANOVA on weight at separation, directly before oral administration, and before perfusion revealed a significant within subject main effect of time at both ages in study 1 (F 2, 316 = 115.967, p < 0.001) and study 2 (F2, 302 = 505.365, p < 0.001). As expected, subjects within all groups lost weight during the 3.5 hour procedure regardless of sex, age, or treatment (supplementary table 2).
Weight loss associated with testing for each subject was calculated by subtracting weight taken at the beginning of the test from the weight directly before perfusion. There were no differences in weight loss between sex or treatment group in study 1 (supplementary figure 4A). However, in study 2, there was a main effect of treatment for weight loss (F2,144 = 4.902, p<0.01), a sex by treatment interaction (F2, 144 = 3.937, p<0.05) and a trending treatment by age interaction (F2, 144 = 2.943 p = 0.056). Following up on the significant sex by treatment interaction revealed a significant main effect of treatment in males (F2, 69 = 10.300, p < 0.001); Milk treated males lost more weight compared to saline (d = 0.79) and Clinical treated males (d = 0.45, supplementary figure 4B). Additionally, there was a significant positive correlation between weight loss and behavioral long distance activity factor 1 scores in study 2 (r(119) = 0.26, p <0.01, supplementary figure 5). There was also a main effect of age on weight loss in study 1 (F1, 154 = 145.493, p< 0.001; d = 1.94) and in study 2 (F1, 144 = 124.32, p < 0.001; d = 1.86) revealing P21 subjects lost more weight than P14 subjects (supplementary figure 4 and supplementary table 2).
Discussion:
The experiments described in this paper aimed to test the hypothesis that orally acquired OXT modulates sensory dependent neural processing and behavior during development in the mouse coinciding with transient cortical OXTR expression. We showed that oral OXT, compared to saline, altered a marker of neural activity, c-Fos, across the trigeminothalamocortical circuit, and the paraventricular nucleus of the hypothalamus (PVN), as well as acute behavior. Specifically, oral OXT compared to saline increased c-Fos densities in the PVN of males and decreased c-Fos in the VPM thalamus in males and females and, when accompanied with sensory stimulation, decreased c-Fos in trigeminal sensorimotor nuclei. These neural circuits are schematized in figure 10. Additionally, oral OXT compared to saline, with sensory stimulation increased pre-weaning males long distance activity behavior and decreased females oromotor behavior. These data implicate oral OXT and trigeminal ganglia afferents in sensory and behavioral development and their functionality should be further studied.
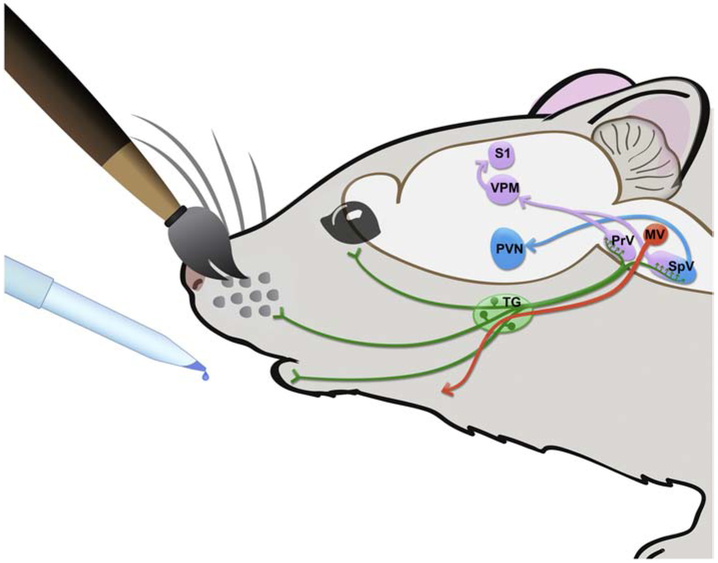
Figure 10: Illustration of experimental model:
Oxytocin receptor positive trigeminal sensory neurons (green) innervate the face. Ninety minutes after oral application of OXT or saline, with or without whisker brushing, c-Fos was measured in CNS regions of interest including the paraventricular nucleus of the hypothalamus (PVN; blue), the motor nucleus of the trigeminal nerve (MV; orange), and the trigeminothalamocortical circuit (purple) carrying information about whisker stimulation (principal trigeminal nucleus, PrV; spinal trigeminal nucleus, SpV; ventroposterior medial nucleus of the thalamus, VPM; somatosensory neocortex in the barrel fields, S1). Anterograde circuit connections are emphasized in these regions of interest. Bidirectional circuitry exists (e.g. descending modulation of the brainstem by the hypothalamus), but it is not depicted in this schematic. Oral application of OXT, regardless of whisker brushing, reduced c-Fos density in the VPM thalamus of males and females and increased c-Fos density in the PVN of males. Oral application of OXT with whisker brushing reduced c-Fos density along the trigeminothalamocortical circuit (PrV, SpV, VPM, S1) of males and females and decreased c-Fos density in the trigeminal motor nucleus (MV) of females.
OXTR: EGFP expression in the trigeminal ganglion and cortex
EGFP signal was present in the trigeminal ganglion of P14 and P21 OXTR:EGFP transgenic males and females. OXTR immunoreactivity in the trigeminal ganglion has been previously reported in adult rats39 while Oxtr mRNA expression in the trigeminal has been observed in mice40 (Hammock et al., unpublished) as well as humans41. Furthermore, applying radioactively tagged OXT to the nose of rats results in dense OXTR binding in the trigeminal nerves. Intranasal OXT has also been shown to alter the neural activity of the trigeminal ganglion38, and somatosensory stimulation has been shown to increase OXTR expression in the trigeminal ganglion39. Therefore, the trigeminal ganglion may transmit information from environmentally obtained OXT during development via oral and nasal peripheral OXTR13,17 on trigeminal nerve endings. OXT has long been known to exist in human mother’s milk, with higher levels in lactating moms versus bottle feeders, and this can be transferred to the infant and may increase the infants endogenous OXT production in a feed-forward mechanism31. Further, since nursing is always accompanied with social sensory stimulation from the mother, it may be that maternal OXT may particularly influence the social sensory dependent organization of the developing brain through orofacial OXTR on the trigeminal nerve. For example, OXTR KO mice, compared to WT littermates, show significantly reduced Oxt gene expression in the PVN, as well as reduced c-Fos activation in the PVN at P14 (Vaidyanathan and Hammock, in review).
We also confirmed the presence of dense OXTR:EGFP expression in the cortex of P14 mice13. The third postnatal week in mice marks the onset of the capacity for multi-sensory neocortical processing as the eyes open and auditory stimuli evoke measurable activity in the neocortex for the first time42. In the neocortex of mice, synapse density peaks during the end of the third postnatal week 43 and the onset of exploration and increased locomotion predominately provides the neural activity that modulates synaptic strength 44. Cross-modal sensory information is integrated for the first time and this integration process is impacted by experience during critical and sensitive periods of development. Therefore, environmental OXT during the third postnatal week in mice may interact with early sensory stimuli and individual genotype to shape neural circuits and generate brain and behavioral responses that, based off sensory input, influence lifelong contextual behavior 45-48. Through the third postnatal week, maternal-offspring and sibling interactions predominate while exploratory behaviors increase. Apart from the mother, social interaction with siblings may be another form of social OXT exchange and orofacial OXTR activation during the third postnatal week of development and beyond in mice.
C-Fos densities and patterns changed by oral OXT
The immediate early gene, c-Fos, revealed unique patterns of brain activation after oral OXT compared to saline that are dependent and independent of whisker stimulation, sex, and age depending on the brain region investigated.
The Clinical dose of OXT increased c-Fos activity in the PVN of males in both studies by approximately 55% (study 1) - 34% (study 2), suggesting that oral OXT may increase neural activity in the PVN independently of sensory experience in males. Interestingly, after accounting for covariance in all the measured brain regions using PCA, both studies 1 and 2 indicated a factor 2 that included primarily positive loadings of c-Fos densities in the PVN and negative loadings of c-Fos densities in the VPM thalamus suggesting a negative relationship between these two brain regions in this experimental context. Without whisker stimulation (study 1), Clinical and Milk treated P21 males had higher factor 2 scores compared to the saline treated average, with a “large” effect size. In study 2, with whisker stimulation, Clinical treated males had an increase of the same PVN factor 2 scores compared to the saline and Milk treated average in males, again with a “large” effect size. On the other hand, oral OXT seemed to reduce c-Fos immunoreactivity in the PVN in females. Clinical treated P21 females had decreased factor 2 PVN scores compared to the saline treated average in study 1, with a “large” effect size. With whisker brushing in study 2, c-Fos cell densities decreased only in the contralateral PVN in P21 Clinical treated females by approximately 77% compared to saline treatment. Due to the location of the PVN on the midline of the brain, it is challenging to collect left and right hemisphere sections of the PVN consistently in sagittal sections. This technical concern reduced the effective sample size for some midline structures, therefore, ipsilateral versus contralateral effects of oral OXT compared to saline reported in the PVN should be interpreted with caution and replicated. Nevertheless, the consistent finding that oral OXT increased PVN c-Fos activity in males implicates a mechanism by which oral OXT can regulate PVN activity with implications for development. The significance of this mechanism may have differential significance on brain and behavior depending on the environmental context and age. For example, whisker stimulation in study 2 seemed to decrease c-Fos densities in the PVN at P14, compared to study 1, regardless of treatment, and oral OXT with whisker stimulation increased c-Fos densities in the PVN of males in study 2 to a lesser extent compared to oral OXT alone in study 1. Further, it is unclear why oral OXT increased c-Fos densities in males, but not females. One possibility could be potential sex differences in OXTR expression on trigeminal nerve terminals innervating the oronasal cavity. Future research is necessary to investigate this possibility.
In brainstem trigeminal nuclei, oral OXT with whisker stimulation (study 2) decreased c-Fos cell densities while oral OXT alone (study 1) had no effect. P14 males in study 2, had a 52% decrease in c-Fos density in the trigeminal sensory nucleus after the Milk dose with whisker stimulation while both P14 males and females had a 52% decrease in the trigeminal spinal nucleus ipsilateral to whisker brushing after a Clinical dose, compared to saline. With PCA, study 1 and study 2 indicated a factor with high loadings in brain regions of the trigeminothalamocortical sensory circuit. Without whisker stimulation (study 1), OXT had no effect on these Factor 1 sensory scores. However, with whisker stimulation in study 2, Clinical treated P14 and P21 males and females had reduced Factor 1 sensory scores, with a “medium” to “large” effect size. These results suggest that sensory dependent oral OXTR activation may regulate the trigeminal to cortex sensory pathway during development in males and females. The functional significance of this remains to be shown, but an OXT-induced decrease in c-Fos in this sensory circuit could reflect a reduction in circuit noise and a boost in sensory-activated signal. In the CNS, OXT has been shown to modulate signal:noise sensory processing via OXTR12,49. A similar network phenomenon may be at work with OXT delivery to the periphery as well.
Oral OXT with whisker stimulation (study 2), but not oral OXT alone (study 1), decreased c-Fos activity in the trigeminal motor nucleus of P14 females by over 50%, but not males. Additionally, PCA in study 2 indicated a factor with a high loading in the trigeminal motor nucleus that was decreased in Milk and Clinical treated P14 and P21 females compared to saline average, with “large” effect sizes. Interestingly, in study 1, PCA did not differentiate c-Fos densities in the trigeminal motor nucleus as a third factor. This suggests that sensory stimulation is necessary for oral OXT to modulate c-Fos in the trigeminal motor nucleus in females, and there are sex differences in sensory dependent OXT modulation of c-Fos in the trigeminal motor nucleus. Although OXT has been shown to modulate oral behavior in male and female rodents and primates50-52, corresponding activity in the trigeminal motor nucleus has not been previously investigated and the sex difference reported here should be replicated and further studied. These results indicate a potential neural mechanism by which oral and intranasal OXT modulate oral behavior in mammals.
Oral OXT decreased c-Fos activity in the VPM thalamus in males and females of both ages in both studies. In study 1 the Milk dose decreased c-Fos immunoreactivity by approximately 70%, while in study 2 the Clinical dose with whisker stimulation decreased c-Fos immunoreactivity in the VPM thalamus by 60%. Therefore, it may be possible that different doses of oral OXT may regulate the VPM thalamus in different contexts. The VPM thalamus processes sensory information from the whisker pads which is directly received via the trigeminal nuclei of the brainstem. The trigeminal nuclei of the brainstem and its projections have been used as a model of somatosensory neural development due to the sensory dependent development of whisker barrelette and jaw representations in the sensory and spinal nuclei53-55 as well as barreloids in the VPM thalamus56. The oral OXT induced reduction of c-Fos cell densities in the VPM thalamus may therefore be a result of oral OXT induced reduction of c-Fos in the trigeminal nuclei. Furthermore, PVN factor 2 scores in both studies 1 and 2 indicate a negative correlation between c-Fos activity in the PVN and VPM implicating neural regulation of the VPM thalamus by the PVN in an opposing direction of trigeminal modulation.
C-Fos densities and patterns associated with age and sex
While not a main focus of our research effort, we observed consistent main effects of age and sex in study 1 and study 2. Most notably, c-Fos activity in the PVN was higher in P21 mice compared to P14. Curiously, this age difference was considerably more robust in study 2 (with whisker brushing): it appears that c-Fos counts were significantly lower in P14 mice in study 2 compared to the P14 mice in study 1 (without whisker brushing), suggesting that whisker brushing may have reduced c-Fos levels in the PVN at P14. Additionally, males had higher c-Fos density in the PVN than females. This baseline sex difference may underly the oral OXT induced enhancement of c-Fos activity in the PVN of males, but not females, although future research is necessary to confirm this. Similar to the PVN, c-Fos cell densities in the trigeminal motor nucleus were higher at P21 compared to P14 in males and females in study 2 with whisker stimulation but only in females in study 1.
In contrast to the PVN, c-Fos densities in sensory processing brain nuclei, including the trigeminal sensory nucleus, trigeminal spinal nucleus, and whisker barrel cortex were higher at P14 compared to P21 in studies 1 and 2. In the VPM thalamus, c-Fos densities were higher at P14 compared to P21 only in study 2 with whisker stimulation. The third postnatal week is the beginning of multi-sensory experience in mice which is critical for experience-dependent refinement of the central nervous system57. Thus, the increased c-Fos activity in sensory processing nuclei at P14 compared to P21 may reflect increased number of neurons and/or increased neuronal responsivity driving the strengths and weakness of synapses. At P21, neurons and synapses may have been eliminated as the brain reaches a more mature state.
Acute behavior response to oral OXT with whisker stimulation
The acute behavioral impact of orally-applied OXT with whisker stimulation was sex specific. In this exploratory behavioral analysis, PCA generated a 3 factor solution to individual differences in patterns of behavior: long distance activity (behaviors where all 4 limbs were moving), proximal activity (behaviors where just the forelimbs were moving), and chew-like oromotor behaviors (just the mouth was moving, even though no food was available during the test). P14 and P21 Milk and Clinical treated males had higher long distance activity factor scores than the saline average, including running, rearing, and climbing behaviors. The effect size for this difference in male long distance behavior was “large”. However, this effect was not observed in female mice. On the other hand, Milk and Clinical treated P14 and P21 females in our study had decreased chew-like oromotor behaviors compared to the saline average, but this effect was not apparent in males. This effect in females was “medium” to “large”. Although there was no food or water provided in the cage during behavioral observations, both male and female mice were often observed chewing bedding and their own feces during the ten minutes after administration. In humans, intranasal OXT given to human infants with Prader-Willi Syndrome (PWS) increased their crawling as toddlers, but sex differences were not investigated although the population consisted of 63% males50. That study also found in humans, that intranasal OXT rescued life-threatening suckling deficits in a sample consisting of both male and female infants with PWS further supporting the idea that oronasal OXT regulates oral sensory-motor behavior. Additionally, intracranial injections of OXT, compared to saline, increased paw sucking while an OXTR antagonist decreased oral behavior in P10 pups51. Similar to our study, OXT effects on oral behavior were observed with vibrissae stimulation in pups51.
The observed behavioral effects might also be informative for mechanisms of weaning. Nursing decreases from postnatal week 1 to week 4 in preparation for weaning58. Oral OXTR decreasing trigeminal motor nucleus activity in females may be one mechanism regulating this decrease in nursing of pre-weanling females. It may be that decreased activity in the trigeminal motor nucleus and corresponding oromotor behavior after oral OXT and whisker stimulation is an important mechanism for weaning in female mice, but not male mice, since females are more likely to stay closer to the nest for longer than males59. A different mechanism may be important for weaning in males which may still be regulated by OXT. Indeed, oral OXT with whisker brushing increased long distance behavior in males in this study. This may be an important mechanism by which OXT transferred from mother to male offspring during nursing increases the likelihood of males leaving the nest. Further research is necessary to investigate this hypothesis.
The trigeminal nerve transmits sensory information from the face to the brain via its three branches which innervate different parts of the face and include the ophthalmic, maxillary, and mandibular branches. These sensory neurons synapse on the trigeminal sensory nuclei of the brainstem and convey tactile information from the whiskers and the rest of the face to the primary whisker barrel cortex, the PVN, and the trigeminal motor nucleus. Additionally, the trigeminal sensory, spinal, and motor nuclei of the brainstem are part of a sensorimotor feedback loop and central pattern generator regulating coordinated facial responses such as suckling, eating, and whisking 60,61. In turn, the whisker barrel cortex also projects to the trigeminal nuclei. The trigeminal nuclei themselves are also heavily interconnected with both inhibitory and excitatory internuclear projections54 and inhibitory interneurons that regulate receptive fields. Neural activity in the lemniscal pathway depends on active versus passive whisking behavior and other exploratory behaviors62-64. Therefore, the increased locomotor behavior in males and decreased oromotor behavior in females may be driving the decrease in c-Fos profiles in the sensory processing brain regions investigated in our study, or vice versa. For example, it reasons that the decrease in oromotor behavior in females with oral OXT treatment is due to decreased neuronal activity in the trigeminal motor nucleus which innervates the lower jaw area and regulates the muscles of mastication.
Caveats
In addition to its action at OXTR, OXT can also signal through additional OXT/vasopressin receptors65-67. While the evidence presented here strongly suggests that OXT can achieve brain and behavior effects when applied to the mouth, the data do not specifically rule out the possibility of OXT binding at other receptors in this neuropeptide receptor family. Future work to specifically test the hypothesis that the OXTR in the oronasal cavity mediates this effect will need to use selective agonists or perhaps evaluate the effects of orally applied OXT in OXTR KO mice. If effects still are apparent, this would suggest a potential role for other receptors to mediate the effects of oronasal application of OXT.
Additionally, in our experiments, we specifically tested the effects of experimenter-delivered oral OXT (vs. saline). In addition to experimenter delivery of OXT, OXT could come from conspecifics, and/or through increased endogenous levels after some event causing OXT release into the periphery. Future experiments based on the results presented here will be needed to test ideas about oral OXT dynamics from conspecifics or endogenous release and their effects on brain and behavior.
Conclusion and future directions
Our study demonstrates that orally applied OXT alters acute behavior and brain activity in developing mice in sex specific ways. Considering that developing mammals receive oral OXT in a social context daily via nursing and that both peripheral and central OXT in rodents and humans alters developmental and adult behaviors68, including suckling in infants and social behavior in mice, oral OXTR signaling may modulate developmental trajectories in species typical ways with the added potential to influence within species experience-dependent individual differences. Indeed, suckling behavior is associated with long term consequences in humans, but the molecular mechanisms are unidentified69. Research in infant macaques has shown that oronasal exposure to OXT by a nebulizer increases prosocial behaviors including oral behaviors in both males and females, such as tongue protrusions and lip smacking52. In humans, suckling can influence the mothers OXT levels, with breastfeeding moms having higher circulating plasma OXT levels compared to bottle feeding moms31. It has been proposed that the OXT system may modulate its own production through a feedforward mechanism between the mother and offspring50. The acute and long-term effects of orofacial OXT exposure in conjunction with sensory stimulation should be further studied.
Supplementary Material
Table 2: Study 1 results for c-Fos principal component analysis (PCA) factor scores after a high (Clinical) or low (Milk) dose of oral oxytocin (OXT) versus saline in postnatal day (P) 14 and P21 male and female OXTR:EGFP mice.
Average c-Fos cell densities were z-scored per IHC batch and z-scores were entered into a PCA. Factor scores are expressed as mean ± standard error of the mean (SEM). Main and interaction effects p – values are from multivariate mixed model analyses on factor scores and effect sizes are expressed in parentheses as partial eta squared values. Bold font emphasizes statistical significance. Post-hoc results are Bonferroni corrected for multiple comparisons. T = treatment; S = sex; A = age; PVN = paraventricular nucleus of the hypothalamus
| Study 1 PCA c-Fos |
Mean ± SEM | p-value (ηp2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treat ment |
Sex | Age | T × S | T × A | A × S |
T × S × A |
post hoc | |||
| P14 | P21 | P14 | P21 | |||||||||
| Factor 1 sensory-motor | ||||||||||||
| Saline | −0.16 ± 0.30 | −0.14 ± 0.48 | 0.36 ± 0.37 | 0.14 ± 0.36 | 0.49 (0.02) |
0.96 (0.00) |
0.93 (0.00) |
0.36 (0.00) |
0.57 (0.01) |
0.83 (0.00) |
0.60 (0.01) |
|
| Milk | 0.14 ± 0.38 | −0.36 ± 0.39 | −0.27 ± 0.32 | –0.10 ± 0.22 | ||||||||
| Clinical | 0.09 ± 0.36 | 0.50 ± 0.27 | −0.12 ± 0.21 | 0.12 ± 0.34 | ||||||||
| Factor 2 PVN | ||||||||||||
| Saline | 0.06 ± 0.28 | −0.52 ± 0.33 | −0.48 ± 0.34 | 0.25 ± 0.29 | 0.29 (0.03) |
0.08 (0.03) |
0.86 (0.00) |
0.17 (0.04) |
0.53 (0.01) |
0.42 (0.01) |
0.01 (0.10) |
♂P21 saline < ♂P21 Milk & Clinical |
| Milk | −0.28 ± 0.36 | 0.64 ± 0.44 | 0.11 ± 0.27 | −0.47 ± 0.18 | ||||||||
| Clinical | 0.57 ± 0.27 | 0.61 ± 0.63 | 0.17 ± 0.27 | −0.55 ± 0.14 | ||||||||
Table 3: Study 2 results for c-Fos cell density in six brain regions after a high (Clinical) or low (Milk) dose of oral oxytocin (OXT) versus saline and whisker brushing in postnatal day (P) 14 and P21 male and female OXTR:EGFP mice.
Average c-Fos cell density is expressed as mean ± standard error of the mean (SEM). Main and interaction effects p – values are from multivariate mixed model analyses on mean c – Fos densities and effect sizes are expressed in parentheses as partial eta squared values. Bold font emphasizes statistical significance. Post-hoc results are Bonferroni corrected for multiple comparisons. * = significance did not survive Bonferroni corrected post-hoc test. T = treatment; S = sex; A = age; PVN = paraventricular nucleus of the hypothalamus
| Study 2 c- Fos |
Mean ± SEM | p-value (ηp2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Treat ment |
Sex | Age | T × S | T × A | A × S |
T × S × A |
post hoc | |||
| P14 | P21 | P14 | P21 | |||||||||
| PVN | ||||||||||||
| Saline | 118 ± 21 | 621 ±104 | 135 ± 30 | 538 ± 140 | 0.26 (0.07) |
0.05 (0.06) |
0.00
(0.45) |
0.04* (0.08) |
0.46 (0.02) |
0.12 (0.03) |
0.18 (0.04) |
P14 < P21 ♂ > ♀ |
| Milk | 141 ± 38 | 439 ± 63 | 139 ± 32 | 471 ± 106 | ||||||||
| Clinical | 217 ± 55 | 873 ± 157 | 138 ± 12 | 371 ± 110 | ||||||||
| Trigeminal sensory | ||||||||||||
| Saline | 202 ± 54 | 47 ± 13 | 122 ± 20 | 47 ± 162 | 0.40 (0.02) |
0.16 (0.02) |
0.00 (0.36) |
0.30 (0.03) |
0.34 (0.02) |
0.84 (0.00) |
0.03 (0.07) |
♂P14 saline > ♂P14 Milk P14 > P21 |
| Milk | 102 ± 19 | 62 ± 16 | 146 ± 25 | 31 ± 8 | ||||||||
| Clinical | 133 ± 10 | 66 ± 11 | 125 ± 27 | 37 ± 8 | ||||||||
| Trigeminal spinal | ||||||||||||
| Saline | 145 ± 28 | 42 ± 10 | 171 ± 24 | 45 ± 7 | 0.07 (0.06) |
0.15 (0.02) |
0.00 (0.59) |
0.57 (0.01) |
0.27 (0.03) |
0.06 (0.04) |
0.84 (0.00) |
P14 > P21 |
| Milk | 114 ± 14 | 42 ± 5 | 159 ± 17 | 41 ± 17 | ||||||||
| Clinical | 110 ± 11 | 43 ± 6 | 124 ± 21 | 29 ± 7 | ||||||||
| Trigeminal motor | ||||||||||||
| Saline | 40 ± 12 | 92 ± 35 | 106 ± 23 | 84 ± 22 | 0.60 (0.01) |
0.70 (0.00) |
0.04 (0.05) |
0.33 (0.03) |
0.69 (0.02) |
0.92 (0.00) |
0.05 (0.05) |
♀P14 saline > ♀P14 Milk & Clinical P14 < P21 |
| Milk | 63 ± 13 | 81 ± 23 | 30 ± 7 | 90 ± 29 | ||||||||
| Clinical | 70 ± 18 | 78 ± 21 | 54 ± 9 | 86 ± 25 | ||||||||
| Ventroposterior medial thalamus | ||||||||||||
| Saline | 83 ± 27 | 27 ± 15 | 64 ± 26 | 6 ± 3 |
0.02 (0.08) |
0.86 (0.00) |
0.00 (0.13) |
0.12 (0.04) |
0.08 (0.06) |
0.10 (0.03) |
0.54 (0.01) |
saline > Clinical P14 > P21 |
| Milk | 17 ± 7 | 27 ± 16 | 46 ± 14 | 14 ± 8 | ||||||||
| Clinical | 11 ± 3 | 5 ± 3 | 48 ± 19 | 3 ± 1 | ||||||||
| Barrel cortex | ||||||||||||
| Saline | 270 ± 47 | 121 ± 45 | 271 ± 35 | 29 ± 8 | 0.31 (0.03) |
0.11 (0.03) |
0.00 (0.54) |
0.83 (0.00) |
0.90 (0.00) |
0.33 (0.01) |
0.53 (0.01) |
P14 > P21 |
| Milk | 259 ± 35 | 93 ± 31 | 248 ± 35 | 60 ± 25 | ||||||||
| Clinical | 247 ± 23 | 51 ± 16 | 222 ± 46 | 33 ± 13 | ||||||||
Highlights:
OXTR on trigeminal sensory ganglia are poised to detect orally applied OXT.
Oral OXT increased c-Fos in the PVN of male pre-weanling mice.
Oral OXT with whisker stimulation decreased c-Fos in the whisker neural circuit in both sexes.
Oral OXT increased males’ locomotor behavior and decreased females’ oromotor behavior.
Socially acquired OXT may be a mechanism to modulate sensory-dependent brain and behavior development.
Acknowledgements:
We thank Alicia Gonzalez and Alexis Coleman for their assistance in perfusions. We also thank Sarah Almoshaikah, Brynn Paulsen, Marielle Gomez, Aurelie Gedeon, Zoe Jones, Emma Lyon, and Nick Wellman for their assistance with histology. Funding for this work provided by The Florida State University (to EADH), T32 MH093311 and T32 DC000044 (to MT), The Good Nature Institute (to EADH) and NIH MH114994 (to EADH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaidyanathan R & Hammock EAD Oxytocin receptor dynamics in the brain across development and species. Dev. Neurobiol 77, 143–157 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Tabbaa M, Paedae B, Liu Y & Wang Z Neuropeptide Regulation of Social Attachment: The Prairie Vole Model. Compr. Physiol 7, 81–104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung LW et al. Gating of social reward by oxytocin in the ventral tegmental area. Science 357, 1406–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosaki Y & Watanabe S Conditioned social preference, but not place preference, produced by intranasal oxytocin in female mice. Behav. Neuroscl 130, 182–195 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Song Z, Borland JM, Larkin TE, O’Malley M & Albers HE Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 74, 164–172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champagne F, Diorio J, Sharma S & Meaney MJ Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Scl. U. S. A 98, 12736–12741 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lukas M, Bredewold R, Neumann ID & Veenema AH Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 58, 78–87 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Carter CS, Boone EM, Pournajafi-Nazarloo H & Bales KL Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev. Neuroscl 31, 332–341 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bales KL & Carter CS Developmental exposure to oxytocin facilitates partner preferences in male prairie voles (Microtus ochrogaster). Behav. Neurosci 117, 854–859 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Miller TV & Caldwell HK Oxytocin during Development: Possible Organizational Effects on Behavior. Front Endocrinol. 6, 76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberts JR Huddling by rat pups: ontogeny of individual and group behavior. Dev. Psychoblol 49, 22–32 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Zheng J-J et al. Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat. Neurosci 17, 391–399 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Hammock EAD & Levitt P Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse. Front. Behav. Neurosci 7, 195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammock EAD Developmental perspectives on oxytocin and vasopressin. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 40, 24–42 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamay-Tsoory SG & Abu-Akel A The Social Salience Hypothesis of Oxytocin. Biol. Psychiatry 79, 194–202 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Hammock EAD Oxytocin and Plasticity of Social Behavior. Oxf. Handb. Dev. Neural Plast (2017). [Google Scholar]
- 17.Greenwood MA & Hammock EAD Oxytocin receptor binding sites in the periphery of the neonatal mouse. PloS One 12, e0172904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barna J et al. Suckling induced activation pattern in the brain of rat pups. Nutr. Neurosci 21, 317–327 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Caba M, Rovirosa MJ & Silver R Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res. Dev. Brain Res 143, 119–128 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Malick A, Strassman RM & Burstein R Trigeminohypothalamic and reticulohypothalamic tract neurons in the upper cervical spinal cord and caudal medulla of the rat. J. Neurophysiol 84, 2078–2112 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Hockfield S & Gobel S An anatomical demonstration of projections to the medullary dorsal horn (trigeminal nucleus caudalis) from rostral trigeminal nuclei and the contralateral caudal medulla. Brain Res. 252, 203–211 (1982). [DOI] [PubMed] [Google Scholar]
- 22.Li J-L, Kaneko T, Shigemoto R & Mizuno N Distribution of trigeminohypothalamic and spinohypothalamic tract neurons displaying substance P receptor-like immunoreactivity in the rat. J. Comp. Neurol 378, 508–521 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Malick A & Burstein R Cells of origin of the trigeminohypothalamic tract in the rat. J. Comp. Neurol 400, 125–144 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Heintz N Gene Expression Nervous System Atlas (GENSAT) ∣ Nature Neuroscience. Nat. Neuroscl 483, (2004). [DOI] [PubMed] [Google Scholar]
- 25.Gong S et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Gerfen CR, Paletzki R & Heintz N GENSAT BAC Cre-Recombinase Driver Lines to Study the Functional Organization of Cerebral Cortical and Basal Ganglia Circuits. Neuron 80, 1368–1383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon M, Adels L, Coopersmith R & Woodside B Diurnal cycle of mother-young contact in Norway rats. Physiol. Behav 32, 999–1003 (1984). [DOI] [PubMed] [Google Scholar]
- 28.Hoshino K, Wakatsuki Y, ligo M & Shibata S Circadian Clock Mutation in Dams Disrupts Nursing Behavior and Growth of Pups. Endocrinology 147, 1916–1923 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Toki S et al. Importance of early lighting conditions in maternal care by dam as well as anxiety and memory later in life of offspring. Eur. J. Neurosci 25, 815–829 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Jensen Peña C & Champagne FA Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behav. Neurosci 127, 33–46 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeda S, Kuwabara Y & Mizuno M Concentrations and origin of oxytocin in breast milk. Endocrinol. Jpn 33, 821–826 (1986). [DOI] [PubMed] [Google Scholar]
- 32.Leake RD, Weitzman RE & Fisher DA Oxytocin concentrations during the neonatal period. Biol. Neonate 39, 127–131 (1981). [DOI] [PubMed] [Google Scholar]
- 33.Feng C et al. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 9, 754–764 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Cardoso C, Ellenbogen MA, Orlando MA, Bacon SL & Joober R Intranasal oxytocin attenuates the cortisol response to physical stress: A dose–response study. Psychoneuroendocrinology 38, 399–407 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Eckstein M et al. Oxytocin increases eye-gaze towards novel social and non-social stimuli. Soc. Neurosci 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumstein DT, Daniel JC & Evans CS JWatcherTM 1.0 An Introductory User’s Guide. 71 [Google Scholar]
- 37.Schneider CA, Rasband WS & Eliceiri KW NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzabazis A et al. Oxytocin and Migraine Headache. Headache J. Head Face Pain 57, 64–75 [DOI] [PubMed] [Google Scholar]
- 39.Tzabazis A et al. Oxytocin receptor: Expression in the trigeminal nociceptive system and potential role in the treatment of headache disorders. Cephalalgia Int. J. Headache 36, 943–950 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Lopes DM, Denk F & McMahon SB The Molecular Fingerprint of Dorsal Root and Trigeminal Ganglion Neurons. Front. Mol. Neurosci 10, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flegel C et al. RNA-Seq Analysis of Human Trigeminal and Dorsal Root Ganglia with a Focus on Chemoreceptors. PloS One 10, e0128951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brust V, Schindler PM & Lewejohann L Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool 12 Suppl 1, S17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandrasekaran S et al. Unbiased, High-Throughput Electron Microscopy Analysis of Experience-Dependent Synaptic Changes in the Neocortex. J. Neurosci. Off. J. Soc. Neurosci 35, 16450–16462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua JY & Smith SJ Neural activity and the dynamics of central nervous system development. Nat. Neurosci 7, 327–332 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Hurlemann R & Scheele D Dissecting the Role of Oxytocin in the Formation and Loss of Social Relationships. Biol. Psychiatry 79, 185–193 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Leknes S et al. Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Soc. Cogn. Affect. Neurosci 8, 741–749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quattrocki E & Friston K Autism, oxytocin and interoception. Neurosci. Biobehav. Rev 47, 410–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brewer R, Happé F, Cook R & Bird G Commentary on ‘Autism, oxytocin and interoception’: Alexithymia, not Autism Spectrum Disorders, is the consequence of interoceptive failure. Neurosci. Biobehav. Rev 56, 348–353 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Ninan I Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J. Neurochem 119, 324–331 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Tauber M et al. The Use of Oxytocin to Improve Feeding and Social Skills in Infants With Prader–Willi Syndrome. Pediatrics 139, e20162976 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Nelson E & Alberts JR Oxytocin-induced paw sucking in infant rats. Ann. N. Y. Acad. Sci 807, 543–545 (1997). [DOI] [PubMed] [Google Scholar]
- 52.Simpson EA et al. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc. Natl. Acad. Sci. U. S. A 111, 6922–6927 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayashi H Distributions of vibrissae afferent fiber collaterals in the trigeminal nuclei as revealed by intra-axonal injection of horseradish peroxidase. Brain Res. 183, 442–446 (1980). [DOI] [PubMed] [Google Scholar]
- 54.Furuta T et al. Inhibitory gating of vibrissal inputs in the brainstem. J. Neurosci. Off. J. Soc. Neurosci 28, 1789–1797 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erzurumlu RS, Murakami Y & Rijli FM Mapping the face in the somatosensory brainstem. Nat. Rev. Neurosci 11, 252–263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Der Loos H Barreloids in mouse somatosensory thalamus. Neurosci. Lett 2, 1–6 (1976). [DOI] [PubMed] [Google Scholar]
- 57.Holtmaat A & Svoboda K Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci 10, 647–658 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Curley JP et al. The Meaning of Weaning: Influence of the Weaning Period on Behavioral Development in Mice. Dev. Neurosci 31, 318–331 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krackow S Motivational and Heritable Determinants of Dispersal Latency in Wild Male House Mice (Mus musculus musculus)-. Ethology 109, 671–689 (2003). [Google Scholar]
- 60.Kubo A et al. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain 158, 649–659 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Moore JD, Kleinfeld D & Wang F How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci. 37, 370–380 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bredewold R, Smith CJW, Dumais KM & Veenema AH Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crochet S & Petersen CCH Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat. Neurosci 9, 608–610 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Lee S, Carvell GE & Simons DJ Motor modulation of afferent somatosensory circuits. Nat. Neurosci 11, 1430–1438 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landgraf R & Neumann ID Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol 25, 150–176 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Song Z et al. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology 50, 14–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manning M et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol 24, 609–628 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meziane H et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol. Psychiatry 78, 85–94 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Wolthuis-Stigter MI et al. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J. Pediatr 166, 26–30 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.