Abstract
Protein hydrolysates were obtained from salmon frame using Alcalase or Flavourzyme at 3% (w/w protein) for 180 min. Protein hydrolysates prepared using Alcalase (HA) and Flavourzyme (HF) had DH and yield of 25.1–26.9% and 28.5–32.3 g/100 g sample, respectively. HF showed lower bitterness score (5.78) than that of HA (8.68) (P < 0.05). When HA and HF were further subjected to debittering with 2-butanol or isopropanol, the recovery of 77.88–81.60% was obtained (P < 0.05). HF and HA debittered with 2-butanol possessed less bitterness score, 3.60 and 3.77, respectively (P < 0.05). Surface hydrophobicity of 81.4 and 124.8 was attained when HF and HA were debittered with 2-butanol (P < 0.05). Selected debittered hydrolysates, produced using Flavourzyme, followed by fractionation using 2-butanol (HF-B) contained glutamic acid/glutamine (15.14 g/100 g), aspartic acid/asparagine (10.07 g/100 g) and glycine (9.30 g/100 g) as the predominant amino acids. HF-B had the decreased ABTS radical scavenging activity and metal chelating activity. A280 of peptides separated by gel filtration was lowered to some extent and coincided with the lower bitterness score and surface hydrophobicity. Thus, debittered protein hydrolysate from salmon frame could serve as a nutritive ingredient at high levels in health promoting foods.
Keywords: Salmon frame, Protein hydrolysate, Alcalase, Flavourzyme, Debittering
Introduction
The global demand for foods from aquaculture surpassed 160 million tons in 2014 (FAO 2016). Salmon is one of popular species owing to its delicacy. Salmon (Salmo salar) has been imported to Thailand to serve for increasing demand for Thai consumers. Generally, salmon is sold as a fillet or whole fish (Idowu et al. 2018). During salmon filleting, by-products obtained from salmon processing include frames, trimmings (containing muscle, bone and skin), heads (containing the gills) and viscera (liver, kidney and roe) (See et al. 2011). Those leftovers contain significant amount of protein, which can be recovered as a source of food ingredients through enzymatic hydrolysis (Idowu et al. 2018). Fish processing by-products were subjected to enzymatic hydrolysis for recovery of valuable components (Nalinanon et al. 2011). Fish protein hydrolysates (FPH) have been reported to exhibit good functional and nutritional properties as well as bioactivities (Idowu et al. 2018). Nevertheless, a major drawback of protein hydrolysate is the sensation of bitter taste. The bitterness of protein hydrolysates inevitably limits their potential use as a nutritive food ingredient. Consequently, they are incorporated into foods at low level to avoid the bitterness (Hou et al. 2011). Bitterness of hydrolysate is influenced by type of proteases used for hydrolysis. Whey protein hydrolysates produced using Alcalase were more bitter than those produced with Prolyve 1000 or Corolase 7089 (Spellman et al. 2009). The bitterness of protein hydrolysate could be as a result of the specificity of enzyme used to produce the hydrolysate (Spellman et al. 2009). Seo et al. (2008) documented that the hydrolysate from soy protein isolate prepared using Flavourzyme showed the lowest bitterness, compared to those prepared using Alcalase, neutrase, protamex and papain. Flavourzyme is a mixture of enzymes containing both endo- and exo-peptidase. The exopeptidase is able to decrease the bitterness of a bitter peptide by removing terminal hydrophobic amino acids (Tavano 2013).
The bitter taste in protein hydrolysate is due to the formation of low molecular weight peptides made up of hydrophobic amino acids (Idowu et al. 2018). In general, most hydrophobic amino acids are positioned towards the interior of the globular proteins. However, peptides containing hydrophobic amino acids are exposed during enzymatic hydrolysis and interact with taste buds, resulting in a bitter taste (Idowu et al. 2018). Hydrophobic amino acid residues become more exposed with increasing degree of hydrolysis, thereby augmenting bitterness (Idowu et al. 2018). To conquer such a problem, debittering processes of protein hydrolysates have been developed, e.g. selective extraction with alcohols, absorption of bitter peptides on activated carbon and chromatographic removal using different matrices (FitzGerald and O’Cuinn 2006).
Protein hydrolysates from salmon frames have been recently produced. However, they had undesirable bitter taste (Idowu et al. 2018). Debittering using some selected alcohols such as 2-butanol and iso-propanol to remove hydrophobic peptides or free amino acids could be a means to lower bitterness of hydrolysate from salmon frame. However, debittering process might impact on the bioactivity, particularly antioxidant activity. A little information regarding the bitterness of protein hydrolysates from salmon frames using different enzymes subjected to subsequent fractionation using various alcohols exists. Therefore, this work aimed to examine the effect of debittering of protein hydrolysates from salmon frame prepared by Alcalase or Flavourzyme using some alcohols and to characterize and determine antioxidative activities of the resulting debittered hydrolysates.
Materials and methods
Enzymes and chemicals
Alcalase (2.4 L enzyme) from Bacillus licheniformis and Flavourzyme (500L) were obtained from Novozyme (Bagsvaerd, Denmark). 2,4,6-trinitrobenzenesulphonic acid (TNBS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-triazine (TPTZ), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt (ferrozine), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Caffein was procured from Merck KGaA Co. (Darmstadt, Germany). SephadexTM G-25, blue dextran and gel filtration calibration kits (vitamin B12, flavin adeninedinucleotide and glycine-tryrosine) were procured from GE Healthcare (Uppsala, Sweden). All the chemicals used were of analytical grade.
Preparation of salmon frames
Frames of salmon (Salmo salar) of about 30–35 cm in length were obtained from Kingfisher Holding Ltd., Songkhla, Thailand. They were packed in a polyethylene bag, placed in a polystyrene box and embedded in ice with sample/ice ratio of 1:2 (w/w). The samples were delivered to Department of Food Technology, Prince of Songkla University, Hat Yai, Songkhla, within approximately 2 h. The samples were stored at − 20 °C until used, but not more than 3 months.
Production of protein hydrolysates from salmon frames using different proteases
Hydrolysis of salmon frame
Protein hydrolysates were prepared from salmon frames as described by Idowu et al. (2018). Frozen salmon frames were firstly thawed overnight at 4 °C and the size was reduced to 4–5 cm in length with the aid of electric cutting machine (W210E, Union Kitchen & Service, Bangkok, Thailand). The salmon frame was mixed with distilled water at a ratio of 1:1 (w/v). Thereafter, the pH of the mixtures was adjusted to 8.0 using either 1.0 M NaOH or 1.0 M HCl. The mixture was pre-heated using a water bath (Model W350, Memmert, Schwabach, Germany) for 15 min at 55 °C or 60 °C for Flavourzyme or Alcalase, respectively. Subsequently, Alcalase or Flavourzyme at a level of 3.0% (w/w) was added to the mixture. Hydrolysis was conducted for 3 h. Thereafter, the mixtures were heated at 90 °C for 15 min to terminate the reaction. All the mixtures obtained were cooled down to room temperature using running water and filtered with two layers of cheesecloth to remove the undigested bones. Then filtrates were centrifuged at 4000 × g at 4 °C using an Avanti® J-E refrigerated centrifuge (Beckman Coulter, Inc., Palo Alto, CA, USA) for 15 min. The supernatants were freeze-dried using a freeze-dryer (CoolSafe 55, ScanLaf A/S, Lynge, Denmark) for 72 h. The hydrolysates from salmon frames with the aid of Alcalase and Flavourzyme were referred to as “HA” and “HF”, respectively. Both hydrolysates were subjected to analyses.
Analyses
Determination of degree of hydrolysis (DH)
DH of the hydrolysates was determined as tailored by Benjakul and Morrissey (1997).
Yield
The yield of hydrolysate was calculated based on the dry weight of initial salmon frames after drying at 105 °C for 12 h in a hot air oven.
Determination of bitterness
Bitterness of hydrolysates was examined by five female and six male panelists aged between 25 and 33 as detailed by Idowu et al. (2018).
Debittering of protein hydrolysates from salmon frames using various alcohols
Debittering of protein hydrolysates
Removal of bitter compounds using various alcohols was carried out following the method of Lalasidis and Sjoberg (1978) with a slight modification. Both HA and HF were solubilized in distilled water to obtain a concentration of 10% (w/v). The alcohols (2-butanol and iso-propanol) were added and mixed with hydrolysate at a ratio of 1:4 (v/v). The mixtures were stirred for 10 min at room temperature. Then the mixtures were centrifuged at 2250 × g at 4 °C using an Avanti® J-E refrigerated centrifuge (Beckman Je-avanti Fullerton, CA, USA) for 30 min. The lower fraction (hydrolysate rich fraction) was collected and subjected to debittering with the same manner for another time. Thereafter, hydrolysate rich phase was collected and freeze-dried using a freeze-dryer. All the hydrolysates obtained were then analyzed.
Analyses
Determination of nitrogen recovery
Nitrogen recovery in the debittered hydrolysates was calculated based on initial nitrogen content of hydrolysate before debittering as follows:
Determination of surface hydrophobicity
Surface hydrophobicity of the hydrolysates was determined as per the method of Quan and Benjakul (2018) using ANS as a probe.
Determination of solubility
Hydrolysates were dissolved in deionized water to obtain a concentration of 1% (w/v). The pH was adjusted to 7 with either 2 N NaOH or 2 N HCl. The samples were centrifuged at 14,000 × g for 15 min at room temperature. Protein content in the supernatant was measured using the Biuret method (Robinson and Hogden 1940). For total protein, the samples were solubilized with 0.5 M NaOH. Solubility was reported as the percentage.
Molecular weight distribution
Molecular weight distribution of hydrolysate samples was analyzed using a Sephadex G-25 gel filtration column (2.5 × 50 cm) (17-0032-01, GE Healthcare Bio-Science AB, Uppsala, Sweden) as detailed by Idowu et al. (2018).
Determination of antioxidative activities
Antioxidative activities of hydrolysate samples were determined as follows: ABTS radical scavenging activity, DPPH radical scavenging activity, ferric reducing antioxidant power (FRAP) and metal chelating activity (Sae-leaw et al. 2016). The activities were expressed as µmol Trolox (TE) equivalent/g sample, except for metal chelating activity, which was reported as µmol EDTA equivalent/g sample.
Amino acid analysis
Hydrolysates without debittering (HA and HF) and those subjected to debittering using 2-butanol (HA-B and HF-B) showing the lowered bitterness were analyzed for amino acid composition as described by Benjakul et al. (2009). Amino acid was calculated and expressed in g/100 g sample.
Statistical analysis
All the experiments were carried out in triplicate. Analysis of variance (ANOVA) was done and mean comparisons were performed using the Duncan’s multiple range test (Steel and Torrie 1980). Completely randomized design (CRD) was used for all studies, except randomized complete block design (RCBD) was used for analysis of bitterness. SPSS software (IBM software, New York, NY, USA) was used for analysis.
Results and discussion
Impact of proteases on characteristics and bitterness of protein hydrolysate from salmon frame
Degree of hydrolysis (DH)
DHs of protein hydrolysate from salmon frame prepared using different proteases are shown in Table 1. DH of HA (26.88%) was not different from that of HF (25.02%) (P > 0.05). Alcalase is alkaline serine endopeptidase with high specificity for sulfur-containing, aliphatic, acidic, hydroxyl, aromatic and basic amino acids, while Flavourzyme acts as an endo- and exoprotease responsible for release of free amino acids mainly from C-terminal (Thiansilakul et al. 2007). The degree of hydrolysis (DH) is quantification of enzymatic break down of protein and represents the percentage of peptide bonds cleaved. However, Alcalase has been reported to exhibit higher efficacy in hydrolysis of yellow stripe trevally muscle proteins and Persian sturgeon (Acipenser persicus) viscera protein (Klompong et al. 2008; Ovissipour et al. 2012). In general, continuous stirring, incubation temperature and hydrolysis time influenced the cleavage of most compact protein when both enzymes were used (Benjakul and Morrissey 1997). Apart from both proteases used, endogenous proteases such as cathepsins, etc. in raw fish muscle have been reported to play a role in hydrolysis (Mackie 1982). This could assist the breakdown of proteins into smaller peptides during hydrolysis. It can be inferred that the type of proteases used affected the degree of hydrolysis of proteins from salmon frame. Therefore, HA and HF most likely possessed the different peptide sizes and amino acid sequences that might determine their antioxidative activities.
Table 1.
Degree of hydrolysis (DH), yield and bitterness score of the hydrolysates from salmon frame prepared using different enzymes
| Samples | DH (%) | Yield (g/100 g sample) | Bitterness score |
|---|---|---|---|
| HA | 26.88 ± 1.45a | 32.27 ± 1.68a | 8.68 ± 1.19a |
| HF | 25.02 ± 1.11a | 28.51 ± 0.96b | 5.78 ± 1.58b |
Values are presented as mean ± SD (n = 3) (dry weight basis)
HA and HF denote hydrolysates produced using Alcalase and Flavourzyme, respectively
Different lowercase letters within the same column indicate significant differences (P < 0.05)
Yield
Yields of HA and HF were 32.3 and 28.5 g/100 g, respectively. Higher yield was obtained for HA than HF (P < 0.05). In general, state of substrate and surface area of proteinaceous matter played an essential role in protein hydrolysis as well as the yield obtained (Benjakul et al. 2009). Both Alcalase and Flavourzyme most likely cleaved the peptide bonds in the salmon frame at different positions, leading to the different products with varying yields and compositions of free amino acids and peptides (Klompong et al. 2008). Nevertheless, Idowu et al. (2018) reported that higher DH led to higher yield. Smaller peptides are more water soluble and recovered in hydrolysate to a higher extent. Overall, yield of hydrolysates varied with the type of proteases used, in which Alcalase rendered the higher yield.
Bitterness
HA possessed higher bitterness score than HF (P < 0.05). Bitterness is related with peptides containing bulky hydrophobic groups toward their C-terminal. Bulky hydrophobic group such as leucine, tryptophan, valine, tyrosine, isoleucine and phenylalanine at C-terminal contributed to bitterness (Idowu et al. 2018). During hydrolysis, the hidden hydrophobic peptides are exposed or liberated. This resulted in the enhanced bitterness sensation. Concomitantly, the higher bitterness score of HA observed could be as a result of higher exposure of buried hydrophobic group of peptides in HA than HF (P < 0.05). Alcalase is endopeptidase capable of hydrolyzing proteins with broad specificity for peptide bonds and prefers to hydrolyze large uncharged residue. Flavourzyme is the endo- and exopeptidase enzyme mixture, which can remove bitter peptides by removing hydrophobic amino acids at the terminal. The levels and compositions of free amino acids and small peptides were changed during the hydrolysis, depending on enzyme specificity (Wu et al. 2003). Thus, the decrease in bitterness of HF observed could be as a result of removal of hydrophobic peptides or free amino acids by exopeptidase in Flavourzyme. Several factors have been documented to influence bitterness. Those include number of carbons in side chain, DH and concentration (Idowu et al. 2018). Thus, bitterness of hydrolysates from salmon frame was directly affected by the type of proteases used.
Effect of debittering process on characteristics and bitterness of protein hydrolysate from salmon frame
Nitrogen recovery (NR)
Nitrogen recovery (NR) of hydrolysates debittered using different alcohols (2-butanol and iso-propanol) is presented in Table 2. HA and HF treated with 2-butanol referred to as ‘HA-B’ and ‘HF-B’, respectively, had NR of 80.2 and 81.6%, respectively. When treated with iso-propanol, NR of 81.3 and 77.9%, respectively, were obtained for HA and HF, named HA-I and HF-I, respectively. Removal of insoluble protein fractions from hydrolyzed soluble fraction is associated with nitrogen recoveries (Kristinsson and Rasco 2000). Removal of some nitrogenous components during debittering could have led to the lower NR. In general, some peptides could be precipitated in the presence of alcohols (Scanu and Edelstein 1971). Also, alcohol could solubilize some small peptides or free amino acids in hydrolysates. Hence, the slightly lower NR of hydrolysate was observed after treatment with both alcohols. It can be deduced that alcohol treatment for debittering decreased NR of HA and HF from salmon frame to some extent.
Table 2.
Characteristics of protein hydrolysates from salmon frame without and with different debittering processes
| Samples | Alcohol | Nitrogen recovery (%) | Bitterness score | Surface hydrophobicity | Solubility (%) |
|---|---|---|---|---|---|
| HA | – | – | 9.13 ± 1.77aA | 208.57 ± 2.64aA | 95.61 ± 0.72aA |
| HA-B | 2-Butanol | 80.21 ± 0.01 | 3.77 ± 1.33cC | 124.79 ± 3.50cD | 92.70 ± 2.10bAB |
| HA-I | Iso-propanol | 81.28 ± 0.01 | 5.47 ± 1.46bB | 141.54 ± 6.61bC | 89.36 ± 1.05cBC |
| HF | – | – | 6.01 ± 2.34aB | 155.40 ± 3.17aB | 93.81 ± 2.01aAB |
| HF-B | 2-Butanol | 81.60 ± 0.01 | 3.60 ± 1.28bC | 81.41 ± 4.88cE | 90.88 ± 1.35abB |
| HF-I | Iso-propanol | 77.88 ± 0.03 | 4.66 ± 1.89abBC | 116.98 ± 4.82bD | 88.31 ± 3.56abC |
Values are presented as mean ± SD (n = 3)
HA; HF denoted hydrolysates produced using Alcalase and Flavourzyme. HA-B;HF-B: hydrolysate obtained from Alcalase and Flavourzyme, debittered using 2-butanol, respectively. HA-I;HF-I: hydrolysate obtained from Alcalase and Flavourzyme, debittered using isopropanol respectively
Different lowercase letters within the same hydrolysate sample in the same column indicate significant differences (P < 0.05). Different uppercase letters in the same column indicate significant differences (P < 0.05)
Bitterness
Bitterness scores of both HA and HF without and with debittering are shown in Table 2. Flavourzyme is a mixture of endo- and exo-peptidases. As a result, exopeptidase could remove some hydrophobic residues, particularly at N- or C-termini, leading to the lower bitterness of resulting hydrolysate (HF) (6.07), compared with HA (9.13). The result reconfirmed the result presented in Table 1. When 2-butanol and iso-propanol were used for debittering, the significant decreases in bitterness scores were noticeable (P < 0.05). For the same protein hydrolysate used, the lower bitterness score was found for those treated with 2-butanol in comparison with iso-propanol (P < 0.05). The result indicated that the former exhibited higher efficiency in debittering both HA and HF than the latter. Alcohols are hydrophobic (hydrocarbon chain) and hydrophilic (presence of hydroxyl group) in nature (Wasswa et al. 2007). Continuous stirring followed by centrifugation employed during debittering with alcohol could have assisted the interaction with bulky hydrophobic group, especially small peptides or free amino acids, in hydrolysates. As a result, interaction existed between the bulky hydrophobic group of peptides with the hydrophobic part of the alcohol to form hydrophobic-hydrophobic interaction. Via this interaction, more hydrophobic groups of the peptides were solubilized in the alcohol used during debittering process, leading to the lower bitterness observed in all hydrolysates treated with alcohols (P < 0.05). Non-polar (hydrophobic) domains of the alcohol interacted strongly with the hydrophobic peptides, especially towards their C-termini. The bitter peptides, particularly those liberated by exopeptidases in Flavourzyme, were leached out. Those peptides with bitterness could be removed by 2-butanol (both HA-B and HF-B). Consequently, both proteases and type of alcohol used for debittering played a role in lowering the bitterness of hydrolysates.
Surface hydrophobicity
Surface hydrophobicity of both HA and HF before and after debittering is shown in Table 2. Higher surface hydrophobicity denotes the presence of bulky hydrophobic/aromatic amino acids (Vandaburg et al. 1994). The bulky hydrophobic/aromatic amino acids could be released from internal loop of the folded protein during hydrolysis (Aspevik et al. 2016). HA and HF (without debittering) showed high surface hydrophobicity of 208.6 and 155.4, respectively. During hydrolysis, the buried hydrophobic/aromatic amino acids were leached out from the interior domain of globular protein and more likely localized at the surface. It was noted that HA possessed higher surface hydrophobicity than HF (P < 0.05). Higher surface hydrophobicity observed in HA could be as a result of high specificity of Alcalase to cleave buried hydrophobic peptides during hydrolysis, which led to their enhanced exposure. Nevertheless, hydrophobic residues at termini of peptides could be further cleaved by exopeptidase in Flavourzyme. This led to the lower surface hydrophobicity of resulting peptides in HF.
Treatment with alcohol was able to extract hydrophobic amino acids or small peptides via hydrophobic-hydrophobic interaction between alcohol and hydrophobic residues of the hydrolysates. HA-B and HF-B had surface hydrophobicity of 124.8 and 81.4, while HA-I and HF-I possessed surface hydrophobicity of 141.5 and 116.9, respectively. This result suggested that bulky hydrophobic amino acids or peptides of HF had higher affinity to alcohol than HA. Hence, surface hydrophobicity of HF was much decreased. Similarly, between the two alcohols used, 2-butanol showed higher efficiency to reduce the surface hydrophobicity of hydrolysates than iso-propanol. 2-Butanol possessed solvent polarity of 0.506 relative to water (Aspevik et al. 2016). Nevertheless, the apolar nature of 2-butanol could be assumed to be sufficient to attract or bind with hydrophobic amino acids or small peptides of hydrolysates. This possibly led to the decrease in surface hydrophobicity of hydrolysates debittered using 2-butanol than those using iso-propanol. Idowu et al. (2018) reported the influence of hydrophobic/aromatic amino acids on bitterness of hydrolysate. In this study, surface hydrophobicity of hydrolysates correlated well with the bitterness of hydrolysates (Table 2). Type of alcohol used for debittering drastically affected the surface hydrophobicity of the resulting hydrolysates.
Solubility of hydrolysates
Solubility of hydrolysates from salmon frame with and without debittering treatment is shown in Table 2. Solubility ranged between 88.3 and 95.6%. Solubility is regarded to as one of the vital requirement for physicochemical and functional properties of protein hydrolysates (Thiansilakul et al. 2007). HA and HF without debittering treatment possessed higher solubility than others (P < 0.05), except HA-B which had solubility similar to those two samples (P > 0.05). Solubility of hydrolysates could be influenced by reduction in molecular size, exposure of more polar and ionizable groups to the aqueous environment and hydrophobic character of proteins or peptides (Nalinanon et al. 2011). The balance of hydrophilic and hydrophobic forces of peptides is another crucial factor determining the solubility of protein hydrolysate (Klompong et al. 2007). During the debittering process, some soluble peptides might be aggregated as induced by alcohols, thus limiting the solubility of the debittered hydrolysates. When comparing alcohols used, hydrolysates debittered using iso-propanol showed less solubility than those debittered by 2-butanol, indicating the loss of more soluble peptides in the former. Overall, solubility of hydrolysates was altered with the debittering treatment used, especially type of alcohol.
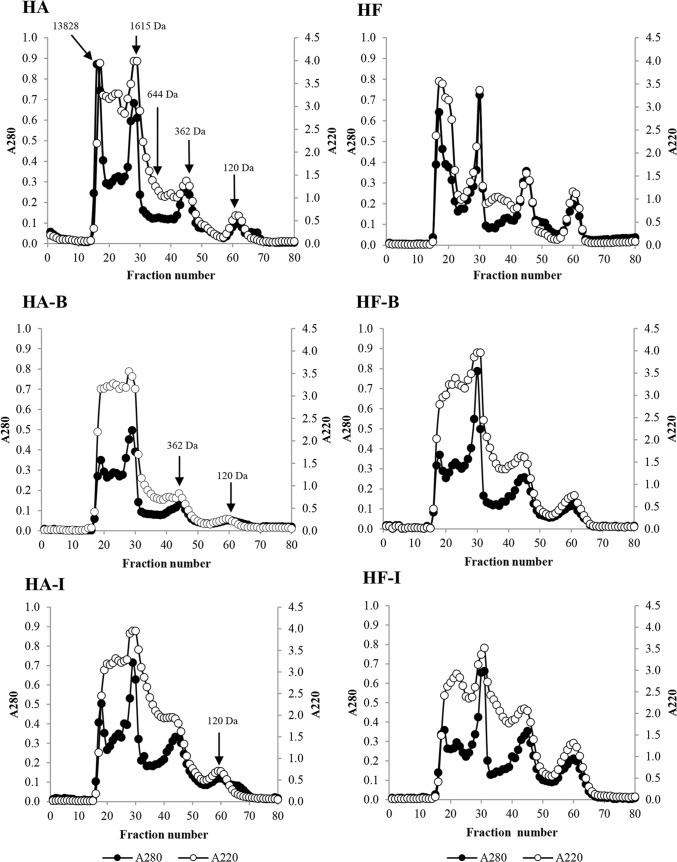
Molecular weight distribution
Elution profiles of hydrolysates obtained from salmon frame without and with debittering treatments are depicted in Fig. 1. A280 measured proteins or peptides, mainly consisting of hydrophobic/aromatic amino acids, while A220 indicated peptide bonds (Karnjanapratum and Benjakul 2015). HA and HF had two major peaks at A280 and A220 with molecular weight (MW) of 13,828 and 1615 Da. However, higher peak heights were observed in HA than in HF. For A220, peak of 644 Da was more pronounced in HF than in HA. At A280 and A220, peptide with MW of 362 Da was observed for both HA and HF, but the latter possessed higher peak height. Peak of peptide with MW of 120 Da was higher in HF than in HA.
Fig. 1.
Elution profile by Sephadex G-25 size exclusion chromatography of hydrolysates from salmon frame using Alcalase and Flavourzyme. HA; HF: hydrolysate obtained from Alcalase and Flavourzyme, respectively. HA-B; HF-B: hydrolysate from Alcalase and Flavourzyme subjected to debittering using 2-butanol, respectively. HA-I; HF-I: hydrolysate from Alcalase and Flavourzyme subjected to debittering using Iso-propanol, respectively
Elution profile of peptides in hydrolysates debittered using 2-butanol was slightly altered. For A280, the decline in peak height of 13,828 Da peptide was observed in HA-B and HF-B. This confirmed that some hydrophobic/aromatic amino acids were more likely removed during debittering process. For A280 and A220, peptides of 644, 362 and 120 Da became less pronounced after debittering in HA-B and HF-B. However, peak heights were higher in HF-B than HA-B. Aggregation of peptides in HA-B and HF-B might be related with the removal of some soluble peptides during debittering.
For A280, there was reduction in peak heights of peak with MW of 13,828 Da in both HA-I and HF-I, suggesting the removal of hydrophobic peptides causing bitterness in hydrolysates. For A280 and A220, similar peak height of peptide with MW of 362 Da was observed between HA-I and HF-I. Peak height of peptide with MW of 120 Da at A280 and A220 in HF-I was more pronounced than HA-I. This result correlated well with surface hydrophobicity and bitterness of hydrolysate (Table 2), which were lower in HA-I than HF-I. Thus, debittering treatment as well as type of alcohol used slightly impacted on size distribution of peptides as indicated by different profiles.
Antioxidative activities
ABTS radical scavenging activities
ABTS activities of hydrolysates without and with debittering are shown in Table 3. ABTS assay is used to determine the antioxidant activity toward lipid peroxyl radicals and hydrophilic radicals (Binsan et al. 2008). Alcalase is the endopeptidase that is able to hydrolyze peptide bonds with broad specificity. Flavourzyme is the endo- and exopeptidase enzyme mixture, in which the latter can release both free amino acids and small peptides from the termini (Ven et al. 2002). Therefore, protein hydrolysates prepared from different enzymes most likely possess the different peptide sizes and amino acid sequences that may determine their antioxidative activities. However, there was no difference in ABTS radical scavenging activity between HA and HF (P > 0.05). Increased hydrophobic domains of peptides have been regarded as a factor that increased the radical scavenging activities of hydrolysates (Ajibola et al. 2011). Furthermore, hydrophobic peptides possessed high efficiency to interact with lipids. This might affect their antioxidative activity (Zhu et al. 2006). Thus, higher surface hydrophobicity of HA and HF (Table 2) could have enhanced their activities. For HA, both alcohols used for debittering had no impact on ABTS radical scavenging activity (P > 0.05). After debittering, the decreases in activity of HF were observed (P < 0.05). Loss of some hydrophobic peptides or free amino acids such as leucine and phenylalanine during debittering of HF could lower its activity. Thus, debittering using both alcohols decreased the activity of HF (P < 0.05).
Table 3.
ABTS and DPPH radical scavenging activities, ferric reducing antioxidant power (FRAP) and metal chelating activity of protein hydrolysates from salmon frame without and with different debittering processes
| Samples | Alcohols | ABTS (µmol TE/g smple) | DPPH (µmol TE/g smple) | FRAP (µmol TE/g smple) | Metal chelating (µmol EDTA/g smple) |
|---|---|---|---|---|---|
| HA | – | 211.87 ± 9.56aAB | 28.52 ± 3.41aA | 26.01 ± 3.07aA | 0.52 ± 0.08aB |
| HA-B | 2-Butanol | 201.54 ± 0.97aBC | 18.99 ± 0.43bB | 23.26 ± 1.62bB | 0.25 ± 0.10bC |
| HA-I | Iso-propanol | 205.47 ± 9.54aABC | 15.49 ± 1.38bB | 21.12 ± 0.39bBC | 0.20 ± 0.03bC |
| HF | – | 216.32 ± 5.71aA | 25.53 ± 3.38aA | 23.11 ± 2.60aB | 0.91 ± 0.40aA |
| HF-B | 2-Butanol | 196.30 ± 5.12bC | 19.20 ± 3.54abB | 22.98 ± 2.68aBC | 0.43 ± 0.08bB |
| HF-I | Iso-propanol | 200.74 ± 9.54bBC | 16.35 ± 5.82bB | 20.25 ± 1.22abC | 0.26 ± 0.05cC |
Values are presented as mean ± SD (n = 3). Different lowercase letters within the same hydrolysate sample in the same column indicate significant differences (P < 0.05). Different uppercase letters in the same column indicate significant differences (P < 0.05). Caption: see Table 2
DPPH radical scavenging activities
DPPH radical scavenging activities of hydrolysates without and with debittering are shown in Table 3. This assay measured the antioxidative activity of compounds as a hydrogen donor or free radical scavenger (Klompong et al. 2007). Both HA and HF showed higher activity than those with debittering (P < 0.05). Kim et al. (2007) reported that hydrophobic peptides function as antioxidants by promoting the solubility of peptides in non-polar solvent, thereby promoting better interaction with free radicals to terminate their activities. This correlated with the higher activities observed in HA and HF without debittering (P < 0.05). Loss of soluble and hydrophobic peptides during debittering could have led to the decrease in DPPH radical scavenging activities of debittered hydrolysates (HA-B, HA-I, HF-B and HF-I) (P < 0.05). Nevertheless, both alcohols used during debittering showed no differences in activities of the resulting HA (P > 0.05). For HF, slight decrease in activity was observed when iso-propanol was used for debittering, compared to that found in HF (P < 0.05). It can be reduced that debittering affected DPPH radical scavenging activities of both HA and HF.
Ferrous reducing antioxidant power (FRAP)
FRAP measures the ability of a compound to donate electron to a free radical in order to reduce TPTZ-Fe(III) complex to TPTZ-Fe(II) complex (Binsan et al. 2008). HA rendered higher activity than those subjected to debittering (P < 0.05). Ajibola et al. (2011) reported that hydrolysate with more hydrophobic peptides could display high reducing activity. This reaffirmed the higher reducing activity of HA without debittering than those with debittering. Similar reducing activities were observed after debittering of HA with both alcohols (P > 0.05). HF, debittered with 2-butanol (HF-B), showed similar FRAP to those without debittering (HF) (P > 0.05). There was no difference in FRAP between HF debittered using 2-butanol and iso-propanol (P > 0.05). This suggested similar electron donating ability of hydrophobic peptides in those hydrolysates to reduce Fe(III) to Fe(II) complex. In general, debittering influenced the activities of HA and HF to some degree.
Metal chelating activity
Metal chelating activity is used to determine the ability of antioxidative peptides to chelate prooxidative metals (Binsan et al. 2008). Concentration of carboxyl and amino groups in the side chains of amino acids has been reported to influence chelating activities of antioxidative peptides (Saiga et al. 2003). HF showed the highest chelating activities than others (P < 0.05). Available carboxyl and amino groups of HF without debittering might play a role in binding with Fe. However, after debittering, activity of both HA and HF decreased (P < 0.05). Activity was similar between HA-B and HA-I when both alcohols were used (P > 0.05). Nevertheless, HF debittered with iso-propanol (HF-I) showed the decrease in activity (P < 0.05). This could be due to loss of some binding groups or domains during debittering with iso-propanol. In summary, debittering process affected the chelating activity of HA and HF.
Amino acid composition
Hydrolysates (HA and HF) without and with debittering using 2-butanol (HA-B and HF-B) with less bitterness scores were selected for amino acid analysis (Table 4). The dominant amino acids in all samples were glutamic acid/glutamine, aspartic acid/asparagine and glycine. HA-B and HF-B possessed higher glutamic acid/glutamine than HA and HF. Removal of hydrophobic amino acids or peptides could increase the proportion of acidic or polar amino acids (Johnson et al. 2003). Glutamic acid is not able to contribute to umami taste (Naknaen et al. 2015). The cleavage of N or C- terminal hydrophobic amino acid by exopeptidase in Flavourzyme, followed by fractionation using 2-butanol in debittering of HF resulted in the decrease in total hydrophobic amino acid as shown in HF-B. Lower hydrophobic amino acids of 34.63 and 35.05 g/100 g sample were obtained for HF-B and HA-B, respectively, compared to those without debittering 35.18 (HF) and 37.69 g/100 g sample (HA), respectively. Thus, less hydrophobic amino acid were related with less bitterness score attained (Table 2). Hydroxyproline was observed in all samples, which indicated the presence of collagen derivatives in hydrolysates. This collagen, particularly from bone or connective tissue, could be solubilized by proteases at a temperature higher than Tmax of fish collagen during hydrolysis (Idowu et al. 2018).
Table 4.
Amino acid profile of selected protein hydrolysates without and with debittering
| Amino acids (g/100 g sample) | HA | HF | HA-B | HF-B |
|---|---|---|---|---|
| Alanine | 6.75 ± 0.04 | 7.19 ± 0.02 | 6.84 ± 0.03 | 6.82 ± 0.01 |
| Arginine | 6.52 ± 0.02 | 6.56 ± 0.04 | 6.87 ± 0.03 | 6.91 ± 0.04 |
| Aspartic acid/asparagine | 9.59 ± 0.00 | 9.22 ± 0.01 | 10.10 ± 0.00 | 10.07 ± 0.01 |
| Cysteine | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Glutamic acid/glutamine | 14.13 ± 0.02 | 14.65 ± 0.01 | 14.98 ± 0.01 | 15.14 ± 0.02 |
| Glycine | 9.03 ± 0.01 | 10.93 ± 0.00 | 9.40 ± 0.00 | 9.30 ± 0.00 |
| Histidine | 3.40 ± 0.02 | 3.58 ± 0.03 | 3.23 ± 0.01 | 3.47 ± 0.03 |
| Isoleucine | 3.76 ± 0.01 | 3.33 ± 0.01 | 3.56 ± 0.01 | 3.37 ± 0.01 |
| Leucine | 7.06 ± 0.03 | 6.39 ± 0.02 | 5.75 ± 0.04 | 5.83 ± 0.03 |
| Lysine | 8.23 ± 0.01 | 8.15 ± 0.02 | 8.67 ± 0.02 | 8.80 ± 0.02 |
| Hydroxylysine | 0.37 ± 0.02 | 0.47 ± 0.02 | 0.41 ± 0.00 | 0.40 ± 0.00 |
| Methionine | 3.14 ± 0.04 | 2.83 ± 0.03 | 2.80 ± 0.04 | 2.79 ± 0.03 |
| Phenylalanine | 3.55 ± 0.01 | 2.99 ± 0.02 | 2.80 ± 0.02 | 2.85 ± 0.01 |
| Hydroxyproline | 1.88 ± 0.00 | 2.51 ± 0.00 | 1.91 ± 0.00 | 2.01 ± 0.01 |
| Proline | 5.14 ± 0.05 | 5.53 ± 0.06 | 5.34 ± 0.06 | 5.33 ± 0.04 |
| Serine | 4.61 ± 0.01 | 4.62 ± 0.01 | 4.75 ± 0.00 | 4.68 ± 0.00 |
| Threonine | 4.56 ± 0.02 | 4.13 ± 0.02 | 4.63 ± 0.01 | 4.59 ± 0.00 |
| Tryptophan | 0.69 ± 0.04 | 0.48 ± 0.03 | 0.52 ± 0.04 | 0.49 ± 0.04 |
| Tyrosine | 3.11 ± 0.03 | 2.27 ± 0.02 | 2.90 ± 0.03 | 2.81 ± 0.02 |
| Valine | 4.48 ± 0.01 | 4.17 ± 0.02 | 4.54 ± 0.01 | 4.33 ± 0.01 |
| Hydrophobic amino acids | 37.69 ± 0.22 | 35.18 ± 0.21 | 35.05 ± 0.24 | 34.63 ± 0.23 |
| Essential amino acids | 38.87 ± 0.18 | 36.04 ± 0.12 | 36.50 ± 0.14 | 36.53 ± 0.16 |
| Non essential amino acids | 59.25 ± 0.15 | 46.80 ± 0.17 | 46.62 ± 0.14 | 46.33 ± 0.18 |
HA; HF: hydrolysates produced using Alcalase and Flavourzyme, respectively. HA-B; HF-B: hydrolysate produced using Alcalase and Flavourzyme and debittered with 2-butanol, respectively
Essential and non-essential amino acids in hydrolysates were in the range of 36.04–38.87 g/100 g sample and 46.33–59.25 g/100 g sample, respectively. Debittering of HF with 2-butanol (HF-B) slightly increased essential amino acids. 2-Butanol plausibly removed non-essential hydrophobic amino acid to a higher degree. As a result, the proportion of essential amino acids could be increased in HF-B. Consequently, amino acid composition of HA and HF were altered slightly by debittering process.
Conclusion
Protein hydrolysate could be derived from salmon frame. Alcalase rendered higher degree of hydrolysis and yield than Flavourzyme. Nevertheless, hydrolysate obtained from Flavourzyme (HF) rendered less bitterness score than those from Alcalase (HA). Debittering of HF with 2-butanol (HF-B) lowered the bitterness and surface hydrophobicity than iso-propanol. In addition, 2-butanol decreased hydrophobic/aromatic peptides as indicated in the elution profile and amino acid composition and markedly decreased bitterness of hydrolysate. However, ABTS radical scavenging activity and metal chelating activity of HF-B were decreased. However, 2-butanol could be recommended to fractionate HF in order to yield hydrolysate with lowered bitterness, which can be used as a nutritive ingredient for food fortification.
Acknowledgements
This research was supported by Food Innovation and Research Institute, Prince of Songkla University, Thailand.
Compliance with ethical standards
Conflict of interest
The authors at this moment declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int J Mol Sci. 2011;12:6685–6702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspevik T, Totland C, Lea P, Oterhals Å. Sensory and surface-active properties of protein hydrolysates based on Atlantic salmon (Salmo salar) by-products. Proc Biochem. 2016;51:1006–1014. doi: 10.1016/j.procbio.2016.04.015. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Benjakul S, Binsan W, Visessanguan W, Osako K, Tanaka M. Effects of flavourzyme on yield and some biological activities of Mungoong, an extract paste from the cephalothorax of white shrimp. J Food Sci. 2009;74:S73–S80. doi: 10.1111/j.1750-3841.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- FAO Food and Agricultural Organization . The state of world fisheries and aquaculture 2016. Contributing to food security and nutrition for all. Rome: Food and Agriculture Organization of the United Nations; 2016. [Google Scholar]
- FitzGerald RJ, O’Cuinn G. Enzymatic debittering of food protein hydrolysates. Biotech Adv. 2006;24:234–237. doi: 10.1016/j.biotechadv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hou H, Li B, Zhao X, Zhang Z, Li P. Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness. LWT Food Sci Technol. 2011;44:421–428. doi: 10.1016/j.lwt.2010.09.009. [DOI] [Google Scholar]
- Idowu AT, Benjakul S, Sinthusamran S, Sookchoo P, Kishimura H. Protein hydrolysate from salmon frames: production, characteristics and antioxidative activity. J Food Biochem. 2018 doi: 10.1111/jfbc.12734. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Xie M, Singh LM, Edge R, Cornell RB. Both acidic and basic amino acids in an amphitropic enzyme, CTP: phosphocholine cytidylyltransferase, dictate its selectivity for anionic membranes. J Biol Chem. 2003;278:514–522. doi: 10.1074/jbc.M206072200. [DOI] [PubMed] [Google Scholar]
- Karnjanapratum S, Benjakul S. Antioxidative gelatin hydrolysate from unicorn leatherjacket skin as affected by prior autolysis. Int Aquat Res. 2015;7:101–114. doi: 10.1007/s40071-014-0088-0. [DOI] [Google Scholar]
- Kim S-Y, Je J-Y, Kim S-K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutr Biochem. 2007;18:31–38. doi: 10.1016/j.jnutbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Hayes KD, Shahidi F. Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. Int J Food Sci Technol. 2008;43:1019–1026. doi: 10.1111/j.1365-2621.2007.01555.x. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J Agric Food Chem. 2000;48:657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- Lalasidis G, Sjoberg LB. Two new methods of debittering protein hydrolysates and a fraction of hydrolysates with exceptionally high content of essential amino acids. J Agric Food Chem. 1978;26:742–749. doi: 10.1021/jf60217a056. [DOI] [PubMed] [Google Scholar]
- Mackie I. Fish protein hydrolysates. Proc Biochem. 1982;17:26–27. [Google Scholar]
- Naknaen P, Itthisoponkul T, Charoenthaikij P. Proximate compositions, nonvolatile taste components and antioxidant capacities of some dried edible mushrooms collected from Thailand. J Food Meas Charact. 2015;9:259–268. doi: 10.1007/s11694-015-9231-x. [DOI] [Google Scholar]
- Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–1362. doi: 10.1016/j.foodchem.2010.07.089. [DOI] [Google Scholar]
- Ovissipour M, Safari R, Motamedzadegan A, Shabanpour B. Chemical and biochemical hydrolysis of Persian sturgeon (Acipenser persicus) visceral protein. Food Bioprocess Technol. 2012;5:460–465. doi: 10.1007/s11947-009-0284-x. [DOI] [Google Scholar]
- Quan TH, Benjakul S. Compositions, protease inhibitor and gelling property of duck egg albumen as affected by salting. Korean J Food Sci Anim Resour. 2018;38:14–25. doi: 10.5851/kosfa.2018.38.1.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HW, Hogden CG. The biuret reaction in the determination of serum proteins: a study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J Biol Chem. 1940;135:707–725. [Google Scholar]
- Sae-leaw T, O’Callaghan YC, Benjakul S, O’Brien NM. Antioxidant activities and selected characteristics of gelatin hydrolysates from seabass (Lates calcarifer) skin as affected by production processes. J Food Sci Technol. 2016;53:197–208. doi: 10.1007/s13197-015-1989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:3661–3667. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- Scanu A, Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971;44:576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- See SF, Hoo LL, Babji A. Optimization of enzymatic hydrolysis of salmon (Salmo salar) skin by Alcalase. Int Food Res J. 2011;18:1359–1365. [Google Scholar]
- Seo WH, Lee HG, Baek HH. Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. J Food Sci. 2008;73:41–46. doi: 10.1111/j.1750-3841.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- Spellman D, O’Cuinn G, FitzGerald RJ. Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem. 2009;114:440–446. doi: 10.1016/j.foodchem.2008.09.067. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. New York: McGraw-Hill; 1980. [Google Scholar]
- Tavano OL. Protein hydrolysis using proteases: an important tool for food biotechnology. J Mol Catal B Enzym. 2013;90(6):1–11. doi: 10.1016/j.molcatb.2013.01.011. [DOI] [Google Scholar]
- Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103:1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- Vandaburg B, Dijkstra BW, Vriend G, Vandarvinne B, Venema G, Eijsink VG. Protein stabilization by hydrophobic interactions at the surface. Eur J Biochem. 1994;220:981–985. doi: 10.1111/j.1432-1033.1994.tb18702.x. [DOI] [PubMed] [Google Scholar]
- Ven CV, Gruppen H, Bont DBA, Voragen G. Correlations between biochemical characteristics and foam-forming and stabilizing ability of whey and casein hydrolysates. J Agric Food Chem. 2002;50:2938–2946. doi: 10.1021/jf011190f. [DOI] [PubMed] [Google Scholar]
- Wasswa J, Tang J, Gu X-H. Desalting fish skin protein hydrolysates using macroporous adsorption resin. Am J Food Technol. 2007;2:406–413. doi: 10.3923/ajft.2007.406.413. [DOI] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Zhu K, Zhou H, Qian H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Proc Biochem. 2006;41:1296–1302. doi: 10.1016/j.procbio.2005.12.029. [DOI] [Google Scholar]