Abstract
The aqueous extract of pomegranate (Punica granatum L.) peel compounds was freeze-dried (FDPOPx) and encapsulated using two wall forming components at two concentrations (maltodextrin: MDX and β-cyclodextrin: βCDX; 5 and 10%) with a mass ratio of 1:5 (extract/wall material). Different properties of the encapsulated powders (bioactive components, physicochemical and morphological properties) and storage stability of prepared microcapsules were evaluated during 42 days of storage at a different relative humidity (52 and 75%) and temperatures (4 and 25 °C). Encapsulated powder with βCDX-10% had the highest total phenolic compounds (TPC: 58.78 mg GA/g) and antioxidant capacity [FRAP: 1414.76 μmol Fe2+/g and DPPH assay (RSA): 77.83%] among other wall materials. The amounts of TPC and their antioxidant capacity decreased during the 42 days of storage. However, the highest TPC was observed in the freeze-dried MDX-10% % encapsulated powder at 4 °C storage temperature and 52% relative humidity with a half-life (t1/2) of 81 days, the reaction rate constant (k) of 0.85 × 10−2 min−1 and the glass transition temperature of 69.73 °C. In addition, the polyphenolic extracts (both free and encapsulated) were able to control the growth of yeasts and molds, and maintaining the sensory properties of cupcakes as the model food system. The lowest growth after 9 days of storage of cupcake was observed in samples prepared with 1.5% of microencapsulated powder (MDX-10%) which was equivalent to the effect of the chemical preservative potassium sorbate.
Keywords: Antifungal, Microencapsulation, Phenolic extract, Pomegranate peel
Introduction
Nowadays, more than 3000 additives and preservatives are used in the food industry to increase the products stability and inherent safety. Consumer studies have shown that they have recently become more informed about food additives and always tend to choose the additives of natural origin than their synthetic analogs (Bearth et al. 2014). Agro-industrial by-products contain peptides, carotenoids, phenolic, and other components, making them a rich source of natural compounds that can potentially be applied in the food industry as food additives sources (Szabo et al. 2018).
Pomegranate peel, as agro-food byproducts, has a potential source of preservatives with several reports on the antimicrobial and antioxidative activity (Sharayei et al. 2019; Al-Zoreky 2009; Akhtar et al. 2015). Pomegranate (Punica granatum), belongs to the Punicaceae family, is native to the semi-warm regions of Iran. It is estimated that the amount of pomegranate production in Iran in 2016–2017 was close to 1,100,000 tons (Anonymous 2017). Therefore, it can be estimated that an amount of about 350,000 tons of pomegranate peel is produced as waste and disposed of annually (pomegranate peel covers about 30–40% of the total fruit weight). The use of pomegranate peel for the production of bioactive compounds will yield a high-value by-product and contribute to the reduction in agricultural waste and environmental problems such as water pollution, unpleasant odors, explosions and combustion, asphyxiation, and greenhouse gas emission (Roy and Lingampeta 2014).
Pomegranate peel extract, as a rich source of bioactive components, is unstable and susceptible to oxidation, polymerization and condensation reactions. Encapsulation technology is used for protecting a bioactive compound from light, oxygen or other unfavorable conditions and improving stability (Munin and Edwards-Levy 2011). There are a number of studies in literature on microencapsulation of phenolic compounds from pomegranate peel with different methods (by double emulsion solvent evaporation, ionic gelation, spray-drying) and wall materials (poly(d,l-lactic-co-glycolic acid)-poly(ethylene glycol), sodium alginate, maltodextrin (DE: 14–17, 18–20, 13–17 and 16.5–19.5), skim milk powder, gum Arabic and chitosan) (Shirode et al. 2015; Zam et al. 2013, 2014; Goula and Lazarides 2015; Cam et al. 2014; Kaderides et al. 2015; Endo et al. 2012). Despite there are studies about the microencapsulation of pomegranate peel extract, there is a lack of knowledge concerning the developing a functional ingredient with potential use in the food industry. Therefore, this study was focused on the encapsulation of the aqueous extract of pomegranate peel by the freeze-drying technique with maltodextrin and β cyclodextrin (as encapsulating materials) in different concentrations and the stability of the microencapsulated powders were evaluated during 42 days of storage at different relative humidity and temperature conditions. Finally, the antifungal activity of aqueous polyphenolic extract of pomegranate peel (free and encapsulated) was evaluated in a food system after incorporating them into a cupcake was investigated in comparison with that of the chemical preservative of potassium sorbate.
Materials and methods
Plant materials
Pomegranate (Punica granatum L. varity Sishe Kape-Ferdos) waste was provided as a byproduct of the fruit juice industry by Anarin company, Ferdos, Iran. Pomegranate peels (POP) were isolated from seeds manually and packed in low-density polyethylene bags with a thickness of 140 microns, and frozen at − 18 °C until further analyses.
Chemicals and reagents
Chemicals and supplies used in this research were: maltodextrin with a high dextrose equivalent (DE = 16.5–19.5), β-cyclodextrin, calcium alginate, 2-4-6-tris (2-pyridyl)-s-triazine (TPTZ), folin–ciocalteu reagent, gallic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH). All chemicals and solvents used in this research were obtained from Sigma-Aldrich (St. Louis, MO) and Merck (Darmstadt, Germany).
Freeze-dried aqueous extract of pomegranate peel (FDPOPx)
Pomegranate peel extract was extracted with water by applying ultrasound process (Heilscher, Germany Ultrasonic Electronic Equipment Co. Ltd, 400 W, 20 kHz) at optimum condition [6.2 min with ultrasonic amplitude 60% at ambient temperature (25 °C)] that identified in previous work (Sharayei et al. 2019). After the sonication treatment, the mixture was agitated in dark at ambient temperature for 48 h. Then the mixture was filtered and the solvent was concentrated in vacuum rotary evaporator (Laborota 4000 efficient, Germany). After what, the concentrate was dried in a freeze drier (Operon- Korea) (− 55 °C, 0.15 mmHg) for 48 h.
Preparation of microencapsulated powders
Wall materials used for microencapsulation were maltodextrin (MDX: 5 and 10%) and β-cyclodextrin (βCDX: 5 and 10%). They were dissolved in distilled water at ambient temperature (25 ± 1 °C) and kept in a refrigerator for 24 h for completion of hydration. Following hydration, the FDPOPx was mixed in a mass ratio of 1:5 (extract: wall material) and centrifuged in a Mikro 220R centrifuge (Hettich Instruments, Tuttlingen, Germany) at 120 rpm, for 30 min at room temperature (~ 22 °C) (Wang et al. 2013). Then, the solutions were dried in a freeze drier (Operon-Korea) (− 55 °C, 0.15 mmHg) for 48 h. Dried materials were ground (using a pestle and mortar) and passed through a 0.71 mm mesh, then stored in brown glass bottles with screwed caps in a freezer (− 18 °C) (Azarpazhooh and Ramaswamy 2012).
Physicochemical analysis
Measurement of moisture content and bulk density
Moisture content (%), wb (wet basis) of FDPOPx and microencapsulated powders was measured using an infrared moisture balance (MX-50, Japan) heating at 105 ± 1 °C until constant weight. Bulk density of FDPOPx and microencapsulated powders was measured by Tonon et al. (2010) method. In brief, 20 g of frozen milled POP was weighed and poured into a 20 mL graduated cylinder up to the mark. The bulk density was then calculated by dividing the sample mass (g) by the sample volume (20 mL).
Determination of total phenolic content (TPC)
The total phenolic content of the FDPOPx and microencapsulated powders was estimated by the Folin-Ciocalteu method of Singleton et al. (1999). TPC was presented as Gallic acid equivalents in mg per g.
Surface phenolic content of microcapsules (SPC)
Surface phenolic content (SPC) was determined following the method of Cilek et al. (2012). An amount of 100 mg of microcapsules was dispersed with 5 mL of ethanol for 3 min. The mixture was filtered with microfilter (0.45 μm) and the total phenolic content of the filtrate was determined. The amounts of surface phenolic compounds were determined and quantified with the same method described in the total phenolic content section.
Antioxidant capacity
The antioxidant activities were estimated by DPPH and FRAP assays. DPPH radical-scavenging assay was carried out according to Koleva et al. (2002). The radical-scavenging activity (RSA) was calculated as a percentage of DPPH discoloration using the Eq. (1):
| 1 |
where AS is the absorbance of the solution when the sample is added at a particular level after remaining for 30 min in the dark at 517 nm and Acont is the absorbance of the DPPH solution.
Ferric reducing-antioxidant power (FRAP) was measured using 2,4,6-tripyridyl-s-triazine (TPTZ) according to Benzie and Strain (1996). The results were expressed in μmol Fe2+ per g at 595 nm, against the control solution.
Colour measurement
For color measurement, FDPOPx and microencapsulated powders were scanned by a photo scanner (HP, Scanjet G3010, resolution of 300 dpi) and the images saved as BMP format. To study the effect of processing parameters on colour components of samples, the RGB colour space images were converted to L*, a*, b* space by Image J software version 1.4.3.67 (National Institute of Health, USA), and the indices of Hue angle (H° = tan−1 (b/a)), chroma (C = (a2 + b2)1/2) were calculated and the mean of three replicates were reported (Karimi et al. 2012).
Determination of encapsulation yield and efficiency
The encapsulation yield (Y) of samples after freeze-drying was calculated according to the following formula (Eq. 2), based on dry matter measurements (Fang and Bhandari 2010):
| 2 |
The encapsulation efficiency (EE) was determined based on the methodology described by Kaderides et al. (2015), as shown in Eq. 3:
| 3 |
where TPC and SPC were the quantity of total phenolic content and surface phenolic content of the freeze-dried microcapsules.
Determination of glass transition temperature
Samples of microencapsulated powders were equilibrated at 75% RH (desiccators containing saturated NaCl) for 1 week. Glass transition temperature (Tg) was determined using a differential scanning calorimeter (DSC1 Mettler Toledo, Switzerland). The temperature ranged from − 40 to 200 °C with a heating rate of 10 °C/min (Cai and Corke 2000). 5 mg of sample powder was weighed directly into a DSC sample pan and then sealed. An empty pan was also used as a reference. Then a temperature scan was done to get the Tg values.
Determination of particle morphology and size distribution
Particles size
A laser diffraction particle size analyzer (SAL, D-2101, Shimadzu, Japan) was used to measure the particle size of powders in terms of diameter. The microencapsulated powders were dispersed in hexane using ultrasonic waves (24 kHz and 20% intensity) for 2 min and then particles size was directly determined (Parrarud and Pranee 2010).
Scanning electron microscopy
Particle structures of the encapsulated powders were evaluated via a scanning electron microscope (LEO-1450, Germany). Using a 2-sided adhesive tape, powders were attached to SEM stubs and then left in desiccators containing phosphorous pentoxide for 48 h. Samples were coated with 200˚A gold under vacuum condition before the examination. SEM was operated at an increasing voltage of 10 kV (Ahmed et al. 2010).
Storage stability evaluation of encapsulated powders
Encapsulated powders were kept at controlled humidity and temperature, in the absence of light for 42 days. Samples of 3 g of each powder were transferred to a closed low-density polyethylene bags (5 cm × 5 cm). The samples were put in sealed desiccators containing saturated NaCl and MgNO3 solutions in order to obtain humidity values of 75 and 52%, then placed in incubators at 4 and 25 °C temperatures (Gradinarua et al. 2003). For determination of total polyphenolic compounds, duplicate bags were removed every 7 days. Core compound without encapsulation was used as a control.
Kinetics of degradation of total polyphenolic compound
Degradation kinetics of total polyphenolic compounds during storage under isothermal heating is obtained by first determining the rate constants at a given temperature against the time. The key parameters of thermal degradation kinetics i.e. half-life (T½) are calculated using (Eqs. 4, 5).
| 4 |
| 5 |
where C0 is initial total phenolic compounds contents and Ct is total phenolic contents after time t (min) while k is the first order kinetic constant (Wang and Xu 2007).
Preparation of cupcake as model food system
The cupcake was prepared and cooked according to A.A.C.C. (2002). Potassium sorbate was added to the mixture as antimicrobial preservative at 0.1% level as well as microencapsulated powder were incorporated in separate samples at concentrations of 0.3 and 1% (values were selected based on the minimum inhibitory concentration test for mold growth using a disk diffusion method in previous work (Sharayei et al. 2019). After the completion of the cooking/baking operation, trays from the oven were transferred to the laboratory and baked cakes were allowed to cool in the laboratory air. The cakes were packed in polyethylene bags of 70 microns thick and stored in medium conditions (temperature 25 °C) for 9 days. The total yeast and mold were counted at different times (after 4 h, 3 and 9 days from the production of the cupcake).
Sensory evaluation of cupcake
The sensory evaluation of cupcakes was carried out one day after baking and at room temperature by Ronda et al. (2005). Samples were offered to 20 panelists, who evaluated the attributes aroma, color, texture, taste, and overall acceptance of the cupcakes using a hedonic scale of 5 points (Poor, Fair, Medium, Good and Excellent).
Microbial quality of cupcake
Total yeast and mold count was determined by using pour plate method on Potato Dextrose Agar (PDA, pH 5.6 ± 0.2) by using pour plate method and plates were incubated at 25 °C for 3 days (BAM 1998).
Statistical analysis
All measurements and experiments were performed in triplicate and all data were of normal distribution. Regression and ANOVA (analysis of variance) analyses were performed using the MSTATC and Excel software. Notable differences between means were determined via Duncan’s multiple range tests. P values of less than 0.05 were considered statistically significant.
Results and discussion
Physical characteristics of the microcapsules
The effects of the type and concentration of the wall material on the physical properties of the microcapsules are shown in Table 1. The average moisture content of the microcapsules produced with βCDX and MDX walls was 4.49 and 3.62%, respectively. By comparing the moisture content of the powders, it can be inferred that the microcapsules prepared with MDX lost more moisture content during drying. This issue is probably related to the difference in the number of water-binding groups in βCDX and MDX molecules (Kanakdande et al. 2007). By increasing the concentration of wall materials from 5 to 10%, the moisture content in the microcapsules decreased, so that the highest moisture content was seen at 5% concentration (4.44%) and the lowest was at 10% concentration (3.67%). Microcapsules produced with βCDX had more yield of encapsulation (average of 86.59%) than MDX (average of 83.48%) and increasing wall material concentration from 5 to 10% significantly improved the yield percentage (P < 0.05). Clearly, the increased ingredient concentration led to an increase in the solids mass fraction, thus increasing the production yield of the microcapsules. Also, Kaderides et al. (2015) reported that the encapsulation efficiency with the MDX wall alone was lower (69.80%) than the mixture of MDX with whey protein isolate (98.64%), skimmed milk powder (97.14%), and gum Arabic (73.82%) or skimmed milk powder as wall materials (91.49%).
Table 1.
Physical properties of microencapsulated aqueous polyphenolic extract of pomegranate peel
| Characteristics | FDPOPx | βCDX | MDX | ||
|---|---|---|---|---|---|
| 5% | 10% | 5% | 10% | ||
| Moisture content (%) | 4.12b | 4.73a | 4.24a | 4.14ab | 3.10c |
| Yield (%) | – | 80.64c | 92.54a | 79.70c | 87.26b |
| Bulk density (kg/m3) | 43.60d | 119.56b | 164.50a | 107.03c | 112.15bc |
| Particle size (μm) | – | 33.14b | 40.14a | 24.16c | 35.10b |
| Glass transition temperature (°C) | – | 65.25b | 65.96b | 65.21b | 69.73a |
| L* | 30.05c | 31.11c | 34.69b | 55.67a | 55.01a |
| a* | 9.56a | 8.79ab | 8.70ab | 7.68bc | 6.01c |
| b* | 20.51a | 11.67c | 12.84c | 17.44b | 17.26b |
| ∆E | – | 22.21b | 22.20c | 23.50d | 25.36d |
| C | 22.63a | 14.62c | 15.51c | 19.18b | 18.28b |
| H◦ | 24.99c | 36.92a | 34.13b | 24.49c | 19.18d |
Each value in the table is represented as mean ± SD (n = 3)
Different letters (a–b) in the same line indicate significant difference between wall materials (P < 0.05)
The bulk density of the microcapsules containing βCDX as a wall material was higher (142.03 kg/m3) than the capsules containing MDX (98.59 kg/m3). Therefore, in terms of equal masses, due to lower porosity (higher bulk density), the microcapsules formed by βCDX will occupy less volume than those prepared with MDX. By increasing the concentration of wall materials from 5 to 10%, the bulk density increased from 113.30 to 138.33 kg/m 3. This trend is probably caused by the effect of increasing the concentration of wall materials resulting in creating more pore spaces for the active ingredient. Also, the size of the solid matrix particles used in encapsulation also may influence the bulk density of the produced powders (Tonon et al. 2010).
The average diameter of the microcapsule particles in the βCDX wall (36.64 μm) was more than maltodextrin (29.63 μm). The average diameter of the microcapsule particles at 10% concentration (37.62 μm) was also higher than at the concentration of 5% (28.65 μm). The size of the microcapsule particles highly depends on the type, the density of the wall and the drying method. The size range of particles produced by freeze-drying has been reported to be from 20 to 5000 μm. The varying particle size range in this technology is due to longitudinal fractures and the sublimation of ice crystals (Zuidam and Shimoni 2010).
One of the best methods for testing the thermal behavior of materials is the use of differential scanning calorimeters (DSC). The glass transition temperature can also be calculated by DSC. In this method, the specimen is subjected to a controlled temperature change, and the changes in its physical properties are continuously measured as a function of temperature (Horiuchi 2004). The glass transition temperature of the microcapsules produced with both types of wall material was higher than the ambient temperature, and the increase of wall material concentration to 10% resulted in a slight increase in the glass transition temperature. Therefore, all of the microcapsules produced at ambient temperature will not reach the glass transition temperature and will therefore not be elastic and soft. The glass transition temperature is the temperature at which the molecular movement of the polymer begins to occur; meaning that the molecules below this temperature are incapable of moving and therefore the material is glass-like and hard. The polymer is elastic and soft at temperatures higher than the glass transition temperature (the beginning of molecular movements in the polymer). In the elastic and soft state of the polymer (higher than the glass transition temperature), it is possible to release and transfer the contents of the core out of the wall material (Biliaderis et al. 1999).
The type of the wall had a significant effect on the L*, a* and b* values of the produced microcapsules. Colour is one of the important physical properties of the microcapsules, which is measured according to the Lab color model (Malien-Aubert et al. 2001). The L*value (brightness) in the microcapsules containing MDX (55.3 units) was higher than the brightness of the microcapsules containing βCDX (32.9 units). The intensity of red (a*) in the produced microcapsules changed in precise opposition to the brightness level. The amount of The b* value varied from 26.1 to 35.2 units in microcapsules containing different wall materials. As shown in Table 1, changes in total color (TCD) in the microcapsules prepared with the βCDX were less than that of the MDX. It is possibly due to the color of the wall materials and the microcapsule drying method (Malien-Aubert et al. 2001). The amount of chroma in the microcapsules produced with the MDX was higher than that of the βCDX, indicating their higher color purity or their higher capacity for colored. There was a significant difference between the Hue angles in the microcapsules. Hue angle expresses minor color differences, and the numbers are as follows: 0°, red–purple, 90°; yellow; 180°, blue; and 270° green–blue (McGuire 1992). The results of Table 1 shows that the microcapsules prepared with the βCDX have a stronger red–purple color compared to the maltodextrin. As shown in Table 1, increasing the concentration of wall materials did not significantly change the color components L*, a*, and b* of the produced microcapsules.
Chemical characteristics of the microcapsules
The effect of the type of wall material on the chemical attributes of the microcapsules is shown in Table 2. The results demonstrated that the type and concentration of the wall material had a significant effect on all chemical attributes of the prepared microcapsules (P < 0.05). The total phenolic compounds of the microcapsules from βCDX were considerably higher than those from MDX, which is probably due to a better protective effect of βCDX on the phenolic compounds of the aqueous extract of pomegranate peel (core compounds). It has been also reported previously that the type of wall and core compounds have significant effects on the volatility of microstructure compounds (Finney et al. 2002). The higher the volatility of the components of the core material, the higher will be the amount of their excretion from inside the microcapsules during the drying process (Soottitantawat et al. 2005). The flexibility and molecular dimensions of wall materials used also play an important role in this process because they directly affect the molecular distribution of components into the shell around the microcapsules and their displacement to the surface (Najafi et al. 2011). The faster the formation of the crust and the lower its permeability towards distributing volatile and sensitive compound, the higher the stability of the core content will be.
Table 2.
Chemical properties and antioxidant capacity of microencapsulated aqueous polyphenolic extract of pomegranate peel
| Parameteres | FDPOPx | βCDX | MDX | ||
|---|---|---|---|---|---|
| 5% | 10% | 5% | 10% | ||
| TPCa (mg GA/g) | 60.19a | 54.96b | 58.78a | 23.75c | 51.58b |
| Efficiency of TPC (%) | – | 82.61b | 89.91a | 28.38d | 72.84c |
| FRAPb (Fe2+, μmol/g) | 1432.35a | 1346.68c | 1414.76b | 1166.1d | 1409.23b |
| RSAc (%) | 78.02a | 50.06b | 77.83a | 39.59c | 52.67b |
aTPC total phenolic compound
bFRAP ferric reducing antioxidant power
cRSA radical scavenging activity
Mean ± SD (standard deviation) within a row with the same lowercase letters are not significantly different (P < 0.05)
The amount of phenolic compounds presents in the microcapsules and their antioxidant capacity was improved by increasing the concentration of wall materials from 5 to 10%. The mechanism of entrapment of dispersed molecules can be explained by the speed of crust formation and selective diffusion theory (Thijssen and Rulkens 1968, Roos 1995). Thijssen and Rulkens (1968) proposed the mechanism of retention of dispersed molecules in spray drying. It was suggested that the diffusion coefficient of volatile components decreases at a higher rate than the diffusion coefficient of water during drying. A similar mechanism relating the entrapment of dispersed molecules inside micro-regions was proposed by Flink and Karel (1970), explaining the retention of dispersed components in freeze-drying via formation of a solid glassy encapsulant matrix around dispersed components (Roos, 1995).
Further, as illustrated in Table 2, microcapsules were able to sequester the DPPH stable radical and to reduce ferric ion and microcapsules containing βCDX- 10% had the highest antioxidant capacity. Antioxidant capacity for DPPH and FRAP assays may have been influenced by bioactive compounds present in the FDPOPx and of reaction mechanisms involved in each of these analyses. The major class of pomegranate peel’s phytochemicals is the polyphenols that include flavonoids (flavonols, flavanols, and anthocyanins), condensed tannins (pro-anthocyanidins) and hydrolyzable tannins (ellagi-tannins and gallo-tannins). hydrolysable polyphenols in pomegranate peel, specifically ellagitannins, are the most active antioxidants amongst the tannins contained therein. These compounds (ellagic acid, punicalagin, punicalin, and gallagic acid) have been shown to hold heightened antioxidant and pleiotropic biological activities and notably, to act synergistically together (Seeram et al. 2005).
Microcapsule surface properties
The scanning electron microscope (SEM) images of different microcapsules are shown in Fig. 1. The microcapsules did not have a definite geometric shape. The reason for this phenomenon is probably the drying mechanism (Abdul-Fattah et al. 2007). The microcapsules containing the βCDX exhibited a higher degree of indentation, porosity, and trachea, In addition, it can be observed that by increasing the concentration of the βCDX, the number of cracks and fractions rose. While, microcapsules containing the MDX (both 5 and 10% concentrations), the walls were thin, uniform, non-porous and unindented. The exact mechanism and the main cause of these changes in appearance and surface properties of the microcapsules are not fully clear and require further studies, but these differences may be caused by the difference between the superficial power and the spatial structure of MDX and βCDX. The formation of superficial wrinkles and cavities at the surface of the microsphere probably indicate the effect of mechanical stresses and drying conditions on the wall material (Lee and Rosenberg 2000).
Fig. 1.
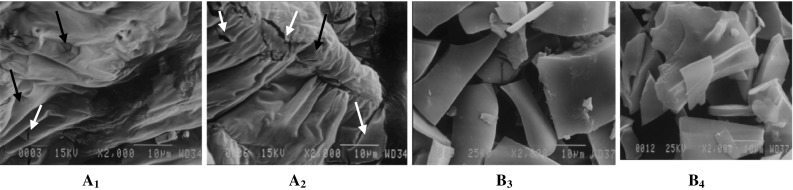
Micrographs of microencapsulated aqueous polyphenolic extract of pomegranate peel (× 3000), A: βCDX; B: MDX and indices 1 and 2: wall concentration 5 and 10%. Red arrows showed and cracks and fractions and blue arrows showed porous (color figure online)
Storage stability of the microcapsules
In order to evaluate the stability of various microcapsules under different storage conditions, different kinetic parameters (rate of degradation (k), half-life (T1/2)) for the loss of total phenolic compounds were calculated (Table 3). The loss of phenolic compounds highly depended on the type and concentration of wall materials, and in addition to the relative humidity and temperature of the storage environment. βCDX had a greater rate of loss of TPC than MDX. By increasing the wall material concentration from 5 to 10%, the protection for the phenolic compounds had a noticeable improvement. The reason for the decrease in the protection of the βCDX is probably the superficial properties and excessive cracking on the surface of the microcapsules (Fig. 1) and a lower glass transition temperature in comparison to the maltodextrin wall especially at 10% (Table 1), which has resulted in the further release of core compounds during the storage period. Soottitantawat et al. (2005) achieved similar results in the microencapsulation of limonene with modified starch/maltodextrin/Arabic gum. They observed that release levels were higher in smaller microcapsules containing a lower percentage of the wall material is larger than in larger microcapsules containing more wall materials, which is consistent with the results of this research. Lower levels of reduction of phenolic compounds during the storage period in microcapsules containing MDX-10%, compared to other microcapsules and the control sample (core compounds with no walls, FDPOPx), indicate better protection of these types of microcapsules compared with other microcapsules and the control sample.
Table 3.
Kinetic degradation data for microencapsulated aqueous polyphenolic extract of pomegranate peelat different storage conditions
| Wall type | Wall concentration (%) | Relative humidity (%) | Temperature (°C) | ka × 10−2 ± SEb (min−1) | Tc1/2 (day) | R2 |
|---|---|---|---|---|---|---|
| βCDX | 5 | 75 | 4 | 1.50 ± 0.01 | 46.21 | 0.93 |
| 25 | 1.80 ± 0.03 | 38.51 | 0.96 | |||
| 52 | 4 | 1.28 ± 0.00 | 54.15 | 0.93 | ||
| 25 | 1.360 ± 0.01 | 50.97 | 0.93 | |||
| 10 | 75 | 4 | 1.300 ± 0.00 | 53.32 | 0.92 | |
| 25 | 1.40 ± 0.02 | 49.51 | 0.91 | |||
| 52 | 4 | 1.22 ± 0.01 | 56.82 | 0.94 | ||
| 25 | 1.28 ± 0.01 | 54.15 | 0.96 | |||
| βCDX | 5 | 75 | 4 | 2.20 ± 0.04 | 31.51 | 0.94 |
| 25 | 2.30 ± 0.00 | 30.14 | 0.90 | |||
| 52 | 4 | 2.00 ± 0.02 | 34.51 | 0.94 | ||
| 25 | 2.20 ± 0.01 | 31.51 | 0.96 | |||
| 10 | 75 | 4 | 0.90 ± 0.01 | 77.02 | 0.94 | |
| 25 | 1.10 ± 0.03 | 63.01 | 0.97 | |||
| 52 | 4 | 0.85 ± 0.00 | 81.51 | 0.90 | ||
| 25 | 0.88 ± 0.01 | 78.77 | 0.93 | |||
| 75 | 4 | 3.30 ± 0.04 | 21.00 | 0.98 | ||
| 25 | 4.30 ± 0.02 | 16.12 | 0.96 | |||
| 52 | 4 | 3.17 ± 0.03 | 21.87 | 0.96 | ||
| 25 | 3.89 ± 0.06 | 17.82 | 0.96 |
aRate of degradation
bStandard error
cHalf-life
Sensory properties of microcapsules incorporated cupcakes
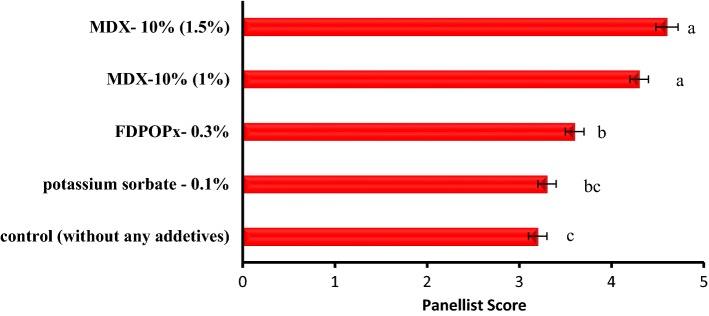
The results of the sensory evaluation of cupcake (overall acceptance), influenced by the addition of microencapsulated aqueous extract (microencapsulated with MDX- 10% at 1 and 1.5% concentration) and freeze-dried aqueous extract (FDPOPx, 0.3%) in comparison with the cupcakes supplemented with potassium sorbate as chemical preservative (0.1%) and the control sample (without any additive substances) are shown in Fig. 2. The results showed that the addition of preservatives (natural and synthetic) increased the desirability of the cupcake in terms of flavor, color, and overall acceptance in comparison to the control sample, and the test samples containing microencapsulated extract at a concentration of 1.5%, achieved the highest score in aroma, taste, color and general acceptance (P < 0.05). Improvement of the sensory characteristics of cupcake through the addition of FDPOPx with or without microencapsulation is due to the effect of their phenolic compounds on shelf life and sensory properties. Avis et al. (2007) also reported an improvement in the flavor and scent of cupcakes by adding orange peel to them.
Fig. 2.
Sensory evaluation (overall acceptance) of cupcake 9 h after baking under the influence of the addition of potassium sorbate (0.1%), FDPOPx (not microencapsulated, 0.3%), and microencapsulated aqueous extract with a MDX-10% (1 and 1.5%)
Anti-fungal properties of microcapsules incorporated cupcakes
The results of the microbial evaluation of cupcake (total mold and yeast count) influenced by the addition of microencapsulated aqueous extract (microencapsulated with MDX-10% at 1 and 1.5% concentrations) and freeze-dried aqueous extract (FDPOPx, 0.3%) compared to the addition of the synthetic preservative, potassium sorbate (0.1%) and the control sample (without any additives) during 9 days of storage under environmental conditions (at 25 °C) are shown in Table 4. With an increase in storage time, the growth rate of yeast and mold increased; however, both natural and synthetic additives reduced the growth of mold and yeast in the samples as compared to the control sample. The lowest growth rate of yeast and mold after 9 days of storage was observed in the sample containing 1.5% of the extract microencapsulated and the sample containing 0.1% potassium sorbate. The inhibitory effect on yeast and mold by potassium sorbate is well recognized and is due to the inhibition of dehydrogenase, sulfhydryl oxidase and catalase enzymes in the microbial cell (Buazzi and Marth 1991). Marín et al. (2002) investigated the effects of weak-acid-based preservatives (potassium sorbate, sodium benzoate, calcium propionate) in various confectionery products, on protecting Aspergillus niger and Aspergillus flavus against decay, and reported that potassium sorbate with 3.0% concentration significantly inhibits fungal growth and decay in these products.
Table 4.
Growth rate of yeast and mold in cupcake (Log (cfu/g)10) during 9 days of storage in environmental conditions influenced by the addition of potassium sorbate (0.1%), freeze-dried aqueous extract (FDPOPx, 0.3%) and microencapsulated aqueous extract with MDX-10% (1 and 1.5%)
| Sample | Storage time | ||
|---|---|---|---|
| After 4 h | 3 days | 9 days | |
| Control | – | 1.18 ± 0.48a | 1.63 ± 0.50a |
| Cupcake + potassium sorbate (0.1%) | – | – | 0.12 ± 0.00d |
| Cupcake + freeze-dried aqueous extract (FDPOPx -0.3%) | – | 0.87 ± 0.18b | 1.24 ± 0.32b |
| Cupcake + microencapsulated aqueous extract with MDX- 10% (1%) | – | – | 0.52 ± 0.18c |
| Cupcake + microencapsulated aqueous extract with MDX- 10% (1.5%) | – | – | 0.12 ± 0.06d |
Each value in the table is represented as mean ± SD (n = 3)
Mean ± SD (standard deviation) within a row with the same lowercase letters are not significantly different at P < 0.05
Protection against the growth of mold and yeast by a microencapsulated and free extract of pomegranate peel is probably due to the existence of phenolic compounds. Phenolic compounds, which have anti-microbial properties, have been shown to penetrate the lipids of the cellular and mitochondrial walls and in addition to causing cellular deformation and increasing the permeability of the cells, leading to the release of ions and other cellular contents. Although the release of certain amounts of intra-bacterial material can be tolerable for the cell, the release of large amounts of cellular contents results in the death of cells by the excretion of vital molecules and ions (Pauli 2006). Moreover, better inhibition of microencapsulated extracts compared to free extract is probably due to the protection of phenolic compounds during the thermal process of preparing the cake, and their gradual release during storage time. Regarding the results of the sensory and microbial test, a free and microencapsulated extract of pomegranate peel can be introduced as a substitute for the potassium sorbate chemical preservative.
Conclusion
The findings of this study clearly showed that the type and concentration of encapsulating material as well as relative humidity, temperature and storage time determine the properties of the final encapsulated powder. Comparing with the two encapsulated material, MDX exhibited superior encapsulating properties and considerable barrier effect against diffusion and loss of core material. The glass transition temperature of the microcapsules prepared with both wall types was higher than the ambient temperature and increased by increasing wall concentration from 5 to 10%. The addition of aqueous polyphenolic extract of pomegranate peel (free and encapsulated) to cupcakes showed that the cupcake contained 1.5% of MDX-10% had the most preferable of sensory preference. Also, 1.5% of MDX-10% approximately equivalent to the synthetic preservative of potassium sorbate increased the shelf life of the cupcake. Therefore, The encapsulated pomegranate peel extract could be used as an innovative and nature active additive, to enrich functional properties and improve preservation of food products. However, further work is required to isolate and characterize the individual phenolic compounds present in extracts and to determine the mechanisms involved in the antioxidant capacity and antifungal effects.
Acknowledgements
The authors thank Iran National Scientific Foundation (INSF) and Agricultural Engineering Research Institute (AERI) for financial support.
Abbreviations
- FDPOPx
Freeze-dried pomegranate peel extract
- MDX
Maltodextrin
- βCDX
β-Cyclodextrin
- Y
Yield
- TPC
Total phenolic compounds
- RSA
2,2-Diphenyl-1-picrylhydrazyl free radical scavenging
- FRAP
Ferric reducing-antioxidant power
- k
Rate of degradation
- T1/2
Half-life
- DSC
Differential scanning calorimeters
- SEM
Scanning electron microscope
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Approved methods of the American Association of cereal chemists. St. Paul: American Association of Cereal Chemists; 2002. [Google Scholar]
- Abdul-Fattah AM, Kalonia DS, Pikal MJ. The challenge of drying method selection for protein pharmaceuticals: product quality implications. J Pharm Sci. 2007;96:1886–1916. doi: 10.1002/jps.20842. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Sorifa Akter MST, Jin-Cheol L, Jong-Bang Eun C. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci Technol. 2010;43:1307–1312. [Google Scholar]
- Akhtar S, Ismail T, Fraternale D, Sestili P. Pomegranate peel and peel extracts: chemistry and food features. Food Chem. 2015;174:417–425. doi: 10.1016/j.foodchem.2014.11.035. [DOI] [PubMed] [Google Scholar]
- Al-Zoreky N. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int J Food Microbiol. 2009;134:244–248. doi: 10.1016/j.ijfoodmicro.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Anonymous (2017) Agricultural Statistics, the crop year 2016–2017. Ministry of Agriculture publication, planning and support deputy, General Directorate of Statistics and Information
- Avis C, Akingbala J, Bassus-Tatlor G. Effect of drying and storage on flavor quality of orange peel for cupcakes. J Food Agric Environ. 2007;5(2):78–82. [Google Scholar]
- Azarpazhooh E, Ramaswamy HS. Modeling and optimization of microwave osmotic dehydration of apple cylinders under continuous-flow spray mode processing conditions. Food Bioprocess Technol. 2012;5(5):1486–1501. [Google Scholar]
- BAM (1998) Bacteriological analytical manual. Food and Drug Administration, 8th edn. A.O.A.C. International, Gaicserburg
- Bearth A, Cousin M, Siegrist M. The consumer’s perception of artificial food additives: influences on acceptance, risk and benefit perceptions. Food Qual Prefer. 2014;38:14–23. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Biliaderis CG, Lazaridou A, Arvanitoyannis I. Glass transition and physical properties of polyol-plasticized pullulan-starch blends at low moisture. Carbohydr Polym. 1999;40:29–47. [Google Scholar]
- Buazzi MM, Marth EH. Mechanism in the inhibition of Listeria monocytogenes by potassium sorbate. Food Microbiol. 1991;8:249–256. [Google Scholar]
- Cai YZ, Corke H. Production and properties of spray-dried Amaranthus Betacyanin pigments. J Food Sci Technol. 2000;65:1248–1252. [Google Scholar]
- Cam M, İcyer NC, Erdogan F. Pomegranate peel phenolics: microencapsulation, storage stability and potential ingredient for functional food development. LWT Food Sci Technol. 2014;55(1):117–123. [Google Scholar]
- Endo EH, Ueda-Nakamura T, Nakamura CV. Activity of spray-dried microparticles containing pomegranate peel extract against Candida albicans. Molecules. 2012;17(9):10094–10107. doi: 10.3390/molecules170910094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z, Bhandari B. Encapsulation of polyphenols: a review. Trends Food Sci Technol. 2010;21:510–523. [Google Scholar]
- Finney J, Buffo R, Reineccius GA. Effects of type of atomization and processing temperatures on the physical properties and stability of spray-dried flavors. J Food Sci. 2002;67:1108–1114. [Google Scholar]
- Flink J, Karel M. Retention of organic volatiles in freeze-dried solutions of carbohydrates. J Agric Food Chem. 1970;18(2):295–297. [Google Scholar]
- Goula AM, Lazarides HN. Integrated processes can turn industrial food waste into valuable food by-products and/or ingredients: the cases of the olive mill and pomegranate wastes. J Food Eng. 2015;167:45–50. [Google Scholar]
- Gradinarua G, Biliaderisb CG, Kallithrakac S, Kefalasa P, Garcia-Viguerad C. Thermal stability of Hibiscus sabdariffa L. anthocyanins in solution and in the solid state: effects of copigmentation and glass transition. Food Chem. 2003;83:423–436. [Google Scholar]
- Horiuchi K. DSC studies on structural phase transitions and molecular motions in some A2MCl4 compounds. Phys Status Solidi. 2004;210:723–726. [Google Scholar]
- Kaderides K, Goula AM, Adamopoulos KG. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov Food Sci Emerg Technol. 2015;31:204–215. [Google Scholar]
- Kanakdande D, Bhosale RS, Singhal R. Stability of cumin oleoresin microencapsulated in different combination of gum arabic, maltodextrin and modified starch. Carbohydr Polym. 2007;67:536–541. [Google Scholar]
- Karimi M, Fathi M, Sheykholeslami Z, Sahraiyan B, Naghipoor F. Effect of different processing parameters on quality factors and image texture features of bread. J Bioprocess Biotech. 2012;2(5):1–7. [Google Scholar]
- Koleva II, van Beek TA, Linssen JP, De Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Rosenberg M. Preparation and some properties of water-insoluble, whey protein-based microcapsules. J Microencapsul. 2000;17:29–44. doi: 10.1080/026520400288535. [DOI] [PubMed] [Google Scholar]
- Malien-Aubert C, Dangles O, Amiot MJ. Colour stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular pigmentation. J Agric Food Chem. 2001;49:170–176. doi: 10.1021/jf000791o. [DOI] [PubMed] [Google Scholar]
- Marín S, Guynot ME, Neira P, Bernadí M, Sanchis V, Ramos AJ. Risk assessment of the use of sub-optimal levels of weak acid preservatives in the control of mould growth on bakery products. Int J Food Microbiol. 2002;79:203–211. doi: 10.1016/s0168-1605(02)00088-0. [DOI] [PubMed] [Google Scholar]
- McGuire RG. Reporting of objective color measurements. Hort Science. 1992;27:254–1255. [Google Scholar]
- Munin A, Edwards-Lévy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3:793–829. doi: 10.3390/pharmaceutics3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi MN, Kadkhodaee R, Mortazavi SA. Effect of the drying process and wall material on the properties of encapsulated cardamom oil. Food Biophys. 2011;6:68–76. [Google Scholar]
- Parrarud S, Pranee A. Microencapsulation of Zn-chlorophyll pigment from pandan leaf by spray drying and its characteristic. Int Food Res J. 2010;17:1031–1042. [Google Scholar]
- Pauli A. α-Bisabolol from chamomile—a specific ergosterol biosynthesis inhibitor. Int J Aromather. 2006;16:5–21. [Google Scholar]
- Ronda F, Gomes M, Blanco CA, Caballero PA. Effects of polyols and nondigestible oligosaccharides on the quality of sugar-free sponge cakes. Food Chem. 2005;90:549–555. [Google Scholar]
- Roos YH. Phase transitions in foods. San Diego: Academic Press; 1995. pp. 1–347. [Google Scholar]
- Roy S, Lingampeta P. Solid wastes of fruits peels as a source of low-cost broad spectrum natural antimicrobial compounds-furanone, furfural, and benezenetriol. Int J Res Eng Technol. 2014;3(7):273–279. [Google Scholar]
- Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep Purif Technol. 2005;41:49–55. [Google Scholar]
- Sharayei P, Azarpazhooh E, Zomorodi SH, Ramaswamy HS. Ultrasound-assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT Food SciTechnol. 2019;101:342–350. [Google Scholar]
- Shirode AB, Bharali DJ, Nallanthighal S, Coon JK, Mousa SA, Reliene R. Nanoencapsulation of pomegranate bioactive compounds for breast cancer chemoprevention. Int J Nanomed. 2015;10:475–484. doi: 10.2147/IJN.S65145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total polyphenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Soottitantawat A, Bigeard F, Yoshii H, Furuta T, Ohkawara M, Linko P. Influence of emulsion and powder size on the stability of encapsulated d-limonene by spray drying. Innov Food Sci Emerg Technol. 2005;6(1):107–114. [Google Scholar]
- Szabo K, Catoi AF, Vodnar DC. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant Foods Hum Nutr. 2018;73:268–277. doi: 10.1007/s11130-018-0691-0. [DOI] [PubMed] [Google Scholar]
- Thijssen HAC, Rulkens WH. Retention of aromas in drying food liquids. DeIngenieur. 1968;47:45–56. [Google Scholar]
- Tonon RV, Brabet C, Hubinger MD. Anthocyanin stability and antioxidant activity of spray-dried acai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res Int. 2010;43(3):907–914. [Google Scholar]
- Wang WD, Xu SY. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Eng. 2007;82(3):271–275. [Google Scholar]
- Wang G, Chen J, Shi Y. Preparation of microencapsulated xanthophyll for improving solubility and stability by nanoencapsulation. J Food Eng. 2013;117:82. [Google Scholar]
- Zam W, Bashour G, Abdelwahed W, Khayata W. Formulation and in vitro release of pomegranate peels’ polyphenols microbeads. Int J Pharm Sci Res. 2013;4:3536–3540. [Google Scholar]
- Zam W, Bashour G, Abdelwahed W, Khayata W. Alginate-pomegranate peels’ polyphenols beads: effects of formulation parameters on loading efficiency. Braz J Pharm Sci. 2014;50:741–748. [Google Scholar]
- Zuidam NJ, Shimoni E. Overview of microencapsulates for use in food products or processes and methods to make them. In: Zuidam NJ, Nedovic VA, editors. Encapsulation technologies for active food ingredients and food processing. New York: Springer; 2010. [Google Scholar]