Abstract
Nanozymes are nanomaterials with intrinsic enzyme-like properties. They can specifically catalyze substrates of natural enzymes under physiological condition with similar catalytic mechanism and kinetics. Compared to natural enzymes, nanozymes exhibit the unique advantages including high catalytic activity, low cost, high stability, easy mass production, and tunable activity. In addition, as a new type of artificial enzymes, nanozymes not only have the enzyme-like catalytic activity, but also exhibit the unique physicochemical properties of nanomaterials, such as photothermal properties, superparamagnetism, and fluorescence, etc. By combining the unique physicochemical properties and enzyme-like catalytic activities, nanozymes have been widely developed for in vitro detection and in vivo disease monitoring and treatment. Here we mainly summarized the applications of nanozymes for disease imaging and detection to explore their potential application in disease diagnosis and precision medicine.
Keywords: nanozyme, natural enzyme, disease imaging, precision medicine, tumor
Graphical Abstract
Nanozymes for disease imaging and diagnosis. Adapted with permission from Fan et al. (2012), Li et al. (2017), Ding et al. (2019).
Introduction
Nanozyme is a new type of artificial enzyme with intrinsic enzyme-like characteristics. In 2007, we reported the landmark paper that Fe3O4 nanoparticles (NPs) have intrinsic peroxidase-like activity (Gao et al., 2007), and since that time nanozymes have increasingly attracted attention from a broad spectrum of scientists and technologists because of their high catalytic activity, low cost, and high stability (Gao and Yan, 2016). To date, there are more than 300 types of nanomaterials that have been found to possess the intrinsic enzyme-like activity, including the peroxidase activity of Fe3O4 (Gao et al., 2007), Co3O4 (Mu et al., 2012), CuO (Liu et al., 2014), V2O5 (André et al., 2011), MnFeO3 (Chi et al., 2018), FeS (Dai et al., 2009), graphene quantum dots (Nirala et al., 2017), CeO2 (Xue et al., 2012), BiFeO3 (Wei et al., 2010), CoFe2O4 (He et al., 2010), FeTe (Roy et al., 2012), gold@carbon dots (Zheng et al., 2016); oxidase activity of Au (Comotti et al., 2004), Pt (Yu et al., 2014), CoFe2O4 (Zhang et al., 2013), MnO2 (Xing et al., 2012), CuO NPs (Hu et al., 2017) and NiCo2O4 (Su et al., 2017); catalase activity of CeO2 NPs (Talib et al., 2010), Pt-Ft NPs (Fan et al., 2011), Ir NPs (Su et al., 2015), MoS2 nanosheets (Chen et al., 2018), Prussian Blue NPs (Zhang W. et al., 2016); superoxide oxidase activity of CeO2 (Tarnuzzer et al., 2005), Fullerene (Ali et al., 2004), FePO4 microflowers (Wang W. et al., 2012), Gly-Cu (OH)2 NPs (Korschelt et al., 2017), N-PCNs (Fan et al., 2018); haloperoxidase activity of V2O5 nanowire (Natalio et al., 2012), CeO2x Nanorods (Herget et al., 2017); sulfite oxidase activity of MoO3 NPs (Ragg et al., 2014); phosphatase activity of CeO2 (Kuchma et al., 2010), Fe2O3 NPs (Huang, 2018); phosphotriesterase activity of Co3O4/GO nanocomposites (Wang et al., 2017), CeO2 NPs (Vernekar et al., 2016), MOF-808 (M = Zr) (Mondal and Holdt, 2016), UiO-66@LiOtBu (M = Zr) (Mondal and Holdt, 2016); CO oxidase activity of Cu2O@CeO2 core@shell nanocubes (Wang et al., 2015); chymotrypsin activity of Cr-MIL-101 (Nath et al., 2016); G-selective DNA cleaving activity of fullerene carboxylic acid (Tokuyama et al., 1993); protease activity of Cu-MOF (Li et al., 2014); restriction endonuclease activity of CdTe NPs (Sun et al., 2018); carbonic anhydrase activity of Co-BBP@Tb-MOF (Sahoo et al., 2013), etc. With the emergence of the new concept of “nanozymology” (Jiang B. et al., 2018), “Nanozymes” have now become an emerging new field bridging nanotechnology and biology.
As a new type of promising artificial enzymes, nanozymes have shown a broad spectrum of applications because of their obvious advantages including high stability, high catalytic activity, low cost, large surface area for functionalization, and tunable activity. In particular, by combining their unique physicochemical properties (such as fluorescence, X-ray absorption, and paramagnetic properties, etc.), nanozymes have been widely explored from in vitro detection (Leng et al., 2009; Liang et al., 2010; Roy et al., 2012) to in vivo disease imaging and therapy (Hyon Bin et al., 2010; Yang et al., 2012; Kwon et al., 2016; Singh et al., 2017; Fan et al., 2018). In this review, we summarized the progress of nanozymes in disease detection and imaging, and discussed the current challenges and future directions of nanozyme development in disease imaging and diagnosis.
Nanozymes for Pathological Disease Diagnosis
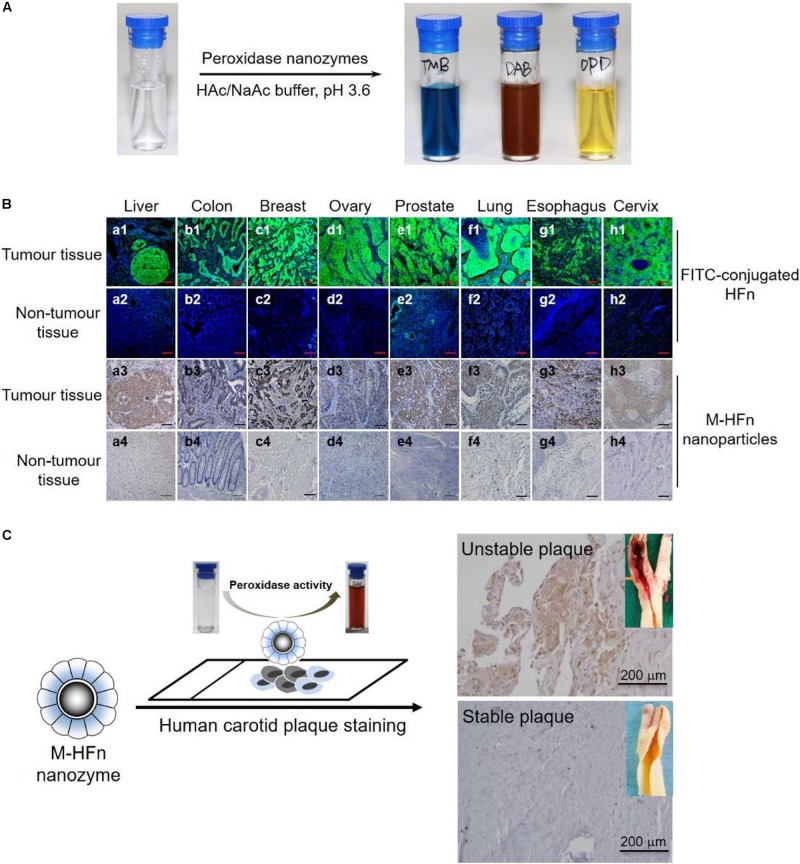
Peroxidase nanozymes catalyze the oxidation of colorimetric substrates, such as 3,3,5,5-tetramethylbenzidine (TMB), diazo-aminobenzene (DAB), and o-phenylenediamine (OPD), to give a color reaction that can be used for imaging the recognized biomarkers within tissue sections for pathological disease diagnosis (Figure 1A). In 2012, Our group developed a magnetoferritin nanozyme (M-HFn) which is composed of a recombinant human heavy-chain ferritin (HFn) protein nanocage encapsulated an iron oxide nanocore for tumor targeting and imaging (Fan et al., 2012). HFn nanocage specifically recognized tumor cells via binding to overexpressed transferrin receptor1 (TfR1) in tumor cells. Iron oxide nanocores catalyzed the oxidation of color substrates in the presence of H2O2 to produce an intense color reaction for visualizing tumor tissues. We examined 474 clinical human specimens including 247 clinical tumor tissues and 227 normal tissues and demonstrated that M-HFn nanozymes could identify nine types of cancer cells with a specificity of over 95% and sensitivity of 98%. The concentration of M-HFn was 1.8 μM, and the reactive time was 1 h for DAB staining (Figure 1B). Likewise, Gu’s groups developed avastin antibody-functionalized Co3O4 nanozymes as target-specific peroxidase mimics for immunohistochemical staining of vascular endothelial growth factor (VEGF) in tumor tissues and the concentration of Ab-Co3O4 was 15 μg/ml, 100 μL, and the reactive time was 30 min for DAB staining (Dong et al., 2014). Due to the high peroxidase-like activity, Co3O4 nanozyme has been proved to be a potential label in place of natural enzymes. So far, numerous of peroxidase nanozyme-based staining methods have been developed for pathological diagnosis of breast cancer, colorectal, stomach, and pancreas (Zhang T. et al., 2016), hepatocellular carcinoma (Hu et al., 2014; Jiang et al., 2019), esophageal cancer (Wu et al., 2011), and bladder cancer (Peng et al., 2019).
FIGURE 1.
Nanozymes for pathological tissue imaging. (A) Peroxidase nanozymes catalyze the oxidation of various peroxidase substrates (TMB, DAB, and OPD) in the presence of H2O2 to produce different color reactions. Adapted with permission from ref (Jiang B. et al., 2018), © 2018, Springer Nature. (B) M-HFn nanozymes specifically stained tumor tissues from different organs. Adapted with permission from ref (Fan et al., 2012), © 2012, Springer Nature. (C) Peroxidase nanozymes for the pathological identification of unstable atherosclerotic plaques from patients with symptomatic carotid disease. Reproduced with permission from ref (Liang and Yan, 2019), © 2019, American Chemical Society.
By compare with the traditional immunohistochemistry, the nanozyme-based pathological staining method is more rapid and sensitive because of their higher catalytic activity than natural enzymes [e.g., horseradish peroxidase (HRP)], which greatly shortens the diagnostic time and reduces the cost and thus has significant implications for clinical pathological diagnosis. In addition, besides tumor pathological diagnosis, peroxidase nanozymes have also been used for pathological identification of human high-risk and ruptured atherosclerotic plaques (Wang et al., 2019). M-HFn nanozymes specifically distinguish the ruptured and high-risk plaque tissues via TfR1, which is highly expressed in plaque-infiltrated macrophages and significantly associated with the increasing risk of plaque rupture. As shown Figure 1C, M-HFn peroxidase nanozymes could specifically distinguish high-risk plaque tissues from patients with symptomatic carotid disease, and M-HFn staining showed a significant correlation with plaque vulnerability (r = 0.89, P < 0.0001).
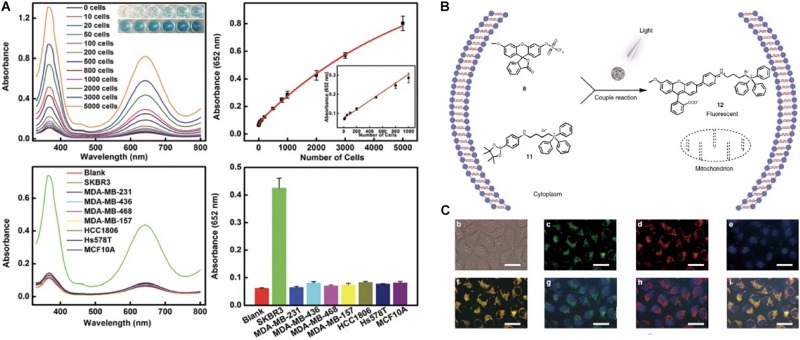
To further improve the detection sensitivity of nanozyme-based pathological staining method, much effort has been expended to improve the enzyme-like catalytic activity of nanozymes, including adjusting their size, shape, composition, surface modification, and heteroatomic doping (Dong et al., 2014; Zhang et al., 2017; Jiang et al., 2019; Li et al., 2019). In 2018, Leong and co-workers engineered a mesoporous silica-gold nanocluster hybrid nanozymes with excellent peroxidase-like catalytic activity for selective detection of HER2-positive (HER2+) breast cancer cell (Li et al., 2019). Owing to their high catalytic performance, the prepared silica-gold hybrid nanozymes achieved the detection limit of 10 cells using colorimetric analysis. The hybrid nanozymes did not stain, or only slightly stained, normal or lesion tissues, but strongly stained cancerous regions. Significantly, there was a clear distinction between cancerous cells and adjacent normal cells in representative sections (Figure 2A).
FIGURE 2.
Nanozymes for live cell and organelle imaging. (A) A multifunctional mesoporous silica-gold nanozyme platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay. Reproduced with permission from ref (Li et al., 2019), © 2019, The Royal Society of Chemistry. (B) Scheme of nanozyme-based methods for mitochondrial fluorescent imaging. (C) Representative nanozyme-based light-mediated reversible catalysis for mitochondrial imaging. Adapted with permission from ref (Wang et al., 2018).
Besides enzyme-like activity, the unique physicochemical properties (such as luminescence, X-ray absorption, and paramagnetic properties) of nanozymes also have been widely developed for pathological tissue imaging. For instance, Cai’s group developed a folate receptor-targeting gold nanocluster as fluorescence enzyme mimetic nanoprobes for tumor tissues fluorescence visualizing detection. In the work, the intravenous dose used was 500 mg/kg for fluorescence imaging, and the concentration of 1.8 mM, 1 h was used for DAB staining (Hu et al., 2014). For the same tumor tissue slice, nanozyme staining and fluorescent staining were obtained simultaneously in a one-step incubation, and the results were mutually complementary. Thus, the developed fluorescence/nanozyme nanoprobes could provide a molecular colocalization diagnosis strategy within clinical tissue specimens, which efficiently avoids false-positive and false-negative results, and greatly improves the detection accuracy, credibility, and repeatability for cancer pathological diagnoses. Likewise, Zhang et al., also developed a gold nanozyme-based dark-field imaging assay as a novel immunohistochemical method for detecting HER2 overexpressed in breast cancer tissues (Lin et al., 2016). By quantitative analysis of the optical property of dark-field imaging, cancerous tissue can be quantitatively divided into four levels: “−, +, ++, and ++.”
Despite the fact that nanozyme-based staining methods have been broadly developed for pathological disease diagnosis, there are still many unresolved issues and challenges. The first is how to improve the enzyme-like activity of nanozymes. Since the catalytic activity of nanozymes is directly correlated with their detection sensitivity, the improvement of enzyme-like activity of nanozymes could help substantially improve the detection sensitivity of nanozyme-based staining methods. However, the issue of false positives would arise along with the improved enzyme-like activity (Wu et al., 2019). In addition, the false positive issue would become even more severe due to the limited substrate specificity of nanozymes. We proposed a strategy to improve both the catalytic activity and the substrate specificity by introducing histidine residues onto the surface of Fe3O4 nanozymes to mimic the natural peroxidase enzymes (Fan et al., 2017). Juewen Liu engineered a specific nanozyme using molecular imprinting method to enhance the substrate selectivity and activity of enzyme mimics (Zhang et al., 2017). In addition, the oriented immobilization of the recognizing moieties to the surface of nanozymes could also reduce false positives greatly. Guo et al., constructed an inorganic/protein hybrid nanozyme by oriented immobilizing nature enzymes on the surface of inorganic graphene NPs (Liu Y. et al., 2019). The prepared nanohybrid nanozymes exhibited outstanding peroxidase-mimicking activity and excellent substrate selectivity. The second challenge for nanozyme staining method is how to quantically analyze the pathological staining results. Currently, the clinical pathological analysis mainly relies on experienced judgment, which is subjective, and variation between observers is high for certain categories of pathological diagnosis (Qian and Jiao, 2017). By combining the unique optical property and enzyme-like catalytic activities, nanozymes hold promise to achieve quantitative analysis for pathological disease staining diagnosis.
Nanozymes for Live Cell and Organelle Imaging
Cytological examination is an important means of clinical disease diagnosis (Bromberg et al., 2007; Mosterd et al., 2008; Hao et al., 2011; Venugopal and Prasad, 2015). Exfoliated cells from blood, cerebrospinal fluid, spinal fluid, chest water and mucous liquid can provide a large amount of clinical information (including cell morphology, cell type, and cell proportion, etc.), which can be used for cancer screening (Schiffman et al., 2000; Dillner et al., 2008), CNS hematologic malignancies (Bromberg et al., 2007) anemia diagnosis (Hao et al., 2011), and Langerhans cell granulomatosis detection (Mosterd et al., 2008).
Currently, the most commonly used cytological detection methods are flow cytometry, cytological smear and nucleic acid testing. These traditional methods are characterized by high technical requirements, time consuming or high cost. Nanozymes-driven color reaction can be used for qualitative and quantitative analysis of cytological features. Trau et al. extended the application of nanozymes to the detection of circulating tumor cells (CTCs) (Li et al., 2017). The targeting antibody-conjugated Fe3O4 nanozymes simultaneously achieved CTC magnetic isolation and visualization by catalyzing the oxidation of colorimetric substrate TMB into blue colored products. In addition, the visualized CTCs can be further quantified using UV-vis measurement. The developed nanozyme platform successfully detected 13 melanoma CTCs per mL blood within 50 min, and the concentration of Fe3O4 nanozymes used was about 0.2 mg/ml for TMB colorimetric development. Later, Wang et al. (2018) also developed an Fe3O4 NPs-based ultrasensitive electrochemical CTCs detection strategy (Tian et al., 2018). Under the optimized experimental conditions, the proposed nanozyme cytosensor exhibited significant analytical performance for MCF-7 CTCs detection with a detection limit of 6 cells mL–1 with a linear range from 15 to 45 cells mL–1 at the acceptable stability condition and reproducibility. Recently, nanozyme-based detection strategies have been broadly developed for the cytological detection of breast cancer cell (Li et al., 2019), cervical cancer cells (Yu et al., 2013; Maji et al., 2015), human chronic myelogenous leukemia cell (Ge et al., 2014), melanoma tumor cell (Li et al., 2017), and squamous cancer (Wang et al., 2014) etc.
In addition to detecting CTCs, researchers also employed the catalytic activity of nanozymes to design real-time detection probes for organelle imaging in living cells. For example, Qu et al., designed a heterogeneous palladium nanozyme that could effectively mediate the bioorthogonal reactions in situ through light and thus realized the specific imaging of mitochondria in living cells (Wang et al., 2018) (Figures 2B,C). Beside CTCs and organelle imaging detection, there are also several other nanozymes-based colorimetric methods for specific disease imaging, including jaundice (Santhosh et al., 2014), acquired immune deficiency syndrome (Lin et al., 2017), diabetes (Tianran et al., 2014), infectious disease (Kim et al., 2014; Duan et al., 2015), and neurodegenerative disease (Wang C. I. et al., 2012; Farhadi et al., 2014). Thus, compared to traditional methods (such as PCR, cell flow cytometry, and ELISA), nanozymes methods exhibit more broaden prospect for live cell and organelle imaging because nanozyme assay is more fast, cost-effective and much easier to operate.
Nanozymes for In Vivo Imaging
In addition to enzyme-mimicking activity, nanozymes also exhibit fluorescence, electricity, paramagnetic properties and other unique physicochemical properties. By employing the unique physicochemical properties, nanozymes also have been broadly developed for in vivo monitoring and imaging of disease. For example, we utilized the unique r2 relaxivity of iron nanozymes and achieved tumor in vivo magnetic resonance imaging (MRI) after we achieved the in vitro tumor tissue imaging by using the peroxidase-like activity of iron-based nanozymes with a single-dose of 125I-M-HFn NPs containing 45 μg HFn, 500 μCi 125I, and 11.2 μg Fe by intravenously injection (Zhao et al., 2016). We also designed a quantum-dot-based nanozyme vesicle for in vivo H2O2-responsive catalytic photoacoustic imaging of nasopharyngeal carcinoma (Ding et al., 2019). In this work, graphene quantum dots showed intense peroxidase activity and effectively catalyzed the peroxidase substrate 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt (ABTS) into its oxidized form. The oxidized ABTS then exhibited strong near-infrared (NIR) absorbance, rendering it to be an ideal contrast agent for photoacoustic imaging. In the study, GQDzyme was at a dose of 100 μg/mL, 100 μL by an intravenously injection (Figure 3A). Jiang X. et al. (2018) achieved tumor phototherapy and simultaneous photoacoustic/thermal imaging and computed tomography by using a developed iridium oxide catalase nanozyme with extraordinary photothermal conversion efficiency and X-ray absorption coefficient showing a typical example of fully exploiting the multifunctional properties of nanozymes for tumor imaging and treatment. The BSA-IrO2 NPs used in the study was 1.5 mM, 200 μL via an intravenously injection (Jiang X. et al., 2018).
FIGURE 3.
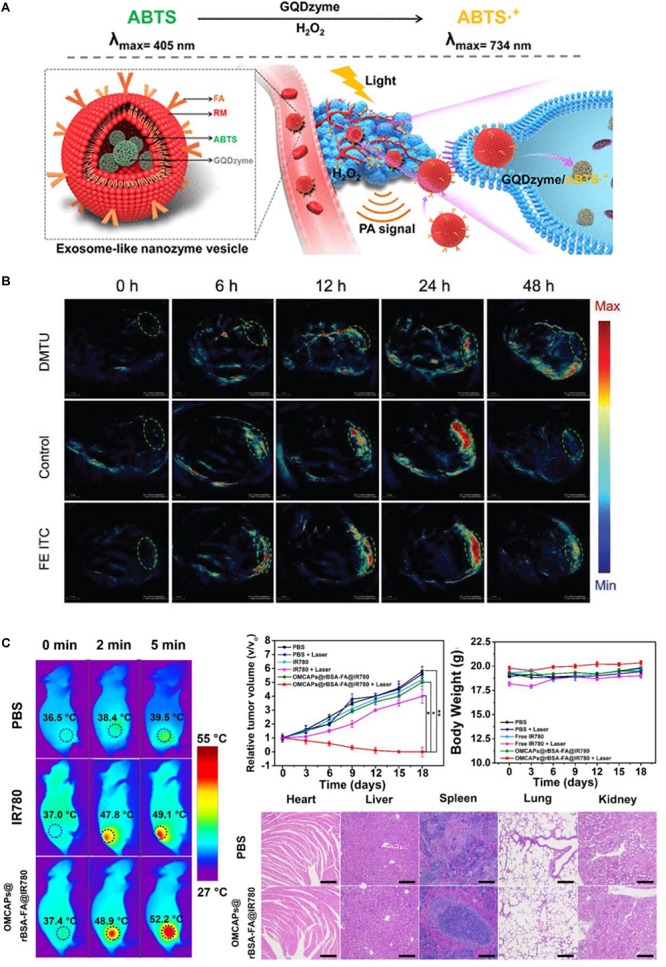
Nanozymes for in vivo imaging of disease progression. (A) Schematic illustration of exosome-like nanozyme vesicles for the H2O2-responsive catalytic photoacoustic imaging of tumors. Reproduced with permission from ref (Ding et al., 2019), © 2019, American Chemical Society. (B) Representative nanozyme-based tumor photoacoustic imaging images. Reproduced with permission from ref (Liu F. et al., 2019), © 2019, John Wiley and Sons. (C) Carbon-gold hybrid nanozymes for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy of tumors. Reproduced with permission from ref (Zhang et al., 2019).
Nanozyme probes have also been broadly developed for disease therapeutic monitoring. For example, in 2019, Chen’s group prepared a tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy (Liu F. et al., 2019). In their work, the constructed activatable nanoreactors achieved non-invasive imaging of tumor progression by using nanozyme-mediated photoacoustic imaging signal and photothermal therapy (PTT) function and the AMP NPs were at a dose of 10 mg/kg, 200 μL (Figure 3B). Cui’s group also prepared a mesoporous carbon-gold hybrid nanozyme probe for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy of tumors (Zhang et al., 2019). The results demonstrated that the synthesized nanozyme probes revealed excellent tumor targeting efficacy, long tumor retention, and favorably diagnostic and therapeutic effect for tumor (Figure 3C).
Besides cancer imaging diagnosis, nanozymes also have been broadly exploited for many other disease imaging such as infections, inflammation and some neurological diseases. For example, Rotello et al., reported a gold NPs-based charge-switchable nanozyme for bioorthogonal imaging of biofilm-associated infections (Tonga et al., 2015). In this work, the developed gold nanozymes could penetrate and accumulate inside the acidic microenvironment of biofilms and achieved imaging detection of the biofilm-associated infections arising from different and/or mixed bacteria species. Zhao et al. fabricated MnO2 nanozymes as the intracellular catalytic DNA circuit generators for versatile imaging of DNA base-excision repair in living cells (Chen et al., 2017). MnO2 nanosheet was used not only as a DNA nano-carrier but also a DNAzyme cofactor supplier. In this study, DNAzyme strands are blocked via the hybridization with the damaged bases-containing excision probes, which could be recognized by the corresponding base-excision repair enzymes in living cell. The detection signal could be 40-fold amplified by integrating several sets of probes with a dose of 20 μg mL–1 MnO2 nanozymes. Likewise, Yang et al. (2018) reported a nanozyme tag enabled chemiluminescence imaging probe for simultaneous multiplex imaging of cytokines. The prepared chemiluminescence nanozyme probe provides a novel and universal nanozyme-labeled multiplex immunoassay strategy for high-throughput detection of relevant biomarkers and further disease diagnosis. Thus, nanozymes open novel avenues for monitoring the initiation and progress of diseases by combining the unique physicochemical properties and enzyme-like catalytic activities of nanozymes.
Summary and Outlook
The emergence of nanozymes uncovers the biological effects of inorganic nanomaterials. Nanozymes thus can be used as an alternative of natural enzymes because of their capability to address the limitations of natural enzymes such as low stability, high cost, and difficult storage. Over the past decade, nanozyme-based probes have been widely developed for disease imaging and diagnosis from in vitro to in vivo. The typical nanozymes for disease imaging diagnosis are summarized in Supplementary Table S1.
Despite the remarkable advantages of nanozymes, there still remains plenty of limitations while put nanozymes into practical clinical application, such as poor dispersibility, easy sedimentation after surface modification, limited catalytic types, poor substrate selectivity, and potential nanotoxicity. To further drive the rapid development of nanozyme-based imaging agents for disease diagnosis, substantial breakthroughs are expected by overcoming the following challenges: (1) Rational design and surface modification are still remain critical challenges to improve their substrate selectivity and dispersibility of nanozymes. Thus, both experimental and computational studies should be combined together to aid in the process of nanozyme design and surface modification. (2) A detailed understanding of the relationship between the catalytic properties and the in vivo biological behaviors of nanozymes is necessary. It is because the size, morphology and surface property of nanozymes have a direct impact on their catalytic activity and thus determine the in vivo biological behaviors of nanozymes. (3) A systematic evaluation of the biological fates and the biocompatibility of nanozyme systems (including the cytotoxicity, in vivo uptake and behavior, biodistribution, immunogenicity, blood compatibility, and tissue compatibility) should be performed when administrated into living organisms, since the biocompatibility and biodegradability are the common concerns when move these systems into clinical practice. (4) Developing standards and reference materials. Although nanozymes have shown a broad range of applications from in vitro biological detection to in vivo imaging diagnosis, there are rare basic concepts and the corresponding standards on nanozyme research. Therefore, nanozymes performance should be fully normalization according to their size, shape, modification to compare with each other when used for disease imaging diagnosis.
Author Contributions
PW, TW, ML, and XY researched the literature and wrote the review. All authors revised and polished the review.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Key R&D Program of China (2017YFA0205501), the National Natural Science Foundation of China (81722024 and 81571728), the Key Research Program of Frontier Sciences (QYZDY-SSWSMC013), and the Youth Innovation Promotion Association (2014078).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00015/full#supplementary-material
References
- Ali S. S., Hardt J. I., Quick K. L., Kim-Han J. S., Erlanger B. F., Huang T. T., et al. (2004). A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free. Radic. Biol. Med. 37 1191–1202. 10.1016/j.freeradbiomed.2004.07.002 [DOI] [PubMed] [Google Scholar]
- André R., Natálio F., Humanes M., Leppin J., Heinze K., Wever R., et al. (2011). V2O5 nanowires with an intrinsic peroxidase−like activity. Adv. Funct. Mater. 21 501–509. 10.1002/adfm.201001302 [DOI] [Google Scholar]
- Bromberg J. E. C., Breems D. A., Kraan J., Bikker G., van der Holt B., Smitt P. S., et al. (2007). CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology 68 1674–1679. 10.1212/01.wnl.0000261909.28915.83 [DOI] [PubMed] [Google Scholar]
- Chen F., Bai M., Cao K., Zhao Y., Wei J., Zhao Y. (2017). Fabricating MnO2 nanozymes as intracellular catalytic DNA circuit generators for versatile imaging of base−excision repair in living cells. Adv. Funct. Mater. 27:1702748 10.1002/adfm.201702748 [DOI] [Google Scholar]
- Chen T., Zou H., Wu X., Liu C., Situ B., Zheng L., et al. (2018). Nanozymatic antioxidant system based on MoS2 nanosheets. ACS Appl. Mater. Interfaces 10 12453–12462. 10.1021/acsami.8b01245 [DOI] [PubMed] [Google Scholar]
- Chi M., Chen S., Zhong M., Wang C., Lu X. (2018). Self-templated fabrication of FeMnO3 nanoparticle-filled polypyrrole nanotubes for peroxidase mimicking with a synergistic effect and their sensitive colorimetric detection of glutathione. Chem. Comm. 54 5827–5830. 10.1039/c8cc01574k [DOI] [PubMed] [Google Scholar]
- Comotti M., Della Pina C., Matarrese R., Rossi M. (2004). The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 43 5812–5815. 10.1002/anie.200460446 [DOI] [PubMed] [Google Scholar]
- Dai Z., Liu S., Bao J., Ju H. (2009). Nanostructured FeS as a mimic peroxidase for biocatalysis and biosensing. Chemistry 15 4321–4326. 10.1002/chem.200802158 [DOI] [PubMed] [Google Scholar]
- Dillner J., Rebolj M., Birembaut P., Petry K. U., Szarewski A., Munk C., et al. (2008). Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 337:a1754. 10.1136/bmj.a1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Cai Y., Gao L., Liang M., Miao B., Wu H., et al. (2019). Exosome-like nanozyme vesicles for H2O2-responsive catalytic photoacoustic imaging of xenograft nasopharyngeal carcinoma. Nano Lett. 19 203–209. 10.1021/acs.nanolett.8b03709 [DOI] [PubMed] [Google Scholar]
- Dong J., Song L., Yin J. J., He W., Wu Y., Gu N., et al. (2014). Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl. Mater. Interfaces 6 1959–1970. 10.1021/am405009f [DOI] [PubMed] [Google Scholar]
- Duan D., Fan K., Zhang D., Tan S., Liang M., Liu Y., et al. (2015). Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 74 134–141. 10.1016/j.bios.2015.05.025 [DOI] [PubMed] [Google Scholar]
- Fan J., Yin J. J., Ning B., Wu X., Hu Y., Ferrari M., et al. (2011). Direct evidence for catalase and peroxidase activities of ferritin–platinum nanoparticles. Biomaterials 32 1611–1618. 10.1016/j.biomaterials.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Fan K., Cao C., Pan Y., Lu D., Yang D., Feng J., et al. (2012). Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 7 459–464. 10.1038/nnano.2012.90 [DOI] [PubMed] [Google Scholar]
- Fan K., Wang H., Xi J., Liu Q., Meng X., Duan D., et al. (2017). Optimization of Fe3O4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem. Comm. 53 424–427. 10.1039/c6cc08542c [DOI] [PubMed] [Google Scholar]
- Fan K., Xi J., Lei F., Wang P., Zhu C., Yan T., et al. (2018). In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 9:1440. 10.1038/s41467-018-03903-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi K., Forough M., Pourhossein A., Molaei R. (2014). Highly sensitive and selective colorimetric probe for determination of L-cysteine in aqueous media based on Ag/Pd bimetallic nanoparticles. Sens. Actuators. B Chem. 202 993–1001. 10.1039/C4TA03990D [DOI] [Google Scholar]
- Gao L., Yan X. (2016). Nanozymes: an emerging field bridging nanotechnology and biology. Sci. China. Life Sci. 59 400–402. 10.1007/s11427-016-5044-3 [DOI] [PubMed] [Google Scholar]
- Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., et al. (2007). Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2 577–583. 10.1038/nnano.2007.260 [DOI] [PubMed] [Google Scholar]
- Ge S., Liu F., Liu W., Yan M., Song X., Yu J. (2014). Colorimetric assay of K-562 cells based on folic acid-conjugated porous bimetallic Pd@Au nanoparticles for point-of-care testing. Chem. Comm. 50 475–477. 10.1039/c3cc47622g [DOI] [PubMed] [Google Scholar]
- Hao J. J., Qiu Y. N., Zhou D. F., Xiao Y., Liu Q., Jin R. M. (2011). Comparisons of clinical features of chronic aplastic anemia and myelodysplastic syndrome in children. China. J. Contem. Pedia 13 867–869. 10.1111/j.1600-0714.2011.01024.x [DOI] [PubMed] [Google Scholar]
- He S., Shi W., Zhang X., Li J., Huang Y. (2010). β− cyclodextrins-based inclusion complexes of CoFe2O4 magnetic nanoparticles as catalyst for the luminol chemiluminescence system and their applications in hydrogen peroxide detection. Talanta 82 377–383. 10.1016/j.talanta.2010.04.055 [DOI] [PubMed] [Google Scholar]
- Herget K., Hubach P., Pusch S., Deglmann P., Götz H., Gorelik T. E., et al. (2017). Haloperoxidase mimicry by CeO2-x nanorods combats biofouling. Adv. Mater. 29:1603823. 10.1002/adma.201603823 [DOI] [PubMed] [Google Scholar]
- Hu A. L., Deng H. H., Zheng X. Q., Wu Y. Y., Lin X. L., Liu A. L., et al. (2017). Self-cascade reaction catalyzed by CuO nanoparticle-based dual-functional enzyme mimics. Biosens. Bioelectron. 97 21–25. 10.1016/j.bios.2017.05.037 [DOI] [PubMed] [Google Scholar]
- Hu D., Sheng Z., Fang S., Wang Y., Gao D., Zhang P., et al. (2014). Folate receptor-targeting gold nanoclusters as fluorescence enzyme mimetic nanoprobes for tumor molecular colocalization diagnosis. Theranostics 4 142–153. 10.7150/thno.7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. L. (2018). Hydrolysis of phosphate esters catalyzed by inorganic iron oxide nanoparticles acting as biocatalysts. Astrobiology 18 294–310. 10.1089/ast.2016.1628 [DOI] [PubMed] [Google Scholar]
- Hyon Bin N., Jung Hee L., Kwangjin A., Il P. Y., Mihyun P., In Su L., et al. (2010). Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. 46 5247–5247. 10.1002/ange.200604775 [DOI] [PubMed] [Google Scholar]
- Jiang B., Duan D., Gao L., Zhou M., Fan K., Tang Y., et al. (2018). Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 13 1506–1520. 10.1038/s41596-018-0001-1 [DOI] [PubMed] [Google Scholar]
- Jiang X., Zhen W., Liu Y., Lin L., Tian H. (2018). BSA-IrO2: Catalase-like nanoparticles with high photothermal conversion efficiency and X-Ray absorption coefficient for anti-inflammation and tumor theranostics. Angew. Chem. 130 10309–10313. 10.1002/ange.201804466 [DOI] [PubMed] [Google Scholar]
- Jiang B., Yan L., Zhang J., Zhou M., Shi G., Tian X., et al. (2019). Biomineralization synthesis of the cobalt nanozyme in SP94-ferritin nanocages for prognostic diagnosis of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 11 9747–9755. 10.1021/acsami.8b20942 [DOI] [PubMed] [Google Scholar]
- Kim M. I., Ye Y., Woo M. A., Lee J., Park H. G. (2014). A highly efficient colorimetric immunoassay using a nanocomposite entrapping magnetic and platinum nanoparticles in ordered mesoporous carbon. Adv. Healthc. Mater. 3 36–41. 10.1002/adhm.201300100 [DOI] [PubMed] [Google Scholar]
- Korschelt K., Ragg R., Metzger C. S., Kluenker M., Oster M., Barton B., et al. (2017). Glycine-functionalized copper (II) hydroxide nanoparticles with high intrinsic superoxide dismutase activity. Nanoscale 9 3952–3960. 10.1039/c6nr09810j [DOI] [PubMed] [Google Scholar]
- Kuchma M. H., Komanski C. B., Colon J., Teblum A., Masunov A. E., Alvarado B., et al. (2010). Phosphate ester hydrolysis of biologically relevant molecules by cerium oxide nanoparticles. Nanomedicine 6 738–744. 10.1016/j.nano.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Cha M. Y., Kim D., Kim D. K., Soh M., Shin K., et al. (2016). Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 10 2860–2870. 10.1021/acsnano.5b08045 [DOI] [PubMed] [Google Scholar]
- Leng B., Zou L., Jiang J., Tian H. (2009). Colorimetric detection of mercuric ion (Hg2+) in aqueous media using chemodosimeter-functionalized gold nanoparticles. Sens. Actuators. B Chem. 140 162–169. 10.1016/j.snb.2009.03.074 [DOI] [Google Scholar]
- Li B., Chen D., Wang J., Yan Z., Jiang L., Duan D., et al. (2014). MOFzyme: intrinsic protease-like activity of Cu-MOF. Sci. Rep. 4:6759. 10.1038/srep06759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang J., Wang Y., Trau M. (2017). Simple and rapid colorimetric detection of melanoma circulating tumor cells using bifunctional magnetic nanoparticles. Analyst 142 4788–4793. 10.1039/c7an01102d [DOI] [PubMed] [Google Scholar]
- Li M., Lao Y. H., Mintz R. L., Chen Z., Shao D., Hu H., et al. (2019). A multifunctional mesoporous silica-gold nanocluster hybrid platform for selective breast cancer cell detection using a catalytic amplification-based colorimetric assay. Nanoscale 11 2631–2636. 10.1039/c8nr08337a [DOI] [PubMed] [Google Scholar]
- Liang F., Musto C. J., Suslick K. S. (2010). A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. J. Am. Chem. Soc. 132 4046–4047. 10.1021/ja910366p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M., Yan X. (2019). Nanozymes: from new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 52 2190–2200. 10.1021/acs.accounts.9b00140 [DOI] [PubMed] [Google Scholar]
- Lin F., Tian Y., Rong Y., Lou D., Zhang X., Meng W., et al. (2016). Enzyme catalysis enhanced dark-field imaging as a novel immunohistochemical method. Nanoscale 8 8553–8558. 10.1039/c5nr08232c [DOI] [PubMed] [Google Scholar]
- Lin X. D., Liu Y. Q., Tao Z. H., Gao J. T., Deng J. K., Yin J. J., et al. (2017). Nanozyme-based bio-barcode assay for high sensitive and logic-controlled specific detection of multiple DNAs. Biosens. Bioelectron. 94 471–477. 10.1016/j.bios.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Liu F., Lin L., Zhang Y., Wang Y., Sheng S., Xu C., et al. (2019). A tumor-microenvironment-activated nanozyme-mediated theranostic nanoreactor for imaging-guided combined tumor therapy. Adv. Mater. 31:1902885. 10.1002/adma.201902885 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zheng Y., Chen Z., Qin Y., Guo R. (2019). High-performance integrated enzyme cascade bioplatform based on protein–BiPt nanochain@graphene oxide hybrid guided one-pot self-assembly strategy. Small 15:1804987. 10.1002/smll.201804987 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhu G., Bao C., Yuan A., Shen X. (2014). Intrinsic peroxidase−like activity of porous CuO micro−/nanostructures with clean surface. Chinese J. Chem. 32 151–156. 10.1002/cjoc.201300683 [DOI] [Google Scholar]
- Maji S. K., Mandal A. K., Nguyen K. T., Borah P., Zhao Y. (2015). Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 7 9807–9816. 10.1021/acsami.5b01758 [DOI] [PubMed] [Google Scholar]
- Mondal S. S., Holdt H. J. (2016). Breaking down chemical weapons by metal–organic frameworks. Angew. Chem. 55 42–44. 10.1002/anie.201508407 [DOI] [PubMed] [Google Scholar]
- Mosterd K., Van Marion A., Van Steensel M. A. (2008). Neonatal langerhans’ cell histiocytosis: a rare and potentially life−threatening disease. Int. J. Dermatol. 47 10–12. 10.1111/j.1365-4632.2008.03950.x [DOI] [PubMed] [Google Scholar]
- Mu J., Wang Y., Zhao M., Zhang L. (2012). Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Comm. 48 2540–2542. 10.1039/c2cc17013b [DOI] [PubMed] [Google Scholar]
- Natalio F., Andre R., Hartog A. F., Stoll B., Jochum K. P., Wever R., et al. (2012). Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat. Nanotechnol. 7 530–535. 10.1038/nnano.2012.91 [DOI] [PubMed] [Google Scholar]
- Nath I., Chakraborty J., Verpoort F. (2016). Metal organic frameworks mimicking natural enzymes: a structural and functional analogy. Chem. Soc. Rev. 45 4127–4170. 10.1039/c6cs00047a [DOI] [PubMed] [Google Scholar]
- Nirala N. R., Khandelwal G., Kumar B., Prakash R., Kumar V. (2017). One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta 173 36–43. 10.1016/j.talanta.2017.05.061 [DOI] [PubMed] [Google Scholar]
- Peng C., Hua M. Y., Li N. S., Hsu Y. P., Chen Y. T., Chuang C. K., et al. (2019). A colorimetric immunosensor based on self-linkable dual-nanozyme for ultrasensitive bladder cancer diagnosis and prognosis monitoring. Biosens. Bioelectron. 126 581–589. 10.1016/j.bios.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Qian B., Jiao L. (2017). Application of multiplexed immunofluorescent staining and multi-spectral imaging in histological studies. China. J. His. Cyt. 26 373–382. 10.16705/j.cnki.1004-1850.04.010 [DOI] [Google Scholar]
- Ragg R., Natalio F., Tahir M. N., Janssen H., Kashyap A., Strand D., et al. (2014). Molybdenum trioxide nanoparticles with intrinsic sulfite oxidase activity. ACS Nano 8 5182–5189. 10.1021/nn501235j [DOI] [PubMed] [Google Scholar]
- Roy P., Lin Z. H., Liang C. T., Chang H. T. (2012). Synthesis of enzyme mimics of iron telluride nanorods for the detection of glucose. Chem. Comm. 48 4079–4081. 10.1039/c2cc30833a [DOI] [PubMed] [Google Scholar]
- Sahoo P. C., Jang Y. N., Lee S. W. (2013). Enhanced biomimetic CO2 sequestration and CaCO3 crystallization using complex encapsulated metal organic framework. J. Cryst. Growth. 373 96–101. 10.1016/j.jcrysgro.2012.11.043 [DOI] [Google Scholar]
- Santhosh M., Chinnadayyala S. R., Kakoti A., Goswami P. (2014). Selective and sensitive detection of free bilirubin in blood serum using human serum albumin stabilized gold nanoclusters as fluorometric and colorimetric probe. Biosens. Bioelectron. 59 370–376. 10.1016/j.bios.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Schiffman M., Herrero R., Hildesheim A., Sherman M. E., Bratti M., Wacholder S., et al. (2000). HPV DNA testing in cervical cancer screening - results from women in a high-risk province of costa rica. JAMA 283 87–93. 10.1001/jama.283.1.87 [DOI] [PubMed] [Google Scholar]
- Singh N., Savanur M. A., Srivastava S., D’Silva P., Mugesh G. (2017). A redox modulatory Mn3O4 nanozyme with multi−enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew. Chem. 56 14267–14271. 10.1002/ange.201708573 [DOI] [PubMed] [Google Scholar]
- Su H., Liu D. D., Zhao M., Hu W. L., Xue S. S., Cao Q., et al. (2015). Dual-enzyme characteristics of polyvinylpyrrolidone-capped iridium nanoparticles and their cellular protective effect against H2O2-induced oxidative damage. ACS Appl. Mater. Interfaces 7 8233–8242. 10.1021/acsami.5b01271 [DOI] [PubMed] [Google Scholar]
- Su L., Dong W., Wu C., Gong Y., Zhang Y., Li L., et al. (2017). The peroxidase and oxidase-like activity of NiCo2O4 mesoporous spheres: Mechanistic understanding and colorimetric biosensing. Anal. Chim. Acta 951 124–132. 10.1016/j.aca.2016.11.035 [DOI] [PubMed] [Google Scholar]
- Sun M., Xu L., Qu A., Zhao P., Hao T., Ma W., et al. (2018). Site-selective photoinduced cleavage and profiling of DNA by chiral semiconductor nanoparticles. Nat. Chem. 10 821–830. 10.1038/s41557-018-0083-y [DOI] [PubMed] [Google Scholar]
- Talib P., Dowding J. M., Sanjay S., Brian W., Eric H., Karakoti A. S., et al. (2010). Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Comm. 46 2736–2738. 10.1039/B922024K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnuzzer R. W., Colon J., Patil S., Seal S. (2005). Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 5 2573–2577. 10.1021/nl052024f [DOI] [PubMed] [Google Scholar]
- Tian L., Qi J. X., Qian K., Oderinde O., Cai Y. Y., Yao C., et al. (2018). An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme. Sens. Actuators. B Chem. 260 676–684. 10.1016/j.snb.2018.01.092 [DOI] [Google Scholar]
- Tianran L., Liangshuang Z., Liangqia G., Fengfu F., Guonan C. (2014). Seeing diabetes: visual detection of glucose based on the intrinsic peroxidase-like activity of MoS2 nanosheets. Nanoscale 6 11856–11862. 10.1039/c4nr03393k [DOI] [PubMed] [Google Scholar]
- Tokuyama H., Yamago S., Nakamura E., Shiraki T., Sugiura Y. (1993). Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 115 7918–7919. 10.1021/ja00070a064 [DOI] [Google Scholar]
- Tonga G. Y., Jeong Y., Duncan B., Mizuhara T., Mout R., Das R., et al. (2015). Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts. Nat. Chem. 7 597–603. 10.1038/nchem.2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal S. B., Prasad S. (2015). Cytological diagnosis of osteoblastoma of cervical spine: a case report with review of literature. Diagn. Cytopathol. 43 218–221. 10.1002/dc.23175 [DOI] [PubMed] [Google Scholar]
- Vernekar A. A., Das T., Mugesh G. (2016). Vacancy−engineered nanoceria: enzyme mimetic hotspots for the degradation of nerve agents. Angew. Chem. 55 1412–1416. 10.1002/ange.201510355 [DOI] [PubMed] [Google Scholar]
- Wang C. I., Chen W. T., Chang H. T. (2012). Enzyme mimics of Au/Ag nanoparticles for fluorescent detection of acetylcholine. Anal. Chem. 84 9706–9712. 10.1021/ac300867s [DOI] [PubMed] [Google Scholar]
- Wang W., Jiang X., Chen K. (2012). Iron phosphate microflowers as peroxidase mimic and superoxide dismutase mimic for biocatalysis and biosensing. Chem. Comm. 48 7289–7291. 10.1039/c2cc32429f [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang Y., Du Z., Ren J., Qu X. (2018). Designed heterogeneous palladium catalysts for reversible light-controlled bioorthogonal catalysis in living cells. Nat. Commun. 9:1209. 10.1038/s41467-018-03617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., He J., Duan D., Jiang B., Wang P., Fan K., et al. (2019). Bioengineered magnetoferritin nanozymes for pathological identification of high-risk and ruptured atherosclerotic plaques in humans. Nano Res. 12 863–868. 10.1021/acsnano.5b07408 [DOI] [Google Scholar]
- Wang T., Wang J., Ye Y., Ping S., Yi Y., Wang T., et al. (2017). Co3O4/reduced graphene oxide nanocomposites as effective phosphotriesterase mimetics for degradation and detection of paraoxon. Ind. Eng. Chem. Res. 56 9762–9769. 10.1021/acs.iecr.7b02223 [DOI] [Google Scholar]
- Wang X., Liu D., Li J., Zhen J., Zhang H. (2015). Clean synthesis of Cu2O@CeO2 core@shell nanocubes with highly active interface. NPG Asia. Mater. 7:e158 10.1038/am.2014.128 [DOI] [Google Scholar]
- Wang Y. L., Zhang Y., Su Y., Li F., Ma H. M., Li H., et al. (2014). Ultrasensitive non-mediator electrochemical immunosensors using Au/Ag/Au core/double shell nanoparticles as enzyme-mimetic labels. Talanta 124 60–66. 10.1016/j.talanta.2014.02.035 [DOI] [PubMed] [Google Scholar]
- Wei L., Yu-Sang L., Jing Y., Lihua Z., Zhengdan L., Heqing T., et al. (2010). Ultrasensitive fluorometric determination of hydrogen peroxide and glucose by using multiferroic BiFeO3 nanoparticles as a catalyst. Talanta 81 901–907. 10.1016/j.talanta.2010.01.035 [DOI] [PubMed] [Google Scholar]
- Wu L., Li G. H., Xu X., Zhu L., Huang R. M., Chen X. Q. (2019). Application of nano-ELISA in food analysis: recent advances and challenges. Trends. Anal. Chem. 113 140–156. 10.1016/j.trac.2019.02.002 [DOI] [Google Scholar]
- Wu Y., Song M., Xin Z., Zhang X., Zhang Y., Wang C., et al. (2011). Ultra-small particles of iron oxide as peroxidase for immunohistochemical detection. Nanotechnology 22:225703. 10.1088/0957-4484/22/22/225703 [DOI] [PubMed] [Google Scholar]
- Xing L., Qi W., Huihui Z., Lichun Z., Yingying S., Yi L. (2012). BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst 137 4552–4558. 10.1039/C2AN35700C [DOI] [PubMed] [Google Scholar]
- Xue J., Song H., Zhao H., Wei B., Zhang L., Yi L. (2012). Well-redispersed ceria nanoparticles: promising peroxidase mimetics for H2O2 and glucose detection. Anal. Methods 4 3261–3267. 10.1039/c2ay25511a [DOI] [Google Scholar]
- Yang F., Hu S., Zhang Y., Cai X., Huang Y., Wang F., et al. (2012). A hydrogen peroxide−responsive O2 nanogenerator for ultrasound and magnetic−resonance dual modality imaging. Adv. Mater. 24 5205–5211. 10.1002/adma.201202367 [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhong Y., Tang X., Li J., Gao W. (2018). Nanozyme tags enabled chemiluminescence imaging immunoassay for multiplexed cytokine monitoring. Chem. Comm. 54 13813–13816. 10.1039/c8cc07779g [DOI] [PubMed] [Google Scholar]
- Yu C. J., Chen T. H., Jiang J. Y., Tseng W. L. (2014). Lysozyme-directed synthesis of platinum nanoclusters as a mimic oxidase. Nanoscale 6 9618–9624. 10.1039/c3nr06896j [DOI] [PubMed] [Google Scholar]
- Yu T., Youhui L., Zhenzhen H., Jinsong R., Xiaogang Q. (2013). Incorporating graphene oxide and gold nanoclusters: a synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv. Mater. 25:2510. 10.1002/adma.201370115 [DOI] [PubMed] [Google Scholar]
- Zhang A., Pan S., Zhang Y., Chang J., Cheng J., Huang Z., et al. (2019). Carbon-gold hybrid nanoprobes for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy. Theranostics 9 3443–3458. 10.7150/thno.33266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Cao C., Tang X., Cai Y., Yang C., Pan Y. (2016). Enhanced peroxidase activity and tumour tissue visualization by cobalt-doped magnetoferritin nanoparticles. Nanotechnology 28:045704. 10.1088/1361-6528/28/4/045704 [DOI] [PubMed] [Google Scholar]
- Zhang W., Hu S., Yin J. J., He W., Lu W., Ma M., et al. (2016). Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138 5860–5865. 10.1021/jacs.5b12070 [DOI] [PubMed] [Google Scholar]
- Zhang X., He S., Chen Z., Huang Y. (2013). CoFe2O4 nanoparticles as oxidase mimic-mediated chemiluminescence of aqueous luminol for sulfite in white wines. J. Agric. Food Chem. 61 840–847. 10.1021/jf3041269 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu B., Liu J. (2017). Molecular imprinting for substrate selectivity and enhanced activity of enzyme mimics. Small 13 10.1002/smll.201602730 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Liang M., Li X., Fan K., Xiao J., Li Y., et al. (2016). Bioengineered magnetoferritin nanoprobes for single-dose nuclear-magnetic resonance tumor imaging. ACS Nano 10 4184–4191. 10.1021/acsnano.5b07408 [DOI] [PubMed] [Google Scholar]
- Zheng C., Ke W., Yin T., An X. (2016). Intrinsic peroxidase-like activity and the catalytic mechanism of gold@ carbon dots nanocomposites. RSC Adv. 6 35280–35286. 10.1039/c6ra01917j [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.