Abstract
Purpose:
To develop an optical coherence tomography angiography (OCTA)-based framework for quantitatively analyzing the spatial distribution of choriocapillaris (CC) impairment around choroidal neovascularization (CNV) secondary to age-related macular degeneration (AMD).
Methods:
In a retrospective, cross-sectional study, 400 kHz swept-source OCTA images from 7 eyes of 6 patients with CNV secondary to AMD were quantitatively analyzed using custom software. A lesion-centered zonal OCTA analysis technique—which portioned the field-of-view into zones relative to CNV boundaries—was developed to quantify the spatial dependence of CC flow deficits.
Results:
Quantitative, lesion-centered zonal analysis of CC OCTA images revealed highest flow deficit percentages near CNV boundaries, decreasing in zones farther from the boundaries. OCTA using shorter (1.5 ms) interscan times revealed more severe flow deficits than OCTA using longer (3.0 ms) interscan times; however, spatial trends were similar for both interscan times. A detailed description of the OCTA processing steps and parameters was provided so as to elucidate their influence on quantitative measurements.
Conclusion:
Impairment of the CC, assessed by flow deficit percentages, was most prominent closest to CNV boundaries. The lesion-centered zonal analysis technique enabled quantitative CC measurements relative to focal lesions. Understanding how processing steps, imaging/processing parameters, and artifacts can affect quantitative CC measurements is important for longitudinal, OCTA-based studies of disease progression and treatment response.
Keywords: choriocapillaris, CNV, OCTA, AMD, blood flow, choroid
Summary Statement:
Using quantitative, spatially-resolved analysis, this study found choriocapillaris flow impairment surrounding choroidal neovascularization secondary to age-related macular degeneration. An extensive discussion of image processing steps and parameters is also presented with the aim of reducing misinterpretations and mitigating artifacts.
Introduction
Neovascular age-related macular degeneration (nAMD), a sub-type of age-related macular degeneration (AMD), is characterized by the presence of choroidal neovascularization (CNV), which are new blood vessels, typically arising from the choriocapillaris (CC), that penetrate through Bruch’s membrane. While the pathogenesis of CNV remains incompletely understood, studies using histology, electron microscopy, and indocyanine green angiography (ICGA) all confirm CC impairment surrounding the CNV boundary.1–6 More recently, studies using optical coherence tomography angiography (OCTA),7,8 a functional extension of optical coherence tomography (OCT) that enables depth resolved visualization of blood flow, have qualitatively corroborated these observations.9–13 Noting the important role of the CC in nAMD pathogenesis, the primary aim of this study is to use swept source OCTA (SS-OCTA) to quantitatively analyze the spatial distribution of CC impairment in eyes with CNV secondary to AMD.
This study also presents a lesion-centered zonal analysis methodology, which we developed in order to quantitatively analyze the distribution of CC impairment. In addition, this study presents a detailed description of OCTA processing steps, imaging/processing parameters, and artifacts—including thresholding, binarization, segmentation, motion correction, image averaging, interscan time, optical resolution, A-scan density, and projections artifacts—and discusses their potential effects on quantitative CC OCTA measurements.
Methods
This was a retrospective study conducted at the New England Eye Center (NEEC) of Tufts Medical Center. The study was approved by the Institutional Review Boards at Tufts Medical Center and the Massachusetts Institute of Technology. This research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996. Written informed consent was obtained prior to imaging. Participants underwent a complete ophthalmic examination by a trained retina specialist at the NEEC. Selected patients with a new diagnosis, or known history, of CNV secondary to AMD were imaged on a prototype SS-OCTA instrument. The analysis steps, summarized schematically in Figure 1, are described in the subsequent sections.
Figure 1.
Analysis workflow.
SS-OCTA Acquisition
OCTA data were acquired using a prototype 400 kHz SS-OCT system operating at a ~1050 nm wavelength. The full-width-at-half-maximum (FWHM) axial and transverse optical resolutions, in tissue, were ~9 μm and 20 μm, respectively. For each eye, 3 mm × 3 mm and 6 mm × 6 mm fields-of-view were acquired and the field size having the best quality, and encompassing the entire lesion, was selected for analysis. For both fields-of-view, the acquisition protocol was 500 A-scans per B-scan, 500 unique B-scan positions per volume, and 5 repeated B-scans per B-scan position. The fundamental interscan time—the time between repeated B-scans—was 1.5 ms.
OCTA Computation
OCTA images from 1.5 ms and 3.0 ms interscan time data were generated using an amplitude-based,7,8,14,15 variable interscan time analysis (VISTA)16 approach. In order to avoid artifacts in regions of low OCT signal, OCTA volumes were thresholded, on the basis of the signal level in their corresponding OCT volumes, at 3 standard deviations above the mean background noise. It is worth noting that within the OCTA literature there are at least two senses in which the term thresholding is used: first, as the step during the computation of the OCTA signal itself in which regions of low OCT signal are set to 0 (black) in the OCTA image (to avoid false positive OCTA signals); and, second, as the process of binarizing an OCTA image to differentiate regions of flow (i.e., vessels) from regions of non-flow (e.g., flow voids or intercapillary areas). To avoid confusion, in this paper we use the term thresholding to describe former, and binarization to describe the latter. Further details of OCTA computation are provided in Appendix I.
Motion Correction
To compensate for motion artifacts, orthogonal, raster scanned (“x-fast” and “y-fast”) volumes were software motion corrected and merged using a previously published algorithm;17,18 this algorithm uses OCT features to compensate motion in all three dimensions on an A-scan basis. Following software motion correction, the OCTA volumes were merged to improve OCT signal and decrease noise. This process is analogous to registering and averaging en face OCTA data, as previously reported;19–21 however, in our study registration and averaging are performed on volumetric (3-D) data, rather than en face (2-D) data.
Segmentation and Exclusion of the CNV Lesion
The CNV boundaries were manually segmented using an orthoplane image viewer. Briefly, at each axial depth, the lesion boundary at that depth was traced in the en face plane; the boundary of the entire lesion was computed as the maximum extent of the projected boundaries from each depth. Because projection artifacts limit the ability to visualize the CC in regions underlying the CNV, all pixels within the CNV boundary were excluded from analysis, as discussed in the CC Flow Deficit Metric section.
CC Slab Formation
A custom, automatic, OCT-OCTA graph-cut segmentation algorithm,22 similar to that proposed by Chiu et al.,23 was used to generate an approximate segmentation of Bruch’s membrane. In short, the algorithm uses the gradient (i.e., the spatial variation in pixel values) of the OCT and OCTA B-scans to locate Bruch’s membrane. The automatic segmentation was then manually corrected on an individual B-scan basis. When Bruch’s membrane was not directly visible, it was identified as the posterior margin of the RPE. For each interscan time, an en face OCTA CC slab was formed by median projection of the OCTA volume through the ~25 μm (6 pixels) immediately below Bruch’s membrane. Median projection was used instead of mean projection to improve robustness to small segmentation errors.
Artifact Identification
OCTA images suffer from a variety of artifacts that can confound analysis.24,25 In our en face OCTA CC slabs, projection artifacts from larger overlying vessels were prominent, as were motion artifacts from saccades (Figure 2). Rather than attempting to compensate for these artifacts, which might itself generate errors, we opted to exclude these regions from analysis (see Discussion). A prerequisite to artifact exclusion is accurate artifact identification, which is discussed below. Artifact exclusion, per se, is discussed in the CC Flow Deficit Metric section.
Figure 2.
OCTA artifact detection and exclusion. (A) OCTA CC slab (6 mm × 6 mm; Eye 4) showing data gaps caused by saccadic motion (white arrow-heads), and projection artifacts (orange arrow-heads). (B) Motion artifact mask generated during the volume registration step. (C) Projection artifact mask. (D) OCTA CC slab of panel A with motion and projection artifacts excluded (teal-colored regions). False-positive and false-negative projection artifact detections (orange and white arrows, respectively) can arise due to imperfections in the automatic identification. (E-H) Processing steps used to identify projection artifacts. (E) Projection of the OCTA volume through the RPE highlights larger retinal vessels. The white arrow points to a projected vessel that is not detected; see corresponding white arrow in panel D. (F) The region within the CNV boundary is removed using the boundary of the manually segmented lesion. (G) Median filtering of panel F with a 60 μm square kernel. (H) Binarization via Otsu’s method; this image is then filtered via morphological opening with a 24 μm diameter circular kernel to remove smaller, disconnected areas generated during binarization, forming the image of panel C.
Projection Artifacts
Projection artifacts24 cause overlying retinal vessels, particularly larger vessels, to appear in the underlying OCTA CC slab, obfuscating CC patterning, and causing artifactually high OCTA signals (Figure 2A and 2C). We automatically identified projection artifacts by generating a projection-vessel image computed by projecting the OCTA volume through the axial positions occupied by the RPE (Figure 2E–2H). The RPE is useful for identifying projected vessels because projection artifacts generate high OCTA signals within the RPE due to its high backscattering. Moreover, under normal conditions, the RPE is avascular; thus, the entirety of the OCTA signal observed in the RPE slab corresponds to projected OCTA signals. In the presence of pathology, including CNV (as in the present study), the assumption that the RPE is avascular breaks down in the region of pathology. However, since we excluded the region of CNV from analysis (Figure 2F), the assumption of avascularity was valid in the analyzed regions. Nevertheless, this is a limitation of the technique that should be considered when applying it for other pathologies.
Motion Artifacts
Saccadic eye motion causes strip-like regions of the fundus to be either un-imaged or repeatedly imaged. If strips of the fundus are un-imaged in both the x-fast and y-fast raster scanned volumes, there will be missing data at the intersection of the two un-imaged strips, resulting in a small rectangular shaped “data gap” (Figure 2A and 2B). These data gaps can be automatically identified in the volume registration and merging processes and excluded from the analysis (Figure 2B).
CC Flow Deficit Metric
In this study we used flow deficits26—also known as flow voids—to measure CC impairment. Throughout, we will use the term flow deficit, rather than flow void, to emphasize that low OCTA signals do not necessarily correspond to a complete absence of blood flow—areas of low OCTA signals can be also be generated by slow/impaired blood flow. CC flow deficits were defined as regions of the OCTA CC having values below an empirically chosen binarization level (see Appendix II).
Spatial Analysis of CC Flow Deficits
Qualitative, Local Analysis
To qualitatively visualize the spatial distribution of flow deficits, we generated a smoothed flow deficit percentage image by spatial averaging with a modified Gaussian kernel (250 μm standard deviation; see Appendix II for details) and displayed the result using a false color scale, with redder colors indicating higher flow deficit percentages, and bluer colors indicating lower flow deficit percentages. This approach is similar to the false color capillary density images used to analyze the spatial distribution of retinal vascular impairment;27 however, here we are analyzing the flow deficit percentage (a measure of blood flow impairment) rather than capillary density (a measure of blood flow). In the smoothed flow deficit percentage image, a given pixel value indicates the flow deficit percentage within that pixel’s local (250 μm) neighborhood.
Quantitative, Lesion-Centered Zonal Analysis
We performed lesion-centered zonal analysis to quantitatively study the spatial distribution of CC flow deficits as a function of distance from the CNV boundary. In particular, we used the CNV boundary—whose delineation was described previously—to partition the field-of-view into 250 μm wide zones positioned at increasing distances from boundary (Figure 3). Specifically, the first zone contains all points less than 250 μm from the CNV boundary; the second zone contains all points between 250 μm and 500 μm from the CNV boundary; and so forth. The smoothed flow deficit percentage image (described in the preceding section) was then averaged within each of these zones, generating an average flow deficit percentage for that zone. This procedure enables quantitative assessment of CC impairment as a function of distance from the CNV lesion. Appendix III provides further details of the spatial analysis.
Figure 3.
Illustration of the lesion-centered zonal approach to spatial analysis. (A) 6 mm × 6 mm en face OCTA formed via mean projection of the OCTA volume through the CNV depths (Eye 1). The white contour indicates the CNV boundary; the OCTA signal outside the boundary has been set to black for clarity. (B) Lesion-centered contours (red) used in this study; each zone is 250 μm in width.
Results
We analyzed 7 eyes (6 patients) with CNV secondary to AMD. The OCTA volumes of CNV eyes were used in a previous study by our group.28 In that study, the selection of 3 mm × 3 mm versus 6 mm × 6 mm field-of-view was decided on the basis of which field size provided the best scan quality while encompassing the entire lesion. Consequentially, we also use data from 3 mm × 3 mm and 6 mm × 6 mm fields-of-view for this study (see Table 1).
Table 1.
Subject Descriptions
| Eye | Age [Years] | No. of Anti-VEGF Injections Before Imaging | Visual Acuity | Field-of-View |
|---|---|---|---|---|
| Treatment Naïve | ||||
| 1 | 65 | 0 | 20/70 | 6 mm × 6 mm |
| 2 | 66 | 0 | 20/40 | 3 mm × 3 mm |
| Chronic | ||||
| 3 | 56 | 2 | 20/100 | 6 mm × 6 mm |
| 4* | 83 | 6 | 20/30 | 6 mm × 6 mm |
| 5 | 63 | 15 | 20/20 | 3 mm × 3 mm |
| 6 | 75 | 18 | 20/30 | 6 mm × 6 mm |
| 7** | 83 | 25 | 20/30 | 6 mm × 6 mm |
| Mean (SD) | 70.1 (10.4) | 13.2 (9.3)† | 20/46 |
SD = standard deviation.
are the right and left eye, respectively, from the same patient.
Only from chronic eyes.
Patients
Table 1 presents demographics of the enrolled subjects, sorted by the number of prior anti-VEGF injections. Detailed anti-VEGF treatment histories are provided in Table 2 in Appendix IV.
Table 2.
Anti-VEGF Treatment Histories Prior to Imaging

|
Spatial Analysis of CC Flow Deficits
Spatial analyses of Eye 1 and Eye 7 are shown in Figures 5 and 6, respectively. Analyses of Eye 2 through Eye 6 are provided online (see Figures, Supplemental Digital Content 1 through Supplemental Digital Content 5). Briefly, in all cases we found CC impairment extending beyond the CNV boundary. Moreover, we found that this impairment was most pronounced in regions closest to the lesion, decreasing away from the lesion. Pooled analysis of the 5 eyes imaged with a 6 mm × 6 mm FOV is shown in Figure 4.
Figure 5.
Analysis of Eye 1. (A) Cropped fluorescein angiogram. (B) 6 mm × 6 mm en face OCTA formed via mean projection of the OCTA volume through the CNV; the white contour indicates the CNV boundary, and the OCTA image outside the boundary has been set to black for clarity. (C) OCT B-scan and (D) OCTA B-scan extracted from the position indicated by the dashed arrow of panel B. The depth range used to project the CC slab is indicated by the red lines (25 μm thick slab). (E) 1.5 ms CC OCTA slab. (F) CC OCTA slab with projection artifacts, motion artifacts, and regions within the lesion excluded (teal). (G) Raw CC flow deficit image, where white indicates flow deficit pixels. (H) CC flow deficit percentage map, where the colors correspond to the local percentage of flow deficits, as indicated by the color bar. (I) Average of the flow deficit percentage image of panel H over 250 μm zones (white contours). (J) Bar plot of panel I, where the x-axis indicates distance of the zone from the CNV boundary (in mm), and the y-axis indicates the average CC flow deficit percentage. Dark gray bars correspond to flow deficit analysis using 1.5 ms interscan time OCTA images; light gray to analysis using 3.0 ms interscan time OCTA images.
Figure 6.
Analysis of Eye 7. (A) Cropped fluorescein angiogram. (B) 6 mm × 6 mm en face OCTA formed via mean projection of the OCTA volume through the CNV; the white contour indicates the CNV boundary, and the OCTA image outside the boundary has been set to black for clarity. (C) OCT B-scan and (D) OCTA B-scan from the position indicated by the dashed arrow of panel B. The depth range used to project the CC slab is indicated by the red lines (25 μm thick slab). (E) 1.5 ms CC OCTA slab. (F) CC OCTA slab with projection artifacts, motion artifacts, and regions within the lesion excluded (teal). (G) Raw CC flow deficit image, where white indicates flow deficit pixels. (H) CC flow deficit percentage map, where the colors correspond to the local percentage of flow deficits, as indicated by the color bar. (I) Average of the flow deficit percentage image of panel H over 250 μm wide zones (white contours). (J) Bar plot of panel I, where the x-axis indicates distance of the zone from the CNV boundary (in mm), and the y-axis indicates the average CC flow deficit percentage. Dark gray bars correspond to flow deficit analysis using 1.5 ms interscan time OCTA images; light gray to analysis using 3.0 ms interscan time OCTA images.
Figure 4.
Pooled bar plots of the 5 CNV eyes imaged with a 6 mm × 6 mm field-of-view. The x-axis indicates the distance of the zones from the CNV boundary (in mm), and the y-axis indicates the CC flow deficit percentage within each zone. Dark gray bars correspond to flow deficit analysis using 1.5 ms interscan time OCTA data; light gray bars correspond to analysis using 3.0 ms interscan time OCTA data. Error bars represent standard deviations amongst the 5 eyes. Shorter interscan time OCTA data detects more flow deficits, but shows a similar average trend to longer interscan time data.
Discussion
Impairment of the CC surrounding CNV has been previously reported. McLeod et al.1 used alkaline phosphatase staining with semi-automatic image analysis to quantitatively study the CC vessel area percentage in regions surrounding CNV lesions. Compared to control eyes, they found a statistically significant decrease in CC vessel area and a trend of increasing CC vessel area with increasing distance from the lesion. A recent study by Seddon et al.,6 using ulex europaeus agglutinin (UEA) lectin staining, found a statistically significant loss of CC vessel area adjacent to CNV lesions compared to normal controls and also reported severe CC impairment in regions >1 mm from the CNV boundary. Qualitative ICGA and OCTA studies have also reported CC impairment extending beyond CNV boundaries.9–13 Our results agree well with these prior studies in that: (1) we found CC impairment extending beyond the CNV boundary, and (2) this impairment was most pronounced in regions closest to the lesion. These observations were consistent across all eyes, irrespective of age, lesion size, and treatment history.
The effect of anti-VEGF injections on CC impairment has been previously discussed in the literature.29–31 While our study design did not allow us to examine potential associations between anti-VEGF treatment and CC impairment, the methods presented in this paper may be useful for future studies aimed at quantitatively assessing the impact of anti-VEGF injections on CC impairment, and for studies aimed at developing markers that predict CNV development and/or treatment response.
Development of Methods for Analyzing CC OCTA Data
A key objective of this study was to develop OCTA analysis techniques for quantitative assessment of CC features in the presence of focal lesion(s). There are two components to the technique presented in this study: (1) choosing a metric for CC impairment, and (2) developing a method to assess the spatial distribution of CC flow impairment relative to focal lesions.
CC Flow Deficits
In this study, flow deficits were used to assess CC impairment. CC flow deficit analysis, described by Spaide26 and used in a number of prior studies,20,32–35 has several advantages: detecting discrete regions of flow impairment may make the analysis more repeatable amongst different instrument manufacturers and OCTA algorithms; flow deficits are physically interpretable and relate directly to previous histological studies; and, flow deficit characteristics, such as size, may have physiological links to the progression of CC impairment in AMD.26,34 However, flow deficit analysis also has limitations: flow deficits may not be as sensitive to early changes in blood flow, such as partial reductions in blood flow speed; and, results may vary depending on the binarization level/method used to detect the deficits. Prior CC studies used a variety of different approaches for flow deficit binarization: Spaide26,34 and Uji et al.20 used the Phansalkar method, a local adaptive scheme; Al-Sheikh et al.33 used Otsu’s method, a global adaptive scheme; Borrelli et al.32 used maximum entropy binarization, a global adaptive scheme, similar to Otsu’s method; and Zhang et al.35 binarized OCTA images using deviation from a group of normal controls. In our study, we opted to use a fixed global binarization level to detect flow deficits, which has the advantage of consistency and simplicity. However, because flow deficit statistics are dependent on the binarization level—and because our binarization level was empirically selected (see Appendix II)—we performed a tolerance analysis by varying the binarization level by ±25% (see Figure, Supplemental Digital Content 6). The tolerance analysis shows that different choices of binarization level produced substantial differences in flow deficit statistics, which should be considered when comparing results from different studies. However, despite these differences, overall trends are still preserved, suggesting that measurements with different algorithms may preserve relative trends even though quantitative values differ. Therefore, we believe the conclusions of this study would be maintained if different algorithm parameters were used for flow deficit detection.
Assessment of Spatial Distribution of CC Flow Impairment
This study analyzed the spatial distribution of CC flow deficits both qualitatively and quantitatively. For qualitative analysis, flow deficit percentage was displayed using a false color scale, analogous to false color perfusion mapping of the retinal vasculature.27 Each pixel in a flow deficit percentage image represents the percentage of flow deficit area in that pixel’s local (250 μm) neighborhood; the averaging range can be adjusted to emphasize finer details (smaller ranges) or larger trends (larger ranges). To quantitatively assess the spatial distribution of CC flow deficits relative to CNV lesions, we used a lesion-centered zonal analysis (related lesion-centered analysis techniques have been previously reported for drusen32). In addition to CNV lesions, lesion-centered analysis is well suited to studying other focal pathologies, including nascent geographic atrophy (nGA), drusen-associated GA (DAGA), and GA. Moreover, lesion-centered analysis is extendible to incorporate both distance and angle (Figure 7). Finally, lesion-centered analysis is also suitable for longitudinal studies, such as predicting the expansion/contraction of a lesion. As an example, by overlaying the lesion-centered sectors of Figure 7 on the registered lesion boundary of a follow-up visit, expansion can be correlated with CC flow impairment. However, special care must be taken because, unlike a standard analysis using fixed positions on the fundus, lesion-centered positions change as the lesion changes.
Figure 7.
Resolving CC impairment as a function of angle (Eye 7; cf. Figure 6). (A) Analysis of spatial geometry, where only the first zone (0 μm, 250 μm] is considered. (B) Flow deficit percentage image (same color scale as in Figure 6). The angle resolved analysis highlights the non-uniformity of CC impairment.
Potential Artifacts and the Role of OCTA Imaging, Processing, and Analysis Parameters
OCTA imaging and analysis is more susceptible to artifacts than structural OCT, and there are multiple parameters involved in OCTA image generation that can affect quantitative CC measurements. In commercial instruments, many image processing steps are performed before OCTA data is analyzed or exported. For this reason, it is helpful to comprehensively describe potential artifacts along with the parameters for OCTA imaging, processing, and analysis. Understanding and reducing these artifacts is especially important for longitudinal studies involving quantitative analysis.
Effect of Wavelength and Swept-Source versus Spectral-Domain Detection
Both the wavelength and detection method (i.e., swept-source versus spectral-domain) influence OCT and OCTA data. Current commercial spectral-domain OCT (SD-OCT) instruments operate at ~840 nm wavelengths, whereas commercial SS-OCT instruments, and the SS-OCT prototype used in this study, operate at ~1050 nm. Compared to shorter wavelengths, longer wavelengths are less attenuated by ocular opacities and the retinal pigment epithelium (RPE).36,37 Moreover, SS-OCT has less sensitivity roll-off (a phenomenon in which detection sensitivity decreases when the retina is further from the system zero delay) than SD-OCT. Unlike wavelength-dependent attenuation, sensitivity roll-off can be mitigated by positioning the choriocapillaris near the zero-delay, as is done in enhanced depth imaging (EDI).38 OCTA image quality is strongly dependent on OCT image quality; as such, attenuation and sensitivity roll-off make SS-OCT advantageous for OCTA imaging of vascular features beneath the RPE.39–41
Effect of Thresholding
OCTA detects blood flow by measuring fluctuations between repeated B-scans; however, noise in OCT data also causes fluctuations, which can generate false OCTA signals that masquerade as blood flow. To avoid noise-related artifacts, regions of low OCT signal are set to zero (black) before OCTA images are displayed—a step known as thresholding. Although thresholding in its strictest sense is only required in “normalized” OCTA computation schemes, such as that used in this paper, low OCT signals also generate low OCTA signals in unnormalized computations in an analogous manner. Thresholding can dramatically affect both qualitative and quantitative OCTA image analysis25 and is a particular concern in regions below the RPE and/or underneath areas of fluid, due to attenuation and/or aberration of the OCT beam. As discussed in the preceding section, compared to shorter wavelength SD-OCT systems, longer wavelength SS-OCT systems are less attenuated and are therefore less susceptible to thresholding artifacts when imaging the choriocapillaris; nevertheless, thresholding can still be a concern for longer wavelength SS-OCT imaging.25 For example, suppose there was insufficient OCT signal around the CNV at the level of the CC—due to, perhaps, fluid and/or RPE attenuation; in such a situation, thresholded OCTA images would not show an OCTA signal in these regions, irrespective of whether CC flow was present. This, in turn, would produce false positive detection of CC impairment.
In our analysis, we thresholded all voxels having OCT signal values less than 3 standard deviations above the noise mean. To confirm this thresholding did not affect the conclusions of our study, we repeated the entire analysis using unthresholded OCTA data (i.e., OCTA data where thresholding was not applied). The results, summarized online (see Figure, Supplemental Digital Content 6), show a negligible difference between the thresholded and unthresholded analyses, suggesting that there was sufficient OCT signal at the level of the CC. As discussed in detail in Cole et al.,25 this confirms that the conclusions of this study are not due to thresholding artifacts.
Understanding and excluding thresholding artifacts is especially important for studies using commercial SD-OCT instruments operating ~840 nm wavelengths. In commercial instruments, in which user adjustment of the threshold is typically unavailable, thresholding artifacts can often be detected by visualizing the en face OCT image alongside the en face OCTA image, and confirming that there is sufficient OCT signal. However, some commercial instruments employ automatic brightness and/or contrast adjustment of en face OCT images, making low OCT signal regions harder to identify. Recently, Zhang et al.35 proposed a scheme to compensate signal attenuation artifacts in OCTA CC images acquired using a PLEX Elite 9000 (Carl Zeiss Meditec Inc., Dublin, CA) SS-OCTA system. Their approach, which scales the CC OCTA signal using a smoothed and inverted version of the CC OCT signal, showed promise for compensating for OCT signal attenuation caused by drusen and may also be useful for compensating attenuation from CNV-related OCT signal attenuation.
Role of the Interscan Time
In this study we used VISTA to generate 1.5 ms and 3.0 ms interscan time OCTA images. For all eyes and zones, shorter (1.5 ms) interscan time OCTA images were more sensitive to flow impairment than longer (3.0 ms) interscan times, and yielded higher CC flow deficit percentages; Figure 8 compares the effect of interscan time on flow deficit analysis for Eye 1. Our finding that longer interscan times have a reduced ability to differentiate between CC blood flow speeds (e.g., in CC flow impairment) agrees with prior studies using VISTA,42 as well as studies using backstitched OCTA43 and two-beam Doppler.44 A corollary of this finding is that special care is required when comparing quantitative OCTA measurements from instruments having different interscan times. Nevertheless, our results show that relative measurements and spatial trends were preserved for both long and short interscan times, suggesting the overall trends observed in this study would be reproducible using commercial instruments, which use longer (~5 ms) interscan times.
Figure 8.
Effect of interscan time on flow deficit analysis (Eye 1). Panels A-D correspond to 1.5 ms interscan time analysis, and panels E-H correspond to 3.0 ms interscan time analysis. (A, E) CC OCTA slab. (B,F) Raw CC flow deficit image, where white indicates flow deficit pixels; projection artifacts, motion artifacts, and regions within the lesion are excluded (teal). (C,G) CC flow deficit percentage map, where the colors correspond to the local percentage of flow deficits, as indicated by the color bar. (D,H) Average of the flow deficit percentage image of panels C and G over 250 μm zones (white contours). Notice that there is a higher percentage of flow deficits—indicated by redder colors—in the 1.5 ms interscan time analysis. (I-L) Closer examination of individual flow deficits. Panels I.1, J.1, K.1, and L.1 are extracted from the dashed boxes of panel A; panels I.2, J.2, K.2, and L.2 are extracted from the dashed boxes of panel B; panels I.3, J.3, K.3, and L.3 are extracted from the dashed boxes of panel E; and panels I.4, J.4, K.4, and L.4 are extracted from the dashed boxes of panel F. Note how there are certain flow deficits that appear in the binarized 1.5 ms interscan time OCTA image, but are absent from the binarized 3.0 ms interscan time OCTA image (yellow arrow of I.2 and I.4); other flow deficits are relatively unchanged with the varying interscan time (white arrow of J.2 and J.4); and other flow deficits are present, but reduced in the binarized 3.0 ms interscan time OCTA image (see panels K and L).
The variation in the flow deficits size with interscan time (Figure 8), supports usage of the term flow deficit, rather than flow void. With reference to panels I-L of Figure 8, we can see that, in some instances, flow deficits visible in 1.5 ms interscan time OCTA images are absent, or smaller, in 3.0 ms interscan time images (see, for example, the flow deficit indicated by the yellow arrow in panels I.2 and I.4). This implies that blood flow is present, but with insufficient speed to be detected in the binarized 1.5 ms OCTA image. In such an instance, the 3.0 ms interscan time allows more time for slowly moving blood cells to travel and, consequentially, to generate a higher OCTA signal. Other regions in Figure 8 may have a complete absence of blood flow: the flow deficit indicated by the white arrow in panels J.2 and J.4 is largely unchanged as the interscan time is changed.
Effect of Segmentation and Slab Generation
An en face OCTA image is only accurate if the segmentation boundaries used to generate it are accurate. In commercial OCT(A) instruments, fully automatic segmentation is typically used due to its speed and ease; however, even state-of-the-art automatic segmentation software can err, introducing local and/or global deviations from the desired result. This is particularly true when retinal features are distorted by pathology, such as in CNV; moreover, the CC is thin, and therefore requires extremely accurate segmentation (a few tens-of-microns). To achieve this accuracy, we manually corrected our automatic Bruch’s membrane segmentation on a B-scan-by-B-scan basis. While achieving optimum accuracy, this approach is not practical for larger scale studies or clinical use, where automatic, or semi-automatic, segmentation methods combined with careful segmentation review are required.
We chose to generate the en face OCTA CC slab images by projecting the OCTA volume from Bruch’s membrane though the 25 μm immediately posterior to Bruch’s membrane. This depth range, which is larger than the ~10 μm depth occupied by the CC, represents a compromise between robustness to segmentation errors (which improves with larger projection ranges) and exclusion of larger choroidal vessels (which improves with smaller projection ranges). Our 25 μm range is comparable to those ranges of prior CC flow deficit studies: Borrelli et al. used a 30 μm slab starting 31 μm posterior to the segmented RPE; Zhang et al. used a 20 μm slab starting at the segmented Bruch’s membrane; Spaide et al. and Al-Sheikh et al. used 10 μm slabs starting 31 μm to 34 μm posterior to the segmented RPE/Bruch’s membrane; and Uji et al. used one of (a) an 8 μm thick slab starting 29 μm posterior to segmented RPE, (b) a 6 μm slab starting 31 μm posterior to the segmented RPE, or (c) a 6 μm slab starting 35 μm posterior to the segmented RPE. We performed additional analysis (not reported) and found that the choice of CC slab thickness and position scaled the CC statistics, but did not alter overall trends. Nevertheless, with the CC slab thickness used in this study, larger choroidal vessels were at times visible in the OCTA CC slab (most notably in Eye 2; see Figure, Supplemental Digital Content 1) and are a source of possible measurement error.
Effect of Projection Artifacts
Projection artifacts, which cause overlying vessels to appear in underlying layers, are an important source of errors in OCTA interpretation and analysis.24 Projection artifacts limit the ability to visualize structures in 3-D, reducing the effective dimensionality of the data to somewhere in between 2-D and 3-D (“2.5-D”). Because projection artifacts pose substantial challenges to qualitative and quantitative analysis, a number of strategies for projection artifact removal have been proposed.45–47 However, if there is a region of the repeated OCT B-scans that becomes completely decorrelated—which can happen in large and/or high flow speed blood vessels—the OCT signal of the underlying structures will also be completely decorrelated. In this limit, it is not possible to measure OCTA signals from underlying vasculature, which creates a missing data problem. Although these missing data can be estimated (e.g., via interpolation), additional constraints, such as vascular continuity, are required. Estimation will be most faithful when the vascular structure is clearly resolved and the regions of missing data are small relative to the vascular structure; otherwise, the missing data cannot be accurately estimated. With typical transverse OCT resolutions, these criteria are met in the retinal vasculature, but not in the CC. For this reason, we opted to exclude—rather than correct—projection artifacts in our OCTA data. Similar approaches should be considered when using commercial instruments.
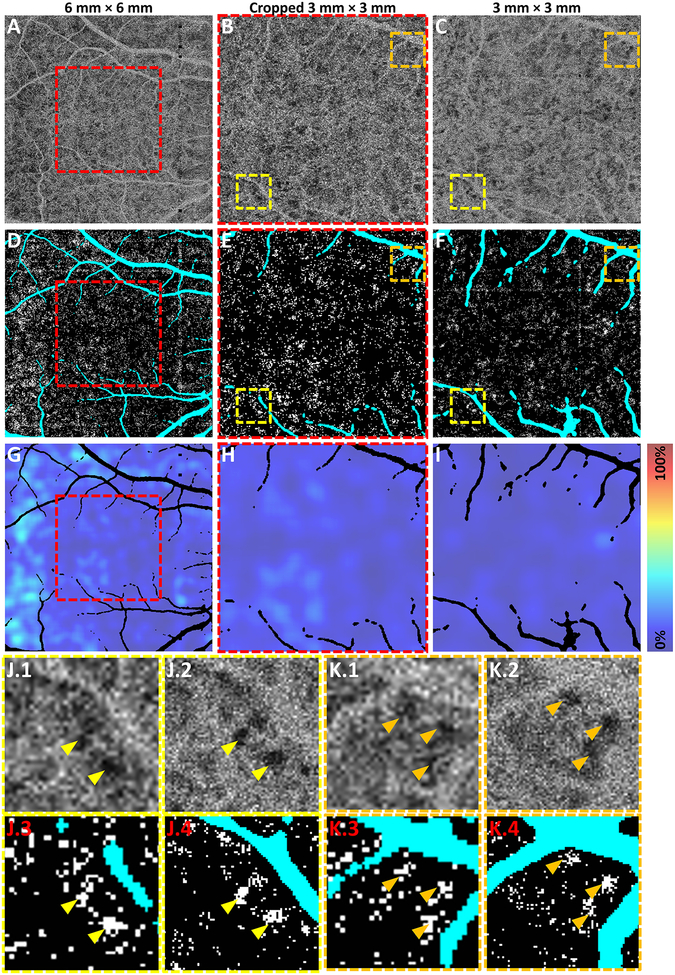
Our treatment of projection artifacts was limited in that we only excluded projection artifacts appearing in the RPE slab (see Methods), which corresponded to relatively large retinal vessels. Smaller retinal vasculature may also generate projection artifacts that affect the OCTA CC signal, which would not be excluded by our method. Errors in automatic vessel identification were another limitation of our projection artifact treatment, which resulted in the occasional inclusion of a projection artifact (false-negative), and/or exclusion of regions not affected by a projection artifact (false-positive; see Figure 2D). Moreover, in additional analysis comparing a 6 mm × 6 mm field-of-view image to a 3 mm × 3 mm field-of-view image in a 64 year-old healthy control eye (Figure 9), we observed that some smaller vessels excluded in the 3 mm × 3 mm field-of-view were not excluded in the corresponding 6 mm × 6 mm field-of-view, likely resulting from the difference in A-scan sampling density. However, these discrepancies were relatively minor, and we do not believe they altered the study conclusions (the study did not compare or combine data from 6 mm × 6 mm and 3 mm × 3 mm fields-of-view).
Figure 9.
Analysis of representative, 64 year-old healthy control eye. (A-C) 1.5 ms CC OCTA slabs from a: (A) 6 mm × 6 mm field-of-view; (B) 3 mm × 3 mm field-of-view, formed by cropping the CC OCTA slab in panel A to the region indicated by the red dashed box; and (C) 3 mm × 3 mm field-of-view, from a separate acquisition (not cropped). (D-F) Raw CC flow deficit images of panels A-C, respectively; white indicates flow deficit pixels and teal indicates projection/motion artifacts (excluded). (G-I) CC flow deficit percentage maps of panels D-F, respectively; the colors correspond to the local flow deficit percentages, as indicated by the color bar. (J, K) Closer examination of individual flow deficits. Panels J.1 and K.1 are extracted from the dashed boxes of panel B; panels J.2 and K.2 are extracted from the dashed boxes of panel C; panels J.3 and K.3 are extracted from the dashed boxes of panel E; and panels J.4 and K.4 are extracted from the dashed boxes of panel F. Arrow-heads in panels J-K point to individual flow deficits.
Effect of Transverse Optical Resolution
The transverse optical resolution in our study, as well as in all studies performed using commercial OCT instruments, is ~20 μm FWHM, comparable to the intercapillary spacing of the CC, particularly in the macula. Scanning electron microscopy48, adaptive optics OCTA,49 and MHz A-scan rate OCTA50 show a mean CC intercapillary spacing of ~20 μm to ~40 μm, with substantial variation about the mean. However, a 40 μm intercapillary spacing is only ~2× larger than the optical spot size; when combined with speckle noise and statistical flow fluctuations, this implies that the CC patterning will not be clearly resolvable near the macula, appearing instead as an average of the intercapillary regions and CC vessels. In the context of CC flow deficit analysis, this averaging effect is likely to be largely inconsequential because, irrespective of the optical spot size, the OCTA signal on average in an diseased eye with CC impairment will still be lower than that in a normal eye without CC impairment; moreover, larger flow deficits, such as those present in the nAMD eyes of this study, are clearly resolvable. Nevertheless, finer resolution of the CC would likely lead to an improved appreciation of pathological features.
Effect of Image Averaging
In addition to the transverse optical resolution, the visibility of the CC is also impacted by the vascular continuity and signal-to-noise ratio of the OCTA data. These two factors can be substantially improved using a variety of image averaging approaches: split-spectrum amplitude-decorrelation angiography (SSADA) generates multiple OCTA images—each of which has a broadened axial resolution—and then forms a single OCTA image via averaging;51 and registration and averaging of en face OCTA images has been demonstrated for retinal19,21 and CC vasculature.20 In our study, two types of image averaging were employed: OCTA B-scan averaging and motion correction with volume merging. Our OCTA protocol acquires 5 repeated OCT B-scans, which yields 4 OCTA B-scans with 1.5 ms interscan times (or 3 OCTA B-scans with 3.0 ms interscan times); these OCTA B-scans are then averaged to generate a single OCTA B-scan. Compared to commercial systems, which use 2–3 repeated B-scans, 5 repeated B-scans improves the vascular continuity and signal-to-noise ratio. Our study also uses software that motion corrects and merges two orthogonally scanned OCTA volumes. This process is similar to registration and merging of en face data, except that it uses volumetric data and therefore is independent of segmentation accuracy. In general, commercial instruments that acquire larger numbers of repeated B-scans or use software motion correction and volume merging will have improved OCTA image quality. However, while some amount of averaging improves CC OCTA imaging, averaging larger numbers of en face or volumetric data sets may blur features because eye motion is not fully corrected, as reported for retinal vasculature.21
Effect of Field Size and Sampling Density
In our study, we isotropically scanned both 3 mm × 3 mm and 6 mm × 6 mm fields-of-view with 500 × 500 A-scans; consequentially, the spacing between adjacent A-scans (spatial sampling period) was 6 μm and 12 μm, respectively. Importantly, for both fields-of-view, the A-scan spacing was comparable to, or less than, ½ the OCT optical spot size; thus, the OCTA image data should preserve the optical resolution. If the A-scan spacing is larger (sparser sampling) than ½ the spot size (the critical sampling period), the image data will have a lower effective resolution than the optical resolution. Comparing the 3 mm × 3 mm and 6 mm × 6 mm fields-of-view in the control eye of Figure 9, we see close agreement, down to the level of individual flow deficits. Although there is a difference in pixel size between the 3 mm × 3 mm and 6 mm × 6 mm images—6 μm and 12 μm, respectively; see panels J and K of Figure 9—this does not affect the measured flow deficit size. Therefore, the 3 mm × 3 mm and 6 mm × 6 mm scan protocols used in this study should give consistent results. Nevertheless, to avoid mixing dissimilar data, the pooled analysis of Figure 4 is restricted to 6 mm × 6 mm data. Further studies are needed to investigate the effects of sampling density on quantitative OCTA analysis of the CC.
More generally, insufficient A-scan density is a potential limitation in commercial OCT instruments, which currently operate at 80 kHz to 100 kHz A-scan rates, slower than the 400 kHz prototype instrument used in this study. With slower A-scan rates, larger fields-of-view will have sparser A-scan sampling, reducing the ability to resolve small features (assuming that total imaging time is held constant). This sparser sampling explains why wider field-of-view retinal OCTA images cannot resolve small capillaries as well as smaller field-of-view OCTA images. In imaging the CC, the A-scan spacing should be sufficiently dense so as to detect the smallest CC flow voids, which limits the field-of-view for a given image acquisition time. Moreover, quantitative analysis of sub-sampled OCTA images with differing A-scan sampling may yield inconsistent results and is not recommended.
Effect of Motion Artifacts
Compared to OCT imaging, OCTA imaging is more susceptible to motion artifacts. There are three predominate causes of this increased susceptibility: acquiring repeated B-scans lengthens imaging time, increasing the likelihood that motion occurs during the scan; en face OCTA images contain fine microvasculature, smaller than the typical features of en face OCT, which are more affected by motion; and, OCTA measures motion between repeated scans—while moving blood cells generate an OCTA signal, so too does bulk motion.52 To mitigate motion artifacts, in this study we used software motion correction of orthogonal x-fast and y-fast raster volumes. This technique has advantages: it is hardware-free; it produces geometrically correct 3-D anatomy; merging/averaging increases the signal-to-noise ratio; and, by combining data from x-fast and y-fast volumes, missing data areas (“data gaps”) are substantially reduced compared to a single x-fast/y-fast volume without eye tracking. This technique also has limitations: repeated volume imaging increases acquisition time; there are residual data gaps at the intersection of saccadic motion artifacts (eye tracking was not used in our study); a region that is covered by two volumes will have a different signal-to-noise ratio than a region that is covered by only one volume; and, if motion correction is sub-optimal, there can be feature blurring and/or distortion.
The data gap limitation can be addressed by acquiring additional volumes or by using hardware-based eye-tracking. While such approaches were not pursued in this study, data gaps typically occupy a small area relative to the field-of-view, are randomly distributed, and can be excluded from analysis; thus, it is unlikely that data gaps influenced our results. The difference in signal-to-noise ratio between regions covered by two volumes and those covered by only one volume can pose challenges for quantitative OCTA measurements. Since merging two volumes increases the signal-to-noise ratio, saccadic strip regions, which are only covered by one volume, have a lower signal-to-noise ratio than other image regions; consequently, saccadic strip regions are likely to generate more flow deficits (Figure 10). In this study we did not compensate for saccadic strips because they typically occupy a small area relative to the field-of-view, are randomly distributed, and have a much small effect on flow deficit detection than the pathology being studied. However, mitigation of saccadic strips may be important for studies of early stage disease, and, consequently, it is useful to briefly mention methods to mitigate the effects of these artifacts: saccadic strip regions could be excluded from the analysis (similar to how the data gaps, projection artifacts, and lesion regions were excluded); saccadic strip regions could be binarized with different binarization levels, adapted to the difference in the signal-to-noise ratios; eye-tracking could be employed, as is done on several commercial OCTA systems; or, more than 2 volumes could be acquired, motion corrected, and then merged in a way that minimizes the differences in signal-to-noise ratios of the resulting OCTA images.
Figure 10.
Effect of motion artifacts (Eye 1). (A) 1.5 ms interscan time CC OCTA slab. (B) Raw CC flow deficit image, where white indicates flow deficit pixels; projection artifacts, motion artifacts, and regions within the lesion are excluded (teal). (C) Enlargement of the dashed box of panel A. (D) Enlargement of the dashed box of panel B. Data gaps, which occur at the intersections of saccade strips, appear rectangular squares (colored black in panels A and C, and colored teal in panels B and D). Saccadic strip regions, which lay along the lines indicated by the arrows in panel D, have data coming from only one volume, and therefore have a different signal-to-noise ratio compared to the regions not along the saccadic strips. This is apparent by looking closely at the texture of the OCTA signal in panel C. As a result, when the CC OCTA slab is binarized to form the raw CC flow deficit image, the saccadic strip regions tend to have more flow deficits; this can be seen by looking at the increased presence of flow deficits along the lines indicated by the black arrows in panel D.
The possibility of blurring features when motion correcting and merging is also a concern, particularly when considering the near- or below-resolution features of the CC. However, analysis of control subjects by our group (not reported) has found that if the input volumes are of reasonable quality, merged volumes preserve the CC patterning (for example, all data in Figure 9 is merged, and flow deficits are clearly visible and reproducible over different fields-of-view). Moreover, we carefully reviewed and compared the x-fast and y-fast volumes to their merged counterparts, and there was no evidence that blurring affected study results. Nevertheless, the potential for blurring artifacts from software motion correction should be carefully considered.
Commercial instruments use a variety of methods to perform motion correction: the Triton (Topcon, Japan) and the Spectralis (Heidelberg Engineering, Germany) use infrared full-field fundus images to track saccadic eye motion and re-scan missed regions; the AngioPlex and PLEX Elite 9000 (Carl Zeiss Meditec Inc., Dublin, CA) use a 20 kHz line scan ophthalmoscope (LSO) for eye tracking; and the AngioVue (Optovue Inc., Fremont, CA) uses a combination of infrared full-field fundus image eye tracking in conjunction with a software motion correction of orthogonal raster scanned volumes. Although the many differences—such as processing algorithms and wavelengths—preclude a direct comparison of these eye tracking and motion correction technologies, some general comparisons can be made. High resolution LSO images are likely superior to infrared fundus images for accurate eye tracking that preserves the visibility of CC structures. For CC imaging, combined eye tracking and software motion correction/merging is likely superior to tracking or software motion correction alone because eye tracking minimizes saccadic motion effects (reducing data gaps and saccadic strip regions), while software motion correction/merging provides sub-pixel accuracy and increases vessel continuity by volume averaging.53 Nevertheless, irrespective of motion correction method, data should be carefully reviewed for artifacts.
Limitations
The small sample size of this study does not allow us to extrapolate our findings beyond our cohort. Moreover, our study lacked an age-matched control cohort. However, since our study analyzed intra-eye trends (i.e., the spatial distribution of CC impairment within an eye), age-matched controls are less critical. Nevertheless, age-matched controls are essential for determining which patients have CC impairment outside the population norms. Another limitation is that CC impairment was assessed using a simple flow deficit percentage, which converts the continuous OCTA signal into a binary one; this approach is limited in that it may not capture subtler reductions in blood flow.
Conclusion
In this study we developed an OCTA-based framework for quantitatively analyzing the spatial distribution of CC flow deficits in eyes with CNV secondary to AMD. A lesion-centered zonal method was used to measure flow deficit percentages in zones at varying distances from the CNV lesion boundaries. CC flow deficit percentages were highest near CNV boundaries, and progressively decreased away from the boundaries.
The development of quantitative OCTA methods to assess CC impairment is important for future longitudinal studies of AMD. The CC is especially challenging to quantify because of its location below the RPE, which makes it susceptible to low-OCT signal artifacts, and its fine intercapillary spacing, which make its appearance on OCTA highly dependent on acquisition and image processing steps. Moreover, as demonstrated in this study, changes in interscan time, OCTA thresholding levels, and flow deficit binarization levels all affect quantitative measurements of flow deficits; OCTA artifacts from segmentation, projection, optical resolution, averaging, A-scan sampling, and motion correction can also affect quantitative results. The use of a prototype SS-OCT instrument enabled comprehensive control of all image processing parameters and provided a framework to understand and mitigate OCTA artifacts—we believe that this framework can be extended to commercial instruments. Understanding OCTA processing steps, parameters, and artifacts will be important for reducing errors and variances in larger scale, longitudinal studies of progression and treatment response.
Supplementary Material
Supplemental Digital Content 6. Effects of thresholding level and binarization level on flow deficit statistics. pdf
Supplemental Digital Content 1. CC Analysis of Eye 2. pdf
Supplemental Digital Content 2. CC Analysis of Eye 3. pdf
Supplemental Digital Content 3. CC Analysis of Eye 4. pdf
Supplemental Digital Content 4. CC Analysis of Eye 5. pdf
Supplemental Digital Content 5. CC Analysis of Eye 6. pdf
Financial Support
NIH 5-R01-EY011289–31, Macula Vision Research Foundation (MVRF), Champalimaud Vision Award, Beckman-Argyros Award in Vision Research, Massachusetts Lions Clubs.
Appendices
OCTA signals are dependent on the details of image processing, and different algorithms have the potential to yield different results. Therefore, in Appendices I–III we provide a mathematical description of how OCTA signals were computed, how CC flow deficits were detected from OCTA images, and how the lesion-centered zonal analysis was performed. These sections are mathematical so as to provide the most accurate and unambiguous description. An understanding of these details is not required to understand the overall findings of the study. However, we present this information to provide a more complete characterization of our methodology, and to encourage documentation for future, larger scale studies.
Appendix IV provides the detailed treatment histories of the 5 patients who were treated with anti-VEGF prior to imaging.
Appendix I: OCTA Computation
The ζ·Δt interscan time OCTA B-scan, Bζ,OCTA, was generated using a variable interscan time analysis (VISTA)16 function of the form:
where k ∈ K is the pixel index of the B-scan, N is the number of repeated B-scans (in this study N = 5), ζ ∈{1,…,N−1} is the interscan time index, Δt is the fundamental interscan time, and Bj,OCT is the j-th repeated OCT B-scan. The decorrelation kernel10 D was:
and T, the thresholding function, was defined as:
where the thresholding level was ℓOCT = μN + 3σN (see Discussion); here, μN and σN are, respectively, the mean and standard deviation of the background noise. For this study we only considered ζ = 1 and ζ = 2, corresponding to 1.5 ms and 3.0 ms interscan time OCTA images, respectively.
Appendix II: CC Flow Deficit Metric
We defined CC flow deficits as all pixels of the OCTA CC slab below the binarization level, ℓb. The smoothed flow deficits percentage image, Iζ,FVP, as:
where P is index set of all pixels in the OCTA CC slab, E is the index set of all excluded pixels, and ℓb is the binarization level. For this study, based on subjective analysis of 4 eyes from 3 controls (60, 64, and 78 years-old; 2 eyes were analyzed from the 64 year-old control), we chose ℓb = 0.21 (OCTA pixel values occupy [0,1]). For our analysis, E = AM ∪ AP ∪ L, where AM is the index set of all pixels belonging to regions of motion artifacts, AP is the index set of all pixels belonging to regions of projection artifacts, and L is the index set of all pixels within the CNV boundary. is an adaptively weighted Gaussian kernel defined as:
For our study, σS was taken to be 250 μm, which matches our zone widths (250 μm); in the sum is taken over non-artifact pixels only so as to ensure consistent normalization.
Appendix III: Spatial Analysis of CC Flow Deficits
To form the lesion-centered coordinate system, for every non-lesion pixel in the image we formed the minimum distance map:
Where ‖p−q‖ is the distance between the pixels indexed by p and q (we used the Euclidean notion of distance), and ∂L is the set of pixels on the lesion boundary. Using M, we then divided the image into a sequence of non-overlapping zones of thickness Δd pixels. In particular, the k-th zone was defined as:
Alternatively stated, Sk consists of all the pixels that are more than (k−1)Δd pixels from the lesion boundary, and less than, or equal to, kΔd pixels from the lesion boundary. In this study we set Δd to correspond to 250 μm, which gives a reasonable balance between resolution and averaging. The flow deficit percentage of the k-th zone was defined as:
Where |Sk \ E| is the number of pixels in the zone Sk that are not in the excluded set E. Note that the excluded regions have no effect on the flow deficit percentage.
Appendix IV: Detailed Anti-VEGF Treatment Histories
Table 2 details the anti-VEGF treatment histories for the 5 treated eyes of this study. All listed injections occurred prior to the date of OCTA imaging.
Footnotes
Competing Interest Statement: None of the authors have any proprietary interest in this work.
References
- 1.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship Between RPE and Choriocapillaris in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLeod DS, Lutty GA. High-Resolution Histologic Analysis of the Human Choroidal Vasculature. Invest Ophthalmol Vis Sci. 1994;35(11):3799–3811. [PubMed] [Google Scholar]
- 3.Hayashi K, de Laey JJ. Indocyanine green angiography of choroidal neovascular membranes. Ophthalmologica. 1985;190(1):30–39. doi: 10.1159/000309489. [DOI] [PubMed] [Google Scholar]
- 4.Bhutto I, Lutty G. Understanding Age-Related Macular Degeneration (AMD): Relationships between the Photoreceptor/Retinal Pigment Epithelium/Bruch’s Membrane/Choriocapillaris Complex. Mol Aspects Med. 2012;33(4):295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris Breakdown Precedes Retinal Degeneration in Age-Related Macular Degeneration. Neurobiol Aging. 2014;35(11):2562–2573. doi: 10.1016/j.neurobiolaging.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JM, McLeod DS, Bhutto IA, et al. Histopathological Insights Into Choroidal Vascular Loss in Clinically Documented Cases of Age-Related Macular Degeneration. JAMA Ophthalmol. 2016;134(11):1272–1280. doi: 10.1001/jamaophthalmol.2016.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical Coherence Tomography Angiography. Prog Retin Eye Res. 2017. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashani AH, Chen C-L, Gahm JK, et al. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog Retin Eye Res. 2017;60(Supplement C):66–100. doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong Y-J, Miura M, Makita S, et al. Noninvasive Investigation of Deep Vascular Pathologies of Exudative Macular Diseases by High-Penetration Optical Coherence Angiography. Invest Ophthalmol Vis Sci. 2013;54(5):3621–3631. 10.1167/iovs.12-11184. [DOI] [PubMed] [Google Scholar]
- 10.Jia Y, Bailey ST, Wilson DJ, et al. Quantitative Optical Coherence Tomography Angiography of Choroidal Neovascularization in Age-Related Macular Degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moult E, Choi W, Waheed NK, et al. Ultrahigh-Speed Swept-Source OCT Angiography in Exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D. Choriocapillaris Loss in Advanced Age-Related Macular Degeneration. J Ophthalmol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuehlewein L, Bansal M, Lenis TL, et al. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am J Ophthalmol. 2015;160(4):739–748.e2. doi: 10.1016/j.ajo.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Gao SS, Jia Y, Zhang M, et al. Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT27–OCT36. 10.1167/iovs.15-19043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorczynska I, Migacz JV, Zawadzki RJ, Capps AG, Werner JS. Comparison of Amplitude-Decorrelation, Speckle-Variance and Phase-Variance OCT Angiography Methods for Imaging the Human Retina and Choroid. Biomed Opt Express. 2016;7(3):911–942. doi: 10.1364/BOE.7.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi W, Moult EM, Waheed NK, et al. Ultrahigh-Speed, Swept-Source Optical Coherence Tomography Angiography in Nonexudative Age-Related Macular Degeneration with Geographic Atrophy. Ophthalmology. 2015;122(12):2532–2544. doi: 10.1016/j.ophtha.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus MF, Potsaid B, Mayer MA, et al. Motion Correction in Optical Coherence Tomography Volumes on a per A-scan Basis using Orthogonal Scan Patterns. Biomed Opt Express. 2012;3(6):1182–1199. doi: 10.1364/BOE.3.001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus MF, Liu JJ, Schottenhamml J, et al. Quantitative 3D-OCT Motion Correction with Tilt and Illumination Correction, Robust Similarity Measure and Regularization. Biomed Opt Express. 2014;5(8):2591–2613. doi: 10.1364/BOE.5.002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Impact of Multiple En Face Image Averaging on Quantitative Assessment from Optical Coherence Tomography Angiography Images. Ophthalmology. 2017;124(7):944–952. doi: 10.1016/j.ophtha.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, SR S. Choriocapillaris Imaging using Multiple En Face Optical Coherence Tomography Angiography Image Averaging. JAMA Ophthalmol. 2017;135(11):1197–1204. 10.1001/jamaophthalmol.2017.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mo S, Phillips E, Krawitz BD, et al. Visualization of Radial Peripapillary Capillaries Using Optical Coherence Tomography Angiography: The Effect of Image Averaging. PLoS One. 2017;12(1):e0169385 10.1371/journal.pone.0169385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schottenhamml J, Moult EM, Novais EA, et al. OCT-OCTA Segmentation: a Novel Framework and an Application to Segment Bruch’s Membrane in the Presence of Drusen In: The Association for Research in Vision and Ophthalmology. 2017:645–645. [Google Scholar]
- 23.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaide RF, Fujimoto JG, Waheed NK. Image Artifacts in Optical Coherence Tomography Angiography. Retina. 2015;35(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole ED, Moult EM, Dang S, et al. The Definition, Rationale, and Effects of Thresholding in OCT Angiography. Ophthalmol Retin. 2017;1(5):435–447. doi: 10.1016/J.ORET.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaide RF. Choriocapillaris Flow Features Follow a Power Law Distribution: Implications for Characterization and Mechanisms of Disease Progression. Am J Ophthalmol. 2016;170:58–67. doi: 10.1016/j.ajo.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Agemy SA, Scripsema NK, Shah CM, et al. Retinal Vascular Perfusion Density Mapping Using Optical Coherence Tomography Angiography in Normals and Diabetic Retinopathy Patients. Retina. 2015;35(11):2353–2363. doi: 10.1097/IAE.0000000000000862. [DOI] [PubMed] [Google Scholar]
- 28.Rebhun CB, Moult EM, Ploner SB, et al. Analyzing Relative Blood Flow Speeds in Choroidal Neovascularization Using Variable Interscan Time Analysis OCT Angiography. Ophthalmol Retin. March 2018. doi: 10.1016/j.oret.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunwald JE, Daniel E, Huang J, et al. Risk of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative Rreatments to Inhibit VEGF in Age-Related Choroidal Neovascularisation: 2-Year Findings of the IVAN Randomised Controlled Trial. Lancet. 2018;382(9900):1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 31.Lois N, McBain V, Abdelkader E, Scott NW, Kumari R. Retinal Pigment Epithelial Atrophy in Patients with Exudative Age-Related Macular Degeneration Undergoing Anti-Vascular Endothelial Growth Factor Therapy. Retina. 2013;33(1):13–22. doi: 10.1097/IAE.0b013e3182657fff. [DOI] [PubMed] [Google Scholar]
- 32.Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2017;58(11):4792–4798. 10.1167/iovs.17-22360. [DOI] [PubMed] [Google Scholar]
- 33.Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, et al. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Invest Ophthalmol Vis Sci. 2017;58(4):2063–2069. 10.1167/iovs.16-21289. [DOI] [PubMed] [Google Scholar]
- 34.Spaide RF. Ising Model of Choriocapillaris Flow. Retina. 2018;38(1). [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Zheng F, Motulsky EH, et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Invest Ophthalmol Vis Sci. 2018;59(1):203–211. 10.1167/iovs.17-22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unterhuber A, Považay B, Hermann B, Sattmann H, Chavez-Pirson A, Drexler W. In Vivo Retinal Optical Coherence Tomography at 1040 nm-Enhanced Penetration into the Choroid. Opt Express. 2005;13(9):3252–3258. doi: 10.1364/OPEX.13.003252. [DOI] [PubMed] [Google Scholar]
- 37.Povazay B, Hermann B, Unterhuber A, et al. Three-Dimensional Optical Coherence Tomography at 1050 nm versus 800 nm in Retinal Pathologies: Enhanced Performance and Choroidal Penetration in Cataract Patients. J Biomed Opt. 2007;12(4):41211. doi: 10.1117/1.2773728. [DOI] [PubMed] [Google Scholar]
- 38.Spaide RF, Koizumi H, Pozonni MC. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Novais EA, Adhi M, Moult EM, et al. Choroidal Neovascularization Analyzed on Ultrahigh-Speed Swept-Source Optical Coherence Tomography Angiography Compared to Spectral-Domain Optical Coherence Tomography Angiography. Am J Ophthalmol. 2018;164:80–88. doi: 10.1016/j.ajo.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane M, Moult EM, Novais EA, et al. Visualizing the Choriocapillaris Under Drusen: Comparing 1050-nm Swept-Source Versus 840-nm Spectral-Domain Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT585–OCT590. 10.1167/iovs.15-18915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AR, Roisman L, Zhang Q, et al. Comparison Between Spectral-Domain and Swept-Source Optical Coherence Tomography Angiographic Imaging of Choroidal Neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499–1505. 10.1167/iovs.16-20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moult EM, Waheed NK, Novais EA, et al. Swept-Source Optical Coherence Tomography Angiography Reveals Choriocapillaris Alterations in Eyes with Nascent Geographic Atrophy and Drusen-Associated Geographic Atrophy. Retina. 2016;36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braaf B, Vermeer KA, Vienola KV, de Boer JF. Angiography of the Retina and the Choroid with Phase-Resolved OCT using Interval-Optimized Backstitched B-scans. Opt Express. 2012;20(18):20516–20534. doi: 10.1364/OE.20.020516. [DOI] [PubMed] [Google Scholar]
- 44.Jaillon F, Makita S, Yasuno Y. Variable Velocity Range Imaging of the Choroid with Dual-Beam Optical Coherence Angiography. Opt Express. 2012;20(1):385–396. doi: 10.1364/OE.20.000385. [DOI] [PubMed] [Google Scholar]
- 45.Zhang M, Hwang TS, Campbell JP, et al. Projection-Resolved Optical Coherence Tomographic Angiography. Biomed Opt Express. 2016;7(3):816–828. doi: 10.1364/BOE.7.000816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep. 2017;7:42201. doi: 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang A, Zhang Q, Wang RK. Minimizing Projection Artifacts for Accurate Presentation of Choroidal Neovascularization in OCT Micro-Angiography. Biomed Opt Express. 2015;6(10):4130–4143. doi: 10.1364/BOE.6.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olver JM. Functional Anatomy of the Choroidal Circulation: Methyl Methacrylate Casting of Human Choroid. Eye. 1990;4:262 10.1038/eye.1990.38. [DOI] [PubMed] [Google Scholar]
- 49.Kurokawa K, Liu Z, Miller DT. Adaptive Optics Optical Coherence Tomography Angiography for Morphometric Analysis of Choriocapillaris [Invited]. Biomed Opt Express. 2017;8(3):1803–1822. doi: 10.1364/BOE.8.001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorczynska I, Migacz JV., Jonnal R, Zawadzki RJ, Poddar R, Werner JS. Imaging of the Human Choroid with a 1.7 MHz A-scan Rate FDML Swept Source OCTSsystem. SPIE Proc. 2017;1004510(February 2017):1004510. doi: 10.1117/12.2251704. [DOI] [Google Scholar]
- 51.Jia Y, Tan O, Tokayer J, et al. Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Camino A, Jia Y, Liu G, Wang J, Huang D. Regression-Based Algorithm for Bulk Motion Subtraction in Optical Coherence Tomography Angiography. Biomed Opt Express. 2017;8(6):3053–3066. doi: 10.1364/BOE.8.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camino A, Zhang M, Gao SS, et al. Evaluation of Artifact Reduction in Optical Coherence Tomography Angiography with Real-Time Tracking and Motion Correction Technology. Biomed Opt Express. 2016;7(10):3905–3915. doi: 10.1364/BOE.7.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 6. Effects of thresholding level and binarization level on flow deficit statistics. pdf
Supplemental Digital Content 1. CC Analysis of Eye 2. pdf
Supplemental Digital Content 2. CC Analysis of Eye 3. pdf
Supplemental Digital Content 3. CC Analysis of Eye 4. pdf
Supplemental Digital Content 4. CC Analysis of Eye 5. pdf
Supplemental Digital Content 5. CC Analysis of Eye 6. pdf