Abstract
Lentinula edodes (shiitake), an edible and medicinal mushroom, was chosen for this study with the aim of evaluating the possibility of release of bioelements into artificial digestive juices and analyzing the anti-inflammatory properties. The extracts were prepared from fruiting bodies and biomass enriched with copper (Cu), zinc (Zn), and selenium (Se). The content of bioelements was analyzed by total reflection X-ray fluorescence method. Relatively low content of elements was observed in the fruiting bodies: Cu–1.6, Zn–7.6, and Se–0.12 mg/100 g d.w. compared to mycelial cultures. The anti-inflammatory properties were evaluated in RAW 264.7 cells. Based on the levels of cyclooxygenase 2 protein, nuclear factor erythroid 2-related factor 2, and peroxisome proliferator-activated receptor γ determined using Western blot technique, it was found that the addition of bioelements enhanced the anti-inflammatory properties of mycelium. This indicates that L. edodes cultured on a suitable medium may be used as a potential component of anti-inflammatory products.
Keywords: Shiitake, Biologically active elements, Extraction in digestive juices, Medicinal properties of mushrooms, RAW 264.7 cells
Introduction
Lentinula edodes (Berk.) Pegler (Basidiomycota), known as shiitake, is the second most popular species of edible mushroom next to Agaricus bisporus (J.E. Lange) Imbach (white mushroom) and is obtained by cultivation. The fruiting bodies of L. edodes are of both dietary and therapeutic significance, and hence, this species is listed as one of the medicinal mushrooms [1–3]. This species owes its popularity to its culinary uses, but above all to the presence of substances with therapeutic properties. The drugs and other medicinal products obtained from L. edodes are used in the treatment of various cancers due to their immunostimulating, anti-inflammatory, and anti-oxidant effects [1, 2, 4, 5]. The polysaccharides present in the fruiting bodies of L. edodes enhance the immune function and eliminate the side effects of chemo- and radiotherapy, besides exhibiting anti-cancer, anti-viral, and anti-bacterial properties. The compounds responsible for the biological activity of L. edodes are the polysaccharides belonging to the group of β-d-glucans, which include lentinan [3–9]. Other important antibiotic substances identified in the fruiting bodies of L. edodes are lentin (protein), lenthionine (exobiopolymer containing sulfur in the molecule), lentinosin (purine compound), and lentinamycin A and B [1]. In 1972, atherosclerotic substances, the most important of which are statins including eritadenine and lovastatin, were also detected in the fruiting bodies of L. edodes [6, 8, 9]. Like other mushroom species, the fruiting bodies of L. edodes are a good source of vitamins and have the ability to accumulate bioelements [1, 2, 5, 8]. Because elements such as copper (Cu), zinc (Zn), and selenium (Se) were detected in the fruiting bodies of L. edodes, which play a key role as anti-inflammatory factors, this study was undertaken to obtain biomass from the in vitro cultures of L. edodes with maximum content of Cu(II), Zn(II), and Se(IV) ions [2, 10–12].
The aim of this study was to investigate whether modifying the composition of the medium used for in vitro culturing of L. edodes will allow obtaining biomass rich in bioelements that may be used as a potential component in natural anti-inflammatory products.
The study also aimed to analyze whether the elements added to the culture media were accumulated in higher amounts by mycelium and were then released into artificial digestive juices in a Gastroel-2014 apparatus which is especially designed for this purpose [13]. It was checked whether the bioelements were released effectively under conditions imitating those prevailing in the human gastrointestinal tract, which could be reflected in their protective effects on the mucous membranes of the stomach and intestines.
The content of Cu, Zn, Se, calcium (Ca), potassium (K), iron (Fe), and manganese (Mn) was determined in mushroom materials. The content of the released bioelements was evaluated by total reflection X-ray fluorescence (TXRF) method. The study also aimed to determine which of the bioelements (Cu, Zn, Se) is the most beneficial to obtain mycelium with a higher anti-inflammatory potential, using RAW 264.7 cell lines (mouse macrophages) activated with lipopolysaccharide (LPS).
Materials and Methods
Reagents
The substances used to mineralization process: HNO3 (65%) and H2O2 (30%) (Suprapur®) were obtained from Merck (Darmstadt, Germany). Four-time distilled water with conductivity below 1 μS/cm was obtained in HLP 5 apparatus from Hydrolab (Straszyn, Poland).
Additives for mushroom media in the form of copper sulfate (CuSO4) and zinc sulfate (ZnSO4) were purchased from the Polish Company of Chemistry (Gliwice, Poland). Zinc hydrogen aspartate (C8H12N2O8Zn) and copper gluconate (C12H22CuO14) were purchased from Farmapol (Poznań, Poland). Selenitriglycerides (Selol)—prepared from Se(IV) and sunflower oil—was obtained from the Department of Bioanalysis and Drug Analysis at the Medical University of Warsaw in the synthesis process as described in Polish patent 76,530 [14]. Selol was used with a declared Se concentration of 5% (w/v).
The substances used to prepare artificial digestive juices were purchased from different companies: CaCl2 and MgCl2 from Chempur (Kraków, Poland); NaHCO3 from PPH Golpharm (Kraków, Poland); NaCl and NH4Cl from Alfa Aesar® (Kandel, Germany); bile salts and pepsin from BTL (Łódź, Poland); pancreatic extract and HCl Suprapur® from Merck (Darmstad, Germany); and KHCO3, C6H8O7, Na2HPO4, and K2HPO4 from Polish Company of Chemistry (Gliwice, Poland).
Materials
Fresh fruiting bodies of L. edodes species of commercial origin were used for the experiments and for establishing in vitro cultures. The young fruiting bodies were taxonomically identified using the MycoKey 4.1 key (http://www.mycokey.com). Mycelial fragments were collected from the hymenial part of fresh fruiting bodies and were degreased with 70% ethyl alcohol. The fragments were then sterilized with 15% sodium hypochlorite (NaClO) solution for several minutes. After rinsing with redistilled water, sterile fragments of fruiting bodies were transferred under laminar flow to Oddoux medium solidified with agar [15]. To obtain a large amount of biomass, it is necessary that the biomass grown on in vitro cultures on solid medium be transferred to a modified Oddoux liquid substrate. Therefore, biomass obtained from in vitro cultures on solid medium was transferred to Erlenmeyer flasks containing 250 mL of liquid medium; the initial mass of the inoculum was 0.1 g. The in vitro cultures were allowed to grow for 21 days on Oddoux medium at 25 ± 2 °C, under a photoperiod of 16 h light (900 lx) and 8 h dark, on an ALTEL shaker (Poland) at 140 rpm.
Experimental In Vitro Cultures of L. edodes
The following materials were prepared for the planned experiments: biomass of L. edodes (control) was obtained on a liquid Oddoux medium and on the same liquid medium with the addition of zinc compounds, such as zinc hydrogen aspartate (C8H12N2O8Zn) at a concentration of 200 mg/L and zinc sulfate (ZnSO4) at a concentration of 174.47 mg/L, and copper compounds, such as copper gluconate (C12H22CuO14) at a concentration of 72.88 mg/L and copper sulfate (CuSO4) at a concentration of 33.60 mg/L. In vitro cultures of L. edodes were prepared on a medium enriched with Se organic compounds too by the addition of Selol (Se(IV) 25 and 50 mg/L medium). The mycelial cultures were transferred to Erlenmeyer flasks and were shaken in order to promote a high growth of biomass. After culturing, mycelium was separated from the medium, frozen, and lyophilized (Freezone 4.5 lyophilizator, Labonco; temperature − 40 °C) for further experiments as in previous study [16, 17].
Analysis of Bioelements in L. edodes Mycelium
The amount of elements in the fruiting bodies and mycelium of L. edodes and extracts obtained by digestion with artificial digestive juices was determined using TXRF method. The TXRF method was chosen for this study due to several significant aspects, such as high sensitivity, precision, accuracy, and repeatability of analyses, as well as possibility to achieve faster measurement with a very large number of samples. The optimization of mineralization conditions for analysis of samples in combination with the TXRF method enabled optimal analysis of bioelements in the fruiting bodies, mycelial cultures, and extracts obtained from L. edodes.
For this analysis, 0.2 g samples of lyophilized mushroom material were weighed, with an accuracy of 0.01 g, and were transferred to Teflon vessels, to which 2 mL of H2O2 solution (30%) and 4 mL of concentrated HNO3 solution (65%) were added. Mineralization was carried out in Magnum II microwave apparatus (ERTEC) in three stages of 10 min each, at a power of 70% and 100%, respectively, maintaining the temperature of the device at 290 °C. After mineralization, the solutions were transferred to quartz evaporators and evaporated on a heating plate at 150 °C to remove excess reagents and water. The residue obtained after evaporation was quantitatively transferred to 10-mL volumetric flasks with four-time distilled water. To analyze the composition of bioelements such as K, Ca, Mn, Fe, Cu, Zn, and Se in the prepared test samples, 1000 ppm gallium was used as an internal standard. The composition of elements was measured using a TXRF spectrometer Nanohunter II (Rigaku) equipped with an X-ray tube containing a molybdenum anode at 50 kV for 1000 s.
Artificial Digestive Juice Extraction
Artificial digestive juices imitating saliva, gastric and intestinal juices were used in the study to extract the bioelements from L. edodes mycelium. These digestive juices were prepared as follows:
Artificial Saliva
Solutions of 100 mL MgCl2 (1.5 mmol/L), 100 mL KH2PO4 (25 mmol/L), 100 mL KHCO3 (150 mmol/L), 100 mL Na2HPO4 (24 mmol/L), 6 mL citric acid (25 mmol/L), and 100 mL CaCl2 (15 mmol/L) were prepared and added successively to a volumetric flask. The volume of the solution was made up to 1000 mL with four-time distilled water [18].
Artificial Gastric Juice
A total of 3.2 g of pepsin and 2.0 g of NaCl were dissolved in four-time distilled water, and then to this solution, 80 mL of 1 mol/L HCl was added. The volume of the solution was finally made up to 1000 mL with water [19].
Artificial Intestinal Juice
A total of 120 mg bile acids, 20 mg pancreatic extract, and 8.4 g NaHCO3 were dissolved in four-time distilled water, and the volume of the solution was made up to 1000 mL with water [20].
Extracts were obtained from lyophilized biomass from fruiting bodies and in vitro cultures of L. edodes and were digested in vitro using artificial digestive juices in the Gastroel-2014 apparatus as follows [13]. The extraction of mushroom material was carried out under conditions imitating those prevailing in the human body (temperature 37 °C and mixing movements imitating peristaltic movements). About 0.3 g of mushroom material was transferred to 100-mL Erlenmeyer flasks and moistened with 3 mL of artificial saliva solution for 1 min. Then, 20 mL of gastric juice was added to the flasks. The flasks were sealed and placed in the Gastroel-2014 apparatus for the digestion process. The process was conducted for 30 and 60 min, respectively. The obtained extracts were filtered using of paper filters and syringe membrane filters. The remaining mushroom material was again transferred to Erlenmeyer flasks, and 20 mL of intestinal juice was added. Digestion was allowed to proceed for 150 min. After digestion, the extracts were filtered to obtain a clear solution. Each sample was subjected to three independent repetitions. The content of elements was also determined by TXRF method described above.
Preparation of Mycelial Extracts for Evaluation of Anti-inflammatory Properties
To prepare methanolic extracts of L. edodes, the lyophilized biomass obtained from in vitro cultures was first homogenized in a mortar, and 2 g of each homogenate sample was then used for extraction. Mycelium was extracted five times with 100 mL of methanol using ultrasound at a frequency of 49 kHz for 30 min (Sonic-2, Polsonic, Poland). Following extraction, the methanol in the extracts was evaporated to dryness. Then, 250 mg of dry extract was dissolved in 96% ethanol, filtered through sterile bacteriological syringe filters with a pore diameter of 0.2 μm, and transferred quantitatively to 5-mL volumetric flasks. The prepared samples were then diluted to the desired concentrations by mixing 100 μL with 900 μL of distilled water. Finally, the extracts were stored at 4 °C until they were used for analysis of anti-inflammatory activity in RAW 264.7 cell lines.
Cell Cultures
Mus musculus macrophages RAW 264.7 (American Type Cell Culture: TIB-71) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 1% antibiotic solution (100 IU/mL penicillin, 0.1 μg/mL streptomycin) and 10% fetal bovine serum (ATCC, Manassas, VA, USA). The cells were maintained in a humidified atmosphere with 5% CO2 in air at 37 °C and were finally seeded into a six-well plate (Sarstedt AG&Co., Nümbrecht, Germany) at a density of 5 × 105 cells/well in 2 mL of medium. Before the experiment, the morphology of cells was observed with an inverted light microscope (Olympus, Tokyo, Japan), and cell viability was assessed by Trypan Blue Exclusion Test. Then, cells were activated with LPS (10 ng/mL) and incubated with the prepared mushroom extracts (100 μL) for 24 h.
No cytotoxic effects or apoptosis were observed in the RAW 264.7 cells activated with LPS and treated with L. edodes extracts. Cell viability varied from 100 to 99% following treatment with the extracts. After incubation, both the media and the cells were collected by scrapping.
Western Blot for Protein Quantification
Cell lysates were prepared using M-PER (Thermo Fisher Scientific, Waltham, MA, USA) buffer with protease inhibitor cocktail set III (Merck, Darmstadt, Germany). The contents of proteins in the lysates were determined using Bradford reaction. Samples (40 μg) were solubilized in Laemmli buffer added with 2% mercaptoethanol (Bio-Rad, Hercules, CA, USA) and were then subjected to 10% SDS (sodium dodecyl sulfate)-polyacrylamide gel electrophoresis as described earlier [21]. Following transfer, membranes were blocked for 1 h at room temperature in the presence of casein in TBS (tris-buffered saline)–1% Tween buffer (Bio-Rad) and subsequently incubated overnight at 4 °C with the following primary antibodies: anti-COX-2, anti-Nrf2, anti-FABP4, anti-β-actin (GeneTex Inc., Irvine, CA, USA), and anti-PPARγ (Cayman Chemical, Ann Arbor, MI, USA), all of which were diluted to the ratio of 1:1000. After incubation, the membranes were washed and incubated with secondary antibodies (anti-rabbit IgG (HRP); Thermo Fisher Scientific, Waltham, MA, USA) for 1 h at room temperature. Then, the membranes were washed again, and proteins were detected using a Clarity Western ECL Luminol Substrate detection kit (Bio-Rad). The integrated optical density of the protein bands was quantified using a Chemi Doc Camera with Image Lab software (Bio-Rad).
Statistical Analysis
The results are shown as mean ± standard deviation (SD), and all experiments were performed six times. Using one-way ANOVA with Tukey’s test was used to elaborate the results of evaluation of bioelements composition as well as proteins level. For all the tests, the value p < 0.05 was accepted as the level of statistical significance (GraphPad InStat).
Results and Discussion
The amount of biomass obtained from L. edodes mycelium cultures grown on control media was 8–9 g d.w. (dry weight)/L medium, whereas the amount obtained from media enriched with bioelements (Cu, Se, Zn) was 9–9.5 g. The dynamics of mycelium growth did not differ from that reported in the earlier studies [16, 22].
In the first stage of the study, the content of selected macro- (K, Ca) and microelements (Mn, Fe, Cu, Zn, Se) in the fruiting bodies of L. edodes of commercial origin was determined, as well as those present in the mycelium of L. edodes obtained from in vitro cultures grown on modified liquid media, which were enriched with Cu, Zn, and Se compounds in inorganic (ZnSO4, CuSO4) and organic forms (C8H12N2O8Zn, C12H22CuO14, and Selol) (Table 1). It is well known that mushroom extracts exhibit anti-inflammatory properties; therefore, the bioelements that also exert similar effects were chosen for fortification of in vitro cultures [5, 22]. As a result, mycelium with the highest possible anti-inflammatory activity was obtained.
Table 1.
Content of elements (mg/100 g d.w.) determined in the fruiting bodies and mycelium, as well as in basic media and the ones enriched with Zn, Cu and Se used for in vitro culturing of L. edodes
| L. edodes | Metals | |||||||
|---|---|---|---|---|---|---|---|---|
| K | Ca | Mn | Fe | Cu | Zn | Se | Ga | |
| L. edodes (fruiting bodies) | 1839.5 ± 89.1a | 312.2 ± 56.2a | 2.9 ± 0.2a | 14.5 ± 2.6a | 1.6 ± 0.3a | 7.6 ± 0.4a | 0.01 ± 0.00 | 50.0 ± 0.0 |
| L. edodes (control mycelium) | 1035.3 ± 273.6a,b | 684.4 ± 127.1a,b | 18.9 ± 3.4a,b | 14.5 ± 2.5b | – | 17.2 ± 2.4b | 0.01 ± 0.00 | 50.0 ± 0.0 |
| L. edodes (control medium) | 1828.6 ± 7.5b,c | 93.4 ± 5.9a,b,c | 17.5 ± 0.1a,c | 12.9 ± 1.1c | – | 6.0 ± 0.1b,c | – | 50.0 ± 0.0 |
| L. edodes + ZnSO4 (mycelium) | 780.8 ± 51.5a,c,d | 234.5 ± 26.1b,c | 9.8 ± 0.8a,b,c,d | 9.7 ± 1.4a,b,d | 3.2 ± 0.1a,d | 231.2 ± 6.5a,b,c,d | 0.01 ± 0.00 | 50.0 ± 0.0 |
| L. edodes + ZnSO4 (medium) | 391.9 ± 17.4a,b,c,d | 123.4 ± 6.9a,b,e | – | 9.7 ± 1.3a,b,e | 14.3 ± 0.3a,d | 0.6 ± 0.3b,d,e | 0.01 ± 0.00 | 50.0 ± 0.0 |
| L. edodes + ZnHasp (mycelium) | 1069.4 ± 257.4a,c,d | 295.7 ± 58.6b,c,e | 13.9 ± 1.3a,b,c,d | 13.2 ± 3.9f | – | 141.2 ± 13.8a,b,c,d,e,f | 0.01 ± 0.00 | 50.0 ± 0.0 |
| L. edodes + ZnHasp (medium) | 5103.9 ± 131.6a,b,c,d | 215.6 ± 55.2b,c | 13.5 ± 0.2a,b,c,d | 23.1 ± 0.5a,b,c,d,e,f | – | 257.3 ± 0.7a,b,c,d,e,f | – | 50.0 ± 0.0 |
| L. edodes + CuSO4 (mycelium) | 1002.5 ± 81.8a,c,d | 362.3 ± 52.1b,c,d | 15.1 ± 0.8a,b,d | 11.9 ± 2.6d | 21.0 ± 1.8a | 11.1 ± 1.7a,b,c,d | 0.5 ± 0.2a,b | 50.0 ± 0.0 |
| L. edodes + CuSO4 (medium) | 2650.7 ± 132.6a,d,e | 35.1 ± 5.0a,b,d,e | 19.5 ± 0.1a,d,e | 21.7 ± 0.6a,b,c,d,e | 97.0 ± 1.0a,d,e | – | 0.4 ± 0.0a,b,e | 50.0 ± 0.0 |
| L. edodes + CuGlu (mycelium) | 1084.0 ± 103.6a,c,e,f | 396.3 ± 118.8b,c | 15.4 ± 0.8a,b,e | 14.6 ± 4.4e,f | 20.4 ± 2.3a,e,f | 9.4 ± 1.5b,c,f | 0.01 ± 0.0d | 50.0 ± 0.0 |
| L. edodes + CuGlu (medium) | 391.9 ± 17.4a,b,c,d | 123.4 ± 6.9a,b,d | – | 9.7 ± 1.3a,b,e,f | 14.3 ± 0.4a,d,e | 0.6 ± 0.3a,b,c,d,f | – | 50.0 ± 0.0 |
| L. edodes + Se(IV) 25 mg/L medium (mycelium) | 803.6 ± 16.5a,c,d | 420.1 ± 39.6a,b,c | 18.7 ± 1.0a,d | 2.71 ± 1.5a,b,c,d | – | 18.5 ± 1.7a,c,d | 114.3 ± 15.3a,b,c | 50.0 ± 0.0 |
| L. edodes + Se(IV) 25 mg/L medium (medium) | 2602.8 ± 155.2a,b,c | 57.1 ± 4.4a,b,d,e | 16.5 ± 0.8a | 8.4 ± 0.1a,b,e,d,f | 28.8 ± 0.2a,b,e | 23.6 ± 1.7a,b,c,d,e | 40.3 ± 3.3b,c,d,e | 50.0 ± 0.0 |
| L. edodes + Se(IV) 50 mg/L medium (mycelium) | 621.8 ± 23.0a,b,c,e,f | 359.7 ± 62.3b,c,e | 14.9 ± 1.3a,b,d | 13.5 ± 1.9d,e,f | 7.9 ± 0.5e,f | 15.1 ± 1.6a,c,d,e,f | 568.6 ± 62.5a,b,c,d | 50.0 ± 0.0 |
| L. edodes + Se(IV) 50 mg/L medium (medium) | 2599.7 ± 149.2a,b,c,d | 56.18 ± 4.2a,b,d | 17.3 ± 1.0.a | 7.3 ± 0.8a,b,c,d,f | 24.0 ± 1.2a,b,f | 27.1 ± 1.3a,b,c,d,e | 112 ± 15.1a,b,c,d | 50.0 ± 0.0 |
Data are presented as mean ± standard deviation; n = 6 repetitions. Gallium was used as an internal standard. Tukey–Kramer test was used to reveal the differences between paired groups of elements in rows compared to fruiting bodies, control mycelium, and control medium; the same letters (a, b, c, d, e, and f) are marked for which the content differences are statistically significant (for p values < 0.05) (GraphPad InStat)
Among all the elements, K was found at the highest content in the fruiting bodies; its content was 1839.5 mg/100 g d.w. The content of Ca was highest in the mycelium cultured on the control medium; its content was 684.4 mg/100 g d.w. The presented results from the analysis of macroelement content in L. edodes mycelium were higher compared to the results obtained for fruiting bodies of the same species (K 537.9–1031.0 mg/100 g d.w., Ca 17.9–60.7 mg/100 g d.w.) [23–26]. The content of Cu was 21.0 mg/100 g d.w. in the mycelium obtained on the medium enriched with CuSO4, whereas the content of Fe was 14.6 mg/100 g d.w. in the mycelium obtained on the medium enriched with C12H22CuO14. The highest content of Mn, 18.9 mg/100 g d.w., was found in the mycelium obtained from non-enriched medium. In the case of Se, an amount of 568.6 mg/100 g d.w. was found in the mycelium obtained on the medium enriched with Selol at a higher concentration (Se(IV) 50 mg/L medium). A high amount of Zn (231.2 mg/100 g d.w.) was found in the mycelium obtained from in vitro cultures enriched with ZnSO4 (Table 1). Analysis of micronutrients showed that obtained L. edodes mycelia were characterized by a higher content of these elements than fruiting bodies of this mushroom species (Mn 0.2–4.8 mg/100 g d.w., Fe 4.4–12.5 mg/100 g d.w., Cu 1.4–18.2 mg/100 g d.w., Zn 5.9–28.4 mg/100 g d.w.) [23–26].
Because Zn, Cu, and Se, which are the elements with a high anti-inflammatory potential, were added to the culture medium, their content in mycelial cultures was analyzed in the next stage of the study, with respect to the type of compound and concentration used [5].
It was identified that the amount of Cu was highest (21.0 mg/100 g d.w.) in the biomass of L. edodes cultured on the medium enriched with inorganic copper salt (CuSO4), while a slightly lesser amount was found in the mycelium obtained from in vitro cultures grown on the media enriched with C12H22CuO14 (20.4 mg/100 g d.w.). A similar trend was observed for the content of Zn: the amount of Zn in the biomass of L. edodes obtained on the medium enriched with inorganic salt (ZnSO4) was 231.2 mg/100 g d.w., which was almost twice as high as the amount in the mycelium obtained from in vitro cultures grown on the medium enriched with C8H12N2O8Zn (141.2 mg/100 g d.w.). Selenium was added to the medium only in organic form due to its proven beneficial effect on the human body compared to the classic sodium selenite (Na2SO3) with a very narrow therapeutic index [12]. The highest amount of Se was obtained from the medium added with the highest dose of Se (568.6 mg/100 g d.w.). Because the elements Cu, Zn, and Se were identified in biomass obtained from in vitro cultures grown on medium enriched with these elements, a particularly valuable property of edible mushroom mycelia, i.e., their natural ability to accumulate elements from the medium on which they grow, was confirmed (Table 1) [16, 17, 26]. Therefore, mushroom mycelia can be successfully used as a natural source of these elements.
In order to determine the actual bioavailability of Cu, Zn, and Se to the human body, the release of these elements from the mushroom material into artificial digestive juices was investigated. Selenium is an essential micronutrient for human, and its deficiency causes harmful effects in living organisms, not only the amount of Zn and Cu released from the fruiting bodies and mycelium obtained from in vitro cultures grown on medium enriched with Zn and Cu salts, but also the amount of Se released from mycelium grown on medium enriched with Selol was evaluated [12]. The evaluation of the effect of addition of Se(IV) compounds on the amount of Cu and Zn released from mycelial cultures of L. edodes was also useful in the analysis of anti-inflammatory activity.
It was confirmed from the experiments that the tested bioelements were effectively released into artificial digestive juices. Thus, these elements may also be bioavailable for the human body (Fig. 1a–f). The previous experiments also presented that the elements from mushroom materials were released effectively [16, 17, 27].
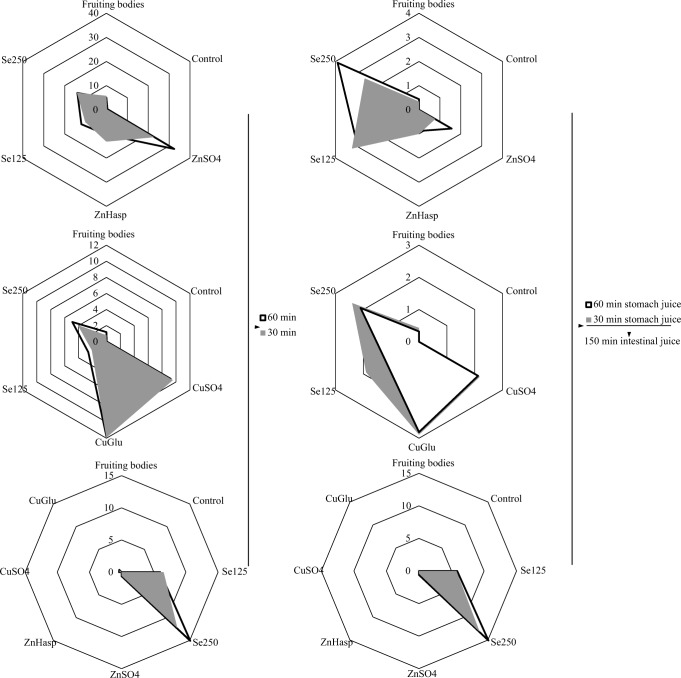
Fig. 1.
Amount (mg/100 g d.w.) of a Zn, b Cu, and c Se released into artificial gastric juice and d Zn, e Cu, and f Se released into artificial intestinal juice. Fruiting bodies—L. edodes fruiting bodies; Control—L. edodes mycelium; ZnSO4—L. edodes mycelium enriched with ZnSO4; ZnHasp—L. edodes mycelium enriched with C8H12N2O8Zn; CuSO4—L. edodes mycelium enriched with CuSO4; CuGlu—L. edodes mycelium enriched with C12H22CuO14; Se125—L. edodes mycelium enriched with Se(IV) 25 mg/L medium; Se250—L. edodes mycelium enriched with Se(IV) 50 mg/L medium
The study showed that the examined elements were released in larger amounts into gastric juice than into intestinal juice, and to a small extent, the amount of elements released was dependent on the duration of the extraction process. The same trends were observed for the Imleria badia mycelium [16]. In the case of Zn, the amount released in artificial gastric juice from the biomass grown on the medium enriched with ZnSO4 was determined to be 22.73 mg/100 g d.w. after 30 min of incubation and 32.46 mg/100 g d.w. after 60 min of incubation. When extracted into intestinal juice, the amounts released were lower and equaled to 0.77 and 1.56 mg/100 g d.w., respectively. In the case of mycelium enriched with C8H12N2O8Zn, the amount of Zn released into gastric juice after 30 min was 13.2 mg/100 g d.w. and after 60 min was 10.3 mg/100 g d.w., and the amount released into intestinal juice was about 1 mg/100 g d.w. In the case of mycelium grown on the control medium, the amount of Zn released into artificial gastric juice was 0.4 mg/100 g d.w. In the case of Zn released into artificial intestinal juice, the amounts were found to be below the level of quantification (Fig. 1a, d). Thus, the results obtained confirmed the ability of the mycelium to accumulate Zn from the medium [16, 17]. What is important, the amounts of Zn released into the artificial gastric juice were higher than those determined in the artificial intestinal juice. This is in line with previous experiments where higher amounts of elements were released into gastric juice [27]. Some differences were observed in the results of the analysis of copper compounds. This element was more effectively released into artificial gastric juice after digestion of biomass obtained on the medium enriched with organic copper salt (C12H22CuO14). The content of Cu released into artificial gastric juice was determined to be 11.91 mg/100 g d.w, whereas the amount of Cu extracted into artificial intestinal juice was only 3 mg/100 g d.w. (Fig. 1b, e). In the case of Se, it was found that higher amounts (13.2 mg/100 g d.w.) were extracted into artificial gastric juice from biomass obtained from media enriched with Selol at higher concentration (Se(IV) 50 mg/L medium) than the amount (5.99 mg/100 g d.w.) obtained with Selol at lower concentration (Se(IV) 25 mg/L medium). The same tendency was observed in the analysis of Se release into artificial intestinal juice (Fig. 1c, f).
Thus, the study on the release of Cu, Zn, and Se confirmed that the fruiting bodies and mycelium of edible mushrooms are the materials from which these elements are released into artificial digestive juices and the released elements may potentially be bioavailable to the human body. The obtained results also corroborated that the biomass obtained from in vitro cultures of L. edodes enriched with the abovementioned microelements is a source of bioelements which ensures an average supply of these elements to meet the daily needs of the human body [28, 29].
The study also evaluated the anti-inflammatory activity of mushroom extracts obtained from the in vitro cultures of L. edodes grown on control media and the ones enriched with Zn, Cu, and Se compounds. The results confirmed anti-inflammatory activity of mushroom material (inflammation was induced with a commonly used agent LPS) which was in line with previous studies on edible mushroom species that demonstrated their potential anti-inflammatory effect [5, 30–34].
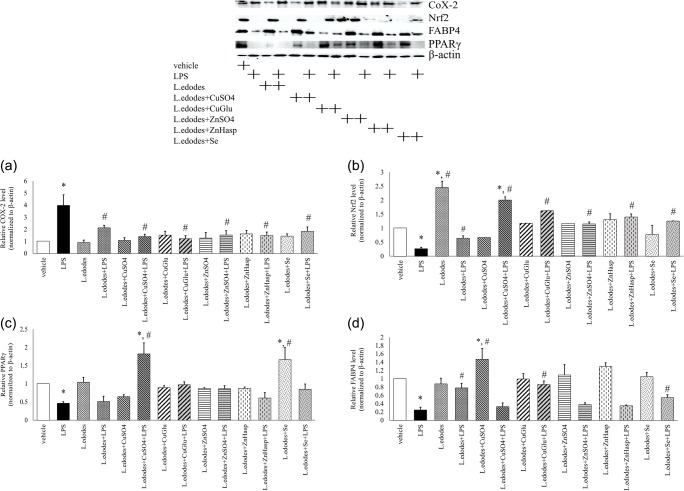
Cyclooxygenase-2 (COX-2) is a pro-inflammatory protein, and in the light of modern knowledge, searching for natural COX-2 agonists is extremely important [32, 35–37]. The results of the present study showed statistically the highest level of COX-2 in RAW 264.7 cells activated with LPS (p = 0.000). A high expression of this protein was also recorded in cells incubated with control mycelium extract activated with LPS (Fig. 2a). Analysis of COX-2 level in macrophages treated with L. edodes extracts from in vitro cultures grown on media enriched with Cu, Zn, or Se compounds revealed that the expression of this protein was comparable to the control cells despite cells activation with LPS. Similar results were observed in the case of I. badia species, in which addition of zinc salt to the medium had a positive effect on the anti-inflammatory effect of mycelium compared to the control mycelium [31]. In addition, it should be emphasized that the mycelium obtained from cultures enriched with bioelements (Cu, Zn, and Se) had a protective effect on the cells, reducing their level of COX-2 in case of inflammation caused by LPS (Fig. 2a). The results observed by other researchers in Antrodia camphorata, Inonotus obliquus, A. bisporus, Cantharellus cibarius, I. badia, Ganoderma lucidum, and Elaphomyces granulates species showed that the signaling pathway of COX-2 was inhibited [31, 38–42]. The present study also revealed the anti-inflammatory properties of the L. edodes extract enriched with vitamin D in C57B1/6 mouse model with inflammatory liver disease. Supplementation of L. edodes extract with vitamin D resulted in a significant reduction of liver damage. In addition, the histopathological features were improved and plasma levels of inflammatory factors such as aminotransferases and interferon gamma (INF-γ) were found to decrease. It was also noted that the anti-inflammatory effect of mushroom extract and vitamin D was synergistic in nature [6].
Fig. 2.
An exemplary blot and relative level of COX-2 (a), Nrf2 (b), PPARγ (c), and FABP4 (d) in RAW 264.7 cells supplemented with L. edodes mycelial extracts and activated with LPS. Vehicle—control cells; LPS—cells activated with lipopolysaccharide; L. edodes—cells incubated with extract from control mycelium; L. edodes+CuSO4—cells incubated with extract enriched with CuSO4; L. edodes + CuGlu—cells incubated with extract enriched with C12H22CuO14; L. edodes + ZnSO4—cells incubated with extract enriched with ZnSO4; L. edodes + ZnHasp—cells incubated with extract enriched with C8H12N2O8Zn; L. edodes + Se—cells incubated with extract enriched with Se(IV) 50 mg/L medium. Values are presented as means ± SD. Data are expressed as in relation to β-actin. *p < 0.001 relative to vehicle group, #p < 0.001 relative to LPS-activated cells
The study also analyzed Nrf2 (nuclear factor erythroid 2-related factor 2), a protein related to detoxification processes and a transcription factor [43]. Activation of Nrf2 may lead to induction of phase II enzymes responsible for inhibiting the activity of potential carcinogens. There is also scientific evidence for the involvement of Nrf2 in extinguishing inflammatory processes [35, 36]. In addition, a direct relationship between Nrf2 activity and reduction of COX-2, which is a pro-inflammatory factor, was identified [37]. Obtaining mycelium in laboratory conditions, which can increase the activity of Nrf2, may contribute to the development of new natural drugs with anti-inflammatory potential in the future. Statistically, the highest expression of Nrf2 was found in cells incubated with control mycelial extracts (p = 0.000), in contrast to LPS activated cells, where each enriched mycelial extracts exhibited better anti-inflammatory activity (Fig. 2b). Especially, high expression of Nrf2 was observed in LPS-activated cells treated with mycelial extract enriched with CuSO4 and C12H22CuO14 (Fig. 2b). Comparing with the results obtained for another species, A. bisporus, it was found that this species also showed anti-inflammatory activity. The highest expression of Nrf2 was observed in cells treated with extracts from mycelial cultures of A. bisporus enriched with α-linolenic acid. This suggests that mushroom extracts enriched with α-linolenic acid may also upregulate Nrf2 signaling [44]. In addition, the results of an in vivo study conducted on Antrodia cinnamomea showed the positive effect of this mushroom species on the expression of Nrf2 [45]. However, the present study on L. edodes species showed that mainly lentinan present in this species exerted an anti-inflammatory effect, causing, inter alia, an increase in Nrf2 expression [46].
In order to confirm the anti-inflammatory effect, the expression of the transcription factor peroxisome proliferator-activated receptor γ (PPARγ) was also studied. PPARγ plays an important role in the differentiation of adipocytes, in carbohydrate and lipid metabolism, and in the regulation of inflammatory processes. It has been proven that specific PPARγ agonists can inhibit the development of insulin resistance while acting simultaneously as anti-diabetic agents, and also have beneficial effects on inflammatory diseases, and even on some cancers [47, 48]. The relationship between the presence of Zn as an anti-inflammatory agent and PPARγ activity was also demonstrated [48]. In the present study, statistically, the highest expression of PPARγ was observed in cells treated with mushroom extracts enriched with CuSO4 after LPS activation (p = 0.000) and in cells treated with extracts enriched with Selol without LPS activation (p = 0.01) (Fig. 2c). However, it should be emphasized that the addition of each mycelium extracts increased the level of PPARγ compared to LPS-activated control cells (Fig. 2c). A study proved that diet rich in fatty acids (FAs), especially edible mushrooms, may be a factor affecting the level of PPARγ [32]. It also proved that extracts from C. cibarius containing FAs exerted agonistic activity against receptors activated by PPARγ [5]. In our study, Western blot analysis of PPARγ level once again confirmed the anti-inflammatory effect of mushroom extracts.
Taking into account that edible mushrooms, including L. edodes, show numerous pro-health activities due to the presence of fatty acids (FAs), the expression of FA-binding protein 4 (FABP4) was studied [5]. FABP4 is mainly found in macrophages and adipose tissues, where it regulates the storage of FAs and the lipolysis process. FABP4 is also an important mediator of inflammation as well as a factor responsible for metabolism [49]. An increase the expression of FABP4 was observed in the RAW 264.7 cells treated with mushroom extract, compared to macrophages activated with LPS. The highest expression of this protein was observed in cells without inflammatory state after treatment with mushroom extracts enriched with CuSO4 again, while the lowest expression was observed in control cells after LPS activation (Fig. 2d). Extracts enriched in bioelements proved to be more effective than control mycelial extracts (Fig. 2d). The following are some of the saccharides in mushrooms that regulate FABP4 activity: trehalose, β-glucans (e.g. lentinan from L. edodes species), and chitosans [5].
Conclusion
The experiment joining the accumulation and release of the examined bioelements into artificial digestive juices from mushroom material, and also the analysis of their anti-inflammatory properties, highlighted the potential benefits of consumption of L. edodes species in the form of fruiting bodies and biomass (being a potential dietary supplement). It was presented that L. edodes in vitro cultures accumulated Cu, Zn, and Se from modified and optimized media and released them effectively into artificial digestive juices, which can be directly translate into the expected results of digestion process in human body. This indicates the possibility and benefits of fortification of L. edodes mycelium with specific bioelements.
It was also demonstrated that the L. edodes species is characterized by anti-inflammatory properties. From the results of Western blot, it was found that addition of Cu, Zn, or Se enhanced the anti-inflammatory properties of L. edodes mycelial extracts, suggesting that the mycelium of L. edodes may be used as a potential component in natural anti-inflammatory dietary supplement. Additionally, obtained results could be used in commercial production of L. edodes.
Funding Information
This work was supported by the National Science Center, Poland (2017/25/N/NZ7/00554).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that there is no conflict of interest.
Footnotes
The original version of this article was revised: Figure 1 is in a wrong way exported to Pdf File (is in black color).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/24/2019
The original version of this article unfortunately contained a mistake.
References
- 1.Bisen PS, Baghel RK, Sanodiya BS, Thakur GS, Prasad GBKS. Lentinula edodes: a macrofungus with pharmacological activities. Curr Med Chem. 2010;17:2419–2430. doi: 10.2174/092986710791698495. [DOI] [PubMed] [Google Scholar]
- 2.Finimundy TC, Dillon AJP, Henriques JAP, Ely MR. A review on general nutritional compounds and pharmacological properties of the Lentinula edodes mushroom. Food Nutr Sci. 2014;5:1095–1105. doi: 10.4236/fns.2014.512119. [DOI] [Google Scholar]
- 3.Roupas P, Keogh J, Noakes M, Margetts C, Taylor P. The role of edible mushrooms in health: evaluation of the evidence. J Funct Foods. 2012;4:687–709. doi: 10.1016/j.jff.2012.05.003. [DOI] [Google Scholar]
- 4.Du B, Zhu F, Xu B. An insight into the anti-inflammatory properties of edible and medicinal mushrooms. J Funct Foods. 2018;47:334–342. doi: 10.1016/j.jff.2018.06.003. [DOI] [Google Scholar]
- 5.Muszyńska B, Grzywacz-Kisielewska A, Kała K, Gdula-Argasińska J. Anti-inflammatory properties of edible mushrooms: a review. Food Chem. 2018;243:373–381. doi: 10.1016/j.foodchem.2017.09.149. [DOI] [PubMed] [Google Scholar]
- 6.Drori A, Shabat Y, Ben Ya'acov A, Danay O, Levanon D, Zolotarov L, Ilan Y. Extracts from Lentinula edodes (Shiitake) edible mushrooms enriched with vitamin D exert an anti-inflammatory hepatoprotective effect. J Med Food. 2016;19:383–389. doi: 10.1089/jmf.2015.0111. [DOI] [PubMed] [Google Scholar]
- 7.Gründemann C, Garcia-Käufer M, Sauer B, Scheer R, Merdivan S, Bettin P, Huber R, Lindequist U. Comparative chemical and biological investigations of β-glucan-containing products from shiitake mushrooms. J Funct Foods. 2015;18:692–702. doi: 10.1016/j.jff.2015.08.022. [DOI] [Google Scholar]
- 8.Morales D, Gil-Ramirez A, Smiderle FR, Piris AJ, Ruiz-Rodriguez A, Soler-Rivas C. Vitamin D-enriched extracts obtained from shiitake mushrooms (Lentinula edodes) by supercritical fluid extraction and UV-irradiation. Innov Food Sci Emerg. 2017;41:330–336. doi: 10.1016/j.ifset.2017.04.008. [DOI] [Google Scholar]
- 9.Rincão VP, Yamamoto KA, Ricardo NMPS, Soares SA, Meirelles LDP, Nozawa C, Linhares REC. Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virol J. 2012;9:37. doi: 10.1186/1743-422X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies KM, Mercer JFB, Chen N, Double KL. Copper dyshomeostasis in Parkinson’s disease: implications for pathogenesis and indications for novel therapeutics. Clin Sci. 2016;130:565–574. doi: 10.1042/CS20150153. [DOI] [PubMed] [Google Scholar]
- 11.Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health. J Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 12.Ślusarczyk J, Dudek M, Wierzbicka M, Suchocki P, Kuraś M. Antimitotic effect of Selol and sodium selenate (IV) on Allium test cells. Caryologia. 2014;67:250–259. doi: 10.1080/0144235X.2014.974353. [DOI] [Google Scholar]
- 13.Opoka W, Muszyńska B, Rojowski J, Rumian J (2016) Gastroel-2014. Poland Patent Application, P 417238
- 14.Fitak B, Grabowski M, Suchocki P (1999) Polish patent 76530 (Cl. A61K31/095)
- 15.Oddoux L (1957) Recherches sur les mycéliums secondaires des Homobasidiés en culture pure. Imprimerie de Trevoux, Lyon
- 16.Kała K, Maślanka A, Sułkowska-Ziaja K, Rojowski J, Opoka W, Muszyńska B. In vitro culture of Boletus badius as a source of indole compounds and zinc ions released in artificial digestive juices. Food Sci Biotechnol. 2016;25:829–837. doi: 10.1007/s10068-016-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakowska A, Reczyński W, Muszyńska B. Optimization of the liquid culture medium composition to obtain the mycelium of Agaricus bisporus rich in essential minerals. Biol Trace Elem Res. 2016;173:231–240. doi: 10.1007/s12011-016-0638-y. [DOI] [PubMed] [Google Scholar]
- 18.Arvidson K, Johasson EG. Galvanic current between dental alloys in vitro. Scand J Dent Res. 1985;93:467–473. doi: 10.1111/j.1600-0722.1985.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 19.Polish Pharmakopeia Edition X (2014) PTFarm, Warszawa
- 20.Neumann M, Goderska K, Grajek K, Grajek W. Modele przewodu pokarmowego in vitro do badań nad biodostępnością składników odżywczych. Food Sci Technol Quality. 2006;1:30–45. [Google Scholar]
- 21.Gdula-Argasińska J, Czepiel J, Woźniakiewicz A, Wojtoń K, Grzywacz A, Woźniakiewicz M, Jurczyszyn A, Perucki W, Librowski T. N-3 fatty acids as resolvents of inflammation in the A549 cells. Pharmacol Rep. 2015;6:610–615. doi: 10.1016/j.pharep.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Muszyńska B, Kała K, Sułkowska-Ziaja K, Krakowska A, Opoka W. Agaricus bisporus and its in vitro culture as a source of indole compounds released into artificial digestive juices. Food Chem. 2016;199:509–515. doi: 10.1016/j.foodchem.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 23.George P, Ranatunga T, Reddy S, Sharma G. A comparative analysis of mineral elements in the mycelia and the fruiting bodies of shiitake mushrooms. Am J Food Technol. 2014;9:360–369. doi: 10.3923/ajft.2014.360.369. [DOI] [Google Scholar]
- 24.Gulati A, Atri N, Sharma S, Sharma B. Nutritional studies on five wild Lentinus species from North-West India. World J Dairy Food Sci. 2011;6:140–145. [Google Scholar]
- 25.Manjunathan J, Kaviyarasan V. Nutrient composition in wild and cultivated edible mushroom, Lentinus tuberregium (Fr.) Tamil Nadu., India. Int Food Res J. 2011;18:809–811. [Google Scholar]
- 26.Mleczek M, Siwulski M, Rzymski P, Niedzielski P, Gąsecka M, Jasińska A, Budzyńska S, Budka A. Multi-elemental analysis of Lentinula edodes mushrooms available in trade. J Environ Sci Health B. 2017;52:196–205. doi: 10.1080/03601234.2017.1261551. [DOI] [PubMed] [Google Scholar]
- 27.Kała K, Krakowska A, Sułkowska-Ziaja K, Szewczyk A, Reczyński W, Opoka W, Muszyńska B. Kinetics of extracted bioactive components from mushrooms in artificial digestive juices. Int J Food Prop. 2017;20:1796–1817. doi: 10.1080/10942912.2016.1219742. [DOI] [Google Scholar]
- 28.Almani Shahneela, Talpur Farah Naz, Memon Najma, Afridi Hassan Imran. Selenium Contents in Different Types of Raw and Processed Meat Products, Consumed Among the General Population of Pakistan. Biological Trace Element Research. 2019;193(2):357–363. doi: 10.1007/s12011-019-01732-4. [DOI] [PubMed] [Google Scholar]
- 29.Szpanowska-Wohn A, Kolarzyk E, Chowaniec E. Estimation of intake of zinc, copper and iron in the diet of patients with chronic renal failure treated by haemodialysis. Biol Trace Elem Res. 2008;124:97–102. doi: 10.1007/s12011-008-8131-x. [DOI] [PubMed] [Google Scholar]
- 30.Chien RC, Yang SC, Lin LM, Mau JL. Anti-inflammatory and antioxidant properties of pulsed light irradiated Lentinula edodes. J Food Process Preserv. 2016;41:e13045. doi: 10.1111/jfpp.13045. [DOI] [Google Scholar]
- 31.Grzywacz A, Gdula-Argasińska J, Kała K, Opoka W, Muszyńska B. Anti-inflammatory activity of biomass extracts of the bay mushroom, Imleria badia (Agaricomycetes), in RAW 264.7 cells. Int J Med Mushrooms. 2016;18:769–779. doi: 10.1615/IntJMedMushrooms.v18.i9.20. [DOI] [PubMed] [Google Scholar]
- 32.Hintze KJ, Tawzer J, Ward RE. Concentration and ratio of essential fatty acids influences the inflammatory response in lipopolysaccharide challenged mice. Prostaglandin Leukot Essent Fatty Acids. 2016;111:37–44. doi: 10.1016/j.plefa.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Moro C, Palacios I, Lozano M, D’Arrigo M, Guillamón E, Villares A, Martínez JA, García-Lafuente A. Anti-inflammatory activity of methanolic extracts from edible mushrooms in LPS activated RAW 264.7 macrophages. Food Chem. 2012;130:350–355. doi: 10.1016/j.foodchem.2011.07.049. [DOI] [Google Scholar]
- 34.Ren G, Yu M, Li K, Hu Y, Wang Y, Xu X, Qu J. Seleno-lentinan prevents chronic pancreatitis development and modulates gut microbiota in mice. J Funct Foods. 2016;22:177–188. doi: 10.1016/j.jff.2016.01.035. [DOI] [Google Scholar]
- 35.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta, Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey MK, Gupta SC, Nabavizadeh A, Aggarwal BB. Regulation of cell signaling pathways by dietary agents for cancer prevention and treatment. Semin Cancer Biol. 2017;46:158–181. doi: 10.1016/j.semcancer.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Gdula-Argasińska J, Grzywacz-Kisielewska A, Krakowska A, Opoka W, Muszyńska B. Anti-inflammatory properties of Cantharellus cibarius from in vitro culture enriched in zinc. Acta Pol Pharm. 2018;75:423–433. [Google Scholar]
- 39.Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013;139:503–508. doi: 10.1016/j.foodchem.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 40.Shie PH, Wang SY, Lay HL, Huang GJ. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int Immunopharmacol. 2016;31:186–194. doi: 10.1016/j.intimp.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Smiderle FR, Alquini G, Tadra-Sfeir MZ, Iacomini M, Wichers HJ, Van Griensven LJLD. Agaricus bisporus and Agaricus brasiliensis (1->6)-ß-D-glucans show immunostimulatory activity on human THP-1 derived macrophages. Carbohydr Polym. 2013;94:91–99. doi: 10.1016/j.carbpol.2012.12.073. [DOI] [PubMed] [Google Scholar]
- 42.Stanikunaite R, Khan SI, Trappe JM, Ross SA. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother Res. 2009;23:575–578. doi: 10.1002/ptr.2698. [DOI] [PubMed] [Google Scholar]
- 43.Krysztofiak A, Krajka-Kuźniak V. When defense becomes dangerous – transcription factor Nrf2 and cancer. Postepy Hig Med Dosw. 2015;69:140–152. doi: 10.5604/01.3001.0009.6486. [DOI] [PubMed] [Google Scholar]
- 44.Muszyńska B, Grzywacz-Kisielewska A, Kała K, Gdula-Argasińska J. Anti-inflammatory potential of in vitro cultures of the white button mushroom, Agaricus bisporus (Agaricomycetes), in Caco-2 cells. Int J Med Mushrooms. 2018;20:129–139. doi: 10.1615/IntJMedMushrooms.2018025408. [DOI] [PubMed] [Google Scholar]
- 45.Lee MT, Lin WC, Wang SY, Lin LJ, Yu B, Lee TT. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-κB-dominated pathways in broiler chickens. Poult Sci. 2018;97:2419–2434. doi: 10.3382/ps/pey076. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, Peng H, Dong L, Chen L, Ma X, Peng Y, Dai S, Liu Q. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int Immunopharmacol. 2016;36:1–8. doi: 10.1016/j.intimp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Hong SS, Lee JH, Jeong W, Kim N, Jin HZ, Hwang BY, Lee HJ, Lee SJ, Jang DS, Lee D. Acetylenic acid analogues from the edible mushroom Chanterelle (Cantharellus cibarius) and their effects on the gene expression of peroxisome proliferator-activated receptor-gamma target genes. Bioorg Med Chem Lett. 2012;22:2347–2349. doi: 10.1016/j.bmcl.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 48.Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Floresta G, Pistarà V, Amata E, Dichiara M, Marrazzo A, Prezzavento O, Rescifina A. Adipocyte fatty acid binding protein 4 (FABP4) inhibitors. A comprehensive systematic review. Eur J Med Chem. 2017;138:854–873. doi: 10.1016/j.ejmech.2017.07.022. [DOI] [PubMed] [Google Scholar]