Abstract
Oncolytic viruses (OVs) constitute a new and promising immunotherapeutic approach toward cancer treatment. This therapy takes advantage of the natural propensity of most tumor cells to be infected by specific OVs. Besides the direct killing potential (oncolysis), what makes OV administration attractive for the present cancer immunotherapeutic scenario is the capacity to induce two new overlapping, but distinct, immunities: anti-tumoral and anti-viral. OV infection and oncolysis naturally elicit both innate and adaptive immune responses (required for long-term anti-tumoral immunity); at the same time, the viral infection prompts an anti-viral response. In this review, we discuss the dynamic interaction between OVs and the triggered responses of the immune system. The anti-OV immunological events that lead to viral clearance and the strategies to deal with such potential loss of the therapeutic virus are discussed. Additionally, we review the immune stimulatory actions induced by OVs through different inherent strategies, such as modulation of the tumor microenvironment, the role of immunogenic cell death, and the consequences of genetically modifying OVs by arming them with therapeutic transgenes. An understanding of the balance between the OV-induced anti-tumoral versus anti-viral immunities will provide insight when choosing the appropriate virotherapy for any specific cancer.
Main Text
Oncolytic Viruses as a Cancer Immunotherapeutic Platform
During the oncogenic process, cancer cells undergo multiple genetic and physiological changes that make them distinguishable from normal cells. Among these cancer-inherent hallmarks, tumor cells evolve to evade immune-mediated recognition and destruction, including the acquisition of defects in cellular anti-viral pathways, such as those mediated by the interferons (IFNs).1, 2, 3 Theoretically, every type of malignant cell is more susceptible to infection by at least some viruses, and therefore this natural propensity has been explored as an emerging anti-cancer therapy by the exploitation of oncolytic viruses (OVs) to selectively infect and kill cancer cells, while exerting minimal or no pathogenicity against the host.4 OVs either occur naturally and are exploited as genetically unmodified isolates (e.g., reovirus), which include wild-type and naturally attenuated strains, or they are genetically engineered (e.g., herpes simplex virus-1 [HSV-1], adenoviruses, vesicular stomatitis virus [VSV], measles virus [MV], vaccinia virus [VV], or myxoma virus [MYXV]), encompassing genetic edits to the virus genome to weaken viral pathogenicity, improve immunogenicity, and/or insert therapeutic genes (transgenes).5, 6, 7, 8, 9, 10
When selecting for the appropriate OV treatment strategy, intrinsic characteristics should be taken into consideration. Each OV family will exhibit unique genome complexities, replication mechanisms, lytic properties, packaging capacities for transgenes, and immune response triggering capabilities to stimulate anti-tumoral immunity. Since different OVs will exhibit distinct tumor tropisms, it has been difficult to identify individual molecular biomarkers that predict specific anti-tumor efficacies for any OV.7,11 Concurrent with the properties of OVs, the tumor biology and immune landscape will also contribute to the outcome of the therapeutic approach. The tumor microenvironment (TME) typically exhibits an immunosuppressive milieu leading to the active subversion of effective anti-tumoral immunity. Tumors generally secrete soluble immunosuppressive mediators, including nitric oxide, and cytokines such as interleukin (IL)-10 and transforming growth factor-β (TGF-β).3,8,12 In addition, regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are recruited to the TME where they co-opt the capacity of the elements of the acquired immune response pathway to recognize and clear the tumor cells.8,11,12 The multiple and complementary mechanisms of action of OVs will be successful only if they ultimately reverse the local immunosuppression within the TME and create a sufficient pro-inflammatory and pro-immune environment within the tumor bed to re-establish acquired anti-tumoral responses to the resident cancer cells.
Besides the recognized anti-tumor qualities of OVs, as a result of their ability to create a favorable microenvironment for the action of the immune system against unique cancer cell determinants, the anti-viral immunity triggered against viral antigens from the resultant infection is also a key player during OV-based therapies. Indeed, induced anti-viral immunity was once considered detrimental for OVs, since the activation of the immune system against the virus itself is expected to restrict the viral replication and spread, leading to a decrease in therapeutic efficacy. However, it has now been recognized that there are undeniably beneficial aspects on the OV infection being detected by the immune system.8 Following administration, the OV will infect tumor cells and hijack the cell’s protein synthesis, promoting the production of viral macromolecules, but it will also trigger the expression and recognition of “danger signals.” These are a consequence of a cascade of signaling events that culminate with the release of cytokines and damage-associated molecular patterns (DAMPs).4,8,9 Additionally, OVs cause cancer cell killing by promoting cell lysis, a process known as oncolysis, followed by the release of infectious viral progeny that spread to surrounding tumor cells (amplification of oncolysis) as well as subproducts, including viral particles, pathogen-associated molecular patterns (PAMPs), DAMPs, tumor cell debris, and tumor-associated antigens (TAAs).3,4,8,13 All of these processes contribute to the stimulation of the innate and adaptive anti-cancer immune responses locally and systemically. Besides oncolysis and anti-tumoral immunity, some OVs have been shown to have potent anti-angiogenic effects by triggering an acute disruption of the tumor vasculature.14, 15, 16 Indeed, successful oncolytic virotherapy relies on a balance between anti-viral pathways that eliminate the virus and pro-immune pathways that recognize cellular epitopes, TAAs, and neoantigens from the virus-infected tumor cells.
Here, we discuss the dynamics between OV monotherapies and the immune system from two contrasting perspectives, as the immune system has a recognized and obligatory role in the outcome of virotherapies. On the one hand, we look at the different challenges that the immune system poses to restrict and impede OVs. On the other hand, we review the anti-cancer immunotherapeutic potential of OVs, particularly for immune barren tumors that are nonresponsive to immune checkpoint inhibitor (ICI) therapies, resulting from their ability to stimulate the anti-tumoral immunity in novel ways. The different therapeutic combinations involving OVs and ICIs are highly relevant for the field and are furthest advanced in the clinic, and the reader is referred to other reviews on this aspect of combinatorial virotherapy.17, 18, 19, 20
Immune Restrictions to OV Therapeutics
OV infection of cancer cells changes how antigens are presented to the immune system and is the key reason why novel anti-tumoral immunity is elicited. The cell-intrinsic aberrations of OV-infected cancer cells are linked to how they are perceived by the elements of the innate and acquired immune systems. However, following the virus colonization of the tumor, the host anti-viral immunity will become activated and mobilized to restrict virus replication and spread, culminating in viral clearance and elimination of the therapeutic effect.8 Thus, the effective “time window” for most OVs to activate anti-tumoral immunity is generally within the first 1–2 weeks of administration, before the virus is cleared. One of the major challenges of OV immunotherapy is therefore to achieve a balance between the desirable triggering of new anti-tumoral immunity and the competing anti-viral immunity, while keeping undesired anti-viral effector processes from becoming the dominant response pathway that overwhelms the acquisition of acquired anti-tumoral immunity. Stated simply, how can one maximize the generation of immune responses directed against tumor antigens revealed from OV-infected cancer cells while minimizing the consequences of anti-viral responses against viral antigens?
Neutralizing Anti-viral Antibodies
For some disseminated cancers and/or micrometastatic lesions that are not readily amenable to intratumoral injection, the delivery of OVs should be systemic (e.g., intravenous infusion), which represents a major technical challenge for OV treatment efficacy.21 Besides the existence of a variety of physical barriers within the circulatory system, a potential obstacle is the presence of anti-viral antibodies that either pre-exist (e.g., patients that have been previously vaccinated with a related virus) or arise from treatment-induced neutralizing anti-viral antibodies (nAbs), since these both reduce the effective virus titer, hinder any repetitive OV systemic delivery regimen, and contribute to patient anti-viral immunity.11,22
Methods involving masking viral surface proteins with polymeric materials have been developed to enhance protection against nAbs and extend viral circulation half-life.23, 24, 25, 26 Recently, Francini et al.27 developed a new class of polyvalent diazonium polymers to coat and shield the oncolytic adenovirus enadenotucirev, resulting in one of the first reports on complete ablation of nAb binding at polymer concentrations 10- to 20-fold lower than what was previously reported. Importantly, coating did not cause permanent inactivation of the OV. Also in the oncolytic adenovirus field, a recent strategy involved redirecting the viral nAbs against the tumor cell surface, showing how one of the limiting factors of using OVs would become reconfigured to become a beneficial feature of cancer immunotherapy.22 This method comprises the use of a recombinant bifunctional adaptor protein with the ability of capturing adenoviral-specific nAbs but also recognizes tumor cells through a polysialic acid-specific single-chain variable fragment (scFv).
To shield OVs from their own induced immunogenicity, which triggers the production of nAbs, encapsulation of the viral agent has been widely applied in the oncolytic adenovirus field. One of these cloaking methods consists of using specialized subcellular structures named extracellular vesicles (EVs) that originate from plasma membrane. Cancer cell-derived EVs transporting oncolytic adenovirus (Ad5D24) exhibited a tumor-selective delivery in a lung carcinoma model.28,29 In addition, tumor cell-derived microparticles, a specific type of vesicle (0.1–1 μM), have proven also to be efficient carriers for oncolytic adenovirus.30 Alternatively to the encapsulation in fragments of the plasma membrane, the use of liposomes to wrap OVs has been widely applied to adenoviruses, and more recently to alphavirus M1.31, 32, 33
All of these are recent reports on different approaches on how oncolytic adenoviruses can ghost the immune system and get protection against nAbs. Nonetheless, it would be relevant to further investigate whether such concepts could be applied to other OVs.
A related strategy applied to diverse OV platforms to counteract nAb inactivation consists of a cell-based carrier system, i.e., using different cell types as “shielding vehicles” pre-loaded ex vivo with OVs. These carrier cells are used as Trojan horses, hiding the OVs from recognition and attack by the immune system, which allows a longer therapeutic window, and they have shown the ability to traffic into tumor beds and release their viral cargo there.34,35 The portfolio of vehicle cells tested to date includes mixed leukocyte populations, such as bone marrow (BM) and peripheral blood mononuclear cells (PBMCs), but also isolated leukocyte types, such as stem cells (mesenchymal stem cells [MSCs], either BM or adipose tissue derived, and neural stem cells [NSCs]) and selected immune cells (e.g., cytokine-induced killer cells, dendritic cells [DCs], monocytes, macrophages, naturally circulating or genetically engineered T cells).35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 In addition to the shielding strategy, the use of different carrier cells loaded with OVs might result in different therapeutic outcomes, as they can as well modulate the anti-viral and anti-tumoral immune responses after being infused with the virus. MSCs, for example, are considered attractive candidates for cancer therapy due to low immunogenicity, strong tumor-tropic homing properties, and lack of stimulation of sentinel lymphocyte proliferation, avoiding in this way immune rejection when the cells are allogeneic.52 MSC carrier cells also possess impressive virus amplification potential and the capacity to immunosuppress both the innate and adaptive arms of anti-viral immunity.53 A recent work reporting the synergistic and potent immunotherapeutic effect of T cell receptor transgenic T cells loaded with VSV is another example of the importance of the cytotoxic effector function, beyond acting merely as inert “shielding vehicles.”49 In addition to the aforementioned normal leukocytes, the potential of irradiated tumor cells and transformed cell lines as carriers for OV systemic delivery has also been explored.37,54, 55, 56, 57
The route of OV administration will greatly define how the immune system recognizes and interferes with the virus, and this interaction will determine the treatment efficacy. Although the presence of interfering nAbs may explain the modest activity of some OV therapies, in a phase I/II clinical trial carried out with Pexa-Vec OV administered intravenously to patients with advanced hepatocellular carcinoma, the virus biological activity was not inhibited by baseline nAbs.58 Furthermore, Berkeley et al.46 recently found that fully neutralized reovirus carried by human monocytes resulted in tumor cell infection and lysis.
Innate Anti-viral Resistance Mechanisms
Upon detecting a viral infection, the host innate immunity is designed to trigger a rapid cascade of actions to control virus replication and dissemination. The production and activation of anti-viral sensors and effectors, as well as the recruitment of immune cells to the site of infection, are among the major host immune early response components.3
The anti-viral IFNs are one of the main players on the dynamic crosstalk between OV therapy and the immune system. The success of this immunotherapeutic approach relies on taking advantage of the frequent dysfunctional IFN signaling in cancer cells, making them more susceptible to OV infection, replication, and oncolysis. However, OVs are a moving target for the IFN-mediated anti-viral response in the host, particularly within the TME.59,60 Several studies report the correlation between an upregulated level of endogenous IFN signaling and resistance to OV therapy.60 As an example, a recent study from Kurokawa et al.61 showed that RSAD2/viperin expression impairs the oncolytic MV therapeutic efficacy in vivo in an ovarian tumor model. RSAD2/viperin is an IFN-stimulated gene (ISG) with anti-viral activity against several enveloped viruses, which is known to be expressed at increased levels in tumor cells with a MV resistance phenotype. Another recent and representative example was the evaluation of STING (stimulator of IFN genes) activity in malignant peripheral nerve sheath tumor (MPNST) cell lines, showing that it is predictive of oncolytic HSV sensitivity. Indeed, the authors claimed that STING downregulation renders MPNSTs more permissive to the OV infection and cell-to-cell spread.62
The role of IFN signaling components and ISGs as key regulators of tumor resistance to OV therapy makes them potential prognostic biomarkers of IFN-induced resistance. Concomitantly, the opposite is also valid: specific defects in the IFN signaling pathway might serve as potential biomarkers for OV sensitivity. In light of this, using anti-viral elements as markers will help to maximize therapeutic outcome by selecting individual cancer patients more likely to benefit from OV treatment.60,63 For tumors expressing high levels of IFNs and ISGs, combination therapy of OVs and IFN signaling modulators/inhibitors is a strategy employed to enhance the success of OV treatment.64, 65, 66, 67 For instance, JAK (Janus kinase)/STAT (signal transducer and activator of transcription) inhibition with ruxolitinib increases the oncolytic efficiency of oncolytic VSV, HSV, and MV.66, 67, 68, 69, 70, 71, 72
Cell-mediated innate immune responses, namely by natural killer (NK) cells, also lead to impairment of OV treatment efficacy. NK cells are a type of innate lymphoid cell that contributes to the cytolytic killing of virus-infected cells, additionally exhibiting a key regulatory role in shaping adaptive immune responses to restrict infection.73 In a glioblastoma model, the administration of oncolytic HSV induced rapid recruitment and activation of NK cells, which resulted in a viral clearance increase and diminished anti-tumoral efficacy.74 In a later study, the authors verified that the combination of TGF-β with oncolytic HSV inhibited NK cell intracranial recruitment, activation, and function, thereby permitting enhanced viral replication and increased survival of mice in both syngeneic and xenograft glioblastoma models.75 The use of different cells to carry the OVs undetected by the immune system, as described previously, comes with extra advantages. Besides protecting naked viruses, MSCs possess strong immunosuppressive properties that also contribute to overcome innate immune barriers against OVs. By delivering oncolytic VV via adipose-derived MSCs, Draganov et al.53 demonstrated the potential of these Trojan horse cells to be immunosuppressive toward NK cells in both autologous and allogeneic settings.
Immune System Stimulation by OV Treatment
Anti-viral responses might not only inhibit the oncolytic activities of OVs, but at the same time they can activate the innate and acquired arms of the immune system. Gujar et al.8 stated that many of the “undesirable” anti-viral immune responses in fact activate the immune response against the tumors, which is necessary for transforming them from immune “cold” to immune “hot.” Thus, anti-viral signaling can prime the tumors for subsequent immune clearance responses, such that the immune system will also collaterally target cancer cells harboring the OV by directing the response specifically at virus replication sites.20
There are several aspects that can be taken into account in a more classic definition of immune system stimulation by OVs: combination therapy of OVs with ICIs;76, 77, 78 adoptive cell therapy (ACT) using OVs as a vaccine or priming of the tumor;76,77,79 transition of a cold tumor to hot by OV infection; and using armed viruses with transgenes that can help configure a more effective activation of the immune system within the TME. Herein, we focus on activation mechanisms that are induced by OVs by themselves or by the inclusion of immune-stimulatory genes or deletion of viral genes.
Virus Induction of Immunogenic Cell Death
Immunogenic cell death (ICD) was first described two decades ago, dividing the recognized elements between “self” and “non-self” via PAMPs.80,81 Over the years, the definition of ICD has evolved to encompass a type of cell death that is sufficient to induce an adaptive immune response against exogenous or endogenous antigens in dying cells elicited by the presence of danger signals.80,82 It is not known if there is a single main induction pathway that leads to ICD, but it has been observed that it is related to endoplasmic reticulum (ER) stress and reactive oxygen species (ROS) generation. ICD is also defined as a type of apoptotic cell death that releases danger signals such as DAMPs and PAMPs. PAMPs and DAMPs determine the type of immune response, and their inducers can be classified as type I and type II. Type I includes most of the ICD inducers that activate cell death through targets that are not directly associated to the ER, and type II selectively targets the ER.80,83 OVs are one of the most described ICD stimulators, and they are more likely to be type II inducers.84 They induce ICD via apoptosis that is derived from ER stress and autophagy, but can also involve virus-stimulated pyroptosis, necroptosis, and necrosis that can also induce ICD to a lesser form. The main hallmarks of ICD that can be measured in culture and have been more described so far include ATP release, ecto-expression of calreticulin (CRT), which is normally within the ER, and late apoptotic HMGB1 release in different redox states, which normally is a DNA-associated protein. There are other ICD hallmarks that have been recently described, such as activation of annexin I, type I IFN, IL-1β, exposure of F-actin, and heat shock proteins (HSP70, HSP90).20,81,84 Pathways of ICD activation differ dramatically between viruses, but in general they all exhibit one or more hallmarks that can be associated with other related types of cell death, such as necroptosis, apoptosis, pyroptosis, or autophagy, depending on the virus used and/or the target cell.81,83,85
In the case of the HSV-1-derived talimogene laherparepvec (T-Vec), although the efficiency of the virus was proven against metastatic melanoma during clinical trials, the immune response activation remains incompletely understood. Bommareddy et al.85 demonstrated that T-Vec not only induces the hallmarks of ICD in vitro, but in an in vivo model the virus was able to increase immune cell infiltration of CD8+ T cells in both injected and collateral tumors that were specific for tumor-associated antigens (TAAs). Moreover, they found that stimulation of IFN response genes (such as STING) mediated inhibition inherent to each cell line that enabled or dampened the activation of the immune system.85 MV has also been found to induce the release of TAAs that allow myeloid and plasmacytoid DCs to mature, and therefore activate an adaptive immune response (CD8+ T cells), but the specific ICD pathway induced by the infection of the virus is still unknown.81,86 The adenovirus dl922-947, which allows the virus to only infect cells with a defective retinoblastoma pathway, has been shown to reduce tumor volume and regress tumors in 30% of mice with malignant pleural mesothelioma.87 Similar ICD activation was observed with less intensity with the adenovirus strain Ad881 in colorectal cancer, but in combination with chemotherapy, it induces a more robust ICD response.88,89 Newcastle disease virus (NDV) has also been shown to induce ICD in lung cancer cells, melanoma, multiple myeloma, and in glioma GL261 cells via autophagy and inflammatory pathways.84,90, 91, 92, 93
Many of these studies have been performed both in in vitro and in vivo mouse models. Nevertheless, in many cases the correlation between the presence of these ICD hallmarks in vitro and an effective induced anti-tumor response in vivo is not always apparent, and a linkage with bona fide ICD responses in vivo has not been proven yet. In the future, more studies should be focused on the pathways by which ICD is measured, particularly in vivo, to potentiate the effect of OVs to activate the immune system.
TME Modulation by OVs
OVs not only can activate ICD, but at the same time it is observed that many can modulate the TME to render it less immunosuppressive, or what is more commonly known as “cold-to-hot” tumor modulation. The TME is composed of tumor cells, resident or infiltrated non-transformed cells (e.g., cancer-associated fibroblasts, vascular endothelial cells, immune recruited cells), secreted factors, and the extracellular matrix, and so clearly the definition of a cold versus hot tumor depends on many variables, only some of which are understood.
One of the first responses against OVs is the classical anti-viral response from normal cells, which can potentially inhibit OV replication and/or spread directly. One of the main drivers of this response is type I IFN (IFN-α and IFN-β).20,94, 95, 96 In addition to mediating the anti-viral state, type I IFN also has an important role in anti-tumor responses by stimulating immune cells within the TME, such as NK cells and CD8+ T cells, and pro-inflammatory cytokines.96 For example, MV activates plasmacytoid DCs, as well as IFN-α that subsequently activates Toll-like receptors (TLRs), RIG-like receptors (RLRs), and expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) for cytotoxic functions.96
Type I IFN promotes anti-tumor immune reactions due to its regulatory role on NK cells and CD8+ T cells. NK cells, when activated, produce type II IFN (IFN-γ), which inhibits angiogenesis, induces apoptosis, and is an immune stimulant (by activating major histocompatibility complex [MHC] class II in DCs, phagocytic activity of macrophages, and CD8+ T cell responses). Type I IFN can also upregulate expression of MHC class I in DCs, co-stimulatory molecules (CD40, CD86), and maintenance of a Th1 polarized response.94,95 Importantly, note that the ability to induce type I IFN in each tumor bed, as well as the intensity by which specific OVs activate type I IFN signaling, varies dramatically among OVs, and these facts all determine the extent of the OV to stimulate the acquired immune system against viral and tumor antigens.95 It is necessary to further investigate the effect for each OV to find the correct balance between anti-viral and anti-tumoral immunity.
Another key regulator of the immunostimulatory versus immunosuppressive phenotype of the TME is the tumor-associated macrophage population. Macrophages can be polarized in at least two states: M1 pro-inflammatory macrophages are associated with anti-viral and anti-tumoral responses, while M2 immunosuppressive macrophages are associated with metastasis, angiogenesis, and suppression of anti-tumoral and anti-viral responses.94,95,97 The macrophage M1 state is induced by IFN-γ and lipopolysaccharides, while the M2 state is induced by polarizing cytokines such as IL-4. The M1 state of macrophages favors the expression of CCL20, CXCL10, CXCL11, CCL15, and CXCL9 and the secretion of TNF and IL-12 to attract and activate NK cells, T cells, and DCs. These activated cells also express paracrine and autocrine IFN-γ, IL-12, and IL-15. Alternatively, the M2 state produces vascular endothelial growth factor (VEGF), IL-8, and TGF-β.94,97 It is tempting to predict that when the TME has a higher population of M1 macrophages versus M2 macrophages, it is more likely to have the desired anti-tumor immune response when the tumor is infected with OVs. However, in reality, this response varies between OVs, even in the same tumor type, and the presence of M1 macrophages does not guarantee a positive response, as summarized by Denton et al.94 Other immune cell types that act as polarizing mediators, such as neutrophils,98 can potentially play an important role, and this is unexplored territory that could make a big impact in the conversion of a cold TME into a hot one.
The fact that there is not necessarily only one preferred state of macrophages that can induce an immunostimulatory response also suggests that cellular heterogeneity plays a role within the tumor beds. Heterogeneous tumors are more difficult to treat because some cells might still be able to detect the PAMPs and DAMPs released, and produce type I IFN to activate an immune response, but some cells might already have lost the capacity and be in a more immunosuppressive state.95 TMEs can be classified in general as inflamed, immune excluded, or even as an immune desert. An inflamed TME is presumed to be the ideal category because this tumor is most likely to be susceptible to OV-induced immune stimulation. The two latter states have an immunosuppressive environment with suppressive immune cells embedded within the tumor that are less likely to be activated, or are refractory in influx of immune cells, either before or after OV treatment. This heterogeneity can be observed not only intratumorally, but also intertumorally within the same host.96
Once the initial OV response has developed within the TME, reversing immunosuppression leads to the release of TAAs or tumor-associated neoantigens (TANs) that are then presented to antigen-presenting cells (APCs) via MHC class I, either by direct presentation from within the virus-infected cells, or by cross-presentation via uninfected APCs. Lymphocyte T cell recruitment can activate an adaptive immune cell response with the help of the chemokines and cytokines mentioned above.20,78,96 Nevertheless, when OVs cause an inflammatory response within the TME, this activates the production of key immunoregulatory cytokines, and Tregs and MDSCs may be recruited too, inhibiting the subsequent immune responses.20 This equilibrium in the TME between the activation of an anti-viral response and an anti-tumoral response is a key dynamic that OVs can manipulate.
Virus Arming Can Enhance OV Effects and Promote the Elimination of Unfavorable Factors in the TME
Besides using OVs as primers to stimulate the immune system by modulation of the TME, another strategy that has been exploited is inserting host cytokines or other immunoregulatory genes within the OVs’ genome, resulting in transgene-armed OVs. Armed OVs are designed to produce locally within the virus-infected TME a specific protein of interest. This helps reduce the systemic toxicity that at least some of these immunostimulatory molecules can generate,11,99 although this is not always the case. Sometimes, as well, arming is used to increase the safety of the OV by reducing the level of virus pathogenicity.100 The type of virus can directly influence the choice of transgene, as well as the number of transgenes that can be incorporated into a single virus construct. RNA viruses have typically a smaller genome and can encode only a limited number of transgenes, unlike DNA viruses, which can generally accommodate more transgenes without affecting replication.11
As mentioned earlier, most transgenes aim to drive an adaptive immune response against tumor antigens and/or to help overcome immune cell-barren tumors. Such transgenes include cytokines, chemokines, inhibitory receptors, co-stimulatory receptors, bispecific cell engagers, immune ligands, and combinations of any of these.11,99,101
Most common cytokines exploited to date in OVs are IL-2, IL-12, IL-15, IL-6, IL-21, IL-18, IL-24, and granulocyte-macrophage colony stimulating factor (GM-CSF), which all stimulate different elements of the immune system.99,101 IL-2 promotes T cell expansion but it induces severe side effects in humans and also stimulates Tregs.99,102 To overcome some of its toxicity, Liu et al. 103 engineered expression of membrane-bound IL-2 expressed in VV, which reduced the toxic side effects, and its anti-tumoral effects were comparable to the virus expressing free IL-2. IL-12 stimulates the polarization of T helper (Th) cells to Th1 and has shown to induce more robust anti-tumoral effects than does GM-CSF.10,99,101 IL-15 can stimulate only T cells and NK cells and is less toxic than IL-2, and Stephenson et al.104 showed that when inserted in VSV, local IL-15 delivered by the OV induced a better immune response than when IL-15 was delivered systemically.10,99,101,105 GM-CSF was used as a transgene in the US Food and Drug Administration (FDA)-approved OV, T-Vec, showing macrophage and DC maturation, as well as improved antigen presentation. It has also been shown to remodel the TME and, when combined with immuno-checkpoint inhibitors, overcome resistance to immunotherapy.11,99,106,107 As observed, cytokines can induce diverse pleiotropic effects to stimulate anti-tumor responses, but none is devoid of toxicity.
Alternatively, some of the most common chemokines incorporated within OV genomes are CCL2, CCL5, CCL19, CXCL11, CXCL9, and CXCL10, which induce higher infiltration of Th1 leukocytes and T cell trafficking, but none of these viruses has shown tumor regression by themselves.10,20
Inhibitory receptors or single-chain antibodies expressed in OVs, such as anti-CTLA-4, and anti-PD-1, have been shown to remodel the TME, but tumors can become resistant to these therapies.10,99,108
Transgenes encoding type I IFN (α, β) or type II IFN (γ) improved OV therapy (via MHC class I and MHC class II expression) in nude mice but not in an immunocompetent model, and IFN-γ, specifically, seems to exert a positive effect in tumor regression in some viruses, but it greatly depends on the OV used.99,101,106
Finally, the bi-specific T cell engagers (BiTe and BiKe) can circumvent the step of TAAs being presented by MHC class I to activate T cells. Alternatively, membrane-integrated T cell engagers (MiTes ) have an effect only in virus-infected cells instead of bystander cells similar to when BiTe or BiKe are used.10,20,99,108 For a more complete summary of transgenes, please refer to the tables found in de Graaf et al.99 and Harrington et al.11
One of the alternative strategies is to express known TAAs to prime a specific T cell response against pre-chosen antigens. For example, a Maraba virus expressing an ovarian TAA showed that it boosts the response only in combination with anti-PD-1 antibody, due to the initial immune pressure of the TAA in the tumor developing an increase in PD-1 in the tumor.20,109 Another way to change an immune response can be enhancing a pathway to activate different TLRs (TLR2) rather than the canonical TLR3.110
One advantage about transgene-armed OVs is that depending on the viral promoter that controls the transgene or by regulating the translation of the protein, timing of the immune activation can be delayed. Effect of transgenes can have a maximum effect if the expression is delayed until the viral oncolysis is highest to avoid a too-rapid immune response.108 Balance between time of expression and lysis of cells depends on the lytic ability of the OV (i.e., highly lytic viruses and less lytic viruses).11,108
To achieve the best results, there is a balance to take into consideration between the transgene to be expressed, the OV to be used, and the tumor target. It is also important to consider the function of the transgene to regulate the time at which it has to be expressed to induce an adaptive immune response as desired. Finally, multiple transgenes expressed by OVs, including cytokines and ICIs, should be explored in the future to have a localized expression with the desired combinatorial effects observed in combination therapies that have shown to improve anti-tumoral efficiency.
Conclusions
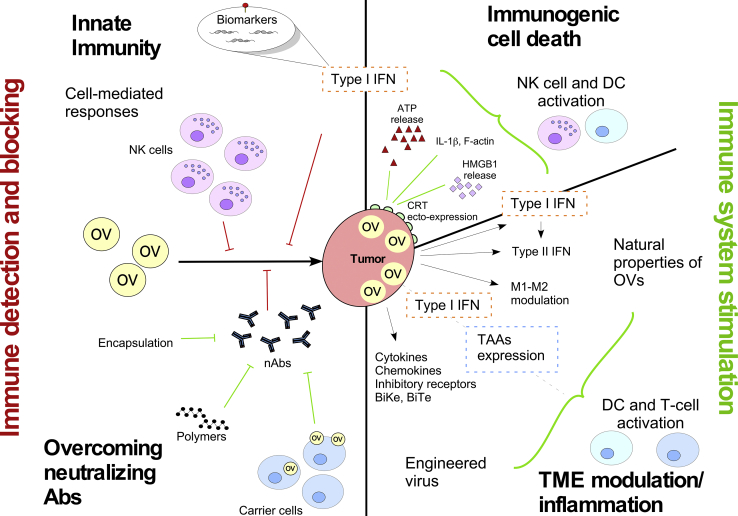
The crosstalk between OVs and the immune system includes both restrictive and stimulatory actions: the immune system fights the OV by triggering anti-viral pathways (e.g., type I IFN) and by imposing blocking strategies (neutralizing antibodies); simultaneously, the OVs have the ability to stimulate the anti-tumoral immunity by activating NK cell and T cells via ICD, by modulating the TME (OV infection inherent effect), or by engineering OVs to activate both DCs and T cells (Figure 1).
Figure 1.
The Dynamic Duo
The interaction between OVs and the immune system can lead to both the inhibition and stimulation of the immune system. OV detection and blocking by the immune system is mainly done via nAbs, type I IFN, and NK cells (red lines, left side). Different strategies have been engineered to overcome the effect of nAbs such as encapsulation, polymers, and carrier cells (green lines, left side). At the same time, some of these inhibitory responses can also lead to stimulation of the immune system via ICD, type I and type II IFN, and cell modulation (green lines, top right side). Alternatively, OVs can be engineered to activate the immune system by inserting immunostimulatory molecules such as TAAs, chemokines, cytokines, BiTe, and BiKe (green lines, center right side).
Failures and successes of oncolytic virotherapy are inseparable from how the OV interacts with the immune system of the tumor-bearing host, and whether the needed immune pathways are still intact after prior anti-cancer therapies.
Patient immune profiling, including pre-existing immunity to the virus and the status of prognostic biomarkers, should be critical for any personalizing OV regimen (either as a monotherapy or in combination therapies).
OV therapy has the potential to shift the clinical outcomes for immunologically barren and ICI-resistant tumors.
When therapeutic transgenes are inserted into any OV, it is important to consider the timing when it is more beneficial to express the transgene, depending on its function, the OV used, and the type of tumor.
Overall, the balance between virus-induced anti-viral versus anti-tumoral responses is vital to be able to generate a positive adaptive immune response that induces durable tumor regression.
Conflicts of Interest
G.M. is a founder and equity holder of Oncomyx Therapeutics, Inc.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Choi A.H., O’Leary M.P., Fong Y., Chen N.G. From benchtop to bedside: a review of oncolytic virotherapy. Biomedicines. 2016;4:E18. doi: 10.3390/biomedicines4030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filley A.C., Dey M. Immune system, friend or foe of oncolytic virotherapy? Front. Oncol. 2017;7:106. doi: 10.3389/fonc.2017.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marelli G., Howells A., Lemoine N.R., Wang Y. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front. Immunol. 2018;9:866. doi: 10.3389/fimmu.2018.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffey M.C., Strong J.E., Forsyth P.A., Lee P.W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 6.Strong J.E., Coffey M.C., Tang D., Sabinin P., Lee P.W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell J., McFadden G. Viruses for tumor therapy. Cell Host Microbe. 2014;15:260–265. doi: 10.1016/j.chom.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gujar S., Pol J.G., Kim Y., Lee P.W., Kroemer G. Antitumor benefits of antiviral immunity: an underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y., Hui P., Du X., Su X. Updates to the antitumor mechanism of oncolytic virus. Thorac. Cancer. 2019;10:1031–1035. doi: 10.1111/1759-7714.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Domínguez L.E., McFadden G. Poxvirus oncolytic virotherapy. Expert Opin. Biol. Ther. 2019;19:561–573. doi: 10.1080/14712598.2019.1600669. [DOI] [PubMed] [Google Scholar]
- 11.Harrington K., Freeman D.J., Kelly B., Harper J., Soria J.C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 12.Prestwich R.J., Harrington K.J., Pandha H.S., Vile R.G., Melcher A.A., Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev. Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davola M.E., Mossman K.L. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? OncoImmunology. 2019;8:e1581528. doi: 10.1080/2162402X.2019.1596006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angarita F.A., Acuna S.A., Ottolino-Perry K., Zerhouni S., McCart J.A. Mounting a strategic offense: fighting tumor vasculature with oncolytic viruses. Trends Mol. Med. 2013;19:378–392. doi: 10.1016/j.molmed.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 16.Toro Bejarano M., Merchan J.R. Targeting tumor vasculature through oncolytic virotherapy: recent advances. Oncolytic Virother. 2015;4:169–181. doi: 10.2147/OV.S66045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C.Y., Hutzen B., Wedekind M.F., Cripe T.P. Oncolytic virus and PD-1/PD-L1 blockade combination therapy. Oncolytic Virother. 2018;7:65–77. doi: 10.2147/OV.S145532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaRocca C.J., Warner S.G. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin. Transl. Med. 2018;7:35. doi: 10.1186/s40169-018-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin N.T., Bell J.C. Oncolytic virus combination therapy: killing one bird with two stones. Mol. Ther. 2018;26:1414–1422. doi: 10.1016/j.ymthe.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell L., Peng K.W., Russell S.J., Diaz R.M. Oncolytic viruses: priming time for cancer immunotherapy. BioDrugs. 2019;33:485–501. doi: 10.1007/s40259-019-00367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson M.S., Lemoine N.R., Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv. Virol. 2012;2012:805629. doi: 10.1155/2012/805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niemann J., Woller N., Brooks J., Fleischmann-Mundt B., Martin N.T., Kloos A., Knocke S., Ernst A.M., Manns M.P., Kubicka S. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy. Nat. Commun. 2019;10:3236. doi: 10.1038/s41467-019-11137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Riordan C.R., Lachapelle A., Delgado C., Parkes V., Wadsworth S.C., Smith A.E., Francis G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 24.Fisher K.D., Stallwood Y., Green N.K., Ulbrich K., Mautner V., Seymour L.W. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Q., Han J., Zhao D., Gong T., Zhang Z., Sun X. Protection of adenovirus from neutralizing antibody by cationic PEG derivative ionically linked to adenovirus. Int. J. Nanomedicine. 2012;7:985–997. doi: 10.2147/IJN.S27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil I.R., Khechara M.P., Kurusamy S., Armesilla A.L., Gupta A., Mendrek B., Khalaf T., Scandola M., Focarete M.L., Kowalczuk M., Radecka I. Poly-gamma-glutamic acid (γ-PGA)-based encapsulation of adenovirus to evade neutralizing antibodies. Molecules. 2018;23:E2565. doi: 10.3390/molecules23102565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francini N., Cochrane D., Illingworth S., Purdie L., Mantovani G., Fisher K., Seymour L.W., Spain S.G., Alexander C. Polyvalent diazonium polymers provide efficient protection of oncolytic adenovirus enadenotucirev from neutralizing antibodies while maintaining biological activity in vitro and in vivo. Bioconjug. Chem. 2019;30:1244–1257. doi: 10.1021/acs.bioconjchem.9b00189. [DOI] [PubMed] [Google Scholar]
- 28.Garofalo M., Saari H., Somersalo P., Crescenti D., Kuryk L., Aksela L., Capasso C., Madetoja M., Koskinen K., Oksanen T. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release. 2018;283:223–234. doi: 10.1016/j.jconrel.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Garofalo M., Villa A., Rizzi N., Kuryk L., Mazzaferro V., Ciana P. Systemic administration and targeted delivery of immunogenic oncolytic adenovirus encapsulated in extracellular vesicles for cancer therapies. Viruses. 2018;10:E568. doi: 10.3390/v10100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ran L., Tan X., Li Y., Zhang H., Ma R., Ji T., Dong W., Tong T., Liu Y., Chen D. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66. doi: 10.1016/j.biomaterials.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Mendez N., Herrera V., Zhang L., Hedjran F., Feuer R., Blair S.L., Trogler W.C., Reid T.R., Kummel A.C. Encapsulation of adenovirus serotype 5 in anionic lecithin liposomes using a bead-based immunoprecipitation technique enhances transfection efficiency. Biomaterials. 2014;35:9554–9561. doi: 10.1016/j.biomaterials.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoyama K., Kuroda S., Morihiro T., Kanaya N., Kubota T., Kakiuchi Y., Kikuchi S., Nishizaki M., Kagawa S., Tazawa H., Fujiwara T. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017;7:14177. doi: 10.1038/s41598-017-14717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Huang H., Zou H., Tian X., Hu J., Qiu P., Hu H., Yan G. Liposome encapsulation of oncolytic virus M1 to reduce immunogenicity and immune clearance in vivo. Mol. Pharm. 2019;16:779–785. doi: 10.1021/acs.molpharmaceut.8b01046. [DOI] [PubMed] [Google Scholar]
- 34.Willmon C., Harrington K., Kottke T., Prestwich R., Melcher A., Vile R. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 2009;17:1667–1676. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramírez M., García-Castro J., Melen G.J., González-Murillo Á., Franco-Luzón L. Patient-derived mesenchymal stem cells as delivery vehicles for oncolytic virotherapy: novel state-of-the-art technology. Oncolytic Virother. 2015;4:149–155. doi: 10.2147/OV.S66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lilly C.L., Villa N.Y., Lemos de Matos A., Ali H.M., Dhillon J.S., Hofland T., Rahman M.M., Chan W., Bogen B., Cogle C., McFadden G. Ex vivo oncolytic virotherapy with myxoma virus arms multiple allogeneic bone marrow transplant leukocytes to enhance graft versus tumor. Mol. Ther. Oncolytics. 2016;4:31–40. doi: 10.1016/j.omto.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iankov I.D., Blechacz B., Liu C., Schmeckpeper J.D., Tarara J.E., Federspiel M.J., Caplice N., Russell S.J. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 38.Dwyer R.M., Kerin M.J. Mesenchymal stem cells and cancer: tumor-specific delivery vehicles or therapeutic targets? Hum. Gene Ther. 2010;21:1506–1512. doi: 10.1089/hum.2010.135. [DOI] [PubMed] [Google Scholar]
- 39.Josiah D.T., Zhu D., Dreher F., Olson J., McFadden G., Caldas H. Adipose-derived stem cells as therapeutic delivery vehicles of an oncolytic virus for glioblastoma. Mol. Ther. 2010;18:377–385. doi: 10.1038/mt.2009.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooney R., Majid A.A., Batalla-Covello J., Machado D., Liu X., Gonzaga J., Tirughana R., Hammad M., Lesniak M.S., Curiel D.T., Aboody K.S. Enhanced delivery of oncolytic adenovirus by neural stem cells for treatment of metastatic ovarian cancer. Mol. Ther. Oncolytics. 2018;12:79–92. doi: 10.1016/j.omto.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A.U., Rolle C.E., Tyler M.A., Han Y., Sengupta S., Wainwright D.A., Balyasnikova I.V., Ulasov I.V., Lesniak M.S. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol. Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed A.U., Tyler M.A., Thaci B., Alexiades N.G., Han Y., Ulasov I.V., Lesniak M.S. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol. Pharm. 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorne S.H., Negrin R.S., Contag C.H. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 44.Ilett E.J., Bárcena M., Errington-Mais F., Griffin S., Harrington K.J., Pandha H.S., Coffey M., Selby P.J., Limpens R.W., Mommaas M. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 2011;17:2767–2776. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunuales M., Garcia-Aragoncillo E., Casado R., Quetglas J.I., Hervas-Stubbs S., Bortolanza S., Benavides-Vallve C., Ortiz-de-Solorzano C., Prieto J., Hernandez-Alcoceba R. Evaluation of monocytes as carriers for armed oncolytic adenoviruses in murine and Syrian hamster models of cancer. Hum. Gene Ther. 2012;23:1258–1268. doi: 10.1089/hum.2012.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkeley R.A., Steele L.P., Mulder A.A., van den Wollenberg D.J.M., Kottke T.J., Thompson J., Coffey M., Hoeben R.C., Vile R.G., Melcher A., Ilett E.J. Antibody-neutralized reovirus is effective in oncolytic virotherapy. Cancer Immunol. Res. 2018;6:1161–1173. doi: 10.1158/2326-6066.CIR-18-0309. [DOI] [PubMed] [Google Scholar]
- 47.Muthana M., Giannoudis A., Scott S.D., Fang H.Y., Coffelt S.B., Morrow F.J., Murdoch C., Burton J., Cross N., Burke B. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71:1805–1815. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- 48.Villa N.Y., Wasserfall C.H., Meacham A.M., Wise E., Chan W., Wingard J.R., McFadden G., Cogle C.R. Myxoma virus suppresses proliferation of activated T lymphocytes yet permits oncolytic virus transfer to cancer cells. Blood. 2015;125:3778–3788. doi: 10.1182/blood-2014-07-587329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melzer M.K., Zeitlinger L., Mall S., Steiger K., Schmid R.M., Ebert O., Krackhardt A., Altomonte J. Enhanced safety and efficacy of oncolytic VSV therapy by combination with T cell receptor transgenic T cells as carriers. Mol. Ther. Oncolytics. 2018;12:26–40. doi: 10.1016/j.omto.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong H.T., Hasegawa K., Dietz A.B., Russell S.J., Peng K.W. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene Ther. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 51.Qiao J., Wang H., Kottke T., Diaz R.M., Willmon C., Hudacek A., Thompson J., Parato K., Bell J., Naik J. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon A.R., Hong J., Li Y., Shin H.C., Lee H., Kim H.S., Yun C.O. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res. 2019;79:4503–4514. doi: 10.1158/0008-5472.CAN-18-3900. [DOI] [PubMed] [Google Scholar]
- 53.Draganov D.D., Santidrian A.F., Minev I., Nguyen D., Kilinc M.O., Petrov I., Vyalkova A., Lander E., Berman M., Minev B., Szalay A.A. Delivery of oncolytic vaccinia virus by matched allogeneic stem cells overcomes critical innate and adaptive immune barriers. J. Transl. Med. 2019;17:100. doi: 10.1186/s12967-019-1829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamada K., Desaki J., Nakagawa K., Zhang T., Shirakawa T., Gotoh A., Tagawa M. Carrier cell-mediated delivery of a replication-competent adenovirus for cancer gene therapy. Mol. Ther. 2007;15:1121–1128. doi: 10.1038/sj.mt.6300128. [DOI] [PubMed] [Google Scholar]
- 55.Alcayaga-Miranda F., Cascallo M., Rojas J.J., Pastor J., Alemany R. Osteosarcoma cells as carriers to allow antitumor activity of canine oncolytic adenovirus in the presence of neutralizing antibodies. Cancer Gene Ther. 2010;17:792–802. doi: 10.1038/cgt.2010.36. [DOI] [PubMed] [Google Scholar]
- 56.Saito A., Morishita N., Mitsuoka C., Kitajima S., Hamada K., Lee K.M., Kawabata M., Fujisawa M., Shirakawa T. Intravenous injection of irradiated tumor cell vaccine carrying oncolytic adenovirus suppressed the growth of multiple lung tumors in a mouse squamous cell carcinoma model. J. Gene Med. 2011;13:353–361. doi: 10.1002/jgm.1578. [DOI] [PubMed] [Google Scholar]
- 57.Hamada K., Takagi S., Kuboshima H., Shimada H., Takagi K., Yasuoka T., Matsubara K., Sassa Y., Furuya T., Suzuki K. Cloning of carrier cells infected with oncolytic adenovirus driven by midkine promoter and biosafety studies. J. Gene Med. 2019;21:e3064. doi: 10.1002/jgm.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breitbach C.J., Heo J., Cho M., Moon A., Kim C.W., Burke J., Hickman T., Dubois K., Lee Y.S., Daneshmand M. 19. Baseline neutralizing antibody status does not affect intravenous delivery of oncolytic vaccinia Pexa-Vec (JX-594) in liver cancer patients. Mol. Ther. 2013;21(Suppl 1):S7–S8. [Google Scholar]
- 59.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 60.Ebrahimi S., Ghorbani E., Khazaei M., Avan A., Ryzhikov M., Azadmanesh K., Hassanian S.M. Interferon-mediated tumor resistance to oncolytic virotherapy. J. Cell. Biochem. 2017;118:1994–1999. doi: 10.1002/jcb.25917. [DOI] [PubMed] [Google Scholar]
- 61.Kurokawa C., Iankov I.D., Galanis E. A key anti-viral protein, RSAD2/VIPERIN, restricts the release of measles virus from infected cells. Virus Res. 2019;263:145–150. doi: 10.1016/j.virusres.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J.M., Ghonime M.G., Cassady K.A. STING restricts oHSV replication and spread in resistant MPNSTs but is dispensable for basal IFN-stimulated gene upregulation. Mol. Ther. Oncolytics. 2019;15:91–100. doi: 10.1016/j.omto.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matveeva O.V., Chumakov P.M. Defects in interferon pathways as potential biomarkers of sensitivity to oncolytic viruses. Rev. Med. Virol. 2018;28:e2008. doi: 10.1002/rmv.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fulci G., Breymann L., Gianni D., Kurozomi K., Rhee S.S., Yu J., Kaur B., Louis D.N., Weissleder R., Caligiuri M.A., Chiocca E.A. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc. Natl. Acad. Sci. USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jha B.K., Dong B., Nguyen C.T., Polyakova I., Silverman R.H. Suppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapy. Mol. Ther. 2013;21:1749–1757. doi: 10.1038/mt.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dold C., Rodriguez Urbiola C., Wollmann G., Egerer L., Muik A., Bellmann L., Fiegl H., Marth C., Kimpel J., von Laer D. Application of interferon modulators to overcome partial resistance of human ovarian cancers to VSV-GP oncolytic viral therapy. Mol. Ther. Oncolytics. 2016;3:16021. doi: 10.1038/mto.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel M.R., Dash A., Jacobson B.A., Ji Y., Baumann D., Ismail K., Kratzke R.A. JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models. Cancer Gene Ther. 2019;26:411–418. doi: 10.1038/s41417-018-0074-6. [DOI] [PubMed] [Google Scholar]
- 68.Ghonime M.G., Cassady K.A. Combination therapy using ruxolitinib and oncolytic HSV renders resistant MPNSTs susceptible to virotherapy. Cancer Immunol. Res. 2018;6:1499–1510. doi: 10.1158/2326-6066.CIR-18-0014. [DOI] [PubMed] [Google Scholar]
- 69.Felt S.A., Droby G.N., Grdzelishvili V.Z. Ruxolitinib and polycation combination treatment overcomes multiple mechanisms of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. J. Virol. 2017;91 doi: 10.1128/JVI.00461-17. e00461-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allagui F., Achard C., Panterne C., Combredet C., Labarrière N., Dréno B., Elgaaied A.B., Pouliquen D., Tangy F., Fonteneau J.F. Modulation of the type I interferon response defines the sensitivity of human melanoma cells to oncolytic measles virus. Curr. Gene Ther. 2017;16:419–428. doi: 10.2174/1566523217666170102110502. [DOI] [PubMed] [Google Scholar]
- 71.Jackson J.D., Markert J.M., Li L., Carroll S.L., Cassady K.A. STAT1 and NF-κB inhibitors diminish basal interferon-stimulated gene expression and improve the productive infection of oncolytic HSV in MPNST cells. Mol. Cancer Res. 2016;14:482–492. doi: 10.1158/1541-7786.MCR-15-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Escobar-Zarate D., Liu Y.P., Suksanpaisan L., Russell S.J., Peng K.W. Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors. Cancer Gene Ther. 2013;20:582–589. doi: 10.1038/cgt.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waggoner S.N., Reighard S.D., Gyurova I.E., Cranert S.A., Mahl S.E., Karmele E.P., McNally J.P., Moran M.T., Brooks T.R., Yaqoob F., Rydyznski C.E. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alvarez-Breckenridge C.A., Yu J., Price R., Wojton J., Pradarelli J., Mao H., Wei M., Wang Y., He S., Hardcastle J. NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nat. Med. 2012;18:1827–1834. doi: 10.1038/nm.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han J., Chen X., Chu J., Xu B., Meisen W.H., Chen L., Zhang L., Zhang J., He X., Wang Q.E. TGFβ treatment enhances glioblastoma virotherapy by inhibiting the innate immune response. Cancer Res. 2015;75:5273–5282. doi: 10.1158/0008-5472.CAN-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., Caux C., Depil S. Cold tumors: a therapeutic challenge for immunotherapy. Front. Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ilett E., Kottke T., Thompson J., Rajani K., Zaidi S., Evgin L., Coffey M., Ralph C., Diaz R., Pandha H. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther. 2017;24:21–30. doi: 10.1038/gt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sivanandam V., LaRocca C.J., Chen N.G., Fong Y., Warner S.G. Oncolytic viruses and immune checkpoint inhibition: the best of both worlds. Mol. Ther. Oncolytics. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031–1032. doi: 10.1016/j.cell.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 80.Kepp O., Senovilla L., Vitale I., Vacchelli E., Adjemian S., Agostinis P., Apetoh L., Aranda F., Barnaba V., Bloy N. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Achard C., Boisgerault N., Delaunay T., Tangy F., Grégoire M., Fonteneau J.F. Induction of immunogenic tumor cell death by attenuated oncolytic measles virus. J. Clin. Cell. Immunol. 2015;6:291. [Google Scholar]
- 82.Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Workenhe S.T., Mossman K.L. Oncolytic virotherapy and immunogenic cancer cell death: sharpening the sword for improved cancer treatment strategies. Mol. Ther. 2014;22:251–256. doi: 10.1038/mt.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serrano-Del Valle A., Anel A., Naval J., Marzo I. Immunogenic cell death and immunotherapy of multiple myeloma. Front. Cell Dev. Biol. 2019;7:50. doi: 10.3389/fcell.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bommareddy P.K., Zloza A., Rabkin S.D., Kaufman H.L. Oncolytic virus immunotherapy induces immunogenic cell death and overcomes STING deficiency in melanoma. OncoImmunology. 2019;8:1591875. doi: 10.1080/2162402X.2019.1591875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donnelly O.G., Errington-Mais F., Steele L., Hadac E., Jennings V., Scott K., Peach H., Phillips R.M., Bond J., Pandha H. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013;20:7–15. doi: 10.1038/gt.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Somma S., Iannuzzi C.A., Passaro C., Forte I.M., Iannone R., Gigantino V., Indovina P., Botti G., Giordano A., Formisano P. The oncolytic virus dl922-947 triggers immunogenic cell death in mesothelioma and reduces xenograft growth. Front. Oncol. 2019;9:564. doi: 10.3389/fonc.2019.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamano T., Kubo S., Fukumoto M., Yano A., Mawatari-Furukawa Y., Okamura H., Tomita N. Whole cell vaccination using immunogenic cell death by an oncolytic adenovirus is effective against a colorectal cancer model. Mol. Ther. Oncolytics. 2016;3:16031. doi: 10.1038/mto.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jessup J.M., Mattoo A.R., Korokhov N. 109. Replicating oncolytic chimeric adenovirus expressing shRNA improves inhibition of human colorectal carcinoma (CRC) growth. Mol. Ther. 2015;23(Suppl 1):S46. [Google Scholar]
- 90.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koks C.A., Garg A.D., Ehrhardt M., Riva M., Vandenberk L., Boon L., De Vleeschouwer S., Agostinis P., Graf N., Van Gool S.W. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int. J. Cancer. 2015;136:E313–E325. doi: 10.1002/ijc.29202. [DOI] [PubMed] [Google Scholar]
- 92.Ye T., Jiang K., Wei L., Barr M.P., Xu Q., Zhang G., Ding C., Meng S., Piao H. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am. J. Cancer Res. 2018;8:1514–1527. [PMC free article] [PubMed] [Google Scholar]
- 93.Shao X., Wang X., Guo X., Jiang K., Ye T., Chen J., Fang J., Gu L., Wang S., Zhang G. STAT3 contributes to oncolytic newcastle disease virus-induced immunogenic cell death in melanoma cells. Front. Oncol. 2019;9:436. doi: 10.3389/fonc.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denton N.L., Chen C.Y., Scott T.R., Cripe T.P. Tumor-associated macrophages in oncolytic virotherapy: friend or foe? Biomedicines. 2016;4:E13. doi: 10.3390/biomedicines4030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marchini A., Daeffler L., Pozdeev V.I., Angelova A., Rommelaere J. Immune conversion of tumor microenvironment by oncolytic viruses: the protoparvovirus H-1PV case study. Front. Immunol. 2019;10:1848. doi: 10.3389/fimmu.2019.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Achard C., Surendran A., Wedge M.E., Ungerechts G., Bell J., Ilkow C.S. Lighting a fire in the tumor microenvironment using oncolytic immunotherapy. EBioMedicine. 2018;31:17–24. doi: 10.1016/j.ebiom.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Denton N.L., Chen C.Y., Hutzen B., Currier M.A., Scott T., Nartker B., Leddon J.L., Wang P.Y., Srinivas R., Cassady K.A. Myelolytic treatments enhance oncolytic herpes virotherapy in models of Ewing sarcoma by modulating the immune microenvironment. Mol. Ther. Oncolytics. 2018;11:62–74. doi: 10.1016/j.omto.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Y., Patel B., Dey A., Ghorani E., Rai L., Elham M., Castleton A.Z., Fielding A.K. Attenuated, oncolytic, but not wild-type measles virus infection has pleiotropic effects on human neutrophil function. J. Immunol. 2012;188:1002–1010. doi: 10.4049/jimmunol.1102262. [DOI] [PubMed] [Google Scholar]
- 99.de Graaf J.F., de Vor L., Fouchier R.A.M., van den Hoogen B.G. Armed oncolytic viruses: a kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018;41:28–39. doi: 10.1016/j.cytogfr.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foloppe J., Kempf J., Futin N., Kintz J., Cordier P., Pichon C., Findeli A., Vorburger F., Quemeneur E., Erbs P. The enhanced tumor specificity of TG6002, an armed oncolytic vaccinia virus deleted in two genes involved in nucleotide metabolism. Mol. Ther. Oncolytics. 2019;14:1–14. doi: 10.1016/j.omto.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pearl T.M., Markert J.M., Cassady K.A., Ghonime M.G. Oncolytic virus-based cytokine expression to improve immune activity in brain and solid tumors. Mol. Ther. Oncolytics. 2019;13:14–21. doi: 10.1016/j.omto.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Havunen R., Santos J.M., Sorsa S., Rantapero T., Lumen D., Siurala M., Airaksinen A.J., Cervera-Carrascon V., Tähtinen S., Kanerva A., Hemminki A. Abscopal effect in non-injected tumors achieved with cytokine-armed oncolytic adenovirus. Mol. Ther. Oncolytics. 2018;11:109–121. doi: 10.1016/j.omto.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Z., Ge Y., Wang H., Ma C., Feist M., Ju S., Guo Z.S., Bartlett D.L. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2. Nat. Commun. 2018;9:4682. doi: 10.1038/s41467-018-06954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stephenson K.B., Barra N.G., Davies E., Ashkar A.A., Lichty B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 105.Kowalsky S.J., Liu Z., Feist M., Berkey S.E., Ma C., Ravindranathan R., Dai E., Roy E.J., Guo Z.S., Bartlett D.L. Superagonist IL-15-armed oncolytic virus elicits potent antitumor immunity and therapy that are enhanced with PD-1 blockade. Mol. Ther. 2018;26:2476–2486. doi: 10.1016/j.ymthe.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haddad D. Genetically engineered vaccinia viruses as agents for cancer treatment, imaging, and transgene delivery. Front. Oncol. 2017;7:96. doi: 10.3389/fonc.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chon H.J., Lee W.S., Yang H., Kong S.J., Lee N.K., Moon E.S., Choi J., Han E.C., Kim J.H., Ahn J.B. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin. Cancer Res. 2019;25:1612–1623. doi: 10.1158/1078-0432.CCR-18-1932. [DOI] [PubMed] [Google Scholar]
- 108.Twumasi-Boateng K., Pettigrew J.L., Kwok Y.Y.E., Bell J.C., Nelson B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer. 2018;18:419–432. doi: 10.1038/s41568-018-0009-4. [DOI] [PubMed] [Google Scholar]
- 109.McGray A.J.R., Huang R.Y., Battaglia S., Eppolito C., Miliotto A., Stephenson K.B., Lugade A.A., Webster G., Lichty B.D., Seshadri M. Oncolytic Maraba virus armed with tumor antigen boosts vaccine priming and reveals diverse therapeutic response patterns when combined with checkpoint blockade in ovarian cancer. J. Immunother. Cancer. 2019;7:189. doi: 10.1186/s40425-019-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rojas J.J., Sampath P., Bonilla B., Ashley A., Hou W., Byrd D., Thorne S.H. Manipulating TLR signaling increases the anti-tumor T cell response induced by viral cancer therapies. Cell Rep. 2016;15:264–273. doi: 10.1016/j.celrep.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]