Graphical abstract
Keywords: Alpha-synuclein, SH-SY5Y cells, Rotenone, Graphene oxide, Parkinson’s disease, Cell senescence
Highlights
-
•
α-Synuclein (αS) is a synaptic protein up-regulated in Parkinson’s disease.
-
•
SH-SY5Y neuroblastoma cells were engineered to overexpress αS at low and high levels.
-
•
High-αS overexpression stimulates cell proliferation and delay senescence.
-
•
Low-αS overexpression causes toxicity, oxidative stress, and accelerates senescence.
-
•
A fine-tuned up-regulation of αS is critical for neuronal maintenance and survival.
Abstract
Alpha-Synuclein (aSyn) is a chameleon-like protein. Its overexpression and intracellular deposition defines neurodegenerative α-synucleinopathies including Parkinson’s disease. Whether aSyn up-regulation is the cause or the protective reaction to α-synucleinopathies remains unresolved. Remarkably, the accumulation of aSyn is involved in cancer. Here, the neuroblastoma SH-SY5Y cell line was genetically engineered to overexpress aSyn at low and at high levels. aSyn cytotoxicity was assessed by the MTT and vital-dye exclusion methods, observed at the beginning of the sub-culture of low-aSyn overexpressing neurons when cells can barely proliferate exponentially. Conversely, high-aSyn overexpressing cultures grew at high rates while showing enhanced colony formation compared to low-aSyn neurons. Cytotoxicity of aSyn overexpression was indirectly revealed by the addition of pro-oxidant rotenone. Pretreatment with partially reduced graphene oxide, an apoptotic agent, increased toxicity of rotenone in low-aSyn neurons, but, it did not in high-aSyn neurons. Consistent with their enhanced proliferation, high-aSyn neurons showed elevated levels of SMP30, a senescence-marker protein, and the mitosis Ki-67 marker. High-aSyn overexpression conferred to the carcinogenic neurons heightened tumorigenicity and resistance to senescence compared to low-aSyn cells, thus pointing to an inadequate level of aSyn stimulation, rather than the aSyn overload itself, as one of the factors contributing to α-synucleinopathy.
Introduction
Alpha-Synuclein (aSyn) is an intrinsically disordered and primarily monomeric protein in its soluble form. Usually 140 amino acids long, it is predominantly expressed in the presynaptic terminals and nuclei of neurons, reflected in its name synuclein [1]. This aggregation-prone protein shows the characteristic of structural plasticity, shifting from a disordered random coil in the cytosol to a tetrameric α-helix [2], [3] when associated with membrane phospholipids [4]. The exact role of aSyn in the nervous system has not yet been completely elucidated. Under physiological conditions, the bulk of aSyn is present in neuronal processes and nerve terminals in close proximity with the plasmatic membrane. Accordingly, aSyn may play a role in synaptic vesicle release, synaptic plasticity, membrane trafficking [5], [6], and neurotransmission [6].
aSyn came into the spotlight when its aggregation and deposition in the form of Lewy bodies (LBs) was found inside neurons and glial cells of a group of neurodegenerative diseases called α-synucleinopathies [7], [8]. LBs are the pathological hallmark of Parkinson disease (PD), Parkinson disease with dementia; dementia with LBs, multiple system atrophy, and Hallervorden-Spatz’s disease [9]. Because multiplicity of the synuclein-alpha gene (i.e., the SNCA) gene coding for aSyn causes a juvenile form of PD [10], [11], it is traditionally believed that abnormal accumulation of aSyn promotes its aggregation and neurotoxicity [12]. However, SCNA gene dosage does not always correlate with α-synucleinopathy [13], [14], [15]. aSyn aggregates and diffuse accumulation occurs with aging in neurologically healthy patients [16]. Although, it is presumed that aging-induced intracellular deposits of aSyn represent a pre-clinical stage of the synucleinopathy [8], [17], [18], some evidence also suggests that aging does not have an additive role in aSyn-induced neurodegeneration [19]. To address this contradiction, a multi-hit hypothesis [20] points to a combination of aSyn aggregation with several factors as the triggering factor of α-synucleinopathy. This raises the question of whether the formation of LBs is adaptive and neuroprotective [21] or by contrast, it is a pathological reaction [22] to the origin of α-synucleinopathy.
A neglected aspect in the analyses of α-synucleinopathies is why aSyn is also overexpressed in CNS tumors [23], [24]. Neurodegeneration shares common pathways with oncogenesis [25], [26], [27], [28], [29], [30], [31] and accordingly aSyn may be involved in both processes [32], [33], [34]. It has been suggested that levels of soluble aSyn may be pivotal for the balance between cell viability and cell death [19], [35], [36], [37]. As a proof of concept, it was investigated if the levels of aSyn overexpression could alter the proliferation rate and cell viability of human SH-SY5Y neuroblastoma cells, a cancer cell line frequently chosen to explore PD mechanisms because of its robust dopaminergic phenotype [38]. With this goal in mind, SH-SY5Y cells were engineered ad hoc to permanently overexpress different quantities of wild-type aSyn.
Material and methods
Reagents
Lipofectamine™ 2000 transfection reagent, Dulbecco’s modified Eagle minimum essential/Ham’s F-12 (DMEM/F12) plus Glutamax™ media, fetal bovine serum (FBS), sodium pyruvate, L-glutamine, penicillin G/streptomycin mix, RIPA buffer, and enzyme-free PBS-based cell dissociation buffer were purchased from Gibco (Carlsbad, CA, USA). Noble agar, Crystal Violet dye, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT); non-essential amino acids and protease inhibitor cocktail were acquired from Roche (Palo Alto, CA, USA). β-Mercaptoethanol and rotenone (Rot) were purchased to Sigma-Aldrich (St Louis, MO, USA). Partially reduced graphene oxide (PRGO) was prepared by Abalonyx AS (Oslo, Norway). Graphene oxide (GO) was prepared from natural graphite powder following the modification of the hummers method as follows. An aqueous slurry of GO was dried on a plastic substrate to prepare a GO film. This film was then heated slowly (1 °C/min) to 300 °C in open air to obtain the PRGO film.
Human SH-SY5Y neuroblastoma cell cultures
In-house stock of the human neuroblastoma SH-SY5Y cell line was routinely grown to reach the necessary confluence (over 80%) in DMEM/F12 + glutamax™ medium containing 10% fetal bovine serum (FBS), 4% non-essential amino acids, 1% penicillin/streptomycin, 4.5 g/l glucose, 0.1% amphotericin B, and sodium pyruvate. Cell cultures were maintained at 37 °C in normoxia (5% of CO2). Medium was exchanged every 3 days during cell growth, and cultures were passed when confluent once or twice per week. Cells were harvested using enzyme-free PBS-based cell dissociation buffer. A collection of images was taken from living cell cultures after 2 weeks under a contrast phase filter using an inverted microscope (PrimoVert; Zeiss GmbH; Overkochen; Germany) and digital imaging software (Axiovision 40 V 4.2.8.0; Zeiss GmbH) when indicated.
Generation of stably aSyn-transfected cell lines
Untagged, full-length human aSyn (SNCA GenBank ID: BC108275) cDNA (Clone ID: 6147966), inserted into pcDNA™ 3.1Zeo (+) plasmid was a generous gift from Prof. José González-Castaño (Universidad Autónoma de Madrid). SH-SY5Y cells that underwent less than 5 passages after thawing of a stock culture were transfected with the wild-type human SNCA gene using the transfection reagent Lipofectamine™ 2000 according to the manufacturer’s protocol. A selection of aSyn stably-transfected SH-SY5Y cells was made with Zeocin (200 ng/mL) according to manufacturer instructions. Zeocine resistant clones were picked up and verified for aSyn overexpression by immunoblotting using empty pcDNA™ 3.1Zeo (+) plasmid-transfected SH-SY5Y cells as a control. The abundant bibliography on the subject focuses on the SNCA-gene transfection as a means to overexpress aSyn and mimic the pathological cellular environment that is characteristic of PD [20]. Herein it was made an ad hoc selection of those clones with the highest (high-aSyn) and the lowest (low-aSyn) expression of aSyn, which significantly differed from the basal aSyn expression found in empty plasmid-transfected cells.
Western blotting
De-attached cells were homogenized in ice-cold RIPA buffer containing sodium orthovanadate (1 mM) and the protease inhibitor cocktail (1% NP-40; 0.5% Na deoxycholate; 0.1% SDS; PMSF 100 μg/mL; aprotinin 30 μl/mL; Na orthovanadate 1 mM; 1% Vol/Vol), incubated on ice for 30 min, and cleared by centrifugation (8000g for 10 min at 4 °C). Protein content was determined using the BCA assay (Bio-Rad, Hercules, CA; USA). Cell extract was heated at 95 °C for 10 min in the Laemmli buffer and β-mercaptoethanol, then loaded onto a 10% dodecyl sulfate (SDS)-polyacrylamide electrophoresis gel (40 µg protein/lane), electrophoresed with TRIS-glycine running buffer at 15 V/cm for 1 h, and finally transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA; USA). This membrane was incubated at room temperature in blocking buffer (0.1% of Tween 20 and 2.5% of bovine serum albumin or BSA in Tris-Buffered Saline (TBS) containing 5% of non-fat dry milk for 4 h. Subsequently, the membrane was incubated at 4 °C overnight with the primary antibodies for each antigen diluted in TBS containing 2.5% of BSA. As the primary antibodies rabbit anti-aSyn (1:1000; Millipore; AB5038P) was used; mouse monoclonal anti-SMP30 (1:500; Santa Cruz Biotechnology, G-10); and polyclonal rabbit anti-Ki-67 (1:5000; Millipore; AB9260). Rabbit anti-β-actin (1:5000; Santa Cruz, sc-130656) was used as the loading control.
Incubation with secondary antibodies was performed for 1 h at room temperature in TBS solution supplemented with 2.5% of BSA. The horseradish-peroxidase (HRP)-coupled anti-rabbit and anti-mouse IgG (1:10,000 in TBS plus 2.5% of BSA; Abcam) was used for secondary antibodies. Protein bands on the membrane were detected by the chemiluminescence method using the horseradish peroxidase SuperSignal™ WEST Dura Extended Duration Substrate (Gibco, Carlsbad, CA, USA). The analysis of bands relied on the optical densitometry using the ChemiDoc™ detector and the Image Lab™ software (Bio-Rad, Hercules, CA; USA). Image analysis and quantification of bands were conducted using the Fiji-ImageJ software (htpp://imagej.nih.gov/ij/).
Cytotoxic effects assessment
Harvested cells were seeded at a density of 5 × 104 cells per cm2 in 48-well microplates and their viability measured 24 h later. The reduction of the thiazolyl blue tetrazolium bromide (MTT) dye to formazan was taken as the initial indicator of cell viability [39]. A stock (5 mg/mL in PBS) of the MTT salt was diluted 10 folds in the cell culture medium. After a 2-hour incubation at 37 °C, the yellow MTT salt was reduced to purple formazan by active mitochondrial reductase enzymes of the living cells. The medium was then aspirated and formazan precipitate dissolved in 100 µl of pure DMSO. Aliquots were transferred to a 48-well microplate, and optical density read at 560 nm with DMSO as a blank using a microplate reader (BioTEK analyzer, Izasa Scientific, Barcelona, Spain). Cell viability was also evaluated by the vital-dye (Trypan blue) exclusion test when necessary. Trypan blue was dissolved directly into the culture medium (0.02% Vol/Vol) to evaluate the fraction of dead (Trypan blue-tangible) cells vs. total cell counts at 200-fold magnification under the light of an inverted microscope (PrimoVert; Zeiss GmbH; Overkochen; Germany). Individual cell counts were conducted using the Fiji-ImageJ software (htpp://imagej.nih.gov/ij/).
Rotenone (Rot) and partially reduced graphene oxide (PRGO) treatments
SH-SY5Y transfected clones were seeded in 96-well microplates at a concentration of 5 × 103 cells per well in phenol red-free DMEM/F12 medium supplemented with FBS (10%) and sodium pyruvate (100 mM). Half of the plates were pretreated with PRGO, which was added fresh to the medium (50 µg/mL). PRGO was chosen because it is a form of graphene that differentiates cells into neuronal lineages [40], [41]. After incubating the cells for 7 days, the medium was removed and the cells treated with Rot, which was diluted from a freshly made stock solution (0.5 mg/mL of pure DMSO) into the medium at 0; 0.1; 0.2; 0.4; and 1.2 µM concentrations (the final concentration of DMSO was 0.1%). After a 24-hour incubation, a stock of MTT reagent (5 mg/mL of PBS) was diluted 10 folds into the medium, the cells were incubated overnight, and the formazan precipitate solubilized in pure DMSO and quantified by colorimetry. Absorbance of wells having either medium or PRGO was set equal to 100% for each cell clone to normalize absorbance values of wells within the experimental treatments.
Proliferation assay
Cells were seeded in 96-well microplates (2 × 103 cells/well) and incubated at 37 °C. Cell proliferation was quantified by the MTT method at 0, 24, 48, and 72 h after seeding cells as described above. Assuming that the metabolic activity of cells is stable within short periods of time, as we determined it to be the case, an increase in the absorbance should accurately reflect changes in cell proliferation. Absorbance at the seeding time (0 h) was therefore set equal to 100% to estimate cell growth at the times set above.
Adherent colony formation assay
This method assesses the reproductive viability (clonogenicity) of single cells. It is based on the capacity of adherent cells to produce progeny in a monolayer; i.e., a colony of approximately 50 cells derived from a single cell [42]. For each experimental condition, a total of three Petri plates, containing 103 cells in 10 mL of medium each, were incubated in triplicate for 10 days at 37 °C in an atmosphere of 5% of CO2 and protected from light. Some of the cells were incubated with a conditioned medium (Cm) obtained after a 24-hour incubation with SH-SY5Y cells showing maximum overexpression of aSyn (i.e., high-aSyn). Prior to the incubation, the medium was centrifuged and filtered using a 0.2 µm Minisart®. Syringe filter (Biotech, Goettingen, Germany). At the end of incubation, plates were washed with PBS, incubated in 4%-paraformaldehyde saline buffer for 30 min, washed with PBS, and stained with crystal violet for 15 min. Once the stain was removed and plates were washed with distilled water, colonies were counted using a Colony Counter 560 (Suntex Instruments Co., Taiwan).
Soft agar colony formation assay
The soft agar assay for tumorigenicity is based on the ability of cells to proliferate in a semi-solid matrix [43]. A key advantage of this technique over the conventional 2D monolayer assay (the clonogenic assay) is that, under conditions of isolation, the lack of anchorage challenges the tumorigenic potential of cells. The plasmid or the SNCA-gene transfected SH-SY5Y cells were mixed with Noble agar prepared at 0.3% in fresh culturing medium or conditioned (Cm) medium when indicated, and seeded into 6-well plates containing a solidified bottom layer (Noble agar dissolved at 0.6% in fresh culturing medium) at a density of 104 cells/well. After 3 weeks of incubation, colonies were stained with crystal violet and counted using the Colony Counter 560 (Suntex Instruments Co., Taiwan).
Statistics
Statistical analyses were carried out using SPSS 16.0 for Windows. One-way ANOVA followed by Bonferroni post hoc test were used to determine protein differences in the WB assays. Viability (MTT) results were assessed by a two-way factorial (clone × passage) ANOVA. Student’s t-test was applied to Vital Dye Exclusion data. Two-way (clone × incubation time) ANOVA was used to analyze cell proliferation. Data from tumorigenicity and clonogenicity assays were analyzed by using one-way ANOVA. Neurotoxic treatments were scrutinized by a two-way factorial ANOVA followed by the Bonferroni test for planned post-hoc comparisons, with clone (plasmid vs. low-aSyn and high-aSyn) and treatment (vehicle & PRGO vs. Rot & Rot + PRGO) as between-subject factors. Alpha value was set at P < 0.05.
Results
Characterization of aSyn-overexpressing SH-SY5Y neurons
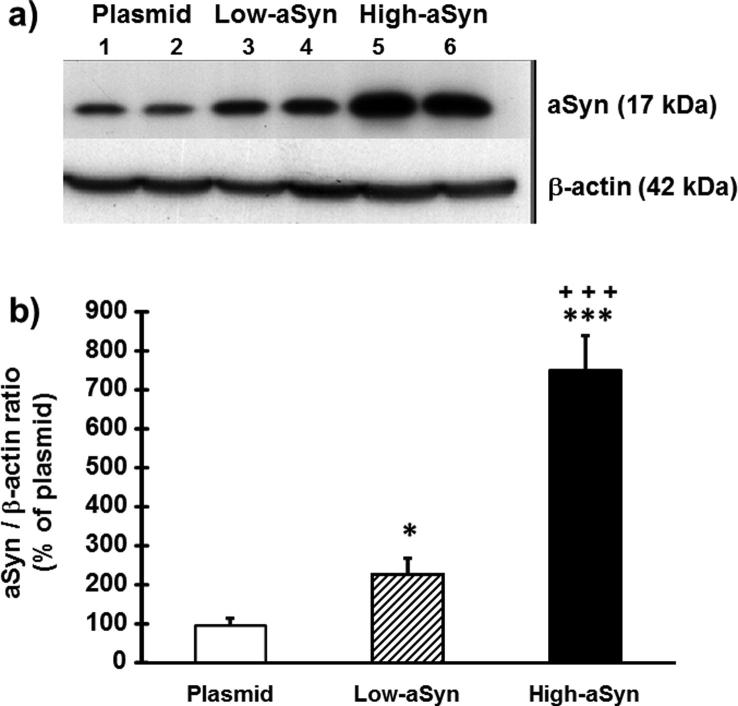
Fig. 1 shows the overexpression levels of aSyn in lysates of SNCA-transgenic SH-SY5Y cells (the aSyn clones) as a percentage of the basal expression shown by control cells transfected with empty plasmids (the plasmid clone). Based on the immunoblots (Fig. 1a), it was confirmed that SH-SY5Y cells were positively transfected with the SNCA gene (F(2,13) = 150.43, P < 0.0001; Fig. 1b). The intensity of the aSyn band changed from moderate to substantial as seen in the low-aSyn clone (125% of the plasmid clone, P < 0.05) and the high-aSyn clone (750% of the plasmid clone, P < 0.0001), respectively. Differences between both aSyn-overexpressing clones were statistically significant (P < 0.0001).
Fig. 1.
aSyn-overexpression in SCNA-gene transfected SH-SY5Y neurons. (a) Representative Western blots of positively SCNA gene transfected clones (lanes 3 and 4, low-aSyn; lanes 5 and 6, high-aSyn) compared to the empty plasmid-transfected clone (lanes 1 and 2, Plasmid). (b) aSyn expression was quantified by optical densitometry, then normalized with loading (β-actin) controls, and finally represented like the percentage of the plasmid value (100%): Values are expressed as the mean ± SD of 5 independent experiments with Bonferroni post hoc tests: **P < 0.01, ***P < 0.0001 vs. Plasmid; +++P < 0.0001 vs. low-aSyn.
Viability of aSyn-overexpressing SH-SY5Y neurons
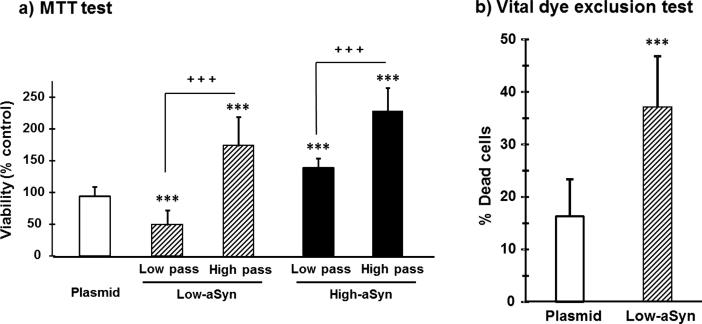
The interest was to know to what extent differences in the levels of aSyn overexpression may affect cell viability and how the period of culturing impacted cell cultures, as the wellbeing of cultured cells may decline as a result of the progressive culture aging. Fig. 2a represents the viability, as determined by the MTT method, of aSyn clones at low passage (a second sub-culture following 1 freeze/thaw cycle) as well as at high passage (over 10 sub-cultures following at least two freeze/thaw cycles) compared to the plasmid clone. The two-way ANOVA revealed a statistically significant clone effect (F(2,97) = 64.98, P < 0.0001), passage effect (F(1,97) = 147.55, P < 0.0001), and passage-clone interaction (F(2,97) = 65.5, P < 0.0001). Cell viability of the low-aSyn clone was reduced by 50%, but only at low passage (P < 0.0001 compared to plasmid). Moderate aSyn expression damaged cells just at low passage as confirmed in a separate set of experiments, where the ratio of Trypan blue-tangible (dead) cells was higher than plasmid-transfected cells (t (45) = 2.58; P < 0.0001; Fig. 2b). Nevertheless, viability of the low-aSyn clone in the MTT test improved as the number of culture passages increased (the high-passage condition) to the point of overcoming the viability of plasmid-transfected cells (P < 0.0001, Fig. 2a). Culture time was a determinant factor of the cytotoxicity of aSyn overexpression.
Fig. 2.
Viability of aSyn-overexpressing SH-SY5Y neurons during subculturing. Moderate levels of aSyn overexpression provoked cytotoxicity at low passage, but not at high passage. High-aSyn overexpression increased cell viability regardless the passage. Values are expressed as the mean ± SD of 24 independent experiments with Bonferroni post hoc tests: ***P < 0.0001 vs. Plasmid; +++P < 0.0001 vs. low-passage aSyn counterparts.
aSyn overexpression enhanced the proliferation rates of SH-SY5Y neurons
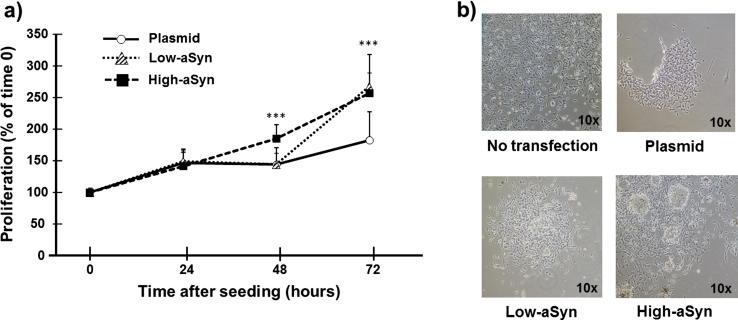
Next, it was measured the time-dependent variability in proliferation rates of the SCNA-gene and plasmid transfected clones. Fig. 3 shows the increment (%) of cell population during a 3-day incubation after one freeze/thaw cycle and two sub-culture rounds. The two-way factorial (clone × incubation time) ANOVA was preferred over the repeated-measures ANOVA analysis because sphericity was not assumed (Mauchly’s test of spehericity: P < 0.05). A two-way ANOVA showed significant differences in the growth rates of clones over time (clone × incubation time interaction, F(6,62) = 10.127, P < 0.0001). Differences were observed following a 48-hour incubation when high-aSyn clone began to proliferate faster than low-aSyn and plasmid clones (P < 0.0001), whereas levels of proliferation of the low-aSyn clone approached those of the high-aSyn clone after a 72-hour incubation (P < 0.0001 compared to plasmid). A delayed proliferating response and subsequent amelioration of the low-aSyn clone during the culture time was thus confirmed.
Fig. 3.
Proliferation of aSyn-overexpressing SH-SY5Y neurons. (a) Proliferation of plasmid and aSyn cells at 0, 24, 48 and 72 h after seeding. Data represent the mean ± SD of 12 independent experiments with Bonferroni post hoc tests: ***P < 0.0001 vs. Plasmid at the same-time. (b) Phase contrast microscopy images (magnification 10×) of non-transfected, plasmid-transfected, aSyn-overexpressing (low-aSyn and high-aSyn) neurons after a 3-day incubation. Note that aSyn-overexpressing cells adhere easily in larger clusters.
aSyn overexpression stimulates the formation of adherent cell colonies
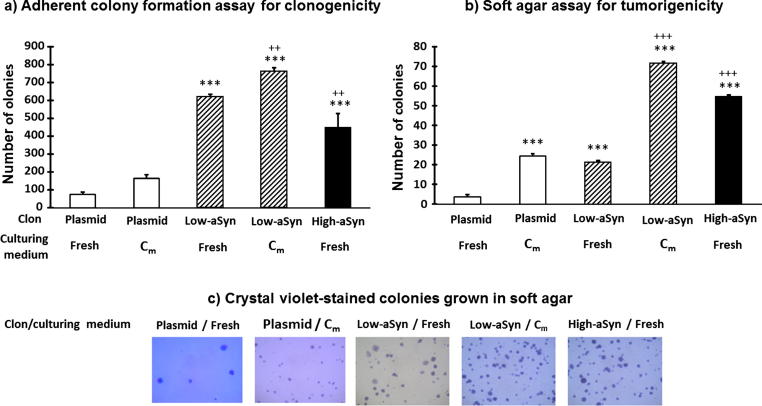
It could be argued that the MTT assay captures cell metabolic activity better than cell proliferation. Therefore, effects of aSyn overexpression in cell viability was reevaluated by measuring the formation of adherent colonies (clonogenicity, Fig. 4a). Conditioned medium (Cm) from high-aSyn cell cultures was introduced as an additional treatment to investigate whether the proliferative effects of high-aSyn overexpression could be controlled by paracrine ways. In attachment-dependent conditions, clonogenicity was linked to the aSyn overexpression in the SH-SY5Y clones (F(4,14) = 184.1, P < 0.0001; low-aSyn and high-aSyn clones were higher compared to plasmid; P < 0.0001). These effects, however, were not specific because replication viability of the low-aSyn clone was moderately higher compared to the high-aSyn clone (P < 0.01), a difference that was even larger after incubation with Cm (low-aSyn clone + Cm compared to the low-aSyn clone; P < 0.01).
Fig. 4.
Colony formation in aSyn-overexpressing SH-SY5Y neurons. Colony counting in (a) attachment-dependent (clonogenicity) conditions and (b) attachment-independent (tumorigenicity) conditions. Cm: conditioned medium from high-aSyn culture. Data represent the mean ± SD of 3 (clonogenicity) to 4 (tumorigenicity) different experiments with Bonferroni post hoc test ***P < 0.001 compared to Plasmid; ++P < 0.01, +++P < 0.001 compared to low-aSyn. (c) Images of crystal violet-stained colonies grown from different clones as observed in agar plates.
Effects of aSyn overexpression in colony formation in agar (CFA)
To assess tumorigenicity, cell cultures were grown on agar to measure proliferation in attachment-independent conditions. The CFA assay rigorously tests cell proliferation and migration, which depend on floating cancer stem cells. This assay confirmed that aSyn overexpression stimulated the production of colony formation units (Fig. 4b; F(4,19) = 140.45, P < 0.0001; low-aSyn and high-aSyn clones compared to the plasmid clone; P < 0.0001). In contrast to clonogenicity, tumorigenicity in the high-aSyn clone was higher than in the low-aSyn clone (P < 0.0001). Likewise clonogenicity, culturing with Cm, increased the tumorigenicity of low-aSyn (the highest CFA rate), and the plasmid clone (P < 0.0001 vs. non-conditioned counterparts) probably by paracrine action.
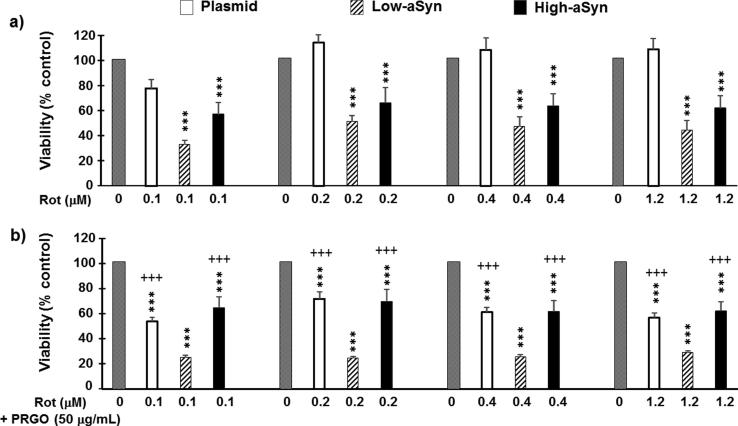
aSyn overexpression precipitates Rot toxicity in SH-SY5Y neurons
Viability of the transfected clones was challenged with Rot, a complex I inhibitor and potent pro-oxidant agent. Pretreatment with PRGO is deemed to promote cell aging. Fig. 5 represents the cytotoxic effects of (a) increasing concentrations of Rot and (b) the effects of 50 µg/mL of PRGO pretreatment in the cytotoxicity produced by Rot. As shown in Fig. 5a, there was a statistically significant clone effect (F(2,302) = 15.32, P < 0.0001), a treatment effect (F(3,302) = 57.63, P < 0.0001), and a clone × treatment interaction (F(6,302) = 15.53, P < 0.0001). The treatment with Rot significantly damaged the low-aSyn clone (P < 0.0001 compared to Vehicle, P < 0.0001 compared to plasmid) and the high-aSyn clone (P < 0.001 compared to Vehicle, P < 0.0001 compared to plasmid), whereas the plasmid clone was not affected.
Fig. 5.
Rotenone-induced neurotoxicity in aSyn overexpressing neurons and worsening effects of PRGO. (a) Cytotoxity of rotetone (Rot) in SH-SY5Y neurons. The 24-hour treatment with Rot damaged only aSyn-overexpressing neurons. (b) Pretreatment with 50 µg/mL of partially reduced graphene oxide (PRGO) triggers Rot cytotoxicity in plasmid-transfected cells while worsening the conditions for low-aSyn cells. Grey bars represent the viability (100%) in vehicle control. Cell viability was calculated as the percentage of vehicle control of each clone type. Data represent the mean ± SD of 8 independent experiments with Bonferroni post hoc tests: ***P < 0.001 compared to Plasmid; +++P < 0.001 compared to low-aSyn.
The combination of the Rot with PRGO treatments (Fig. 5b) exhibited synergy and exacerbated cytotoxicity in the low-aSyn clone (P < 0.0001 compared to the PRGO, plasmid, and high-aSyn groups), as well as it finally caused damage to the plasmid clone (P < 0.0001 compared to PRGO). The Rot + PRGO treatment, however, showed no evident changes in the viability of the high-aSyn clone compared to the Rot treatment. Absence of a clear dose-toxicity relationship was likely due to floor effects.
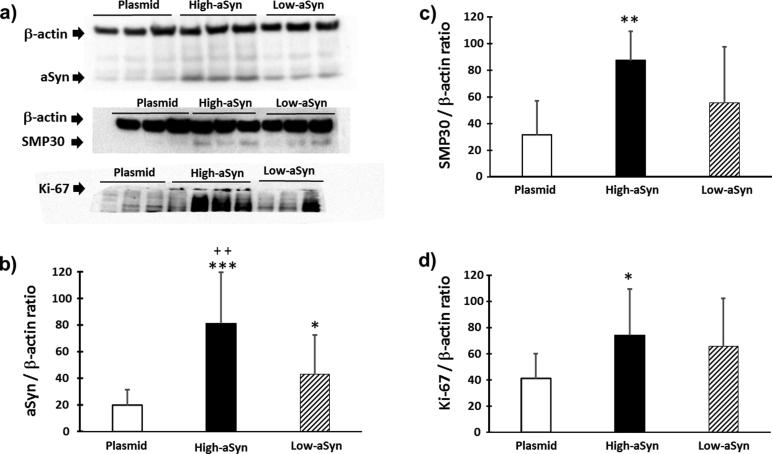
Cell senescence is retarded in high-aSyn SH-SY5Y neurons
Finally, aSyn overexpression effects in specific markers of senescence (SMP30) and mitosis (Ki-67) were investigated. At the same time, it was double checked if both aSyn clones kept overexpressing the transfected SCNA gene. As shown in the Fig. 6a and b, aSyn transgenesis was not lost throughout freeze/thaw cycling (clone effect; F(2,37) = 15.74, P < 0.0001; high-aSyn: P < 0.01 compared to plasmid; low-aSyn: P < 0.05 compared to plasmid; and high-aSyn: P < 0.01 compared to low-aSyn). aSyn overload increased the expression of SMP30, a protein deemed to be decreased by aging (clone effect: F(2,20) = 6.02, P < 0.05; high-aSyn: P < 0.01 compared to plasmid; low-aSyn: P = 0.089 compared to plasmid). In parallel, the cell proliferation marker Ki-67 differed across clones (F(2,26) = 3.773, P < 0.05) and its expression was increased in high-aSyn neurons (P < 0.05 compared to plasmid).
Fig. 6.
SMP30 and Ki-67 protein levels in aSyn overexpressing neurons. (a) Representative Western blots of aSyn, SMP30, and Ki-67 proteins. Note that Western blot for Ki-67 has 10 instead of 9 lanes. Lanes are as follows: 1–3 were plasmid cells, 4–6 (4–7 in Ki-67 western blot) were high-aSyn cells, and lanes 7–9 (8–10 in Ki-67 western blot) were low-aSyn cells. (b) Differences in aSyn expression across clones were replicated. (c) Expression of the senescence SPM30 marker which increased mainly in high-aSyn cells. (d) Expression of the mitosis Ki-67 marker that was also significantly increased in high-aSyn cells. Data represent the mean ± SD of 6–12 independent experiments with Bonferroni post hoc tests tests: *P < 0.05; **P < 0.01 compared to Plasmid; ++P < 0.01 compared to low-aSyn.
Discussion
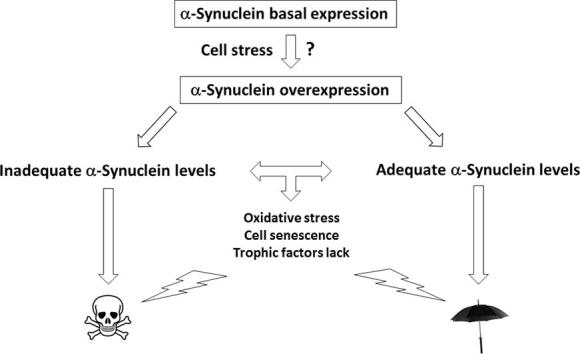
This research showed the dual consequences (cell toxicity and cell proliferation) of aSyn overexpression in the same in vitro model of human dopaminergic-like SH-SY5Y neurons. While aSyn overexpression made cells be more susceptible to oxidative stress, especially at moderate increments of aSyn, a larger amount of protein stimulated tumorigenicity of this cancer cell line, thus rejuvenating the culture. Dependence of the above effects on aSyn levels implies a critical role for aSyn overexpression in neuronal regenerative vigor and aging.
SNCA (or PARK1) was the first gene linked to autosomal-dominant PD [44]. Up-regulation of the wild-type aSyn as a result of abnormal gene dosage contributes to PD [10]. Point-mutations in the SNCA gene (A53T and A30P) linked to idiopathic PD, consistently yield neurotoxicity [45] and increased aSyn fibril formation [46] when overexpressed in SH-SY5Y cells. In contrast, the viability of cells overexpressing wild-type aSyn hardly differs from non-transgenic cells [45]. Likewise, wild-type aSyn overexpression in transgenic mice cannot efficiently mimic synucleinopathies [47], [48]. Only doxyclycline-inducible gene expression and transient transfection [49], [50], [51] with the SCNA gene in SH-SY5Y cells, and adenovirus-mediated SCNA gene delivery in whole animals [52] reliably induce neurotoxicity, but only after switching on the expression of the wild-type aSyn. In agreement with Kanda et al. [45], we found that wild-type aSyn overexpression was not directly neurotoxic. Moderate aSyn overexpression triggered levels of cell death (~20% of the total cells) similar to those of other reports [49], [50], [51], [53], but only when cells were still recovering from thawing. In this vein, low-aSyn and plasmid-transfected cells showed delayed growth at logarithmic rate with respect to high-aSyn cells (the proliferation assay), likely because they fell short of releasing enough growth factor to the culture medium.
This hypothesis was confirmed in the tumorigenicity experiment, in which low-aSyn and plasmid-transfected cells responded to the medium drawn from actively growing high-aSyn cells. Although, replication viability (clonogenicity) was not directly proportional to the levels of aSyn overexpression, pro-invasive capacity (tumorigenicity) of high-aSyn cells support the role of enhanced aSyn via paracrine, in tumorigenic progression found in some human malignancies [31], [54], [55], [56]. The discrepancies in the effects of aSyn overexpression across clonogenicity (low-aSyn > high-aSyn) and tumorigenicity (high-aSyn > low-aSyn) assays may imply different forms of aSyn fibrils, depending on its concentration, which is important to aSyn cytotoxicity [7], [57]. aSyn possesses chaperone activity, which is greater in the fibrillary form [58]. A limited overexpression of aSyn could then alter the dynamics of the protein and its chaperone activity. Characterization of aggregated forms of aSyn needs further research.
Up-regulation of wo proteins may explain why cell proliferation and senesce occurred at the same time in high-aSyn overexpressing cells, the Ki-67 protein, which is strictly associated with cell proliferation [59] and the senescence marker protein-30 (SMP30 or regucalcin) and which is down-regulated by aging [60]. Similarly, to aSyn, both proteins can be found in membranes [61], [62] and they play regulatory roles in intracellular calcium signaling [61], [63]. Because of the augmented Ki-67 expression and tumorigenicity of the high-aSyn cell lineage, and to a somewhat lesser extent in low-aSyn cells, aSyn may stimulate mitosis in neuroblastoma stem cells in the same way it does in neural stem cells in the adult brain [64]. SMP30 and aSyn share some functional features. Extracellular aSyn, which is not always lethal to neurons [57], promotes dopaminergic neuronal survival [65] whereas SMP30 prevents cells from senescing [60]. Similarly, aSyn overexpression favored tumorigenesis, and to a lesser degree clonogenicity, of neuroblastoma cells, probably by paracrine control. There is evidence that aSyn is secreted to the extracellular milieu [66], [67] by means of exosomes [68]. Our results therefore agree with the hypothesis that a rise in aSyn expression over basal levels could have a protective effect against aging, rather than being its detrimental consequence [25], [65], [69], [70]. Our results also are in accordance with evidence that suggests that aging-induced accumulation of aSyn is not linked to the progression of PD [16].
To confirm that aSyn overexpression represents a two-edged sword to neural survival, the neurotoxicity of the pro-oxidant Rot was evaluated [71], either alone or in combination with PRGO, a graphene-based nanomaterial known to be a potent accelerator and inductor of neuronal differentiation [72]. aSyn overexpression reduces intracellular antioxidant-defense systems [73] making SH-SY5Y cells more vulnerable to oxidative stress and cell aging [45], [74], [75], [76]. Cytotoxicity by Rot [71], [77] only affected aSyn-transgenic cells. While Rot works by interfering with the electron transport chain in mitochondria, aSyn overexpression could also have a direct impact on mitochondrial integrity and the cellular energy status [78]. GO and its reduced form PRGO induce apoptosis in cancer stem cells [40], [79] and neuronal cell lines [41] by arresting the cell cycle at the G0/G1 phase. The PRGO treatment prior to the addition of Rot severely worsened the viability of the low-aSyn cells, but not of the high-aSyn cells, a finding accounted for by the protective role of wild-type aSyn against cell senescence in the presence of oxidative stress [48], [80], [81], [82], [83], [84], [85]. Indeed, the inhibition of aSyn basal production can even kill SH-SY5Y cells [86]. The neurotoxic consequences of the aSyn overexpression in PD may not be as direct as initially thought [13], [14], [15], [86], [87], [88].
Conclusion
The present study demonstrates that aSyn overexpressed at different levels produce different effects in the same model of human dopaminergic-like SH-SY5Y neurons. It is suggested that the maintenance of adequate levels of aSyn overexpression with aging rather than aSyn up-regulation itself should be the target in PD research.
Acknowledgments
Acknowledgements
We thank Prof. María Javier Ramirez, and Prof. José González-Castaño for their technical assistance. We also thank David Montenegro for the proofreading. This work was supported by the Fundación Universidad de Navarra, Universidad de Malaga, Campus de Excelencia Internacional Andalucía Tech, and Andalucía Government PAIDI-CTS156, Spain. Javier de la Rosa was granted with a predoctoral fellowship from the Asociación de Amigos de la Universidad de Navarra, Spain.
Compliance with Ethics requirements
The authors have read and abided by the statement of Ethics in publishing and Ethical guidelines for journal publication.
Declaration of Competing Interest
Author Rune Wendelbo is the CEO of the Abalonyx Co. All other the authors have no conflict of interests to report.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Maroteaux L., Campanelli J.T., Scheller R.H. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dedmon M.M., Lindorff-Larsen K., Christodoulou J., Vendruscolo M., Dobson C.M. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 3.Deleersnijder A., Gerard M., Debyser Z., Baekelandt V. The remarkable conformational plasticity of alpha-synuclein: blessing or curse? Trends Mol Med. 2013;19:368–377. doi: 10.1016/j.molmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Fusco G., Chen S.W., Williamson P.T.F., Cascella R., Perni M., Jarvis J.A. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science. 2017;358:1440–1443. doi: 10.1126/science.aan6160. [DOI] [PubMed] [Google Scholar]
- 5.Fortin D.L., Nemani V.M., Nakamura K., Edwards R.H. The behavior of α-Synuclein in neurons. Mov Disord. 2010;25:S21–S26. doi: 10.1002/mds.22722. [DOI] [PubMed] [Google Scholar]
- 6.Emanuele M., Chieregatti E. Mechanisms of alpha-synuclein action on neurotransmission: cell-autonomous and non-cell autonomous role. Biomolecules. 2015;5:865–892. doi: 10.3390/biom5020865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duda J.E., Lee V.M., Trojanowski J.Q. Neuropathology of synuclein aggregates. J Neurosci Res. 2000;1561:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.McCann H., Stevens C.H., Cartwright H., Halliday G.M. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014;20(Suppl. 1):S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 9.Jellinger K.A. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;Suppl. 6:S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 10.Eriksen J.L., Przedborski S., Petrucelli L. Gene dosage and pathogenesis of Parkinson's disease. Trends Mol Med. 2005;11:91–96. doi: 10.1016/j.molmed.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Konno T., Ross O.A., Puschmann A., Dickson D.W., Wszolek Z.K. Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord. 2016;Suppl. 1:S1–S6. doi: 10.1016/j.parkreldis.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waxman E.A., Giasson B.I. Molecular mechanisms of alpha-synuclein neurodegeneration. Biochem Biophys Acta. 2009;1792:616–624. doi: 10.1016/j.bbadis.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J., Hague S.M., Hanson M., Gibson A., Wilson K.E., Evans E.W. SNCA multiplication is not a common cause of Parkinson disease or dementia with Lewy bodies. Neurology. 2004;63:554–556. doi: 10.1212/01.wnl.0000133401.09043.44. [DOI] [PubMed] [Google Scholar]
- 14.Hofer A., Berg D., Asmus F., Niwar M., Ransmayr G., Riemenschneider M. The role of alpha-synuclein gene multiplications in early-onset Parkinson's disease and dementia with Lewy bodies. J Neural Transmit (Vienna) 2005;112:1249–1254. doi: 10.1007/s00702-004-0263-3. [DOI] [PubMed] [Google Scholar]
- 15.Nishioka K., Hayashi S., Farrer M.J., Singleton A.B., Yoshino H., Imai H. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 16.Jellinger K.A. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Trans (Vienna) 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 17.Markesbery W.R., Jicha G.A., Liu H., Schmitt F.A. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frigerio R., Fujishiro H., Ahn T.B., Josephs K.A., Maraganore D.M., DelleDonne A. Incidental Lewy body disease: do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging. 2011;32:857–863. doi: 10.1016/j.neurobiolaging.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y., Kordower J.H. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: is this the target for Parkinson's disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Wong Y.C., Krainc D. α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M., Kim Y.M., Lee G., Junn E., Iwatsubo T., Mouradian M.M. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. J Biol Chem. 2004;279:4625–4631. doi: 10.1074/jbc.M310994200. [DOI] [PubMed] [Google Scholar]
- 22.Shults C.W. Lewy bodies. Proc Natl Acad Sci USA. 2007;103:1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawashima M., Suzuki S.O., Dohura K., Iwaki T. α-Synuclein is expressed in a variety of brain tumors showing neuronal differentiation. Acta Neuropathol. 2000;99:154–160. doi: 10.1007/pl00007419. [DOI] [PubMed] [Google Scholar]
- 24.Fung K.M., Rorke L.B., Giasson B., Lee V.M., Trojanowski J.Q. Expression of α-, β-, and γ-synuclein in glial tumors and medulloblastomas. Acta Neuropathol. 2003;106:167–175. doi: 10.1007/s00401-003-0718-x. [DOI] [PubMed] [Google Scholar]
- 25.da Costa C.A., Ancolio K., Checler F. Wild-type but not Parkinson's disease-related ala-53 –> Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 26.El-Guendy N., Rangnekar V.M. Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res. 2003;283:51–66. doi: 10.1016/s0014-4827(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 27.Bajaj A., Driver J.A., Schernhammer E.S. Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer Causes Control. 2010;21:697–707. doi: 10.1007/s10552-009-9497-6. [DOI] [PubMed] [Google Scholar]
- 28.Morris L.G., Veeriah S., Chan T.A. Genetic determinants at the interface of cancer and neurodegenerative disease. Oncogene. 2010;29:3453–3464. doi: 10.1038/onc.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H.B., Tang B., Liu Y.W., Wang X.F., Chen G.J. Alzheimer disease and cancer risk: a meta-analysis. J Cancer Res Clin Oncol. 2015;141:485–494. doi: 10.1007/s00432-014-1773-5. [DOI] [PubMed] [Google Scholar]
- 30.Engel P.A. Is age-related failure of metabolic reprogramming a principal mediator in idiopathic Parkinson's disease? Implications for treatment and inverse cancer risk. Med Hypotheses. 2016;93:154–160. doi: 10.1016/j.mehy.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 31.Inzelberg R., Flash S., Friedman E., Azizi E. Cutaneous malignant melanoma and Parkinson disease: common pathways? Ann Neurol. 2016;80:811–820. doi: 10.1002/ana.24802. [DOI] [PubMed] [Google Scholar]
- 32.Israeli E., Yakunin E., Zarbiv Y., Hacohen-Solovich A., Kisos H., Loeb V. α-Synuclein expression selectively affects tumorigenesis in mice modeling Parkinson's disease. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan T., Zhu J., Hwu W.J., Jankovic J. The role of alpha-synuclein in melanin synthesis in melanoma and dopaminergic neuronal cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.da Costa C.A., Duplan E., Checler F. α-Synuclein and p53 functional interplay in physiopathological contexts. Oncotarget. 2017;8:9001–9002. doi: 10.18632/oncotarget.14385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo J.H., Rah J.C., Choi S.H., Shin J.K., Min K., Kim H.S. Alpha-synuclein regulates neuronal survival via Bcl-2 family expression and PI3/Akt kinase pathway. FASEB J. 2002;16:1826–1828. doi: 10.1096/fj.02-0041fje. [DOI] [PubMed] [Google Scholar]
- 36.Musgrove R.E., King A.E., Dickson T.C. Neuroprotective upregulation of endogenous α-synuclein precedes ubiquitination in cultured dopaminergic neurons. Neurotox Res. 2011;19:592–602. doi: 10.1007/s12640-010-9207-x. [DOI] [PubMed] [Google Scholar]
- 37.Kim H.J., Jeon B.S., Yoon M.Y., Park S.S., Lee K.W. Increased expression of α-synuclein by SNCA duplication is associated with resistance to toxic stimuli. J Mol Neurosci. 2012;47:249–255. doi: 10.1007/s12031-012-9732-6. [DOI] [PubMed] [Google Scholar]
- 38.Xicoy H., Wieringa B., Martens G.J. The SH-SY5Y cell line in Parkinson's disease research: a systematic review. Mol Neurodegener. 2017;12:10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:53–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 40.Jaworski S., Sawosz E., Kutwin M., Wierzbicki M., Hinzmann M., Grodzik M. In vitro and in vivo effects of graphene oxide and reduced graphene oxide on glioblastoma. Int J Nanomed. 2015;10:1585–1596. doi: 10.2147/IJN.S77591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang Y., Liu J., Wu J., Yin Q., Liang H., Chen A. Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int J Nanomed. 2017;12:5501–5510. doi: 10.2147/IJN.S141032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 43.Borowicz S., Van Scoyk M., Avasarala S., Karuppusamy Rathinam M.K., Tauler J. The soft agar colony formation assay. J Vis Exp. 2014;92 doi: 10.3791/51998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaser T. Mendelian forms of Parkinson’s disease. Biochem Biophys Acta. 2009;1792:587–596. doi: 10.1016/j.bbadis.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Kanda S., Bishop J.F., Eglitis M.A., Yang Y., Mouradian M.M. Enhanced vulnerability to oxidative stress by alpha-synuclein mutations and C-terminal truncation. Neuroscience. 2000;97:279–284. doi: 10.1016/s0306-4522(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 46.Narhi L., Wood S.J., Steavenson S., Jiang Y., Wu G.M., Anafi D. Both familial Parkinson's disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 47.Buchman V., Ninkina N. Modulation of α-synuclein expression in transgenic animals for modelling synucleinopathies-Is the juice worth the squeeze? Neurotox Res. 2008;14:329–341. doi: 10.1007/BF03033857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Sánchez F., Milán M., Buendía P., Cano-Jaimez M., Ambrosio S., Rosenthal A. Pro-survival effect of human wild-type alpha-synuclein on MPTP-induced toxicity to central but not peripheral catecholaminergic neurons isolated from transgenic mice. Neuroscience. 2010;167:261–276. doi: 10.1016/j.neuroscience.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Vekrellis K., Xilouri M., Emmanouilidou E., Stefanis L. Inducible over-expression of wild-type α-synuclein in human neuronal cells leads to caspase dependent non-apoptotic death. J Neurochem. 2009;109:1348–1362. doi: 10.1111/j.1471-4159.2009.06054.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J.X., Zhang H.B., Huang Y., He Y., Zheng Y., Anderson J.P. Tenuigenin attenuates α-synuclein-induced cytotoxicity by down-regulating polo-like kinase 3. CNS Neurosci Ther. 2013;19:688–694. doi: 10.1111/cns.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dulovic M., Jovanovic M., Xilouri M., Stefanis L., Harhaji-Trajkovic L., Kravic-Stevovic T. The neuroprotective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro. Neurobiol Dis. 2014;63:1–11. doi: 10.1016/j.nbd.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Mochizuki H., Yamada M., Mizuno Y. Alpha-synuclein overexpression model. J Neural Transm. 2006;Suppl. 70:281–284. [PubMed] [Google Scholar]
- 53.Dadakhujaev S., Noh H.S., Jung E.J., Cha J.Y., Baek S.M., Ha J.H. Autophagy protects the rotenone-induced cell death in alpha-synuclein overexpressing SH-SY5Ycells. Neurosci Lett. 2010;472:47–52. doi: 10.1016/j.neulet.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 54.Bruening W., Giasson B.I., Klein-Szanto A.J., Lee V.M., Trojanowski J.Q., Godwin A.K. Synucleins are expressed in the majority of breast and ovarian carcinomas and in preneoplastic lesions of the ovary. Cancer. 2000;88:2154–2163. [PubMed] [Google Scholar]
- 55.Matsuo Y., Kamitani T. Parkinson’s disease-related protein, alpha synuclein, in malignant melanoma. PLoS ONE. 2005;5 doi: 10.1371/journal.pone.0010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge Y., Xu K. Alpha-synuclein contributes to malignant progression of human of human meningioma via Akt/mTOR pathway. Cancer Cell Int. 2016;16:86. doi: 10.1186/s12935-016-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Froula J.M., Henderson B.W., Gonzalez J.C., Vaden J.H., Mclean J.W., Wu Y. α-Synuclein fibril-induced paradoxical structural and functional defects in hippocampal neurons. Acta Neuropathol Commun. 2018;6:35. doi: 10.1186/s40478-018-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rekas A., Ahn K.J., Kim J., Carver J.A. The chaperone activity of α-synuclein: utilizing deletion mutants to map its interaction with target proteins. Proteins. 2012;80:1316–1325. doi: 10.1002/prot.24028. [DOI] [PubMed] [Google Scholar]
- 59.Scholzen T., Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 60.Marques R., Maia C.J., Vaz C., Correia S., Socorro S. The diverse roles of calcium-binding protein regucalcin in cell biology: from tissue expression and signaling to disease. Cell Mol Life Sci. 2014;71:93–111. doi: 10.1007/s00018-013-1323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auluck P.K., Caraveo G., Lindquist S. α-Synuclein: membrane interactions and toxicity in Parkinson's disease. Annu Rev Cell Dev Biol. 2010;26:211–233. doi: 10.1146/annurev.cellbio.042308.113313. [DOI] [PubMed] [Google Scholar]
- 62.Arun P., Aleti V., Parikh K., Manne V., Chilukuri N. Senescence marker protein 30 (SMP30) expression in eukaryotic cells: existence of multiple species and membrane localization. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0016545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi X., Sun Y., Wang P., Gu L., Wang L., Yang H. The interaction between calcineurin and α-synuclein is regulated by calcium and calmodulin. Biochem Biophys Res Commun. 2018;496:1109–1114. doi: 10.1016/j.bbrc.2018.01.148. [DOI] [PubMed] [Google Scholar]
- 64.Pérez-Villalba A., Sirerol-Piquer M.S., Belenguer G., Soriano-Cantón R., Muñoz-Manchado A.B., Villadiego J. Synaptic regulator α-synuclein in dopaminergic fibers is essentially required for the maintenance of subependymal neural stem cells. J Neurosci. 2018;38:814–825. doi: 10.1523/JNEUROSCI.2276-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim H.J., Jeon B.S., Kim H.J., Ahn T.B. Nanomolar concentration of α-synuclein enhances dopaminergic neuronal survival via Akt pathway. Neural Regen Res. 2013;8:3269–3274. doi: 10.3969/j.issn.1673-5374.2013.35.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borghi R., Marchese R., Negro A., Marinelli L., Forloni G., Zaccheo D. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 67.El-Agnaf O.M., Salem S.A., Paleologou K.E., Cooper L.J., Fullwood N.J., Gibson M.J. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 68.Emmanouilidou E., Melachroinou K., Roumeliotis T., Garbis S.D., Ntzouni M., Margaritis L.H. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kholodilov N.G., Neystat M., Oo T.F., Lo S.E., Larsen K.E., Sulzer D. Increased expression of rat synuclein in the substantia nigra pars compacta identified by mRNA differential display in a model of developmental target injury. J Neurochem. 1999;73:2586–2599. doi: 10.1046/j.1471-4159.1999.0732586.x. [DOI] [PubMed] [Google Scholar]
- 70.Chandra S., Gallardo G., Fernández-Chacón R., Schlüter O.M., Südhof T.C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;12:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 71.Hongo H., Kihara T., Kume T., Izumi Y., Niidome T., Sugimoto H. Glycogen synthase kinase-3β activation mediates rotenone-induced cytotoxicity with the involvement of microtubule destabilization. Biochem Biophys Res Commun. 2012;426:94–99. doi: 10.1016/j.bbrc.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 72.Defterali C., Verdejo R., Peponi L., Martin E.D., Martínez-Murillo R., López-Machado M.A. Thermally reduced graphene is a permissible material for neurons and astrocytes and de novo neurogenesis in the adult olfactory bulb in vivo. Biomaterials. 2014;82:84–93. doi: 10.1016/j.biomaterials.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Perfeito R., Ribeiro M., Rego A.C. Alpha-synuclein-induced oxidative stress correlates with altered superoxide dismutase and glutathione synthesis in human neuroblastoma SH-SY5Y cells. Arch Toxicol. 2017;91:1245–1259. doi: 10.1007/s00204-016-1788-6. [DOI] [PubMed] [Google Scholar]
- 74.Byers B., Cord B., Nguyen H.N., Schule B., Fehno L., Lee P.C. SCNA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate alpha-synuclein and are susceptible to oxidadite stress. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perfeito R., Lázaro D.F., Outeiro T.F., Rego A.C. Linking alpha-synuclein phosphorylation to reactive oxygen species formation and mitochondrial dysfunction in SH-SY5Y cells. Mol Cell Neurosci. 2014;62:51–59. doi: 10.1016/j.mcn.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Noronha C., Perfeito R., Laço M., Wüllner U., Rego A.C. Expanded and wild-type ataxin-3 modify the redox Status of SH-SY5Y cells overexpressing α-synuclein. Neurochem Res. 2017;42:1430–1437. doi: 10.1007/s11064-017-2199-7. [DOI] [PubMed] [Google Scholar]
- 77.Sánchez-Reus M.I., Peinado I.I., Molina-Jiménez M.F., Benedí J. Fraxetin prevents rotenone-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Neurosci Res. 2005;53:48–56. doi: 10.1016/j.neures.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Ganjam G.K., Bolte K., Matschke L.A., Neitemeier S., Dolga A.M., Höllerhage M. Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis. 2019;10:1–6. doi: 10.1038/s41419-019-2091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fiorillo M., Verre A.F., Iliut M., Peiris-Pagés M., Ozsvari B., Gandara R. Graphene oxide selectively targets cancer stem cells across multiple tumor types: implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget. 2015;6:3553–3562. doi: 10.18632/oncotarget.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee M., Hyun D., Halliwell B., Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- 81.Hashimoto M., Hsu L.J., Rockenstein E., Takenouchi T., Mallory M., Masliah E. alpha-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem. 2002;277:11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- 82.Manning-Bog A.B., McCormack A.L., Purisai M.G., Bolin L.M., Di Monte D.A. α-Synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaul S., Anantharam V., Kanthasamy A., Kanthasamy A.G. Wild-type alpha-synuclein interacts with pro-apoptotic proteins PKCdelta and BAD to protect dopaminergic neuronal cells against MPP+-induced apoptotic cell death. Brain Res Mol Brain Res. 2005;139:137–152. doi: 10.1016/j.molbrainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Chung WG, Miranda CL, Maier CS. Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res 2007;1176:133–42. [DOI] [PMC free article] [PubMed]
- 85.Menges S., Minakaki G., Schaefer P.M., Meixner H., Prots I., Schlötzer-Schrehardt U. Alpha-synuclein prevents the formation of spherical mitochondria and apoptosis under oxidative stress. Sci Rep. 2017;7:42942. doi: 10.1038/srep42942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collier T.J., Redmon D.E., Steece-Collier K., Lipton J.W., Manfredsson F.P. Is alpha-synuclein loss-of-function a contributor to parkinsonian pathology? Evidence from non-human primates. Front Neurosci. 2016;10:12. doi: 10.3389/fnins.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sironi F., Trotta L., Antonini A., Zini M., Ciccone R., Della Mina E. Alpha-synuclein multiplication analysis in Italian familial Parkinson disease. Parkinsonism Relat Disord. 2010;16:228–231. doi: 10.1016/j.parkreldis.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Bourdenx M., Dovero S., Engeln M., Bido S., Bastide M.F., Dutheil N. Lack of additive role of ageing in nigrostriatal neurodegeneration triggered by α-synuclein overexpression. Acta Neuropathol Commun. 2015;3:46. doi: 10.1186/s40478-015-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]