Abstract
The present study aimed at investigating the effect of enzymatic hydrolysis times (40-240 min) with alcalase and pancreatin in enzyme-substrate ratio (2% w/w) on the hydrolysis degree, electrophoresis bands, antioxidant properties and chelating activities of iron and copper ions of bioactive peptides derived from defatted Bunium persicum Bioss. (black cumin) press cake. The hydrolysis degree was enhanced by increasing the process time using both enzymes. Both hydrolysis of the enzymes were led to producing peptides with low molecular weight (less of 10 kDa). The DPPH• radical scavenging activity was more influenced by peptides hydrolyzed by alcalase. But, the products hydrolyzed by pancreatin had a higher inhibitory effect on the ABTS•+ cationic radical than alcalase hydrolysis. The primary protein reducing power was reached the highest level after enzymatic hydrolysis by alcalase and pancreatin, respectively, for 200 and 240 min. Following the use of proteins hydrolyzed by alcalase and pancreatin, the production of thiobarbituric acid reactive substances was also diminished from 0.45 to 0.42 and 0.38 (mg MDA/L emulsion), respectively. After assessing the iron ion chelating, a higher level of activity was observed in the alkaline-derived enzyme hydrolysis samples. Furthermore, the highest amount of copper ion chelating was obtained after hydrolyzing the enzymes for 200 min.
Keywords: Food science, Antioxidant, Extraction, Enzyme hydrolysis, Degree of hydrolysis, Chelating, Black cumin
Food science, Antioxidant, Extraction, Enzyme hydrolysis, Degree of hydrolysis, Chelating, Black cumin.
1. Introduction
Oxidation is considered as one of the fundamental variables influencing food perishing and lessening its storage stability. This procedure is especially perceptible in formulations containing lipid or oily phases (Jamdar et al., 2010). In order to prevent lipid oxidation, particularly in food products and emulsions, synthetic antioxidants (BHA, BHT and propyl gallates) are used in various industries. However, give to to the potential risks, the use of these antioxidants is limited or banned in some food products and in some countries. Thus, natural antioxidants are widely used among the people (Shahidi and Zhong, 2015).
Antioxidant peptides are regarded as healthy and safe compounds with properties, such as low molecular weight, low cost, high activity and easy to absorb. In contrast to the synthetic antioxidants, these antioxidants can also be ascribed to the higher stability in different conditions, safety, nutritional value and high functionality. The antioxidant activity of bioactive peptides can be assigned to the capability to inhibit free radicals, to prevent the peroxidation of lipids, and the chelating of metallic ions (Shahidi and Zhong, 2015). Likewise, the antioxidant activity of these peptides is affected by their structure and amino acid sequence. Among plant and animal sources appropriate for producing hydrolyzed proteins, plants can be considered as the most accessible sources with respect to their lower cost and less allergenicity (Sarmadi and Ismail, 2010).
Nowadays, there is a drastically increasing demand for the use of food processing waste along with a variety of health and medical compounds. The products and waste arisen out of the seed oil are considered as one of these sources (Feyzi et al., 2017). Even though the waste and press cake resulted from seed oils are extremely practical source of protein, they are mostly discarded or utilized for livestock. There are a considerable number of studies conducted in this field. After investigating the impact of the enzymatic hydrolysis of black bean protein concentrates utilizing pepsin and alcalase on the antioxidant activity of hydrolyzates, Evangelho et al. (2016) concluded that there was a higher level of antioxidant activity for inhibiting the radical ABTS•+ by hydrolyzing the enzyme with alcalase. Likewise, the hydrolyzates obtained by hydrolysis with pepsin had the higher radical DPPH• scavenging. Mao et al. (2011) assessed the anti-inflammatory and inhibition of free radicals (DPPH•, superoxide and hydrogen peroxide) of yak milk casein prior or subsequent to the enzymatic hydrolysis (Mao et al., 2011). Their results demonstrated that the casein hydrolyzed by alcalase had the highest radical DPPH• scavenging compared to other treatments. The evaluation of free radical scavenging activity of papain-catalyzed casein plasteins performed by Zhao et al. (2010) indicated that radical scavenging activities was expanded by all casein plasteins.
The Black cumin (Bunium persicum Bioss.) is believed to be a common medicinal seeds, which has been used extensively for centuries. The proteins embodied in the cumin seeds alongside other compounds assume a significant role in protecting the skin from free radicals. With regard to the chemical composition, there is a substantial amount of protein content, around 3-20%, in the black cumin (Al-Jasass and Al-Jasser, 2012). The black cumin is comprised of 5-4% essential oil, in which 45-65% of carvone is found. Furthermore, there is a blend of ketone, carbon, a terpen and a modest quantity of carvacrol in the black cumin essential oil. Recent studies have revealed that there was approximately 5.8–10.3% essential oil in the black cumin (the effect of a specific planting climate), which was planted in Poland, and there existed around 7.49–6.70% ash, 4.10–5.85% essential oil and 9.95%–22.14% fatty oil and 9.23–18.21% protein nitrogen content in the fruit composition of the plant, and about 56.0–58.9 percent carvone was observed in its essential oil (Ramadan, 2007). The particular function and activity of enzymes under delicate reaction conditions can be regarded as their most significant characteristic (Sarmadi and Ismail, 2010). On the contrary, considering that the cold pressed oil is disposed of as waste or used for livestock despite its high nutritional composition and rich protein content, it is critically important to extract protein and reuse it as a source of nutritional, antioxidant and bioactive compounds. Consequently, with regard to the advantages and applications of producing and using bioactive peptides derived from hydrolysis of enzymes, the present study aimed at assessing the feasibility of protein extraction and the use of waste resulted from defatted Bunium persicum Bioss. press cake, as well as investigating the effect of enzymatic hydrolysis and characteristics of the antioxidant of hydrolyzed peptide.
2. Materials and methods
2.1. Materials
The waste of oil extracted from Bunium persicum Bioss. (Iranian black cumin) was prepared from the oil extraction centers in Gorgan, Iran. All chemicals used in this study were also purchased from Merck and Sigma (Germany).
2.2. Sample preparation
So as to defat it, the dried waste was blended with hexane at a ratio of 1:4 (w/v) and was stirred at the room temperature for 3 h. Then, the hexane was isolated by the buccaneer. The resulting waste powder was dried at the room temperature and went through a 40 mesh sieve (Feyzi et al., 2017).
2.3. Protein extraction
The following procedure explains the extraction of protein resulted from defatted powder. The cumin powder was blended (1:10 ratio) with a 0.33 M solution of sodium chloride (pH = 9.25) and then stirred for 2 h. Afterwards, the solution was centrifuged at 4500 g for 30 min. In the next step, the pH of the supernatant was adjusted to pH 4.5 (pH of isoelectric protein) with 1 mol.L−1 HCl. Then, in order to precipitate the proteins, the solution was centrifuged at 4500 rpm for 20 min. The precipitated protein was washed twice with distilled water and centrifuged at 4500 g for 5 min. Afterwards, by adjusting to pH = 7.2 and adding 1 M sodium hydroxide, it was transformed into soluble form. In subsequent, the isolated protein was dried with a freeze-drying and was kept at 4 °C. All processes were carried out at the room temperature (Feyzi et al., 2017).
2.4. Protein extraction efficiency
With the aim of assessing the effectiveness of protein extracted from Iranian black cumin press cake, the amount of protein existed in the extracted material was estimated after that freeze-drying was performed and then its weight ratio to the protein found in the press cake (before being extracted) was determined by using micro-Kjeldahl (Jamdar et al., 2010).
2.5. Preparation of hydrolyzed protein
In the enzymatic hydrolysis process, the protein extracted from the cumin waste at a concentration of 5% w/v was dissolved in 0.2 M phosphate buffer (pH = 7.4) and hydrated during constant stirring for 30 min at the room temperature. Then, the primary solution of the pancreatin enzyme in the aforementioned buffer was added to the enzyme-substrate ratio of 2% w/w. The reaction temperature was 40 °C for pancreatin and the reaction time was viewed as steady under stirred conditions at 200 rpm between 40-240 min. Once the hydrolysis process was completed, the reaction medium was put in a 90 °C water bath for 15 min so as to disable the reaction and activity of the enzyme. Afterwards, the solution was cooled to the ambient temperature. The solution was centrifuged at 5000 rpm for 10 min. The supernatant was isolated and lyophilized until it being used at -20 °C. All of the above steps were carried out for enzymatic hydrolysis using alcalase in a buffer with pH = 8 and a temperature at 50 °C (Chen et al., 2012).
2.6. Determine the degree of hydrolysis
To determine the degree of hydrolysis, the hydrolyzed suspension and trichloroacetic acid (0.44 M) were mixed in a 1:1 volume ratio and incubated for 15 min at 4 °C. Then, the mixture was centrifuged (Heidolph, Germany) at 10000 rpm for 10 min. The Bradford method (1976) was used to determine the amount of protein in supernatant containing trichloroacetic acid (0.22 M). Finally, the degree of hydrolysis was estimated with regard to the amount of protein embodied in supernatant containing TCA to the protein presented in the suspension (after dilution with distilled water in an equal volume) and they were reported in percentages.
2.7. Electrophoresis test
For electrophoresis, the samples were mixed with buffer containing bromophenol blue in a 1:4 ratio. The resulting samples were boiled for 5 min. 12.5% polyacrylamide gel was prepared between two glass plates. After setting lower gel, the upper gel (4%) was prepared and injected into the glass plates. The amount of 20 μl of each sample was poured into the gel wells. The gel was placed inside the electrophoresis tank. The first fixed voltage of 70 V was adjusted to reach the ends of the gel (about 20 min). Then, the voltage of the device was adjusted to 120 V and the electrophoresis (V16, LabRepCo, USA) was performed for 80 min until the blue color of bromophenol blue reached to the bottom of the glass plates. Afterward, the electrophoresis were terminated and the gel was separated gently from the plates and placed in a brilliant blue R 250 coat solution to spend a night in the shaker. The next day, the gel was removed from the staining solution and in order to eliminate the background color, it was placed in a bleached solution. After a few hours, the background color was disappeared and protein bands appeared, which their molecular weights was estimated with regard to the bands (Abeyrathne et al., 2016).
2.8. DPPH• radicals scavenging assay
The method presented by Wu et al. (2003) with insufficient modifications was applied to determine the percentage of DPPH• free radical scavenging (Wu et al., 2003). The hydrolyzed powders were primarily dissolved in distilled water (at a concentration of 40 mg/ml). Then, 1.5 ml of each sample was blended with 1.5 ml of ethanolic DPPH• (0.15 mM) solution and the vortex operation performed in 20 s. The resulting mixture was then centrifuged at 2500 rpm for 10 min and stored in darkness for 20 min. The absorbance of the supernatant was read at 517 nm. The percentage of DPPH• radical containment was estimated using the following formula [1]:
| I (%) = [A blank- A sample/ A blank] × 100 | [1] |
Where, Ablank was control absorbance (the same volume of distilled water is blended with the DPPH• solution instead of the sample solution). Asample was sample absorbance.
2.9. Determination of ABTS•+ radical scavenging ability
The methods introduced by You et al. with some modifications were applied to measure the ABTS•+ inhibitory activity of the hydrolyzates (You et al., 2009). ABTS•+ radical solution was prepared by combining a similar volume ratio of ABTS (at a concentration of 7 mM) with 2.45 mM potassium persulfate. The mixture was placed in a dark place and at room temperature for 12-16 h prior to use. During this time, the oxidation and ABTS•+ radical production was carried out by potassium persulfate. Prior to testing, the ABTS•+ solution was diluted with PBS (pH 7.4, 0.2 M) to get an absorbance of 0.7 ± 0.02 at 734 nm. Then, 40 μl of each sample (at a concentration of 4 mg/ml) was added to 4 ml of diluted ABTS•+ solution. The mixture was stirred for 30 s and placed in a dark place for 6 min. The absorbance of the final solution was measured at 734 nm. The standard curve was prepared by reacting 40 μl of trolox (50, 100, 250, 500, 750 and 1000 μM) along with 4 ml of diluted ABTS•+ solution. The percentage of ABTS•+ radical scavenging of the samples was calculated in accordance with the following equation. Moreover, the ABTS•+ radical inhibitory activity was expressed based on the standard trolox curve, with respect to the equivalent antioxidant capacity of the trolox.
| %AA = [A blank- A sample/ A blank] × 100 | [2] |
Where, Ablank is control absorbance and Asample is absorption of the hydrolyzed sample.
2.10. Antioxidant activity in emulsion
Antioxidant activity was performed in the emulsion by homogenizing 1 g of corn oil and 100 μl of Tween 20 with 100 ml of distilled water using a homogenizer at a speed of 22000 rpm for 2 min in an ice bath. So as to assess the lipid oxidation by blending 8 ml of oil emulsion, 0.5 ml of 0.2% ascorbic acid solution, 0.5 ml of FeSO4 ppm and 1 ml of soluble of the hydrolyzed protein, the sample preparation was performed.
Then, the treatments were incubated at 37 °C for 16 h. After incubation, 1 ml of each sample was added to 2 ml of thiobarbituric acid/trichloroacetic acid solution (20 mM TBA/15% TCA) and 50 μl of BHA 10% solution in 90% ethanol, and then it was cooled. The resulting blend was incubated for 15 min in a 90 °C water bath. Afterward, the samples were cooled in an ice-water bath for 10 min and then centrifuged at 3000 rpm for 15 min at 5 °C. The absorbance of the solution was measured at a wavelength of 532 nm. To prepare a control sample, 1 ml of distilled water was mixed with 2 ml of TBA/TCA solution. The amount of TBARS was expressed as milligram of malondialdehyde (MDA) per liter of emulsion (Abeyrathne et al., 2016).
2.11. Ferric reducing power determination
So as to specify the reducing power of hydrolyzed samples, 0.5 ml of a hydrolyzed sample dissolved in distilled water (at a concentration of 40 mg/ml) along with 0.5 ml of 0.2 mM phosphorus (pH 6.6) and 0.5 ml of potassium ferricyanide of 1% w/v mixture were incubated at 50 °C for 20 min. Then, 0.5 ml of 10% trichloroacetic acid solution was added to the mixture and centrifuged for 10 min at 2500 rpm. Finally, 1 ml supernatant was mixed with 1 ml distilled water and 0.2 ml ferric chloride (0.1% w/w). Sample adsorption was read at 700 nm after mixing for 10 min at ambient temperature. The same volume of distilled water was used as a sample for the preparation of control samples. It was indicated that reducing power was enhanced by increasing the absorbance of the reactive mixture (Ahmadi et al., 2007).
2.12. Hydroxyl radical scavenging activity
The method developed by Kim and Minamikawa (1997) was applied to examine the hydroxyl radical scavenging activity. For this test, 0.2 ml of 10 mM FeSO4-EDTA were mixed with 0.5 ml 2-deoxy-d-ribose (10 mM), 0.2 ml hydrolyzed samples, 0.9 ml sodium phosphate buffer (0.2 M, pH 7.4) and 0.2 ml of 10 mM hydrogen peroxide. The mixture was incubated at 37 °C for 1 h. Then, 1 ml of 2.8% trichloroacetic acid (TCA) and 1 ml of 1% thiobarbituric acid were added to the mixture and consequently, the reaction was stopped. The mixture was placed in a boiling water bath for 15 min, then it was cooled in ice. A spectrophotometer was used to read the sample absorption, which it was at532 nm (Ultrospec 2000, England). The results that were expressed in the percentage of hydroxyl radical scavenging were measured by using the following equation:
| %Inhibition = (1- As/Ab) × 100 | [3] |
Where, Ab is the absorption of the control sample and As is sample absorption.
2.13. Iron chelating activity
The iron ion chelating activity was determined according to Decker and Welch (1990) method [28]. First, 1 ml of the sample dissolved in distilled water (at a concentration of 40 mg/ml) was mixed with 0.05 ml of iron (II) chloride (2 mM) and 1.85ml of double distilled water. Afterward, 0.1 ml of ferrozine solution (5 mM) was added and the mixture was strongly stirred. The adsorption was measured after mixing for 10 min at an ambient temperature at 562 nm. Double distillation water was used as a control sample. The chelating activity of the samples was calculated using the following equation:
| Chelating activity = [(A control – A sample/A control)] × 100 | [4] |
Where, Ablank is absorption of the control sample without the active compound and Asample is absorption of the hydrolyzed sample.
2.14. Copper ion chelating activity
Copper ion chelating activity was measured by using Kong and Xiong method for the hydrolyzed proteins (Kong and Xiong, 2006). First, 1 ml of 0.2 mM copper sulfate solution was mixed with 1ml of the hydrolyzed solution in 15 ml of falcon and incubated for 5 min at room temperature. Then, 1 ml of trichloroacetic acid solution was added 11.3 % and the samples were centrifuged at 2500 rpm for 10 min. Subsequently, 2 ml of supernatant was added to 1 ml of 10% pyridine and 20 μL of pyrocathechol violet. The vortex mixture was incubated for 5 min at room temperature. The absorbance of the samples was read at 632 nm and the activity of copper ion was determined using the following equation:
| Copper chelating activity (%) = [1 – (A sample/A blank)] × 10 | [5] |
Where, Ablank is the absorption of the control sample without the active compound and Asample is the absorption of the hydrolyzed sample.
2.15. Statistical analysis
Three replications of all tests were performed. Mean and standard deviation (SD) of the data were calculated. In order to identify the effective factors statistically, the effects of each treatment and variables were evaluated using one-way ANOVA and SPSS software version 19. Afterward, comparison of the meanings was done by using Duncan's multiple range test to examine the significance of the variables effect (P < 0.05).
3. Results and discussion
3.1. Extraction efficiency and degree of hydrolysis
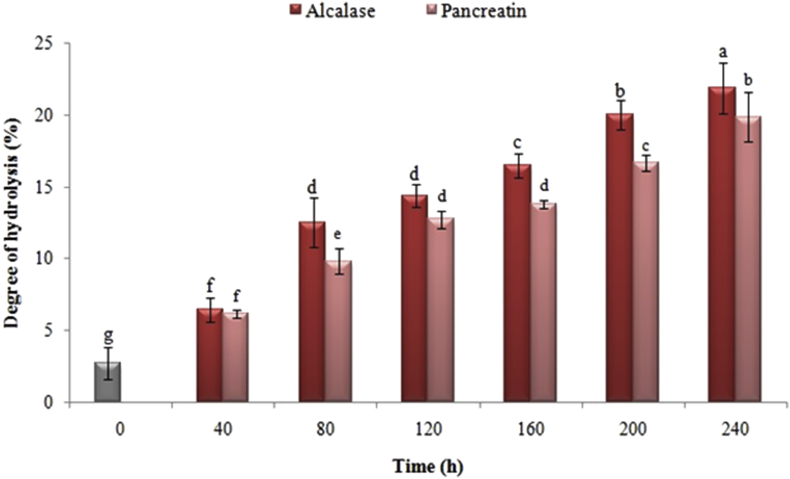
In this research, the extraction efficiency was around 45% with respect to the initial protein content in Iranian black cumin and the extracted samples. Figure 1 shows the effect of time and type of enzyme on the degree of hydrolysis of Iranian black cumin protein. The sample at 0 h was associated with the non-hydrolyzed black cumin protein. The positive effect of hydrolysis at 0 h was due to the use of HCl during the process of protein extraction that very minor hydrolysis was happened and also a small amount of amino acids was released (Feyzi et al., 2017). As it is shown in Figure 1, the degree of hydrolysis was significantly influenced by the process time. By increasing the activity time of each of the enzymes up to 240 min, the degree of hydrolysis was particularly increased. Also, at certain time intervals, the index for the samples hydrolyzed by alcalase was higher than pancreatin, although this trend was not observed at all intervals.
Figure 1.
Effect of enzyme hydrolysis time of black cumin proteins with pancreatin and alcalase on degree of hydrolysis. Averages with the same letters indicate that there is no significant difference at the 5% level.
The degree of hydrolysis is a significant indicator in determining the functional properties of hydrolyzed proteins (Kristinsson and Rasco, 2000). The degree of hydrolysis was significantly influenced by the process time (You et al., 2009). Thus, the chain length of the peptides became shorter and the molecular weight distribution decreased by increasing the degree of hydrolysis and as a result of the break of the peptide bands. Therefore, the amount of free amino acids increased (Ahmadi et al., 2007). This finding was in accordance with the results of research by You et al. (2009), Mao et al. (2011), Jamdar et al. (2010), Zhao et al. (2010) and Chen et al. (2011), in which the effect of various proteases on the degree of hydrolysis of loach fish proteins (papain and protamix), yak milk casein (trypsin, pepsin, alcalase and papain), peanut (alcalase), casein plasteins (papain) and soy protein isolate (pancreatin) was respectively investigated.
3.2. Effect of the enzymatic hydrolysis process on electrophoretic bands
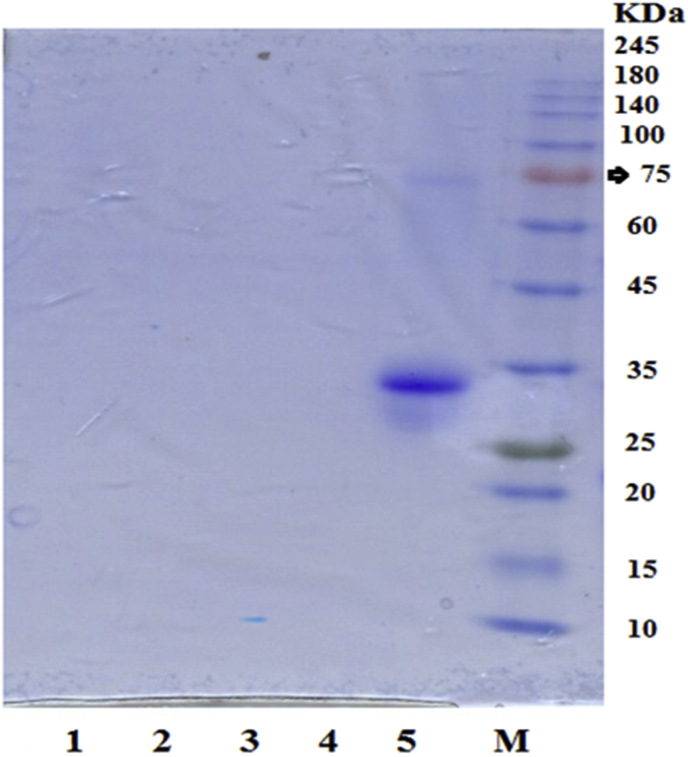
Figure 2 shows the gel electrophoresis image obtained from the initial black cumin protein samples and its samples hydrolyzed by alcalase and pancreatin during different periods. The results indicated the presence of bonds of primary protein from black cumin in the molecular weights, respectively, within the range of 75 kDa (strength bonds), 60-70 kDa (weak bands), 32 kDa (strength band) and weak bands between 25-30 kDa. In the hydrolyzed samples, there was observed a weak band in the molecular weight within the range of 10 kDa at 80 min just for the proteins hydrolyzed by pancreatin. In other samples, no bands were observed, suggesting a significant reduction in the molecular weight and the breakdown of peptide chains to di-peptides and amino acids.
Figure 2.
Image of gel electrophoresis (SDS-PAGE) and bands formed from samples in molecular weight. M) Marker 15% Tris-Glycine, 5) Hydrolyzed cumin protein; hydrolyzed proteins; 4) alkaline 80 min; 3) pancreatin 80 min; 2) alcalase 200 min; 1) pancreatin 200 min.
The study of electrophoresis images is considered as another indicator of the breakdown and hydrolysis of peptides (Abeyrathne et al., 2016). For instance, the hydrolysis of ovomucin, under different enzymatic and thermal treatments, was conducted by Abeyrathne et al. (2016). In their study, the degree of hydrolysis and the conditions for the formation of peptide bands in gel electrophoresis was evaluated. In ovomucin hydrolyzed by pepsin and trypsin, small and pale bands were found, implying the incomplete hydrolysis and the production of small peptides. Though, there was no bond in the ovomuci samples hydrolyzed by alkaline during 3–24 h, which implies the complete hydrolysis of the protein. In another study, Luo et al. (2014) observed small, uniform, and pale areas, along with the transfer of bands to lower molecular weight fractions after sodium caseinate was hydrolyzed by papain. However, in the case of pancreatin-treated and trypsin-treated treatments, there were several distinct bands, which indicate a peptide break in several parts, and a distribution of different molecular weights. The results of this study also confirmed the findings of the degree of hydrolysis.
3.3. Effect of enzyme type and process time on DPPH• free radical scavenging
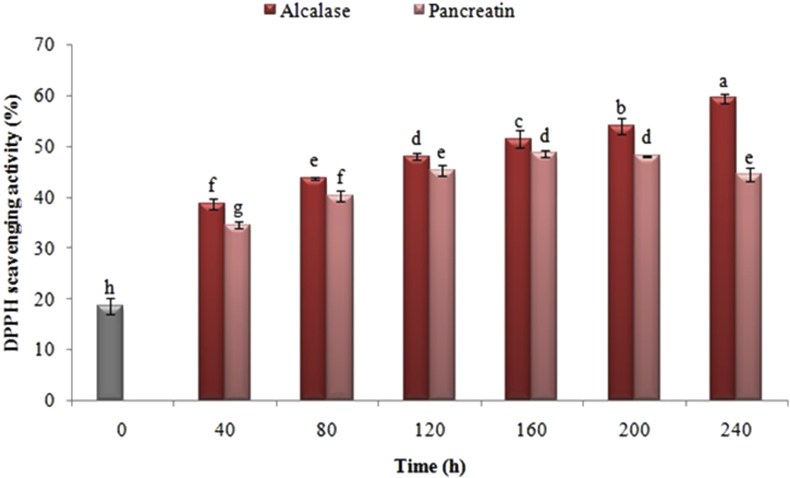
The results of DPPH• radical inhibitory activity measurement in protein extracted of black cumin and its hydrolyzed products are presented in Figure 3. The findings proposed that non-hydrolyzed black cumin protein had a little ability in the DPPH• radical inhibition. Nevertheless, the free radical scavenging was enhanced to roughly 2 times (P < 0.05) through the enzymatic treatment of cumin protein for 40 min. In the hydrolyzed treatments with pancreatin, DPPH• free radical inhibitory activity was enhanced by increasing hydrolysis time to 160 min, yet the extension of hydrolysis of enzyme represented a decreasing impact on this indicator. As opposed to the hydrolysis of the enzyme with pancreatin, extending the time of hydrolysis with alcalase constantly brought about increased DPPH• free radical inhibitory activity. Among the used enzymes, the samples produced with alcalase had a higher activity than pancreatin (P < 0.05).
Figure 3.
Effect of time of black cumin protein enzymatic hydrolysis by alcalase and pancreatin on DPPH• Free radical scavenging. The same letters indicate that there is no significant difference at the 5% level.
The capacity to respond with free radicals and create stable species, which reduces oxidation, is regarded as one of the characteristics of antioxidants (You et al., 2009). Subsequently, there are numerous researches applied DPPH• free radical to assess the antioxidant activity of different reducing agents. The antioxidant activity of the samples was enhanced by intensifying the hydrolysis process time following increasing the degree of hydrolysis and releasing more hydrophobic and active peptides and amino acids. Considering that the peptides and hydrophobic amino acids had shown a faster response with DPPH• radicals, these compounds have a higher capacity to inhibit this radical in comparison to various hydrophilic types. The reason for this activity can be ascribed to an enhancement in the groups of lateral chains containing hydrophobic amino acids, which makes it easier for these peptides to be promptly accessible to respond with DPPH• free radicals (Je et al., 2005). Nevertheless, the reason for the decrease in the free DPPH• free radical capability, which is caused by the high hydrolysis rate, can be ascribed to the complete hydrolysis of peptides, leading to the complete release and the high availability of hydrophilic amino acids (You et al., 2009). Moreover, polarization reduces the reaction of amino acids to DPPH• radical (Zhao et al., 2010).
3.4. Effect of the enzymatic hydrolysis process on free radical inhibitors of ABTS•+
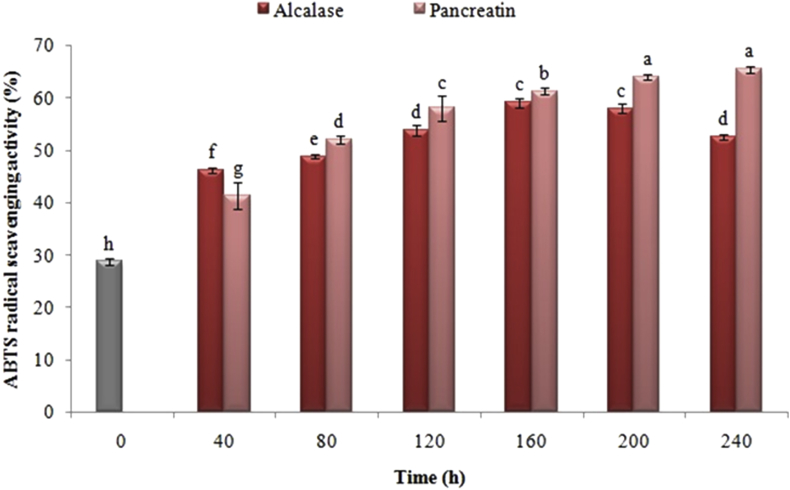
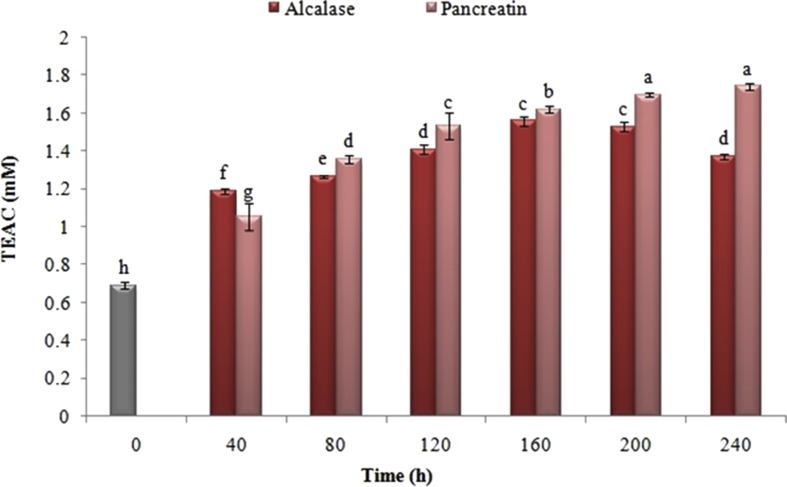
Figures 4 and 5 exhibit the effect of the enzymatic hydrolysis time and the type of enzyme utilized in the ABTS•+ inhibitory and the equivalent antioxidant capacity of the Trolox, respectively. The radical scavenging ability of ABTS•+ was increased substantially following the enzymatic hydrolysis for 40 min by using any of pancreatin and alcalase enzymes (P < 0.05).
Figure 4.
Effect of enzymatic hydrolysis time by pancreatin and alcalase on free radical absorption activity of ABTS•+ of black cumin proteins. The same letters indicate that there is no significant difference at the 5% level.
Figure 5.
Effect of enzymatic hydrolysis time with pancreatin and alcalase on antioxidant capacity equivalent to Trolox of black cumin proteins. The same letters indicate that there is no significant difference at the 5% level.
In contrast to the hydrolyzed treatments with alcalase, the samples hydrolyzed with pancreatin had higher ABTS•+ cationic radical inhibitory activity and Trolox equivalent antioxidant capacity during all of the enzymatic hydrolysis period. Additionally, the amount of these indicators was influenced by the time of hydrolysis process. Contrary to the results of the DPPH• assay, the proteins hydrolyzed with pancreatin were increased the ABTS•+ radical scavenging activity due to increased release of hydrophilic amino acids and antioxidant. However, among the samples hydrolyzed by alkaline, the hydrolyzed samples following 160 min had the highest antioxidant activity and radical scavenging of ABTS•+ (P < 0.05), which was diminished by increasing the hydrolysis time.
The assessment of the ABTS•+ radical scavenging capacity to be used in both types of hydrophilic and lipophilic compounds is broadly applied as an antioxidant activity assay (Miliauskas et al., 2004). Antioxidant activity, cationic and water-soluble radical inhibitory ability of ABTS•+, like the other indices, rely upon the type of protease enzyme, the degree of hydrolysis, and the amino acid composition of the peptides. Considering that the difference in the type of solubility between DPPH• radicals (fat soluble) and ABTS•+ (water soluble) radicals was significant, the type of amino acid composition is influential on inhibiting each of these radicals. Accordingly, the reason for these changes can be ascribed to the enhancement in the release of hydrophobic amino acids as well as to the reduction in the accessibility of hydrophilic antioxidants to react with the ABTS•+ radical (You et al., 2009).
3.5. Effect of the enzymatic hydrolysis process on antioxidant activity in emulsion
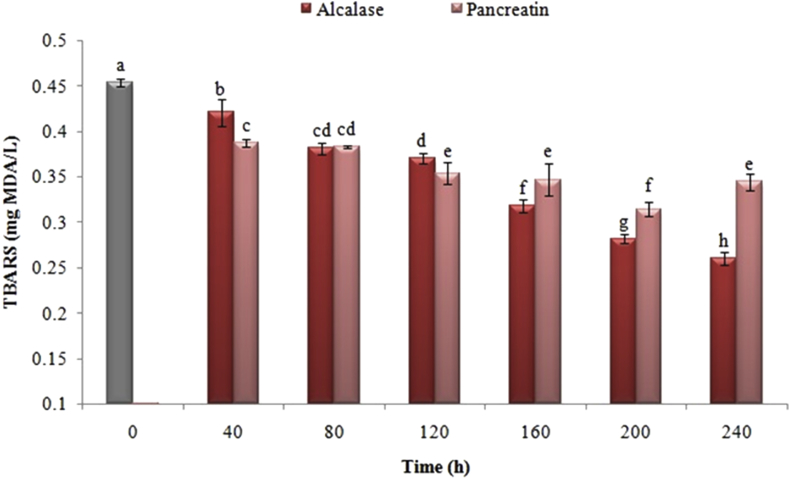
Figure 6 illustrates the impact of different enzymatic hydrolysis of Iranian black cumin protein with pancreatin and alcalase on the production of reactive tiobarbituric acid (TBARS) compounds in the emulsion system of oil in water. The results indicated that enzymatic hydrolysis had a significant impact on decreasing the production of TBARS. Among the samples hydrolyzed by pancreatin and alcalase, the highest scavenging activity of the secondary compounds of oxidation were observed at 200 and 240 min, respectively.
Figure 6.
Effect of time of enzymatic hydrolysis of black cumin protein with pancreatin and alcalase on inhibition activity of secondary oxidation products. The same letters indicate that there is no significant difference at the 5% level.
Measuring the reactive compounds of thiobarbituric acid or TBARS (as secondary oxidation products based on the measurement of malondialdehyde) is a common way to assess the standard methods so as to estimate the amount of oxidation in lipids (Abeyrathne et al., 2016). In the food industry, synthetic antioxidants (BHA, BHT and propyl gallate) are utilized to avoid lipid oxidation. However, there is an increasing inclination toward using natural antioxidants such as peptides from enzyme hydrolysis due to the potential risks of using the synthetic antioxidants (Pihlanto, 2006). There is an enormous number of studies conducted on the impact and mechanism of peptides actions, which taken from the enzymatic hydrolysis of various proteins on the oxidative stability of food emulsions. For instance, there is an assessment performed on the inhibitory impact of lipoxygenase activity by various casein sections (Wang and Xiong, 2005). The results indicated that beta-casein digested by trypsin or casein digested by both trypsin and subtilisin kept its inhibitory properties. As a whole, the production of TBARS was diminished the peptides derived from hydrolysis of enzymes through two methods, including inhibiting the various free radicals and chelating of metal, as well as the formation of membranes and films around droplets of emulsions and preventing the access of lipids to oxidative agents (Wang and Xiong, 2005). Besides, there are various factors such as the amino acid composition of peptides and chain length, as well as the ability to inhibit free radicals, the chelating of metal ions of proxidants in peptides derived from hydrolyzed black cumin affecting on the stability of food emulsions and the production of secondary compounds derived from lipid oxidation.
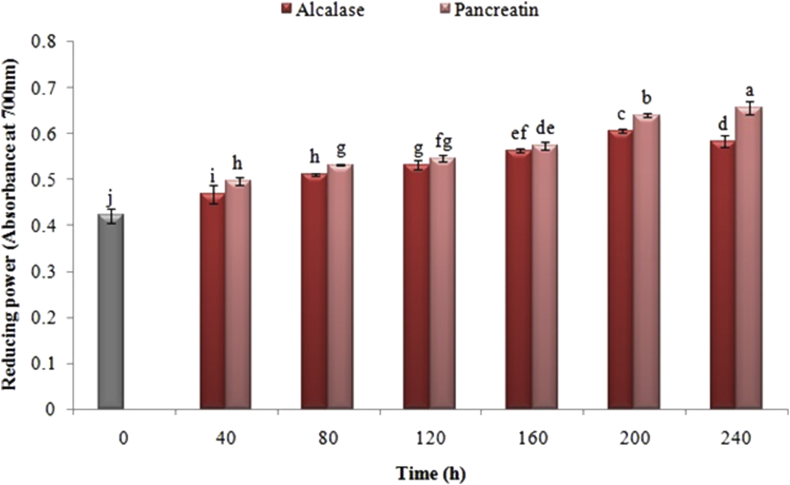
3.6. Effect of enzyme type and process time on ferric reducing power
Figure 7 represents the reducing power of black cumin proteins hydrolyzed by pancreatin and alcalase enzymes at different times. The statistical results showed that there was a significant increase in Fe+3 reducing power following just hydrolysis for 40 min. The amount of this index was enhanced by increasing the enzyme hydrolysis time up to 200 min by using both enzymes. Although this amount in hydrolyzed alkaline samples was reduced when the hydrolysis time was increased to more than 200 min, there was no effect on the hydrolyzed samples by pancreatin.
Figure 7.
Effects of time of enzymatic hydrolysis of black cumin protein by pancreatin and alcalase on hydrolyzed reducing power. The same letters indicate that there is no significant difference at the 5% level.
The reduction of ferric (Fe+3) in the form of ferrous (Fe+2) can be considered as one of the most important properties of antioxidants in food samples (Dorman et al., 2003). The antioxidant activity of peptides taken from the enzymatic hydrolysis of various proteins has been reported by various studies. The reason for the enhancement in the ferric reducing power of hydrolyzed proteins can be ascribed to the breakdown of peptide chains, increased release of amino acids with antioxidant activity (such as tryptophan, methionine, lysine, histidine and tyrosine), and the capacity for hindering free radicals (Jamdar et al., 2010). In the same vein, You et al. (2009) were evaluated the impact of enzymatic digestion on the hydrolyzed proteins of loach fish by papain and Protamex. The most noteworthy reducing power was identified with the proteins hydrolyzed by papain. The increased regenerative capacity can be ascribed to the increased hydrogen or electron capability of peptides and amino acids (You et al., 2009).
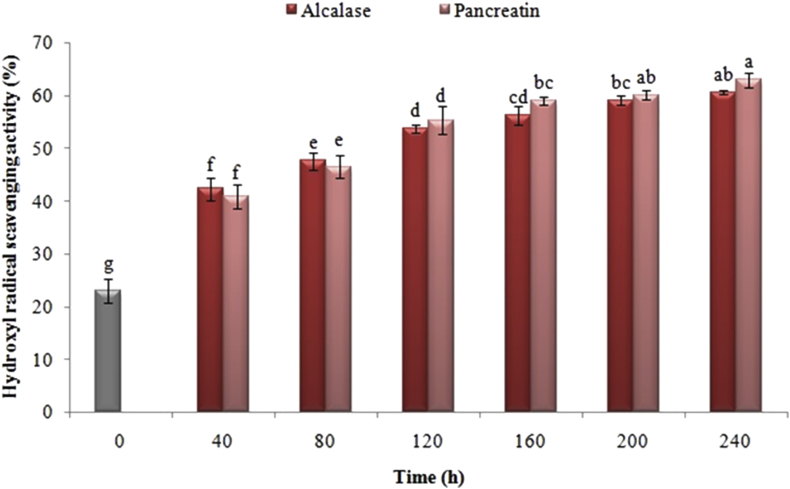
3.7. Effect of the enzymatic hydrolysis process on radical hydroxyl scavenging activity
Figure 8 illustrates the impact of enzymatic hydrolysis by pancreatin and alcalase on Iranian black cumin at various time intervals on the radical hydroxyl inhibition activity. According to the results of this study, there was an increase of roughly 2 times in the value of this index following hydrolyzing the sample for 40 min through any of the aforementioned enzymes. In addition, the radical hydroxyl inhibition activity in the samples hydrolyzed with pancreatin and alcalase was enhanced by increasing hydrolysis time. Moreover, there was no difference between the samples hydrolyzed with pancreatin when compared with alcalase regarding the radical hydroxyl inhibition activity (P < 0.05).
Figure 8.
Effects of time of enzymatic hydrolysis of black cumin protein with pancreatin and alcalase on radical hydroxyl scavenging activity of black cumin proteins. The same letters indicate that there is no significant difference at the 5% level.
Radical hydroxyl can be regarded as one of the active oxygen species produced in the human body, which it can quickly respond with biological molecules such as amino acids, proteins, and DNA, consequently, led to physiological disorders (Cacciuttolo et al., 1993). Accordingly, the body can be protected against the radical hydroxyl by eliminating them (Je et al., 2005). The antioxidant property of proteins was enhanced by increasing the amino acids and active groups after enzymatic hydrolysis. As an example, free radicals existed in the milk and whey can be scavenged by amino acids such as tyrosine and cysteine (Pihlanto, 2006). Moreover, there are some researches conducted on histidine-containing peptides, which concluded that these peptides are utilized as metallic ion chelating agents, active oxygen scavengers, and radical hydroxyl inhibitors. Besides, the capacity to free radicals with the antioxidant activity in food systems was likewise protonated by aromatic amino acids like tryptophan (Chen et al., 2012). In the same vein, Chen et al. (2012) has studied the impact of time and degree of hydrolysis of egg white proteins on hydroxyl radicals, which is in accordance with the results of the present study (Chen et al., 2012). Furthermore, this study yielded results that were in conformity with those of You et al. (2009), in which the extensive hydrolysis of Misgurnus anguillicaudatus proteins by papain and Protamex was reported. They concluded that the enhancement in hydrolysis degree typically caused an improvement in the activity of radical hydroxyl scavenging (You et al., 2009).
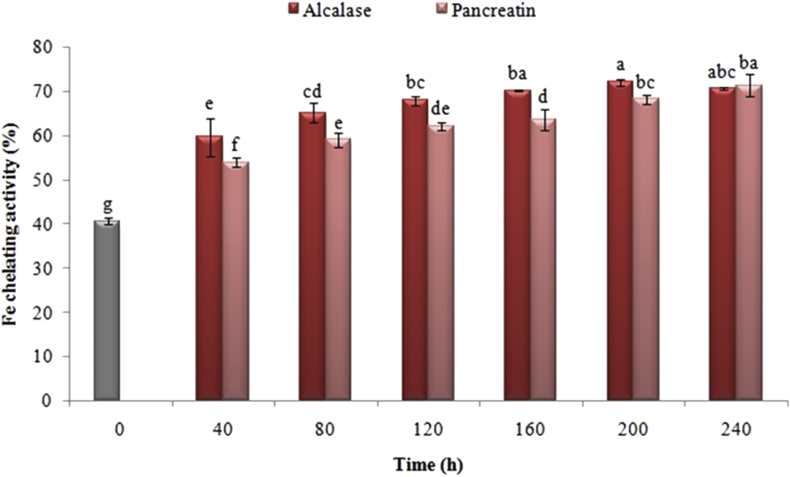
3.8. Effect of enzyme type and process time on iron ion chelating activity
Figure 9 illustrates the impact of various enzymatic hydrolysis of the extracted proteins by pancreatin and alcalase on iron ion chelating activity. The results indicated that iron chelating activity in the protein that extracted, respectively, from 40.57% to 53.86% and 59.54% be enhanced just following the enzymatic hydrolysis of black cumin protein with pancreatin and alcalase for 40 min. As a whole, the iron ion chelating activity was extended by intensifying the hydrolysis time in both enzymes. Among the hydrolyzed treatments, the samples hydrolyzed by alkaline-derived enzyme were more active and had more chelating activity (P < 0.05).
Figure 9.
Effects of time of enzymatic hydrolysis of black cumin protein with pancreatin and alcalase on hydrolyzed iron ion chelating activity. The same letters indicate that there is no significant difference at the 5% level.
Due to the accelerated procedure of lipid hydroperoxides breakdown into highly reactive alkoxyl radicals, iron and other metals perform as proxidants in numerous food systems (McClements and Decker, 2000). The capacity of hydrolyzed proteins, which taken from various sources in iron chelating, relies upon the kind of utilized enzyme, the nature of the initial protein and its degree of hydrolysis (Pihlanto, 2006). Each of the protein sources has amino acid sequences and these sequences had different impacts on the chelating activity of metal ions. For instance, among various protein sources, caseins that contain polar portions is comprised of phosphorylated residues and serine-serine-serine-glutamic acid-glutamic acid sequences. These parts perform as efficient cleavers through creating a complex of calcium, iron, and zinc (Wang and Xiong, 2005). Similar results were reported by Jamdar et al. (2010) in studying the effect of conditions and degree of hydrolysis on the iron ion chelating of peanut hydrolyzed proteins, but no mechanism was found for describing the function of peptides and products derived from hydrolysis of enzymes (Jamdar et al., 2010).
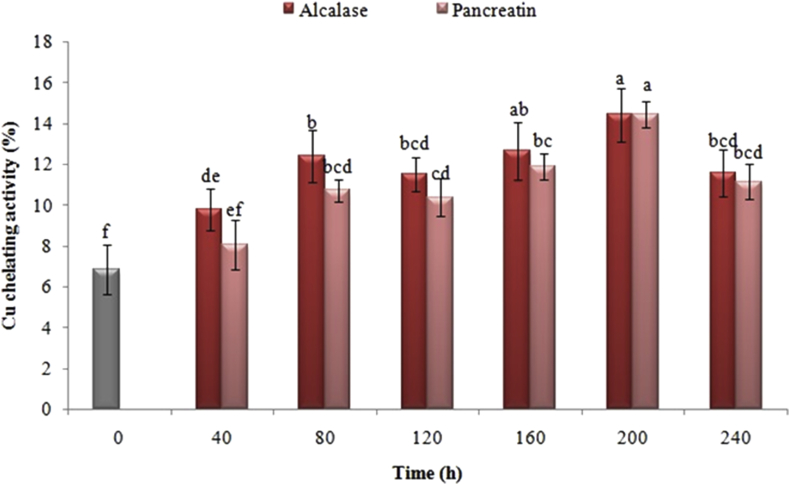
3.9. Effect of the enzymatic hydrolysis process on copper ion chelating activity
As it is illustrated in Figure 10, the hydrolysis time and type of enzyme affected on the copper ion chelating activity in the hydrolyzed proteins. The results indicated that enzyme hydrolysis had a significant impact on improving this capacity. Following the hydrolysis with pancreatin and alcalase for 40 min, the activity of Cu2+chelating in black cumin protein was subsequently enhanced from 6.82% to 8.04 and 9.78%, respectively. Moreover, this capacity was improved by enzyme hydrolysis with pancreatin in the given period. Following the hydrolysis for 200 min, the highest percentage of chelating activity was acquired just in the event that the hydrolyzed samples done by alcalase, and consquently, the hydrolyzed activity was diminished by intensifying the hydrolysis time. Among the examined enzymes during the same periods of hydrolysis, there was also no significant difference between the copper ion chelating activity (P < 0.05).
Figure 10.
Effects of time of enzymatic hydrolysis of black cumin proteins with pancreatin and alcalase on hydrolyzed copper ion chelating activity. The same letters indicate that there is no significant difference at the 5% level.
As a whole, the chelating of metals relies upon the kind of enzyme, the degree of hydrolysis and the amino acid composition of the raw material. For instance, peptides that obtained by enzymatic hydrolysis of ovomucin indicated a lower chelating of copper ion than the control sample, in spite of the high iron ion chelating activity (optimal treatment of more than 80%) (Abeyrathne et al., 2016). In accordance with You et al. (2009), the results of the present study showed that there was an increase in the percentage of Cu2+ chelating due to rising the degree of hydrolysis of pancreatin-digested loach proteins (You et al., 2009). There is a possibility to ascribe the mechanism of action of the peptides, which are produced by enzymatic hydrolysis in copper ion chelating to the enhancement in the carboxylic free groups, effective amino acids like histidine comprising the imidazole ring and ionic reactions (Zhu et al., 2008). Neverthelese, there are some studies that revealed an enhancement in the degree of hydrolysis and protein breaking can diminish the antioxidant activity of peptides. In short, peptides with 5–19 amino acids assume a more prominent role in avoidance and antioxidant activity in hindering linoleic acid and lipids from auto-oxidation (Chen et al., 2012).
4. Conclusions
The results indicated that enzymatic hydrolysis of protein from Bunium persicum Biosswaste had a significant impact on the antioxidant properties and metal chelating activity. Moreover, each of the alcalase and pancreatin enzymes had various impacts on the studied indices. The DPPH• radical scavenging activity was more influenced by peptides, which were taken from hydrolysis of alcalase. On the contrary, the products resulted from the enzymatic hydrolysis with pancreatin exhibited higher ABTS•+ inhibitory effect than the alkaline hydrolysis. Hydroxyl radical scavenging activity and reducing power were additionally influenced by the activity of both enzymes. For instance, these parameters were negatively affected by long-term hydrolysis (more than 200 min) with alcalase. Furthermore, the production of reactive thiobarbituric acid compounds (TBARS) was diminished following the use of alkaline and pancreatin in hydrolyzing the black cumin protein. On the contrary, the chelating activity of metal ions (iron and copper) was likewise influenced by the activity of both enzymes. After assessing these indices, it can be concluded that the highest activity of chelating, particularly in iron ion inhibition, was associated with the hydrolysis by alcalase. Consequently, bioactive peptides taken from enzymatic hydrolysis of Iranian black cumin waste protein were suitable for the antioxidant properties and the chelating activity of metal ions, along with the capacity to produce, enrich, and formulate various food products in an effort to improve the general health.
Declarations
Author contribution statement
Zahra Shahi: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Seyyedeh Z. Sayyed-Alangi: Analyzed and interpreted the data; Wrote the paper.
Leila Najafian: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors gratefully acknowledge the support of Sari Branch, Islamic Azad University, Sari, Iran.
References
- Abeyrathne E.D., Lee H.Y., Jo C., Suh J.W., Ahn D.U. Enzymatic hydrolysis of ovomucin and the functional and structural characteristics of peptides in the hydrolysates. Food Chem. 2016;192:107–113. doi: 10.1016/j.foodchem.2015.06.055. [DOI] [PubMed] [Google Scholar]
- Ahmadi F., Kadivar M., Shahedi M. Antioxidant activity of Kelussia odoratissima Mozaff. in model and food systems. Food Chem. 2007;105(1):57–64. [Google Scholar]
- Al-Jasass F.M., Al-Jasser M.S. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci. World J. 2012 doi: 10.1100/2012/859892. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cacciuttolo M.A., Trinh L., Lumpkin J.A., Rao G. Hyperoxia induces DNA damage in mammalian cells. Free Radic. Biol. Med. 1993;14(3):267–276. doi: 10.1016/0891-5849(93)90023-n. [DOI] [PubMed] [Google Scholar]
- Chen C., Chi Y.J., Zhao M.Y., Xu W. Influence of degree of hydrolysis on functional properties, antioxidant and ACE inhibitory activities of egg white protein hydrolysate. Food Sci. Biotechnol. 2012;21(1):27–34. [Google Scholar]
- Chen L., Chen J., Ren J., Zhao M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J. Agric. Food Chem. 2011;59(6):2600–2609. doi: 10.1021/jf103771x. [DOI] [PubMed] [Google Scholar]
- Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38(3):674–677. [Google Scholar]
- Dorman H.J.D., Koşar M., Kahlos K., Holm Y., Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003;51(16):4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- Evangelho J.A.d., Berrios J.d.J., Pinto V.Z., Antunes M.D., Vanier N.L., Zavareze E.d.R. Antioxidant activity of black bean (Phaseolus vulgaris L.) protein hydrolysates. Food Sci. Technol. 2016;36:23–27. [Google Scholar]
- Feyzi S., Varidi M., Zare F., Varidi M.J. Effect of drying methods on the structure, thermo and functional properties of fenugreek (Trigonella foenum graecum) protein isolate. J. Sci. Food Agric. 2017;98(5):1880–1888. doi: 10.1002/jsfa.8669. [DOI] [PubMed] [Google Scholar]
- Jamdar S., Rajalakshmi V., Pednekar M., Juan F., Yardi V., Sharma A.J.F.C. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121(1):178–184. [Google Scholar]
- Je J.Y., Park P.J., Kim S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005;38(1):45–50. [Google Scholar]
- Kim J.W., Minamikawa T. Hydroxyl radical-scavenging effects of spices and scavengers from brown mustard (Brassica nigra) Biosc. Biotech. Biochem. 1997;61(1):118–123. [Google Scholar]
- Kong B., Xiong Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006;54(16):6059–6068. doi: 10.1021/jf060632q. [DOI] [PubMed] [Google Scholar]
- Kristinsson H.G., Rasco B.A. Fish protein hydrolysates: production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000;40(1):43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- Luo Y., Pan K., Zhong Q. Physical, chemical and biochemical properties of casein hydrolyzed by three proteases: partial characterizations. Food Chem. 2014;155:146–154. doi: 10.1016/j.foodchem.2014.01.048. [DOI] [PubMed] [Google Scholar]
- Mao X.Y., Cheng X., Wang X., Wu S.J.J.F.C. Free-radical-scavenging and anti-inflammatory effect of yak milk casein before and after enzymatic hydrolysis. Food Chem. 2011;126(2):484–490. [Google Scholar]
- McClements D.J., Decker E.A. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65(8):1270–1282. [Google Scholar]
- Miliauskas G., Venskutonis P.R., Van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85(2):231–237. [Google Scholar]
- Pihlanto A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006;16(11):1306–1314. [Google Scholar]
- Ramadan M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): an overview. Int. J. Food Sci. Technol. 2007;42(10):1208–1218. [Google Scholar]
- Sarmadi B.H., Ismail A.J.P. Antioxidative peptides from food proteins: a review. Peptides. 2010;31(10):1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Zhong Y. Measurement of antioxidant activity. Journal of Functional Foods. 2015;18:757–781. [Google Scholar]
- Wang L.L., Xiong Y.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food Chem. 2005;53(23):9186–9192. doi: 10.1021/jf051213g. [DOI] [PubMed] [Google Scholar]
- Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36(9-10):949–957. [Google Scholar]
- You L., Zhao M., Cui C., Zhao H., Yang B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innovat. Food Sci. Emerg. Technol. 2009;10(2):235–240. [Google Scholar]
- Zhao X.H., Wu D., Li T.J. Preparation and radical scavenging activity of papain-catalyzed casein plasteins. Dairy Sci. Technol. 2010;90(5):521–535. [Google Scholar]
- Zhu L.J., Chen J., Tang X., Xiong Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008;56(8):2714–2721. doi: 10.1021/jf703697e. [DOI] [PubMed] [Google Scholar]