Abstract
Hypertension is a risk factor for premature death and roughly 50% of hypertensive patients are salt-sensitive. The incidence of salt-sensitive hypertension increases with age. However, the mechanisms of salt-sensitive hypertension are not well understood. We had demonstrated decreased renal sodium-hydrogen exchanger regulatory factor 1 (NHERF1) expression in old salt-resistant F344 rats. Based on those studies we hypothesized that NHERF1 expression is required for the development of some forms of salt-sensitive hypertension. To address this hypothesis, we measured blood pressure in NHERF1 expressing salt-sensitive 4-mo and 24-mo-old male and female Fischer Brown Norway (FBN) rats and male and female 18-mo-old NHERF1 knock-out (NHERF1−/−) mice and wild-type (WT) littermates on C57Bl/6J background after feeding high salt (8% NaCl) diet for 7 days. Our data demonstrate that 8% salt diet increased blood pressure in both male and female 24-mo-old FBN rats but not in 4-mo-old FBN rats and in 18-mo-old male and female WT mice but not in NHERF1−/− mice. Renal dopamine 1 receptor (D1R) expression was decreased in 24-mo-old rats, compared with 4-mo-old FBN rats. However, sodium chloride cotransporter (NCC) expression increased in 24-mo-old FBN rats. In FBN rats, age had no effect on Na-K ATPase α1 and NKCC2 expression. By contrast, high salt diet increased the renal expressions of NKCC2, and NCC in 24-mo-old FBN rats. High salt diet also increased NKCC2 and NCC expression in WT mice but not NHERF1−/− mice. Our data suggest that renal NHERF1 expression confers salt sensitivity with aging, associated with increased expression of sodium transporters.
Keywords: NHERF1, salt sensitive hypertension, aging, kidney, transporters
Introduction
Hypertension is endemic and a major risk factor for premature death due to cardiovascular and renal complications. The majority of patients have essential hypertension, which is of unknown cause [1]. Dietary salt is widely accepted as a risk factor for the development of hypertension [2]. About 50% of patients with essential hypertension are salt-sensitive, the prevalence of which increases with age [3,4]. Several genetic and environmental factors are linked to salt-sensitivity [5,6]. However, the mechanisms causing salt-sensitive hypertension and the differences between sexes are still evolving [7–10].
Recently, our laboratory reported an age-related decrease in the renal expression of sodium hydrogen exchanger regulatory factor-1 (NHERF1) in a widely used rat model of aging, Fisher 344 (F344) rats [11]. F344 rats are normotensive and salt-resistant at 4-mo of age but develop hypertension with age independent of salt intake [12]. NHERF-1, a scaffolding protein with two canonical PDZ (postsynaptic protein PSD-95/SAP 90, Drosophila septate junction protein Discs-large, tight junction protein ZO-1) domains and an ezrin binding domain [13], is highly expressed in both brush border membranes (BBM) and basolateral membranes (BLM) of the renal proximal tubule [14]. NHERF-1 also associates with several G protein-coupled receptors (GPCR), including the α-adrenergic receptor, parathyroid hormone receptor, and dopamine 1 receptor in renal proximal tubule cells to regulate ion transport [15–19]. Our studies in OK cells demonstrated that sodium-potassium ATPase (NKA) α1 and dopamine 1 receptor (D1R) associate with NHERF-1 through its PDZ2 domain [19]. NHERF-1 also plays an important role in the regulation of the expression, trafficking, and activity of several ion transporters in the renal proximal tubule, including sodium-phosphate cotransporter type IIa (NpT2a) [20,21], sodium-hydrogen exchanger-3 (NHE3) [22], and sodium bicarbonate cotransporter type 1 (NBCe1) [23]. Similar to our findings in F344 rats [11], renal NHERF1 expression has been shown to be decreased in a genetically hypertensive rat, the spontaneously hypertensive rat (SHR), compared with its normotensive control, the Wistar Kyoto (WKY) rat [24]. The SHR is genetically hypertensive and develop hypertension independent of salt intake [25]. These studies suggest that NHERF1 is a reasonable candidate for mediating salt-sensitive hypertension through its effects on sodium transport [26], although the potential mechanisms are unknown.
Based on our previous studies that renal NHERF1 expression decreases in a well-studied salt-resistant rat model of aging, F344 [11], we hypothesized that the increased expression of NHERF1 mediates some forms of age-related, salt-sensitive hypertension. To address this hypothesis, we examined the effects of high salt intake in male and female FBN rat, another rat model of salt-sensitive hypertension that develops with aging [26] and in a mouse model with germline, global deletion of NHERF1 expression. We found that similar to F344 rats, 18-mo-old mice lacking NHERF1 expression (NHERF1−/− mice) were resistant to hypertension, as opposed to their salt-sensitive wild type littermates. Our data also show that feeding a high salt diet for a week increased mean arterial pressure (MAP) in 24-mo-old FBN rats but not in 4-moold FBN rats. The increase in MAP was accompanied by an increase in the renal expression of NKCC2 and NCC. These results demonstrate, for the first time, that NHERF1 expression may be one of the factors that confers salt sensitivity in rodent models of aging.
Materials and Methods:
Animals:
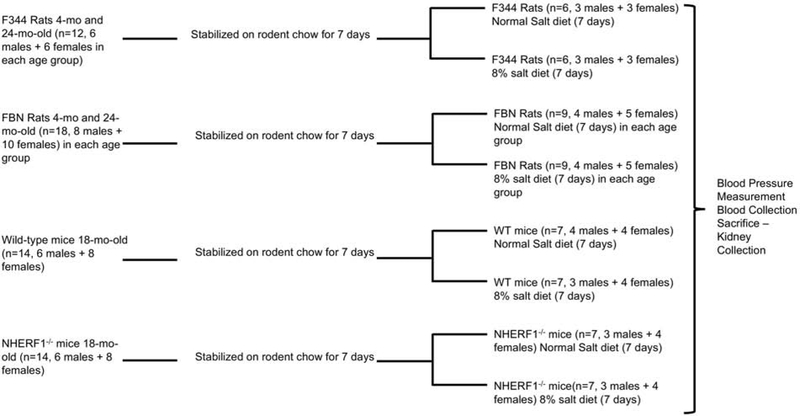
All animal experiments were performed according to the US Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the Howard University and the University of Louisville. F344 rats (n=12, 6 males and 6 females) and Fisher Brown Norway (FBN) rats of ages 4 (n=18, 8 males and 10 females) and 24-month (n=18, 8 males and 10 females) were provided by the National Institute of Aging. NHERF1−/− mice (18-mo old) (n=14, 6 males and 8 females) and their wild type (WT) littermates on C57Bl/6J (n=14, 6 males and 8 females) background were provided by Dr. Weinman. The animals were stabilized on standard rodent chow and water ad libitum for one week prior to experiments. The animals were then equally divided into two groups, one group of animals were fed with normal rodent chow containing 0.9% NaCl, and another group were fed with rodent chow containing 8% NaCl diet (Harlan Laboratories Inc., Indianapolis, IN) for 7 days (Figure 1). In preliminary experiments we observed an increase in MAP and achievement of salt balance with 7 days of 8% salt diet. The remaining experiments were performed with the same protocol.
Figure 1: Animal Protocol.
Figure describes the animal protocol. Numbers in parenthesis represents animals used in each experiment.
Measurement of sodium, chloride, and potassium in serum and urine:
Sodium, chloride, and potassium were measured in serum and urine (diluted 1:10 with Medica urine diluent), using EasyLyte Plus analyzer (Medica, Bedford, MA), following manufacturer’s protocol. Briefly, on day 6 of the salt treatment the animals were housed in metabolic cages (Tecniplast, West Chester, PA). Food and water were measured by weight before and 6 days after the salt treatment to determine 24 hr. water and food intake. Urine was collected overnight for 24 hr. and measured using micropipettes. After blood pressure measurement, blood was collected from the femoral artery (rats) or carotid artery (mice); serum was collected after overnight coagulation.
Blood Pressure Measurement:
Blood pressure was measured in pentobarbital (50 mg/kg body weight)-anesthetized rats by placing a catheter in the right femoral artery (rats) or carotid artery (mice) and data were analyzed using customized Micro-Med software, as described previously [27].
Preparation of Crude membrane:
The kidney cortex was homogenized in 50 mM mannitol, 5 mM Tris-HCl, pH 7.4. The homogenates were centrifuged at 5000x g to remove cell debris and the supernatant was centrifuged at 100,000x g for 1 h. The pellet (membrane-enriched fraction) was resuspended in 300 mM mannitol, 5 mM Tris-HCl, pH 7.2.
Western Blotting:
Western blot was used to quantify the expression of NHERF1, D1R, NKA α1 subunit, NKCC2, and NCC in membrane-enriched fractions from kidney cortex exactly as described previously [14] using NKA (α6F) from (DHSB Iowa University Hybridoma facilities), D1R (P. Jose), NHERF1 [14], NKCC2, and NCC (M. Knepper) antibodies. Briefly, 25 μg protein samples were separated on a 10% SDS-PAGE in Tris-Glycine-SDS buffer at 120 volts for 2 hr. using BioRad’s minigel apparatus. The proteins were electrophoretically transferred onto nitrocellulose membranes at 100 volts for 90 min at 4°C, using the wet transfer system from BioRad. The nitrocellulose membranes were incubated with 5% milk in TBS-T (blocking buffer) for 1 hr. at room temperature to block non-specific proteins. The nitrocellulose membranes were washed with TBS-T twice for 5 min and the membranes were incubated overnight at 4°C with primary antibodies (1:1000 dilution) in blocking buffer. The membranes were washed 4 times with TBS-T for 10 min each time. The membranes were then incubated with secondary mouse (NKA) or rabbit (NHERF1, NKCC2, NCC, and D1R) antibodies, together with HRP-linked anti-actin (1:10000) antibodies for 1 hr. at room temperature. The signal was detected using Super-signal (Thermo Fischer) in BioRad Universal Hood II Gel documentation system.
Protein Determination:
Protein concentration was measured by bicinchoninic acid method using bovine serum albumin as standard.
Statistics:
Lamorte’s Power Calculation Spreadsheet (https://www.bu.edu/researchsupport/compliance/animal-care/workingwith-animals/research/sample-size-calculations-iacuc/) was used to estimate sample sizes. Data are shown as mean ± SD. The n values represent the number of animals. P values were calculated using GraphPad Prism software utilizing two-way ANOVA, followed by Bonferroni post-hoc test. A p value <0.05 was a priori considered statistically significant. All raw data along with statistical analyses are presented in supplemental data files.
Results
Effect of high salt diet on blood pressure in F344 rats:
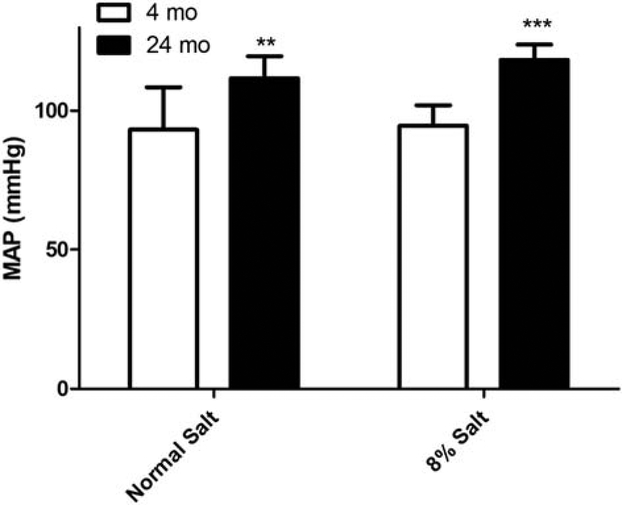
The F344 rat is a normotensive and salt-resistant model of aging [12]. Our laboratory has shown that in F344 rats, renal proximal tubule cell NHERF1 expression decreased with aging [11]. To confirm that old F344 rats are salt-resistant, we fed normal rodent chow (0.9% NaCl) or chow containing 8% NaCl to 4-mo, and 24-mo-old to F344 rats. On day 7 blood pressure was measured as described in Methods. As shown in Fig. 2, the mean arterial pressure (MAP) was significantly higher in 24-mo-old F344 rats than 4-mo-old rats on normal salt diet. However, 8% salt diet did not increase MAP in either 4-mo or 24-mo-old F344 rats.
Figure 2: Effect of dietary salt on mean arterial pressure (MAP, mm Hg) in 4-mo and 24-mo old F344 rats.
Blood pressure was measured in anesthetized 4-mo or 24-mo old F344 rats as described in Methods. Each data point represents (red=normal salt diet; blue=8% salt diet) MAP (mm Hg) from a single animal and the horizontal lines are data as mean±SD from 6 animals in each group (3 males and 3 females). Because there were no significant differences between male and female rats, the data was pooled together. ** indicates p value < 0.01 and *** indicates p value < 0.001 from 4-mo old rats by two-way ANOVA followed by Bonferroni post-hoc test.
Effect of high salt diet on body weight and urinary volume, sodium, chloride, and potassium in FBN rats:
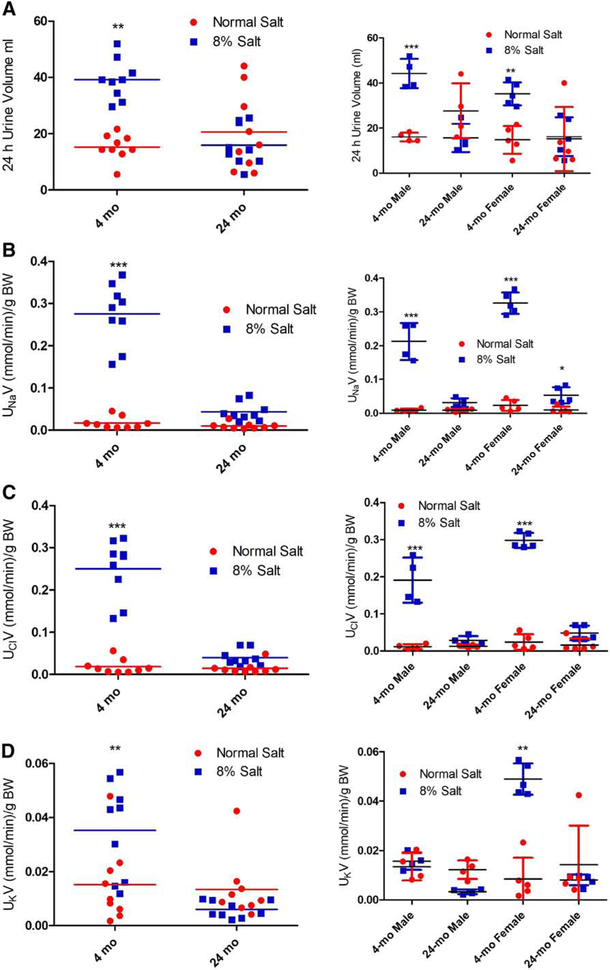
The FBN rat, a cross between F344 and Brown Norway rats, is a salt-sensitive model of aging [26]. To determine the effect of a high salt diet on renal handling of salt and water, we fed normal (0.9%) or 8% NaCl diet to 4-mo and 24-mo-old FBN rats. On the 6th day of the experiment the FBN rats were individually housed in metabolic cages for 24 hr. to measure food and water intake and urine volume and electrolytes. Body weight was determined before the start of the experiment and on the day of sacrifice. As shown in Table 1, there were no differences in body weight and food and water intake between the 0.9% and 8% salt-fed groups. However, the body weight of male rats was significantly higher than female rats at both 4-mo and 24-mo of age. 8% salt diet, relative to 0.9% salt diet, increased urine volume in both male and female 4-mo-old rats but not in 24-mo-old rats, regardless of sex (Fig. 3A). Similarly, 8% salt diet, relative to 0.9% salt diet, increased sodium (Fig. 3B) and chloride (Fig. 3C) excretions in both male and female 4-mo-old rats but not in 24-mo-old rats. By contrast, the increase in potassium excretion caused by 8% salt diet in 4-mo-old rats was exclusively due to the increase in 4-mo-old female rats (Fig. 3D).
Table 1:
Body weight before starting the salt diet and on day 6 of normal (0.9% NaCl) or high (8% NaCl) salt diet in FBN rats.
| (Normal Salt) Before | (Normal Salt) After | (8% Salt) Before | (8% Salt) After | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Body Wt. Mean±SD | Body Wt. Mean±SD | Food Intake (g/d) Mean±SD | Water Intake (mL/d) Mean±SD | N | Body Wt. Mean±SD | Body Wt. Mean±SD | Food Intake (g/d) Mean± SD | Water Intake (mL/d) Mean±SD | N | |
| 4-mo Male | 327.00±18.13 | 327.88±17.88 | 18.89±3.5 | 18.25±1.7 | 4 | 319.75±10.52 | 326.00±9.65 | 16.08±1.38 | 22.25±3.1 | 4 |
| 4-mo Female | 197.60±6.82 | 197.32±5.83 | 10.01±0.8 | 14.8±2.28 | 5 | 192.80±7.14 | 194.22±7.24 | 11.01±1.3 | 17.2±1.92 | 5 |
| 24-mo Male | 460.75±36.37 | 464.93±38.11 | 20.36±3.8 | 20.75±1.5 | 4 | 431.25± 27.31 | 433.30±28.39 | 19.00±4.3 | 23.6±1.9 | 4 |
| 24-mo Female | 262.34±6.95 | 263.26±7.96 | 11.13±1.8 | 17.6±2.07 | 5 | 248.44±14.23 | 251.70± 16.43 | 11.05±1.4 | 21.6±2.34 | 5 |
Figure 3: Effect of dietary salt on urinary volume, sodium, chloride, and potassium in FBN rats.
FBN rats (4-mo and 24-mo-old) were individually housed in metabolic cages for 24 hr. on day 6 of 8% dietary salt (a day before sacrifice). Urinary (A) volume; (B) sodium; (C) chloride; and (D) potassium was measured. The left panel in each figure shows data pooled from both male and female rats. The right panels show data from 4-mo and 24-mo-old male and female rats. Each data point (red=normal salt diet; blue=8% salt diet) represents values from a single animal and the horizontal lines are data as mean±SD (n=9; 4 males and 5 females in each group). * indicates p < 0.05, ** indicates p < 0.01 and *** indicates p < 0.001 from the respective normal salt fed rats by two-way ANOVA followed by Bonferroni post-hoc test.
Effect of high salt diet on mean arterial blood pressure in FBN rats:
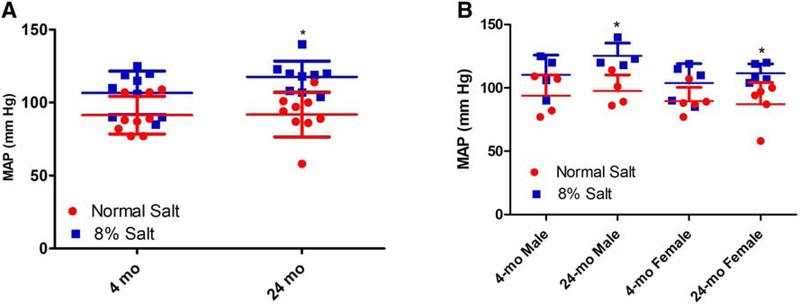
To determine the effect of 8% salt diet on mean arterial pressure, blood pressure was measured in anesthetized FBN rats. As shown in Fig.4, in contrast to the F344 rats, 8% salt diet, relative to normal (0.9%) salt diet, slightly but significantly increased mean arterial blood pressure in 24-mo-old FBN rats but not in 4-mo-old FBN rats, regardless of sex. On 0.9% salt diet, there were no differences in MAP between 4-mo and 24-mo-old FBN rats, regardless of sex (Fig 4 A–B).
Figure 4: Effect of dietary salt on mean arterial pressure (MAP, mm Hg) in 4-mo and 24-mo-old FBN rats.
FBN rats (4-mo and 24-mo-old) were fed normal (0.9%) or 8% dietary salt for 7 days. Blood pressure was measured in anesthetized 4-mo and 24-mo-old FBN rats on day 7 as described in Methods. Each data point represents MAP (mm Hg) from a single animal (red=normal salt diet; blue=8% salt diet) and the horizontal lines are data as mean±SD from 9 animals in each group (4 males and 5 females). A, shows data pooled from both male and female rats. B, shows data from 4-mo and 24-mo-old male and female rats. * indicates p < 0.05 from the respective normal salt fed rats by two-way ANOVA followed by Bonferroni post-hoc test.
Effect of age and salt intake on the renal expression of NHERF1, NHE3, NKCC3, and NCC in FBN rats:
Recently we demonstrated that NHERF1 expression decreases with age in F344 rats [11]. To determine if renal NHERF1 expression plays a role in salt-sensitive hypertension, we measured NHERF1 expression in crude membranes from kidney cortex of FBN rats. Renal NHERF1 expression was higher in 24-mo-old FBN male rats than in 4-mo-old male rats (Fig 5A, top panel), in contrast to the reduced expression in 22-mo-old F344 rats [11]. On normal salt (0.9% NaCl) diet, renal NHERF1 expression was significantly lower in 4-mo-old and 24-mo-old female rats than age-matched male rats on normal salt diet (Supplemental Data). High salt (8% NaCl) diet had no effect on renal NHERF1 expression in either 4-mo-old or 24-mo-old male rats but increased NHERF1 expression in female rats regardless of age, to the same level observed in 4-mo-old male FBN rats.
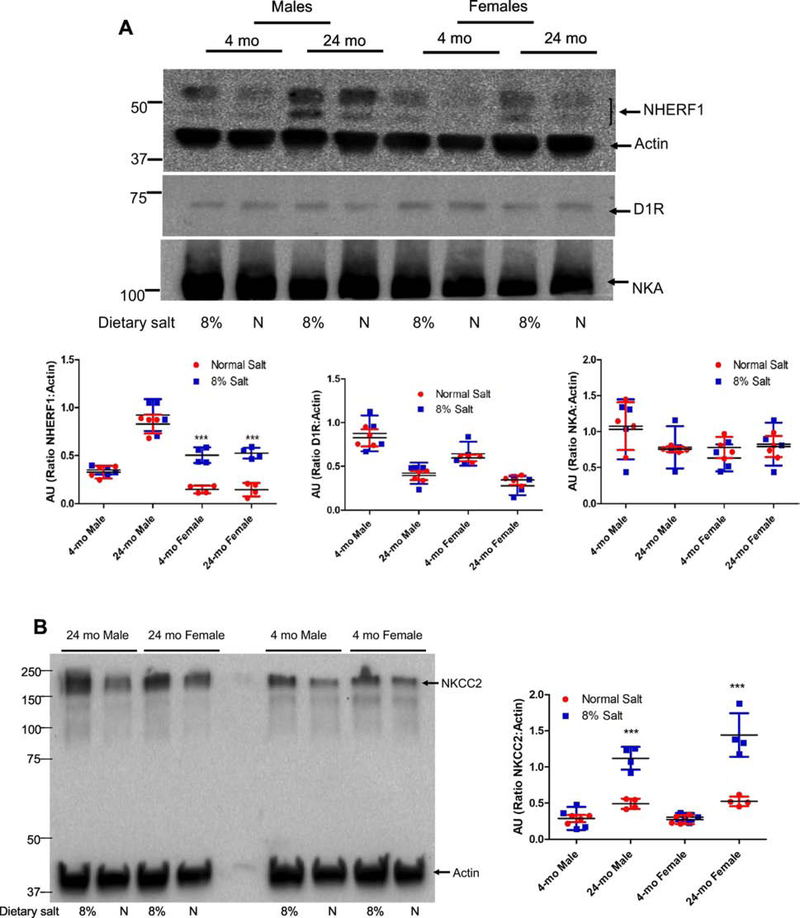
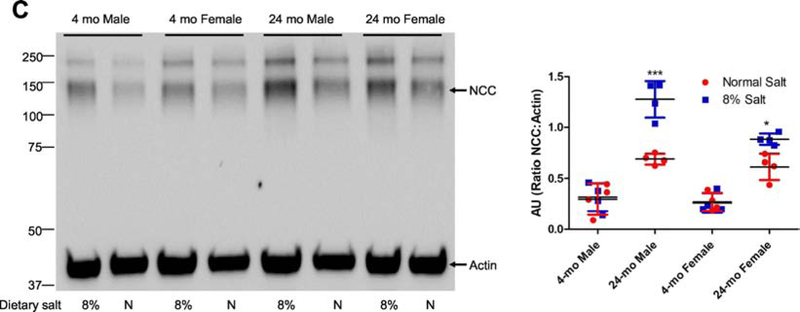
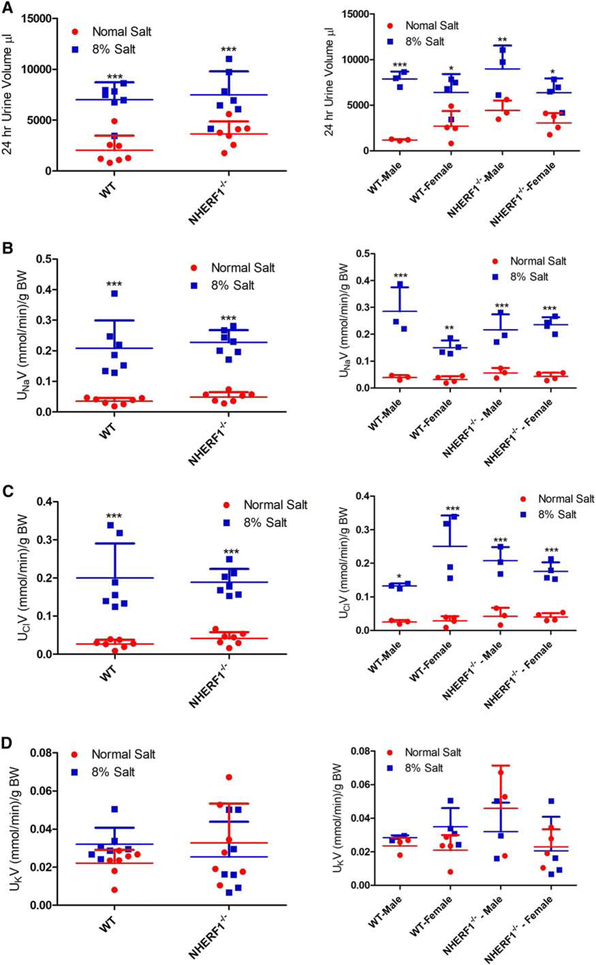
Figure 5: Effect of dietary salt on expression of NHERF1, dopamine 1 receptor (D1R), NKA α1 subunit, NKCC2, and NCC in FBN rats.
FBN rats were fed normal (0.9%) or 8% dietary salt for 7 days. The animals were euthanized following blood pressure measurement on day 7 and the kidneys were removed, decapsulated, and crude membrane homogenates were prepared as described in methods. Expression of (A) NHERF1 (top panel); D1R (middle panel); and NKA α1 (bottom panel). One gel was used, and the nitrocellulose paper was cut below the 100 KDa marker (for NKA α1), at 60 KDa (for D1R) and the bottom portion below 60 KDa was used for NHERF1 together with Actin (B) NKCC2; and (C) NCC was determined by western blot. A representative western blot from one male and one female rat groups is shown. To determine equal loading the nitrocellulose membrane was simultaneously blotted on the same blot for actin using an HRP-actin antibody together with HRP-secondary antibodies. Each data point represents AU (ratio of specific protein to actin) from a single animal (red=normal salt-diet; blue=8% salt diet) and the horizontal lines are data as mean±SD from 9 animals in each group (4 males and 5 females). *** indicates p < 0.001 and * indicates p < 0.05 from normal salt fed rats, by two-way ANOVA followed by Bonferroni post-hoc test.
Renal D1R receptor expression decreased with age in both male and female FBN rats, although the expression was lower in male than in female rats at any age (Supplemental Data). Increasing dietary salt from normal (0.9% NaCl) to high (8% NaCl) had no effect on renal D1R expression in both 4-mo old and 24-mo old FBN rats, regardless of sex (Fig. 5A).
The expression and/or activity of sodium transporters/pumps/channels are involved in the regulation of blood pressure [28]. However, in FBN rats, neither age, salt intake, nor sex had any effect on renal NKA α1 subunit expression (Fig. 5A, lowest panel). On normal (0.9% NaCl) diet, neither age nor sex had any effect on renal NKCC2 expression (Fig. 5B). However, high salt (8% NaCl) diet increased the renal expression of NKCC2 in 24-mo-old rats, regardless of sex.
In contrast to the absence of an effect of age and sex on NKA α1 subunit and NKCC2 expression, renal NCC expression increased with age, regardless of sex. In addition, NCC expression was increased by 8% salt diet in 24-mo-old but not in 4-moold rats, regardless of sex (Fig. 5C).
Effect of NHERF1 deletion on the development of salt sensitive hypertension in aging:
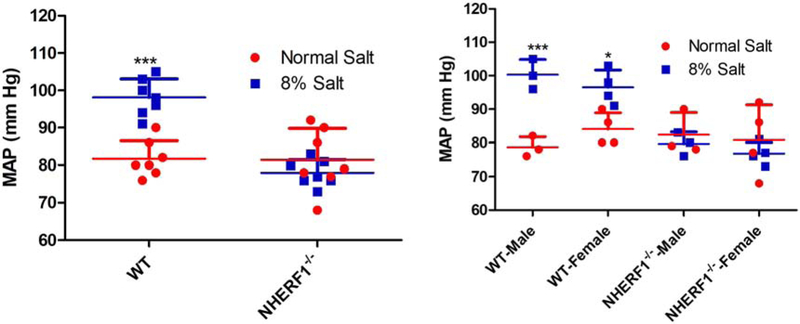
As aforementioned, renal NHERF1 expression was significantly higher in 24-mo old male than 4-mo old male FBN rats (Fig 5A), in contrast to the decreased renal NHERF1 expression in 24-mo-old F344 rats [11]; blood pressure increases with age in F344 (Fig. 2) independent of dietary salt but dependent on salt intake in FBN rats (Fig. 4). To determine if NHERF1 expression were required for the development of salt sensitive hypertension of aging, we fed 18-mo-old WT (C57Bl/6J) and NHERF1−/− mice rodent chow containing normal (0.9% NaCl) or high (8% NaCl) salt-diet for 7 days. On day 6 of the experiment, the mice were individually housed in metabolic cages for 24 hr. to measure food and water intake and urine volume and electrolytes. Body weight was determined before the start of the experiment and on the day of sacrifice. As shown in Table 2, there were no differences in body weight among the groups. Similarly, food and water intakes among the groups were not different (Food: 2.8±0.8 (N) vs. 2.12±0.54 (8%) (WT) and 2.13±0.53 (N) vs. 2.11±0.72 (8%) (NHERF1−/−) g/d; Water: 2.3±0.14 (N) vs. 2.6±0.21 (8%) (WT) and 2.4±0.32 (N) vs. 2.49±0.28 (8%) (NHERF1−/−) mL/d). Feeding 8% NaCl diet increased 24 hr. urinary volume, and Na and Cl excretion in both WT and NHERF1−/− groups, regardless of sex (Fig 6A, 6B, 6C). However, urinary volume and sodium excretion with 8% NaCl was higher in WT males than in WT females (Fig. 6B). There were no significant differences in urinary potassium among the groups, regardless of sex or salt intake (Fig. 6D).
Table 2:
Body weight before starting the specified salt diet and at the end of the experiment in WT and NHERF1−/− mice
| (Normal Salt) Before | (Normal Salt) After | (8% Salt) Before | (8% Salt) After | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | N | Mean | SEM | N | Mean | SEM | N | Mean | SEM | N | |
| WT-Male | 28.03 | 1.36 | 3 | 28.4 | 1.33 | 3 | 26.3 | 0.85 | 3 | 26.83 | 0.96 | 3 |
| WT-Female | 26.43 | 1.92 | 4 | 27.13 | 1.95 | 4 | 25.78 | 1.03 | 4 | 26.23 | 0.89 | 4 |
| NHERF1−/− -Male | 29.33 | 1.76 | 3 | 30.17 | 1.73 | 3 | 27.67 | 3.71 | 3 | 28.27 | 3.58 | 3 |
| NHERF1−/− -Female | 32 | 1.47 | 4 | 32.5 | 1.48 | 4 | 33.75 | 1.11 | 4 | 35.1 | 1.14 | 4 |
Figure 6: Effect of dietary salt on urinary volume, sodium, chloride, and potassium in WT and NHERF1−/− mice.
WT and NHERF−/− mice were individually housed in metabolic cages for 24 h on day 6 of 8% dietary salt (a day before sacrifice). Urinary (A) volume; (B) sodium; (C) chloride; and (D) potassium was measured. The left panel in each figure shows data pooled from both male and female rats. The right panels show data from WT and NHERF1−/− male and female mice. Each data point represents values from a single animal (red=normal salt diet; blue=8% salt diet) and the horizontal lines are data as mean±SD (n=7; 3 males and 4 females in each group). * indicates p < 0.05, ** indicates p < 0.01, and *** indicates p < 0.001 from the respective normal salt fed mice by two-way ANOVA followed by Bonferroni post-hoc test.
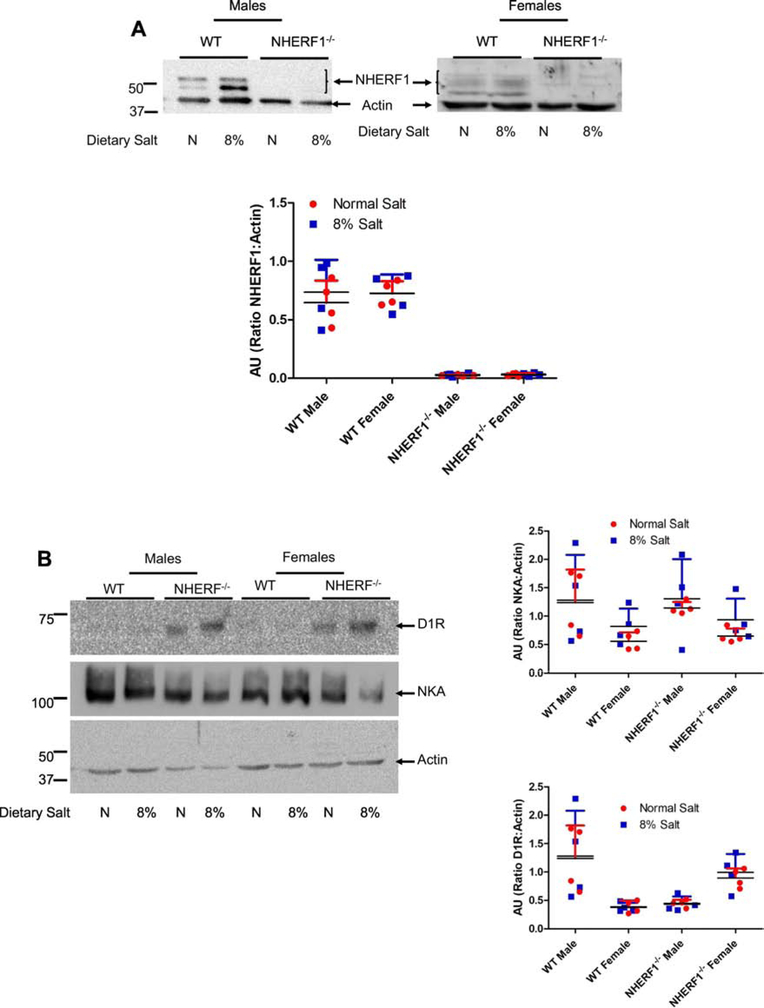
Feeding 8% salt diet increased MAP in male and female WT but not in NHERF1−/− mice (Fig. 7). There were no differences in MAP between male and female WT or NHERF1−/− mice both on normal and 8% salt diet (Fig. 7). We have previously demonstrated that NHERF1 associates with D1R and that the association between NHERF1 and D1R increased upon treatment with dopamine [19]. Increasing salt intake also increases renal dopamine production [29]. To determine if the increase in MAP is due to a change in D1R expression in NHERF1−/− mice, we measured NHERF1, D1R, and NKA α1 subunit expression in whole kidney homogenates from WT and NHERF1−/− mice. Fig. 8A confirms the absence of NHERF1 expression in NHERF1−/− mice. Renal NHERF1 expression in WT mice was not affected by high salt diet, regardless of sex. On normal (0.9%) salt diet, renal D1R expression was not significantly different between WT and NHERF1−/− mice. High salt diet had no effect on renal D1R expression in both WT and NHERF1−/− mice, regardless of sex (Fig. 8B, top panel). However, renal D1R expression was lower in WT females than WT males, regardless of the amount of salt intake. By contrast, renal D1R expression tended to be higher in female NHERF1−/− mice than male NHERF1−/− mice (Fig. 8B). There was no significant difference in NKA α1 subunit expression in any of the groups, regardless of sex or NHERF1 genotype (Fig. 8B, middle panel).
Figure 7: Effect of dietary salt on mean arterial pressure (MAP, mm Hg) in WT and NHERF1−/− mice.
WT and NHERF1−/− mice were fed normal (0.9%) or 8% dietary salt for 7 days. Blood pressure was measured in anesthetized WT and NHERF−/− mice as described in Methods. Each data point represents MAP from a single animal (red=normal salt-diet; blue=8% salt diet) and the horizontal lines are data as mean±SD from 7 animals in each group (3 males and 4 females). * indicates p < 0.05 and *** indicates p < 0.001 from the respective normal salt fed mice by two-way ANOVA followed by Bonferroni post-hoc test.
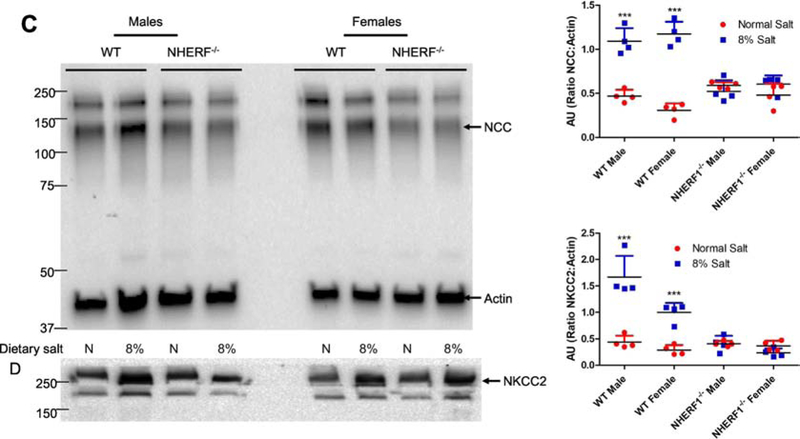
Figure 8: Effect of dietary salt on expression of NHERF1, dopamine 1 receptor (D1R), NKA α1 subunit, NKCC2, and NCC in WT and NHERF1−/− mice.
WT and NHERF−/− mice were fed normal (0.9%) or 8% dietary salt for 7 days. The mice were euthanized on day 7 and the kidneys were removed, decapsulated, and crude membrane homogenates were prepared as described in methods. Expression of (A) NHERF1; (B) D1R (top) and NKA α1 (middle); (C) NCC; and (D) NKCC2 was determined by western blot. NCC blot was stripped and blotted for NKCC2. In Figure B, one gel was used, and the nitrocellulose paper was cut below the 100 KDa marker (for NKA α1), at 60 KDa (for D1R) and the bottom portion below 60 KDa was used for Actin. A representative western blot from one male and one female mice in each group is shown. To determine equal loading the nitrocellulose membrane was simultaneously blotted on the same blot for actin using an HRP-actin antibody. Each data point represents AU (ratio of specific protein to actin) from a single animal (red=normal salt-diet; blue=8% salt diet) and the horizontal lines are data as mean±SD from 8 animals in each group (4 males and 4 females). *** indicates p < 0.001 from respective the respective normal salt fed mice by two-way ANOVA followed by Bonferroni post-hoc test.
To determine if dietary salt changes the expression of sodium transporters, we measured the expression of NCC and NKCC2 in crude membrane preparations from whole kidneys. As shown in Figs. 8C and 8D, 8% dietary salt increased the renal expression of NCC (Fig. 8C) and NKCC2 (Fig. 8D) in WT mice but not in the NHERF1−/− mice, regardless of sex. The increase in renal NKCC2 but not renal NCC expression associated with an increase in salt intake was higher in WT male than WT female mice (Fig. 8D). Renal NCC expression was similar in male and female mice regardless of salt intake or NHERF1 genotype (Figs. 8C and 8D).
Discussion:
The present study, for the first time, demonstrates an association between renal NHERF1 expression and salt-sensitive hypertension in a rat model of aging, the FBN rat. In an earlier study, we demonstrated decreased NHERF1 expression in aged F344 rats, a salt-resistant model, suggesting that its expression may be a requirement for the development of age-related salt-sensitive hypertension. We confirmed this hypothesis in a NHERF1−/− mouse model.
Both F344 and the Fisher 344 X Brown Norway (FBN) rats are well studied models of aging. The F344 rats develop severe inflammatory kidney disease [30] with age while FBN rats are considered a model of healthy aging [31,32]. F344 rats are resistant to the pro-hypertensive effect of increased salt intake while adult FBN rats develop hypertension in response to increased dietary salt [6,26,33,34]. The age-related decrease in NHERF1 in F344 rats may be an adaptive mechanism to blunt the development of age-related, salt-induced hypertension. A decrease in renal NHERF1 expression has been shown, as well, in a model of genetic, non-salt-sensitive model of hypertension, the SHR [24,25]. However, the role of NHERF1 expression in the development of salt-sensitive hypertension is not known and has not been studied in any animal model of hypertension. We speculate that NHERF1 may play a role in the membrane expression and/or trafficking of G-protein-coupled receptors involved in development of salt-sensitive hypertension, including the D1R [35–37] and AT1R [26,33,34]. We have previously demonstrated that NHERF1 associates with D1R and is required for dopamine-mediated inhibition of NKA in opossum kidney cells [19] and in renal proximal tubule cells from Wistar-Kyoto and SHRs [11]. Our data show an increase in total D1R expression in kidney homogenates of female NHERF1−/− mice, relative to WT female mice, both on normal and 8% salt diet. Intrarenal dopamine has been demonstrated to inhibit sodium reabsorption in renal proximal tubules [38]. The increased expression of D1R in the female NHERF1−/− mice and 4-mo old male and female FBN rats may explain the lack of salt-dependent increase in BP in these animals. However, we cannot rule out other mechanisms for the salt resistance in NHERF1−/− mice, especially in male NHERF1−/− mice, including decreased activity of pro-hypertensive systems, such as the renin-angiotensin-aldosterone system or increased activity of anti-hypertensive systems, such as the atrial natriuretic peptide pathway. Recent GWAS demonstrated association of polymorphisms of RGS14 with salt sensitivity [5]. More recently, RGS14 was demonstrated to associate with NHERF1 [39], and inhibition of RGS14-NHERF1 interaction was associated with alterations in PTH receptor signaling. Whether alterations in RGS14-NHERF1-receptor interactions contribute toward salt sensitivity in our models remain to be determined.
The decrease in D1R expression in 24-mo-old FBN rats, in which renal NHERF1 expression increases with age, at least in male FBN rats, was surprising because our previous data in cell culture models suggested that NHERF1 is required for NKA regulation by dopamine and that the association of D1R increases with NHERF1 in dopamine-treated opossum kidney cells [19]. We speculate that the in vitro studies may not have recapitulated the in vivo effects of salt intake and systems that interact with D1R, e.g., renin-angiotensin-aldosterone system. In fact, recent reports suggest that there is a yin-yang interaction between the expression of AT1R and D1R and regulation of blood pressure [40]. Chugh et al [33,34] has demonstrated an increase in AT1R expression at the mRNA level and a decrease in D1R expression in 22-mo old FBN rats as compared with 4-mo old FBN rats. NHERF1 has reported to be important in the angiotensin II-induced activation of NHE3 in opossum kidney proximal tubule cells in culture [41]. We speculate that the yin-yang between D1R and AT1R may be regulated by NHERF1.
The roles of sodium and potassium transporters in the kidney have been extensively studied in several models of hypertension [42–44]. Tian et al [45] reported an increase in the renal expression of NKCC2 and NCC in response to water restriction in old FBN rats. Our data show that there were no differences in basal expression of renal NKCC2 between young and old FBN rats but basal renal NCC expression was higher in old (24-mo) than young (4-mo) FBN rats. High salt diet-dependent increase in the renal expressions of NKCC2 and NCC in aging FBN rats and NHERF1 WT mice confirms the increase in renal NKCC2 and NCC expressions in C57Bl/6 mice [46] and Wistar [28] but not Sprague-Dawley rats [47]. By contrast, in NHERF1−/− mice, high salt diet did not affect the renal expressions of NKCC2 and NCC. Phosphorylation of NKCC2 and NCC leads to their activation and increase in blood pressure [48–51]. It remains to be determined if NHERF1 has any direct effects on the expression and/or phosphorylation of these proteins or that NHERF1 facilitates the effects of hormones that regulate renal sodium transport.
Sexual dimorphism in blood pressure has been observed in rodents and humans. Blood pressure is higher in men than age-matched premenopausal women.[52]. However, women may be more salt-sensitive than men [53–55], although mortality and complications of hypertension remain higher in men than women. In the present study we did not observe any significant differences in blood pressure between young (4-mo) and old (24-mo) male and female FBN rats, although renal NHERF1 was higher in old male than old female FBN rats. There were also no sex differences in blood pressure in NHERF1 WT and NHERF1−/− mice. Regarding sexual dimorphism in renal sodium transporter, Veiras et al [56] reported that female Sprague-Dawley rats excreted a saline load more rapidly than male rats, related to a greater decrease in renal proximal sodium reabsorption. This was associated with a redistribution of NHE3 to the base of the microvilli of renal proximal tubules and decreased expression of NpT2a. Under basal conditions, these authors also reported increased renal cortical NHERF1 and NCC expression in female, relative to male, Sprague Dawley rats. NCC expression was also higher in female than male C57Bl/6J mice; NHERF1 expression was not different. In contrast to that report, our study shows lower renal NHERF1 expression in female FBN than male FBN rats, regardless of age. However, we did not find any difference in renal NHERF1 expression between male and female NHERF1 wild-type mice on C57Bl/6 background. The mechanisms for the differences in NHERF1 expression between male and female rats are unknown. Some studies suggest that estrogen may increase the expression of NHERF1 in lungs [57] and breast epithelia [58] but not in placenta [59]. The reasons of these discordant results are unknown. However, these differences may be due to differences in rat models and the age of the animals used in the studies. Here, we used young (4-mo) and old (24-mo) FBN rats and old (18-mo) C57Bl/6J mice while in other studies the adult SHR, Dahl, or Sprague-Dawley rats were used. Further studies are required to determine the differences in different species.
Perspective:
The sodium-hydrogen exchanger regulatory factor-1 (NHERF1) regulates trafficking of GPCRs and transporters to the plasma membrane. In this study, we show that the absence of NHERF1 results in resistance to the hypertensive effects of a high salt diet in aging animals. The present study showing differences in renal expression of D1R, NKCC2, and NCC may explain disparities in humans to develop hypertension in response to dietary salt. We suggest that NHERF1 PDZ domains could represent novel therapeutic targets for salt-sensitive hypertension.
Conclusion:
In summary, we report here for the first time a novel role for NHERF1 in the expression of salt-sensitive hypertensive phenotype. Our data demonstrate that lack of NHERF1 may confer salt resistance. By contrast, an increase in NHERF1 expression may contribute to the development of salt sensitivity in 24-mo old FBN rats that exhibit salt resistance at an early age. Further studies are required to confirm the role of NHERF1 in trafficking, phosphorylation, and/or expression of the sodium transporters along the nephron.
Supplementary Material
Acknowledgments
“The opinions expressed in this manuscript do not reflect the opinions of the Department of Veteran Affairs or NIH”.
We acknowledge the technical assistance of Caryl Conklin.
We thank Dr. Mark A. Knepper, NHLBI, NIH, Bethesda, MD for providing the antibodies and providing facilities in his laboratory to perform western blots.
Sources of Funding: The work was supported by NIH-5R21AG047474 from NIA and 16GRNT31030019 from AHA to SJK, R01DK039308 and P01HL074940 from NIH to PAJ, Veteran Affairs Merit Review grant (NEPH-016-09S) to EDL and R25AG047843-NIH to Howard University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References:
- [1].Kanbay M, Chen Y, Solak Y, Sanders PW, Mechanisms and consequences of salt sensitivity and dietary salt intake., Curr. Opin. Nephrol. Hypertens 20 (2011) 37–43. doi: 10.1097/MNH.0b013e32834122f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rust P, Ekmekcioglu C, Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension, in: 2016: pp. 61–84. doi: 10.1007/5584_2016_147. [DOI] [PubMed] [Google Scholar]
- [3].Luft FC, Weinberger MH, Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm, Am. J. Clin. Nutr 65 (1997) 612S–617S. doi: 10.1093/ajcn/65.2.612S. [DOI] [PubMed] [Google Scholar]
- [4].Zemel MB, Sowers JR, Salt sensitivity and systemic hypertension in the elderly., Am. J. Cardiol 61 (1988) 7H–12H. http://www.ncbi.nlm.nih.gov/pubmed/3289354 (accessed April 25, 2019). [DOI] [PubMed] [Google Scholar]
- [5].Mahajan A, Rodan AR, Le TH, Gaulton KJ, Haessler J, Stilp AM, et al. , Trans-ethnic Fine Mapping Highlights Kidney-Function Genes Linked to Salt Sensitivity, Am. J. Hum. Genet 99 (2016) 636–646. doi: 10.1016/j.ajhg.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sanders PW, Salt-sensitive hypertension: lessons from animal models., Am. J. Kidney Dis 28 (1996) 775–782. doi:S0272–6386(96)90265–6 [pii]. [DOI] [PubMed] [Google Scholar]
- [7].Marketou ME, Maragkoudakis S, Anastasiou I, Nakou H, Plataki M, Vardas PE, et al. , Salt-induced effects on microvascular function: A critical factor in hypertension mediated organ damage., J. Clin. Hypertens. (Greenwich) (2019) jch.13535. doi: 10.1111/jch.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kurtz TW, DiCarlo SE, Pravenec M, Morris RC, The pivotal role of renal vasodysfunction in salt sensitivity and the initiation of salt-induced hypertension., Curr. Opin. Nephrol. Hypertens 27 (2018) 83–92. doi: 10.1097/MNH.0000000000000394. [DOI] [PubMed] [Google Scholar]
- [9].Ellison DH, Treatment of Disorders of Sodium Balance in Chronic Kidney Disease., Adv. Chronic Kidney Dis 24 (2017) 332–341. doi: 10.1053/j.ackd.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ali A, Abu Zar M, Kamal A, Faquih AE, Bhan C, Iftikhar W, et al. , American Heart Association High Blood Pressure Protocol 2017: A Literature Review., Cureus. 10 (2018) e3230. doi: 10.7759/cureus.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barati MT, Ketchem CJ, Merchant ML, Kusiak WB, Jose PA, Weinman EJ, et al. , Loss of NHERF-1 expression prevents dopamine-mediated Na-K-ATPase regulation in renal proximal tubule cells from rat models of hypertension: Aged F344 rats and spontaneously hypertensive rats, Am. J. Physiol. - Cell Physiol 313 (2017). doi: 10.1152/ajpcell.00219.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT, High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats., Am. J. Hypertens 5 (1992) 585–91. doi: 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- [13].Shenolikar S, Weinman EJ, NHERF: targeting and trafficking membrane proteins., Am J Physiol Ren. Physiol 280 (2001) F389–95. http://www.ncbi.nlm.nih.gov/pubmed/11181400 (accessed March 18, 2016). [DOI] [PubMed] [Google Scholar]
- [14].Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, et al. , Parathyroid hormone regulation of Na+,K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells, J. Am. Soc. Nephrol 16 (2005). doi: 10.1681/ASN.2004121049. [DOI] [PubMed] [Google Scholar]
- [15].Rendina D, De Filippo G, Strazzullo P, Bergwitz C, Bastepe M, Karim Z, et al. , NHERF1 mutations and responsiveness of renal parathyroid hormone, N Engl J Med 359 (2008) 1128–1135. doi: 10.1056/NEJMc086284. [DOI] [PubMed] [Google Scholar]
- [16].Donowitz M, Singh S, Singh P, Chakraborty M, Chen Y, Murtazina R, et al. , Alterations in the proteome of the NHERF2 knockout mouse jejunal brush border membrane vesicles., Physiol. Genomics 43 (2011) 674–84. doi: 10.1152/physiolgenomics.00258.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ardura JA, Friedman PA, Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors., Pharmacol. Rev 63 (2011) 882–900. doi: 10.1124/pr.110.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Romero G, von Zastrow M, Friedman PA, Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity., Adv. Pharmacol 62 (2011) 279–314. doi: 10.1016/B978-0-12-385952-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Salyer S, Lesousky N, Weinman EJ, Clark BJ, Lederer ED, Khundmiri SJ, Dopamine regulation of Na+-K+-ATPase requires the PDZ-2 domain of sodium hydrogen regulatory factor-1 (NHERF-1) in opossum kidney cells, Am. J. Physiol. - Cell Physiol 300 (2011). doi: 10.1152/ajpcell.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ketchem CJ, Khundmiri SJ, Gaweda AE, Murray R, Clark BJ, Weinman EJ, et al. , Role of the sodium hydrogen exchanger regulatory factor 1 (NHERF1) in forward trafficking of the type IIa sodium phosphate cotransporter (NpT2a)., Am. J. Physiol. Renal Physiol 309 (2015) ajprenal.00133.2015. doi: 10.1152/ajprenal.00133.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Weinman EJ, Lederer ED, NHERF-1 and the regulation of renal phosphate reabsoption: a tale of three hormones., Am J Physiol Ren. Physiol 303 (2012) F321–7. doi: 10.1152/ajprenal.00093.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murtazina R, Kovbasnjuk O, Donowitz M, Li X, Na+/H+ exchanger NHE3 activity and trafficking are lipid Raft-dependent., J. Biol. Chem 281 (2006) 17845–55. doi: 10.1074/jbc.M601740200. [DOI] [PubMed] [Google Scholar]
- [23].Lee HJ, Kwon MH, Lee S, Hall RA, Yun CC, Choi I, Systematic family-wide analysis of sodium bicarbonate cotransporter NBCn1/SLC4A7 interactions with PDZ scaffold proteins., Physiol. Rep 2 (2014) e12016. doi: 10.14814/phy2.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kobayashi K, Monkawa T, Hayashi M, Saruta T, Expression of the Na+/H+ exchanger regulatory protein family in genetically hypertensive rats, J. Hypertens 22 (2004) 1723–30. http://www.ncbi.nlm.nih.gov/pubmed/15311100 (accessed July 12, 2018). [DOI] [PubMed] [Google Scholar]
- [25].Griffin KA, Churchill PC, Picken M, Webb RC, Kurtz TW, Bidani AK, Differential salt-sensitivity in the pathogenesis of renal damage in SHR and stroke prone SHR., Am. J. Hypertens 14 (2001) 311–20. doi: 10.1016/s0895-7061(00)01282-6. [DOI] [PubMed] [Google Scholar]
- [26].Pokkunuri I, Chugh G, Rizvi I, Asghar M, Age-related hypertension and salt sensitivity are associated with unique cortico-medullary distribution of D1R, AT1R, and NADPH-oxidase in FBN rats., Clin. Exp. Hypertens 37 (2015) 1–7. doi: 10.3109/10641963.2014.977489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holthouser KA, Mandal A, Merchant ML, Schelling JR, Delamere NA, Valdes RR Jr., et al. , Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE-1)-dependent mechanism in human kidney proximal tubule cells, Am J Physiol Ren. Physiol 299 (2010) F77–90. doi: 10.1152/ajprenal.00581.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li J, Wang DH, Function and regulation of epithelial sodium transporters in the kidney of a salt-sensitive hypertensive rat model., J. Hypertens 25 (2007) 1065–72. doi: 10.1097/HJH.0b013e3280a8b87d. [DOI] [PubMed] [Google Scholar]
- [29].Baines AD, Effects of salt intake and renal denervation on catecholamine catabolism and excretion., Kidney Int. 21 (1982) 316–22. http://www.ncbi.nlm.nih.gov/pubmed/7069996 (accessed June 12, 2019). [DOI] [PubMed] [Google Scholar]
- [30].Kwekel JC, Vijay V, Desai VG, Moland CL, Fuscoe JC, Age and sex differences in kidney microRNA expression during the life span of F344 rats, Biol. Sex Differ 6 (2015) 1. doi: 10.1186/s13293-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sprott RL, Ramirez I, Current Inbred and Hybrid Rat and Mouse Models for Gereontological Research., ILAR J 38 (1997) 104–109. http://www.ncbi.nlm.nih.gov/pubmed/11528051 (accessed May 21, 2016). [DOI] [PubMed] [Google Scholar]
- [32].Sprott RL, Development of animal models of aging at the National Institute of Aging., Neurobiol. Aging 12 635–8. http://www.ncbi.nlm.nih.gov/pubmed/1791897 (accessed May 21, 2016). [DOI] [PubMed] [Google Scholar]
- [33].Chugh G, Pokkunuri I, Asghar M, Renal dopamine and angiotensin II receptor signaling in age-related hypertension., Am. J. Physiol. Renal Physiol 304 (2013) F1–7. doi: 10.1152/ajprenal.00441.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chugh G, Lokhandwala MF, Asghar M, Altered functioning of both renal dopamine D1 and angiotensin II type 1 receptors causes hypertension in old rats., Hypertension. 59 (2012) 1029–36. doi: 10.1161/HYPERTENSIONAHA.112.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Felder RA, Eisner GM, Jose PA, D1 dopamine receptor signalling defect in spontaneous hypertension, Acta Physiol Scand. 168 (2000) 245–250. doi: 10.1046/j.1365-201x.2000.00634.x. [DOI] [PubMed] [Google Scholar]
- [36].Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, et al. , Single-nucleotide polymorphisms of the dopamine d2 receptor increase inflammation and fibrosis in human renal proximal tubule cells, Hypertension. 63 (2014). doi: 10.1161/HYPERTENSIONAHA.113.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pinto V, Amaral J, Silva E, Simão S, Cabral JM, Afonso J, et al. , Age-related changes in the renal dopaminergic system and expression of renal amino acid transporters in WKY and SHR rats., Mech. Ageing Dev 132 298–304. doi: 10.1016/j.mad.2011.06.003. [DOI] [PubMed] [Google Scholar]
- [38].Armando I, Konkalmatt P, Felder RA, Jose PA, The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease., Transl. Res 165 (2014) 505–11. doi: 10.1016/j.trsl.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Friedman PA, Mamonova T, Magyar CE, Squires KE, Sneddon WB, Emlet DR, et al. , Genetic variants disrupt human RGS14 binding to NHERF1 and regulation of NPT2A-mediated phosphate transport, BioRxiv. (2019) 540781. doi: 10.1101/540781. [DOI] [Google Scholar]
- [40].Wang Z, Zeng C, Villar VAM, Chen S-Y, Konkalmatt P, Wang X, et al. , Human GRK4γ 142V Variant Promotes Angiotensin II Type I Receptor–Mediated Hypertension via Renal Histone Deacetylase Type 1 Inhibition, Hypertension. 67 (2016) 325–334. doi: 10.1161/HYPERTENSIONAHA.115.05962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].He P, Zhao L, No YR, Karvar S, Yun CC, The NHERF1 PDZ1 domain and IRBIT interact and mediate the activation of Na+/H+ exchanger 3 by ANG II., Am. J. Physiol. Renal Physiol 311 (2016) F343–51. doi: 10.1152/ajprenal.00247.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mutig K, Trafficking and regulation of the NKCC2 cotransporter in the thick ascending limb., Curr. Opin. Nephrol. Hypertens 26 (2017) 392–397. doi: 10.1097/MNH.0000000000000351. [DOI] [PubMed] [Google Scholar]
- [43].Budzikowski AS, Huang BS, Leenen FH, Brain “ouabain”, a neurosteroid, mediates sympathetic hyperactivity in salt-sensitive hypertension., Clin. Exp. Hypertens 20 (1998) 119–40. http://www.ncbi.nlm.nih.gov/pubmed/9533610 (accessed February 22, 2016). [DOI] [PubMed] [Google Scholar]
- [44].Welling PA, Rare mutations in renal sodium and potassium transporter genes exhibit impaired transport function., Curr. Opin. Nephrol. Hypertens 23 (2014) 1–8. doi: 10.1097/01.mnh.0000437204.84826.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tian Y, Riazi S, Khan O, Klein JD, Sugimura Y, Verbalis JG, et al. , Renal ENaC subunit, Na–K–2Cl and Na–Cl cotransporter abundances in aged, water-restricted F344 × Brown Norway rats, Kidney Int. 69 (2006) 304–312. doi: 10.1038/SJ.KI.5000076. [DOI] [PubMed] [Google Scholar]
- [46].Udwan K, Abed A, Roth I, Dizin E, Maillard M, Bettoni C, et al. , Dietary sodium induces a redistribution of the tubular metabolic workload., J. Physiol 595 (2017) 6905–6922. doi: 10.1113/JP274927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Frindt G, Palmer LG, Surface expression of sodium channels and transporters in rat kidney: effects of dietary sodium., Am. J. Physiol. Renal Physiol 297 (2009) F1249–55. doi: 10.1152/ajprenal.00401.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ares GR, Caceres PS, Ortiz PA, Molecular regulation of NKCC2 in the thick ascending limb., Am. J. Physiol. Renal Physiol 301 (2011) F1143–59. doi: 10.1152/ajprenal.00396.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT, WNK Kinase Signaling in Ion Homeostasis and Human Disease., Cell Metab. 25 (2017) 285–299. doi: 10.1016/j.cmet.2017.01.007. [DOI] [PubMed] [Google Scholar]
- [50].Hadchouel J, Ellison DH, Gamba G, Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases., Annu. Rev. Physiol 78 (2016) 367–89. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- [51].Ko B, Hoover RS, Molecular physiology of the thiazide-sensitive sodium-chloride cotransporter., Curr. Opin. Nephrol. Hypertens 18 (2009) 421–7. doi: 10.1097/MNH.0b013e32832f2fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lopez-Ruiz A, Sartori-Valinotti J, Yanes LL, Iliescu R, Reckelhoff JF, Sex differences in control of blood pressure: Role of oxidative stress in hypertension in females, Am. J. Physiol. - Hear. Circ. Physiol 295 (2008) H466–74. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, et al. , National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: Building on the Current Scientific Evidence., Hypertens. (Dallas, Tex. 1979) 68 (2016) 281–8. doi: 10.1161/HYPERTENSIONAHA.116.07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bursztyn M, Ben-Dov IZ, Sex differences in salt-sensitivity risk approximated from ambulatory blood pressure monitoring and mortality., J. Hypertens 31 (2013) 900–5. doi: 10.1097/HJH.0b013e32835f29f4. [DOI] [PubMed] [Google Scholar]
- [55].He J, Gu D, Chen J, Jaquish CE, Rao DC, Hixson JE, et al. , Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study, J. Hypertens 27 (2009) 48–54. doi: 10.1097/HJH.0b013e328316bb87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, et al. , Sexual dimorphic pattern of renal transporters and electrolyte homeostasis, J. Am. Soc. Nephrol 28 (2017) 3504–3517. doi: 10.1681/ASN.2017030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fanelli T, Cardone RA, Favia M, Guerra L, Zaccolo M, Monterisi S, et al. , β-Oestradiol rescues ΔF508CFTR functional expression in human cystic fibrosis airway CFBE41o− cells through the up-regulation of NHERF1, Biol. Cell 100 (2008) 399–412. doi: 10.1042/bc20070095. [DOI] [PubMed] [Google Scholar]
- [58].Meng R, Qin Q, Xiong Y, Wang Y, Zheng J, Zhao Y, et al. , NHERF1, a novel GPER associated protein, increases stability and activation of GPER in ER-positive breast cancer, Oncotarget. 7 (2016) 54983–54997. doi: 10.18632/oncotarget.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pepe GJ, Burch MG, Albrecht ED, Regulation of Expression and Localisation of the Na+/H+ Exchanger (NHE) 3 and the NHE Regulatory Factor 2 in Baboon Placental Syncytiotrophoblast by Oestrogen, Placenta. 28 (2007) 878–888. doi: 10.1016/j.placenta.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.