Abstract
The care cascade—which evaluates outcomes across stages of patient engagement in a health system—is an important framework for assessing quality of tuberculosis (TB) care. In recent years, there has been progress in measuring care cascades in high TB burden countries; however, there are still shortcomings in our knowledge of how to reduce poor patient outcomes. In this paper, we outline a research agenda for understanding why patients fall through the cracks in the care cascade. The pathway for evidence generation will require new systematic reviews, observational cohort studies, intervention development and testing, and continuous quality improvement initiatives embedded within national TB programs. Certain gaps, such as pretreatment loss to follow-up and post-treatment disease recurrence, should be a priority given a relative paucity of high-quality research to understand and address poor outcomes. Research on interventions to reduce death and loss to follow-up during treatment should move beyond a focus on monitoring (or observation) strategies, to address patient needs including psychosocial and nutritional support. While key research questions vary for each gap, some patient populations may experience disparities across multiple stages of care and should be a priority for research, including men, individuals with a prior treatment history, and individuals with drug-resistant TB. Closing gaps in the care cascade will require investments in a bold and innovative action-oriented research agenda.
Keywords: Tuberculosis, Cascade of care, Continuum of care, Research agenda, Pretreatment loss to follow-up, Medication adherence
1. Introduction
The care cascade evaluates patient outcomes for a disease across stages of care. National-level care cascade analyses have identified that large numbers of individuals with active tuberculosis (TB) experience poor outcomes at critical points in health system engagement, highlighting foundational problems in quality of TB care [1,2]. We recently outlined guidelines for estimating the number of individuals with active TB in a population who successfully reach (or drop out at) different care cascade stages [3]. While such analyses help quantify gaps in care delivery, they do not illuminate why patients fall through the cracks—information that is critical for developing interventions to improve outcomes in TB programs.
Reasons for poor outcomes—and interventions to address these problems—may vary at each care cascade stage. Closing gaps in the care cascade may require interventions at the level of the population or health system (including the private sector), at the level of TB diagnostic and treatment centers, and at the level of the TB patient-health provider interaction. Rectifying gaps at different scales will require diverse interventions—potentially including large-scale public education, increased access to health facilities, initiatives in the private sector, integration of new diagnostic and monitoring technologies, and interventions to address patients’ psychosocial needs. In addition, some patients may be at higher risk for poor outcomes, thereby meriting greater attention and specialized interventions. In light of these complexities, in this manuscript, we outline an agenda to start answering key questions regarding poor patient outcomes in the TB care cascade.
2. Frameworks and research questions
2.1. Framework for the TB care cascade
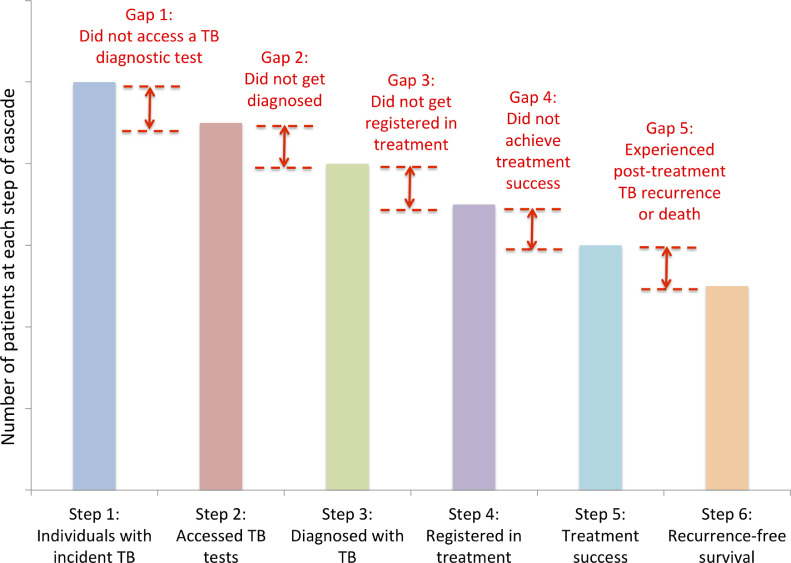
We previously described a care cascade model for individuals with active TB, in which each stage contains a step (number of individuals who reach that point in care) and a gap (those with poor outcomes, quantified as the difference between steps) (Fig. 1) [3]. Key gaps include: individuals with active TB in the population who do not reach health facilities and access a TB diagnostic test (Gap 1), those who access locations where diagnostic tests are available but do not get successfully diagnosed (Gap 2), those successfully diagnosed who do not get registered in treatment (Gap 3), those who start therapy but do not achieve treatment success (Gap 4), and those who finish therapy but experience death or TB recurrence within a year (Gap 5). We describe a research agenda to address each of these gaps below.
Fig. 1.
Generic care cascade model for individuals with active TB in a population [3].
2.2. Research questions
The research agenda below is guided by three broad questions. First, who is disproportionately falling out of the TB care cascade? Understanding the types of individuals who are at higher risk for poor outcomes at each stage may help to develop and refine interventions that focus on these specific populations, although we acknowledge that quality of care can and should be improved for all people with TB. Risk of dropping out of care may vary by demographics (e.g., age, gender), type of tuberculosis (e.g., pulmonary, extrapulmonary, prior treatment history), microbiological susceptibility (e.g., drug-resistant forms of TB), comorbidities (e.g., HIV, diabetes), or other social factors (e.g., living in migrant, urban slum, or indigenous communities).
Second, why are patients falling out of the cascade? Understanding barriers to engaging in TB care that contribute to poor outcomes are important to inform intervention development. Such barriers may occur at the level of the health system (e.g., poor quality of care or user experience), the patient (e.g., substance use, depression), the patient's family and community (e.g., TB-related stigma), or society (e.g., structural barriers).
Third, what interventions are needed to reduce gaps in the care cascade? Beneficial interventions might involve using novel technologies to address health system or patient barriers, social and behavioral interventions to address psychosocial barriers, social protection schemes for patients, or incentives to change healthcare provider (HCP) behavior, including in the private health sector. Intervention development would ideally be informed by research on the first two questions described above.
2.3. Pathway for generating evidence
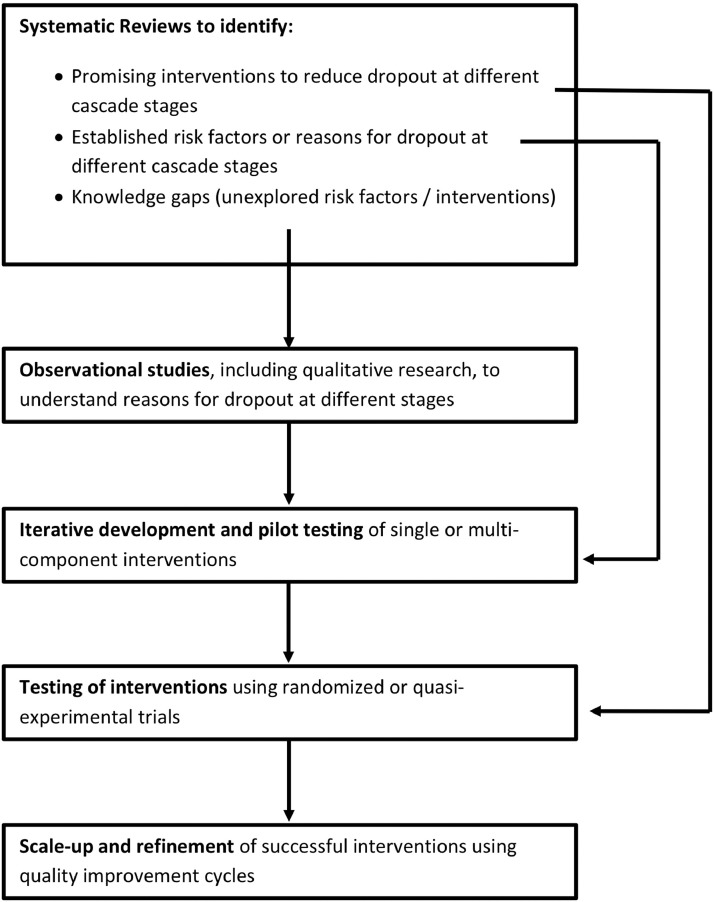
Diverse research approaches will be required to understand which patients are being lost, identify reasons for these losses, develop interventions, and implement these interventions in routine clinical practice (Fig. 2). Systematic reviews help to aggregate evidence about reasons for patient dropout across care cascade stages and the effectiveness of interventions to reduce these gaps. For example, systematic reviews have synthesized evidence on barriers to TB medication adherence from qualitative studies [4] and assessed the effectiveness (or lack thereof) of directly observed therapy (DOT) [5], [6], [7], [8], [9] and other interventions for improving adherence, including digital adherence technologies (DATs) [8,10]. Systematic reviews have not evaluated reasons or interventions for other care cascade gaps, such as pretreatment loss to follow-up (PTLFU) (Gap 3) and post-treatment relapse or death (Gap 5). Studies of HIV care delivery provide helpful examples to guide similar systematic reviews for TB [11], [12], [13], [14], [15].
Fig. 2.
Evidence generation pathway to address gaps in the TB care cascade.
By identifying research gaps, systematic reviews may guide further qualitative and quantitative observational research to identify novel risk factors for patient dropout. Findings of observational studies may in turn guide theory-informed intervention development to address risk factors, using iterative implementation and refinement. Implementation research frameworks—including the Unified Theory of Acceptance and Use of Technology (for technology-based interventions) [16], the RE-AIM framework, and the Consolidated Framework for Implementation Research—may guide approaches to designing, evaluating, and implementing interventions.
Intervention testing can take a variety of approaches. Since interventions to retain patients often require health system changes, cluster-randomized trials may facilitate rigorous evaluations of such interventions. However, such resource-intensive research approaches may not always be practical. Due to poor quality of care at later care cascade stages, interventions that address one gap may improve surrogate endpoints without translating into benefits in long-term outcomes, such as TB cure or recurrence-free survival, which does not necessarily mean that the intervention is not beneficial [17]. In addition, multicomponent interventions are more likely to improve long-term outcomes, but development and assessment of such interventions may be more amenable to quality improvement cycles (e.g., plan-do-study-act) and observational studies embedded in routine clinical practice, rather than randomized trials, to enable real-time iterative improvements [18]. Ideally, such quality improvement initiatives would be aligned to the TB care cascade—as an organizing framework and outcome measure—and be informed by theories of change aimed at strengthening health systems. Such initiatives, if well implemented, have the potential to continuously generate ideas and interventions for health system change while allowing assessment of the feasibility of those ideas.
3. Research to address key gaps in the care cascade
In the following sections, we describe specific questions that may be relevant to each gap in the cascade (Table 1).
Table 1.
Research questions relevant to each gap in the TB care cascade.
| Research questions | Potential research approaches | Relevance |
|---|---|---|
| Gap 1: Case-finding | ||
| Which populations do not have access to TB services? | • Analysis of data from national demographic and health surveys • Local exercises mapping the geographic distribution of notified patients in relation to the availability of TB services |
• May help to identify locations where TB services need to be expanded to ensure access to high-risk populations • May identify populations that would benefit from novel community-based strategies such as use of health extension workers for TB screening |
| Why do some individuals with active TB in the population not seek care or delay seeking care? | • Interviews with individuals diagnosed with TB in prevalence surveys who have not sought care • Interviews with individuals with symptoms concerning for TB in the community who have not sought care • Interviews with TB patients who had substantial delay in seeking care |
• May help guide targeting of public education strategies via radio, television, or social media • May help identify the types of individuals who should be prioritized in community active case-finding activities |
| Why do some healthcare providers (HCPs) not refer individuals for TB testing? | • Questionnaires using clinical vignettes to assess HCP knowledge • Standardized patient studies to assess actual HCP behavior • Qualitative research to understand HCPs’ clinical decision-making |
• May help identify types of HCPs who lack necessary knowledge or provide suboptimal care with regard to TB evaluation and testing • Standardized patient and knowledge assessments provide approaches for testing the benefits of interventions aimed at modifying behavior, including education of HCPs, use of incentives, and provision of support through public-private initiatives • Understanding HCPs clinical decision-making may facilitate educational strategies targeted at shifting their behavior |
| How can case detection rates of active case-finding (ACF) initiatives be increased? | • ACF trials focusing on high-risk groups, such as household contacts, people living with HIV (PLHIV), or individuals with silica exposure • ACF trials using identification of geographic TB hotspots to facilitate spatial targeting of case-finding approaches |
• May help identify the most efficient approaches for focusing ACF initiatives to increase the case detection and therefore the number of individuals entering the TB care cascade |
| Gap 2: Diagnosis | ||
| Which patients disproportionately do not get diagnosed with TB? | • Cross-sectional studies using exit interviews with structured or qualitative data collection to identify patients presenting to different health system levels who have not been tested for TB despite having symptoms • Cohort studies to understand which patients are not being appropriately tested |
• May help to identify whether certain groups are being disproportionately missed |
| Why do some patients not get appropriately diagnosed with TB, despite getting evaluated and tested? | • Patient pathways analyses to understand where TB tests are available in relation to patient care-seeking • Cohort studies to understand risk factors for patient attrition during the TB diagnostic workup • Qualitative research to understand barriers in the TB evaluation process |
• May help identify types of health facilities where World Health Organization (WHO)-approved TB tests are not accessible or feasible to implement, requiring a triage and referral mechanism • May help to identify patient characteristics that predict attrition during TB evaluation to facilitate development of targeted interventions • May help to identify health system barriers that need to be addressed to facilitate completion of the TB diagnostic process or whether the appropriate diagnostic algorithms are being used |
| How do we improve diagnosis of TB test-negative (i.e., smear-negative, Xpert-negative) TB patients? | • Cohort studies to understand patient attrition during the TB diagnostic workup, with a specific focus on TB diagnostic test-negative patients • Qualitative research to understand barriers in the TB evaluation process |
• May facilitate approaches for simplifying algorithms for the diagnostic workup of test-negative TB to reduce patient attrition |
| Gap 3: Linkage to care | ||
| Why do some diagnosed TB patients experience pretreatment loss to follow-up (PTLFU)? | • Cohort studies to understand patient attrition during linkage to care • Qualitative research to understand challenges in the process of linkage to care |
• May help to identify patient characteristics that predict PTLFU • May help to identify health system barriers contributing to PTLFU • May inform development of technology- and human-resource-based interventions to improve linkage to care |
| Gap 4: Retention on therapy and medication adherence | ||
| Why do some patients experience suboptimal TB treatment outcomes or medication non-adherence? | • Cohort studies to understand patient attrition during TB treatment or non-adherence to medications • Qualitative research to understand barriers to completing TB treatment or adhering to medications |
• May help to identify patient characteristics that predict suboptimal treatment outcomes or medication non-adherence • May help to identify health system barriers contributing to suboptimal treatment outcomes or medication non-adherence • May inform development of technology- and human-resource-based interventions to TB treatment outcomes and medication adherence |
| Gap 5: Post-treatment TB recurrence-free survival | ||
| Why do some TB patients experience post-treatment disease recurrence or death after finishing treatment? | • Cohort studies to understand post-treatment TB recurrence or mortality • Studies assessing post-treatment disability, mental health, pulmonary function, and emerging chronic diseases |
• May help to identify patient characteristics that predict post-treatment disease recurrence and mortality • May help to inform the development of approaches to post-treatment care for TB patients that would aim to achieve early identification of disease recurrence while facilitating treatment of post-TB sequelae, such as chronic lung disease |
3.1. Gap 1. Case-finding
Addressing the case-finding gap is contingent on understanding who is missed by current case finding efforts and how to decrease delays faced by those who are eventually diagnosed. There are a few reasons why individuals with TB in the community may not get evaluated and access a TB test. First, they may not have access to TB services, due to distance or other barriers. Second, they may not seek care for their symptoms, even if services are available. Finally, even if they do seek care, HCPs might not recognize their symptoms as being concerning for TB and initiate appropriate evaluation.
Identifying high-risk populations that have poor access to TB services is an initial step to reducing Gap 1. For example, historically marginalized populations—such as indigenous people living in the Brazilian and Peruvian Amazon, rural Canada, and rural India—have particularly poor access to TB services [19], [20], [21], [22]. At the national level, demographic and health surveys may provide insights into populations that have poor access to TB services [23]; however, addressing this problem at a local level may require mapping exercises to understand health service availability in an area relative to the geographical distribution of notified TB patients. Such exercises would also need to account for biases that may result from notifications being higher in areas with better access to health facilities [24].
In settings where TB services are relatively accessible, it is critical to understand why some individuals with TB in the population may not seek care. TB prevalence surveys provide an opportunity to study this problem. Individuals diagnosed with TB during prevalence surveys can be interviewed to understand whether they have sought care, and, if they have not, what prevented them from seeking further care [3]. Prevalence surveys also provide quantitative information that may shed light on disparities in care-seeking behavior. For example, findings from prevalence surveys show that a meaningful proportion of individuals with TB may not seek care because they are asymptomatic, suggesting that the only way to identify such individuals early may be by using chest X-ray or novel biomarker-based screening as part of active case-finding [25]. In addition, a recent systematic review found discrepancies in prevalence and notification data that suggest men may be less likely to seek or access care in many settings [26]. In situations where care-seeking data are unavailable from prevalence surveys, similar data may be available for individuals in the population with TB-related symptoms [1]. Studies examining factors associated with delays in TB care seeking may also provide valuable information [27], since patients experiencing long delays may serve as a surrogate for understanding those who do not seek care at all.
Understanding why HCPs do not refer individuals with symptoms for TB testing requires research into HCP knowledge and behavior. Recent studies using standardized patients in India, China, Kenya, and South Africa have provided insights into HCP behavior when evaluating individuals with TB symptoms [28]. In addition to revealing universally low TB testing rates by HCPs, these studies show that patient characteristics—including gender, age, and biometric characteristics (e.g., body mass index)—have little association with HCPs’ decisions to test for TB [28,29], although male standardized patients reported significantly shorter interactions with providers and felt providers were less likely to take their worries seriously [29]. In contrast, HCP characteristics did influence rates of TB testing and correct management. HCPs with MBBS degrees perform better than non-MBBS providers in India [30]. Public sector HCPs perform better than private sector HCPs in Kenya [31]. Qualitative studies also provide unique insights into HCP behavior with regard to TB evaluation [32], [33], [34]. For example, in India, HCPs often defer or delay bacteriological TB testing in favor of empirical treatment [34]. Patient-pathway analyses (PPA) may help identify not only where patients seek care but also gaps in diagnostic capacity in public versus private or lower- versus higher-level healthcare facilities [35,36].
Each of these problems in Gap 1 has different solutions that warrant evaluation. Increasing availability of TB services may be possible in geographic areas that are unconnected to health facilities using novel approaches, such as health extension workers [37]. Care-seeking behavior at the population level may be modified by public education strategies disseminated by radio, television, or social media. HCP knowledge of appropriate TB evaluation is low in many contexts [38,39]; however, even when HCPs have adequate knowledge, they often still do not appropriately evaluate for TB (the “know-do” gap) [40]. As such, increasing TB testing rates may require supporting HCPs, including ancillary providers such as community pharmacists, through public-private collaborations or provision of incentives [41,42].
Active or enhanced case-finding (ACF) strategies can circumvent the challenges of these other interventions by bringing TB screening to the doorstep of high-risk individuals; however, the optimal ACF approach remains elusive and will likely vary across settings. For example, a community-randomized trial of household-level enhanced case-finding in Zambia and South Africa did not demonstrate a decrease in TB incidence [43], while a trial in Vietnam demonstrated that conducting ACF on household contacts of TB patients was more effective in detecting TB than passive case finding alone [44]. With the advent of digital radiography with automated computer evaluation, there is renewed interest in community-based mass chest radiography screening campaigns, which were used with relative success in high-income countries in the 1930s-1960s [45].
There is also increasing recognition that people who have previously had TB are an important risk group, such that longitudinal follow-up of these individuals may increase case detection [46]. Refining ACF strategies in high-risk groups—such as household contacts, people living with HIV (PLHIV), or people exposed to silicosis—is a critical area for implementation research. Research is also needed to understand the benefits of spatial targeting of ACF by focusing on geographical hotspots with high TB incidence, which may also increase case detection [47].
3.2. Gap 2. Diagnosis
In the Indian and South African care cascades, Gap 2 revealed that many TB patients did not get successfully diagnosed, despite reaching health facilities and accessing TB diagnostic tests [1,2]. Certain groups are known to be at higher risk of missed diagnoses, often due to the imperfect sensitivity of existing diagnostic tests. These groups include PLHIV or those who are immunosuppressed for other reasons and children. Of note, these groups are more likely to have extra-pulmonary TB, which is more challenging to diagnose due to the need for biopsies and lower sensitivity of diagnostic tests on non-sputum specimens [48]. Studies that have used exit interviews with patients who present to healthcare settings in high-incidence settings identify missed opportunities for TB screening [49]. Further research is needed to identify whether certain groups, for example, women versus men [50], or patients with substance use are less likely to undergo recommended diagnostic evaluation.
The diagnostic gap may occur for several reasons. Sputum microscopy, which has relatively poor sensitivity, remains the dominant diagnostic modality in many high TB burden countries. Diagnosis of smear-negative pulmonary TB often relies on patients finishing multistep diagnostic algorithms associated with high rates of patient attrition [51,52]. Few high TB incidence countries have made higher-sensitivity WHO-approved TB tests (e.g., Xpert MTB/RIF) available at the most decentralized level (L0), which consists of care provided at health posts or by community health workers [53]. As such, patient pathways analyses suggest that TB patients are likely not accessing the best WHO-approved tests [36]. There has also been wide variability in the way in which Xpert MTB/RIF has been implemented in terms of indications for testing as well as geographic availability (e.g., urban versus rural settings [54]). In high incidence countries, such as India, where patients are more likely to initially seek private sector care, a modeling study suggests that rolling out Xpert MTB/RIF with restricted testing indications in the public sector alone might have limited impact on TB incidence [55]. Further research will help to understand test- and location-specific differences in diagnostic gaps in different contexts.
In order to close Gap 2, it is essential to understand that a diagnostic test in isolation cannot improve patient outcomes without efforts to strengthen the entire care cascade [17]. Several high-profile randomized trials of the implementation of diagnostic tests such as Xpert and urine LAM have not demonstrated mortality benefit [56], [57], [58]. Research is critical to understand the limitations, unrelated to a diagnostic test's accuracy, which may result when a new test is implemented in real world settings. For example, the benefits of TB diagnostic tests have been undermined in South Africa by high rates of empirical treatment [59], centralized laboratory testing, and challenges in obtaining sputum samples [60]. Qualitative research can provide insights into how patients navigate diagnostic ecosystems, including understanding why tests may not function as intended in real world settings [61].
While improving access to existing WHO-endorsed diagnostic tests is critical for closing the diagnostic gap, there is also need for new TB diagnostic tests that could help close Gaps 1 and 2 by allowing for more rapid TB diagnosis, facilitating identification of drug-resistant TB via rapid susceptibility testing, and facilitating triage and disease rule out in the community [62,63]. Research should also investigate how diagnostic algorithms for bacteriological test-negative TB can be simplified—for example, by earlier use of radiological studies—to ensure patients get diagnosed before being lost to follow-up.
3.3. Gap 3. Linkage to care
Systematic reviews suggest that patient losses from PTLFU (Gap 4) may be more substantial than those during the entire TB treatment course in some high TB burden settings [1,2,64]. The reasons that patients diagnosed with TB do not start treatment are diverse and include patient and health system factors [64]. For example, some studies suggest that particular patient characteristics predict higher risk of PTLFU, including having previously been treated for TB [65], older age [65,66], male sex [66], and weakness due to advanced TB [66], [67], [68]. Of these, having a prior TB treatment history is of particular concern, since these patients often also have poorer treatment outcomes and are at higher risk for having drug-resistant TB [1,65]. Health system factors found to contribute to PTLFU include: site of diagnosis (e.g., hospitals [69] or tertiary and TB specialty centers [65]), failure to communicate sputum test results to patients [64,68], challenges in navigating between health facilities [68], and dissatisfaction with waiting times [64]. In Indian studies, missing patient contact information in health records was a major barrier to being able to track these “lost” patients [65,66,70,71]. There is a notable paucity of qualitative research evaluating PTLFU, highlighting an area where further studies are needed. The high-quality qualitative studies that have been conducted emphasize the role of health system barriers in contributing to PTLFU [68,72].
The literature suggests that interventions should at least partly focus on addressing health system barriers. Technology may have a role in improving efficiency of care delivery after diagnosis. For example, electronic medical records may improve recording of patient contact information, so that patients can more easily be tracked, and automated SMS texts may help notify patients of their TB diagnoses [73]. Human resource-based solutions are perhaps even more critical. For example, patient navigators (i.e., individuals tasked with helping patients reach next steps in care) and patient tracking interventions may help prevent loss to follow-up, especially from high-volume tertiary hospitals [74]. The literature on interventions to reduce PTLFU is sparse, highlighting a need for high-quality implementation studies. Few studies have looked at PTLFU in higher-risk patients, such as those with drug-resistant TB.
3.4. Gap 4. Retention on therapy and medication adherence
Gap 4 comprises poor outcomes during TB therapy, due to treatment failure, loss to follow-up or death [3]. This has historically been the only gap routinely reported by national TB programs. As a result, Gap 4 has been a central focus of TB care delivery research in recent decades. Systematic reviews have evaluated studies on TB treatment outcomes to understand reasons for mortality [75], medication non-adherence [4], and interventions to improve adherence and reduce loss to follow-up [8].
One systematic review showed that, in high TB burden settings, individuals with drug-resistant TB, HIV co-infection (especially with advanced immunosuppression), older age, and undernutrition have higher TB case-fatality rates [75]; other studies from high burden settings have highlighted strong associations between tobacco or alcohol use and poor TB treatment outcomes [76,77]. In lower burden settings, non-infectious comorbidities (e.g., diabetes, chronic lung disease, renal disease, malignancy) and injection drug use were additional factors associated with increased TB mortality [75].
Research has helped define approaches for addressing some of these risk factors to improve TB patient outcomes. For example, randomized trials have shown that early initiation of antiretroviral therapy in PLHIV with active TB and advanced immunosuppression is associated with improved survival [78], [79], [80], and rigorous evidence affirms the benefits of drug-susceptibility testing and treatment with individualized drug regimens for patients with multidrug-resistant (MDR) TB [81].
In spite of the research already conducted to understand and address poor treatment outcomes, we argue that there is need for new research—particularly on risk factors that have been less actively studied—to inform development of novel interventions to reduce Gap 4. For example, undernutrition is a major predictor of poor treatment outcomes; however, trials to assess benefits of macro- and micro-nutrient supplementation are relatively sparse and inconclusive [82,83]. A recent study from Ethiopia found that 54% of TB patients had probable depression at treatment initiation [84]. Untreated depression was associated with three times increased relative risk of death and nine times increased risk of loss to follow-up [84]. And yet, few studies have assessed the impact of treating depression on TB outcomes. Studies showing promising benefits of treating alcohol use disorder [85], social protection schemes (e.g., cash transfer) for TB patients [86], [87], [88], and using community-based strategies (e.g., psychosocial support groups [89]) merit broader evaluation.
Approaches to monitoring medication adherence, particularly DOT, have been a major focus of research, under the assumption that such monitoring is critical for ensuring optimal treatment outcomes. However, systematic reviews have found conflicting results regarding whether DOT yields better outcomes than self-administered therapy, although most suggest little benefit of most DOT approaches [[5], [6], [7], [8], [9],90,91]. More recently, research has focused on using DATs to “electronically observe” pill-taking [10,92]. Findings regarding the accuracy and impact of DATs on treatment outcomes remain mixed [10,92]. Studies suggest that some DATs have poor accuracy for measuring TB medication adherence [93,94] and show no benefit for improving treatment outcomes [95,96], while others have found higher accuracy or improvements in adherence or treatment outcomes with use of these technologies [97], [98], [99]. These mixed findings regarding both DOT and DATs suggest that benefits of these interventions are dependent on the local context, technology used, design of the monitoring strategy, approach to intervening upon non-adherence, and quality of implementation. Using DATs to facilitate human interaction (e.g., provider-patient communication, early identification of medication adverse effects)—rather than simply using the technology to observe patients—may be associated with improvements in outcomes [98,99]. In general, future research on Gap 4 should focus on interventions that move beyond simply monitoring TB patients and towards actually providing support to address their needs.
3.5. Gap 5. Post-treatment TB recurrence-free survival
Gap 5 comprises TB recurrence or death after completing treatment. Assessing Gap 5 is most important during the initial year after a patient completes TB treatment, because most disease recurrence occurs within 12 months of finishing therapy [100]. Post-treatment deaths are also relevant to capture as part of this gap, because TB patients who achieve cure or treatment completion continue to have elevated mortality, part of which may be due to undiagnosed disease recurrence or TB related sequelae [101].
Patient characteristics that may predict TB recurrence include male sex [102] and prior treatment history [103], highlighting groups who may benefit from additional support during treatment. For all TB patients, the risk of disease recurrence partly reflects quality of care received during therapy. For example, undiagnosed drug resistance [104,105], suboptimal medication adherence [103,104,106], and smoking [76,104] are independently associated with increased risk of disease recurrence. These findings suggest that improvements in diagnostics (to facilitate early identification of drug resistance), support for patient adherence, and treatment of comorbidities could potentially reduce TB recurrence.
Gap 5 has implications not only for patient management during therapy but also for post-treatment care. One potential implication of high TB recurrence rates in some contexts [105,107] is that ensuring regular post-treatment follow-up with ongoing screening for TB symptoms may facilitate early identification of disease recurrence, which could effectively serve as a form of ACF. For example, a recent modeling study suggests that ACF among previously treated TB patients, as well as secondary prophylaxis with isoniazid therapy for some patients, could accelerate reduction in TB incidence in South Africa [46]. Routine post-treatment follow-up could also facilitate management of emerging chronic conditions, including post-TB lung disease [108], [109], [110] and increased cardiovascular risk seen in individuals with recent TB [101]. Involving affected communities to provide insights regarding wellbeing after TB is critical to inform the post-TB research agenda, given the breadth of TB-related complications, which range from psychological ill-health to disabilities (e.g., hearing loss) to catastrophic socioeconomic consequences [111].
3.6. Risk factors that contribute to multiple gaps in the care cascade
Some patient characteristics are associated with poor outcomes across multiple care cascade gaps. For example, a recent systematic review suggests that men with TB in the community are less likely to reach care and get notified (i.e., started on treatment) by national programs [26]. Studies from a variety of settings also suggest that men may be at higher risk of death while on TB treatment [75] and for experiencing post-treatment disease recurrence [102]. In some settings, patients with a prior treatment history may be more likely to suffer from PTLFU [65], suboptimal treatment outcomes [1,112], and post-treatment disease recurrence [103]. Individuals with drug-resistant TB in particular suffer from disproportionately poor outcomes at every care cascade gap [1,2,113]. Given that many TB patients lack social support to engage in care, community-based care and strategies for facilitating social support may be beneficial to TB patients at multiple care cascade stages, based on evidence from the HIV and maternal health literature [114], [115], [116]. Patients with these various characteristics may benefit from dedicated interventions to address their needs at every stage of care.
4. Applying this research agenda in different geographic scales and populations
The multifaceted agenda described above is meant to highlight key gaps in knowledge that may be addressed through studies conducted at different levels of geographic scale and in diverse populations (Table 2). For example, we have previously advocated that national TB programs could use multisite prospective cohort studies, with representative sampling of health facilities, to achieve nationally-representative estimates of patient losses at key care cascade stages—from diagnosis to recurrence-free survival [3]. Rigorous measurement of clinical, psychosocial, and health system factors for patients included in such studies might simultaneously identify characteristics that predict patient attrition to inform national-level interventions and policies.
Table 2.
Potential geographic scales or population focuses of interest for the care cascade research agenda.
| Geographic scale or population of interest | Potential research approaches | Limitations of the research approaches when applied in a given geographic scale or population |
|---|---|---|
| National TB programs / country-level studies | • Nationally-representative cohort studies to identify predictors of poor care cascade outcomes • Systematic mapping of populations without access to TB services • Assessing reasons individuals with active TB have not sought care in large-scale TB prevalence surveys |
• National service mapping may identify major service gaps but miss barriers to health facility accessibility for local subpopulations • Reasons for not seeking care or dropping out of care by may vary for sub-populations in local contexts |
| Key high-risk populations (e.g., people living with HIV, people who inject drugs, slum residents, tribal populations, migrants, refugees, miners, individuals with silicosis, healthcare workers) | • Cohort studies to identify predictors of poor care cascade outcomes by screening and follow-up of affected individuals at specific sites (e.g., HIV clinics, opioid agonist therapy centers, etc.) or using unique sampling methods (e.g., respondent-driven sampling) • Qualitative studies may provide rich information that is generalizable to others in the affected sub-population |
• Findings in a given high-risk population may have limited generalizability outside of that sub-population |
| Local city or district TB programs, hospitals, or clinics | • Cohort and qualitative studies to understand reasons for poor care cascade outcomes | • Findings may directly inform local changes in care delivery but may have limited generalizability |
However, nationally-representative studies may provide suboptimal information regarding barriers to engagement in the TB care cascade for key high-risk populations—such as PLHIV (particularly in low HIV prevalence settings) [117], people who live in slums [118], people who inject drugs [119,120], prisoners [121], migrants [122], miners [123,124], individuals with silicosis [125], and healthcare workers [126] to name a few. Unique sampling methods may be required for these sub-populations. For example, finding people who inject drugs with TB—to understand care-seeking behavior and care cascade dropout—may require screening and follow-up of individuals recruited by respondent-driven sampling or from opioid agonist therapy centers [127]. Finally, cohort and qualitative studies to understand care cascade outcomes at the local city, district, hospital, or clinic level may help to directly inform local interventions and quality improvement initiatives.
5. Conclusion: Need for bold and innovative research on the care cascade
In the last few years, there has been substantial progress in developing approaches for measuring care cascades for active TB disease in high TB burden countries [[1], [2], [3],128]. However, while the TB community has gained a better understanding of the scale of patient losses throughout the cascade, we still have major shortcomings in our knowledge of how to reduce these gaps in care [129]. For some gaps, such as PTLFU and post-treatment disease recurrence, there has been a paucity of research given the scale of these problems. Even for interventions aimed at addressing gaps that have historically been dynamic areas of research—such as ACF approaches or strategies for promoting TB medication adherence—there are important limitations in our knowledge regarding their efficacy or optimal approaches to implementation. As such, research needs to be expanded across all levels of the evidence generation pathway (Fig. 2). TB researchers can take inspiration from the extensive research on the care cascade that has been conducted by the HIV community. Closing gaps in the care cascade has the potential to more rapidly accelerate reduction in TB incidence [128,130]; however, achieving this goal will require urgent investments in a bold and innovative action-oriented research agenda.
Ethics statement
Note that this is a review article for which no ethical clearances are required.
Declaration of Competing Interest
None.
Funding
RS is supported by a Doris Duke Clinical Scientist Development Award. RRN is supported by a National Institutes of Health Career Development Award (NIAID K23 AI132648-02) and an American Society of Tropical Medicine and Hygiene Burroughs Wellcome Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Subbaraman R., Nathavitharana R.R., Satyanarayana S., Pai M., Thomas B.E., Chadha V.K. The tuberculosis cascade of care in india's public sector: a systematic review and meta-analysis. PLoS Med. 2016;13(10) doi: 10.1371/journal.pmed.1002149. PMID: 27780217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naidoo P., Theron G., Rangaka M.X., Chihota V.N., Vaughan L., Brey Z.O. The south african tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl_7):S702–SS13. doi: 10.1093/infdis/jix335. PMID: 29117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbaraman R., Nathavitharana R.R., Mayer K.H., Satyanarayana S., Chadha V.K., Arinaminpathy N. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019;16(2) doi: 10.1371/journal.pmed.1002754. PMID: 30811385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munro S.A., Lewin S.A., Smith H.J., Engel M.E., Fretheim A., Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238. doi: 10.1371/journal.pmed.0040238. PMID: 17676945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karumbi J., Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015;(5) doi: 10.1002/14651858.CD003343.pub4. Cd003343PMID: 26022367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasipanodya J.G., Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57(1):21–31. doi: 10.1093/cid/cit167. PMID: 23487389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian J.H., Lu Z.X., Bachmann M.O., Song F.J. Effectiveness of directly observed treatment of tuberculosis: a systematic review of controlled studies. Int J Tuberc Lung Dis. 2014;18(9):1092–1098. doi: 10.5588/ijtld.13.0867. PMID: 25189558. [DOI] [PubMed] [Google Scholar]
- 8.Alipanah N., Jarlsberg L., Miller C., Linh N.N., Falzon D., Jaramillo E. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7) doi: 10.1371/journal.pmed.1002595. PMID: 29969463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay B., Castellanos M., Ebell M., Whalen C.C., Handel A. An attempt to reproduce a previous meta-analysis and a new analysis regarding the impact of directly observed therapy on tuberculosis treatment outcomes. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0217219. PMID: 31120965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngwatu B.K., Nsengiyumva N.P., Oxlade O., Mappin-Kasirer B., Nguyen N.L., Jaramillo E. The impact of digital health technologies on tuberculosis treatment: a systematic review. Eur Respir J. 2018;51(1) doi: 10.1183/13993003.01596-2017. PMID: 29326332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genberg B.L., Shangani S., Sabatino K., Rachlis B., Wachira J., Braitstein P. Improving Engagement in the HIV care cascade: a systematic review of interventions involving people living with HIV/AIDS as peers. AIDS Behav. 2016;20(10):2452–2463. doi: 10.1007/s10461-016-1307-z. PMID: 26837630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacPherson P., Munthali C., Ferguson J., Armstrong A., Kranzer K., Ferrand R.A. Service delivery interventions to improve adolescents' linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health. 2015;20(8):1015–1032. doi: 10.1111/tmi.12517. PMID: 25877007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govindasamy D., Meghij J., Kebede Negussi E., Clare Baggaley R., Ford N., Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings–a systematic review. J Int AIDS Soc. 2014;17:19032. doi: 10.7448/IAS.17.1.19032. PMID: 25095831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox M.P., Rosen S., Geldsetzer P., Barnighausen T., Negussie E., Beanland R. Interventions to improve the rate or timing of initiation of antiretroviral therapy for HIV in sub-Saharan Africa: meta-analyses of effectiveness. J Int AIDS Soc. 2016;19(1):20888. doi: 10.7448/IAS.19.1.20888. PMID: 27507249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed S., Autrey J., Katz I.T., Fox M.P., Rosen S., Onoya D. Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries. Soc Sci Med. 2018;213:72–84. doi: 10.1016/j.socscimed.2018.05.048. PMID: 30059900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesh V., Thong J., Xu X. Unified theory of acceptance and use of technology: a synthesis and the road ahead. J Assoc Inf Syst. 2016;17(5):328–376. [Google Scholar]
- 17.Pai M., Schumacher S.G., Abimbola S. Surrogate endpoints in global health research: still searching for killer apps and silver bullets? BMJ Glob Health. 2018;3(2) doi: 10.1136/bmjgh-2018-000755. PMID: 29607104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berwick D. The science of improvement. J American Med Assoc. 2008;299(10):1182–1184. doi: 10.1001/jama.299.10.1182. [DOI] [PubMed] [Google Scholar]
- 19.Malacarne J., Gava C., Escobar A.L., Souza-Santos R., Basta P.C. Health service access for tuberculosis diagnosis and treatment among indigenous peoples in Rondonia state, Brazilian Amazon, 2009-2011: a cross-sectional study. Epidemiol Serv Saude. 2019;28(3) doi: 10.5123/S1679-49742019000300002. PMID: 31508714. [DOI] [PubMed] [Google Scholar]
- 20.Gianella C., Ugarte-Gil C., Caro G., Aylas R., Castro C., Lema C. TB in vulnerable populations: the case of an indigenous community in the peruvian Amazon. Health Hum Rights. 2016;18(1):55–68. PMID: 27780999. [PMC free article] [PubMed] [Google Scholar]
- 21.Patel S., Paulsen C., Heffernan C., Saunders D., Sharma M., King M. Tuberculosis transmission in the Indigenous peoples of the Canadian prairies. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188189. PMID: 29136652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniyandi M., Rao V.G., Bhat J., Yadav R. Performance of Revised National Tuberculosis Control Programme (RNTCP) in tribal areas in India. Indian J Med Res. 2015;141(5):624–629. doi: 10.4103/0971-5916.159553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardeshi G., Deluca A., Agarwal S., Kishore J. Tuberculosis patients not covered by treatment in public health services: findings from India's National Family Health Survey 2015-16. Trop Med Int Health. 2018;23(8):886–895. doi: 10.1111/tmi.13086. PMID: 29851437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang S., Ogbudebe C., Chijioke-Akaniro O., Igbabul S.-A., Abdur-Razzaq H., Okorie O. Defining novel TB risk groups for intensified case finding based on state-level case detection gaps in Nigeria (Abstract OA-01-302-31) Int J Tuberc Lung Dis. 2019;23(10):S64. [Google Scholar]
- 25.Onozaki I., Law I., Sismanidis C., Zignol M., Glaziou P., Floyd K. National tuberculosis prevalence surveys in Asia, 1990-2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(9):1128–1145. doi: 10.1111/tmi.12534. PMID: 25943163. [DOI] [PubMed] [Google Scholar]
- 26.Horton K.C., MacPherson P., Houben R.M., White R.G., Corbett E.L. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13(9) doi: 10.1371/journal.pmed.1002119. PMID: 27598345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sreeramareddy C.T., Qin Z.Z., Satyanarayana S., Subbaraman R., Pai M. Delays in diagnosis and treatment of pulmonary tuberculosis in India: a systematic review. Int J Tuberc Lung Dis. 2014;18(3):255–266. doi: 10.5588/ijtld.13.0585. PMID: 24670558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels B., Kwan A., Pai M., Das J. Lessons on the quality of tuberculosis diagnosis from standardized patients in China, India, Kenya, and South Africa. J Clin Tuberc Other Mycobact Dis. 2019;16 doi: 10.1016/j.jctube.2019.100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels B., Kwan A., Satyanarayana S., Subbaraman R., Das R.K., Das V. Use of standardised patients to assess gender differences in quality of tuberculosis care in urban India: a two-city, cross-sectional study. Lancet Glob Health. 2019;7(5):e633–ee43. doi: 10.1016/S2214-109X(19)30031-2. PMID: 30928341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan A., Daniels B., Saria V., Satyanarayana S., Subbaraman R., McDowell A. Variations in the quality of tuberculosis care in urban India: a cross-sectional, standardized patient study in two cities. PLoS Med. 2018;15(9) doi: 10.1371/journal.pmed.1002653. PMID: 30252849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels B., Dolinger A., Bedoya G., Rogo K., Goicoechea A., Coarasa J. Use of standardised patients to assess quality of healthcare in Nairobi, Kenya: a pilot, cross-sectional study with international comparisons. BMJ Glob Health. 2017;2(2) doi: 10.1136/bmjgh-2017-000333. PMID: 29225937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDowell A., Engel N., Daftary A. In the eye of the multiple beholders: qualitative research perspectives on studying and encouraging quality of TB care in India. J Clin Tuberc Other Mycobact Dis. 2019;16 doi: 10.1016/j.jctube.2019.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDowell A., Pai M. Alternative medicine: an ethnographic study of how practitioners of Indian medical systems manage TB in Mumbai. Trans R Soc Trop Med Hyg. 2016;110(3):192–198. doi: 10.1093/trstmh/trw009. PMID: 26884500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDowell A., Pai M. Treatment as diagnosis and diagnosis as treatment: empirical management of presumptive tuberculosis in India. Int J Tuberc Lung Dis. 2016;20(4):536–543. doi: 10.5588/ijtld.15.0562. PMID: 26970165. [DOI] [PubMed] [Google Scholar]
- 35.Hanson C.L., Osberg M., Brown J., Durham G., Chin D.P. Conducting patient-pathway analysis to inform programming of tuberculosis services: methods. J Infect Dis. 2017;216(suppl_7):S679–SS85. doi: 10.1093/infdis/jix387. PMID: 29117350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson C., Osberg M., Brown J., Durham G., Chin D.P. Finding the missing patients with tuberculosis: lessons learned from patient-pathway analyses in 5 countries. J Infect Dis. 2017;216(suppl_7):S686–SS95. doi: 10.1093/infdis/jix388. PMID: 29117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fekadu L., Hanson C., Osberg M., Makayova J., Mingkwan P., Chin D. Increasing access to tuberculosis services in Ethiopia: findings from a patient-pathway analysis. J Infect Dis. 2017;216(suppl_7):S696–S701. doi: 10.1093/infdis/jix378. PMID: 29117346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satyanarayana S., Subbaraman R., Shete P., Gore G., Das J., Cattamanchi A. Quality of tuberculosis care in India: a systematic review. Int J Tuberc Lung Dis. 2015;19(7):751–763. doi: 10.5588/ijtld.15.0186. PMID: 26056098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braham C.A., White P.J., Arinaminpathy N. Management of tuberculosis by healthcare practitioners in Pakistan: a systematic review. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0199413. PMID: 29928031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das J., Kwan A., Daniels B., Satyanarayana S., Subbaraman R., Bergkvist S. Use of standardised patients to assess quality of tuberculosis care: a pilot, cross-sectional study. Lancet Infect Dis. 2015;15(11):1305–1313. doi: 10.1016/S1473-3099(15)00077-8. PMID: 26268690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daftary A., Satyanarayana S., Jha N., Singh M., Mondal S., Vadnais C. Can community pharmacists improve tuberculosis case finding? A mixed methods intervention study in India. BMJ Glob Health. 2019;4(3) doi: 10.1136/bmjgh-2019-001417. PMID: 31179037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arinaminpathy N., Deo S., Singh S., Khaparde S., Rao R., Vadera B. Modelling the impact of effective private provider engagement on tuberculosis control in urban India. Sci Rep. 2019;9(1):3810. doi: 10.1038/s41598-019-39799-7. PMID: 30846709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayles H., Muyoyeta M., Du Toit E., Schaap A., Floyd S., Simwinga M. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382(9899):1183–1194. doi: 10.1016/S0140-6736(13)61131-9. PMID: 23915882. [DOI] [PubMed] [Google Scholar]
- 44.Fox G.J., Nhung N.V., Sy D.N., Hoa N.L.P., Anh L.T.N., Anh N.T. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med. 2018;378(3):221–229. doi: 10.1056/NEJMoa1700209. PMID: 29342390. [DOI] [PubMed] [Google Scholar]
- 45.Golub J.E., Mohan C.I., Comstock G.W., Chaisson R.E. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9(11):1183–1203. PMID: 16333924. [PMC free article] [PubMed] [Google Scholar]
- 46.Marx F.M., Yaesoubi R., Menzies N.A., Salomon J.A., Bilinski A., Beyers N. Tuberculosis control interventions targeted to previously treated people in a high-incidence setting: a modelling study. Lancet Glob Health. 2018;6(4):e426–ee35. doi: 10.1016/S2214-109X(18)30022-6. PMID: 29472018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cudahy P.G.T., Andrews J.R., Bilinski A., Dowdy D.W., Mathema B., Menzies N.A. Spatially targeted screening to reduce tuberculosis transmission in high-incidence settings. Lancet Infect Dis. 2018;19(3):e89–e95. doi: 10.1016/S1473-3099(18)30443-2. PMID: 30554997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohli M., Schiller I., Dendukuri N., Dheda K., Denkinger C.M., Schumacher S.G. Xpert((R)) MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;(8) doi: 10.1002/14651858.CD012768.pub2. Cd012768PMID: 30148542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chihota V.N., Ginindza S., McCarthy K., Grant A.D., Churchyard G., Fielding K. Missed opportunities for TB Investigation in primary care clinics in South Africa: experience from the XTEND Trial. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0138149. PMID: 26383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mhalu G., Weiss M.G., Hella J., Mhimbira F., Mahongo E., Schindler C. Explaining patient delay in healthcare seeking and loss to diagnostic follow-up among patients with presumptive tuberculosis in Tanzania: a mixed-methods study. BMC Health Serv Res. 2019;19(1):217. doi: 10.1186/s12913-019-4030-4. PMID: 30953502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chadha V.K., Praseeja P., Hemanthkumar N.K., Shivshankara B.A., Sharada M.A., Nagendra N. Implementation efficiency of a diagnostic algorithm in sputum smear-negative presumptive tuberculosis patients. Int J Tuberc Lung Dis. 2014;18(10):1237–1242. doi: 10.5588/ijtld.14.0218. PMID: 25216839. [DOI] [PubMed] [Google Scholar]
- 52.Thomas A., Gopi P.G., Santha T., Jaggarajamma K., Charles N., Prabhakaran E. Course of action taken by smear negative chest symptomatics: a report from a rural area in South India. Indian J Tuberc. 2006;53:4–6. [Google Scholar]
- 53.Huddart S., MacLean E., Pai M. Location, location, location: tuberculosis services in highest burden countries. Lancet Glob Health. 2016;4(12):e907–e908. doi: 10.1016/S2214-109X(16)30248-0. PMID: 27855868. [DOI] [PubMed] [Google Scholar]
- 54.Qin Z.Z., Pai M., Van Gemert W., Sahu S., Ghiasi M., Creswell J. How is Xpert MTB/RIF being implemented in 22 high tuberculosis burden countries? Eur Respir J. 2015;45(2):549–554. doi: 10.1183/09031936.00147714. PMID: 25359338. [DOI] [PubMed] [Google Scholar]
- 55.Salje H., Andrews J.R., Deo S., Satyanarayana S., Sun A.Y., Pai M. The importance of implementation strategy in scaling up Xpert MTB/RIF for diagnosis of tuberculosis in the Indian health-care system: a transmission model. PLoS Med. 2014;11(7) doi: 10.1371/journal.pmed.1001674. PMID: 25025235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Theron G., Zijenah L., Chanda D., Clowes P., Rachow A., Lesosky M. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(9915):424–435. doi: 10.1016/S0140-6736(13)62073-5. PMID: 24176144. [DOI] [PubMed] [Google Scholar]
- 57.Churchyard G.J., Stevens W.S., Mametja L.D., McCarthy K.M., Chihota V., Nicol M.P. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health. 2015;3(8):e450–e457. doi: 10.1016/S2214-109X(15)00100-X. PMID: 26187490. [DOI] [PubMed] [Google Scholar]
- 58.Gupta-Wright A., Corbett E.L., van Oosterhout J.J., Wilson D., Grint D., Alufandika-Moyo M. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet. 2018;392(10144):292–301. doi: 10.1016/S0140-6736(18)31267-4. PMID: 30032978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theron G., Peter J., Dowdy D., Langley I., Squire S.B., Dheda K. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis. 2014;14(6):527–532. doi: 10.1016/S1473-3099(13)70360-8. PMID: 24438820. [DOI] [PubMed] [Google Scholar]
- 60.Davids M., Dheda K., Pant Pai N., Cogill D., Pai M., Engel N. A survey on use of rapid tests and tuberculosis diagnostic practices by primary health care providers in South Africa: implications for the development of new point-of-care tests. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0141453. PMID: 26509894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yellappa V., Lefevre P., Battaglioli T., Devadasan N., Van der Stuyft P. Patients pathways to tuberculosis diagnosis and treatment in a fragmented health system: a qualitative study from a south Indian district. BMC Public Health. 2017;17(1):635. doi: 10.1186/s12889-017-4627-7. PMID: 28778192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pai M., Schito M. Tuberculosis diagnostics in 2015: landscape, priorities, needs, and prospects. J Infect Dis. 2015;211(Suppl 2):S21–S28. doi: 10.1093/infdis/jiu803. PMID: 25765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kik S.V., Denkinger C.M., Casenghi M., Vadnais C., Pai M. Tuberculosis diagnostics: which target product profiles should be prioritised? Eur Respir J. 2014;44(2):537–540. doi: 10.1183/09031936.00027714. PMID: 24696110. [DOI] [PubMed] [Google Scholar]
- 64.MacPherson P., Houben R., Glynn J.R., Corbett E.L., Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92(2):126–138. doi: 10.2471/BLT.13.124800. PMID: 24623906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas B.E., Subbaraman R., Sellappan S., Suresh C., Lavanya J., Lincy S. Pretreatment loss to follow-up of tuberculosis patients in Chennai, India: a cohort study with implications for health systems strengthening. BMC Infect Dis. 2018;18(1):142. doi: 10.1186/s12879-018-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gopi P.G., Chandrasekaran V., Subramani R., Narayanan P.R. Failure to initiate treatment for tuberculosis patients diagnosed in a community survey and at health facilities under a DOTS program in a district of south India. Indian J Tuberc. 2005;52:153–156. [Google Scholar]
- 67.Nyirenda T., Harries A.D., Banerjee A., Salaniponi F.M. Registration and treatment of patients with smear-positive pulmonary tuberculosis. Int J Tuberc Lung Dis. 1998;2(11):944–945. PMID: 9848620. [PubMed] [Google Scholar]
- 68.Thomas B.E., Suresh C., Lavanya J., Lindsley M.M., Galivanche A.T., Sellappan S. Understanding pretreatment loss to follow-up of tuberculosis patients: an explanatory qualitative study in Chennai, India (Preprint) medRxiv. 2019 doi: 10.1101/19006312. 19006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Botha E., den Boon S., Lawrence K.A., Reuter H., Verver S., Lombard C.J. From suspect to patient: tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis. 2008;12(8):936–941. PMID: 18647454. [PubMed] [Google Scholar]
- 70.Mehra D., Kaushik R.M., Kaushik R., Rawat J., Kakkar R. Initial default among sputum-positive pulmonary TB patients at a referral hospital in Uttarakhand. India. Trans R Soc Trop Med Hyg. 2013;107(9):558–565. doi: 10.1093/trstmh/trt065. PMID: 23920324. [DOI] [PubMed] [Google Scholar]
- 71.Sai Babu B., Satyanarayana A.V., Venkateshwaralu G., Ramakrishna U., Vikram P., Sahu S. Initial default among diagnosed sputum smear-positive pulmonary tuberculosis patients in Andhra Pradesh. India. Int J Tuberc Lung Dis. 2008;12(9):1055–1058. PMID: 18713504. [PubMed] [Google Scholar]
- 72.Squire S.B., Belaye A.K., Kashoti A., Salaniponi F.M., Mundy C.J., Theobald S. 'Lost' smear-positive pulmonary tuberculosis cases: where are they and why did we lose them? Int J Tuberc Lung Dis. 2005;9(1):25–31. PMID: 15675546. [PubMed] [Google Scholar]
- 73.Mehta K., Kumar A.M.V., Chawla S., Chavda P., Selvaraj K., Shringarpure K.S. 'M-TRACK' (mobile phone reminders and electronic tracking tool) cuts the risk of pre-treatment loss to follow-up by 80% among people living with HIV under programme settings: a mixed-methods study from Gujarat. India. Glob Health Action. 2018;11(1) doi: 10.1080/16549716.2018.1438239. PMID: 29482468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McBrien K.A., Ivers N., Barnieh L., Bailey J.J., Lorenzetti D.L., Nicholas D. Patient navigators for people with chronic disease: a systematic review. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0191980. PMID: 29462179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waitt C.J., Squire S.B. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15(7):871–885. doi: 10.5588/ijtld.10.0352. PMID: 21496360. [DOI] [PubMed] [Google Scholar]
- 76.Thomas B.E., Thiruvengadam K., Kadam D S.R., Ovung S., Sivakumar S. Smoking, alcohol use disorder and tuberculosis treatment outcomes: a dual co-morbidity burden that cannot be ignored. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0220507. PMID: 31365583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pednekar M.S., Hakama M., Gupta P.C. Tobacco use or body mass–do they predict tuberculosis mortality in Mumbai, India? Results from a population-based cohort study. PLoS One. 2012;7(7):e39443. doi: 10.1371/journal.pone.0039443. PMID: 22848354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Havlir D.V., Kendall M.A., Ive P., Kumwenda J., Swindells S., Qasba S.S. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–1491. doi: 10.1056/NEJMoa1013607. PMID: 22010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blanc F.X., Sok T., Laureillard D., Borand L., Rekacewicz C., Nerrienet E. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–1481. doi: 10.1056/NEJMoa1013911. PMID: 22010913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdool Karim S.S., Naidoo K., Grobler A., Padayatchi N., Baxter C., Gray A.L. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. PMID: 22010915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad N., Ahuja S.D., Akkerman O.W., Alffenaar J.C., Anderson L.F., Baghaei P. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi: 10.1016/S0140-6736(18)31644-1. PMID: 30215381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Subbaraman R., Andrews J. Nutrition and tuberculosis. In: Sharma SK, editor. Textbook of Tuberculosis & Nontuberculous Mycobacterial Diseases. 3rd ed. Jaypee Brothers Medical Publishers; New Delhi: 2019. pp. 529–539. editor. [Google Scholar]
- 83.Sinha P., Davis J., Saag L., Wanke C., Salgame P., Mesick J. Undernutrition and Tuberculosis: Public Health Implications. J Infect Dis. 2019;219(9):1356–1363. doi: 10.1093/infdis/jiy675. PMID: 30476125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ambaw F., Mayston R., Hanlon C., Medhin G., Alem A. Untreated depression and tuberculosis treatment outcomes, quality of life and disability, Ethiopia. Bull World Health Organ. 2018;96(4):243–255. doi: 10.2471/BLT.17.192658. PMID: 29695881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas B., Watson B., Senthil E.K., Deepalakshmi A., Balaji G., Chandra S. Alcohol intervention strategy among tuberculosis patients: a pilot study from South India. Int J Tuberc Lung Dis. 2017;21(8):947–952. doi: 10.5588/ijtld.16.0693. PMID: 28786805. [DOI] [PubMed] [Google Scholar]
- 86.Reis-Santos B., Shete P., Bertolde A., Sales C.M., Sanchez M.N., Arakaki-Sanchez D. Tuberculosis in Brazil and cash transfer programs: a longitudinal database study of the effect of cash transfer on cure rates. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212617. PMID: 30794615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carter D.J., Daniel R., Torrens A.W., M N.S., Maciel E.L.N., Bartholomay P. The impact of a cash transfer programme on tuberculosis treatment success rate: a quasi-experimental study in Brazil. BMJ Glob Health. 2019;4(1) doi: 10.1136/bmjgh-2018-001029. PMID: 30740248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klein K., Bernachea M.P., Irribarren S., Gibbons L., Chirico C., Rubinstein F. Evaluation of a social protection policy on tuberculosis treatment outcomes: a prospective cohort study. PLoS Med. 2019;16(4) doi: 10.1371/journal.pmed.1002788. PMID: 31039158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Acha J., Sweetland A., Guerra D., Chalco K., Castillo H., Palacios E. Psychosocial support groups for patients with multidrug-resistant tuberculosis: five years of experience. Glob Public Health. 2007;2(4):404–417. doi: 10.1080/17441690701191610. PMID: 19283636. [DOI] [PubMed] [Google Scholar]
- 90.Yin J., Yuan J., Hu Y., Wei X. Association between directly observed therapy and treatment outcomes in multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150511. PMID: 26930287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H., Ehiri J., Yang H., Tang S., Li Y. Impact of community-based DOT on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0147744. PMID: 26849656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Subbaraman R., de Mondesert L., Musiimenta A., Pai M., Mayer K.H., Thomas B.E. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health. 2018;3 doi: 10.1136/bmjgh-2018-001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thomas B., Kumar V., Chiranjeevi M., Ramachandran G., Periyasamy M., Khandewale A.S. Updated findings from an evaluation of the accuracy of 99DOTS a digital technology for monitoring tuberculosis medication adherence in HIV co-infected and uninfected patients (Abstract SOA-13-1132-01) Int J Tuberc Lung Dis. 2019;23(10):S318. [Google Scholar]
- 94.Thomas B., Kumar V., Chiranjeevi M., Ramachandran G., Murugesan P., Khandewale A.S. Understanding challenges TB patients face in using digital adherence technologies (Abstract PS-11-616-31) Int J Tuberc Lung Dis. 2019;23(10):S236. [Google Scholar]
- 95.Mohammed S., Glennerster R., Khan A.J. Impact of a daily SMS medication reminder system on tuberculosis treatment outcomes: a randomized controlled trial. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0162944. PMID: 27802283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iribarren S., Beck S., Pearce P.F., Chirico C., Etchevarria M., Cardinale D. TextTB: a mixed method pilot study evaluating acceptance, feasibility, and exploring initial efficacy of a text messaging intervention to support TB treatment adherence. Tuberc Res Treat. 2013;2013 doi: 10.1155/2013/349394. PMID: 24455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu X., Lewis J.J., Zhang H., Lu W., Zhang S., Zheng G. Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients: a cluster-randomised trial. PLoS Med. 2015;12(9) doi: 10.1371/journal.pmed.1001876. PMID: 26372470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoeli E., Rathauser J., Bhanot S.P., Kimenye M.K., Mailu E., Masini E. Digital health support in treatment for tuberculosis. N Engl J Med. 2019;381(10):986–987. doi: 10.1056/NEJMc1806550. PMID: 31483974. [DOI] [PubMed] [Google Scholar]
- 99.Story A., Aldridge R.W., Smith C.M., Garber E., Hall J., Ferenando G. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393(10177):1216–1224. doi: 10.1016/S0140-6736(18)32993-3. PMID: 30799062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nunn A.J., Phillips P.P., Mitchison D.A. Timing of relapse in short-course chemotherapy trials for tuberculosis. Int J Tuberc Lung Dis. 2010;14(2):241–242. PMID: 20074418. [PubMed] [Google Scholar]
- 101.Romanowski K., Baumann B., Basham C.A., Ahmad Khan F., Fox G.J., Johnston J.C. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19(10):1129–1137. doi: 10.1016/S1473-3099(19)30309-3. PMID: 31324519. [DOI] [PubMed] [Google Scholar]
- 102.Velayutham B., Chadha V.K., Singla N., Narang P., Gangadhar Rao V., Nair S. Recurrence of tuberculosis among newly diagnosed sputum positive pulmonary tuberculosis patients treated under the Revised National Tuberculosis Control Programme, India: a multi-centric prospective study. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200150. PMID: 29979738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bestrashniy J., Nguyen V.N., Nguyen T.L., Pham T.L., Nguyen T.A., Pham D.C. Recurrence of tuberculosis among patients following treatment completion in eight provinces of Vietnam: a nested case-control study. Int J Infect Dis. 2018;74:31–33. doi: 10.1016/j.ijid.2018.06.013. PMID: 29944930. [DOI] [PubMed] [Google Scholar]
- 104.Thomas A., Gopi P.G., Santha T., Chandrasekaran V., Subramani R., Selvakumar N. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis. 2005;9(5):556–561. PMID: 15875929. [PubMed] [Google Scholar]
- 105.Cox H., Kebede Y., Allamuratova S., Ismailov G., Davletmuratova Z., Byrnes G. Tuberculosis recurrence and mortality after successful treatment: impact of drug resistance. PLoS Med. 2006;3(10):1836–1843. doi: 10.1371/journal.pmed.0030384. PMID: 17020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Imperial M.Z., Nahid P., Phillips P.P.J., Davies G.R., Fielding K., Hanna D. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708–1715. doi: 10.1038/s41591-018-0224-2. PMID: 30397355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marx F.M., Floyd S., Ayles H., Godfrey-Faussett P., Beyers N., Cohen T. High burden of prevalent tuberculosis among previously treated people in Southern Africa suggests potential for targeted control interventions. Eur Respir J. 2016;48(4):1227–1230. doi: 10.1183/13993003.00716-2016. PMID: 27390274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pasipanodya J.G., McNabb S.J., Hilsenrath P., Bae S., Lykens K., Vecino E. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. doi: 10.1186/1471-2458-10-259. PMID: 20482835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pasipanodya J.G., Miller T.L., Vecino M., Munguia G., Garmon R., Bae S. Pulmonary impairment after tuberculosis. Chest. 2007;131(6):1817–1824. doi: 10.1378/chest.06-2949. PMID: 17400690. [DOI] [PubMed] [Google Scholar]
- 110.Hnizdo E., Singh T., Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55(1):32–38. doi: 10.1136/thorax.55.1.32. PMID: 10607799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Allwood B., van der Zalm M., Makanda G., Mortimer K. The long shadow post-tuberculosis. Lancet Infect Dis. 2019;19(11):1170–1171. doi: 10.1016/S1473-3099(19)30564-X. PMID: 31657778. [DOI] [PubMed] [Google Scholar]
- 112.Marx F.M., Dunbar R., Hesseling A.C., Enarson D.A., Fielding K., Beyers N. Increased risk of default among previously treated tuberculosis cases in the Western Cape Province, South Africa. Int J Tuberc Lung Dis. 2012;16(8):1059–1065. doi: 10.5588/ijtld.11.0506. PMID: WOS:000306678800013. [DOI] [PubMed] [Google Scholar]
- 113.Cox V., Cox H., Pai M., Stillo J., Citro B., Brigden G. Health care gaps in the global burden of drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23(2):125–135. doi: 10.5588/ijtld.18.0866. PMID: 30808447. [DOI] [PubMed] [Google Scholar]
- 114.Bateganya M.H., Amanyeiwe U., Roxo U., Dong M. Impact of support groups for people living with HIV on clinical outcomes: a systematic review of the literature. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S368–S374. doi: 10.1097/QAI.0000000000000519. PMID: 25768876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bekker L.G., Myer L., Orrell C., Lawn S., Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96(4):315–320. PMID: 16670804. [PubMed] [Google Scholar]
- 116.Prost A., Colbourn T., Seward N., Azad K., Coomarasamy A., Copas A. Women's groups practising participatory learning and action to improve maternal and newborn health in low-resource settings: a systematic review and meta-analysis. Lancet. 2013;381(9879):1736–1746. doi: 10.1016/S0140-6736(13)60685-6. PMID: 23683640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization (WHO). Global tuberculosis report. Geneva: World Health Organization, 2019. Contract No.: WHO/CDS/TB/2019.15.
- 118.Noykhovich E., Mookherji S., Roess A. The risk of tuberculosis among populations living in slum settings: a systematic review and meta-analysis. J Urban Health. 2018 doi: 10.1007/s11524-018-0319-6. PMID: 30341562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tahseen S., Shahnawaz H., Riaz U., Khanzada F.M., Hussain A., Aslam W. Systematic case finding for tuberculosis in HIV-infected people who inject drugs: experience from Pakistan. Int J Tuberc Lung Dis. 2018;22(2):187–193. doi: 10.5588/ijtld.17.0390. PMID: 29506615. [DOI] [PubMed] [Google Scholar]
- 120.Gupta A., Mbwambo J., Mteza I., Shenoi S., Lambdin B., Nyandindi C. Active case finding for tuberculosis among people who inject drugs on methadone treatment in Dar es Salaam. Tanzania. Int J Tuberc Lung Dis. 2014;18(7):793–798. doi: 10.5588/ijtld.13.0208. PMID: 24902554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bourdillon P.M., Goncalves C.C., Pelissari D.M., Arakaki-Sanchez D., Ko A.I., Croda J. Increase in tuberculosis cases among Prisoners, Brazil, 2009-2014. Emerg Infect Dis. 2017;23(3):496–499. doi: 10.3201/eid2303.161006. PMID: 28221118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dhavan P., Dias H.M., Creswell J., Weil D. An overview of tuberculosis and migration. Int J Tuberc Lung Dis. 2017;21(6):610–623. doi: 10.5588/ijtld.16.0917. PMID: 28482955. [DOI] [PubMed] [Google Scholar]
- 123.Ndlovu N., Musenge E., Park S.K., Girdler-Brown B., Richards G., Murray J. Four decades of pulmonary tuberculosis in deceased South African miners: trends and determinants. Occup Environ Med. 2018;75(11):767–775. doi: 10.1136/oemed-2017-104806. PMID: 29934377. [DOI] [PubMed] [Google Scholar]
- 124.Adams L.V., Basu D., Grande S.W., Craig S.R., Patridge M.T., Panth N. Barriers to tuberculosis care delivery among miners and their families in South Africa: an ethnographic study. Int J Tuberc Lung Dis. 2017;21(5):571–578. doi: 10.5588/ijtld.16.0669. PMID: 28399973. [DOI] [PubMed] [Google Scholar]
- 125.Sharma N., Kundu D., Dhaked S., Das A. Silicosis and silicotuberculosis in India. Bull World Health Organ. 2016;94(10):777–778. doi: 10.2471/BLT.15.163550. PMID: 27843169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alele F.O., Franklin R.C., Emeto T.I., Leggat P. Occupational tuberculosis in healthcare workers in sub-Saharan Africa: a systematic review. Arch Environ Occup Health. 2019;74(3):95–108. doi: 10.1080/19338244.2018.1461600. PMID: 29702035. [DOI] [PubMed] [Google Scholar]
- 127.Solomon S.S., McFall A.M., Lucas G.M., Srikrishnan A.K., Kumar M.S., Anand S. Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: a cross-sectional, community-based analysis. PLoS Med. 2017;14(11) doi: 10.1371/journal.pmed.1002460. PMID: 29182638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Reid M.J.A., Arinaminpathy N., Bloom A., Bloom B.R., Boehme C., Chaisson R. Building a tuberculosis-free world: the Lancet Commission on tuberculosis. Lancet. 2019;393(10178):1331–1384. doi: 10.1016/S0140-6736(19)30024-8. PMID: 30904263. [DOI] [PubMed] [Google Scholar]
- 129.Agins B.D., Ikeda D.J., Reid M.J.A., Goosby E., Pai M., Cattamanchi A. Improving the cascade of global tuberculosis care: moving from the "what" to the "how" of quality improvement. Lancet Infect Dis. 2019 doi: 10.1016/S1473-3099(19)30420-7. PMID: 31447305. [DOI] [PubMed] [Google Scholar]
- 130.Vesga J.F., Hallett T.B., Reid M.J.A., Sachdeva K.S., Rao R., Khaparde S. Assessing tuberculosis control priorities in high-burden settings: a modelling approach. Lancet Glob Health. 2019;7(5):e585–e595. doi: 10.1016/S2214-109X(19)30037-3. PMID: 30904521. [DOI] [PubMed] [Google Scholar]