Abstract
c-Jun N-terminal kinases (JNKs) are members of the mitogen-activated protein kinase (MAPK) family and are derived from three genes, Jnk1-3. These kinases are involved in cellular responses to homeostatic insults, such as inflammation and apoptosis. Furthermore, increased JNK expression and activation are associated with debilitating neurodegenerative diseases, including Alzheimer’s and Parkinson’s. We previously reported elevated levels of phosphorylated JNK (pJNK), indicative of JNK hyperactivation, in the CA1 hippocampus of chronically epileptic rats. We also showed that pharmacological inhibition of JNK activity reduced seizure frequency in a dose-dependent fashion (Tai TY et al., Neuroscience, 2017). Building on these observations, the objectives of this current study were to investigate the timeline of JNK activation during epileptogenesis, and to identify the JNK isoform(s) that undergo hyperactivation in the chronic epilepsy stage. Western blotting analysis of CA1 hippocampal homogenates showed JNK hyperactivation only during the chronic phase of epilepsy (6–9 weeks post-status epilepticus), and not in earlier stages of epileptogenesis (1 h, 1 day, and 1 week post-status epilepticus). After enrichment for pJNK by immunoprecipitation, we identified JNK2 as the only significantly hyperactivated JNK isoform, with expression of the 54 kDa pJNK2 variant elevated to a greater extent than the 46 kDa pJNK2 variant. Expression of the total amounts of both JNK2 variants (phosphorylated plus non-phosphorylated) was reduced in epilepsy, however, suggesting that activation of upstream phosphorylation pathways was responsible for JNK2 hyperactivation. Since our prior work demonstrated that pharmacological inhibition of JNK activation had an antiepileptic effect, JNK2 hyperactivation is therefore likely a pathological event that promotes seizure occurrences. This investigation provides evidence that JNK2 is selectively hyperactivated in epilepsy and thus may be a novel and selective antiepileptic target.
Keywords: Epilepsy, Epileptogenesis, JNK, Phosphorylation, Status epilepticus, Pilocarpine
Introduction
c-Jun N-terminal kinases (JNKs) are members of the mitogen-activated kinase family that includes p38 mitogen-activated kinase (p38 MAPK) and extracellular signal-regulated kinase (ERK). These kinases have in common activation by phosphorylation via upstream kinases, and subsequent phosphorylation of downstream targets, altering target function. JNKs are also referred to as stress-activated protein kinases (SAPKs) since they are activated by cellular metabolic stress, such as hypoxia or inflammation. JNKs in particular are known for their role in promoting neuronal apoptosis in the setting of excitotoxic insults or neurodegenerative conditions (Mehan et al., 2011). The JNK family consists of three isoforms (JNK1, JNK2, and JNK3), and each in turn is expressed by mRNA splice variants (10 in total), occurring within the N-terminal or the C-terminal of JNK mRNA. JNK splice variants translated into protein migrate electrophoretically at either 54 or 46 kilodaltons (kDa) (Coffey, 2014).
The role of JNK activation in excitotoxic neuronal apoptosis and neurodegenerative disease is well established. Several studies have shown that constitutive deletion of JNK3, the brain-specific isoform, or JNK1, which is globally expressed, conferred resistance to hippocampal pyramidal neuron death following challenge with kainic acid (Yang et al., 1997; de Lemos et al., 2018). Other work showed that JNK2, which is also expressed globally, and JNK3 are hyperactivated in dopaminergic neurons in Parkinson’s disease and contribute to the apoptotic loss of neurons in that disease (Huang et al., 2016). JNKs also mediate in part the hyperphosphorylation of tau in Alzheimer’s disease (AD), with JNK2 responsible for the largest number of tau phosphosites (Yoshida et al., 2004; Mehan et al., 2011; Ploia et al., 2011). The hyperphosphorylation of tau by JNKs promotes its aggregation into insoluble fibrils, leading to loss of its physiological function. Activated JNK is detectable in the cerebrospinal fluid of human patients with AD, and may serve as a biomarker of that condition (Paquet et al., 2015).
There are few studies of the role of JNK in epilepsy. As mentioned, JNK3−/− mice fail to show neurodegeneration following acute provocation of seizures by kainic acid (Yang et al., 1997; de Lemos et al., 2018). We published one of the first studies to examine JNK activation in chronically epileptic animals generated by pilocarpine-induced status epilepticus (SE) (Tai et al., 2017). This is a well-established animal model for human acquired temporal lobe epilepsy that we and others have characterized previously (Turski et al., 1983; Jung et al., 2007; Toyoda et al., 2013). Our prior study demonstrated aggregate elevated activation levels, or hyperactivation, of JNK isoforms in the CA1 region of the hippocampus of chronically epileptic rats experiencing frequent convulsive seizures. Interestingly, pharmacologic inhibition of JNK activity reduced spontaneous seizure frequency in a dose-dependent fashion, suggesting that JNK activation plays a role in sustaining the epileptic state.
Our prior study left several unanswered questions about the role of JNK activation in epilepsy. First, what is the time course of JNK activation during epileptogenesis, the development of epilepsy following a brain insult? It is possible that JNK activation precedes the development of spontaneously recurrent seizures, which would suggest that JNK activation plays a causative role in epileptogenesis. Conversely, JNK activation might occur concomitantly with the development of epilepsy, and thus may be a consequence of recurrent seizures. Second, are all JNK isoforms activated equally in the development of epilepsy, or do some isoforms and splice variants play a more prominent role? The answer to this question might identify JNK isoforms that could be specifically targeted as a novel antiepileptic pharmacologic strategy to reduce seizure frequency.
To address these questions, we measured activated (phosphorylated) and total (phosphorylated and non-phosphorylated) JNK expression in the CA1 hippocampal region of rats over a range of time points following the induction of epilepsy with pilocarpine-induced SE. We then quantitated the relative activation of each JNK isoform in the chronic phase of epilepsy. Our results demonstrate that JNK activation is increased only during chronic epilepsy and not during earlier time points of epileptogenesis. Further, only the JNK2 isoform is hyperactivated in chronic epilepsy, with greater activation of the 54 kDa splice variant. These results suggest that JNK2 is a specific molecular target that may be involved in the maintenance of the chronic epileptic state, as manifested by recurrent seizures and hippocampal neurodegeneration.
Experimental procedures
Pilocarpine-induced status epilepticus (SE)
Experimental animals were generated using the pilocarpine protocol as previously described (Jung et al., 2007). The University of Washington Institutional Animal Care and Use Committee approved all animal procedures. In brief, 6-week-old male Sprague Dawley rats underwent induction of SE with pilocarpine hydrochloride (385 mg/kg intraperitoneal [i.p.]) after pretreatment with scopolamine methylnitrate (1 mg/kg i.p.). After 60 min of convulsive SE, seizures were terminated with repeated doses of phenobarbital (PB; 15 mg/kg i.p.) every 30−45 min until cessation of convulsive motor activity. The rats comprising the 1 h post-SE cohort were sacrificed instead of receiving PB. For other study time points representing different stages of epileptogenesis (1 day, 1 week, and six-to-nine weeks post-SE), animals were singly housed until time of sacrifice. At sacrifice, these rats were exposed to isoflurane (5 %) and injected with ketamine/xylazine anesthesia (87/13 mg/kg i.p.). Age-matched, singly housed naïve rats were used as controls and were sacrificed at the same time points described above. After brain slicing using previously described procedures (Williams et al., 2015), the rat brain slices were frozen and stored at −80 °C. We focused on the CA1 hippocampus for two reasons: 1) it is the most affected region both in the mesial temporal lobe in human temporal lobe epilepsy (TLE) and in the rodent pilocarpine model of TLE (Najm et al., 2006; Curia et al., 2008); and 2) our long history of published work on the CA1 hippocampus consistently showed alterations in both expression of ion channels and phosphorylation signaling in CA1 pyramidal neurons (e.g. (Jung et al., 2007, 2010)).
Western analyses
Quantification of protein expression from CA1 hippocampus via western blotting was performed as previously described (Williams et al., 2015; Tai et al., 2017). CA1 hippocampal regions were microdissected from frozen rat brain slices on dry ice, pooled, and homogenized in the following buffer: 50 mM Tris, 5 mM EDTA, 50 mM NaCl, 1 mM sodium orthovanadate, 10 mM EGTA, 2 mM sodium pyrophosphate, 4 mM para-nitrophenylphosphate, 4 ug/ml aprotinin, 20 ug/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 % Triton X-100 (Roberson et al., 1999). After centrifuging at 20,800 x g at 4 °C for 15 min, the supernatant was transferred to a clean tube. Protein concentration of each supernatant sample was determined by a bicinchoninic acid (BCA) assay (Pierce, ThermoFisher Scientific, Waltham, MA), and samples were mixed with an equal volume of 2x Laemmli buffer (5 % β-mercaptoethanol). After boiling, each sample was loaded in multiple lanes in doubling succession (three or four different amounts) to ensure signal detection occurred within a linear range (Jung et al., 2010).
Each 4–15 % polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA) contained one experimental sample and one age-matched control sample. After transferring proteins to a nitrocellulose membrane, we performed western blotting. The following antibodies (Abs) were used: anti-JNK1 (Cell Signaling Technology, Danvers, MA; catalog #3708); anti-JNK2 (#9258); anti-JNK3 (#2305); pan-specific anti-JNK (#9252); and anti-phosphoJNK (pJNK) (#9251). An antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Life Technologies, ThermoFisher Scientific, Waltham, MA; catalog #AM4300) served as a protein sample loading control. When necessary, stripping of membranes was done before re-probing with a subsequent primary antibody. Detection and analyses of signals from fluorescent secondary antibodies (anti-rabbit IgG (heavy and light chains)-Dylight800 and the anti-mouse-IgG (heavy and light chains)-Dylight680; ThermoFisher Scientific, Waltham, MA) were achieved with the Odyssey CLx Image Studio software (Li-Cor Biosciences, Lincoln, NE). Protein level comparisons were accomplished as previously described (Jung et al., 2010). Briefly, we loaded multiple lanes in doubling succession for each rat homogenate sample, and band intensity for each lane was quantified. A linear function was fit for band intensity vs. protein amount (including the origin), and the slope of the line (m) estimated. Comparisons between the test and control conditions were calculated as the ratio of the slopes (mtest / mctrl) for samples loaded within the same blot. Normalization was achieved by the ratio of GAPDH quantifications from that same homogenate pairing. GAPDH expression on average varied no more than 1–2 % between control and test conditions (data not shown). This technique provides added rigor in quantitating western blots in that readings from multiple lanes are incorporated, and only blots showing a linear relationship of band intensity vs. protein amount were analyzed, ensuring that signal detection was within the linear range of the imaging device.
Enrichment of pJNK by immunoprecipitation (IP)
Supernatants from each pooled microdissected CA1 region were prepared the same as described above. After adding anti-pJNK Ab (CST #9251), samples were incubated with gentle agitation overnight at 4 °C. Protein A/G ultralink resin (ThermoFisher Scientific) was added, and the samples were incubated with gentle agitation at 4 °C for 4−6 h. After washing three times with homogenization buffer, we eluted the mixture with 1x Laemmli buffer with 5 % sodium dodecyl sulfate and 25 mM dithiothreitol.
We performed western blotting on these eluates similarly to the method described above. However, two major differences must be noted. Firstly, because the enrichment process used an anti-pJNK Ab, pJNK levels were used as the protein sample loading control. Secondly, the anti-pJNK Ab used for IP co-elutes with the enriched pJNK and is present as 50 kDa and 25 kDa electrophoretic bands (the two known components for IgG Abs). The contaminating 50 kDa electrophoretic band would interfere with the 54 kDa JNK band analyses if the secondary Ab used in the western protocol was of the same (rabbit) host species as the pJNK Ab. This was the case for IP of JNK2 and JNK3. We therefore conjugated an anti-rabbit IgG-light chain Ab to an infrared (IR, 800 nm) dye (IR Dye 800CW Protein labeling kit-HMW, Li-Cor Biosciences). The resulting IR-secondary Ab functioned as expected to recognize the primary Abs used in the western blotting experiments but also recognized only the 25 kDa band and not the 50 kDa band from the eluate-contaminating Ab used for IP (see Fig. 3C as an example). There exists an additional band that is of higher molecular weight and distinguishable from the 54 kDa JNK band. This band is artifactual, since IP controls that did not contain lysates showed this band but not the 46 and 54 kDa JNK bands (data not shown); this artifactual band has also been reported in IP experiments (Lee et al., 1999). With westerns that did not involve IP, although the secondary IR-labelled anti-rabbit IgG Ab recognized both the heavy and light chains, there were no contaminating Abs within the samples that would interfere with the detection of the JNK electrophoretic bands (see Fig. 1, Fig. 2). For JNK1 detection, the anti-JNK1 Ab was of mouse host. Therefore, the secondary Ab used for JNK1 staining did not recognize the contaminating anti-pJNK Ab used in IP (see Fig. 3B). Lastly, protein level comparisons between the post-SE rat homogenates and their controls were achieved by the same procedure as described in “Western analyses” above. However, instead of GAPDH, pJNK was used for normalization.
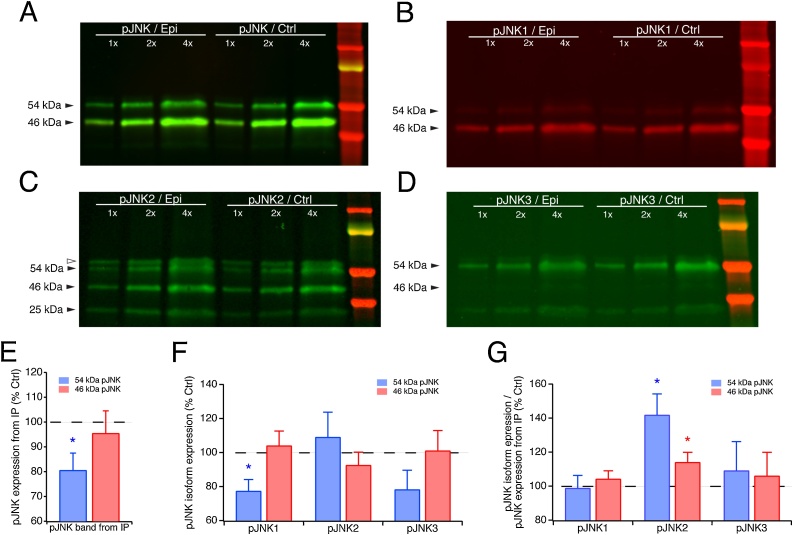
Fig. 3.
Upregulation of pJNK isoform expression in chronic epilepsy. A. Western blot of immunoprecipitated pJNK from the CA1 hippocampus of chronically epileptic animals and age-matched naïve controls. Shown are three different protein loading amounts under each condition and a lane containing molecular weight standards. Epileptic tissue showed less total pJNK expression than control, most likely due to hippocampal neuronal loss in chronic epilepsy. B. When immunoprecipitated pJNK was probed for expression of the JNK1 isoform, pJNK1 expression normalized to overall pJNK expression was unchanged compared to control conditions. C. Expression of pJNK2 showed a different pattern from pJNK1 and pJNK3, with higher expression in epileptic compared to control conditions. Of note, immunoprecipitation with the anti-pJNK antibody produced two artifactual electrophoretic bands as shown in Fig. 3C. One is the band at a slightly larger molecular weight than the 54 kDa JNK band and is demarcated by the open triangle, while the other is labeled as a 25 kDa band. The identities of these artifactual bands are discussed in Experimental procedures. D. pJNK3 expression, like pJNK1, was unchanged compared to control conditions. E. Quantification of the two pJNK electrophoretic bands, in which the 54 kDa band is significantly decreased in chronic epileptic rats but not the 46 kDa band. (F & G) Quantification of pJNK isoform-specific expression before (F) and after (G) normalization to overall immunoprecipitated pJNK. Only pJNK2 showed increased expression under epileptic conditions, while pJNK1 and pJNK3 showed no significant change. The 54 kDa variant of pJNK2 showed the larger increase in epilepsy.
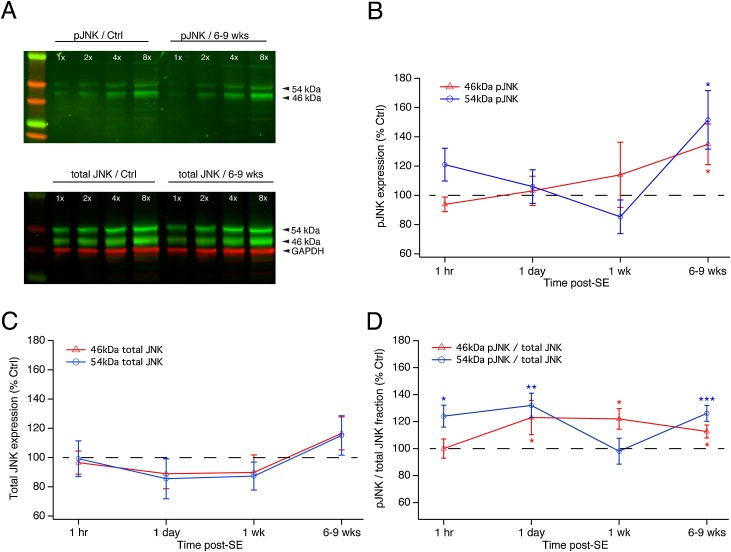
Fig. 1.
Time course of JNK expression during epileptogenesis. A. Example western blot of JNK expression in rat CA1 hippocampus during the chronic epilepsy phase (6–9 weeks post-status epilepticus [post-SE]) compared to age-matched controls. When probed with a primary antibody that recognizes either all phosphorylated JNK isoforms (pJNK) or JNK isoforms regardless of phosphorylation state (total JNK), JNK expression is seen in two electrophoretic bands at 54 and 46 kDa. Shown are four different protein loading amounts in each condition, and a molecular weight standard in the leftmost lane. GAPDH expression was measured as a control for protein loading. B. Time course of pJNK expression during epileptogenesis shows that there is no significant change at times from 1 h to 1 week post-SE. In chronic epilepsy 6–9 weeks post-SE, there is significant elevation of pJNK expression in both 54 and 46 kDa bands compared to control. C. Total JNK expression during epileptogenesis shows no significant change at any time point post-SE. D. The phosphorylated fraction of JNK expression shows significant increases for both 54 and 46 kDa bands in chronic epilepsy and at some intermediate time points during epileptogenesis.
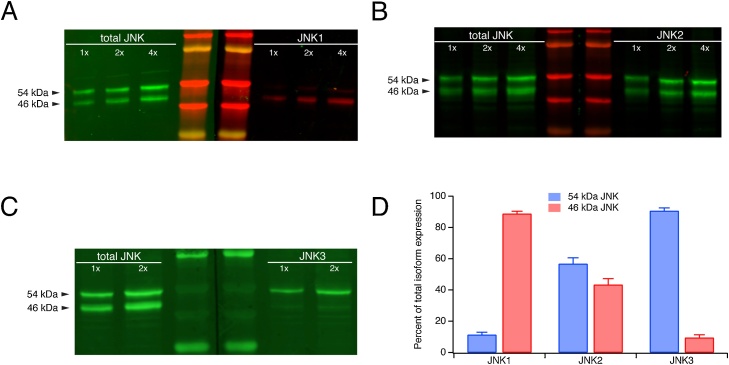
Fig. 2.
Segregation of JNK isoforms into 54 and 46 kDa bands. A. Western blots from a single sample probed with a pan-specific antibody against total JNK (all three isoforms, irrespective of phosphorylation state) and with an isoform-specific JNK1 antibody. Shown are three different protein loading amounts in each western, and two lanes with molecular weight standards. The JNK1 isoform migrated predominantly but not completely in the 46 kDa band. B. The JNK2 isoform migrated nearly equally in the 54 and 46 kDa bands. C. The JNK3 isoform was found almost but not entirely in the 54 kDa band. D. Quantification of the percentage of expression of each JNK isoform in the 54 and 46 kDa bands.
Statistics
Statistics are reported as means ± SEM. With α = 0.05, significance was determined by two-tailed t-tests by using either InStat v3.b or Prism 8 (both from GraphPad, La Jolla, CA). All data sets passed normality testing excepting 54 kDa pJNK1 in Fig. 3G. In that case, both parametric and non-parametric (Wilcoxon) testing resulted in non-significant changes in the post-SE rat cohort compared to the age-matched controls.
Results
Previously, we reported elevated levels of phosphorylated JNK (pJNK) in the chronic epilepsy phase of the rat pilocarpine-induced epilepsy model (Tai et al., 2017), indicating increased overall JNK activation (termed throughout as “hyperactivation”). In that study, pJNK levels were determined by quantifying the activation in chronically epileptic rats of all three JNK isoforms in aggregate that electrophoretically migrate at 54 and 46 kDa. In the current study, we began by measuring expression and activation of each of the two JNK bands over four time points post-SE: 1 h, 1 day, 1 week, and 6–9 weeks. The rationale for choosing these post-SE time points is supported by prior work that methodically evaluated seizure activity in pilocarpine-induced rats using continuous video-EEG monitoring (Jung et al., 2007). The 1 h time point comprises the period when the animal is still actively seizing while in SE. At 1 day, SE has completely abated, but from this time point extending for several days (known as the “latent period”) spontaneous seizures have not yet begun. At 1 week, many animals will begin having spontaneous seizures, but at low frequency. By the 6–9 week time point, virtually all animals will be epileptic, with spontaneous convulsive seizures occurring at increased frequency (on average 3/day) compared to the one-week time point. These time points thus encompass the entire range of epileptogenesis, from induction by SE, through the latent period, and into the chronic phase of epilepsy.
For the first experiment, as in the previous study (Tai et al., 2017), we assayed expression of JNK and its activated form using western blotting. We measured total JNK levels with an Ab that recognizes both phosphorylated and non-phosphorylated forms. We also determined the activation levels of JNK with a phosphospecific Ab that recognizes phosphorylated JNKs. Each antibody is pan-specific and recognizes JNKs irrespective of isoform identity. CA1 hippocampal tissue was collected from pilocarpine-injected rats at the four stages of epileptogenesis and from age-matched naïve controls. As shown in Fig. 1A, both pJNK and total JNK migrate in 54 and 46 kDa bands. GAPDH expression was also measured and used to normalize protein loading between epilepsy and naïve conditions. As described in the Experimental Procedures, quantification of protein expression in each group was achieved by calculating a linear fit of band intensity vs. protein amount for the multiple protein loading amounts in each condition. The ratio of the slopes of the linear functions found for post-SE and their age-matched controls yielded the change in protein expression between the two conditions. This method ensures more accurate determination of changes in protein expression in that multiple lanes are quantified within each blot, and that signal detection of band intensity is verified to be within the linear range of the imaging device.
Fig. 1B shows the expression of activated pJNK during epileptogenesis. Neither the 54 nor the 46 kDa isoforms showed significant changes at the 1 h through 1 week time points post-SE. However, in chronic epilepsy at 6–9 weeks post-SE, both isoforms showed significant elevation of pJNK levels: the 54 kDa pJNK band showed a 152 ± 20.0 % of control increase (n = 16, p = 0.021), while the 46 kDa pJNK band was increased to 135 ± 13.9 % of control (p = 0.025). This result confirmed our previous finding of higher aggregate JNK activation in the chronic phase of epilepsy, and showed that JNKs in both electrophoretic bands underwent hyperactivation.
We next examined if the total expression of JNK (both phosphorylated and nonphosphorylated forms) was altered during any of the epileptogenesis stages. Quantification of total JNK protein levels in each of the electrophoretic bands showed no significant differences between the post-SE rats and their age-matched naïve controls at any time point (Fig. 1C). Our previous study did show that aggregate total JNK levels, when the 54 and 46 kDa bands were quantified together, were modestly yet significantly elevated in our chronically epileptic animals, even though that was not seen here (Tai et al., 2017). We then measured the fractional activation of JNK during epileptogenesis, as quantified by the ratio of phosphorylated JNK to total JNK expression within each animal (Fig. 1D). We reasoned that the percentage of activated JNK from the available pool of total JNK may differ in the post-SE rats, and would reflect activity of phosphorylation pathways upstream of JNK. Significant elevations of JNK fractional activation were seen at all time points and were different for the two electrophoretic bands. Fractional activation of the 54 kDa band was increased at the 1 h post-SE time point (124 ± 8.1 % of control, n = 7, p = 0.030); 1 day (132 ± 9.2 %, n = 14, p = 0.004); and 6–9 weeks (126 ± 5.9 %, n = 16, p < 0.001). For the 46 kDa band, increases in fractional JNK activation occurred at all time points following 1 h post-SE: 1 day (123 ± 12.8 %, n = 12, p = 0.048); 1 week (122 ± 7.6 %, n = 10, p = 0.018); and 6–9 weeks (113 ± 4.8 %, n = 16, p = 0.017). These results demonstrate that the increased expression of pJNK in chronic epilepsy represents an upregulation of upstream phosphorylation pathways that increase the fractional activation of the total JNK pool, and is not mediated by increased total JNK expression. At earlier time points during epileptogenesis, however, the increased fractional activation of JNK by upstream phosphorylation did not produce significant increases in pJNK expression; this is likely due to a modest reduction of total JNK expression (which as shown in Fig. 1C was not statistically significant).
Because overall JNK protein expression in our rat CA1 hippocampal homogenates consists of a mixture of three isoforms migrating electrophoretically at 54 and 46 kDa, we next sought to determine the distribution of JNK isoforms within those two electrophoretic bands in our chronically epileptic rats. We employed JNK isoform-specific antibodies recognizing total (phosphorylated and non-phosphorylated) JNK (Fig. 2A–C), and quantified the expression within each band as a percentage of total expression (sum of the two bands) for each isoform (Fig. 2D). We also probed each sample with a pan-specific antibody recognizing overall (i.e. all isoforms) JNK expression for comparison. When JNK expression in chronic epilepsy was probed with a JNK1-specific antibody (Fig. 2A and D), 11.3 ± 1.7 % (n = 4) of total expression resided in the 54 kDa band, while 86.7 % was found in the 46 kDa band. For JNK2 (Fig. 2B and D), 56.7 ± 3.9 % (n = 3) was found in the 54 kDa band while 43.3 % was in the 46 kDa band. These patterns for JNK1 and JNK2 are congruous with previous studies involving rodent hippocampal tissues (Waetzig and Herdegen, 2004; Brecht et al., 2005; Eminel et al., 2008; Coffey, 2014). For JNK3 (Fig. 2C and D), 90.6 ± 1.9 % (n = 3) was in the 54 kDa band, with 9.4 % residing in the 46 kDa band. This distribution is consistent with several investigations of JNK3 (Waetzig and Herdegen, 2004; Björkblom et al., 2008; Yoon et al., 2012; Liu et al., 2018), although two studies reported that JNK3 is predominantly found in the 46 kDa band (Brecht et al., 2005; Eminel et al., 2008). We observed similar distribution patterns for each JNK isoform in brain lysates from naïve rats and from wildtype C57Bl6 mice (data not shown), indicating that the above JNK patterns are not disease- nor species-related.
In summary, the 54 kDa band is composed predominantly of JNK2 and JNK3, while the 46 kDa band mainly is composed of JNK1 and JNK2. Considered differently, JNK1 largely migrates in the 46 kDa band; JNK3 mostly in the 54 kDa band; and JNK2 expression is split roughly equally between the two bands.
Having determined that significant JNK hyperactivation occurs only in the chronic epilepsy stage of our animal model, and having confirmed the relative distribution of each JNK isoform in the two electrophoretic bands, we then asked which JNK isoforms are hyperactivated. Our approach to this question involved immunoprecipitating pJNK with the pan-specific pJNK antibody used in the previous experiments, then probing that enriched pJNK homogenate with isoform-specific total JNK antibodies. Because only pJNK is pulled down from tissue lysates, we could not normalize pJNK expression between epileptic and control conditions using a different housekeeping protein. Lesser amounts of pJNK were pulled down from epileptic tissue: as shown in Fig. 3A and quantified in Fig. 3E, lesser amounts of pJNK migrating at 54 kDa (80.5 ± 7.0 % of control, n = 8, p = 0.027) but not pJNK migrating at 46 kDa (95.4 ± 9.1 % of control, n = 8, p > 0.05) were pulled down from CA1 hippocampal homogenates in epileptic compared to naïve conditions. This decrease in the 54 kDa pJNK band could be related to the neuronal loss seen in the hippocampus of the rodent pilocarpine model of TLE (Fujikawa, 1996; Curia et al., 2008). We then probed each blot with a JNK isoform-specific antibody to determine whether there were changes in the JNK isoform composition of overall pJNK expression. Before normalization by pJNK, none of pJNK isoforms showed a statistically significant change in pJNK exression, except the 54 kDa pJNK1 isoform (Fig. 3F; 77.3 ± 6.8 % of control, n = 8, p = 0.027). It was apparent however, that the expression of pJNK1 and pJNK3 shown in Fig. 3F followed the pattern of overall pJNK seen in Fig. 3E, such that after normalization of JNK isoform expression in each electrophoretic band by overall pJNK expression in each electrophoretic band, there was no significant change in pJNK1 or pJNK3 expression in epilepsy (Fig. 3G). However, pJNK2 expression after normalization was significantly increased in chronic epilepsy, demonstrating an upregulation in JNK2 activation (Fig. 3G). This elevated JNK2 phosphorylation was observed in both electrophoretic bands, with the greater increase in phosphorylation occurring in the 54 kDa variant (54 kDa: 142 ± 12.4 %, n = 8, p = 0.012; 46 kDa: 114 ± 5.9 %, n = 8, p = 0.048). Interestingly, this demonstrates that the pJNK hyperactivation seen in chronic epilepsy in the 54 and 46 kDa bands is mediated only by the JNK2 isoform (which migrates approximately equally in both bands), and not by the JNK3 and JNK1 isoforms, which predominantly migrate in the 54 and 46 kDa bands, respectively.
We then asked whether the increased expression of pJNK2 in chronic epilepsy was due in part to an increase in the total (phosphorylated and non-phosphorylated) expression of JNK2.
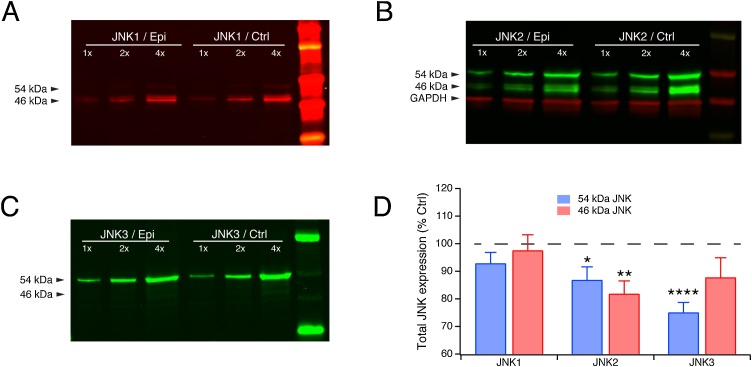
Using isoform-specific total JNK antibodies, we quantified expression of the three JNK isoforms in raw (non-immunoprecipitated) homogenates from animals in the chronic epilepsy condition compared to naïve control conditions. Sample western blots for each JNK isoforms are shown in Fig. 4A–C. Quantification of the change for each JNK isoform expression in epilepsy compared to control after normalization by GAPDH is shown in Fig. 4D. We determined that none of the JNK isoforms showed increased total expression in epilepsy. In fact, both variants of JNK2 and the 54 kDa variant of JNK3 showed modest but significantly downregulated total expression. JNK2 54 kDa expression in chronic epilepsy as a percentage of control expression was 86.8 ± 4.8 % (n = 13, p = 0.019); 46 kDa expression was 81.8 ± 4.7 % (n = 13, p = 0.002). JNK3 expression for the 54 kDa variant was 75.0 ± 3.7 % of control (n = 13, p < 0.0001), while the 46 kDa variant was unchanged at 87.7 ± 7.2 % (n = 13, p > 0.05). JNK1 expression in epilepsy was unchanged from control (54 kDa: 92.8 ± 4.0 %; 46 kDa: 97.5 ± 5.7 %; both n = 13, p > 0.05). These results appear to be at odds with those shown in Fig. 1C showing unchanged aggregate expression of JNK isoforms in chronic epilepsy when probed with a pan-specific JNK antibody; however, the 54 and 46 kDa bands comprise a mixture of JNK isoforms and splice variants of varying relative abundances, and the changes in expression of one isoform variant may be obscured by the relative abundance of the other JNK isoforms and splice variants. Also, since the three antibodies used for staining each of the three JNK isoforms were monoclonal, while the antibody used to detect total JNK was polyclonal (which may lower specificity), measurements of JNK expression using isoform-specific monoclonal antibodies should provide a more accurate analysis than those using the polyclonal pan-specific JNK antibody. With regard to the significantly increased pJNK2 expression seen in chronic epilepsy, we conclude that it is a result of hyperactivation of upstream phosphorylation pathways alone, given that total expression of the JNK2 isoform in epilepsy is decreased.
Fig. 4.
Expression of total JNK isoforms in chronic epilepsy. A. Western blot showing total expression of JNK1 isoform (phosphorylated plus non-phosphorylated) in chronic epilepsy and naïve control conditions. JNK1 expression was not significantly changed in the epileptic condition. B. Expression of JNK2 was reduced in epilepsy for both the 54 and 46 kDa variants. C. Expression of only the 54 kDa variant of JNK3 was significantly reduced under epileptic conditions. D. Quantification of JNK isoform expression in the epileptic condition as a percentage of control expression shows significant downregulation of both JNK2 variants as well as the 54 kDa JNK3 variant.
Discussion
We studied the hyperactivation of JNK signaling during the development of epilepsy in an animal model of acquired temporal lobe epilepsy. The motivation for this project was to follow up on our prior finding that pJNK expression in the aggregate was hyperactivated in chronic epilepsy. We sought to characterize the time course of JNK hyperactivation so to understand whether it was contributing to epileptogenesis or was more likely a consequence of chronic epilepsy, as well as to understand which of the three JNK isoforms were significantly hyperactivated. We found that JNK hyperactivation did not occur early in epileptogenesis, but rather at a time point when animals were experiencing frequent convulsive seizures. We also found that of the three JNK isoforms, only JNK2 showed hyperactivation, with an ∼140 % of control increase in expression of the 54 kDa pJNK2 variant, and an ∼115 % of control increase in the 46 kDa variant. The increase in the activated form of JNK2 occurred without an increase in total JNK2 expression, implying that increased activation of upstream phosphorylation signaling is responsible for JNK2 hyperactivation.
We have previously characterized the course of epileptogenesis in the pilocarpine-induced post-SE model of acquired epilepsy. Using continuous video-EEG monitoring over multiple weeks post-SE, we have found that the first several days post-SE represent a quiescent “latent” period when the animals experience few if any spontaneous seizures, whereas by 1 week post-SE, around 40 % of animals show a low rate of convulsive seizures. By 4–5 weeks post-SE, the proportion of epileptic animals is >80 %, and by 6–9 weeks post-SE, animals are experiencing an average of three convulsive (Racine class 3–5) seizures per 24 h (Jung et al., 2007; Tai et al., 2017). Our previous work showed that at this 6–9 week post-SE time point, which we describe as “chronic epilepsy,” JNK isoforms in aggregate showed increased expression of phosphorylated or activated forms, which we refer to as “hyperactivation.”
The current study shows that this JNK hyperactivation does not begin at the induction of epilepsy with SE, and also is not present during the early stages of epileptogenesis when the animals are either not yet epileptic or epileptic with a low rate of seizures. Rather, JNK hyperactivation is not manifested until animals are chronically epileptic at the 6–9 week post-SE time point. Because seizures begin at least by 1 week post-SE, this implies that JNK activation is not responsible for the process of epileptogenesis itself, but may be a consequence of frequent convulsive seizures. Interestingly, our prior work showed that pharmacological inhibition of JNK activation using the kinase inhibitor SP600125 reduced seizure frequency in a dose-dependent fashion (Tai et al., 2017). It may be that JNK activation contributes to the maintenance of the epileptic state, even if not contributing to epileptogenesis itself.
Our finding that only the JNK2 isoform is hyperactivated in chronic epilepsy is somewhat surprising, given that JNK3 is the (largely) brain-specific isoform, and constitutive deletion of the jnk3 gene had been previously shown to reduce the severity of SE induced by kainic acid, and also to reduce resultant post-SE apoptotic loss of hippocampal neurons (Yang et al., 1997). JNK1—but not JNK2—deletion has likewise been shown to reduce post-SE neuronal loss in the hippocampus, although the effects of JNK1 deletion on seizure severity were not documented in that study (de Lemos et al., 2018). Both of these prior studies examined the role of JNKs only following acutely provoked SE, and not in the chronic phase of epilepsy when seizures spontaneously occur. Our prior study was one of the first to examine the role of JNK signaling in chronic epilepsy, and the current results represent the first to specifically link JNK2 hyperactivation and epilepsy. JNK2 and JNK3 have been implicated in the pathogenesis of other neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseases (Ploia et al., 2011; Zhou et al., 2015; Huang et al., 2016), even though JNK2 expression is not confined to brain but is widespread in other tissues such as liver and skeletal muscle (Pal et al., 2016). JNK2’s proven role in human neurodegenerative disease suggests that it may play a common role in neuronal degeneration in human epilepsy as well.
Since total JNK2 expression was not elevated in our experiments (and in fact was modestly decreased), we conclude that an isoform-specific upregulation of upstream phosphorylation signaling pathways mediates the significant JNK2 hyperactivation. The upstream MAPK kinases MKK4 and MKK7 both activate JNKs by dual threonine-tyrosine phosphorylation (Davis, 2000); we did not test whether either of these signaling pathways themselves was hyperactivated in epilepsy, nor how they produced selective hyperactivation of JNK2 while activation levels of JNK1 and JNK3 remained unchanged from the naïve condition, but this could be a subject for future investigation.
Temporal lobe epilepsy in humans, when medically refractory and manifesting frequent seizures, causes progressive degeneration of hippocampal pyramidal neurons, particularly in the CA1 and CA3 subfields of the hippocampus proper (Najm et al., 2006). This process is clinically termed “mesial temporal sclerosis (MTS),” and results in progressive deficits in verbal memory, naming, and other aspects of cognition (Tai et al., 2018). Given the involvement of JNK isoforms, particularly JNK2 and JNK3, in other neurodegenerative disorders, it is possible that JNK2 hyperactivation in chronic epilepsy plays a role in the progressive loss of pyramidal neurons. Since MTS is associated with loss of episodic memory and naming abilities, prevention of temporal lobe neurodegeneration would by itself have significant clinical benefits. Future experiments to test chronic inhibition of JNK2 hyperactivation in animal models could test this hypothesis.
It is also possible that JNK2 inhibition would have significant antiepileptic potential. Our previous work demonstrated that pharmacological inhibition of JNK activation reduced seizure frequency in our animal model in a dose-dependent manner (Tai et al., 2017). A limitation of this prior study was that only ∼50 % inhibition of JNK activation was achieved using a small molecule inhibitor that affected all JNK isoforms. Having identified that only the JNK2 isoform is hyperactivated in our model, it may be possible to devise molecular means to selectively and more potently knock down JNK2 activation in order to test its antiepileptic therapeutic potential. Since JNKs have been implicated in normal physiological processes such as long-term potentiation and depression (Sherrin et al., 2011), a selective approach to JNK inhibition would minimize adverse cognitive effects of such an antiepileptic therapy.
Ethics statement
The authors certify that animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). Approval to conduct the experiments described was obtained from University of Washington Institutional Animal Care and Use Committee (IACUC) and can be provided upon request. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Conflicts of interest
None.
CRediT authorship contribution statement
A.N. Parikh: Investigation, Formal analysis, Writing - review & editing. F.A. Concepcion: Methodology, Investigation, Formal analysis, Supervision, Writing - original draft. M.N. Khan: Investigation, Formal analysis, Supervision, Validation, Writing - review & editing. R.D. Boehm: Validation, Writing - review & editing, Investigation. O.C. Poolos: Validation, Writing - review & editing, Investigation. A. Dhami: Investigation, Writing - review & editing. N.P. Poolos: Conceptualization, Methodology, Formal analysis, Writing - original draft, Visualization, Supervision, Project administration, Funding acquisition.
Acknowledgement
This study was supported by the National Institutes of Health, R01 grant NS050229.
References
- Björkblom B., Vainio J.C., Hongisto V., Herdegen T., Courtney M.J., Coffey E.T. All JNKs can kill, but nuclear localization is critical for neuronal death. J. Biol. Chem. 2008;283:19704–19713. doi: 10.1074/jbc.M707744200. [DOI] [PubMed] [Google Scholar]
- Brecht S., Kirchhof R., Chromik A., Willesen M., Nicolaus T., Raivich G., Wessig J., Waetzig V., Goetz M., Claussen M., Pearse D., Kuan C.Y., Vaudano E., Behrens A., Wagner E., Flavell R.A., Davis R.J., Herdegen T. Specific pathophysiological functions of JNK isoforms in the brain. Eur. J. Neurosci. 2005;21:363–377. doi: 10.1111/j.1460-9568.2005.03857.x. [DOI] [PubMed] [Google Scholar]
- Coffey E.T. Nuclear and cytosolic JNK signalling in neurons. Nat. Rev. Neurosci. 2014;15:285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- Curia G., Longo D., Biagini G., Jones R.S., Avoli M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- de Lemos L., Junyent F., Camins A., Castro-Torres R.D., Folch J., Olloquequi J., Beas-Zarate C., Verdaguer E., Auladell C. Neuroprotective effects of the absence of JNK1 or JNK3 isoforms on kainic acid-induced temporal lobe epilepsy-like symptoms. Mol. Neurobiol. 2018;55:4437–4452. doi: 10.1007/s12035-017-0669-1. [DOI] [PubMed] [Google Scholar]
- Eminel S., Roemer L., Waetzig V., Herdegen T. c-Jun N-terminal kinases trigger both degeneration and neurite outgrowth in primary hippocampal and cortical neurons. J. Neurochem. 2008;104:957–969. doi: 10.1111/j.1471-4159.2007.05101.x. [DOI] [PubMed] [Google Scholar]
- Fujikawa D.G. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- Huang Q., Du X., He X., Yu Q., Hu K., Breitwieser W., Shen Q., Ma S., Li M. JNK-mediated activation of ATF2 contributes to dopaminergic neurodegeneration in the MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2016;277:296–304. doi: 10.1016/j.expneurol.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Jung S., Jones T.D., Lugo J., Jr., Sheerin A.H., Miller J.W., D’Ambrosio R., Anderson A.E., Poolos N.P. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J. Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S., Bullis J.B., Lau I.H., Jones T.D., Warner L.N., Poolos N.P. Downregulation of dendritic HCN channel gating in epilepsy is mediated by altered phosphorylation signalling. J. Neurosci. 2010;30:6678–6688. doi: 10.1523/JNEUROSCI.1290-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.K., Park J., Lee Y.D., Lee S.H., Han P.L. Distinct localization of SAPK isoforms in neurons of adult mouse brain implies multiple signaling modes of SAPK pathway. Brain Res. Mol. Brain Res. 1999;70:116–124. doi: 10.1016/s0169-328x(99)00136-9. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang C.W., Zhou Y., Wong W.Q., Lee L.C., Ong W.Y., Yoon S.O., Hong W., Fu X.Y., Soong T.W., Koo E.H., Stanton L.W., Lim K.L., Xiao Z.C., Dawe G.S. APP upregulation contributes to retinal ganglion cell degeneration via JNK3. Cell Death Differ. 2018;25:663–678. doi: 10.1038/s41418-017-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehan S., Meena H., Sharma D., Sankhla R. JNK: a stress-activated protein kinase therapeutic strategies and involvement in Alzheimer’s and various neurodegenerative abnormalities. J. Mol. Neurosci. 2011;43:376–390. doi: 10.1007/s12031-010-9454-6. [DOI] [PubMed] [Google Scholar]
- Najm I., Duvernoy H., Schuele S. Hippocampal anatomy and hippocampal sclerosis. In: Wyllie E., Gupta A., lachhwani D.K., editors. The Treatment of Epilepsy. Lippincott Williams & Wilkins, Philadelphia; 2006. [Google Scholar]
- Pal M., Febbraio M.A., Lancaster G.I. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J. Physiol. 2016;594:267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet C., Dumurgier J., Hugon J. Pro-apoptotic kinase levels in cerebrospinal fluid as potential future biomarkers in Alzheimer’s disease. Front. Neurol. 2015;6:168. doi: 10.3389/fneur.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploia C., Antoniou X., Sclip A., Grande V., Cardinetti D., Colombo A., Canu N., Benussi L., Ghidoni R., Forloni G., Borsello T. JNK plays a key role in tau hyperphosphorylation in Alzheimer’s disease models. J. Alzheimers Dis. 2011;26:315–329. doi: 10.3233/JAD-2011-110320. [DOI] [PubMed] [Google Scholar]
- Roberson E.D., English J.D., Adams J.P., Selcher J.C., Kondratick C., Sweatt J.D. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J. Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrin T., Blank T., Todorovic C. c-Jun N-terminal kinases in memory and synaptic plasticity. Rev. Neurosci. 2011;22:403–410. doi: 10.1515/RNS.2011.032. [DOI] [PubMed] [Google Scholar]
- Tai T.Y., Warner L.N., Jones T.D., Jung S., Skyrud D.W., Fender J., Liu Y., Williams A.D., Neumaier J.F., D’Ambrosio R., Poolos N.P. Antiepileptic action of c-Jun-N-terminal kinase (JNK) inhibition in an animal model of epilepsy. Neuroscience. 2017;349:35–47. doi: 10.1016/j.neuroscience.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai X.Y., Bernhardt B., Thom M., Thompson P., Baxendale S., Koepp M., Bernasconi N. Review: neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: clinical, pathological and neuroimaging evidence. Neuropathol. Appl. Neurobiol. 2018;44:70–90. doi: 10.1111/nan.12458. [DOI] [PubMed] [Google Scholar]
- Toyoda I., Bower M.R., Leyva F., Buckmaster P.S. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J. Neurosci. 2013;33:11100–11115. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski W.A., Cavalheiro E.A., Schwarz M., Czuczwar S.J., Kleinrok Z., Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Waetzig V., Herdegen T. Neurodegenerative and physiological actions of c-Jun N-terminal kinases in the mammalian brain. Neurosci. Lett. 2004;361:64–67. doi: 10.1016/j.neulet.2004.02.041. [DOI] [PubMed] [Google Scholar]
- Williams A.D., Jung S., Poolos N.P. Protein kinase C bidirectionally modulates Ih and hyperpolarization-activated cyclic nucleotide-gated (HCN) channel surface expression in hippocampal pyramidal neurons. J. Physiol. 2015;593:2779–2792. doi: 10.1113/JP270453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.D., Kuan C.Y., Whitmarsh A.J., Rincon M., Zheng T.S., Davis R.J., Rakic P., Flavell R.A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- Yoon S.O., Park D.J., Ryu J.C., Ozer H.G., Tep C., Shin Y.J., Lim T.H., Pastorino L., Kunwar A.J., Walton J.C., Nagahara A.H., Lu K.P., Nelson R.J., Tuszynski M.H., Huang K. JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron. 2012;75:824–837. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hastie C.J., McLauchlan H., Cohen P., Goedert M. Phosphorylation of microtubule-associated protein tau by isoforms of c-Jun N-terminal kinase (JNK) J. Neurochem. 2004;90:352–358. doi: 10.1111/j.1471-4159.2004.02479.x. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wang M., Du Y., Zhang W., Bai M., Zhang Z., Li Z., Miao J. Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Ann. Neurol. 2015;77:637–654. doi: 10.1002/ana.24361. [DOI] [PubMed] [Google Scholar]