Abstract
Ultrasonication technology was used to enhance the solubility and availability of lipophilic compounds as curcumin. This study aimed to know the optimal conditions to produce ultrasonication curcumin nanoemulsions stabilized with hydroxylated lecithin using response surface methodology and to evaluate some physical characteristics. Nanoemulsions were produced according to a Central Composite Face-center Design: surfactant oil ratio (SOR, 0.33–1.17), amplitude (A, 8–92%), and ultrasonication time (t, 2–18.4 min). Dynamic light scattering was used to measure the droplet size and polydispersity index of the nanoemulsions. Our results showed that a second-order polynomial function of amplitude and ultrasonication time model fitted well with the mean droplet size and polydispersity of the emulsions. Predicted droplet size was 122.2 nm and polydispersity index was 0.13 obtained at optimal conditions: SOR = 0.72, A = 92%, and t = 12 min. The nanoemulsions remained stable during 15 days of storage at 20 °C. Nanoemulsion remained stable to the aggregation in the pH range from 7.0 to 3.0, while the droplet size increased at lower pH values due to a loss of charge of the lecithin. Nanoemulsion applied in a sugar-beverage showed a yellow-green translucent color, showing better stability on the droplet size than the beverage with the coarse emulsion. Nanoemulsion could be used as a natural colorant in beverages.
Keywords: Nanoemulsion, Ultrasonication, Hydroxylated lecithin, Curcumin, Surface response methodology
Introduction
Curcumin is an oil-soluble phytochemical compound of the rhizome of turmeric recognized mainly for their antioxidant, anti-inflammatory, and anticancer activities. Commercial purified samples contain three significant components, curcumin I (77% diferuloylmethane), curcumin II (17% demethoxycurcumin), and curcumin III (6% bisdemethoxycurcumin) (Lee et al. 2013). These compounds are highly sensitive to pH, light, and oxygen. From the last decades, food researchers are working to improve their technological properties and therapeutic effectiveness as a functional food, which are limited by their low compatibility with the food matrix, low solubility, poor absorption, and limited bioavailability (Naksuriya et al. 2014). Recently, nanoemulsions have emerged as a promising technology to improve these problems (McClements 2012). The droplet size distribution is a critical property of food emulsions to enhance their appearance, solubility, and shelf life (McClements 2016; McClements and Xiao 2017). Structurally, the main difference between nanoemulsions (d < 200 nm) and emulsions (d > 200 nm) is the dimensions of the droplets (McClements 2016). Nanoemulsions are produced by high-intensity methods, including high-pressure homogenization, microfluidization, and ultrasonication (Donsì and Ferrari 2016; Edris 2012; Páez-Hernández et al. 2019). Páez-Hernández et al. (2019) found that the ultrasonication process produced smaller emulsions with a narrower distribution than microfluidization. Ultrasonication is a fast and efficient technique to produce emulsions with small droplet size distribution (Abbas et al. 2014; Carpenter and Saharan 2017; Páez-Hernández et al. 2019). The emulsion droplets are broken down by ultrasound waves of high frequency through mechanical vibrations and acoustic cavitation (Carpenter and Saharan 2017; Gaikwad and Pandit 2008; Gharibzahedi and Jafari 2018). The efficacy of ultrasonication process to reduce the droplet size depends on the processing parameters such as acoustic power, the amplitude of applied waves, and ultrasonication time and emulsion formulation such as emulsifier type and surfactant-oil ratio (Abbas et al. 2014; Carpenter and Saharan 2017; Gaikwad and Pandit 2008; Gharibzahedi and Jafari 2018; Ochoa et al. 2016; Páez-Hernández et al. 2019).
Soybean lecithin is a complex natural surfactant composed of a mixture of phospholipids, glycolipids, triglycerides, sterols, and fatty acids used as an emulsifier, stabilizer, and dispersing agent in the food, feed, and pharmaceutical industries (Klang and Valenta 2011; van Nieuwenhuyzen and Szuhaj 1998). The quantity and ratio of phospholipids in the lecithin (phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and phosphatidic acid) are related to the affinity of the dispersed phase and emulsifying properties (van Nieuwenhuyzen and Szuhaj 1998). The hydrophilic–lipophilic balance (HLB) number indicates the relative affinity of a surfactant molecule for the oil and water phases, and its tendency to form different kinds of association colloids and emulsions (McClements 2015). Standard lecithins are used as emulsifiers in water-in-oil emulsion due to their low HLB numbers. For this reason, standard lecithin is mixed with emulsifiers of higher HLB number to stabilize oil-in-water emulsions (Kim et al. 2016). Modifying lecithins have been developed to increase the HLB number of lecithins, e.g., acetylated lecithins have an HLB number from 7 to 9, or hydroxylated lecithins have a high HLB number from 10 to 12 (Carpenter and Saharan 2017; Klang and Valenta 2011; McClements 2015). Hydroxylated lecithin is produced by hydroxylation of the unsaturated acyl groups in the presence of hydrogen peroxide and a weak acid. Hydroxylated lecithin is predominantly hydrophilic, used to stabilize oil-in-water emulsions or help to prevent the tablet coating from cracking (van Nieuwenhuyzen and Tomás 2008). Thus, this study aimed to optimize the ultrasonication conditions (amplitude, ultrasonication time, and surfactant oil ratio) to produce curcumin nanoemulsions using hydroxylated lecithin as an emulsifier and to evaluate the physical stability during storage. Finally, coarse emulsion and nanoemulsion produced under the optimal conditions were applied in a model beverage, and their physicochemical properties were compared.
Materials and methods
Materials
Curcumin (purity > 95%) was provided by Future Foods (Mexico City, Mexico). Hydroxylated soy lecithin Emulfluid® HL 66 (HLB = 11) was supplied by Lallemand Mexico (Queretaro, Mexico) and medium-chain triglycerides was obtained from Gomas Naturales (Mexico City, Mexico). Absolute ethanol was purchased from “Reactivos Química Meyer” (Mexico City, Mexico). Deionized water was used in all experiments.
Preparation of curcumin nanoemulsions by ultrasonication
Four hundred milligrams of curcumin crystals were dispersed in 40 mL of ethanol. This solution was added to 40 mL of medium-chain triglycerides. Then, ethanol was removed using a rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) (Ochoa et al. 2016). Two grams of curcumin oil phase was mixed with the emulsifier (hydroxylated lecithin) and stored for 12 h in the dark. After that, the continuous phase was added until 20 g of total mass was achieved. Coarse emulsions were prepared using a T25 Ultra-Turrax (IKA Works, Inc., NC, USA) high-speed mixer at 6500 rpm for 4 min. Then, each coarse emulsion was homogenized using a VCX 130 PB (Sonics & Materials, Inc., Newtown CT, USA) ultrasonic processor with a 6 mm stainless steel probe. Nineteen emulsions were produced based on a central composite design (23 + star) including five replicates of the central point. Independent variables were surfactant oil ratio (SOR, 0.33–1.17); the amplitude (A, 8–92%); and ultrasonication time (t, 2–18.4 min). Finally, the emulsions were stored in a climatic chamber ICH-110L (Memmert GmbH + Co.KG, Germany) at 20 °C for 15 days.
Emulsions droplet size
The droplet size (DS) and polydispersity index (PdI) of curcumin emulsions were measured using a Zetasizer NanoZS90 (Malvern Instrument, UK). The Stokes–Einstein equation calculated the droplet size: DS = kBT/3πηsD; where kB is the Boltzmann constant, T is the temperature (K), ηs is the viscosity of the solvent, and D is the Z-average translational diffusion coefficient. Each emulsion was diluted to a concentration of 0.01 g/mL. All measurements were made at 20 °C. The polydispersity index is a metric for distribution width and was used for comparative purposes, i.e., narrow distributions, PdI ≤ 0.2; broader distributions, PdI > 0.7 (ASTM E2490-09 2009). Polydispersity index for DLS depicts the intensity of light scattered by various fractions of the particles differing in their sizes and is calculated by (width/mean)2 for each peak (Bhattacharjee 2016). The values reported were measured in triplicate.
Statistical analysis
Response surface methodology was used to know the effect of amplitude, ultrasonication time, and surfactant oil ratio on the droplet size and the polydispersity index of nanoemulsions. These effects were performed by a three-factor, two-level central composite design, including five replicates of the central point (Table 1). The experimental results were analyzed using the Statgraphics Centurion XV statistical software (v. 2.15.06), fitting a quadratic model to establish a relationship between independent variables (SOR, A, and t) with response variables (Y ≡ DS or PdI), as follows:
| 1 |
Table 1.
Droplet size and polydispersity index of curcumin emulsions produced by ultrasonication
| Experiment | SOR | A (%) | t (min) | DS (nm) | PdI |
|---|---|---|---|---|---|
| E1 | 0.75 | 50 | 1.6 | 436.5 | 0.419 |
| M1 | 0.75 | 8 | 10.0 | 303.1 | 0.279 |
| M2 | 1.17 | 50 | 10.0 | 182.6 | 0.191 |
| L1 | 0.50 | 75 | 5.0 | 208.1 | 0.185 |
| M3 | 0.33 | 50 | 10.0 | 202.9 | 0.191 |
| M4 | 0.75 | 92 | 10.0 | 139.5 | 0.148 |
| L2 | 0.50 | 25 | 5.0 | 291.7 | 0.324 |
| M5 | 0.75 | 50 | 10.0 | 182.8 | 0.143 |
| M6 | 0.75 | 50 | 10.0 | 179.8 | 0.173 |
| H1 | 0.50 | 75 | 15.0 | 152.4 | 0.162 |
| M7 | 0.75 | 50 | 10.0 | 180.6 | 0.176 |
| L3 | 1.00 | 25 | 5.0 | 370.8 | 0.422 |
| H2 | 1.00 | 75 | 15.0 | 144.8 | 0.165 |
| E2 | 0.75 | 50 | 18.4 | 154.1 | 0.182 |
| M8 | 0.75 | 50 | 10.0 | 178.1 | 0.182 |
| L4 | 1.00 | 75 | 5.0 | 191.9 | 0.206 |
| M9 | 0.75 | 50 | 10.0 | 180.1 | 0.180 |
| H3 | 1.00 | 25 | 15.0 | 219.8 | 0.243 |
| H4 | 0.50 | 25 | 15.0 | 220.1 | 0.208 |
The estimated regression coefficients of the polynomial are represented by b0 (constant term), b1, b2 and b3 (linear effects), b11, b22 and b33 (quadratic effects), and b12, b13 and b23 (interaction effects). The quality of the fit was expressed with the coefficient of the determination (R2):
| 2 |
The response surface and contour plots were used to specify the interrelationships between significant variables (Li and Chiang 2012). Multiple responses were minimized according to desirable characteristics of the nanoemulsions, i.e., smaller droplet size (< 200 nm) and lower polydispersity index (< 0.2). Analysis of variance (ANOVA) test was used to determine the differences between treatments mean (P < 0.05) according to Tukey’s test.
Evaluation of the nanoemulsion at different pH
The droplet size and zeta potential were evaluated for the curcumin nanoemulsion produced with the optimal conditions at different pH values. This nanoemulsion was diluted and transferred to an autotitrator MPT-2 coupled to the Zetasizer equipment. Zeta potential was determined, measuring the direction and velocity of the biopolymers dispersion as they moved along the applied electric field. The Zetasizer software changed the electrophoretic mobility measurements into zeta potential values using the Helmholtz–Smoluchowski model. Experiments were performed at pH ranging from 7 to 2 every 0.5 units with a pH resolution of 0.1 units. The pH of the sample was adjusted using either 0.1 N HCl or 0.1 N NaOH. Titration measurements were reported as the average ± standard deviation of measurements made on two independent samples with three measurements made at 20 °C.
Evaluation of the physicochemical properties in a beverage model
The coarse emulsion and optimal nanoemulsion were applied in a sugar-beverage (pH 3 and 10% sugar). One gram of each emulsion was diluted in 100 g of sugar beverage. The CIELAB color space was used to measure the color of the beverage. Samples were measured using a CM-5 spectrophotometer (Konica Minolta, Tokyo, Japan). The standard illuminant D65 and an 8° standard observer were used to measure the samples. The value of L* is lightness, and a* and b* are color coordinates: where L* = 0 is black, L* = 100 is white, +a* is the red direction, − a* is the green direction, +b* is the yellow direction and − b* is the blue direction.
Results and discussion
Optimization of conditions to produce curcumin nanoemulsion
Surface response methodology was applied to know the effect of the amplitude, ultrasonication time, and surfactant oil ratio on the droplet size and polydispersity index of the nanoemulsions. A two-step procedure prepared the curcumin nanoemulsions. In the first step, the coarse emulsions showed multimodal droplet size distributions characterized by droplet sizes above 2.0 µm with polydispersity indexes higher than 0.65. Droplet size distribution offers essential information about the inherent stability and functional performance of the overall system (McClements 2015). Then, the ultrasonication process decreased the mean droplet size between 139.5 nm (E1) and 436.5 nm (M4), showing polydispersity indexes lower than 0.42 (Table 1). Experimental data were used to estimate the quadratic polynomial coefficients’ (Eq. 2) to predict the droplet size and polydispersity index values of the curcumin nanoemulsions (Table 2). Analysis of variance was used to evaluate the significance of the coefficients (P < 0.05). The determination coefficient of the optimized quadratic-models for droplet size was 0.9251 and for polydispersity index, 0.9505. These results indicated that the quadratic models were acceptable to predict the droplet size and polydispersity index of curcumin nanoemulsions. In the regression equation for the DS model, the surfactant/oil ratio linear term (SOR), surfactant/oil ratio × ultrasonication time interaction (SOR·t), surfactant/oil ratio × amplitude interaction (SOR·A), and the surfactant/oil ratio quadratic term (SOR2) had no significant impact among the nanoemulsions produced (P > 0.05). Donsì and Ferrari (2016) suggested that high SOR values favored the production of nanoemulsions by ultrasonication. Besides, it was found that the amplitude linear term (A) and ultrasonication time linear term (t) showed a negative effect on the droplet size values, while the quadratic coefficients (A2 and t2) had a positive significant effect (P < 0.001) on this parameter. After reducing the significant terms, the reduced determination coefficient was of 0.9075 for the DS quadratic model. The adjusted regression model is represented below:
| 3 |
Table 2.
Analysis of variance of the regression coefficients of the fitted quadratic equations for the droplet size and polydispersity index of curcumin emulsions
| Variable | DS | PdI | ||||
|---|---|---|---|---|---|---|
| Coefficient | F-value | P value | Coefficient | F-value | P value | |
| 529.28 | 6.32E−01 | |||||
| SOR | 149.99 | 0.03 | 0.8580 | 6.21E−02 | 2.63 | 0.1390 |
| A | − 3.46 | 36.04 | 0.0002 | − 5.16E−03 | 52.27 | 0.0000 |
| t | − 42.39 | 49.88 | 0.0001 | − 5.33E−02 | 61.35 | 0.0000 |
| SOR2 | 19.68 | 0.02 | 0.8854 | 1.16E−01 | 1.05 | 0.3332 |
| SORA | − 2.05 | 1.40 | 0.2671 | − 2.18E−03 | 2.17 | 0.1750 |
| SORt | − 7.08 | 0.67 | 0.4354 | − 8.10E−03 | 1.20 | 0.3023 |
| A2 | 0.02 | 1.86 | 0.2056 | 2.43E−05 | 4.60 | 0.0605 |
| At | 0.12 | 1.91 | 0.2005 | 2.31E−04 | 9.74 | 0.0123 |
| t2 | 1.50 | 20.40 | 0.0015 | 1.84E−03 | 42.09 | 0.0001 |
| R2 (%) | 92.51 | 95.05 | ||||
The bold letters represent P values < 0.05
From this, it can be observed that the amplitude and the ultrasonication time influenced the droplet size of the emulsions. The influence of the ultrasonication time linear coefficient (t) was 9.5 times higher than the amplitude linear coefficient (A). Moreover, the positive values of the quadratic coefficients predicted a minimum region for the nanoemulsion droplet size. These results suggest that moderate amplitudes produce nanoemulsions using a long ultrasonication time. For example, the predicted droplet size was 158 nm using an amplitude of 50% for 14 min. Minimum mean droplet size predicted was of 121 nm using the amplitude of 99% for 12 min. This result was expected because of as the amplitude increases, the energy that breaks the large droplets into smaller increases too. Gaikwad and Pandit (2008) and Lane et al. (2016) observed that the maximum ultrasonication intensity produced the maximum droplet disruption.
The contour plot of the nanoemulsions droplet size displayed an elliptical boundary of the amplitude and ultrasonication time (Fig. 1a). In this, the gray shaded region shows the conditions to produce nanoemulsions (DS < 200 nm). Based on the response surface plot for droplet size, the quadratic model predicted a significant increase in droplet size for high amplitude and ultrasonication time values. Many authors have been suggested that the small droplet size was produced using a moderate energy-input, and then, when a higher energy-input was used, larger droplet sizes were observed, a behavior known as “over-processing” (Jafari et al. 2007; Kentish et al. 2008; Lane et al. 2016; Lu et al. 2018).
Fig. 1.
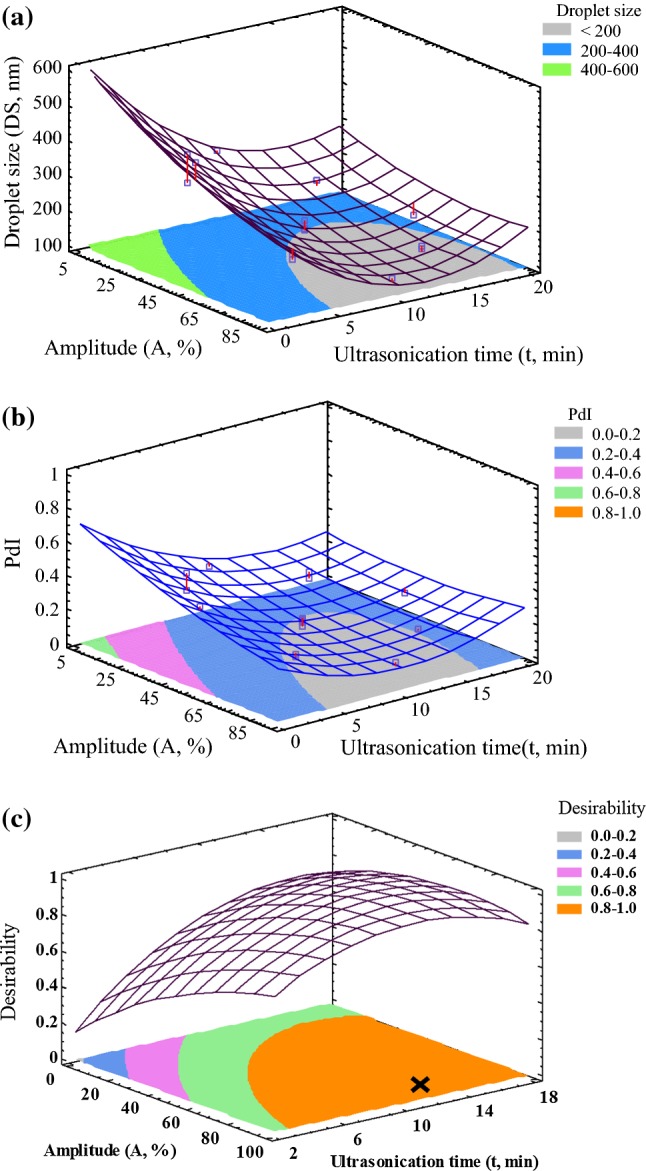
Response surface contour plots of the combined effects of amplitude (A) and ultrasonication time (t) on the curcumin nanoemulsions: a droplet size, b polydispersity index, and c overall desirability function
The effects of the amplitude and ultrasonication time on the polydispersity index of nanoemulsion are shown in Fig. 1b. Polydispersity index values lower than 0.2 specifying uniformity in the droplet size distribution (gray shadow), and consequently, excellent physical stability is feasible (García-Márquez et al. 2017). The coefficients of the surfactant/oil ratio (SOR), amplitude × ultrasonication time interaction (A·t), and the quadratic coefficients of the amplitude (A2) and ultrasonication time (t2) had positive effects on the polydispersity index value. While the amplitude linear coefficient (A) and ultrasonication time coefficient (t) showed a negative impact on the polydispersity index value. The reduced determination coefficient was 0.9260. The regression model is represented below:
| 4 |
The quadratic model of the polydispersity index predicted a small increase in the polydispersity index for long ultrasonication time. In general, the narrowed droplet size distribution by ultrasonication depends on the energy density supplied to the sample and shear forces created by the ultrasonic processor. Abbas et al. (2014) suggested that the droplet size distribution of nanoemulsions could be modified by increasing the ultrasonication time due to small changes in viscosity and interfacial tension of the sample. These changes could increase the collision rate between the nanoemulsion droplets, increasing the probability to flocculate or coalescence of the sample. All the experimental conditions produced fine emulsions, but only a few experimental conditions were suitable to produce the nanoemulsions. A minimum level of the amplitude of ultrasonication and a minimum level of ultrasonication time were set for the maximum desirability. The effect of the amplitude and the ultrasonication time on the combined desirability values to produce nanoemulsions is shown in Fig. 1c. The cross mark represents the optimal conditions predicted to produce curcumin nanoemulsions (98.0 ± 0.5% of desirability). A nanoemulsion of 122.2 nm with a polydispersity index of 0.13 was produced employing a surfactant/oil ratio of 0.72, an amplitude of 92% for 12 min according to the statistical model.
Statistical models were validated by performing a confirmation experiment using the predicted optimal conditions. Figure 2 was generated by varying the ultrasonication time while holding constant the surfactant oil ratio at 0.72 and the amplitude at 92%. When the optimal conditions were used, the experimental droplet size was 126 nm, and the experimental polydispersity index was 0.15, less than 0.5% error was observed in comparison to the predicted data, indicating the accuracy of the models. However, it was necessary to point out that the increase of ultrasonication time higher than 12 min results in a droplet size reduction, instead of the overprocessing of the sample predicted by the statistical models. This could be explained by the high affinity of the hydroxylated lecithin for curcumin-oil phase resulted in high stability of the nanoemulsion under the shear forces produced by ultrasonication processor. Páez-Hernández et al. (2019) and Herrera-Rodríguez et al. (2019) did not observe the overprocessing of the nanoemulsions stabilized with hydroxylated lecithin produced by ultrasonication processors. Ochoa et al. (2016) reported that curcumin nanoemulsions stabilized with phosphatidylcholine presented an overprocessing using an amplitude greater than 40% with an ultrasonic processor of 450 W.
Fig. 2.
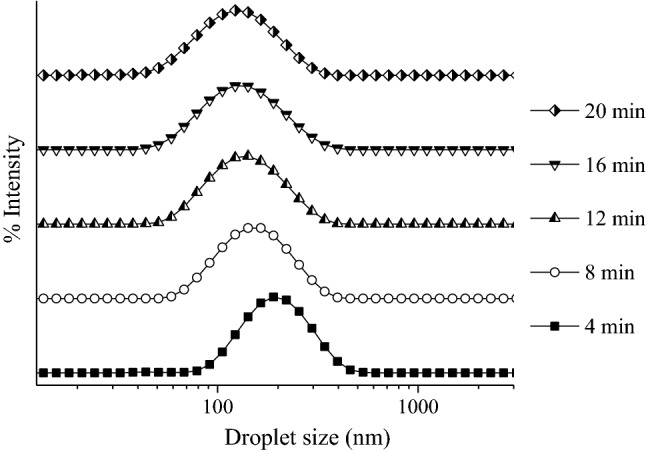
Droplet size distributions of nanoemulsion produced with a surfactant/oil ratio of 0.72 and amplitude of 92% a different ultrasonication time: 4 min (square), 8 min (circle), 12 min (up triangle), 16 min (down triangle), and 20 min (diamond)
Time stability of nanoemulsions at storage conditions
The storage stability of the nanoemulsions was evaluated after 15 days of storage at 20 °C (Fig. 3). Under these conditions, gravitational separation or Ostwald ripening was not observed. Kim et al. (2016) found that curcumin loaded in MCT oils acted as an Ostwald ripening inhibitor, mainly, when it was emulsified with a surfactant mixture (6% Tween-20 and 4% sorbitan monooleate) with an HLB number of 11.7, instead surfactant mixtures of higher HLB number. Similarly, hydroxylate lecithin used in this work has an HLB number of approximately 11, near to those HLB number suggested by Kim et al. (2016). The highest stability of nanoemulsions was associated with the smaller droplet size and lower polydispersity index values. However, the mean droplet size values of nanoemulsions produced with the lowest amplitude and ultrasonication time (L2, L3, and M1) shifted to larger ones after 15 days of storage, as shown in Fig. 3.
Fig. 3.
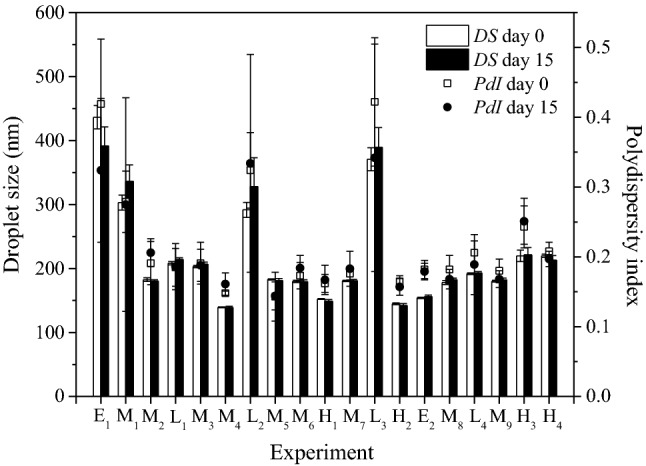
Droplet size (bars) and polydispersity index (square) of the nanoemulsions stored at 20 °C. Stored time: 0 days (empty symbols) and 15 days (filled symbols)
On the other hand, nanoemulsions produced with the highest amplitude and ultrasonication time values were stable. Edris (2012) observed that the droplet size of nanoemulsions (79.8 ± 1.0 nm) formed with defatted lecithin using high-pressure microfluidization remained stable for 3 months. Herrera-Rodríguez et al. (2019) reported that the nanoemulsion stabilized with hydroxylated lecithin showed excellent stability for 28 days at 25 and 40 °C. These findings support the idea that a more stable nanoemulsion is obtained when smaller droplet size and lower polydispersity index values were produced.
Stability of nanoemulsions at different pH
Some emulsions are physically unstable during the manufacturing, transport, or storage affecting product quality (McClements 2016). The changes in droplet size and zeta potential of the nanoemulsion produced with the optimal conditions at different pH values are shown in Fig. 4. Zeta potential is a measure of the emulsion charge density and offers an indication of the inherent stability of emulsions to aggregation or interaction with other molecules in the food matrix (Sui et al. 2017). The zeta potential of the emulsion decreased slightly from − 66 to − 45 mV as the pH decreased from 7.0 to 3.0, but then the zeta potential was reduced dramatically until reach − 1 mV at pH 2.0. According to the zeta potential values, the nanoemulsion droplet size remained stable to aggregation or coalescence under the pH range from 7.0 to 3.0. Below this pH range, the nanoemulsion droplets increased up to 1 μm, showing the flocculation of the nanoemulsion due to the loss of charge of lecithin molecules. Flocculation is the process in which emulsion droplets aggregate without rupture of the surfactant layer between them. Flocculation was accompanied by changes on emulsion droplet size, allowing a phase separation. The results are consistent with those reported by Ozturk et al. (2014) and Wu et al. (2017). They observed that lecithin emulsions had low pH stability in an acidic environment due to a loss of charge of the lecithin.
Fig. 4.
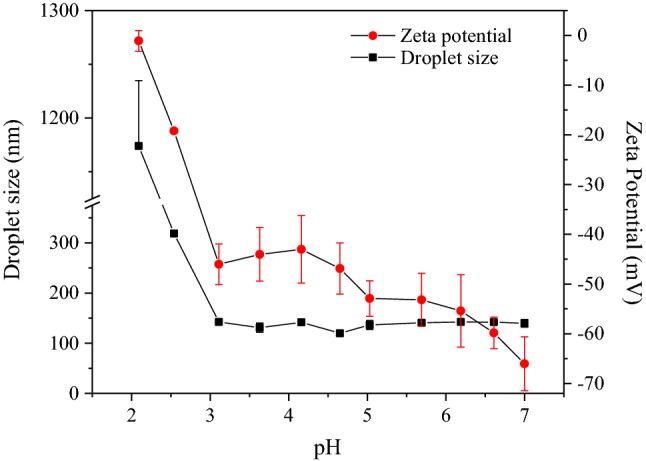
Droplet size (square) and zeta potential (circle) of the nanoemulsion produced with the optimal conditions at different pH values
The optimal nanoemulsion and their respective coarse emulsion were applied in a sugar-beverage (pH 3 and 10% sugar). Droplet size, zeta potential, and color of the resulted beverage are shown in Table 3. The optical properties of beverages are principally determined by their opacity and color (McClements 2015). The beverage with the coarse emulsion showed low stability due to their larger droplet size and lower zeta potential value inducing a rapid phase separation. Also, the beverage was a yellowish-green color with low turbidity produced by the droplet size distribution. When the droplet size is similar to the wavelength of the visible light (380–780 nm), the emulsion appears turbid or opaque (Zhang and McClements 2018). In contrast, the nanoemulsion showed a better performance in the sugar-beverage due to high stability displaying a yellow-green translucent color generated by a less lightness, less green, and more yellow in comparison with the beverage with the coarse emulsion. These results show that curcumin nanoemulsions could be applied in foods with a pH value equal to or higher than 3.0, such as dairy foods, beverages, or low acid fruit juices.
Table 3.
Physicochemical properties of the curcumin emulsions applied in a sugar-beverage base
| Type of emulsion | DS (nm) | PdI | Zeta potential (mV) | L* | a* | b* | Color |
|---|---|---|---|---|---|---|---|
| Coarse emulsion | 590 ± 93a | 0.60 ± 0.064a | − 14.8 ± 0.5a | 95.31a | − 13.58a | 57.35a |  |
| Nanoemulsion | 126 ± 2b | 0.16 ± 0.005b | − 26.4 ± 0.7b | 83.97b | − 8.82b | 70.16b |
Mean value ± standard deviation of three measurements. Different letters in the same column are significantly different (P < 0.05)
Conclusion
Stable curcumin nanoemulsion can be obtained using hydroxylated lecithin as emulsifier agent using the ultrasonication technology. The results showed that the amplitude and ultrasonication time had significant effects on the droplet size and polydispersity index of the nanoemulsions, while the surfactant/oil ratio did not have a considerable impact on both response variables. Second-order polynomial models predicted-well the drop size and polydispersity index of the nanoemulsions at moderate ultrasonication time. However, these did not show an appropriate adjustment to considerable ultrasonication time. Nanoemulsion of 122.2 nm and a polydispersity index of 0.13 was obtained using a surfactant/oil ratio of 0.72, 92% of amplitude for 12 min. The statistical models predict an over-processing of the emulsion with longer ultrasonication times. However, the experimental data did not show a significant variation of the droplet size and the polydispersity index of the nanoemulsions. Nanoemulsions exhibit excellent physical stability when stored at 20 °C for 15 days and a broad range of pH from 3.0 to 7.0. Nanoemulsion provides an overall appearance in an acid beverage. These results provide useful information for developing curcumin nanoemulsion for nutraceutical foods with suitable stability.
Acknowledgements
This work was supported by the “SEP-CONACYT Investigación Básica” under Grant CB-2015-01-258118.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hugo Espinosa-Andrews, Email: hespinosa@ciatej.mx.
Gladys Páez-Hernández, Email: gpaez@ciatej.mx.
References
- Abbas S, Bashari M, Akhtar W, Li WW, Zhang X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason Sonochem. 2014;21:1265–1274. doi: 10.1016/j.ultsonch.2013.12.017. [DOI] [PubMed] [Google Scholar]
- ASTM E2490-09 (2009) Standard guide for measurement of particle size distribution of nanomaterials in suspension by photon correlation spectroscopy (PCS). ASTM International, West Conshohocken, PA. 10.1520/E2490-09
- Bhattacharjee S. DLS and zeta potential: what they are and what they are not? J Control Release. 2016;235:337–351. doi: 10.1016/j.jconrel.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Carpenter J, Saharan VK. Ultrasonic assisted formation and stability of mustard oil in water nanoemulsion: effect of process parameters and their optimization. Ultrason Sonochem. 2017;35:422–430. doi: 10.1016/j.ultsonch.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Edris AE. Formulation and shelf life stability of water-borne lecithin nanoparticles for potential application in dietary supplements field. J Diet Suppl. 2012;9:211–222. doi: 10.3109/19390211.2012.708717. [DOI] [PubMed] [Google Scholar]
- Gaikwad SG, Pandit AB. Ultrasound emulsification: effect of ultrasonic and physicochemical properties on dispersed phase volume and droplet size. Ultrason Sonochem. 2008;15:554–563. doi: 10.1016/j.ultsonch.2007.06.011. [DOI] [PubMed] [Google Scholar]
- García-Márquez E, Higuera-Ciapara I, Espinosa-Andrews H. Design of fish oil-in-water nanoemulsion by microfluidization. Innov Food Sci Emerg Technol. 2017;40:87–91. doi: 10.1016/j.ifset.2016.11.007. [DOI] [Google Scholar]
- Gharibzahedi SMT, Jafari SM. Chapter 9: fabrication of nanoemulsions by ultrasonication. In: Jafari SM, Mcclements DJ, editors. Nanoemulsions. Cambridge: Academic Press; 2018. pp. 233–285. [Google Scholar]
- Herrera-Rodríguez SE, López-Rivera RJ, García-Márquez E, Estarrón-Espinosa M, Espinosa-Andrews H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci Biotechnol. 2019;28:441–448. doi: 10.1007/s10068-018-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488. doi: 10.1016/j.jfoodeng.2007.03.007. [DOI] [Google Scholar]
- Kentish S, Wooster TJ, Ashokkumar M, Balachandran S, Mawson R, Simons L. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol. 2008;9:170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- Kim S-H, Ji Y-S, Lee E-S, Hong S-T. Ostwald ripening stability of curcumin-loaded MCT nanoemulsion: influence of various emulsifiers. Prev Nutr Food Sci. 2016;21:289–295. doi: 10.3746/pnf.2016.21.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klang V, Valenta C. Lecithin-based nanoemulsions. J Drug Deliv Sci Technol. 2011;21:55–76. doi: 10.1016/S1773-2247(11)50006-1. [DOI] [Google Scholar]
- Lane KE, Li W, Smith CJ, Derbyshire EJ. The development of vegetarian omega-3 oil in water nanoemulsions suitable for integration into functional food products. J Funct Foods. 2016;23:306–314. doi: 10.1016/j.jff.2016.02.043. [DOI] [Google Scholar]
- Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol. 2013;11:338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P-H, Chiang B-H. Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem. 2012;19:192–197. doi: 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Lu W-C, Huang D-W, Wang C-CR, Yeh C-H, Tsai J-C, Huang Y-T, Li P-H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J Food Drug Anal. 2018;26:82–89. doi: 10.1016/j.jfda.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- McClements DJ. Nanoparticle and microparticle based delivery systems. Boca Raton: CRC Press; 2015. pp. 149–165. [Google Scholar]
- McClements DJ. Emulsion ingredients. Boca Raton: Taylor & Francis Group; 2016. [Google Scholar]
- McClements DJ, Xiao H. Designing food structure and composition to enhance nutraceutical bioactivity to support cancer inhibition. Semin Cancer Biol. 2017;46:215–226. doi: 10.1016/j.semcancer.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- Ochoa AA, Hernández-Becerra JA, Cavazos-Garduño A, Vernon-Carter EJ, García HS. Preparation and characterization of curcumin nanoemulsions obtained by thin-film hydration emulsification and ultrasonication methods. Revista Mexicana de Ingeniería Química. 2016;15:79–90. [Google Scholar]
- Ozturk B, Argin S, Ozilgen M, McClements DJ. Formation and stabilization of nanoemulsion-based vitamin E delivery systems using natural surfactants: quillaja saponin and lecithin. J Food Eng. 2014;142:57–63. doi: 10.1016/j.jfoodeng.2014.06.015. [DOI] [Google Scholar]
- Páez-Hernández G, Mondragón-Cortez P, Espinosa-Andrews H. Developing curcumin nanoemulsions by high-intensity methods: impact of ultrasonication and microfluidization parameters. LWT. 2019;111:291–300. doi: 10.1016/j.lwt.2019.05.012. [DOI] [Google Scholar]
- Sui X, Bi S, Qi B, Wang Z, Zhang M, Li Y, Jiang L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: its emulsifying property and emulsion stability. Food Hydrocoll. 2017;63:727–734. doi: 10.1016/j.foodhyd.2016.10.024. [DOI] [Google Scholar]
- van Nieuwenhuyzen W, Szuhaj BF. Effects of lecithins and proteins on the stability of emulsions. Lipid/Fett. 1998;100:282–291. doi: 10.1002/(sici)1521-4133(199807)100:7<282::aid-lipi282>3.0.co;2-w. [DOI] [Google Scholar]
- van Nieuwenhuyzen W, Tomás MC. Update on vegetable lecithin and phospholipid technologies. Eur J Lipid Sci Technol. 2008;110:472–486. doi: 10.1002/ejlt.200800041. [DOI] [Google Scholar]
- Wu M-H, Yan HH, Chen Z-Q, He M. Effects of emulsifier type and environmental stress on the stability of curcumin emulsion. J Dispers Sci Technol. 2017;38:1375–1380. doi: 10.1080/01932691.2016.1227713. [DOI] [Google Scholar]
- Zhang Z, McClements DJ. Chapter 2: overview of nanoemulsion properties: stability, rheology, and appearance. In: Jafari SM, Mcclements DJ, editors. Nanoemulsions. Cambridge: Academic Press; 2018. pp. 21–49. [Google Scholar]


