Abstract
Gastric ulcer is a chronic health problem world over. Spirulina is known to contain significant amounts of vitamin B12 hence Spirulina was evaluated for gastroprotective properties against gastric ulcerations. Spirulina biomass (SB) and Spirulina extract (SE) were evaluated in swim stress induced gastric ulcers in adult male albino rats. Both SB and SE inhibited 45% and 60% of ulcers formation induced by swim stress respectively. Gastroprotection has been elucidated to be due to inhibition of (a) upregulated H+, K+-ATPase activity that induced ulcer condition; (b) lipid peroxidation and (c) altered antioxidant enzyme levels. The data highlighted the importance of vitamin B12 in protecting the gut against gastric ulcerations and suggested that both Spirulina biomass and Spirulina extract contain bioavailable B12. Spirulina based product/food can be used as alternatives to gastroprotective agents that are known to cause acidity themselves upon long term use.
Keywords: Spirulina, Vitamin B12, Gastric ulcer, Deficiency of vitamin B12
Introduction
The usual dietary sources of vitamin B12 are animal-derived foods, although a few plant-based foods contain substantial amounts of vitamin B12. Vitamin B12 deficiency thus results in high-risk of vitamin B12 associated disorders in vegetarian population.
Deficiency of vitamin B12 is one of the emerging global problems (Allen 2010), since this deficiency is affecting infants to elders, and both the genders throughout the lifespan. Vitamin B12 deficiency is more common in pregnant and lactating women and young children in developing countries. Elderly persons above the age of 60 are at equally greater risk as performance of gut decreases and vitamin B12 deficiency is known to affect the gut epithelium, there by affecting the regular absorption of food (Schjonsby 1989).
Vitamin B12 deficiency manifested symptoms in humans includes neurologic injury, disability, demyelinating nervous system disease, dementia, psychiatric illness, anemia, vascular occlusions and suppressed immune system (Durand et al. 2003). The biochemical abnormalities include low serum B12, holo-transcobalamin (holo TC), elevated plasma total homocysteine (tHcy), urinary methylmalonic acid.
Microalgae are rich source of several nutrients like vitamins, minerals, essential fatty acids, protein, chlorophyll, carotenoids and a vast spectrum of phytochemicals. Vitamins are found in their natural forms in microalgae (Andrade et al. 2018). Recent reports have emphasized the necessity to identify plant-derived foods that contain significant amount of vitamin B12 to prevent its deficiency in vegetarians (Watanabe et al. 2014). Cyanobacterial species are reported to contain a good amount of vitamin B12, but the forms of vitamin or their bioavailability is not elucidated. Among green algae, Chlorella vulgaris is reported to contain the true/active form of vitamin B12 (Watanabe et al. 2014). Dried purple laver is one of the good sources of vitamin B12. However, many of the studies have focused on the identification of the vitamin B12 in these algae, and none of these studies have analyzed the bioactivity aspects, except in the macro alga Nori, the purple laver (Helliwell et al. 2016).
Radiological studies of vitamin B12 deficient experimental animals indicated the damages in the gastrointestinal lining (Briani et al. 2013). Indeed, intact gastrointestinal lining by gastric epithelium is one of the robust factors in gut defense and hence gut health (Pastorelli et al. 2013). A cohort study conducted in Japan indicated that most patients with complicated peptic ulcers may have deficiency of one or more water-soluble vitamins including vitamin B12 in the early phase of the disease before the onset of ulcer complications (Miyake et al. 2013). It becomes therefore imperative to address the effect of vitamin B12 deficiency during gastric ulcer condition and the status of ulcer during the treatment with vitamin B12 enriched source, in this case, Spirulina platensis.
Current study thus focusses on (1) effect of vitamin B12 on ulcer condition in albino Wistar male rats; (2) ulcer status in normal and vitamin B12 deficient Wistar rats; (3) modulatory effect if any upon feeding vitamin B12 containing Spirulina biomass and extract in comparison with standard vitamin B12 at same concentrations. The present study provided the impact of vitamin B12 effect on gastric ulcerations and the potential anti ulcerative effect of Spirulina biomass and Spirulina extract.
Materials and methods
Spirulina platensis biomass and extract
Spirulina platensis CFTRI strain was used for the experiment. Spirulina was grown in outdoor raceway pond in Zarrouk’s medium. The biomass was harvested by gravity filtration after the culture reached stationary phase. The biomass was acid washed to reduce the ash content and later washed twice with distilled water. The harvested biomass was dried by lyophilization and stored at − 80 °C in air tight container until further use. Spirulina extract was prepared in the aqueous medium from 100 g biomass in batches for the oral intubation to rats.
Reagents
Corn starch, cane sugar and refined groundnut oil were procured from local market. Vitamins, minerals, cellulose, choline chloride and l-cystine were obtained from Himedia Laboratories (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India). Clinical enzyme kits namely SGOT, SGPT, were obtained from Agape Diagnostics, Kerala (India).
Extraction and analysis of vitamin B12
HPLC analysis was carried out to estimate the vitamin B12 levels in the Spirulina biomass (SB) and the extract (SE). Since vitamin B12 is water soluble, water extraction method (Kumudha et al. 2010) was adopted wherein Spirulina biomass was continuously stirred in water at high temperature followed by centrifugation and collected the supernatant. Further, it was purified with XAD and sep-pak columns. After purification, vitamin B12 content of the Spirulina was determined by both HPLC and microbiological assay.
Microbiological assay
For microbiological assay Lactobacillus delbrueckii MTCC 911 strain procured from Microbial Type Culture Collection, CSIR-IMTECH, Chandigarh, India) was used (Kumudha and Sarada 2015).
HPLC analysis
Purified vitamin B12 extract from Spirulina sample and serum samples were analysed by C18 column. Serum samples were incubated in water bath for 20 min at 45 °C, centrifuged and supernatant were analyzed by HPLC that was equipped with C-18 Column (4.6 × 300 mm, particle size 10 µm) procured from Waters Corp., USA. The absorbance was measured at 546 nm. The solvent systems used were (A) 50% methanol containing 0.1% (v/v) acetic acid (B) water, for 40 min with a flow rate of 1 ml/min. Vitamin B12 was eluted with a linear gradient of methanol (from 0 to 90% of a 50% (v/v) methanol solution).
Assessment of gastric ulcers; protection by Spirulina biomass and extract
Healthy albino wistar male rats (120 ± 10 g) were used for the experiments, and the animals were fed with SB and SE for 30 days through guavage. All the experimental animals were maintained under standard conditions of temperature, humidity and light. The study was permitted by the institutional ethical committee, that follows the guidelines of “CPCSEA (Committee for the purpose of control and supervision of experiments on animals, reg. No. 49, 1999), Government of India, New Delhi, India”. All the experimental animals were classified into 9 groups of 6 animals each. Group 1: Rats were given AIN-93 diet (Reeves 1997), which is designated as normal diet. Group 2 and 3 serve as Spirulina Biomass (SB) and standard vitamin B12 controls along with normal diet. Group 4 and 6 are ulcer control groups where group 4 with normal diet; while group 6 with vitamin B12 deficient diet. Group 5 received Spirulina Extract (SE) along with normal diet, followed by ulcer induction (UI). Group 7–9 are vitamin B12 deficient groups fed with standard vitamin B12, SB and SE respectively. The amount of Spirulina biomass and Spirulina extract fed to rats was based on vitamin B12 equivalent to the amount as defined by “The Nutritional requirements of rats” set by the National Research Council, USA. At the end of the experiment, rats were sacrificed.
Induction of ulcer and determination of ulcer index
Ulcer was induced by forced swim stress as per Srikanta et al. 2007 where the rats were subjected to forced swim stress by taking them in a jar of 30-cm high and 10 cm diameter. The jar contained water up to 15 cm height and animals were allowed to swim for 5 h. Rats were sacrificed under deep ether anesthesia. The inner layer of the stomach was examined for the incidence of ulcers. The stomach was removed and opened along the greater curvature. It was washed with normal saline, stretched and flattened on a piece of cardboard. The inner surface was examined for mucosal integrity and occurrence of the ulcer. The total number of mucosal lesions/ulcers per stomach was counted and expressed as ulcer index (UI) (Srikanta et al. 2007). “Lower to higher grade was assigned to milder to severe symptoms. The following are the ulcer scores: 0.5 = red coloration, 1.0 = spot ulcers, 2.0 = hemorrhagic streaks more than 3 mm and less than 5 mm, 3.0 = ulcers/hemorrhagic streaks more than 5 mm. The total ulcer score calculated for each experimental group and mean ulcer score of each experimental group were expressed as the ulcer index (UI)” (Kamath et al. 2008; Kulkarni and Goel 1996). Stomach and liver tissue homogenate and serum were collected from all experimental animals and analyzed for various biochemical parameters.
Estimation of gastric mucin
Gastric wall content of mucin was estimated by Alcian blue-binding method (Corne 1974). A sample of 100 mg of stomach tissues from animals of each group was taken and incubated for 2 h in acetate buffer (pH 5.8, 0.05 M) containing 0.16 M sucrose and 1.0% Alcian blue dye. The absorbance of the supernatant was read at 498 nm.
Histopathological studies
Gastric tissue samples were fixed in 10% buffered formalin for 24 h. The processed tissues were fixed in paraffin blocks and sections made were stained with hematoxylin and eosin dye (Sibilia et al. 2003). The histochemical sections were analyzed by observing under light microscope at 10× magnification.
Preparation of tissue homogenate for biochemical analysis
The stomach and the liver tissues collected were weighed and homogenized in chilled phosphate buffer 20 mM, pH 7.4. The homogenates were centrifuged at 1000g at 4 °C for 20 min using a high-speed cooling centrifuge (Remi C 24, Mumbai, India). The clear supernatants were used for various biochemical parameters.
Estimation of H+, K+-ATPase
Known weight of gastric tissue from animals of each group was homogenized in Tris-HCl buffer 16 mM, pH 6.5. The homogenates were centrifuged at 6000g at 4 °C for 20 min, and the parietal cell extract thus prepared was used to determine the H+, K+-ATPase. The reaction mixture contained Tris-HCl, 2 mM MgCl2, 2 mM KCl. The reaction was initiated with the addition of 2 mM ATP, and the incubation was continued for 30 min at 37 °C. The reaction was terminated by adding ammonium molybdate and trichloroacetic acid mixture followed by centrifugation at 2000g. The amount of inorganic phosphate released from ATP was estimated spectrophotometrically as described by Yoda and Hokin (1970).
TBARS assay
Lipid peroxidation products of serum, stomach liver homogenates were determined as thiobarbituric acid reactive substance (TBARS) as described by Buege and Aust (1978). Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) to form a diadduct that is measured spectrophotometrically at 532 nm. To 1 ml of the above homogenates, 1 ml of 0.15 M KCl and 0.1 ml of 0.2 mM ferric chloride were added to initiate peroxidation and the solution was incubated for 37 °C for 30 min. 2 ml of ice-cold mixture of TCA–TBA–BHT (15% trichloroacetic acid, 0.3% thiobarbituric acid, 0.05% butylated hyroxytoluene in 0.25 N HCl) was added to terminate the reaction and was heated at 80 °C for 60 min. The reaction mixture was cooled, centrifuged and the absorbance of the supernatant was measured at 532 nm.
Antioxidant enzyme assays
Catalase activity
Catalase activity was determined as described by Aebi (1984). H2O2 decomposition on the addition of sample was followed at 240 nm. A unit of catalase was defined as the amount of enzyme required to decompose 1 µmol H2O2 per minute at 25 °C at 7.0 pH.
Glutathione peroxidase activity
Glutathione peroxidase activity was estimated as described by Flohe and Gunzler (1984). The mixture containing the sample, 0.1 ml of 10 mM glutathione reductase and 0.1 ml of 10 mM glutathione were preincubated for 10 min at 37 °C and 0.1 ml of NADPH solution was added. The hydroperoxide independent consumption of NADPH was monitored for 3 min. The reaction was started by adding 0.1 ml of the pre warmed hydroperoxide solution and the decrease in absorption at 340 nm was monitored for 3 min.
Superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed by measuring the reduction in the NBT in the presence of SOD. Activity was expressed as unit per milligram protein per min. (Flohe and Otting 1984).
Results
Analysis of vitamin B12 content in Spirulina biomass and Spirulina extract
Total vitamin B12 content in Spirulina biomass and Spirulina extract was found to be 220 ± 1.8 µg/100 g (Kumudha and Sarada 2015). The true form of vitamin B12 in Spirulina is found to be methyl cobalamin.
Assessment of gastric ulcers, protection by Spirulina biomass and extract
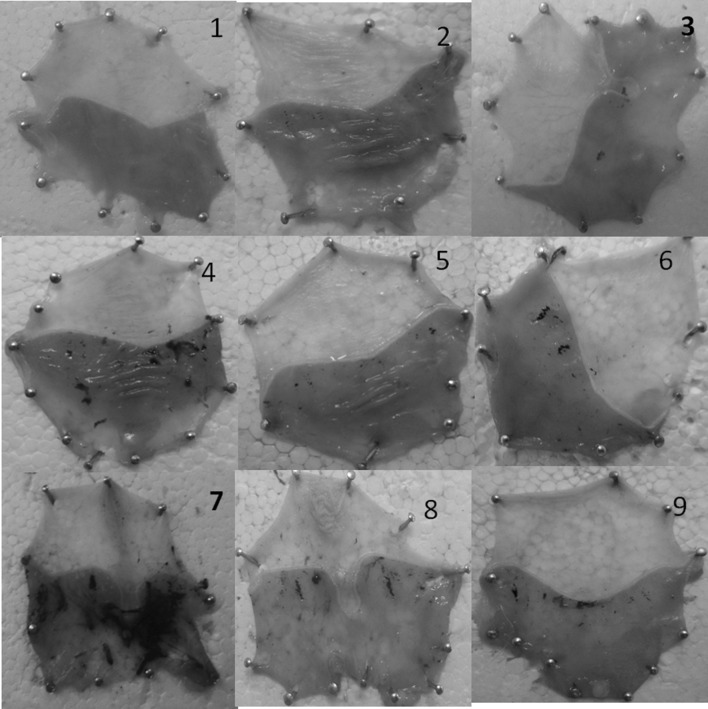
Ulcer score was calculated based on number and intensity of ulcers in each rat in the group as described under materials and methods. Group-7 animals that were induced with ulcers under vitamin B12 deficient diet were considered to have 100% ulceration and relative percent ulceration was calculated in other groups. Relative percent protection by SB and SE was calculated taking the group of animals that have received normal diet as well as vitamin B12 deficient diet as base. In sample and standard control groups, no ulcers were observed suggesting that samples selected for treatment were not causing ulcers per se (Fig. 1).
Fig. 1.
Macroscopic observation of ulcers in the stomach from ulcer induced and Spirulina biomass/Spirulina extract treated animals. Group 1: Healthy with normal diet. Group 2 and 3 serves as Spirulina biomass (SBM) and standard vitamin B12 controls. Group 4 and 6 are ulcer control groups. Group 5 with (SE), followed by ulcer induction. Group 7–9 are vitamin B12 deficient groups with standard vitamin B12, SBM and SE
Animals treated with Spirulina biomass and Spirulina extract showed significant reduction (2.5 folds) in ulcer index as opposed to untreated animals. Rats treated with Spirulina biomass and Spirulina extract under normal diet condition showed ulcer score of 12% and 23% respectively suggesting 88% and 77% gastro protection respectively. Under B12 deficient condition Spirulina biomass showed 75% protection as opposed to 68% by standard vitamin B12 suggesting increased gastro protective potential by SB in addition to vitamin B12. It is possible that SB may contain some additional compounds that can contribute to enhanced gastroprotection in addition to vitamin B12 in SB. It is interesting to observe that animals that were receiving vitamin B12 deficient diet showed ~ 10–13% less than those which were receiving normal diet, indicating that vitamin B12 may play a crucial role in terms of ulcer/tissue healing (Fig. 1).
Swim stress induces severe lesions including inflammatory patches, bleeding in mucosa and ulcers with different size and degree in ulcerated rats. In the present study also the rats treated with forced swim stress showed damage in the gastric wall with a hemorrhagic form of lesions and intraluminal bleeding (Fig. 1) while no such gastric lesions and bleeding were noted in healthy controls. Results were substantiated by histopathological studies. In addition, in ulcerous rats the gastric mucin levels reduced compared to control. Rats that were treated with SB and SE showed increase in mucin level (Table 1).
Table 1.
Gastric mucin levels in healthy, ulcerated and Spirulina biomass and extract treated rats
| S. no. | Group name | mg/g tissue |
|---|---|---|
| 1 | Normal diet | 58.09 ± 7.2 |
| 2 | Normal diet + Spirulina biomass | 57.75 ± 4.2 |
| 3 | Normal diet + Std Vit B12 | 59.68 ± 3.8 |
| 4 | Ul + normal diet | 18.95 ± 2.5 |
| 5 | Ul + Spirulina extract | 43.33 ± 5.2 |
| 6 | Ul + B12 deficient | 18.73 ± 3.8 |
| 7 | UI + B12 deficient +Std B12 | 39.44 ± 2.9 |
| 8 | UI + B12 deficient + Spirulina extract | 38.48 ± 4.3 |
| 9 | UI + B12 deficient + Spirulina biomass | 41.68 ± 3.7 |
UI ulcer induced, Std B12 standard B12
Histopathological studies
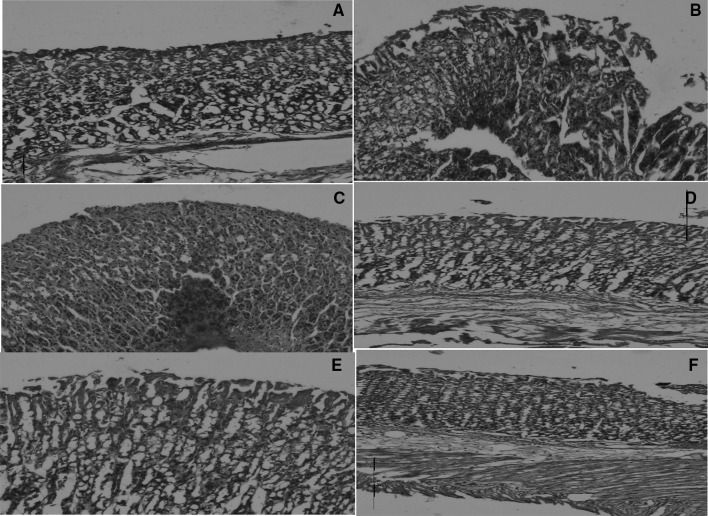
Deep erosions with discontinuous mucosal layer were observed in ulcer-induced rats. Rats pre-treated with Spirulina extract and biomass showed normal histology or only very superficial lesions. Healthy controls showed intact mucosal epithelium. The recovery of the mucosal layer was observed in Spirulina extract and biomass treated groups when compared to ulcer-induced stomach (Fig. 1). Results also showed that tissue regeneration and formation of mucosal epithelium was complete with complete layer formation and intact mucosal glandular tissues in sections from animals treated with SB and Standard vitamin B12 along with normal diet. However the treatment of the same in mucosal gland and epithelium recovery was not efficient as evidenced in Fig. 2.
Fig. 2.
Histopathological observation of stomach from ulcer induced and Spirulina biomass Spirulina extract treated animals. a Control, b ulcerated showing damaged mucosal epithelium, c, d and e, f showed recovery in mucosal epithelium treated with Spirulina biomass and Spirulina extract respectively
H+, K+-ATPase, activity
H+, K+-ATPase activity was increased in ulcerous animals when compared to healthy controls. Spirulina biomass and Spirulina extract could normalize the levels in the stress induced models (Table 2). Around threefold increase in H+K+-ATPase activity during ulcerations were normalized (~ 50%) in both normal diet as well as vitamin B12 deficient groups of animals supplemented with Spirulina biomass and Spirulina extract.
Table 2.
H+, K+-ATPase levels in healthy, ulcerated and treated rats
| S. no. | Group name | µ moles pi release/mg protein/h |
|---|---|---|
| 1 | Normal diet | 1.39 ± 0.12 |
| 2 | Normal diet + Spirulina biomass | 1.22 ± 0.07 |
| 3 | Normal diet + Std Vit B12 | 1.27 ± 0.12 |
| 4 | Ul + normal diet | 3.65 ± 0.28 |
| 5 | Ul + Spirulina extract | 1.83 ± 0.52 |
| 6 | Ul + B12 deficient | 3.87 ± 0.38 |
| 7 | UI + B12 deficient +Std B12 | 1.92 ± 0.17 |
| 8 | UI + B12 deficient + Spirulina extract | 2.18 ± 0.11 |
| 9 | UI + B12 deficient + Spirulina biomass | 1.93 ± 0.18 |
UI ulcer induced, Std B12 standard B12
Changes in antioxidant enzymes
The increase in TBARS levels shown in the stomach homogenate in ulcer condition was normalized by Spirulina biomass as well as Spirulina extract. Decrease in the antioxidant enzymes such as SOD, CAT and glutathione peroxide during ulcer condition were normalized with the treatment of rats with Spirulina biomass and Spirulina extract as evident from Tables 3 and 4.
Table 3.
SOD activity and TBARS level in serum of healthy, ulcerous and treated rats
| S. no. | Group name | SOD U/mg (protein) | TBARS µmol/mg protein |
|---|---|---|---|
| 1 | Normal diet | 16.6 ± 1.2 | 3.77 ± 0.3 |
| 2 | Normal diet + Spirulina biomass | 16.65 ± 0.8 | 4.16 ± 0.3 |
| 3 | Normal diet + Std Vit B12 | 17.32 ± 2.5 | 3.59 ± 0.6 |
| 4 | Ul + normal diet | 8.2 ± 0.5 | 8.62 ± 0.0.6 |
| 5 | Ul + Spirulina extract | 13.46 ± 1.7 | 5.17 ± 0.5 |
| 6 | Ul + B12 deficient | 7.66 ± 1.4 | 9.39 ± 0.8 |
| 7 | UI + B12 deficient + Std B12 | 14.75 ± 0.8 | 5.55 ± 0.7 |
| 8 | UI + B12 deficient + Spirulina extract | 13.85 ± 1.6 | 4.81 ± 0.3 |
| 9 | UI + B12 deficient + Spirulina biomass | 13.65 ± 0.9 | 5.14 ± 0.8 |
UI ulcer induced, Std B12 standard B12
Table 4.
Catalase and Glutathione peroxidase levels in healthy, ulcerous and treated rats
| S. no. | Group name | Catalase µmol/min/mg/protein | Glutathione peroxidase µmol/min/mg protein |
|---|---|---|---|
| 1 | Normal diet | 35.9 ± 6 | 1.17 ± 0.08 |
| 2 | Normal diet + Spirulina biomass | 32.3 ± 4.5 | 1.07 ± 0.6 |
| 3 | Normal diet + Std Vit B12 | 36.7 ± 5 | 1.02 ± 0.06 |
| 4 | Ul + Normal diet | 15.9 ± 2.1 | 3.18 ± 0.1 |
| 5 | Ul + Spirulina extract | 28.8 ± 5.1 | 1.37 ± 0.06 |
| 6 | Ul + B12 deficient | 14.1 ± 3.5 | 3.28 ± 0.09 |
| 7 | UI + B12 deficient + Std B12 | 29.1 ± 9.6 | 1.38 ± 0.06 |
| 8 | UI + B12 deficient + Spirulina extract | 26.4 ± 4.1 | 1.48 ± 0.08 |
| 9 | UI + B12 deficient + Spirulina biomass | 24 ± 3.5 | 1.57 ± 0.1 |
UI ulcer induced, Std B12 standard B12
Discussion
Deficiency of vitamin B12 is observed among people of all ages who consume vegetarian diet. Reason for malabsorption appears to be many. Lack of intrinsic factors in the parietal cells and some genetic factors are known to affect vitamin B12 absorption the most and appear to be the important cause for vitamin B12 malabsorption (Pocock et al. 2013). In this context it is important to understand the absorption levels of vitamin B12 during gastric ulceration condition, where parietal cellular damage and hence loss of regulation of H+K+-ATPase that controls acidity in the lumen of the stomach has been observed.
Vitamin B12 is required as a coenzyme for the metabolism of the amino acids methionine, threonine and valine and for the transformation of methyl-tetrahydrofolate to tetrahydrofolate, which is necessary for DNA synthesis (Al Aisari et al. 2010). During ulcer conditions, there is a lot of erosion of gastric mucosal cells and this has to be regenerated in order to complete the repair process during ulcer healing process. DNA synthesis obviously is essential for ulcer healing process. In the current study therefore, we evaluated the susceptibility to gastric ulcerations under normal and vitamin B12 deficient and the extent of ulcer prevention.
Our results showed that under B12 deficient condition, the gastroprotective potential of Spirulina biomass as well as standard vitamin B12 is reduced and this could be attributed to the delayed ulcer healing processes. Results were also evident in histopathological analysis, where Spirulina biomass (SB) and standard vitamin B12, could regenerate gastric mucosal cells better with the normal diet than with vitamin B12 deficient diet. This can be attributed to the role of vitamin B12 in cellular proliferation. Proliferation is an important phenomenon for regeneration. Supplementation of vitamin B12 thus can augment the process of mucosal proliferation as evidenced by histopathological analysis and production of gastric mucin that can envelope the gastric epithelium for effective defense.
Antiulcer agent together with vitamin B12 may offer speedy recovery of gastric ulcerations, which is evident, when we observed better ulcer protective effect by Spirulina biomass (SB) than vitamin B12 per se. Better effect with SB could be due to the presence of additional probable gastroprotective compounds in them.
Upregulation of H+K+-ATPase, mucosal damage, decreased antioxidant status and increased oxidative stress conditions are common events observed during gastric ulcerations (Suzuki et al. 2012). Generally all ulcer inducing conditions such as stress, ethanol intake or drug like indomethacin have been known to encompass these disease pathogenic steps. Vitamin B12 has direct influence on all these since its absorption is dependent on the intrinsic factor which is present in the parietal cell, perturbation of this cellular integrity has been known to affect H+K+-ATpase, which releases acid, that acts directly on the gastric mucosal epithelium in addition to imbalance in oxidant and antioxidant condition. Various studies have also suggested that vitamin B12 deficiency correlates with lowering of antioxidant status (Herrmann et al. 2001). Our studies thus were directed to evaluate oxidant and antioxidant status in gastric ulceration condition in group of animals that had normal diet as well as the group that had vitamin B12 deficient diet. There is increase in oxidative stress condition in vitamin B12 deficient group than those getting normal diet. Since vitamin B12 fed group also recovered all antioxidants to similar extent, data may suggest that vitamin B12 alone possesses the capability to regulate antioxidant status of cells or tissue. Results were supported by the effective balancing of antioxidant enzymes by vitamin B12 supplementation.
Overall data suggests that although, there is effective normalization of antioxidant enzymes, after vitamin B12 treatment, gastro protection under deficient condition is slightly lesser than the group of animals, which were getting normal diet. Marginal reduction in gastro protective efficiency could be attributed to reduced mucosal recovery as evidenced also by histopathological analysis (Fig. 2). Reduced mucosal recovery could be assigned to reduced proliferation of damaged mucosal cells, which is due to vitamin B12 deficiency. Even upon feeding of vitamin B12, comparatively slow recovery in the vitamin B12 deficient group suggests that the proliferative programme is affected with vitamin B12 deficiency. Current data has direct relevance on its role in increasing susceptibility to gastric ulcerations in experimental animals besides its delayed recovery effect.
Conclusion
The stressful lifestyles, inadequate intake of nutritious food/nutraceuticals, continuous use of non-steroidal anti-inflammatory drugs by the global population are some of the reasons for the increased incidence of ulcers worldwide (Langman et al. 1991; Miller 1987). More than 30 million people in the world are known to take non-steroidal anti-inflammatory drugs. Drugs that are commercially available for treatment, when used for long term are known to cause unpredictable side effects and hence this warranted a safer alternative source for ulcer management. The study demonstrated for the first time that orally administered Spirulina biomass and Spirulina extract containing vitamin B12 exerts gastro protective effect on swim-stress induced gastric lesions in rats.
Acknowledgements
Kumudha A is grateful to Council of Scientific and Industrial Research for providing Senior Research fellowship.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Al Aisari F, Al-Hashmi H, Mula-Abed WA. Comparison between serum holotranscobalamin and total vitamin B12 as indicators of vitamin B12 status. Oman Med J. 2010;25:9–12. doi: 10.5001/omj.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LH. Bioavailability of vitamin B12. Int J Vitam Nutr Res. 2010;80:330–335. doi: 10.1024/0300-9831/a000041. [DOI] [PubMed] [Google Scholar]
- Briani C, Torre CD, Citton V, Manara R, Pompanin S, Binotto G, Adami Fausto. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;11:4521–4539. doi: 10.3390/nu5114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Corne SJ. A method for quantitative estimation of gastric barrier mucus. J Physiol Lond. 1974;242:1169–1179. [PubMed] [Google Scholar]
- Durand C, Mary S, Brazo P, Dollfus S. Psychiatric manifestations of vitamin B12 deficiency: a case report. L’Encephale. 2003;29:560–565. [PubMed] [Google Scholar]
- Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–120. doi: 10.1016/S0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- Flohe L, Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/S0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- Helliwell KE, Lawrence AD, Holzer A, Kudahl UJ, Sasso S, Krautler B, Scanlan DJ, Warren MJ, Smith AG. Cyanobacteria and eukaryotic algae use different chemical variants of vitamin B12. Curr Biol. 2016;26:999–1008. doi: 10.1016/j.cub.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann W, Schorr H, Purschwitz K, Rassoul F, Richter V. Total homocysteine, vitamin B12, and total antioxidant status in vegetarians. Clin Chem. 2001;47:1094–1101. doi: 10.1093/clinchem/47.6.1094. [DOI] [PubMed] [Google Scholar]
- Kamath BS, Srikanta BM, Dharmesh SM, Sarada R, Ravishankar GA. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur J Pharmacol. 2008;590:387–395. doi: 10.1016/j.ejphar.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Goel RK. Gastric antiulcer activity of UL-409 in rats. Indian J Exp Biol. 1996;34:683–688. [PubMed] [Google Scholar]
- Kumuda A, Sarada R. Effect of different extraction methods on vitamin B12 from blue green algae, Spirulina platensis. Pharm Anal Acta. 2015;6:337. [Google Scholar]
- Kumudha A, Kumar SS, Thakur MS, Ravishankar GA, Sarada R. Purification, identification, and characterization of methylcobalamin from Spirulina platensis. J Agric Food Chem. 2010;58:9925–9930. doi: 10.1021/jf102159j. [DOI] [PubMed] [Google Scholar]
- Langman MJS, Brooks P, Hawkey CJ, Silverstein F, Yeomans N. Non-steroidal anti-inflammatory drug associated ulcer: epidemiology, causation and treatment. J Gastroenterol Hepatol. 1991;6:442–449. doi: 10.1111/j.1440-1746.1991.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Andrade LM, Andrade CJ, Dias M, Nascimento CAO, Mendes AM. Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements an overview. Food Process Technol. 2018;6:45–58. [Google Scholar]
- Miller TA. Mechanisms of stress-related mucosal damage. Am J Med. 1987;83:8–14. doi: 10.1016/0002-9343(87)90805-9. [DOI] [PubMed] [Google Scholar]
- Miyake K, Akimoto T, Kusakabe M, Sato W, Yamada A, Yamawaki H, Kodaka Y, Shinpuku M, Nagoya H, Shindo T, Ueki N, Kusunoki M, Kawagoe T, Futagami S, Tsukui T, Sakamoto C. Water soluble vitamin deficiencies in complicated peptic ulcer patients soon after ulcer onset in Japan. J Nutr Sci Vitaminol (Tokyo) 2013;59:503–508. doi: 10.3177/jnsv.59.503. [DOI] [PubMed] [Google Scholar]
- Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics. Front Immunol. 2013;4:280. doi: 10.3389/fimmu.2013.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock G, Richards CD, Richards D. Human physiology: the basis of medicine. Br J Sports Med. 2013;40:880–883. [Google Scholar]
- Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838–841. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- Schjonsby H. Vitamin B12 absorption and malabsorption. Gut. 1989;30:1686–1691. doi: 10.1136/gut.30.12.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia V, Rindi G, Pagani F, Rapetti D, Locatelli V, Torsello A, Campanini N, Deghenghi R, Netti C. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- Srikanta B, Siddaraju M, Dharmesh S. A novel phenol-bound pectic polysaccharide from Decalepis hamiltonii with multi-step ulcer preventive activity. World J Gastroenterol. 2007;13:5196–5207. doi: 10.3748/wjg.v13.i39.5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi Toshifumi. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;1:35–39. doi: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014;6:1861–1873. doi: 10.3390/nu6051861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda A, Hokin LE. On the reversibility of binding of cardiotonic steroids to a partially purified (Na + K)-activated adenosinetriphosphatase from beef brain. Biochem Biophys Res Commun. 1970;40:880–886. doi: 10.1016/0006-291X(70)90985-X. [DOI] [PubMed] [Google Scholar]