Abstract
In response to concerns raised by communities surrounding the New Bedford Harbor Superfund site, we completed a field and modeling study that concluded the harbor is the primary source of polychlorinated biphenyls (PCBs) in air around the harbor. The follow-up question from residents was whether the PCBs measured in air pose a risk to their health. The US Environmental Protection Agency focuses their site-specific, risk-based decisions for site clean-up on cancers. We focused our assessment on the non-cancer effects on the thyroid based on the congener specific patterns and concentrations of PCBs measured in air near and distant to the harbor. Human and animal studies of PCB-induced effects on the thyroid provide evidence to support our analysis. Drawing from the published toxicological data, we used a Margin of Exposure (MOE) approach to derive a human-equivalent concentration in air associated with human health effects on the thyroid. Based on the MOEs calculated herein, evaluation of the MOE indicates that changes in thyroid hormone levels are possible among people living adjacent to the Harbor. Changes in thyroid hormone levels are more likely among people who live near the harbor compared to residents living in areas distant from the harbor. This risk assessment documents potential health risks associated with proximity to a marine Superfund Site using site-specific ambient air PCB congener data.
Keywords: Superfund, polychlorinated biphenyls, ambient air, Margin of Exposure, PCB congener, thyroid
1. Introduction
New Bedford Harbor (NBH), located in southeastern Massachusetts, is surrounded by the towns of Acushnet, Dartmouth, Fairhaven, and the city of New Bedford, which have a combined population of approximately 150,000 (Census Bureau, 2015). In 1982, the US Environmental Protection Agency (EPA) placed the 18,000-acre harbor on the National Priorities List due to high concentrations of PCBs and metals measured in the water and sediments. The PCBs in New Bedford Harbor originated from two electronic capacitor manufacturers—Aerovox Corporation and Cornell Dublier. The companies discharged PCB-containing waste into the harbor and local sewer system beginning in the early 1940s (Pesch et al., 2001). Since becoming a Superfund site, the harbor has undergone multiple federal and state dredging operations, including “hot spot” clean-up for the most contaminated sediments in the 1990s and the current hydraulic dredging that began in 2004, with major removal operations in 2016-2018 (U.S. Environmental Protection Agency, 2014). These dredging activities involve moving contaminated sediment from the bottom of the harbor to disposal facilities, including the on-site confined aquatic disposal (CAD) cells located in the inner harbor. Dredging activities introduce the potential for increased release of airborne PCBs and subsequent exposure through inhalation of ambient PCBs (Vorhees, et al., 1997; Wilson, 2015). For this reason, EPA has been monitoring air concentrations of total PCBs near the harbor since 2004 and comparing these levels to EPA-derived screening levels based on cancer risks. As of April 2018, the EPA reports that all of their monitored concentrations have been below the risk-based screening levels (U.S. EPA, 2017). However, the EPA screening levels are not based on the congeners measured in the air and the monitoring methods do not report congener-specific data. Rather, EPA reports data for groups of PCBs known as homologues following 24-hour air monitoring, reflecting the groups, but not individual congeners. Furthermore, until 2016, the EPA air monitors were located for convenience or where concentrations were anticipated to be highest. These locations were not necessarily reflective of where people are likely to spend time or be exposed. Beginning in late 2016 and extending through late 2017, EPA placed air monitors in locations closer to where people spend time.
People living and working near New Bedford Harbor are potentially exposed to PCBs through contact with water and sediments in the harbor, air around the harbor, indoor air and indoor dust, and consumption of seafood caught from the harbor. Cancer risks associated with seafood consumption are relatively well-characterized and have been the basis for closures of areas to fishing and fish consumption (Basra, Fabian, & Scammell, 2018). We assessed inhalation of PCBs in ambient air by NBH-area residents, both adults and children. The primary exposure medium is the surface water of New Bedford Harbor, from which PCB congeners are volatilized (present in gas phase) or aerosolized (suspended in water droplets or with particulate matter) into the surrounding air. While there are other sources of ambient PCBs around New Bedford Harbor, our field and modeling study showed that the harbor is the primary source of PCBs in air around the harbor (Martinez et al., 2017).
We are now able to estimate residential exposures and health risk for residents of the towns and cities surrounding New Bedford Harbor: Acushnet, Dartmouth, Fairhaven and New Bedford. We initially sought to assess risk using the approach used by regulatory authorities for decision-making, which would allow for comparison of risk from consumption of PCB-contaminated fish with inhalation of PCBs in air.
While this is possible for assessment of cancer risk, it is not possible for non-cancer risk due to the lack of a reference concentration (RfC) (U.S. Environmental Protection Agency, 1994). EPA defines the RfC as the concentration of PCBs in air that sensitive populations can breathe all day of every day and not expect to experience ill health related to PCB exposure. The need for a PCB RfC has been documented, along with the acknowledgement of need for more research to form the scientific foundation of a RfC (Lehmann, Christensen, Maddaloni, & Phillips, 2015).
There is strong evidence supporting the thyroid as a target of PCB toxicity. We relied on the existing toxicological literature from which we derived a human equivalent concentration (HEC) of the airborne PCBs associated with adverse effects in rodent studies. We then compared the HEC with the ambient air concentrations of PCBs measured near homes around NBH to estimate a Margin of Exposure (MOE) for inhalation to PCBs at the Superfund Site. Rather than establishing a concentration deemed acceptable, such as a RfC, the MOE tells us about the likelihood of exposure to cause “unreasonable” risk (U.S. Environmental Protection Agency, 2012). It is a ratio of the toxicity effect level to the estimated exposure dose. The specific objectives of this analysis were as follows:
Determine which PCB congeners are prevalent in ambient air measured around NBH and assess exposure to residents of surrounding communities (exposure assessment)
Provide evidence for the thyroid as a target organ for PCB toxicity for the most prevalent congeners and two relevant Aroclors in the NBH region and/or lower-chlorinated, inhaled PCB congeners (hazard assessment)
Calculate Margin of Exposure for residents (risk assessment)
2. Materials and Methods
2.1. Exposure Assessment
In response to requests from residents to monitor the air closer to where they work and live, the Boston University Superfund Research Program (BUSRP) and our Community Engagement Core partner Toxics Action Center worked with the Iowa Superfund Research Program (ISRP) and residents to identify locations and design a monitoring program to assess congener-specific ambient PCB concentrations in the air around New Bedford Harbor (Figure S1). While EPA monitors airborne PCBs using high volume sampling for 24 hour periods, we used passive air samplers that integrate air concentrations over four to six weeks. We conducted four rounds of air monitoring in 2015 and 2016, one of which occurred during a period of hydraulic dredging and has not been previously reported. Details on sampling, analysis and community engagement methods are described elsewhere (BU Superfund Research Program, 2015; Martinez et al., 2017; Tomsho et al., 2018). Our measurements show that concentrations did not differ significantly across sampling events. Congener-specific concentrations can be found in the Supporting Information. The full PCB congener dataset with associated metadata is available in a freely accessible data repository at https://doi.pangaea.de/10.1594/PANGAEA.902925 (Martinez et al., 2019).
2.2. Hazard Assessment
The Margin-of Exposure analysis is based on changes in thyroid hormone levels as a function of exposure to PCB congeners around NBH, therefore, in the hazard assessment we support the basis for selection of the thyroid as a target of PCB toxicity. We provide evidence from the epidemiology literature and reviewed studies of oral exposure to PCBs relevant to NBH to assess the overall weight of evidence supporting a connection between PCB exposure and the health effects. We then identified key studies that rely on inhalation exposures to PCBs, especially those congeners most abundant in the air around NBH. From this literature, we selected the key studies from which a Point of Departure (POD) air concentration could be taken.
2.3. Margin of Exposure Determination
Using the published toxicological literature, we evaluated the dose-response relationship between PCB congeners and thyroid-related adverse health effects and identified a Point of Departure (POD) from which we derive a human-equivalent concentration (PODHEC) in air. The POD is defined as the point on a dose-response curve established from toxicological or epidemiologic data that corresponds to an estimated low effect level or no effect level, marking the beginning of extrapolation to a relevant human dose or concentration. The principal studies used to develop the dose-response curve for POD determination were selected using an approach similar to that described in Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (Canter et al., 1998; U.S. Environmental Protection Agency, 1994). Using EPA default assumptions, we convert the rodent-based POD to the human equivalent concentration (PODHEC). The Margin of Exposure (MOE) (U.S. EPA, 2012) is then calculated as the ratio of the PODHEC to the geometric mean of the measured ambient airborne PCB concentrations surrounding NBH.
3. Results
3.1. Exposure Assessment
PCB concentration measurements were averaged over the four rounds of sampling to determine an exposure metric for each sampling location. Over the study period at the 18 sampling locations, total PCB air concentrations ranged from 0.4 ng/m3 to 38.6 ng/m3 with an average across sampling locations of 7.0 ng/m3 (standard deviation: 9.3 ng/m3) and a geometric mean of 3.1 ng/m3. Using non-parametric statistical tests, we concluded that the median concentration of total PCBs at each site and overall was not significantly different (p< 0.05) across the four rounds of testing. Therefore, the four rounds were combined for the risk estimate calculations.
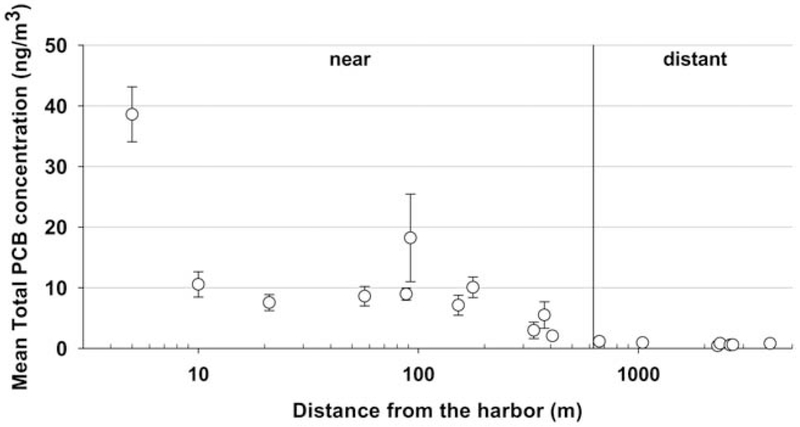
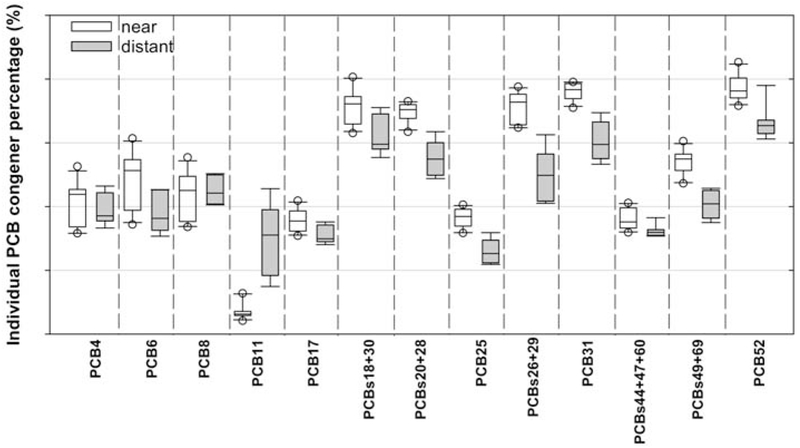
As the distance from the harbor increases, total ambient airborne PCB concentrations decrease out to 625 meters (Figure 1). The pattern illustrates exponential decrease due to dispersion. The arithmetic and geometric mean of the 11 sampling locations within 625 meters of the harbor are 10.9 ng/m3 and 8.1 ng/m3. respectively. We consider a distance further than 625 meters to be background concentrations of total PCBs since concentrations remain relatively flat between 625 and 4000 meters. The arithmetic and geometric means of the seven sampling locations further than 625 meters from NBH are 0.7 ng/m3. We observed a decrease of the 12 most present congeners in the air, from “near’ to “distant” sites, whereas PCB11 increased in the air (Figure 2).
Figure 1.
Relationship between total PCB ambient air concentrations (ng/m3) and distance (m) from New Bedford Harbor
Figure 2.
Comparison of most dominant congeners/congener groupings as percentage of total PCBs at “near” and “distant” locations from New Bedford Harbor
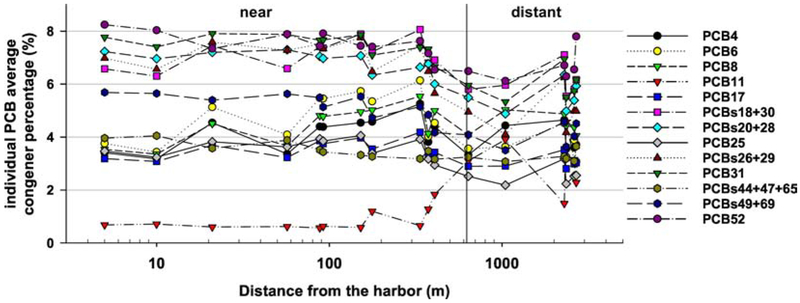
Our previous analysis of data collected in the first three rounds of this study found that the PCB concentrations measured at all 18 locations had a very strong Aroclor 1242/1016 signal (Martinez et al., 2017). This is consistent with the historical use of these two Aroclor mixtures in the vicinity The analysis also found that the Aroclor 1242/1016 signal was the most pronounced at locations closest to the harbor across all sampling rounds. Although all congeners were measured, we identified twelve dominant PCB congeners or congener groupings in air surrounding NBH. PCBs 4, 6, 8, 17, 18+30, 20+28, 25, 26+29, 31, 44+47+65, 49+69 and 52 account for, on average, 63% of the total PCB ambient air exposure at NBH (54% minimum, 71% maximum). The relationship between dominant congeners and distance to the shoreline of NBH is shown in Figure 3. Overall there does not appear to be a relationship between dominant congeners/congener groupings as percent of total PCBs, and distance to the shoreline of NBH. There is very little spatial variation in relative abundance of PCBs between sampling locations, suggesting that all twelve of these congeners/congener groupings are important contributors. It is noteworthy that the relative contribution of PCB11 to total PCBs increases with distance from NBH unlike the other congeners shown. Subsequent drops and spikes in the percent of PCB11 to total PCBs results in relative increases and decreases of the other twelve congeners/congener groupings. The presence of PCB11 at these distances may be due to its presence in, and volatilization from, modern paint pigment (Hu et al., 2008; Jahnke & Hornbuckle, 2019). NBH does not appear to be a source of PCB11. These findings provide evidence that these twelve congeners or congener groupings are important tracers of PCBs released from NBH, and may also be tracers of PCBs associated with production and use of electronic capacitors, the original source of PCBs in NBH.
Figure 3:
Relationship between most dominant congeners/congener groupings as percent of total PCBs and distance from New Bedford Harbor
Analysis of the air monitoring data suggest that PCBs are correlated with distance to the harbor and that concentrations significantly leveled off, reaching background levels, at approximately 625 meters from the harbor. Therefore, we conducted the risk assessment with two separate exposure areas, dividing the sampling locations into those near to the harbor (<625 meters from the harbor) and those distant to the harbor (>625 meters from the harbor). Separate risk calculations were conducted for these two areas, based on the geometric means and are referred to as “near” and “distant”.
3.2. Hazard Assessment
To derive a relevant Point of Departure (POD) from which to estimate health risk, we identified evidence from the epidemiology and toxicological studies that support the thyroid as a target organ of PCB toxicity, with a focus on PCB congeners or mixtures relevant to those measured in the air at NBH. The availability of toxicological studies that are conducted with human-relevant mixtures of PCBs in air is limited, although there are multiple in vivo oral exposure and human epidemiological studies that are used to provide supporting evidence of an association between PCB exposure and adverse health effects.
Evidence is accumulating for a thyroid-dependent mechanism for effects of PCBs on the developing hypothalamic neuroendocrine systems (reviewed in Gauger et al., 2007 and Gore, Krishnan, & Reilly, 2019). Epidemiologic studies provide evidence of association between reduced thyroid hormone levels, or impaired thyroid hormone action, in infants and children as a result of prenatal or early life exposure (Curtis et al., 2019; Dallaire et al., 2008 2009a, 2009b; Dirinck et al., 2016). As previously stated, EPA-derived screening levels used to assess PCBs near NBH are based on cancer risks. However, adverse health effects of PCB exposures, in addition to cancers have been extensively studied and evidence is available for outcomes that are influenced by thyroid hormone physiology, including impaired neurodevelopment (Forns et al., 2018; Grandjean et al., 2001; Sagiv, Thurston, Bellinger, Altshul, & Korrick, 2012; Verner et al., 2015), increase in metabolic diseases (Aminov, Haase, Rej, et al., 2016; Zani et al., 2019) and decreased birthweight (Govarts et al., 2012). Commonly observed health outcomes in epidemiologic studies, including those in which inhalation is a documented route of exposure to mixtures of PCBs, support endocrine-related outcomes including diabetes (Aminov et al., 2016a; Jorgensen et al., 2008; Lee et al., 2010; Philibert et al., 2009; Rylander et al. 2015; Wang et al. 2008), and altered thyroid volume and function (Alvarez-Pedrerol et al., 2008; Darnerud et al., 2010; Langer et al., 2007, 2008; Leijs et al., 2012; Sandau et al., 2002; Turyk et al., 2006). A number of these toxicants may also interfere with the hypothalamic-pituitary-thyroid regulatory axis and be associated with a reduced serum thyroxine (T4 or T3) concentration, but with a normal range of thyroid stimulating hormone (TSH). Animal and epidemiologic studies that examine brain development, indicate the potential for more subtle disruption of local thyroid hormone production or action that may be difficult to detect based on circulating thyroid hormone levels (Brent, 2010). The thyroid is a target organ of PCB toxicity, as documented by an expanding body of evidence that demonstrates that PCBs and other organochlorines influence thyroid function.
There is also a body of literature providing evidence that several environmental toxicants including PCBs can interfere with thyroid hormone, resulting in elevation in serum TSH or a reduction in serum T4 or T3 in animal models (Bansal & Zoeller, 2008; Lau, Walter, Kass, & Puschner, 2017; L. Martin & Klaassen, 2010). A number of these toxicants may also interfere with the hypothalamic–pituitary–thyroid regulatory axis and be associated with a reduced serum T3 or T4 concentration, but with a normal range of TSH.
Most of the animal studies of lower chlorinated congeners and thyroid effects have been conducted through oral administration of Aroclors. Of the twelve most dominant congeners in NBH air samples, only PCB congeners 28 and 52 have been studied in the context of thyroid effects. Most relevant oral exposure studies have been conducted using mixtures of lower chlorinated PCBs: Aroclor 1016, Aroclor 1221, and Aroclor 1242 and we present some of the evidence for PCBs effects on thyroid here. Of the papers that examined changes in thyroid hormone levels, only one examined thyroid stimulating hormone (TSH), a biologically relevant measure of thyroid function (L. Martin & Klaassen, 2010) . Mayes et al., (1998) administered Aroclors 1016, 1242, 1254, and 1260 for 24 months to male and female Sprague-Dawley rats. Aroclors were suspended in hexane, combined with a small amount of diet (the “premix”). The hexane was evaporated, and the premix was blended with diet to achieve the desired final PCB concentrations ranging from 25 to 200 ppm. The authors report that the thyroid glands of male animals receiving Aroclors 1242, 1254, and 1260 were enlarged in a non-dose-related manner. This study showed a dose-dependent incidence of thyroid gland follicular cell adenomas in males for Aroclors 1242, 1254, and 1260, with the incidence being uniform across dose groups and Aroclor mixtures. While the authors report that the neoplasms are likely due to hypersecretion of TSH, they do not discuss the possibility that changes in circulating levels of thyroid hormones may occur as a direct effect of the PCBs administered.
Crofton et al. (2005) dosed 23-day old female Long Evans rats by oral gavage for four consecutive days with 12 individual PCBs, including PCB-126, PCB-153, NBH-relevant PCB-28 and PCB-52, as well as mixtures of the 12. Serum total T4 was measured via radioimmunoassay in samples collected 24 hours after the last dose. Doses of individual chemicals were associated with a 30% thyroid hormone decrease from control (ED30). In particular, both PCB-126 and PCB-153 demonstrated dose-dependent decreases in total T4, although PCB-126 was able to do this at a lower dose than PCB-153 and the mixture was shown to cause decreases in T4 concentrations.
In order to determine the effects of individual PCB congeners on serum T3, T4 and TSH, Martin and Klaassen, (2010) administered Aroclor 1254 or 1242, PCB-95, PCB-99, PCB-118 or PCB-126 in corn oil via oral gavage to male Sprague-Dawley rats for seven consecutive days. Rats were necropsied 24 h after the last dose. Serum total T4 and free T4 hormone levels were evaluated by radioimmunoassay and both were dramatically reduced in response to each of the seven treatments in a dose-dependent manner. Marked T4 reductions occurred in response to Aroclor 1254, PCB-99, and PCB-118. Serum TSH was not significantly affected by any of the compounds administered.
Martin et al. (2012) examined the effects of oral administration of 32 mg/kg-day of Aroclor 1242 to male Sprague-Dawley rats. Aroclor was administered via corn oil solution through oral gavage for seven consecutive days. Effects documented in the treated groups included decreases in serum thyroid hormones T4 and T3, although TSH was not measured.
Four in vivo rat inhalation studies evaluated Aroclor mixtures of direct relevance to NBH communities. One examined the uptake and elimination of Aroclor 1242 (Hu et al., 2010). Another (Casey et al., 1999), compared the adverse outcomes of inhalation versus ingestion of Aroclor 1242. The third, Hu et al., (2012), exposed female Sprague-Dawley rats via nose-only inhalation to a mixture of Aroclors 1242/1254 and PCB11 at an average air concentration of 520±10 μg/m3. The final inhalation-relevant study examined the difference in adverse outcomes of whole body exposure as compared to nose-only exposure to an Aroclor 1242/1254 + PCB11 mixture (Hu et al., 2015). Of the 12 most dominant congeners in NBH air samples, only PCB congeners 28 and 52 have associated toxicology studies.
Casey et al. (1999) compared whole body-inhalation of 0.9 μg/m3 and oral administration of 0.436 ppm of Aroclor 1242 to male Sprague-Dawley rats over 30 days and examined the impacts on thyroid hormones and behavioral outcomes. Both exposure groups displayed decreases in rearing activity and ambulation and increases in T4 and T3 as compared with the control group, with the group exposed via inhalation showing larger increases as compared to the oral exposure group. Additionally, the group exposed via inhalation displayed an increase in intracellular vacuolization of follicular epithelial cells in the thyroid gland. TSH was not measured. Casey et al. (1999), reported elevated T3 and T4 accompanied by increased intracellular vacuolization of follicular epithelial cells of thyroid gland after inhalation exposure to 0.9 ug/m3 volatilized Aroclor 1242. Casey et al. (1999) does not adequately describe exposure information including breathing zone concentrations and evidence supporting a uniform distribution of chemical within the exposure chamber. Furthermore, control animals were not exposed to air under the same conditions as animals exposed to PCBs by inhalation. Compared to animals in the control group, inhalation-exposed animals lived with reduced air flow (three changes of chamber air/h for exposed animals compared to fifteen for control animals) as well as increased humidity and temperature. These factors could all potentially contribute to differences in the baseline level of stress experienced by animals in these two groups, which could translate into differences in thyroid hormone levels and behavioral outcomes beyond those related to the PCB exposure. It is important to note that the animals may very well have been exposed to higher levels of PCBs than are reported by the study and may have been exposed not only by inhalation, but also by oral exposure as a result of grooming after PCB deposition on their fur.
In a short-term study intended to determine pharmacokinetic data for inhaled PCBs, Hu et al. (2010) administered Aroclor 1242 to male Sprague-Dawley rats in a nose-only inhalation system at 2.4 mg/m3 for 2 hours or at 8.2 mg/m3 for 2 h each of 10 consecutive days. Pulmonary immune markers and PCB tissue levels were measured and demonstrated that the airborne PCB mixture contributed significantly to the body burden of lower-chlorinated congeners. Rats exposed in the multi-day study gained significantly less weight over the 10-day exposure period compared to sham-exposed animals. However, there were no significant changes observed in pulmonary immune markers. PCB levels were similar in lung, liver, and adipose tissue, lower in brain, and lowest in blood.
Hu et al. (2012) exposed female Sprague-Dawley rats via nose-only inhalation to a mixture of Aroclors 1242/1254 and PCB11 at an average air concentration of 520±10 μg/m3. The rats were exposed two hours per day for the first week of exposure, and then 1.5 hours per day for weeks 2-4. The dose was estimated to be 134 μg total PCBs per rat [reported as 446 μg/kg-day by Hu et al. (2015)]. The rats did not display any difference in their overall growth rate as compared to controls, nor an increase in enzymes that metabolize PCBs in the livers of the exposed animals, nor an increase in enzymes that metabolize PCBs in the livers of the exposed animals, nor a change in plasma thyroid hormone levels [as reported by (Hu et al., 2015)]. In the 2012 study by Hu et al., female rats exposed to a dose of 446 μg/kg-b.w. were found to have no changes in weight gain or plasma thyroid hormone levels compared with control, although the authors. Reduced glutathione (GSH) is considered to be one of the most important scavengers of reactive oxygen species and its ratio with oxidized glutathione (GSSG) may be used as a marker of oxidative stress. The GSSG/GSH ratio was slightly elevated in the exposed group as compared to the controls, and the total hepatic glutathione was decreased (Hu et al., 2012).
Hu et al. (2015) exposed 11 week old female Sprague-Dawley rats through whole body and nose-only inhalation to a mixture of Aroclor 1242 and Aroclor 1254 supplemented with PCB 11 (64.0%:34.5%:1.5%, w/w). Nose-only exposed rats gained significantly less weight over the 28-day exposure period compared to controls. Plasma total thyroxine (T4) levels were significantly reduced in exposed animals compared to controls (p = 0.0006) and compared to both sham- and PCB-exposed female Sprague–Dawley rats. No change of free T4, total triiodothyronine (T3) or free T3 was observed. TSH levels were not measured. The nose-only group exposure was estimated at 1980 ug/kg-day and also displayed an increase in the formation of malondialdeyede (MDA) in the liver. The whole-body exposure was estimated at 1320 ug/kg-day and displayed paler cytoplasm in centrilobular hepatocytes as compared to the periportal hepatocytes compared with the nose-only exposures (Hu et al., 2015).
Most studies are limited in scope to toxicodynamics and few examine both toxicokinetics and toxicodynamics of PCB mixtures. This is an important distinction when attempting to understand the interaction of PCBs with the multiple components of the thyroid signaling pathways. Of the relevant toxicological literature, only four of the in vivo animal studies administered the PCBs via inhalation (Casey et al., 1999; X. Hu et al., 2010, 2012, 2015). Of the relevant congeners in NBH air, those most studied were PCB 28 and PCB 52, both of which are considered to be indicator PCBs because of their prevalence in both environmental samples and human biospecimens (Boalt et al., 2013). We conclude that changes in thyroid hormone levels in response to PCBs reported in the inhalation studies are supported by observations made in epidemiologic studies and in toxicological studies of oral exposure, which identify the thyroid gland as a target organ for PCB toxicity.
3.3. Margin of Exposure Estimates
We used a margin of exposure approach to determine the health risk to humans exposed to airborne PCBs from NBH. This margin relates the range of concentrations shown to cause adverse effects in animal models with airborne PCB concentrations measured in the “near” and “distant” areas surrounding New Bedford Harbor. MOEs are calculated as the ratio of a POD (e.g., Lowest Observable Adverse Effect Level) to an exposure concentration (e.g., a representative concentration of airborne PCBs surrounding NBH). Large MOEs, those that are several orders of magnitude of difference between the POD and the exposure concentrations indicate less concern that current exposure levels may be causing adverse health effects in the exposed population. Smaller MOEs indicate a greater level of concern that adverse effects may occur.
Using an approach similar to that described in Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry (Canter et al., 1998; U.S. Environmental Protection Agency, 1994), we identified the POD based on the principal studies relevant to airborne PCB exposure and adverse effects on the thyroid, which included Casey et al. (1999), Hu et al. (2012), and Hu et al. (2015). Effects on thyroid hormone levels were found to be the most consistent in inhalation studies and were well-supported by the results of oral exposure studies; thus, PODs based on changes in thyroid hormone levels were used to estimate the MOEs. While each of these studies has limitations (discussed above and in the Discussion section), nevertheless, all three studies identified changes to the thyroid, which supports the thyroid as sensitive to disruption by airborne PCBs.
These studies were selected because they are most relevant to community inhalation of PCBs in ambient air at NBH. The concentrations (in air) used in these studies range from [(0.9 ug/m3) to (520 +/− 10 ug/m3 - 533 ug/m3)] and reflect LOAELs as the PODs since effects on the thyroid in exposed versus unexposed animals were observed at all experimental airborne PCB concentrations (except in Hu et al., 2012). We derived a range of human equivalent PODs (PODHEC) from the PODs identified in the animal studies, as shown in Table S2. These range from 0.9 ug/m3 as the minimum PODHEC (POD HEC 1) to the maximum PODHEC (POD HEC 2) of 76 ug/m3 . Additionally, we chose not to rely on data from Hu et al. (2010) because this study used high doses of PCBs and very short exposure periods, neither of which are representative of airborne PCBs surrounding NBH.
The Margin of Exposure (MOE) is determined by dividing a point of departure (POD), derived from dose–response data by human exposure concentrations using the following equation:
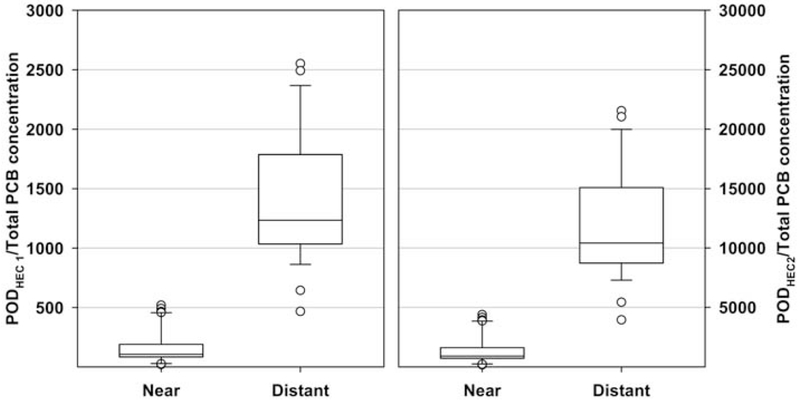
As human exposure concentration declines, MOEs become larger, likewise, as human exposure concentration increases, MOEs become smaller. The “near” and “distant” designations are described previously and reflect the concentrations of total PCBs measured in air as a function of geographic distance from NBH. The MOEs generated for the lower and upper values from the ranges of PODHECs and exposure concentrations for the “near” and “distant” areas at New Bedford Harbor are presented in Figure 4 to provide a range of risks posed to humans exposed to airborne PCBs from NBH. As the PODHEC increases, the MOE increases yet as the exposure concentration increases, the MOE decreases. The minimum PODHEC (POD HEC 1) with the maximum PCB concentration measured shows an MOE of 20, just over one order of magnitude higher relative to “1”. Additionally, the maximum PODHEC (POD HEC 2) compared with the minimum PCB concentration measured shows an MOE of 10,000.
Figure 4:
Margin of Exposure (MOE) at “Near” and “Distant” Locations. Left Panel: PODHEC 1 and Right Panel: PODHEC 2
4. Discussion
Based on the concentrations of airborne PCBs measured around NBH, decreases in thyroid hormone levels are possible in people living adjacent to the harbor, particularly in “near” harbor locations. Thyroid hormones are essential for normal behavioral, intellectual, and neurologic development and inadequate levels of these hormones has a negative effect on brain development. In a cohort of children born to mothers residing adjacent to NBH, moderate associations between PCB and p,p′-DDE levels and Activity Deficit Hypertension Disorder (ADHD)-like behaviors have been reported (Sagiv et al., 2010). Our findings are based on: (1) robust, empirically-measured exposure concentrations and (2) less certain toxicological evidence from published studies. We compared measured concentrations of PCBs in air around NBH with estimated concentrations of PCBs that are associated with adverse outcomes in previously published literature. The scientific evidence to date strongly supports changes to thyroid hormone levels as an important mode of action by which these (and the more heavily chlorinated) PCBs act on mammalian systems (Miller, Crofton, Rice, & Zoeller, 2009). While a more detailed evaluation of the adverse outcome pathway(s) for inhaled PCBs is warranted, it is beyond the scope of this paper
The dose-response calculations were subject to key limitations that contribute uncertainty to the MOEs. The most important limitation is the selected toxicological studies. While the study by Hu et al (2012) is methodologically stronger than the study by Casey et al (1999) it did not identify changes in thyroid hormones levels. Casey et al (1999) demonstrate an increase in T3 and T4 levels in adolescent rats, rather than a decrease as has been reported in the other studies. Elevation in these hormone levels may be transient, as suggested by the authors. Had TSH been measured, more information about the thyroid hormone regulation could have been determined. The implications of elevated thyroid hormones would be borne out in observed rates of hyperthyroidism, or transient increases in T3/T4 and may not be as severe in the developing fetus or child. In contrast, Hu et al (2015) does identify decreases in T4 levels compared with controls, suggesting that the reduction in T4 level may be associated with a PCB dose level between the doses of 446 and 1320 μg/kg body weight. All of the studies rely on limited dose ranges and durations that may be less accurate than reported. We used a default dosimetric adjustment factor for respiratory tract region for a complex mixture that may be an aerosol, rather than a gas. By using the default adjustment factor, we may have over-estimated the PODHEC, resulting in an overestimation of the MOE. In addition, the PCB concentration reported by Hu et al. (2012) may have been underestimated, in which case the resultant PODHEC would be lower, resulting in a lower MOE. The lowest POD identified may in fact not be a LOAEL, but a NOAEL, because the dose at which this occurs did not result in a change in plasma thyroid hormone levels and the POD from Hu et al (2015) has less uncertainty associated with it. In all cases, the implication would be that risk is over-estimated. Our straight-forward approach to derivation provides a starting point for a more data-driven POD that could be based on a Benchmark Dose or one that uses pharmacokinetic data to better estimate a POD. In addition, existing body burdens of PCBs should also be considered as an on-going source of internal exposure, as should consumption of foods contaminated with PCBs. Human exposures to PCBs in air occur through inhalation and dermal contact (Weschler & Nazaroff, 2012) and PCBs are absorbed through the skin (Garner & Matthews, 1998). It is beyond the scope of this analysis to evaluate the predicted uptake through skin based on simple pharmacokinetic models, but the total uptake of PCBs from the ambient air may be under-estimated for people living adjacent to NBH for decades.
We have shown the relevance of non-cancer outcomes for PCBs, and yet we are unable to assess risk using EPA methodologies because there is no non-cancer toxicity value (RfC) published by the US EPA for inhalation of PCBs. The exposure calculations in this risk assessment were subject to key limitations that contribute uncertainty to the estimates. First, the exposure calculations are based on the assumption that concentrations of ambient PCBs around New Bedford Harbor are constant over time. However, if concentrations decrease over time as a result of clean-up of the harbor, the risk estimates in this assessment will be overestimates. Conversely, if remediation activities create additional exposures, these will be underestimates. There is evidence of the former, but not the latter. Active dredging does not appear to affect airborne PCBs emitted from NBH. We measured airborne PCBs were measured during active dredging during the fourth four-week sampling period (July-August 2016) and they were not statistically different from those measured in air samples collected during the previous three sampling periods (July-November 2015). This finding begins to address community concerns about air emissions during this period of dredging (Tomsho et al., 2018). Our modeling studies indicate that removal of contaminated sediment over the last ten years has actually reduced emissions of airborne PCBs (Martinez et al., 2017).
Exposure estimates for individuals living farther than 2,700 meters from the harbor are not assessed in this risk assessment. However, the risk for these individuals (based on exposure to PCBs from the harbor alone) is unlikely to be greater than the estimates described for people living near the harbor given the concentrations of PCBs reported previously (Martinez et al., 2017). While most individuals spend approximately 80-90% of their day indoors (U.S. EPA, 2011), we assume that people were exposed to outdoor air concentrations every day for 24 hours each day. However, this assumption is not necessarily an overestimate of exposure, since indoor and ambient air exchange is highly variable and difficult to predict (Weschler & Nazaroff, 2008; Yamamoto et al., 2010). We recognize that this assessment is not a cumulative risk assessment as described by Payne-Sturges et al. (2015) and therefore does not evaluate the full suite of stressors impacting the residents living and working in this Environmental Justice community adjacent to the harbor.
A major gap in the assessment of risk posed by PCBs is the lack of a RfC used for evaluation of non-cancer outcomes from inhalation of PCBs. In lieu of the RfC, we calculated the MOE for people living near NBH. While this risk assessment is limited in scope, we have attempted to use a site-specific, data-driven approach to calculating our MOE. The strengths of the calculated MOEs includes the relevance and study design of the critical studies on which the PODHECs are based and the data-driven exposure assessment. Notably, we incorporated previously unavailable ambient air concentrations of PCB congeners and used longer duration of residence reflective of many local residents. Our analysis focused on the subset of PCB congeners most prevalent in air samples collected from around NBH. However, prevalence by weight does not necessarily mean that potential health impacts are limited to these congeners. This is especially relevant since existing body burdens of bioaccumulated PCBs and ongoing exposures through diet may play an integral role in the physiological response to additional inhalation exposure to PCBs. Despite these limitations, we show that non-cancer outcomes, specifically changes in thyroid hormone levels are possible in people who reside closest to the harbor. As NBH clean-up continues and the major source of PCBs is removed, it is expected that exposures to PCBs in ambient air will decrease with time.
5. Conclusion
Residents living near New Bedford Harbor are exposed to PCBs through multiple pathways, including consumption of fish and shellfish caught from the harbor, inhalation of volatile PCB congeners in ambient (outdoor) air, and possibly via indoor air. This risk assessment estimates the health risk to people living around New Bedford Harbor with a focus on ambient air exposures (Martinez et al., 2017) . Specifically, PCB congeners that are present in ambient air in NBH communities may result in thyroid hormone level changes in response to inhaled PCBs. The estimated MOEs closest to NBH indicate the potential for a community at risk of thyroid mediated outcomes. This finding is consistent with observations made in previously published epidemiologic studies, which identify the thyroid as a target organ for PCB toxicity.
Supplementary Material
Highlights:
Community concerns focus on health risks associated with PCBs in the air.
PCBs were highest in ambient air closest to the New Bedford Harbor Superfund Site.
Toxicological data were used to derive a thyroid-based effect level for a Margin of Exposure (MOE) evaluation.
Health risks are highest among people living adjacent to New Bedford Harbor.
Acknowledgements
The authors thank Karen Vilandry of Hands Across the River Coalition (HARC) and the residents of New Bedford, Fairhaven, Dartmouth, and Acushnet, MA, who hosted the air samplers on their properties and who actively participated in this work. We thank Sylvia Broude and Claire Miller with Toxics Action Center who helped recruit and communicate with residents throughout the project, and attorneys Staci Rubin and Richard Juang, formerly/representing Alternatives for Community and Environment. We thank D. Lederer at US EPA Region 1 and P. Craffey at MassDEP for sharing information about the dredging processes and data related to the NBH Superfund Site, and the following reviewers of our data and analyses, listed in alphabetical order of last name: David Carpenter, PhD, Stephen Lester, MSc, Harlee Strauss, PhD
Funding: This study was supported by grants from the National Institute of Environmental Health Sciences to the Boston University Superfund Research Program NIEHS/NIH P42 ES007381-19S1 and the University of Iowa Superfund Research Program NIEHS/NIH P42 ES013661. ZEP and KB are supported by a grant from the National Institute of Environmental Health Sciences (T32 ES014562).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All of the authors declare that they do not have any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence this work.
Citations
- Alvarez-Pedrerol M, Ribas-Fito N, Torrent M, Carrizo D, Grimalt JO, & Sunyer J (2008). Effects of PCBs, p,p’-DDT, p,p’-DDE, HCB and -HCH on thyroid function in preschool children. Occupational and Environmental Medicine, 65(7), 452–457. 10.1136/oem.2007.032763 [DOI] [PubMed] [Google Scholar]
- Aminov Z, Haase R, & Carpenter DO (2016). Diabetes in Native Americans: elevated risk as a result of exposure to polychlorinated biphenyls (PCBs). Reviews on Environmental Health, 31(1). 10.1515/reveh-2015-0054 [DOI] [PubMed] [Google Scholar]
- Aminov Z, Haase R, Rej R, Schymura MJ, Santiago-Rivera A, Morse G, … Carpenter DO. (2016). Diabetes prevalence in relation to serum concentrations of polychlorinated biphenyl (PCB) congener groups and three chlorinated pesticides in a Native American population. Environmental Health Perspectives, 124(9), 1376–1383. 10.1289/ehp.1509902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, & Zoeller RT (2008). Polychlorinated biphenyls (Aroclor 1254) do not uniformly produce agonist actions on thyroid hormone responses in the developing rat brain. Endocrinology, 149(8), 4001–4008. 10.1210/en.2007-1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra K, Fabian MP, & Scammell MK (2018). Consumption of Contaminated Seafood in an Environmental Justice Community: A Qualitative and Spatial Analysis of Fishing Controls. Environmental Justice, 11(1), 6–14. 10.1089/env.2017.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boalt E, Nyberg E, Bignert A, Hedman J, Danielson S, & Group C (2013). HELCOM Core Indicator of Hazardous Substances Polychlorinated biphenyls (PCB) and dioxins and furans.
- Brent GA (2010). Environmental exposures and autoimmune thyroid disease. Thyroid: Official Journal of the American Thyroid Association, 20(7), 755–761. 10.1089/thy.2010.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BU Superfund Research Program. (2015). SRPs and partners meet with the community to plan air monitoring study » SPH | Boston University. [Google Scholar]
- Canter D, Lorber M, Braverman C, Warwick R, Walsh J, & Washburn S (1998). Determining the “Margin of Incremental Exposure” An Approach to Assessing Non-Cancer Health Effects of Dioxins. Organohalogen Compounds, 38. [Google Scholar]
- Casey AC, Berger DF, Lombardo JP, Hunt A, & Quimby F (1999). AROCLOR 1242 INHALATION AND INGESTION BY SPRAGUE-DAWLEY RATS. Journal of Toxicology and Environmental Health, Part A, 56(5), 311–342. 10.1080/009841099158033 [DOI] [PubMed] [Google Scholar]
- Census Bureau, U. (2015). 2011-2015 American Community Survey 5-Year Estimates.
- Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, … DeVito MJ. (2005). Thyroid-hormone-disrupting chemicals: Evidence for dose-dependent additivity or synergism. Environmental Health Perspectives, 113(11), 1549–1554. 10.1289/ehp.8195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis SW, Terrell ML, Jacobson MH, Cobb DO, Jiang VS, Neblett MF, … Marcus M. (2019). Thyroid hormone levels associate with exposure to polychlorinated biphenyls and polybrominated biphenyls in adults exposed as children. Environmental Health, 18(1). 10.1186/s12940-019-0509-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, Dewailly É, Ayotte P, Muckle G, Laliberté C, & Bruneau S (2008). Effects of prenatal exposure to organochlorines on thyroid hormone status in newborns from two remote coastal regions in Quebec, Canada. Environmental Research, 108(3), 387–392. 10.1016/j.envres.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Dallaire R, Dewailly É, Pereg D, Dery S, & Ayotte P (2009). Thyroid function and plasma concentrations of polyhalogenated compounds in inuit adults. Environmental Health Perspectives, 117(9), 1380–1386. 10.1289/ehp.0900633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire R, Muckle G, Dewailly É, Jacobson SW, Jacobson JL, Sandanger TM, … Ayotte P. (2009). Thyroid hormone levels of pregnant inuit women and their infants exposed to environmental contaminants. Environmental Health Perspectives, 117(6), 1014–1020. 10.1289/ehp.0800219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Lignell S, Glynn A, Aune M, Tomkvist A, & Stridsberg M (2010). POP levels in breast milk and maternal serum and thyroid hormone levels in mother-child pairs from Uppsala, Sweden. ENVIRONMENT INTERNATIONAL, 36(2), 180–187. 10.1016/j.envint.2009.11.001 [DOI] [PubMed] [Google Scholar]
- Dirinck E, Dirtu AC, Malarvannan G, Covaci A, Jorens PG, & Van Gaal LF (2016). A preliminary link between hydroxylated metabolites of polychlorinated biphenyls and free thyroxin in humans. International Journal of Environmental Research and Public Health, 13(4). 10.3390/ijerph13040421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Stigum H, Høyer BB, Sioen I, Sovcikova E, Nowack N, … Eggesbø M. (2018). Prenatal and postnatal exposure to persistent organic pollutants and attention-deficit and hyperactivity disorder: A pooled analysis of seven European birth cohort studies. International Journal of Epidemiology, 47(4), 1082–1097. 10.1093/ije/dyy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CE, & Matthews HB (1998). The effect of chlorine substitution on the dermal absorption of polychlorinated biphenyls. Toxicology and Applied Pharmacology, 149(2), 150–158. 10.1006/taap.1998.8370 [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, & Zoeller RT (2007). Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environmental Health Perspectives, 115(11), 1623–1630. 10.1289/ehp.10328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Krishnan K, & Reilly MP (2019). Endocrine-disrupting chemicals: Effects on neuroendocrine systems and the neurobiology of social behavior. Hormones and Behavior, 111, 7–22. 10.1016/j.yhbeh.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, … Bonde JP. (2012). Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): A meta-analysis within 12 European birth cohorts. Environmental Health Perspectives, 120(2), 162–170. 10.1289/ehp.1103767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, Burse VW, Needham LL, Storr-Hansen E, Heinzow B, … White RF. (2001). Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicology and Teratology, 23(4), 305–317. 10.1016/S0892-0362(01)00155-6 [DOI] [PubMed] [Google Scholar]
- Hu D, Martinez A, & Hombuckle KC (2008). Discovery of Non-Aroclor PCB (3,3’-Dichlorobiphenyl) in Chicago Air. Environmental Science & Technology, 42(21), 7873–7877. 10.1021/es801823r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler H-J, Gibson-Corley K, & Thome PS (2015). Toxicity Evaluation of Exposure to an Atmospheric Mixture of Polychlorinated Biphenyls by Nose-Only and Whole-Body Inhalation Regimens. Environmental Science & Technology!, 49(19), 11875–11883. 10.1021/acs.est.5b02865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler H-J, Hu D, Hornbuckle K, & Thome PS (2012). Subchronic inhalation exposure study of an airborne polychlorinated biphenyl mixture resembling the Chicago ambient air congener profile. Environmental Science {&} Technology, 46(17), 9653–9662. 10.1021/es301129h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler HJ, Hu D, Kania-Korwel I, Hombuckle KC, & Thome PS (2010). Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environmental Science and Technology, 44(17), 6893–6900. 10.1021/es101274b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen ME, Borch-Johnsen K, & Bjerregaard P (2008). A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia, 51(8), 1416–1422. 10.1007/s00125-008-1066-0 [DOI] [PubMed] [Google Scholar]
- Langer P, Kocan A, Tajtakova M, Petrik J, Chovancova J, Drobna B, … Klimes I. (2007). Fish from industrially polluted freshwater as the main source of organochlorinated pollutants and increased frequency of thyroid disorders and dysglycemia. Chemosphere, 67(9), S379–85. 10.1016/j.chemosphere.2006.05.132 [DOI] [PubMed] [Google Scholar]
- Lau G, Walter K, Kass P, & Puschner B (2017). Comparison of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in the semm of hypothyroxinemic and euthyroid dogs. Peer J, 2017(9). 10.7717/peeq.3780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, & Jacobs DR (2010). Low Dose of Some Persistent Organic Pollutants Predicts Type 2 Diabetes: A Nested Case-Control Study. Environmental Health Perspectives, 118(9), 1235–1242. 10.1289/ehp.0901480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GM, Christensen K, Maddaloni M, & Phillips LJ (2015). Evaluating health risks from inhaled polychlorinated biphenyls: Research needs for addressing uncertainty. Environmental Health Perspectives, 123(2), 109–113. 10.1289/ehp.1408564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijs MM, ten Tusscher GW, Olie K, van Teunenbroek T, van Aalderen WMC, de Voogt P, … Koppe JG. (2012). Thyroid hormone metabolism and environmental chemical exposure. Environmental Health : A Global Access Science Source, 11 Suppl 1, S10 10.1186/1476-069X-11-S1-S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Wilson DT, Reuhl KR, Gallo MA, & Klaassen CD (2012). Polychlorinated biphenyl congeners that increase the glucuronidation and biliary excretion of thyroxine are distinct from the congeners that enhance the serum disappearance of thyroxine. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 40(3), 588–595. 10.1124/dmd.111.042796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, & Klaassen CD (2010). Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicological Sciences, 117(1), 36–44. 10.1093/toxsci/kfq187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Awad AM, Herkert NJ, Heiger-Bemays W, Scammell MK, & Hombuckle KC (2019). Airborne polychlorinated biphenyl congener congener concentrations from Bedford New Bedford, MA, 2015-2016. Retrieved November 11, 2019, from https://doi.pangaea.de/10.1594/PANGAEA.902925
- Martinez A, Hadnott BN, Awad AM, Herkert NJ, Tomsho K, Basra K, … Hombuckle KC. (2017). Release of Airborne Polychlorinated Biphenyls from New Bedford Harbor Results in Elevated Concentrations in the Surrounding Air. Environmental Science & Technology Letters, acs.estlett.7b00047 10.1021/acs.estlett.7b00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes BA, McConnell EE, Neal BH, Brunner MJ, Hamilton SB, Sullivan TM, … Moore JA. (1998). Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures aroclors 1016, 1242, 1254, and 1260. Toxicological Sciences, 41(1), 62–76. 10.1006/toxs.1997.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Crofton KM, Rice DC, & Zoeller RT (2009). Thyroid-disrupting chemicals: Interpreting upstream biomarkers of adverse outcomes. Environmental Health Perspectives, 117(7), 1033–1041. 10.1289/ehp.0800247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne-Sturges DC, Korfmacher KS, Cory-Slechta DA, Jimenez M, Symanski E, Carr Shmool JL, … Scammell MK. (2015). Engaging Communities in Research on Cumulative Risk and Social Stress-Environment Interactions: Lessons Learned from EPA’s STAR Program. Environmental Justice, 8(6), 203–212. 10.1089/env.2015.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch CE, Voyer RA, Latimer JS, Copeland J, Morrison G, Mcgovem D, … Walker H. (2001). Imprint of the Past: Ecological History> of New Bedford Harbor.
- Philibert A, Schwartz H, & Mergler D (2009). An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p’-DDE and PCBs and fish consumption. International Journal of Environmental Research and Public Health, 6(12), 3179–3189. 10.3390/ijerph6123179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander C, Sandanger TM, Nost TH, Breivik K, & Lund E (2015). Combining plasma measurements and mechanistic modeling to explore the effect of POPs on type 2 diabetes mellitus in Norwegian women. Environmental Research, 142, 365–373. 10.1016/j.envres.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, & Korrick SA (2012). Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environmental Health Perspectives, 120(6), 904–909. 10.1289/ehp.1104372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, & Korrick SA (2010). Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. American Journal of Epidemiology, 171(5), 593–601. 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau CD, Ayotte P, Dewailly E, Duffe J, &Norstrom RJ. (2002). Pentachlorophenol and hydroxylated polychlorinated biphenyl metabolites in umbilical cord plasma of neonates from coastal populations in Quebec. Environmental Health Perspectives, 110(4), 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Morgan RK, Feng W, Lin Y, Li X, Luna C, … Lein PJ. (2019). Comparative Analyses of the 12 Most Abundant PCB Congeners Detected in Human Maternal Serum for Activity at the Thyroid Hormone Receptor and Ryanodine Receptor. Environmental Science and Technology, 53(7), 3948–3958. 10.1021/acs.est.9b00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsho KS, Basra K, Rubin SM, Miller CB, Juang R, Broude S, … Scammell MK. (2018). Community reporting of ambient air polychlorinated biphenyl concentrations near a Superfund site. Environmental Science and Pollution Research, 25(17), 16389–16400. 10.1007/s11356-017-0286-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Freels S, Chatterton RJ, Needham LL, Patterson DGJ, … Persky VW. (2006). Associations of organochlorines with endogenous hormones in male Great Lakes fish consumers and nonconsumers. Environmental Research, 102(3), 299–307. 10.1016/j.envres.2006.01.009 [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. (1994). Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. (October), 1–409. https://doi.org/EPA/600/8-90/066F
- U.S. Environmental Protection Agency. (2011). Exposure Factors Handbook 2011 Edition (Final Report). [Google Scholar]
- U.S. Environmental Protection Agency. (2012). Sustainable Futures / P2 Framework Manual 2012. EPA-748-B12-001 13. Retrieved from https://www.epa.gov/sites/production/files/2015-05/documents/13.pdf
- U.S. Environmental Protection Agency. (2014). New Bedford Harbor Superfund Site, Massachusetts. 1–2. Retrieved from https://semspub.epa.gov/work/01/538674.pdf
- U.S. Environmental Protection Agency. (2017). Table E-l Ambient Air Monitoring Program -Total Detectable PCB Homologues New Bedford Harbor Superfund Site.
- Verner MA, Hart JE, Sagiv SK, Bellinger DC, Altshul LM, & Korrick SA (2015). Measured prenatal and estimated postnatal levels of polychlorinated biphenyls (PCBs) and ADHD-related behaviors in 8-year-old children. Environmental Health Perspectives, 123(9), 888–894. 10.1289/ehp.1408084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira VM, Fabian MP, Webster TF, Levy JL, & Korrick SA (2017). Spatial Variability in ADHD-Related Behaviors among Children Bom to Mothers Residing Near the New Bedford Harbor Superfund Site. American Journal of Epidemiology, 185(10), 924–932. 10.1093/aje/kww208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees DJ, Cullen AC, & Altshul LM (1997). Exposure to Polychlorinated Biphenyls in Residential Indoor Air and Outdoor Air near a Superfund Site, 10.1021/ES9703710 [DOI]
- Wang S-L, Tsai P-C, Yang C-Y, & Guo YL (2008). Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care, 31(8), 1574–1579. 10.2337/dc07-2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, & Nazaroff WW (2008). Semivolatile organic compounds in indoor environments. Atmospheric Environment, 42(40), 9018–9040. 10.1016/j.atmosenv.2008.09.052 [DOI] [Google Scholar]
- Weschler CJ, & Nazaroff WW (2012). SVOC exposure indoors: Fresh look at dermal pathways. Indoor Air, 22(5), 356–377. 10.1111/j.1600-0668.2012.00772.x [DOI] [PubMed] [Google Scholar]
- Wilson C (2015). New Bedford, Draft Final Ambient Air Monitoring Plan for Remediation Activities.
- Yamamoto N, Shendell DG, Winer AM, & Zhang J (2010). Residential air exchange rates in three major US metropolitan areas: Results from the Relationship among Indoor, Outdoor, and Personal Air Study 1999-2001. Indoor Air, 20(1), 85–90. 10.1111/j.1600-0668.2009.00622.x [DOI] [PubMed] [Google Scholar]
- Zani C, Magoni M, Speziani F, Leonardi L, Orizio G, Scarcella C, … Donato F. (2019). Polychlorinated biphenyl serum levels, thyroid hormones and endocrine and metabolic diseases in people living in a highly polluted area in North Italy: A population-based study. Heliyon, 5(6). 10.1016/j.heliyon.2019.e01870 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.