Abstract
This study investigated the optimization of chicken skin gelatin film production using different concentrations of glycerol as plasticizer, specifically for use as biodegradable food packaging. Response surface methodology (RSM) was used to optimize the production of gelatin films by following a central composite design (CCD) for two quantitative modulators—(A) gelatin at 1.0, 2.5 and 4.0 g; and (B) glycerol at 0, 0.75, 1.50 g—with outcome metrics to predict tensile strength (TS), elongation at break (EAB) and water vapor permeability (WVP). Potentially optimized conditions were experimentally validated to confirm the model’s suggestions of 4.0 g for gelatin and 1.5 g for glycerol. Experimental results yielded a TS of 3.81 N/mm, which was higher than the RSM predicted value of 3.09 N/mm (p < 0.05). Both EAB and WVP experimental results were slightly lower than predicted values (3.04 vs. 3.14%) (p < 0.05); and (1.27 × 10−9 vs. 1.31 × 10−9 kPa) (p < 0.05), respectively. Overall, experimental results agreed with the model’s predicted values. Hence, this study demonstrates that optimized conditions for the production of chicken skin gelatin films are affected by glycerol concentration and gelatin quantity. Results also suggest that chicken skin gelatin–glycerol film blends have excellent potential for the production of biodegradable food packaging with improved properties.
Keywords: Optimization, Gelatin film, Chicken skin gelatin, Glycerol, Response surface methodology (RSM)
Introduction
Biodegradable films have attracted considerable attention from the food packaging industry because they can partly substitute and/or replace traditional non-biodegradable plastic films. They also provide barrier protection from moisture, gas and lipids and thus enhance food quality (Rhim et al. 2013). These films are generally prepared from polysaccharides, lipids and proteins such as gelatin, milk protein, whey protein and soy protein isolates, and corn zein (Suderman et al. 2016).
Proteins from different sources, especially from extracted gelatins, have been commonly used to develop biodegradable films due to their relative abundance, biodegradability, good film-forming ability, and impressive mechanical properties as moisture and gas barriers (Tongnuanchan et al. 2012). Gelatin is unique among hydrocolloids because it forms thermo-reversible films with melting points that approach human body temperature, which is particularly significant for edible, biodegradable and pharmaceutical applications (Gómez-Guillén et al. 2011). Different sources provide raw material for biodegradable film production such as gelatins from Nile perch skin (Tongnuanchan et al. 2012); pigskin (Hanani et al. 2012) and bovine skin (Voon et al. 2012).
Gelatin forms a three-dimensional network that contains zones of intermolecular microcrystalline junctions, the dehydration of which can produce brittle films (Fakhouri et al. 2013). However, the addition of plasticizers decreases inherent brittleness by reducing intermolecular forces that enhance polymer-chain mobility, which improves flexibility (Jiang et al. 2010). Plasticizers with smaller, highly polar groups per molecule and with greater intra-molecular distance between polar groups, impart superior plasticity to a polymeric system (Taghian Dinani et al. 2014). Thus, the selection of a suitable plasticizer for a specific system is normally based on the compatibility and permanence of the plasticizer, as well as upon the quantity required for plasticization and the desired physical properties of the film product (Leerahawong et al. 2011). Recently, polyols have proved particularly effective for plasticizing gelatin films because they reduce intermolecular hydrogen bonding while also increasing intermolecular spacing. For this reason, many studies have focused on polyols such as glycerol and sorbitol (Al-Hassan and Norziah 2012).
Response surface methodology (RSM) involves the pooling of statistical techniques to design experiments and build models that evaluate effects deriving from process parameters (Taghian Dinani et al. 2014). Central composite design contains embedded or fractional factorial designs with center points that allow the estimation of curvature (Taghian Dinani et al. 2014). As a tool for experimental product development design, RSM has been applied to optimize several food processing operations including food film packaging (Maran et al. 2013a). Tapia-Blacido et al. (2011) reported that RSM’s predictive analysis for amarath flour film yielded better EAB and film solubility when plasticized by 29.6 g sorbitol. Maran et al. (2013a) reported that RSM characterizations of barrier and optical properties for maize starch film allowed the production of film with low water vapour permeability and transparency when plasticized by 1 ml of sorbitol and 0.5 ml of tween-80. RSM optimization of tapioca flour film also led to the production of films with improved TS, EAB, Puncture Force and Puncture Deformation when plasticized by 0.5 to 1 ml of glycerol, as reported by Maran et al. (2013b). Ozdemir et al. (2008) reported RSM optimized results for mixed proportions of protein (0.53 g), sorbitol (0.38 g), beeswax (0.08 g) and potassium sorbate (0.01 g) that yielded an edible film with minimum tackiness as well as WVP ≤ 9 gmm m2 h−1 kPa−1, water solubility ≥ 39%, and an appearance score ≥ 80.
The potential of chicken skin gelatin become the alternative source of gelatin based film was due to it’s possessed better physico-chemical and films forming properties as compared to mammalian gelatin as reported by Sarbon et al. (2013). Therefore, the purposes of this study included the extraction of alternative gelatin from chicken skin and the RSM mediated optimization of a formula for chicken skin gelatin film production using different amounts of glycerol and gelatin content. Consequently, we posited that chicken skin waste from poultry processing has appreciable potential as an economic source of raw material for the production of biodegradable film packaging.
Materials and methods
Materials
The chicken skins were obtained from a local supplier (TD Poultry Sdn. Bhd). The skins were kept in ice during transport to the laboratory and then thoroughly/copiously washed in water and stored at − 80 °C until further use. Glycerol was purchased from Sigma Aldrich. Co. UK. All chemicals used in this study were of analytical grade.
Methods
Sample preparation
Visible fat was mechanically removed and the skins were thoroughly washed again to eliminate gross impurities and then cut into 2–3 cm2 pieces before being freeze-dried. After complete dehydration, samples were ground before being defatted using the Soxhlet method (AOAC 2006).
Gelatin extraction
Using an acid–alkaline pretreatment, the authors prepared chicken skin gelatin following the method described by Sarbon et al. (2013). Defatted chicken skin was ground and then sequentially soaked in sodium hydroxide (0.15%, w/w), sulphuric acid (0.15%, w/v) and citric acid (0.7%, w/w). Each solution was shaken and stirred slowly at room temperature for 30 min before centrifugation at 3500×g for 10 min. The supernatant was discarded and the wash was repeated three times to remove all non-collagenous proteins and pigments. Resultant pellets were thoroughly rinsed in distilled water to remove residual salts and placed in distilled water overnight at 45 °C. The clear extract was filtered and then concentrated by evaporation under a vacuum and thereafter, freeze-dried; thus comprising dried matter that was ground into ‘gelatin powder’ and then weighed and stored for further use.
Production of films
Gelatin film were prepared by employing the casting technique described by Suderman et al. (2016), with slight modifications. Thirteen (13) filmogenic solutions of gelatin and glycerol were prepared according to RSM predictive formulations. Gelatin powder portions weighing 1.0, 2.5 and 4.0 g, respectively, were each mixed with 100 ml of distilled water using mechanical stirring until completely dissolved; thus forming primary film solutions. Respective portions of glycerol at 0 (control), 0.75 and 1.5 g were then added to the samples. All mixtures were stirred at 45 °C for 20 min to obtain homogeneous solutions. Finally, approximately 25 g of each filmogenic solution were poured into clean Petri dishes and oven dried at 45 °C for two days. Dried film samples were then removed and conditioned in a desiccator prior to metrics for tensile strength (TS), elongation at break (EAB) and water vapor permeability (WVP).
RSM optimization of chicken skin gelatin–glycerol film blends
Using response surface methodology (RSM), the authors optimized a formula used to produce a chicken skin gelatin–glycerol film blend. This involved different glycerol concentrations and quantities of gelatin with a view to optimize specific metrics including tensile strength (TS), elongation at break (EAB) and water vapour permeability (WVP). The design comprised thirteen film formulations that were randomly run by central composite design (CCD) (Table 1). Two independent variables were applied at three equidistant levels (− 1, 0, + 1) for (A) gelatin at 1.0, 2.5 and 4.0 g, and (B) glycerol at 0 g, 0.75, and 1.5 g.
Table 1.
Coded values of chicken skin gelatin films condition and responses of 13 experimental runs
| Standard | Factor 1: gelatin (g) | Factor 2: glycerol (g) | Response 1: TS (N/mm) | Response 2: EAB (%) | Response 3: WVP (g m−1 s−1 Pa−1) × 10−9 |
|---|---|---|---|---|---|
| 1 | 1.00 | 0.00 | 0.08 | 1.01 | 3.87 |
| 11 | 2.50 | 0.75 | 1.21 | 2.32 | 4.77 |
| 7 | 2.50 | 0.00 | 0.12 | 1.05 | 3.99 |
| 9 | 2.50 | 0.75 | 2.13 | 3.02 | 5.06 |
| 6 | 4.00 | 0.75 | 2.84 | 3.84 | 1.32 |
| 13 | 2.50 | 0.75 | 1.2 | 2.34 | 4.59 |
| 3 | 1.00 | 1.50 | 0.08 | 2.21 | 3.48 |
| 5 | 1.00 | 0.75 | 0.32 | 2.66 | 3.75 |
| 12 | 2.50 | 0.75 | 1.22 | 2.79 | 4.07 |
| 8 | 2.50 | 1.50 | 0.81 | 2.31 | 3.51 |
| 2 | 4.00 | 0.00 | 0.09 | 1.09 | 3.32 |
| 4 | 4.00 | 1.50 | 3.22 | 3.28 | 1.59 |
| 10 | 2.50 | 0.75 | 1.19 | 2.31 | 4.62 |
Tensile strength (TS) and elongation at break (EAB)
TS and EAB values were determined by texture analyzer (TA.TX Plus, Stable Micro System, USA) using the ASTM 0882-97 method (ASTM 1997). Filmstrips measuring 20 × 100 mm were prepared with a cutting blade and placed on the AT/G grips/probe attached to the texture analyzer with its 5 kg load cell. The initial upper and lower gap between grips was set at 20 mm and filmstrips were stretched at 50 mm/min until rupture. TS (MPa) was calculated as follows:
where Fmax is the maximum load (N) required for rupture and (A) is the cross sectional area (m2) of the film.
Percent EAB was calculated as follows:
where lmax is film elongation (mm) at the moment of rupture; and l0 is the initial grip length (mm) of the sample.
Water vapour permeability (WVP)
The authors applied a modified ASTM method (Suderman et al. 2016) to measure WVP. Silicone vacuum grease sealed filmstrip samples across the tops of cups containing silica gel (0% RH). A rubber band secured every placement. Each cup was weighed and placed in a desiccator with distilled water at 30 °C and then re-weighed at 1 h intervals for 8 h. Three separate film trials were used to determine the WVP mean, calculated as follows:
where w is the cup’s weight gain (g), x is film thickness (m), A is the exposed area of the film (m2), t is time of gain (s), (P2 − P1)−1 is the difference in vapour pressure across the film (Pa).
Statistical analysis
The authors used RSM Design-Expert 6.0.10 software (Stat-Ease 2003) to assess optimized the chicken skin gelatin–glycerol film blend formula. Results are expressed as a mean (± SD) of three trials for each assay. Minitab 14.0 was utilized to calculate all comparative statistical analyses (ANOVA) between means to assess significant differences.
Results and discussion
RSM optimized chicken skin gelatin–glycerol film blend with different concentrations of glycerol, tested for tensile strength (TS), elongation at break (EAB) and water vapor permeability (WVP).
Adequate mechanical strength and elasticity are required for film packaging materials to withstand external stress while maintaining integrity and barrier properties during packaging applications (Rao et al. 2010). TS results for the thirteen trial runs ranged from 0.08 to 3.22 N/mm (Table 1), similar to results for gelatin–glycerol film TS (0.99 N/mm) reported by Bergo and Sobral (2007). This results confirmed that gelatin films plasticized with glycerol have better TS attributed to hydrogen bonding between gelatin and glycerol. Su et al. (2010) concluded that glycerol plasticization weakened interactions between polymer materials in proteins film; thus increasing flexibility. Differences in tensile values likely reflect plasticizer concentrations and variance in gelatin due to sourcing. Diverse gelatin and plasticizer sources necessarily generate dissimilar molecular interactions. Moreover, variance in the ratio of gelatin to glycerol also affects TS value. Increased plasticizer concentrations from 25 to 55 g/100 g of gelatin increases flexibility while reducing resistance to water vapor. Thomazine et al. (2005) reported that increasing the proportion of glycerol reduced tensile strength due to its high plasticizing effect. Such behavior was explained in terms of plasticizer molecular weight; hence, film properties can be treated a function of the number of plasticizer molecules in a given film.
EAB is also known as ‘fracture strain’, i.e., the ratio between changed length and initial length after rupture of the tested sample. EAB is an important property of food packaging films because it expresses a film’s ability to resist changes in shape without crack formation (Cheng et al. 2003). EAB results from the present study’s thirteen trials ranged from 1.01 to 3.84%, similar to results reported for bovine hide film (3.11%) by Vanin et al. (2005). The results showed that EAB increased while tensile strength decreased with increasing concentrations of glycerol. This behavior is likely due to glycerol’s plasticizing effect, as plasticizers reduce interactions between adjacent chains in the biopolymer, which leads to increased mobility and film flexibility. Glycerol plasticized films increase EAB compared to films plasticized by sorbitol, mannitol, polyethylene glycol and sucrose (Vanin et al. 2005). This is attributed to glycerol’s smaller molecular structure, which decreases intermolecular forces along polymer chains and thus imparts increased film flexibility even as barrier properties decrease (Vanin et al. 2005).
For use as food packaging, manufacturers must necessarily understand characteristic permeability features of a film that affect moisture content. One such characteristic is WVP. A film’s water vapour barrier properties can affect physical and chemical deterioration of packaged products. This is related to moisture content equilibrium and is of great importance to the maintainence and extension of a food product’s shelf-life (Siracusa 2012). WVP results for the present work ranged from 1.32 to 5.06 kPa, similar to findings (1.78 kPa) for films plasticized with glycerol reported by Bergo and Sobral (2007). Plasticization effects from glycerol yield gelatin films with lower WVP values due to its hygroscopic character and smaller molecular structure, which disrupt a film’s network. Glycerol also produces more compact films with a denser polymeric structure that inhibits the passage of water vapour. Moreover, glycerol might also enhance gelatin cross-linking and thus decrease the available free volume within the polymeric matrix and thereby decrease water’s molecular diffusion rate through a film, resulting in lower WVP. In addition, storage time also affects WVP values because the longer a film is stored prior to testing, the higher the film’s WVP becomes as it absorbs moisture from the storage environment, which increases WVP. The present results concurred with a study by Suderman et al. (2016), who suggested that longer storage time prior to testing increased WVP for chitosan gelatin film plasticized with glycerol.
Tensile Strength (TS) analysis of chicken skin gelatin films fabricated with different concentrations of glycerol and gelatin.
Summary statistics model
The quadratic RSM model had been suggested to summarize TS predictive analyses for chicken skin gelatin film fabrication with different concentrations of chicken skin gelatin and glycerol because it projects interactions that occur between variables used in diverse film formulations. Results obtained in the present investigation agreed with a previous study that reported optimized TS for Achira flour biodegradable film (Andrade-Mahecha et al. 2012). However, the two-factor quadratic interaction model suggested for TS analyses contradicted a prior study on the optimization of gelatin film blends with glycerol and poly-vinyl alcohol (PVA) (Carvalho et al. 2009). The Carvalho study found that a linear predictive model for TS was more suitable. This difference in modeling for TS is likely due to variance in raw materials as well as in crosslinking agents and plasticizers used in film fabrication.
Analysis of TS variance (ANOVA)
Table 2 shows the ANOVA analysis of TS results from the Response Surface Quadratic model after model reduction. Fisher’s exact test (F test) of experimental data makes it possible to estimate statistically significant results from the proposed model (Maache-Rezzoug et al. 2011). Table 2 shows an F value of 15.77 and a p value of 0.0007, indicating robust significance. The ‘Model F-value’ of 1.20 implied ‘lack of fit’ insignificance relative to pure error. Hence, a ‘Model F-value’ this large had a 43.27% chance of occurring due to noise. The ‘lack of fit’ test predicts a model’s reliablity. For TS, we calculated an insignificant p value for ‘lack of fit’ (p > 0.05; i.e., 0.4327). Thus, this model can be used to navigate the design space.
Table 2.
Analysis of variance (ANOVA): significant model for tensile strength (TS)
| Source | Sum of squares | DF | Mean square | F value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 11.87 | 4 | 2.97 | 15.77 | 0.0007 | Significant |
| A | 5.36 | 1 | 5.36 | 28.47 | 0.0007 | |
| B | 2.43 | 1 | 2.43 | 12.92 | 0.0070 | |
| B2 | 1.63 | 1 | 1.63 | 8.68 | 0.0185 | |
| AB | 2.45 | 1 | 2.45 | 13.02 | 0.0069 | |
| Residual | 1.51 | 8 | 0.19 | |||
| Lack of fit | 0.82 | 4 | 0.21 | 1.20 | 0.4327 | Not significant |
| Pure error | 0.68 | 4 | 0.17 | |||
| Cor total | 13.38 | 12 |
R2 = 0.8875; Adj R2 = 0.8312; Pred R2 = 0.5352; A = gelatin (g), B = glycerol (g)
Based on the cited results, the coefficient of determination (R2 = 0.8875) indicated an 88.75% chance that experimental results were explained by the fitted model over a range of factors to be tested for the chicken skin gelatin–glycerol film blend. Guan and Yao (2008) suggested that R2 should be > 0.80 for a good fit; hence, a ‘Pred R2’ of 0.8312 reasonably agreed with an ‘Adj R2’ of 0.5325. ‘Adeq Precision’ measures the signal to noise ratio and a ratio > 4 is generally acceptable (Canettieri et al. 2007). Present TS model results for the ‘Adeq Precision’ ratio equalled 12.842, indicating a more than adequate signal to noise ratio. Therefore, this model is acceptable for design space navigation.
ANOVA results established that the quadratic model’s terms for gelatin (A) and glycerol (B) had significant effects on the TS of chicken skin gelatin–glycerol film blends (p < 0.05). Interaction terms (B2, AB) also had significant effects on the film’s TS (p < 0.05). Thus, a significant model (p < 0.05) with a non-significant ‘lack of fit’ test validated the model for TS.
Projected RSM surface plot of effects on tensile strength from chicken skin gelatin films fabricated with different concentrations of glycerol as plasticizer.
Using regression coefficients terms to fit a full RSM, the authors tested linear and quadratic equations for predictive interactive effects on TS for the chicken-skin–gelatin–glycerol film blends. In regression analysis, the best descriptive equation derived for TS is as follows, with (A) the response variable for gelatin, and (B) the response variable for glycerol:
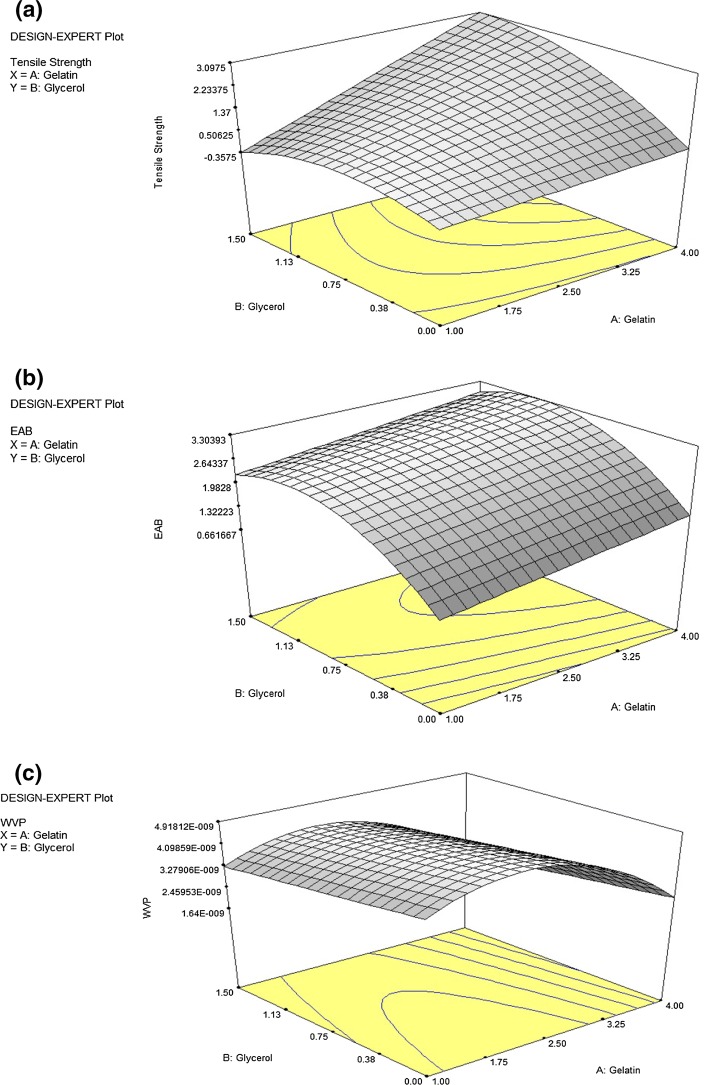
To aid understanding of effects from interactions between all factors, a three dimensional (3D) response surface plot were developed to evaluate both variables simultaneously. Figure 1a shows the 3D response surface graph of the regression coefficient, which projects effects from gelatin and glycerol concentrations on TS, showing that as glycerol concentration increases, TS also increases. Films with higher glycerol content have greater flexibility, as reported by Su et al. (2010). They also suggested that glycerol’s smaller molecular size allows its insertion between polymer chains where it weakens interactions between polymer materials such as polysaccharides and proteins, which increases a film’s flexibility.
Fig. 1.
Response surface plots of interaction effects of process variables on a tensile strength, b elongation at break and (c) water vapor permeability
According to Vanin et al. (2005), plasticizers like glycerol weaken intermolecular forces between adjacent polymer chains and also reduce the material’s glass transition temperature (Tg). Consequently, film flexibility and extensibility increase at the same time material resistance decreases. In this manner, films obtained in the present work reduced puncture force and tensile strength with majority glycerol content while at the same time they acquired greater flexibility and increased EAB. Al-Hassan and Norziah (2012) established glycerol’s smaller molecular weight and greater hygroscopic nature compared to other plasticizers; thus contributing superior plasticizing effects that increase polymer chain mobility leading to increased elasticity and flexibility. Gelatin films also exhibit better tensile strength when plasticized with glycerol, likely due to hydrogen bonding between gelatin and glycerol. Hence, this findings showed that glycerol concentration is a major factor contributing to the TS of chicken skin gelatin–glycerol film blends, and that films plasticized with glycerol generally have good TS.
Analysis of elongation at break (EAB) of chicken-skin-gelatin–glycerol film blends with different glycerol and gelatin content.
RSM summary statistics for EAB
As recommended and previously cited, the quadratic model were used to obtain results that concurred with a study by Andrade-Mahecha et al. (2012), who also used the quadratic model to predict EAB for Achira flour biodegradable and chitosan films with glycerol as the plasticizer for both.
Analysis of EAB variance (ANOVA) for chicken-skin-gelatin–glycerol film blends with different concentrations of glycerol and gelatin.
Table 3 shows ANOVA analyses results for RSM quadratic EAB predictions after model reduction. An F value of (15.48) together with a p value of (0.0008) indicated the model’s significance. A ‘Model F-value’ of (1.36) implied the ‘lack of fit’ was not significantly related to pure error with a 38.77% chance that a ‘Model F-value’ this large could occur due to noise. In this case, the ‘lack of fit’ index for the model’s fitness to predict TS was 0.3877 (p > 0.05), considered ‘not significant’. Hence, the model was deemed fit to optimize parameters.
Table 3.
Analysis of variance (ANOVA): significant model for elongation at break (EAB)
| Source | Sum of squares | DF | Mean square | F value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 7.89 | 4 | 1.97 | 15.48 | 0.0008 | Significant |
| A | 0.90 | 1 | 0.90 | 7.10 | 0.0286 | |
| B | 3.60 | 1 | 3.60 | 28.28 | 0.0007 | |
| A2 | 0.59 | 1 | 0.59 | 4.66 | 0.0628 | |
| B2 | 3.38 | 1 | 3.38 | 26.51 | 0.0009 | |
| Residual | 1.02 | 8 | 0.13 | |||
| Lack of fit | 0.59 | 4 | 0.15 | 1.36 | 0.3877 | Not significant |
| Pure error | 0.43 | 4 | 0.11 | |||
| Cor total | 8.91 | 12 |
R2 = 0.8856; Adj R2 = 0.8284; Pred R2 = 0.6469; A = gelatin (g), B = glycerol (g)
Based on the cited results, the coefficient of determination (R2) equalled 0.8856, indicating an 88.56% chance that experimental results could be projected by the fitted model over a range of test factors for EAB. Moreover, a ‘Pred R2’ value of (0.8284) reasonably agreed with the ‘Adj R2’ value of (0.6469). Furthermore, the ‘Adeq Precision’ ratio was 12.345, indicating an adequate signal-to-noise ratio. Hence, the model was deemed suitable for design space navigation.
ANOVA results indicated that the quadratic model’s terms, (A) for gelatin and (B) for glycerol had significant effects on EAB (p < 0.05) for chicken-skin-gelatin/glycerol film blends with different concentrations of glycerol. Interaction terms (A2, B2) also had significant effects on EAB (p < 0.05). Thus, both a significant model (p < 0 .05) and a non-significant ‘lack of fit’ test validated the model for EAB analyses.
RSM surface plot of effects on elongation at break (EAB) from chicken-skin gelatin–glycerol film blends with different concentrations of glycerol and gelatin.
The RSM model’s equation were derived for elongation at break (EAB) and response variables by using a regression coefficient for linear and interaction terms to fit a full response surface model. The analysis produced the optimized descriptive equation for EAB that follows:
A three dimensional (3D) response surface model was developed to assist understanding of effects on EAB from interactions between all factors by evaluating both variables simultaneously. Figure 1b shows the 3D response surface graph of the regression’s coefficient predicting effects from gelatin and glycerol concentrations on EAB; thus, EAB increased as glycerol concentration increased, predicting that increased glycerol concentrations could result in higher EAB values for the film. This finding agreed with Hoque et al. (2011), who also reported that the addition of glycerol increased EAB for gelatin–glycerol film blends.
Numerous literature reports agree that gelatin films plasticized with glycerol improve EAB (Ahmad et al. 2012). Higher protein content of chicken skin gelatin most likely results in greater protein aggregation during film formation, which improves the film’s mechanical properties. This conclusion concurs with reports on brownstripe red snapper films (Jongjareonrak et al. 2006), in which TS and EAB both increased with increasing protein concentrations. This finding will further suggest that glycerol increases EAB for chicken skin gelatin–glycerol film blends due to low molecular hydrophilic properties that expand the gelatin network so that its structural continuum is not significantly disrupted. Consequently, EAB increases due to increased polymer chain mobility in the presence of glycerol.
Analysis of Water Vapor Permeability (WVP) for chicken-skin gelatin–glycerol film blends with different concentrations of glycerol and gelatin.
RSM summary statistics for WVP
As suggested, the quadratic model were used to obtain WVP results that concurred with a study on the optimization of water vapor permeability for a tapioca starch-based edible film with glycerol as plasticizer (Maran et al. 2013b). Similarly, Maran et al. (2013a) also reported that the quadratic model predicted WVP for a maize-starch edible film with sorbitol as plasticizer.
Analysis of WVP variance (ANOVA) for chicken-skin gelatin–glycerol film blends with different concentrations of glycerol and gelatin.
Table 4 summarizes the ANOVA analysis derived from the RS Quadratic Model for WVP. Both F value (9.79) and p value (0.0036) indicated significance. The ‘Model F-value’ of 3.98 implied that the ‘lack of fit’ was not significantly related to pure error, with a 10.49% chance a ‘Model F-value’ this large could occur due to noise. As the ‘lack of fit’ test predicts the model’s reliability and as its p value was not significant (p > 0.05 for 0.1049), we deemed the model fit to determine optimal prameters. Moreover, the coefficient of determination (R2 = 0.8303) indicated that 83.03% of experimental results for WVP could be described by the fitted model for the range of factors tested. The ‘Pred R2’ value (0.7455) also reasonably agreed with the ‘Adj R2’ (0.3805), and the model’s ‘Adeq Precision’ ratio equalled 9.926, indicating an acceptable signal to noise ratio. Hence, the model was deemed adequate for design space navigation.
Table 4.
Analysis of variance (ANOVA): significant model for water vapor permeability (WVP)
| Source | Sum of squares | DF | Mean square | F value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1.265 × 10−17 | 4 | 3.163 × 10−18 | 9.79 | 0.0036 | Significant |
| A | 3.920 × 10−18 | 1 | 3.920 × 10−18 | 12.13 | 0.0083 | |
| B | 1.144 × 10−18 | 1 | 1.144 × 10−18 | 3.54 | 0.0967 | |
| A2 | 7.152 × 10−18 | 1 | 7.152 × 10−18 | 22.13 | 0.0015 | |
| AB | 4.356 × 10−19 | 1 | 4.356 × 10−19 | 1.35 | 0.2791 | |
| Residual | 2.585 × 10−18 | 8 | 3.232 × 10−19 | |||
| Lack of Fit | 2.066 × 10−18 | 4 | 5.165 × 10−19 | 3.98 | 0.1049 | Not significant |
| Pure Error | 5.195 × 10−19 | 4 | 1.299 × 10−19 | |||
| Cor Total | 1.524 × 10−17 | 12 |
R2 = 0.8303; Adj R2 = 0.7455; Pred R2 = 0.3805; A = gelatin (g), B = glycerol (g)
ANOVA results also verified that the quadratic model’s terms [gelatin (A) and glycerol (B)] had significant effects on WVP (p < 0.05), and interaction terms (A2, AB) also proved significant (p < 0.05). Thus, the model’s statistical significance (p < 0.05) plus it’s non-significant ‘lack of fit’ test validated the model for the projection of WVP effects from varied content in chicken skin gelatin–glycerol film blends.
Response surface plot of effects on WVP for chicken skin gelatin–glycerol film blends with different concentrations of glycerol and gelatin.
The RSM model’s equation and response variables were derived for WVP by using regression coefficients for linear, quadratic and interaction terms to fit a full response surface model. The analysis produced the following optimized descriptive equation for WVP:
A three dimensional (3D) response surface model was developed to assist the understanding of effects on WVP from interactions between all factors by evaluating both variables simultaneously. Figure 1c shows the 3D response surface graph of the regression’s coefficient that predicts effects from gelatin and glycerol concentrations on WVP, demonstrating that WVP would decrease along with increased glycerol concentration; thus indicating that films plasticized with high concentrations of glycerol have lower WVP.
Generally the addition of a plasticizer increases WVP of gelatin films by reducing intermolecular bonds between polymer chains. However, in this study, WVP gradually declined with increasing glycerol content, probably due to glycerol’s weaker effect on the reduction of intermolecular hydrogen bonding between protein molecules, as suggested by Maran et al. (2013a). This finding might also be due to greater aggregations of protein chains between gelatin and glycerol molecules. Previously, Bhat and Karim (2014) reported that additional glycerol might enhance the cross linking of gelatin, a phenomenon that decreases free volume within the polymeric matrix, which then reduces water’s diffusion rate through the film, resulting in lower WVP. Present findings certainly suggest that a denser and more organized polymeric gelatin matrix, formed in the presence of increased glycerol content, will limit the passage of water vapor; thus lowering WVP.
Optimizing tensile strength (TS), elongation at break (EAB) and water vapor permeability (WVP) for a chicken skin gelatin–glycerol film blend.
Optimal response conditions
Table 5 shows the desired profile of optimized fabrication conditions suggested by RSM for TS, EAB and WVP. The model’s ideal hydrolysis conditions suggested 4.00 g of gelatin and 1.5 g of glycerol, which concurred with previous reports recommending weights ranging from (2.0 to 4.0 g) and (0 to 4.5 g) of glycerol, respectively (Cao et al. 2009; Hanani et al. 2014).
Table 5.
Recommended solution for optimized chicken skin gelatin–glycerol film blend
| No | Gelatin | Glycerol | Tensile strength | Elongation at break | Water vapor permeability | Desirability | |
|---|---|---|---|---|---|---|---|
| 1 | 4.00 | 1.50 | 3.0975 | 3.1448 | 1.3109 × 10−9 | 0.980 | Suggested |
| 2 | 4.00 | 1.49 | 3.0974 | 3.1577 | 1.3179 × 10−9 | 0.980 |
Validation test
To confirm the model’s strength, the three fabrication runs to produce films under the suggested optimized conditions were undertook. Observed TS measured 3.81 N/mm, which was higher than the predicted value (3.09 N/mm) (p < 0.05). EAB measured 3.04%, slightly lower than the predicted value (3.14%) (p < 0.05); and WVP measured 1.27 kPa, also lower than the predicted value (1.31 × 10−9 kPa) (p < 0.05). Hence, experimental results closely approximated predicted values for the production of a biodegradable film with superior properties suitable for food packaging.
Conclusion
The RSM generated quadratic model presented for all responses-and as experimentally validated-indicates that chicken skin gelatin films are significantly affected by different concentrations of glycerol. This study demonstrates a successful application of RSM for the investigation, optimization and fabrication of an industry-suitable, plasticized chicken skin gelatin–glycerol film blend. Overall, plasticization by glycerol appears to impart better than projected TS, EAB and WVP properties to chicken skin gelatin film.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad M, Benjakul S, Prodpran T, Agustini TW. Physico-mechanical and antimicrobial properties of gelatin film from the skin of unicorn leatherjacket incorporated with essential oils. Food Hydrocoll. 2012;28(1):189–199. doi: 10.1016/j.foodhyd.2011.12.003. [DOI] [Google Scholar]
- Al-Hassan AA, Norziah MH. Starch–gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll. 2012;26(1):108–177. doi: 10.1016/j.foodhyd.2011.04.015. [DOI] [Google Scholar]
- Andrade-Mahecha MM, Tapia-Blácido DR, Menegalli FC. Development and optimization of biodegradable films based on achira flour. Carbohydr Polym. 2012;88(2):449–458. doi: 10.1016/j.carbpol.2011.12.024. [DOI] [Google Scholar]
- AOAC . Official methods of analysis of AOAC international. 18. Virginia: Association of Official and Analytical Chemists International; 2006. [Google Scholar]
- ASTM (1997) Designation D 882-97: standard test method for tensile properties of thin plastic sheeting. In: Annual book of ASTM standards. American Society for Testing and Materials, Philadelphia, p 159
- Bergo P, Sobral PJA. Effects of plasticizer on physical properties of pigskin gelatin films. Food Hydrocoll. 2007;21(8):1285–1289. doi: 10.1016/j.foodhyd.2006.09.014. [DOI] [Google Scholar]
- Bhat R, Karim AA. Towards producing novel fish gelatin films by combination treatments of ultraviolet radiation and sugars (ribose and lactose) as cross-linking agents. J Food Sci Technol. 2014;51(7):1326–1333. doi: 10.1007/s13197-012-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canettieri EV, De Moraes Rocha GJ, De Carvalho JA, De Silva JBDA. Optimization of acid hydrolysis from the hemicellulosic fraction of Eucalyptus grandis residue using response surface methodology. Bioresour Technol. 2007;98(2):422–428. doi: 10.1016/j.biortech.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Cao N, Yang X, Fu Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009;23(3):729–735. doi: 10.1016/j.foodhyd.2008.07.017. [DOI] [Google Scholar]
- Carvalho RA, Maria TMC, Moraes ICF, Bergo PVA, Kamimura ES, Habitante AMQB, Sobral PJA. Study of some physical properties of biodegradable films based on blends of gelatin and poly(vinyl alcohol) using response surface methodology. Mater Sci Eng. 2009;29(2):485–491. doi: 10.1016/j.msec.2008.08.030. [DOI] [Google Scholar]
- Cheng M, Deng J, Yang F, Gong Y, Zhao N, Zhang X. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24(17):2871–2880. doi: 10.1016/S0142-9612(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Fakhouri FM, Costa D, Yamashita F, Martelli SM, Jesus RC, Alganer K, Innocentini-Mei LH. Comparative study of processing methods for starch/gelatin films. Carbohydr Polym. 2013;95(2):681–689. doi: 10.1016/j.carbpol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Gómez-Guillén MC, Giménez B, López-Caballero MA, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25(8):1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- Guan X, Yao H. Optimization of Viscozyme L-assisted extraction of oat bran protein using response surface methodology. Food Chem. 2008;106(1):345–351. doi: 10.1016/j.foodchem.2007.05.041. [DOI] [Google Scholar]
- Hanani ZN, Roos YH, Kerry JP. Use of beef, pork and fish gelatin sources in the manufacture of films and assessment of their composition and mechanical properties. Food Hydrocoll. 2012;29(1):144–151. doi: 10.1016/j.foodhyd.2012.01.015. [DOI] [Google Scholar]
- Hanani ZN, Roos YH, Kerry JP. Use and application of gelatin as potential biodegradable packaging materials for food products. Int J Biol Macromol. 2014;71:94–102. doi: 10.1016/j.ijbiomac.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Effects of partial hydrolysis and plasticizer content on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. Food Hydrocoll. 2011;25(1):82–90. doi: 10.1016/j.foodhyd.2010.05.008. [DOI] [Google Scholar]
- Jiang M, Liu S, Du X, Wang Y. Physical properties and internal microstructures of films made from catfish skin gelatin and triacetin mixtures. Food Hydrocoll. 2010;24(1):105–110. doi: 10.1016/j.foodhyd.2009.08.011. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006;20(4):492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Leerahawong A, Tanaka M, Okazaki E, Osako K. Effects of plasticizer type and concentration on the physicochemical properties of edible film from squid (Todarodes pacificus) mantle muscle. Fish Sci. 2011;77(6):1061–1068. doi: 10.1007/s12562-011-0398-8. [DOI] [PubMed] [Google Scholar]
- Maache-Rezzoug Z, Pierre G, Nouviaire A, Maugard T, Rezzoug SA. Optimizing thermomechanical pretreatment conditions to enhance enzymatic hydrolysis of wheat straw by response surface methodology. Biomass Bioenergy. 2011;35(7):3129–3138. doi: 10.1016/j.biombioe.2011.04.012. [DOI] [Google Scholar]
- Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K. Response surface modeling and analysis of barrier and optical properties of maize starch edible films. Int J Biol Macromol. 2013;60:412–421. doi: 10.1016/j.ijbiomac.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K. Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym. 2013;92(2):1335–1347. doi: 10.1016/j.carbpol.2012.09.069. [DOI] [PubMed] [Google Scholar]
- Ozdemir M, Ozen BF, Dock LL, Floros JD. Optimization of osmotic dehydration of diced green peppers by response surface methodology. LWT Food Sci Technol. 2008;41(10):2044–2050. doi: 10.1016/j.lwt.2008.01.010. [DOI] [Google Scholar]
- Rao MS, Kanatt SR, Chawla SP, Sharma A. Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohydr Polym. 2010;82(4):1243–1247. doi: 10.1016/j.carbpol.2010.06.058. [DOI] [Google Scholar]
- Rhim JW, Park HM, Ha CS. Bio-nanocomposites for food packaging applications. Progr Polym Sci. 2013;38(10):1629–1652. doi: 10.1016/j.progpolymsci.2013.05.008. [DOI] [Google Scholar]
- Sarbon NM, Badii F, Howell NK. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013;30(1):143–151. doi: 10.1016/j.foodhyd.2012.05.009. [DOI] [Google Scholar]
- Siracusa V. Food packaging permeability behavior: a report. Int J Polym Sci. 2012;1:1–11. doi: 10.1155/2012/302029. [DOI] [Google Scholar]
- Su JF, Huang Z, Yuan XY, Wang XY, Li M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr Polym. 2010;79(1):145–153. doi: 10.1016/j.carbpol.2009.07.035. [DOI] [Google Scholar]
- Suderman N, Isa MIN, Sarbon NM. Effect of drying temperature on the functional properties of biodegradable CMC-based film for potential food packaging. Int Food Res J. 2016;23(3):1075–1084. [Google Scholar]
- Taghian Dinani S, Hamdami N, Shahedi M, Keramat J. Optimization of carboxymethyl cellulose and calcium chloride dip-coating on mushroom slices prior to hot air drying using response surface methodology. J Food Process Preserv. 2014;38(3):1269–1278. doi: 10.1111/jfpp.12088. [DOI] [Google Scholar]
- Tapia-Blacido DR, Do Amaral Sobral PJ, Menegalli FC. Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT Food Sci Technol. 2011;44:1731–1738. doi: 10.1016/j.lwt.2011.04.004. [DOI] [Google Scholar]
- Thomazine M, Carvalho RA, Sobral PJ. Physical properties of gelatin films plasticized by blends of glycerol and sorbitol. J Food Scie. 2005;70(3):E172–E176. doi: 10.1111/j.1365-2621.2005.tb07132.x. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012;134(3):1571–1579. doi: 10.1016/j.foodchem.2012.03.094. [DOI] [PubMed] [Google Scholar]
- Vanin FM, Sobral PJA, Menegalli FC, Carvalho RA, Habitante AMQB. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005;19(5):899–907. doi: 10.1016/j.foodhyd.2004.12.003. [DOI] [Google Scholar]
- Voon HC, Bhat R, Easa AM, Liong MT, Karim AA. Effect of addition of halloysite nanoclay and SiO2 nanoparticles on barrier and mechanical properties of bovine gelatin films. Food Bioprocess Technol. 2012;5(5):1766–1774. doi: 10.1007/s11947-010-0461-y. [DOI] [Google Scholar]