Summary
The zebrafish (Danio rerio) has become a widely used vertebrate model for bacterial, fungal, viral, and protozoan infections. Due to its genetic tractability, large clutch sizes, ease of manipulation, and optical transparency during early life stages, it is a particularly useful model to address questions about the cellular microbiology of host-microbe interactions. Although its use as a model for systemic infections, as well as infections localized to the hindbrain and swimbladder have been thoroughly reviewed, studies focusing on host-microbe interactions in the zebrafish gastrointestinal tract have been neglected. Here, we summarize recent findings regarding the developmental and immune biology of the gastrointestinal tract, drawing parallels to mammalian systems. We discuss the use of adult and larval zebrafish as models for gastrointestinal infections, and more generally, for studies of host-microbe interactions in the gut.
Keywords: Zebrafish, Danio rerio, microbiome, microbiota, host-pathogen interactions, infection model, gastrointestinal tract
1. Introduction
Due to its fecundity, genetic tractability, small size, rapid development, and optical transparency during early development, the zebrafish (Danio rerio) has emerged as one of the most well-used vertebrate model organisms in cellular microbiology. Several reviews have focused on zebrafish as a model for infectious disease (Tobin et al., 2012; Sullivan et al., 2017; Duggan and Mostowy, 2018), but all were focused on systemic disease models or localized infections of the swim bladder or hindbrain; none discussed infections of the gastrointestinal (GI) tract. Over the last few years however, literature in this area has grown considerably, as has literature focusing on the microbiota, and characterization of the transcriptional profiles and cell types of the zebrafish GI tract, allowing us to draw comparisons to the mammalian GI tract. These studies have made it clear that the development and physiology of the zebrafish GI tract bear many similarities to humans. For example, ~70% of human genes have a corresponding orthologue in zebrafish (Howe et al., 2013), and the transcriptional regulatory networks controlling intestinal development and physiology are highly conserved between fish and mammals (Lickwar et al., 2017). These findings highlight the utility of the zebrafish model to study in vivo host-microbe and host-pathogen interactions in the GI tract. With this review, we summarize recent literature highlighting structural and molecular similarities between zebrafish and mammalian GI tract architecture and immune surveillance. We further discuss examples of studies looking at host-microbe and host-pathogen interactions in the zebrafish GI tract, highlighting experimental features and summarizing the most important findings. We hope this review will highlight the utility of the zebrafish model for GI tract- microbe studies, bridging the gap from whole animal studies to single cell and molecular, mechanistic experiments.
2. Organization and development of the zebrafish intestine
2.1. Intestinal segmentation and architecture in zebrafish
The primary functions of the intestine include digestion and absorption of nutrients, and the elimination of waste products. The zebrafish intestine is highly homologous with the mammalian intestine in its development, organization, and function (Pack et al., 1996; Wallace et al., 2005; Ng et al., 2005; Carten and Farber, 2009). Gene expression and transcriptional regulation in intestinal epithelial cells are highly conserved along the segmental regions of the mammalian and zebrafish intestines (Lickwar et al., 2017), and many metabolic functions are also conserved (Schlegel and Gut, 2015; Quinlivan and Farber, 2017). Both zebrafish and mammalian GI tracts are covered by a protective mucus layer that predominantly consists of the gel-forming mucin Muc2 as its structural component (Johansson et al., 2011; Jevtov et al., 2014). Mucin is primarily secreted by goblet cells, which in adult zebrafish are distributed throughout the gut (albeit more abundant in the middle and posterior gut) (Wallace et al., 2005; Ng et al., 2005).
There are, however, some key functional and architectural differences between zebrafish and mammalian GI systems to consider (Figure 1). The mammalian gastrointestinal tract is generally composed of five distinct parts: (1) the stomach, which partially digests food by mixing it with acid and digestive enzymes, (2) the duodenum, which aids chemical digestion, (3) the jejunum, which absorbs nutrients, (4) the ileum, which absorbs bile salts, and (5) the colon, which absorbs water and salts. The zebrafish intestine is divided into three histologically defined segments, including (1) the anterior intestinal bulb, (2) the middle intestine, and (3) the posterior intestine. This nomenclature for the zebrafish gut is typically maintained from larval to adult stages. In contrast to mammals, zebrafish lack the typical signatures of a stomach organ and the intestinal bulb undergoes no acidification. The passage of microbes through the acidic environment of the human stomach, which can reach pH values as low as 1.4 (Dressman et al., 1990), serves as a regulatory cue for some GI pathogens (Ramos-Morales, 2012), and its absence may impact the outcome of infection. Zebrafish do, however, express several digestive enzymes that are functionally equivalent to mammalian gastric markers, including rennin, nothepsin, several cathepsins, and the lipase Lipf (Wallace et al., 2005; Wang et al., 2010). The different regions of the adult zebrafish gut have distinct functions analogous to the mammalian small intestine and colon. Functionally, the anterior intestinal bulb likely plays a role in bile salt recovery (Lickwar et al., 2017), while the anterior and middle regions may aid in lipid and protein absorption (Ng et al., 2005; Brugman, 2016). Like the mammalian colon, the posterior region of the zebrafish gut is responsible for ion and water absorption (Wallace et al., 2005). Established markers of the mammalian small and large intestine (e.g., villin and fabp2) are differentially expressed along the length of the zebrafish intestine (Wang et al., 2010). Transcriptional domains identified in larval zebrafish are maintained in adults, and a model delineating five transcriptional and functional domains has been proposed, and includes ada for the anterior, duodenum-like region, fabp2 and rbp2a for the anterior, jejunum-like region, fabp6 and slc10a2 for the middle, ileum-like region, and lamp2 for the middle to posterior, colon-like region (Lickwar et al., 2017).
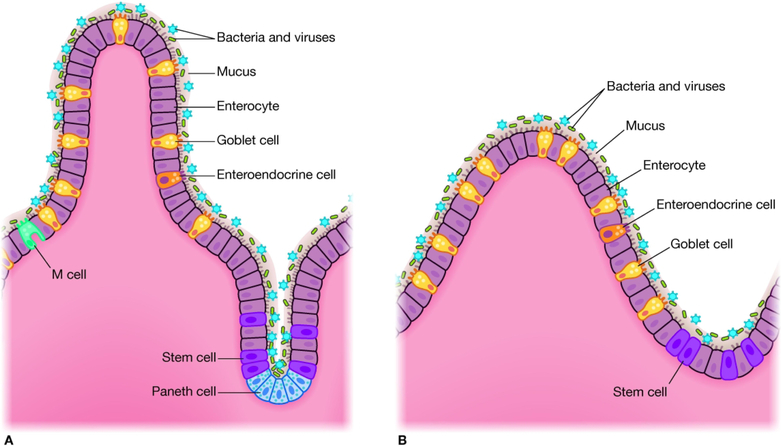
Figure 1.
Comparison of cell types and structures between the mammalian (A) and zebrafish (B) gastrointestinal tract. The zebrafish intestine is organized into irregular folds as opposed to villi, and lacks crypts. Mammalian and zebrafish intestines share stem cells, enterocytes, enteroendocrine cells, and goblet cells. Paneth cells and M-cells are absent in zebrafish.
The intestinal architecture of zebrafish is also less complex than that of mammals as it consists only of the mucosa, muscularis externa, and serosa layers. Underneath the mucosa and epithelium is the lamina propria containing blood capillaries, muscle fibers, and lymphatic vessels. Surrounding this layer is the muscularis externa composed of circular and longitudinal smooth muscle fibers and enteric neurons (Wallace and Pack, 2003). The lining of the mucosa is folded into large, randomly-shaped intestinal folds instead of the mammalian villi and intestinal crypts (crypts of Lieberkühn) (Pack et al., 1996; Wallace et al., 2005; Wang et al., 2010). Since zebrafish lack crypts of Lieberkühn, which in mammals are the source of intestinal stem cells, cell division instead occurs at the base of the folds and cells migrate to the tip of the folds where they become apoptotic (Wallace et al., 2005; Wang et al., 2010). The time course for cell migration in the anterior intestine is 5–7 days, while migration takes 7–10 days in the middle intestine (Wallace et al., 2005). Goblet cells are still present in zebrafish, but distributed throughout the mucosa rather than localized to crypts (Pack et al., 1996; Wang et al., 2010).
The enteric nervous system (ENS) is a functionally important component of the GI system both in zebrafish and mammals, and consists of enteric neurons and glial cells. These cells modulate several key functions including gut peristalsis, hormone secretion, water balance, and absorption. In contrast to the more architecturally complex mammalian ENS which is composed of two plexuses (i.e., myenteric and submucosal), each with their own interconnected ganglia, the zebrafish ENS develops into a single myenteric layer of neurons, glia, and other cell types (e.g., interstitial cells of Cajal) without any clearly defined ganglia (Wallace et al., 2005).
2.2. Development of the zebrafish gastrointestinal tract
Intestinal development in zebrafish larvae can be categorized into 3 major stages (Ng et al., 2005). Stage 1 is defined by formation of the lumen; a thin rod of endodermal cells that undergo anterior-to-posterior differentiation and proliferation (Wallace and Pack, 2003); by 60 hours post-fertilization (hpf) the lumen has expanded rostrocaudal (Kimmel et al., 1995; Alvers et al., 2014). During stage 2, the intestinal epithelium becomes polarized via cell type differentiation (Pack et al., 1996; Wallace et al., 2005), and by 76 hpf, enteroendocrine cells are present throughout the anterior to posterior regions of the intestine. Enteroendrocrine cells produce and release active compounds (e.g., hormones) into the surrounding tissues as well as modulate enzyme secretions and muscle contractions (Wallace et al., 2005; Takashima et al., 2013). At 76 hpf, the beginning of stage 3, intestinal folds have developed in the anterior and middle intestinal regions, and peristaltic contractions have begun (Pack et al., 1996; Wallace et al., 2005). Growth and differentiation of the digestive tract continues, and by 5 days post-fertilization (dpf), the majority of the system is functional (Wallace and Pack, 2003; Ng et al., 2005); at this point the larval zebrafish digestive tract is comprised of the mouth, pharynx, esophagus, intestinal bulb, intestine, and anal opening. Extensive folding can be found within the anterior intestine, but at 5 dpf the posterior intestinal regions have no folds (Ng et al., 2005), and by 6 to 8 dpf, cell proliferation begins to decrease (Cheesman et al., 2011). As the larval zebrafish ages, folding continues and the folds themselves become shorter in the caudal direction (Menke et al., 2011; Li et al., 2019), and the lumen begins to widen at the anterior end and becomes progressively smaller towards the posterior region (Wallace et al., 2005).
Enteric precursors originating from the neural crest migrate into the intestinal tract at 32 hpf and reach the posterior end by 66 hpf (Olden et al., 2008; Heanue et al., 2016). By 4 dpf the zebrafish gut exhibits spontaneous, coordinated contractions and the digestive system is fully functional by 7 dpf (Holmberg et al., 2007). We refer readers to excellent recent reviews of ENS function and its interactions with the various other cells types of the zebrafish intestine (Ganz et al., 2016; Ganz, 2018).
3. Immune surveillance of the gastrointestinal tract
Zebrafish have made valuable contributions to our understanding of vertebrate immunity (Martins et al., 2019). Multiple comprehensive reviews discuss the teleost immune system (Traver et al., 2003; Trede et al., 2004; Sullivan and Kim, 2008a; Meeker and Trede, 2008; Uribe et al., 2011; Renshaw and Trede, 2012; Rauta et al., 2012), and zebrafish immunity in the context of infectious disease (Sullivan and Kim, 2008b; Meijer and Spaink, 2011; Masud et al., 2017). The zebrafish and human immune systems share many similarities, including both innate and adaptive components (Traver et al., 2003; Renshaw and Trede, 2012).
3.1. Innate immunity and the GI tract
Larval zebrafish solely depend on their innate immune system to fend off invading microbes since the adaptive immune system is not functional until 4–6 weeks post-fertilization (Willett et al., 1999; Lam et al., 2004). During this time, colonization of the larval gut by commensals can prime innate immune cells against infections through a Toll-like receptor (TLR) and myeloid differentiation primary response 88 (MyD88) dependent pathway (Galindo-Villegas et al., 2012). One of the benefits of zebrafish is their optical transparency, allowing for the use of transgenic lines with fluorescently-marked innate immune cells like macrophages (Ward, 2003; Redd et al., 2006; Hall et al., 2007; Ellett et al., 2011; Walton et al., 2015; Sanderson et al., 2015; Nguyen-Chi et al., 2017), neutrophils (Mathias et al., 2006; Renshaw et al., 2006; Buchan et al., 2019), and eosinophils (Balla et al., 2010). These resources have given valuable insights into the role of innate immune cells in intestinal homeostasis and pathogenesis.
Intestinal macrophages play a role in microbiome homeostasis and complement regulation. Loss of intestinal macrophages in adult zebrafish irf8 mutants causes dysbiosis of the resident gut microbiota, and a reduction in the complement component C1q (Earley et al., 2018). Neutrophils are recruited to the intestine in response to inflammatory stimuli such as the pathogen-associated molecular pattern (PAMP) lipopolysaccharide (LPS). Immersion of larvae in LPS activates MyD88 and tumor necrosis factor-alpha (TNF-α), and leads to intestinal neutrophil influx (Bates et al., 2007). Colonization of larvae by a conventional microbiota promotes production of alkaline phosphatase by the gut epithelium, which detoxifies microbiota-derived LPS and prevents excessive inflammation to maintain intestinal homeostasis (Bates et al., 2007). Zebrafish TLRs that recognize PAMPs share key structural similarities with mammalian TLRs, but some TLRs may differ in functional aspects (Palti, 2011). For example, in mammals TLR4 responds to LPS, while zebrafish TLR4 paralogs (Tlr4a and Tlr4b) fail to recognize and respond to LPS (Sullivan et al., 2009). Since zebrafish are, however, able to mount a MyD88-dependent inflammatory response upon LPS stimulation (Bates et al., 2007; Yang et al., 2017), it is likely that a hitherto unidentified TLR could be functionally equivalent to mammalian TLR4. At least 17 TLRs have been identified in zebrafish (Jault et al., 2004; Meijer et al., 2004), suggesting redundant and potentially overlapping functions; we refer readers to another review of TLRs and their known functions in teleost species (Palti, 2011).
Zebrafish neutrophils typically migrate to sites of infection or injury at a faster rate than macrophages (Ellett et al., 2011), thus forming the first line of defense against pathogen insult. The presence of a commensal intestinal microbiota increases the number of circulating neutrophils, enhances neutrophil migratory velocity, and recruitment to extra-intestinal sites of injury (Kanther et al., 2014). Zebrafish neutrophils are functionally largely equivalent to mammalian neutrophils: they are capable of phagocytosis and degranulation, produce cytokines and reactive oxygen species (ROS), and can form neutrophil extracellular traps (NETs) (Lieschke et al., 2001; Harvie and Huttenlocher, 2015; Palie et al., 2007).
The precise role of eosinophils has been difficult to delineate due to a lack of specific markers, but they may play a role in responding to intestinal helminth infections (Balla et al., 2010). Adult zebrafish eosinophils share morphology and differential gene expression with mammalian eosinophils (Lieschke et al., 2001). When challenged with helminth antigens or - infection, eosinophil numbers within the zebrafish intestine increase, indicating conservation of eosinophil-mediated immune responses between zebrafish and mammals (Balla et al., 2010).
Secretion of antimicrobial molecules into the intestinal mucosal layer, both constitutive and in response to TLR activation by PAMPs, is also conserved from mammals to zebrafish. Zebrafish produce antimicrobial peptides (AMPs), of which β-defensins, cathelicidins, hepcidins, and histone-derived peptides are also found in mammals, and piscidins are fish specific (Zou et al., 2007; Katzenback, 2015). Secreted peptidoglycan recognition proteins (PGRPs) (Chang et al., 2007) and antibacterial lectins functionally similar to mammalian pore forming C-type lectins (Brinchmann et al., 2018) also contribute to zebrafish intestinal mucosal immunity. Secreted antimicrobial factors are expressed throughout the zebrafish intestine, both in larvae and adults (Oehlers et al., 2011a).
3.2. Adaptive immunity and the GI tract
The zebrafish adaptive immune system reaches morphological and functional maturity by 4–6 weeks post-fertilization (Willett et al., 1999; Lam et al., 2004) and consists of B- and T-cells capable of antigen receptor rearrangement in response to pathogens via recombination activating genes 1 and 2 (ragl and rag2) (Trede and Zon, 1998; Langenau and Zon, 2005). Other initiators of the adaptive immune response, like major histocompatibility complex class I and II molecules, are also present in zebrafish (Sültmann et al., 1993; Sültmann et al., 1994; Takeuchi et al., 1995; Rauta et al., 2012).
The zebrafish lymphatic system shares many morphological, molecular, and functional features with those of mammals, but lacks some lymphoid tissues, like lymph nodes (Jung et al., 2017) and Peyer’s patches (Boehm et al., 2003; Brugman, 2016). However, there is evidence that the enterocytes of the larval and adult zebrafish mid-intestine are highly endocytic (Oehlers et al., 2011a) and perform a specialized function in luminal antigen sampling. Consistent with that, a labeled antigen derived from a Yersinia ruckeri immersion-vaccine is initially detected in the mid-intestine enterocytes of zebrafish larvae, followed by the spleen (Korbut et al., 2016). In adult zebrafish orally infected with Mycobacterium marinum, bacteria are taken up into vacuoles by antigen-sampling cells, which make up the majority of the epithelium of the posterior mid-intestine. Bacteria are trafficked to the basal side of the intestine, where they subsequently co-localize with leukocytes, before eventually travelling to the liver and spleen (Løvmo et al., 2017).
Teleost B-cells exhibit additional phagocytic and microbicidal functions not typically seen in higher vertebrates (Li et al., 2006; Øverland et al., 2010), and can also function as initiating antigen-presenting cells (APCs), linking the innate and adaptive immune systems in zebrafish (Zhu et al., 2014; Lewis et al., 2014). In teleosts, intestinal mucosal immunity is largely dependent on B-cells acting as the primary responders to perturbation, but our understanding of their origins and full spectrum of functions in maintaining gut homeostasis is still limited (see Parra et al., 2016 for review).
4. Zebrafish infection models of gastrointestinal pathogens
4.1. Common routes of infection
Several approaches are currently in use to establish infections with GI pathogens in the zebrafish host, each with distinct advantages and disadvantages to consider. One of the most commonly used routes of infection is by immersion of larvae or adults in a suspension containing a defined pathogen concentration (for detailed protocols see e.g. Varas et al., 2019 for larvae and Mitchell et al., 2017 for adults). The former also offers a side-by-side comparison of infection resulting from immersion vs. caudal vein injection. Immersion infection can be used for larvae beginning when the mouth first opens at around 3 dpf (Ng et al., 2005). It is advantageous due to its extremely high throughput and does not require much specialized equipment. Its disadvantage is that the exact dose of pathogen taken up by the fish, although it can be measured, can only be indirectly controlled by varying the concentration of pathogen in the suspension. Immersion cannot be applied to strictly anaerobic microbes. For some GI pathogens, in particular those for which zebrafish are not a natural host, immersion does not lead to robust intestinal colonization (e.g., Tysnes et al., 2012; Stones et al., 2017 etc.). More recently, the protozoan Paramecium caudatum, a natural prey for larval zebrafish, has been adapted as a vehicle for food-borne infection models (Stones et al., 2017; Flores et al., 2019; Fan et al., 2019). The protozoan internalizes bacteria into storage vacuoles and following uptake of Paramecia by the zebrafish and degradation of the protozoan in the foregut, the bacterial load is released into the middle intestine. This approach, although more laborious than simple immersion, still allows for high throughput and mimics human exposure to GI pathogens by consumption of contaminated food. Another advantage is that P. caudatum passages the pathogen through acidifying storage vacuoles prior to its release into the zebrafish gut, and the low pH may prime the pathogen similarly to the low pH environment of the human stomach, as discussed in section 2. A third, more labor intensive route of infection is oral gavage of adults or microgavage of larval zebrafish (see Cocchiaro and Rawls, 2013 for a detailed protocol). A defined amount of pathogen suspension is directly delivered into the foregut via a fine capillary that is inserted into the mouth and esophagus of the animal. This technique comes with several costs: it is time consuming, requires specialized equipment, and a skilled experimenter is necessary to prevent injury or death of the infected animal. Sufficient sample sizes are necessary to compensate for attrition or failure of the inoculum to reach the intestine due to regurgitation (Runft et al., 2014). Lastly, some studies inject GI pathogens into the caudal vein or peritoneum of zebrafish, which introduces the pathogen into the blood stream. Despite the difference in administration, these studies have contributed valuable insights into microbial factors modulating inflammation and tissue damage, which often drive mortality as a result of infection (Dong et al., 2013; Okuda et al., 2014).
Below, we discuss GI pathogens studied in zebrafish to date, along with relevant outcomes from those studies. The developmental stage and route of infection used in each case are listed in Table 1.
Table 1. Summary - Examples of gastrointestinal pathogens and their disease profiles in the zebrafish host.
The table is organized first by pathogen (in alphabetical order), followed by developmental stage, followed by route of infection. Some of the studies summarized below discuss additional infection models which were not included in the table due to space constraints.
| Pathogen | Route of infection | Zebrafish developmental stage | Disease phenotype/ finding | Reference |
|---|---|---|---|---|
| Aeromonas hydrophila | Immersion | Larvae, 4 dpf | Colonization and increased mortality | Saraceni et al., 2016 |
| Aeromonas hydrophila | Immersion | Adults, 4 months | Change in microbiota composition; induction of innate immune response | Yang et al., 2017 |
| Aeromonas hydrophila, Aeromonas veronii | Immersion | Germ-free larvae, 4–5 dpf; Adults | Intestinal colonization; increased mortality; intestinal lesions in adults; Aerolysin-dependent virulence | Ran et al., 2018 |
| Edwardsiella ictaluri | Co-housing | Adults | Skin hemorrhage; abdominal hemorrhage; nephritis; septicaemia; mortality | Hawke et al., 2013 |
| Edwardsilla piscicida | Immersion | Larvae, 5 dpf | Type III secretion system- and type VI secretion system-dependent increase in mortality | Hu et al., 2019 |
| Edwardsilla piscicida | Immersion | Larvae, 4 dpf | Histone H2A and RIP2 are required for induction of antibacterial genes and decrease bacterial burden and mortality in response to infection | Wu et al., 2019 |
| Edwardsiella tarda | Immersion | Larvae, 1 dpf | Increased mortality; systemic infection | Pressley et al., 2005 |
| Edwardsiella tarda | Immersion | Larvae, 1 dpf | Flagella-dependent increase in mortality | Xu et al., 2014 |
| Edwardsiella tarda | Immersion | Healthy adults; Wounded adults (skin abrasion) | (Healthy) Colonization; no mortality (Wounded) Increased mortality; septicaemia; colonization of intestinal smooth muscle tissue |
Pressley et al., 2005 |
| Edwardsiella tarda | Injection, i.p. | Adults | Type III secretion system-dependent increase in mortality | Okuda et al., 2014 |
| Edwardsiella tarda | Injection, i.p. | Adults | Invasin-dependent increase in mortality (LD50) | Dong et al., 2013 |
| Escherichia coli (EHEC) | Food-borne | Larvae, 4 dpf | Intestinal colonization; neutrophil recruitment; type III secretion system induction; increased mortality; transmission to naïve fish | Stones et al., 2017 |
| Escherichia coli (Nissle, strain 40) | Immersion | Adults | Intestinal colonization; metabolize glucose and decrease intestinal pH; decrease burden of V. cholerae during co-infection | Nag et al., 2018 |
| Giardia duodenalis | Oral gavage | Adults | Intestinal deposition and retention of cysts, excretion of cysts; no detection of trophozoites; no intestinal damage | Tysnes et al., 2012 |
| Human norovirus (HuNoV GI and GII) | Injection, yolk sac | Larvae, 3 dpf | Viral replication; viral persistence in the intestine and hematopoietic cells; transmission to naïve fish; antiviral treatment | Van Dycke et al., 2019 |
| Picornavirus-1 | Naturally occuring | Adults | Intestinal colonization; viral shedding | Altan et al., 2019 |
| Salmonella enterica serovar Typhimurium | Injection, otic vesicle; Injection, yolk | Larvae, 2 dpf | Increased SPI-1- and SPI-2-dependent mortality; caspase-1 activation (WT); Gbp4 inflammasome mediated response to infection | Tyrkalska et al., 2016 |
| Salmonella enterica serovar Typhimurium | Food-borne | Larvae, 5 dpf | Colonization of intestine; neutrophil recruitment; translational fidelity-dependent bacterial fitness in vivo | Fan et al., 2019 |
| Salmonella enterica serovar Typhimurium | Immersion | Larvae, 6 dpf | Colonization of intestine and cloaca; inflammation and swelling of the cloaca; neutrophil recruitment to site of infection | Varas et al., 2017 |
| Salmonella enterica serovars Typhimurium, Enteritidis, Weltevreden | Immersion | Adults | Colonization and replication; shedding and transmission to naïve fish; intestinal inflammation and destruction of intestinal epithelium; diarrhea; increased mortality; protective immunity via bath vaccination with heat-killed bacteria | Howlader et al., 2016 |
| Salmonella enterica serovar Typhimurium | Oral gavage | Adults, 8 months | Colonization of intestine; intestinal inflammation and ulceration; macrophage and lymphocyte infiltration; tissue damage; Increased mortality; spv-dependent pathology | Wu et al., 2017 |
| Vibrio cholerae | Immersion | Larvae, 4 dpf | CRP- and TcpA-dependent intestinal colonization and mortality; rtx, hlyA induction during colonization | Manneh-Roussel et al., 2018 |
| Vibrio cholerae | Immersion | Larvae, 5 dpf | Colonization of the intestine | Runft et al., 2014 |
| Vibrio cholerae | Immersion | Germ-free larvae, 5 dpf | Intestinal colonization; decrease in commensal Aeromonas; type VI secretion system dependent increase in gut peristalsis | Logan et al., 2018 |
| Vibrio cholerae | Oral gavage; Immersion | Adults, 6–9 months | Colonization of intestine; microcolony formation; transmission to naïve fish | Runft et al., 2014 |
| Vibrio cholerae | Immersion | Adults | Intestinal colonization; diarrhea; increased mucin production and excretion | Mitchell et al., 2017 |
| Vibrio parahaemolyticus | Injection, i.p. | Adults, 7–8 months | Increased mortality; necrosis of kidneys, liver and intestinal villi; intestinal bleeding | Zhang et al., 2016 |
| Vibrio vulnificus | Injection, i.p. | Adults | Increased mortality; inflammatory response; protection by AMP epinecidin-1 | Pan et al., 2011 |
4.2. Edwardsiella and Aeromonas infections in zebrafish
Major aquaculture and opportunistic human pathogens include members of the Edwardsiella and Aeromonas genera (Lee and Wendy, 2017). The consumption of food contaminated with these bacteria can cause gastroenteritis in healthy persons, and more severe diarrheal disease in elderly and immunocompromised individuals (Clarridge et al., 1980; Gracey et al., 1982). Edwardsiella species known to infect fish include E. ictaluri, E. hoshinae, E. piscicida, and E. tarda, but only the latter is known to affect humans (Jordan and Hadley, 1969; Hawke et al., 1981; Nomura and Aoki, 1985). Like other fish pathogens, the Edwardsiella and Aeromonas species enter their host through the skin and/or gills (Ventura and Grizzle, 1987; Menanteau-Ledouble et al., 2011). Infection of zebrafish with E. tarda (Pressley et al., 2005) or E. ictaluri (Hawke et al., 2013) induces the pro-inflammatory cytokines IL-β and TNF-α, leads to the development of hemorrhagic septicemia and increases mortality. Furthermore, studies using zebrafish infection models have elucidated E. tarda virulence factors such as (1) the type III secretion system essential for invasion and intracellular replication in phagocytic cells (Okuda et al., 2014), (2) an invasin protein important for haemolytic activity and biofilm formation (Dong et al., 2013), and (3) flagellar components involved in motility, biofilm formation, and adhesion (Xu et al., 2014). As with E. tarda, a type III secretion system dependent increase in mortality is seen with E. piscicida, although a type VI secretion system was identified as an additional virulence factor in the latter (Hu et al., 2019). The same model was used to show that the NOD-like receptor mediated immune response is required for upregulation of antibacterial genes, including beta-defensins and major histocompatibility complex (MHC) related genes, in response to E. piscicida infection (Wu et al., 2019). Failure to induce the pathway leads to increased bacterial burden and mortality in response to infection. Several studies have used zebrafish to investigate the efficacy and mechanism of action of potential novel therapeutics to combat Edwardsiella infections, including vaccines (Guo et al., 2015) and immunomodulatory nanoparticles that fortify resistance against pathogens (Udayangani et al., 2017).
Members of the genus Aeromonas that affect humans include A. hydrophila, A. caviae, A. veronii, and A. dhakensis, while A. salmonicida is fish-specific (Clarridge et al., 1980; Sacho et al., 1990; Joseph and Carnahan, 1994). Immersing larval or injured adult zebrafish in A. hydrophila upregulates the production of IL-ip and TNF-a in response to colonization, and increases neutrophil recruitment to the wound site (Saraceni et al., 2016). Aeromonas sp. isolated from the zebrafish gut exhibit intrinsic antibiotic resistance and harbor extracellular enzymes such as lipase, hemolysin, proteases, and DNase with the potential to degrade host cells, indicative of their potential to cause disease in the zebrafish host (Hossain et al., 2018). Gut colonization with A. hydrophila alters the adult zebrafish intestinal microbiota composition, concomitantly increasing pathogen abundance and decreasing beneficial intestinal bacteria (Yang et al., 2017). Although both A. hydrophila and A. veronii are able to colonize the intestine and cause mortality (Saraceni et al., 2016; Ran et al., 2018), A. veronii expresses more aerolysin toxin, which causes intestinal lesions and invasion of the intestinal barrier, and is associated with increased virulence compared to A. hydrophila (Ran et al., 2018).
4.3. Vibrio infections in zebrafish
Members of the Vibrio genus are natural inhabitants of warm coastal waters. The consumption of raw or contaminated seafood often leads to vibriosis in humans, a disease characterized by diarrhea, nausea, and/or abdominal cramps (Johnston et al., 1986). Though gastroenteritis is most closely associated with V. cholerae, V. parahaemolyticus and V. vulnificus can also cause intestinal disease, serious wound and soft tissue infections, as well as bacteremia (Johnston et al., 1986; Lee et al., 2003; Tsai et al., 2009). Various groups have attempted to model Vibrio-induced diseases in both mammalian and non-mammalian systems, however the animals used were not natural hosts and required tedious manipulation (Rowe et al., 2014). Vibrios naturally colonize zebrafish, and some strains are natural fish pathogens, so using zebrafish as a host to model disease may offer valuable insights into Vibrio pathogenesis and aid the identification of therapies to combat infection (Runft et al., 2014). The V. cholerae serogroup O1 biotype El Tor responsible for cholera efficiently colonizes the zebrafish intestine and forms microcolonies in close contact with the intestinal epithelial surface similar to those seen in human specimen (Runft et al., 2014). The pathogen is shed into the water and can be transmitted to naïve fish (Runft et al., 2014). Adults immersed in V. cholerae develop cholera toxin-independent diarrhea characterized by increased mucin production and excretion (Mitchell et al., 2017). Although cholera toxin is dispensable for colonization and pathogenesis in zebrafish, accessory toxins including RTX and HlyA are induced during intestinal colonization, and the metabolic regulator CRP is required for their activation inside the zebrafish host (Manneh-Roussel et al., 2018). V. cholerae uses a host-directed type VI secretion system to enhance intestinal contractions in the zebrafish gut and to expel resident intestinal microbiota to allow for pathogen colonization (Logan et al., 2018). Treating zebrafish infected with V. vulnificus with the AMP epinecidin-1 increases host survival, possibly through modulation of immunomodulatory genes (Pan et al., 2011). Inoculating zebrafish with V. parahaemolyticus via intraperitoneal injection causes abdominal hemorrhaging, swelling, and secretion of two major markers of host sepsis, TNF-α and IL-1β (Zhang et al., 2016).
4.4. Salmonella infections in zebrafish
More recently, the use of zebrafish has been expanded to model infection of non-fish pathogens in the GI tract. Salmonella enterica serovar Typhimurium is a major causative agent of human foodborne gastroenteritis (Hoelzer et al., 2011). Immersing zebrafish larvae in S. Typhimurium leads to gut colonization and inflammation of the intestine and cloaca (Howlader et al., 2016; Varas et al., 2017). While the precise molecular mechanisms responsible for inflammation are not fully understood, expression of Salmonella virulence plasmid (spv) genes in adult zebrafish suppresses protective host responses such as expression of type II IFN-γ, IL-12, and TNF-α, and promotes expression of cytokines known to facilitate intracellular pathogen survival such as IL-4, IL-10, and IL-13 (Wu et al., 2017). Furthermore, in vivo clearance of S. Typhimurium is mediated by inflammasome activation in neutrophils (Tyrkalska et al., 2016). Mechanistically, infected zebrafish release CXC chemokine 18 and leukotriene B4 that trigger both the recruitment of neutrophils to the infected site and phagocytosis of S. Typhimurium. Once engulfed, S. Typhimurium activates the Gbp4 inflammasome that modulates the activity of cytosolic phospholipase A2 and production of prostaglandins, ultimately leading to bacterial clearance (Tyrkalska et al., 2016). Strains of S. Typhimurium with non-optimal translational fidelity recruit fewer neutrophils following colonization via the food-borne route, and are outcompeted by a wild-type strain in vivo (Fan et al., 2019).
4.5. E. coli infections in zebrafish
Another human enteric pathogen successfully modeled in zebrafish is enterohemorrhagic Escherichia coli (EHEC), which colonizes the intestine and causes bloody diarrhea in the human host. Following foodborne delivery via P. caudatum, EHEC colonizes the middle intestine of zebrafish larvae despite the presence of endogenous microbiota, and by 4 days post-infection survival rates decrease by ~40% (Stones et al., 2017). In humans and cattle, intestinal colonization by EHEC and the formation of attaching and effacing lesions on enterocytes is mediated by a set of virulence genes encoded by the locus of enterocyte effacement (LEE), (Phillips et al., 2000; Elliott et al., 2000). Following foodborne infection of larval zebrafish, EHEC induce the LEE during early colonization of the GI tract, and LEE induction is required for optimal colonization and pathogenesis (Stones et al., 2017). The ability to colonize the zebrafish intestine, however, is not limited to pathogenic E. coli strains; Commensal E. coli isolates from the gut of healthy human volunteers also colonize zebrafish, and can reduce colonization by V. cholerae by decreasing the intestinal pH as a result of glucose metabolism (Nag et al., 2018).
4.6. Non-bacterial GI infections in zebrafish
Gastrointestinal microbes studied in zebrafish are not limited to bacteria; Eukaryotic microorganisms, such as the protozoan Giardia duodenalis can cause intestinal damage in humans and a zebrafish model of inoculation with cysts has been established (Tysnes et al., 2012). Excystation and infection of the zebrafish intestine by trophozoites, however, remains to be shown. Altan et al. recently described a naturally occurring picornavirus in zebrafish (Altan et al., 2019). Picornavirus-1 ZfPV-1 selectively infects a subset of enterocytes and cells in the lamina propria, providing a natural model to study virus-GI tract interactions in a vertebrate host. Human norovirus persists in the larval zebrafish intestine and hematopoietic cells following yolk injection, replicates, and can be transmitted to naïve hosts (Van Dycke et al., 2019).
Zebrafish were initially used to study natural fish pathogens with the intention to limit the impact of infection on aquaculture, but the studies discussed above highlight a rapidly expanding movement to use zebrafish to model zoonotic and human bacterial infections, as well as protozoan and viral infections.
5. Zebrafish as a model to study the intestinal microbiome
The gut microbiome is the collective population of microorganisms that reside in the host GI tract. Many members of the microbiome have beneficial roles, such as regulating digestion, nutrient absorption, and immune system maturation (Umesaki et al., 1999; Semova et al., 2012; Thaiss et al., 2016). Perturbation of the gut microbiome has been linked to a wide range of disease states including neurodevelopmental disorders, obesity, and inflammatory bowel diseases (IBD) (Turnbaugh et al., 2008; Frank et al., 2011; Luna et al., 2017; Felice and O’Mahony, 2017). The application of metagenomics has enabled the differentiation of microbes preferentially residing in healthy versus diseased hosts, but it is presently unclear if there is a conserved ‘core’ microbiome, or how host genetics, the microbiome, and environmental factors interact to determine physiological and pathophysiological states of the host.
Zebrafish are a powerful model to address these unknowns and begin to unravel complex host-microbiota interactions in a more controlled, simple model system compared to other vertebrates used in microbiome studies. For example, derivation of germ-free (GF) larvae is simpler, more expedient, and more cost-effective than using murine GF models (Pham et al., 2008). Studies using conventionally raised (i.e., born and raised in the presence of their normal microbiota), GF, and conventionalized (i.e., derived GF and then colonized with microbiota) larvae are beginning to elucidate the mechanisms governing host-microbiota homeostasis as well as the effects of the microbiota on host organ development and differentiation. Comparative studies of conventional and GF zebrafish larvae demonstrate that the microbiota influences host gene expression, particularly genes related to intestinal epithelial proliferation, nutrient metabolism, and the innate immune response (Rawls et al., 2004; Reikvam et al., 2011). Other studies support the hypothesis that host factors have a dominant effect on determining the intestinal microbiome composition (Rawls et al., 2006; Roeselers et al., 2011). For example, transplantation of intestinal microbiota from donor mice into GF zebrafish larvae reveals that, while the resulting GI microbiota in zebrafish resembles that of the donor mice, the number of microbial lineages present in the recipient community changes drastically. This suggests host-mediated selective pressures restrict the microbiome composition (Rawls et al., 2006). This observation is further supported by microbiome profiling of recently caught and domesticated zebrafish reared in multiple research facilities; there exists a ‘core’ microbiome that is shared among zebrafish lineages regardless of environmental exposure (Roeselers et al., 2011). In vivo analyses indicate the intestinal microbiome is required for intestinal epithelium development and differentiation in zebrafish (Bates et al., 2006). Several developmental deficiencies have been observed in GF larvae, including the absence of brush border intestinal alkaline phosphatase activity, immature glycan expression, a decrease in secretory cell numbers, and altered gut motility. These effects can be reversed with the re-introduction of microbiota from conventional donors (Bates et al., 2006). For an in-depth review of the gut microbiome and its analysis in zebrafish and other teleosts, see Tarnecki et al., 2017.
The mammalian and zebrafish gut microbiome share six bacterial divisions, but the human GI tract is primarily dominated by Firmicutes, Bacteroidetes, and Actinobacteria while Proteobacteria are the predominant phylum in zebrafish (Rawls et al., 2004; Eckburg et al., 2005; Bates et al., 2006). Several studies demonstrate the GI tract of zebrafish can be colonized with aerobic and anaerobic species derived from human fecal communities (Toh et al., 2013; Arias- Jayo et al., 2018; Valenzuela et al., 2018). The successful colonization of the larval gut with anaerobic species, including Lactobacillus paracasei and strict anaerobes such as Eubacterium limosum and Akkermansia muciniphila, in particular, was an important stepping stone in establishing zebrafish as a surrogate host for studies on the human gut microbiome (Toh et al., 2013; Arias-Jayo et al., 2018).
The emerging consumer interest in probiotics as a supplement to promote health and well-being has further propelled microbiome studies in zebrafish. The lactic acid bacteria Lactobacillus plantarum is of particular interest and is currently commercialized as a beneficial probiotic that alleviates stress and anxiety in humans (Chong et al., 2019). In zebrafish, L. plantarum protects against stress-induced dysbiosis of the gut microbiota and ameliorates anxiety-related behavior. These changes in microbiome composition and behavior correlate with an increase in expression of gamma-aminobutyric acid receptors, and of serotonin transporters in zebrafish brains following administration of L. plantarum (Davis et al., 2016). Colonization of adult zebrafish with the probiotic Lactobacillus rhamnosus decreases the expression of host genes associated with dietary lipid metabolism (Falcinelli et al., 2017). Consequently, shifts in microbiome community structure resulting from high dietary fat intake and associated weight gain are ameliorated by supplementation with L. rhamnosus, highlighting its potential to attenuate diet-related metabolic disorder (Falcinelli et al., 2017).
6. Zebrafish models of inflammatory bowel diseases
While the etiologies of multifactorial IBDs like ulcerative colitis and Crohn’s disease are unknown, two key features of the pathology are intestinal epithelial damage and intestinal dysbiosis. The intestinal epithelium provides a physical barrier between the intestinal lumen and surrounding tissues, and works in concert with immune cells to sense and respond to both normal microbiota and opportunistic infection. Therefore, factors such as host genetics and environmental conditions that impair intestinal epithelial homeostasis and disrupt the interplay with resident microbiota can significantly impact IBDs (Bellaguarda and Chang, 2015). Novel murine models of intestinal epithelial damage and inflammation have advanced our understanding of IBDs, but several limitations associated with murine models such as cost, imaging capacity, and genetic manipulation still constrict elucidation of how the aforementioned disease modifiers converge and affect pathogenesis in vivo (Kiesler et al., 2015).
The zebrafish model circumvents murine-associated limitations and provides the opportunity to rapidly explore factors associated with increased inflammation and dysbiosis in response to intestinal epithelial damage and is thus relevant to IBDs in humans. Zebrafish models of chemically-induced IBD mimic some key aspects of the pathological condition in humans, including enterocolitis, intestinal epithelial damage, disruption of intestinal architecture, and shifts in microbiome composition (He et al., 2013). For example, intra-rectal administration of oxazolone in adult zebrafish causes disruption of the intestinal fold architecture, depletes goblet cells, increases immune cell infiltration of the gut, and upregulates pro-inflammatory cytokines (Brugman et al., 2009). Likewise, immersion of larval and adult zebrafish in 2,4,6-trinitrobenzenesulfonic acid (TNBS) induces intestinal inflammation, inhibits peristalsis, and disrupts epithelial integrity by impairing the tight junctions between cells. This suggests TNBS disrupts intestinal barrier function and promotes features observed in IBD patients (Fleming et al., 2010; Oehlers et al., 2011b). It has been proposed that increased intestinal epithelial cell shedding after damage compromises barrier integrity, which in turn fuels more inflammation (Blander, 2016). In support of this, leukocytes mobilize from the caudal hematopoietic tissue to the periphery and accumulate around the damaged intestine following TNBS insult in zebrafish (Oehlers et al., 2011b). Disruption of the intestinal barrier by TNBS promotes microbial infiltration of the lamina propria, initiating a cascading pro-inflammatory response characterized by secretion of TNF-α, via activation of TLR3, MyD88, TIR-domain-containing adapter-inducing interferon-β (TRIF), and nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-kB) (He et al., 2013). Together, these data support the idea that zebrafish provide a high-throughput model to interrogate genetic pathways and drug candidates that can restore epithelial barrier function and modulate immune cell function to protect against microbial infiltration during IBD.
7. Perspective
Zebrafish have been a well-used model for infection biology for many years. More recent research highlighting similarities between the zebrafish gut and the GI tract of mammals has expanded to include studies on GI tract-pathogen interactions. The zebrafish as a model organism particularly lends itself to questions that cannot be addressed easily in other model systems thanks in part to the ease of intravital imaging and raw statistical power. Its use in this context may help unravel new details of the cellular microbiology underpinning infection, lead to the identification of novel virulence factors and aspects of their regulation within the GI niche, and aid the discovery of effective therapies by enabling high-throughput drug screening in the live host. Future studies that use zebrafish to study host-microbiome interactions will continue to identify host-intrinsic and - extrinsic factors and selective pressures critical for establishing and shaping the host microbiome. Such discoveries may aid in the development of novel therapies for combating microbiome- associated diseases.
Acknowledgements
We thank members of the Krachler and Eisenhoffer labs for useful comments on earlier versions of the manuscript. We thank Jordan Pietz (UT MD Anderson Cancer Center) for designing Figure 1. We would like to apologize to everyone whose work we could not include it the current version of the review due to space limitations.
Funding and Conflicts of Interest
This work was supported by the NIH, National Institute of Allergy and Infectious Diseases (Grant R01 AI132354-01A1 to AMK and a Diversity Supplement supporting EF (3R01AI132354-02S1), a John S. Dunn Foundation Grant supporting MAO, a MBID Fellowship supporting ATN, and the Cancer Prevention Institute of Texas, RR14007, National Institutes of General Medical Sciences, R01GM124043, and the Linda and Mark Quick Award for Basic Science to GTE.
References
- Altan E, Kubiski SV, Boros Á, Reuter G, Sadeghi M, Deng X, et al. (2019) A Highly Divergent Picornavirus Infecting the Gut Epithelia of Zebrafish ( Danio rerio ) in Research Institutions Worldwide. Zebrafish 16: 291–299. [DOI] [PubMed] [Google Scholar]
- Alvers AL, Ryan S, Scherz PJ, Huisken J, and Bagnat M (2014) Single continuous lumen formation in the zebrafish gut is mediated by smoothened-dependent tissue remodeling. Development 141: 1110–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Jayo N, Alonso-Saez L, Ramirez-Garcia A, and Pardo MA (2018) Zebrafish Axenic Larvae Colonization with Human Intestinal Microbiota. Zebrafish 15: 96–106. [DOI] [PubMed] [Google Scholar]
- Balla KM, Lugo-Villarino G, Spitsbergen JM, Stachura DL, Hu Y, Banuelos K, et al. (2010) Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116: 3944–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, and Guillemin K (2006) Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Developmental Biology 297: 374–386. [DOI] [PubMed] [Google Scholar]
- Bellaguarda E, and Chang EB (2015) IBD and the Gut Microbiota—from Bench to Personalized Medicine. Current Gastroenterology Reports 17: 15–15. [DOI] [PubMed] [Google Scholar]
- Blander JM (2016) Death in the intestinal epithelium-basic biology and implications for inflammatory bowel disease. FEBS J 283: 2720–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Bleul CC, and Schorpp M (2003) Genetic dissection of thymus development in mouse and zebrafish: Thymus development in mouse and zebrafish. Immunological Reviews 195: 15–27. [DOI] [PubMed] [Google Scholar]
- Brinchmann FM, Patel MD, Pinto N, and Iversen HM (2018) Functional Aspects of Fish Mucosal Lectins—Interaction with Non-Self. Molecules 23: e1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S (2016) The zebrafish as a model to study intestinal inflammation. Developmental & Comparative Immunology 64: 82–92. [DOI] [PubMed] [Google Scholar]
- Brugman S, Liu K, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA, et al. (2009) Oxazolone-Induced Enterocolitis in Zebrafish Depends on the Composition of the Intestinal Microbiota. Gastroenterology 137: 1757–1767.e1. [DOI] [PubMed] [Google Scholar]
- Buchan KD, Prajsnar TK, Ogryzko NV, de Jong NWM, van Gent M, Kolata J, et al. (2019) A transgenic zebrafish line for in vivo visualisation of neutrophil myeloperoxidase. PLoS One 14: e0215592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carten JD, and Farber SA (2009) A new model system swims into focus: using the zebrafish to visualize intestinal lipid metabolism in vivo. Clinical Lipidology 4: 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MX, Nie P, and Wei LL (2007) Short and long peptidoglycan recognition proteins (PGRPs) in zebrafish, with findings of multiple PGRP homologs in teleost fish. Molecular Immunology 44: 3005–3023. [DOI] [PubMed] [Google Scholar]
- Cheesman SE, Neal JT, Mittge E, Seredick BM, and Guillemin K (2011) Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proceedings of the National Academy of Sciences 108: 4570–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong HX, Yusoff NAA, Hor Y-Y, Lew L-C, Jaafar MH, Choi S-B, et al. (2019) Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Beneficial Microbes 10: 355–373. [DOI] [PubMed] [Google Scholar]
- Clarridge JE, Musher DM, Fainstein V, Wallace RJ Jr, and (1980) Extraintestinal human infection caused by Edwardsiella tarda. Journal of clinical microbiology 11: 511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchiaro JL, and Rawls JF (2013) Microgavage of Zebrafish Larvae. Journal of Visualized Experiments 72: e4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-Guyon E, Tinevez J-Y, Renshaw SA, and Herbomel P (2011) Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface- associated microbes. Journal of Cell Science 124: 3053–3059. [DOI] [PubMed] [Google Scholar]
- Dabrowski K (1986) Protein digestion and amino acid absorption along the intestine of the common carp (Cyprinus carpio L.), a stomachless fish: an in vivo study. Reprod Nutr Dev 26: 755–766. [DOI] [PubMed] [Google Scholar]
- Davis DJ, Doerr HM, Grzelak AK, Busi SB, Jasarevic E, Ericsson AC, and Bryda EC (2016) Lactobacillus plantarum attenuates anxiety-related behavior and protects against stress-induced dysbiosis in adult zebrafish. Scientific Reports 6: 33726–33726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Fan X, Wang B, Shi X, and Zhang X-H (2013) Invasin of Edwardsiella tarda is essential for its haemolytic activity, biofilm formation and virulence towards fish. Journal of Applied Microbiology 115: 12–19. [DOI] [PubMed] [Google Scholar]
- Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, et al. (1990) Upper gastrointestinal (GI) pH in young, healthy men and women. Pharmaceutical Research 7: 756–61. [DOI] [PubMed] [Google Scholar]
- Duggan GM, and Mostowy S (2018) Use of zebrafish to study Shigella infection. Dis Models Mech 11: dmm032151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley AM, Graves CL, and Shiau CE (2018) Critical Role for a Subset of Intestinal Macrophages in Shaping Gut Microbiota in Adult Zebrafish. Cell Reports 25: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. (2005) Diversity of the human intestinal microbial flora. Science (New York, NY) 308: 1635–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F, Pase L, Hayman JW, Andrianopoulos A, and Lieschke GJ (2011) mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117: e49–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott SJ, Sperandio V, Girón JA, Shin S, Mellies JL, Wainwright L, et al. (2000) The Locus of Enterocyte Effacement (LEE)-Encoded Regulator Controls Expression of Both LEE- and Non-LEE-Encoded Virulence Factors in Enteropathogenic and EnterohemorrhagicEscherichia coli. Infection and Immunity 68: 6115–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcinelli S, Rodiles A, Hatef A, Piccietti S, Cossignani L, Merrifield DL, et al. (2017) Dietary lipid content reorganizes gut microbiota and probiotic L. rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Scientific Reports 7: 5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Thompson L, Lyu Z, Cameron TA, De Lay NR, Krachler AM, and Ling J (2019) Optimal translational fidelity is critical for Salmonella virulence and host interactions. Nucleic Acids Research 47: 5356–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice VD, and O’Mahony SM (2017) The microbiome and disorders of the central nervous system. Pharmacology Biochemistry and Behavior 160: 1–13. [DOI] [PubMed] [Google Scholar]
- Fleming A, Jankowski J, and Goldsmith P (2010) In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: A feasibility study. Inflammatory Bowel Diseases 16: 1162–1172. [DOI] [PubMed] [Google Scholar]
- Flores E, Thompson L, Sirisaengtaksin N, Nguyen AT, Ballard A, and Krachler AM (2019) Using the Protozoan Paramecium caudatum as a Vehicle for Food-borne Infections in Zebrafish Larvae. Journal of Visualized Experiments 143: e58949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang T, Chen H, et al. (2011) Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflammatory Bowel Diseases 17: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Villegas J, Garcia-Moreno D, Oliveira S. de, Meseguer J, and Mulero V (2012) Regulation of immunity and disease resistance by commensal microbes and chromatin modifications during zebrafish development. Proceedings of the National Academy of Sciences 109: E2605–E2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz J (2018) Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system. Developmental Dynamics 247: 268–278. [DOI] [PubMed] [Google Scholar]
- Ganz J, Melancon E, and Eisen JS (2016) Zebrafish as a model for understanding enteric nervous system interactions in the developing intestinal tract. Methods in Cell Biology. 134: 139164. [DOI] [PubMed] [Google Scholar]
- Gracey M, Burke V, and Robinson J (1982) Aeromonas-associated gastroenteritis. The Lancet 320: 1304–1306. [DOI] [PubMed] [Google Scholar]
- Guo C, Peng B, Song M, Wu C, Yang M, Zhang J-Y, and Li H (2015) Live Edwardsiella tarda vaccine enhances innate immunity by metabolic modulation in zebrafish. Fish & Shellfish Immunology 47: 664–673. [DOI] [PubMed] [Google Scholar]
- Meijer AH, and P. Spaink H (2011) Host-Pathogen Interactions Made Transparent with the Zebrafish Model. Current Drug Targets 12: 1000–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Flores M, Storm T, Crosier K, and Crosier P (2007) The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Developmental Biology 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie EA, and Huttenlocher A (2015) Neutrophils in host defense: new insights from zebrafish. Journal of Leukocyte Biology 98: 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke JP, Kent M, Rogge M, Baumgartner W, Wiles J, Shelley J, et al. (2013) Edwardsiellosis caused by Edwardsiella ictaluri in laboratory populations of Zebrafish Danio rerio. Journal of aquatic animal health 25: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke JP, McWhorter AC, Steigerwalt AG, and Brenner DJ (1981) Edwardsiella ictaluri sp. nov., the Causative Agent of Enteric Septicemia of Catfish. International Journal of Systematic and Evolutionary Microbiology, 31: 396–400. [Google Scholar]
- He Q, Wang L, Wang F, Wang C, Tang C, Li Q, et al. (2013) Microbial fingerprinting detects intestinal microbiota dysbiosis in Zebrafish models with chemically-induced enterocolitis. BMC Microbiology 13: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Boesmans W, Bell DM, Kawakami K, Vanden Berghe P, and Pachnis V (2016) A Novel Zebrafish ret Heterozygous Model of Hirschsprung Disease Identifies a Functional Role for mapk10 as a Modifier of Enteric Nervous System Phenotype Severity. PLOS Genetics 12: e1006439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer K, Moreno Switt AI, and Wiedmann M (2011) Animal contact as a source of human non-typhoidal salmonellosis. Veterinary Research 42: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg A, Olsson C, and Hennig GW (2007) TTX-sensitive and TTX-insensitive control of spontaneous gut motility in the developing zebrafish (Danio rerio) larvae. J Exp Biol 210: 1084–1091. [DOI] [PubMed] [Google Scholar]
- Hossain S, De Silva BCJ, Dahanayake PS, and Heo G-J (2018) Characterization of virulence properties and multi-drug resistance profiles in motile Aeromonas spp. isolated from zebrafish ( Danio rerio ). Letters in Applied Microbiology 67: 598–605. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader DR, Sinha R, Nag D, Majumder N, Mukherjee P, Bhaumik U, et al. (2016) Zebrafish as a novel model for non-typhoidal Salmonella pathogenesis, transmission and vaccine efficacy. Vaccine 34: 5099–5106. [DOI] [PubMed] [Google Scholar]
- Hu T, Chen R, Zhang L, Wang Z, Yang D, Zhang Y, et al. (2019) Balanced role of T3SS and T6SS in contribution to the full virulence of Edwardsiella piscicida. Fish & Shellfish Immunology 93: 871–878. [DOI] [PubMed] [Google Scholar]
- Jault C, Pichon L, and Chluba J (2004) Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Molecular Immunology 40: 759–771. [DOI] [PubMed] [Google Scholar]
- Jevtov I, Samuelsson T, Yao G, Amsterdam A, and Ribbeck K (2014) Zebrafish as a model to study live mucus physiology. Scientific Reports 4: 6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Larsson JMH, and Hansson GC (2011) The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108: 4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Becker SF, and McFarland LM (1986) Gastroenteritis in patients with stool isolates of vibrio vulnificus. The American Journal of Medicine 80: 336–338. [DOI] [PubMed] [Google Scholar]
- Jordan GW, and Hadley WK (1969) Human Infection with Edwardsieila tarda. Annals of Internal Medicine 70: 283–288. [DOI] [PubMed] [Google Scholar]
- Joseph SW, and Carnahan A (1994) The isolation, identification, and systematics of the motile Aeromonas species. Annual Review of Fish Diseases 4: 315–343. [Google Scholar]
- Jung HM, Castranova D, Swift MR, Pham VN, Venero Galanternik M, Isogai S, et al. (2017) Development of the larval lymphatic system in zebrafish. Development 144: 2070–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanther M, Tomkovich S, Xiaolun S, Grosser MR, Koo J, Flynn EJ, et al. (2014) Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cellular Microbiology 16: 1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenback BA (2015) Antimicrobial Peptides as Mediators of Innate Immunity in Teleosts. Biology 4: 607–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesler P, Fuss IJ, and Strober W (2015) Experimental Models of Inflammatory Bowel Diseases. Cellular and Molecular Gastroenterology and Hepatology 1: 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, and Ho SB (2010) Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Current Gastroenterology Reports 12: 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, and Schilling TF (1995) Stages of embryonic development of the zebrafish. Developmental Dynamics 203: 253–310. [DOI] [PubMed] [Google Scholar]
- Korbut R, Mehrdana F, Kania PW, Larsen MH, Frees D, Dalsgaard I, et al. (2016) Antigen Uptake during Different Life Stages of Zebrafish (Danio rerio) Using a GFP-Tagged Yersinia ruckeri. PLoS One 11: e0158968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa T, Uji S, and Suzuki T (2005) Identification of pepsinogen gene in the genome of stomachless fish, Takifugu rubripes. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 140: 133–140. [DOI] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, and Sin YM (2004) Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Developmental & Comparative Immunology 28: 9–28. [DOI] [PubMed] [Google Scholar]
- Langenau DM, and Zon LI (2005) The zebrafish: a new model of T-cell and thymic development. Nature Reviews Immunology 5: 307–317. [DOI] [PubMed] [Google Scholar]
- Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. (2008) Origins and unconventional behavior of neutrophils in developing zebrafish. Blood 111: 132–141. [DOI] [PubMed] [Google Scholar]
- Lee K-K, Liu P-C, and Huang C-Y (2003) Vibrio parahaemolyticus infectious for both humans and edible mollusk abalone. Microbes and Infection 5: 481–485. [DOI] [PubMed] [Google Scholar]
- Lee SW, and Wendy W (2017) Antibiotic and heavy metal resistance of Aeromonas hydrophila and Edwardsiella tarda isolated from red hybrid tilapia (Oreochromis spp.) coinfected with motile aeromonas septicemia and edwardsiellosis. Veterinary world 10: 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KL, Del Cid N, and Traver D (2014) Perspectives on antigen presenting cells in zebrafish. Developmental & Comparative Immunology 46: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Barreda DR, Zhang Y-A, Boshra H, Gelman AE, LaPatra S, et al. (2006) B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nature Immunology 7: 1116–1124. [DOI] [PubMed] [Google Scholar]
- Li J, Prochaska M, Maney L, and Wallace KN (2019) Development and organization of the zebrafish intestinal epithelial stem cell niche. Developmental Dynamics doi: 10.1002/dvdy.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang S, Qi J, Echtenkamp SF, Chatterjee R, Wang M, et al. (2007) Zebrafish Peptidoglycan Recognition Proteins Are Bactericidal Amidases Essential for Defense against Bacterial Infections. Immunity 27: 518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickwar CR, Camp JG, Weiser M, Cocchiaro JL, Kingsley DM, Furey TS, et al. (2017) Genomic dissection of conserved transcriptional regulation in intestinal epithelial cells. PlOs Biology 15: e2002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ (2001) Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98: 3087–3096. [DOI] [PubMed] [Google Scholar]
- Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, et al. (2018) The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proceedings of the National Academy of Sciences 115: E3779–E3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovmo SD, Speth MT, Repnik U, Koppang EO, Griffiths GW, and Hildahl JP (2017) Translocation of nanoparticles and Mycobacterium marinum across the intestinal epithelium in zebrafish and the role of the mucosal immune system. Developmental and Comparative Immunology 67: 508–518. [DOI] [PubMed] [Google Scholar]
- Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, et al. (2017) Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cellular and Molecular Gastroenterology and Hepatology 3: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manneh-Roussel J, Haycocks JRJ, Magán A, Perez-Soto N, Voelz K, Camilli A, et al. (2018) cAMP Receptor Protein Controls Vibrio cholerae Gene Expression in Response to Host Colonization. MBio 9: e00966–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RR, Ellis PS, MacDonald RB, Richardson RJ, and Henriques CM (2019) Resident Immunity in Tissue Repair and Maintenance: The Zebrafish Model Coming of Age. Frontiers in Cell and Developmental Biology 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud S, Torraca V, and Meijer AH (2017) Modeling Infectious Diseases in the Context of a Developing Immune System. In Current Topics in Developmental Biology 124: 277–329. [DOI] [PubMed] [Google Scholar]
- Mathias JR, Perrin BJ, Liu T-X, Kanki J, Look AT, and Huttenlocher A (2006) Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. Journal of Leukocyte Biology 80: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Meeker ND, and Trede NS (2008) Immunology and zebrafish: Spawning new models of human disease. Developmental & Comparative Immunology 32: 745–757. [DOI] [PubMed] [Google Scholar]
- Meijer AH, Gabby Krens SF, Medina Rodriguez IA, He S, Bitter W, Ewa Snaar-Jagalska B, and Spaink HP (2004) Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Molecular Immunology 40: 773–783. [DOI] [PubMed] [Google Scholar]
- Menanteau-Ledouble S, Karsi A, and Lawrence ML (2011) Importance of skin abrasion as a primary site of adhesion for Edwardsiella ictaluri and impact on invasion and systematic infection in channel catfish Ictalurus punctatus. Veterinary Microbiology 148: 425–430. [DOI] [PubMed] [Google Scholar]
- Menke AL, Spitsbergen JM, Wolterbeek APM, and Woutersen RA (2011) Normal Anatomy and Histology of the Adult Zebrafish. Toxicologic Pathology 39: 759–775. [DOI] [PubMed] [Google Scholar]
- Mitchell KC, Breen P, Britton S, Neely MN, and Withey JH (2017) Quantifying Vibrio cholerae Enterotoxicity in a Zebrafish Infection Model. Appl Environ Microbiol 83: e00783–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D, Breen P, Raychaudhuri S, and Withey JH (2018) Glucose Metabolism by Escherichia coli Inhibits Vibrio cholerae Intestinal Colonization of Zebrafish. Infection and Immunity 86: e00486–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ANY, Jong-Curtain T.A. de, Mawdsley DJ, White SJ, Shin J, Appel B, et al. (2005) Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Developmental Biology 286: 114–135. [DOI] [PubMed] [Google Scholar]
- Nguyen-Chi M, Laplace-Builhé B, Travnickova J, Luz-Crawford P, Tejedor G, Lutfalla G, et al. (2017) TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death and Disease 8: e2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura J, and Aoki T (1985) Morphological analysis of lipopolysaccharide from gramnegative fish pathogenic bacteria. Fish Pathology 20: 193–197. [Google Scholar]
- Oehlers SH, Flores MV, Chen T, Hall CJ, Crosier KE, and Crosier PS (2011) Topographical distribution of antimicrobial genes in the zebrafish intestine. Developmental and Comparative Immunology 35: 385–91. [DOI] [PubMed] [Google Scholar]
- Oehlers SH, Flores MV, Okuda KS, Hall CJ, Crosier KE, and Crosier PS (2011) A chemical enterocolitis model in zebrafish larvae that is dependent on microbiota and responsive to pharmacological agents. Developmental Dynamics 240: 288–298. [DOI] [PubMed] [Google Scholar]
- Okuda J, Takeuchi Y, and Nakai T (2014) Type III secretion system genes of Edwardsiella tarda associated with intracellular replication and virulence in zebrafish. Diseases of Aquatic Organisms 111: 31–39. [DOI] [PubMed] [Google Scholar]
- Olden T, Akhtar T, Beckman SA, and Wallace KN (2008) Differentiation of the zebrafish enteric nervous system and intestinal smooth muscle. Genesis 46: 484–498. [DOI] [PubMed] [Google Scholar]
- Øverland HS, Pettersen EF, Rønneseth A, and Wergeland HI (2010) Phagocytosis by B- cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.). Fish & Shellfish Immunology 28: 193–204. [DOI] [PubMed] [Google Scholar]
- Pack M, Solnica-Krezel L, Malicki J, Neuhauss SCF, Schier AF, Stemple DL, et al. (1996) Mutations affecting development of zebrafish digestive organs. Development 123: 321–328. [DOI] [PubMed] [Google Scholar]
- Palie D, Andreasen CB, Ostojie J, Tell RM, and Roth JA (2007) Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. Journal of Immunological Methods 319: 87–97. [DOI] [PubMed] [Google Scholar]
- Palti Y (2011) Toll-like receptors in bony fish: From genomics to function. Developmental & Comparative Immunology 35: 1263–1272. [DOI] [PubMed] [Google Scholar]
- Pan C-Y, Wu J-L, Hui C-F, Lin C-H, and Chen J-Y (2011) Insights into the antibacterial and immunomodulatory functions of the antimicrobial peptide, epinecidin-1, against Vibrio vulnificus infection in zebrafish. Fish & Shellfish Immunology 31: 1019–1025. [DOI] [PubMed] [Google Scholar]
- Parra D, Korytár T, Takizawa F, and Sunyer JO (2016) B cells and their role in the teleost gut. Developmental & Comparative Immunology 64: 150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AD, Navabpour S, Hicks S, Dougan G, Wallis T, and Frankel G (2000) Enterohaemorrhagic Escherichia coliO157:H7 target Peyer’s patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut 47: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressley ME, Phelan PE, Eckhard Witten P, Mellon MT, and Kim CH (2005) Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Developmental & Comparative Immunology 29: 501–513. [DOI] [PubMed] [Google Scholar]
- Quinlivan VH, and Farber SA (2017) Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front Endocrinol (Lausanne) 8: 319–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Morales F (2012) Acidic pH: enemy or ally for enteric bacteria? Virulence 3: 103–6. [DOI] [PubMed] [Google Scholar]
- Ran C, Qin C, Xie M, Zhang J, Li J, Xie Y, et al. (2018) Aeromonas veronii and aerolysin are important for the pathogenesis of motile aeromonad septicemia in cyprinid fish. Environmental Microbiology 20: 3442–3456. [DOI] [PubMed] [Google Scholar]
- Rauta PR, Nayak B, and Das S (2012) Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunology Letters 148: 23–33. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Mahowald MA, Ley RE, and Gordon JI (2006) Reciprocal Gut Microbiota Transplants from Zebrafish and Mice to Germ-free Recipients Reveal Host Habitat Selection. Cell 127: 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, and Gordon JI (2004) From The Cover: Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proceedings of the National Academy of Sciences 101: 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd MJ, Kelly G, Dunn G, Way M, and Martin P (2006) Imaging macrophage chemotaxis in vivo: Studies of microtubule function in zebrafish wound inflammation. Cell Motility and the Cytoskeleton 63: 415–422. [DOI] [PubMed] [Google Scholar]
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, et al. (2011) Depletion of Murine Intestinal Microbiota: Effects on Gut Mucosa and Epithelial Gene Expression. PLoS ONE 6: e17996–e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw SA, Loynes CA, Trushell DMI, Elworthy S, Ingham PW, and Whyte MKB (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108: 3976–3978. [DOI] [PubMed] [Google Scholar]
- Renshaw SA, and Trede NS (2012) A model 450 million years in the making: zebrafish and vertebrate immunity. Disease Models & Mechanisms 5: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, and Rawls JF (2011) Evidence for a core gut microbiota in the zebrafish. The ISME journal 5: 1595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe HM, Withey JH, and Neely MN (2014) Zebrafish as a model for zoonotic aquatic pathogens. Developmental and comparative immunology 46: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, et al. (2014) Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Applied and environmental microbiology 80: 1710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacho H, Moore PJ, and Hide GR (1990) Aquatically acquired Aeromonas hydrophila wound infection A report of 3 cases. South African Medical Journal 78: 339–340. [PubMed] [Google Scholar]
- Sanderson LE, Chien A-T, Astin JW, Crosier KE, Crosier PS, and Hall CJ (2015) An inducible transgene reports activation of macrophages in live zebrafish larvae. Developmental & Comparative Immunology 53: 63–69. [DOI] [PubMed] [Google Scholar]
- van der Sar A.M., Stockhammer OW, van der Laan C., Spaink HP, Bitter W, and Meijer AH (2006) MyD88 Innate Immune Function in a Zebrafish Embryo Infection Model. Infection and Immunity 74: 2436–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraceni PR, Romero A, Figueras A, and Novoa B (2016) Establishment of Infection Models in Zebrafish Larvae (Danio rerio) to Study the Pathogenesis of Aeromonas hydrophila. Frontiers in Microbiology 7: 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A, and Gut P (2015) Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cellular and Molecular Life Sciences 72: 2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, and Rawls JF (2012) Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host & Microbe 12: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stones DH, Fehr AGJ, Thompson L, Rocha J, Perez-Soto N, Madhavan VTP, et al. (2017) Zebrafish (Danio rerio) as a Vertebrate Model Host To Study Colonization, Pathogenesis, and Transmission of Foodborne Escherichia coli O157. mSphere 2: e00365–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C, Charette J, Catchen J, Lage CR, Giasson G, Postlethwait JH, et al. (2009) The Gene History of Zebrafish tlr4a and tlr4b Is Predictive of Their Divergent Functions. J Immunol 183: 5896–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan C, and Kim CH (2008a) Innate Immune System of the Zebrafish, Danio rerio In Innate Immunity of Plants, Animals, and Humans. Heine H (ed.). Springer Berlin Heidelberg, Berlin, Heidelberg: pp. 113–133. [Google Scholar]
- Sullivan C, and Kim CH (2008b) Zebrafish as a model for infectious disease and immune function. Fish & Shellfish Immunology 25: 341–350. [DOI] [PubMed] [Google Scholar]
- Sullivan C, Matty MA, Jurczyszak D, Gabor KA, Millard PJ, Tobin DM, and Kim CH (2017) Infectious disease models in zebrafish. Methods in Cell Biology 138: 101–136. [DOI] [PubMed] [Google Scholar]
- Sültmann H, Mayer WE, Figueroa F, O’hUigin C, and Klein J (1993) Zebrafish Mhc class II a chain-encoding genes: polymorphism, expression, and function. Immunogenetics 38: 408–420. [DOI] [PubMed] [Google Scholar]
- Sültmann H, Mayer WE, Figueroa F, O’Huigin C, and Klein J (1994) Organization of Mhc Class II B Genes in the Zebrafish (Brachydanio rerio). Genomics 23: 1–14. [DOI] [PubMed] [Google Scholar]
- Takashima S, Gold D, and Hartenstein V (2013) Stem cells and lineages of the intestine: a developmental and evolutionary perspective. Development Genes and Evolution 223: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Figueroa F, O’hUigin C, and Klein J (1995) Cloning and characterization of class I Mhc genes of the zebrafish, Brachydanio rerio. Immunogenetics 42: 77–84. [DOI] [PubMed] [Google Scholar]
- Tarnecki AM, Burgos FA, Ray CL, and Arias CR (2017) Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. Journal of Applied Microbiology 123: 2–17. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, et al. (2016) Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 540: 544–551. [DOI] [PubMed] [Google Scholar]
- Tobin DM, May RC, and Wheeler RT (2012) Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host-Pathogen Interaction. PLOS Pathogens 8: e1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh MC, Goodyear M, Daigneault M, Allen-Vercoe E, and Van Raay TJ (2013) Colonizing the Embryonic Zebrafish Gut with Anaerobic Bacteria Derived from the Human Gastrointestinal Tract. Zebrafish 10: 194–198. [DOI] [PubMed] [Google Scholar]
- Tokunaga Y, Shirouzu M, Sugahara R, Yoshiura Y, Kiryu I, Ototake M, et al. (2017) Comprehensive validation of T- and B-cell deficiency in rag1-null zebrafish: Implication for the robust innate defense mechanisms of teleosts. Sci Rep 7: 7536–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, et al. (2003) The Zebrafish as a Model Organism to Study Development of the Immune System. Advances in Immunology 81: 253–330. [PubMed] [Google Scholar]
- Trede NS, Langenau DM, Traver D, Look AT, and Zon LI (2004) The Use of Zebrafish to Understand Immunity. Immunity 20: 367–379. [DOI] [PubMed] [Google Scholar]
- Trede NS, and Zon LI (1998) Development of T-cells during fish embryogenesis. Developmental & Comparative Immunology 22: 253–263. [DOI] [PubMed] [Google Scholar]
- Tsai Y-H, Huang T-J, Hsu RW-W, Weng Y-J, Hsu W-H, Huang K-C, and Peng KT (2009) Necrotizing Soft-Tissue Infections and Primary Sepsis Caused by Vibrio vulnificus and Vibrio cholerae non-O1. The Journal of Trauma: Injury, Infection, and Critical Care 66: 899–905. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, and Gordon JI (2008) Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host & Microbe 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrkalska SD, Candel S, Angosto D, Gómez-Abellán V, Martín-Sánchez F, García-Moreno D, et al. (2016) Neutrophils mediate Salmonella Typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nature communications 7: 12077–12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysnes K, Jorgensen A, Poppe T, Midtlyng P, and Robertson L (2012) Preliminary experiments on use of zebrafish as a laboratory model for Giardia duodenalis infection. Acta Parasitologica 57: 1–6. [DOI] [PubMed] [Google Scholar]
- Udayangani RMC, Dananjaya SHS, Fronte B, Kim C-H, Lee J, and De Zoysa M (2017) Feeding of nano scale oats β-glucan enhances the host resistance against Edwardsiella tarda and protective immune modulation in zebrafish larvae. Fish & Shellfish Immunology 60: 72–77. [DOI] [PubMed] [Google Scholar]
- Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, and Itoh K (1999) Differential Roles of Segmented Filamentous Bacteria and Clostridia in Development of the Intestinal Immune System. Infection and Immunity 67: 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe C, Folch H, Enriquez R, and Moran G (2011) Innate and adaptive immunity in teleost fish: a review. Veterinární Medicína 56: 486–503. [Google Scholar]
- Van Dycke J, Ny A, Conceiçào-Neto N, Maes J, Hosmillo M, Cuvry A, et al. (2019) A robust human norovirus replication model in zebrafish larvae. PLOS Pathogens 15: e1008009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M-J, Caruffo M, Herrera Y, Medina DA, Coronado M, Feijóo CG, et al. (2018) Evaluating the Capacity of Human Gut Microorganisms to Colonize the Zebrafish Larvae (Danio rerio). Frontiers in Microbiology 9: 1032–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas M, Ortíz-Severín J, Marcoleta AE, Díaz-Pascual F, Allende ML, Santiviago CA, and Chávez FP (2017) Salmonella Typhimurium induces cloacitis-like symptomsin zebrafish larvae. Microbial Pathogenesis 107: 317–320. [DOI] [PubMed] [Google Scholar]
- Varas MA, Ortiz-Severin J, Marcoleta AE, Santiviago CA, Allende ML, and Chávez FP (2019) Static Immersion and Injection Methods for Live Cell Imaging of Foodborne Pathogen Infections in Zebrafish Larvae. Methods in Molecular Biology 1918: 183–190. [DOI] [PubMed] [Google Scholar]
- Ventura MT, and Grizzle JM (1987) Evaluation of portals of entry of Aeromonas hydrophila in channel catfish. Aquaculture 65: 205–214. [Google Scholar]
- Wallace KN, Akhter S, Smith EM, Lorent K, and Pack M (2005) Intestinal growth and differentiation in zebrafish. Mechanisms of Development 122: 157–173. [DOI] [PubMed] [Google Scholar]
- Wallace KN, and Pack M (2003) Unique and conserved aspects of gut development in zebrafish. Developmental Biology 255: 12–29. [DOI] [PubMed] [Google Scholar]
- Walton EM, Cronan MR, Beerman RW, and Tobin DM (2015) The Macrophage-Specific Promoter mfap4 Allows Live, Long-Term Analysis of Macrophage Behavior during Mycobacterial Infection in Zebrafish. PLOS ONE 10: e0138949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Du J, Lam SH, Mathavan S, Matsudaira P, and Gong Z (2010) Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genomics 11: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AC (2003) The zebrafish spi1 promoter drives myeloid-specific expression in stable transgenic fish. Blood 102: 3238–3240. [DOI] [PubMed] [Google Scholar]
- Willett CE, Cortes A, Zuasti A, and Zapata AG (1999) Early hematopoiesis and developing lymphoid organs in the zebrafish. Developmental Dynamics 214: 323–336. [DOI] [PubMed] [Google Scholar]
- Wu XM, Cao L, Nie P, Chang MX (2019) Histone H2A cooperates with RIP2 to induce the expression of antibacterial genes and MHC related genes. Developmental & Comparative Immunology 101: 103455. [DOI] [PubMed] [Google Scholar]
- Wu S, Wang L, Xu G, Yang S, Deng Q, Li Y, and Huang R (2017) spv locus aggravates Salmonella infection of zebrafish adult by inducing Th1/Th2 shift to Th2 polarization. Fish & Shellfish Immunology 67: 684–691. [DOI] [PubMed] [Google Scholar]
- Xu T, Su Y, Xu Y, He Y, Wang B, Dong X, et al. (2014) Mutations of flagellar genes fliC12, fliA and flhDC of Edwardsiella tarda attenuated bacterial motility, biofilm formation and virulence to fish. Journal of Applied Microbiology 116: 236–244. [DOI] [PubMed] [Google Scholar]
- Yang H-T, Zou S-S, Zhai L-J, Wang Y, Zhang F-M, An L-G, and Yang G-W (2017) Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish & Shellfish Immunology 71: 35–42. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Dong X, Chen B, Zhang Y, Zu Y, and Li W (2016) Zebrafish as a useful model for zoonotic Vibrio parahaemolyticus pathogenicity in fish and human. Developmental & Comparative Immunology 55: 159–168. [DOI] [PubMed] [Google Scholar]
- Zhu L, Lin A, Shao T, Nie L, Dong W, Xiang L, and Shao J (2014) B Cells in Teleost Fish Act as Pivotal Initiating APCs in Priming Adaptive Immunity: An Evolutionary Perspective on the Origin of the B-1 Cell Subset and B7 Molecules. J Immunol 192: 2699. [DOI] [PubMed] [Google Scholar]
- Zou J, Mercier C, Koussounadis A, and Secombes C (2007) Discovery of multiple beta-defensin like homologues in teleost fish. Molecular Immunology 44: 638–647. [DOI] [PubMed] [Google Scholar]