Abstract
The aim of the study was to screen the metabolite profile of phalsa (Grewia asiatica), an underutilized fruit crop, using liquid chromatography-high resolution mass spectrometric analysis. A total of 50 compounds were tentatively identified based on their molecular mass and characteristic fragment ions, each with less than 5 ppm of mass error. These compounds included 21 flavonols, 2 dihydroflavonols, 7 flavones, 3 flavanols, 6 anthocyanins, 3 isoflavonoids, 2 phenolic acids, 2 flavanones, and 4 other phenolics. Flavonols were the predominant group of compounds, representing around 52.6% of the total phenolics. The paper has also discussed the potentiality of phalsa as an emerging functional food for the management of various human diseases in relation to the existing literature.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04092-y) contains supplementary material, which is available to authorized users.
Keywords: Phalsa (Grewia asiatica L, Tiliaceae); Phenolic profiling; Antioxidant activity; High resolution LC–MS
Introduction
In the new millennium providing food and nutrition security to a growing population is a great challenge. It has often been noted that many of the underutilized crops do not only have a higher nutritional level compared to commonly cultivated ones, but also can adapt better to changing climatic conditions. Such an underutilized crop is phalsa (Grewia asiatica L, family—Tiliaceae), which bears fruits that are small in size. Its fruits develop from purple to black color at maturity, indicating the presence of many phenolic constituents, including anthocyanins, flavonols, etc. Consumed predominantly as a fresh fruit, phalsa is native to tropical Southern Asian countries, including India, Pakistan, Nepal, Thailand, and others. As it can endure and sustain under adverse climatic condition, e.g., frost and drought, it can be used as an alternative food crop (Lim 2012) for sustaining food security.
In India, the demand of phalsa has been steadily increasing in markets due to its health benefits. Usually produced in the months of March–April in India, its fruits are reported to have astringent, stomachic and cooling effects in traditional Indian folklore medicine (Kirtikar and Basu 1989; Paul 2015). As the unripe fruit is known to have anti-inflammatory properties, it has been used for treating respiratory and cardiac diseases, blood disorders, fever, and throat infections (Lim 2012). Pharmacological research also revealed that the ripe fruits have potential antioxidant (Asghar et al. 2008), anticancerous (Marya et al. 2011), and radioprotective (Sharma and Sisodia 2009) properties. The polyphenolic fraction of the crude extract of phalsa has componentssuch as phenolic acid, flavanol, flavonol, and anthocyanins, which have shown significant anti-fungal/anti-microbial activities (Siddiqi et al. 2011). Additionally, this fruit demonstrated hepato-protective (Sharma and Sisodia 2010), analgesic, antipyretic (Das et al. 2012), and antihyperglycemic effects (Khattab et al. 2015).
Due to the above-mentioned health beneficial properties, phalsa fruit is often considered as a promising functional food (Khan et al. 2019) and has been a subject of many phytochemical investigations. Previously, Agarwal and Mishra (1979) tentatively identified the presence of pelargonidin-3,5-diglucoside, naringenin-7-O-β-d-glucoside, quercetin, quercetin-3-O-β-d-glucoside, tannins, catechins, and cyanidin-3-glucoside in this fruit. During an investigation on the nature of anthocyanin pigments using paper chromatography, Khurdiya and Anand (1981) reported delphinidin-3-glucoside and cyanidin-3-glucoside as the main pigments, responsible for its coloration. In both cases, the compounds were identified based on their retention factors (RF) and certain chemical principles, and therefore, did not have compound-specific selectivity in confirmation. Later, by using reverse phase HPLC, Taskeen et al. (2010) noted the presence of only rhamnetin and luteolin in phalsa. In their study, identifications were based on matching the retention time of the signals against the corresponding reference standards, and hence, was limited within the scope of the targeted flavonoids. Nevertheless, drawing on our earlier experience, we can say that the HPLC–UV based analysis of such complex samples often results in false detections due to complex coelutions and difficulties in peak integration.
When studying the phenolics utilizing LC–MS/MS analysis, Sharma and Gupta (2013) reported 8 polyphenols, viz., gallic acid, ellagic acid, chlorogenic acid, p-coumaric acid, catechin, quercetin, myricetin, and rutin in the phalsa pomace. While examining the suitability of the ripe phalsa as a source of natural colorants and also to explore its neutraceutical potential, Talpur et al. (2017) had identified 7 anthocyanins by LC–MS/MS analysis, which included 2 non-acylated anthocyanins (delphinidin-3-O-glucoside, peonidin-3-O-glucoside), 4 acylated anthocyanins (pelargonidin-3-O-malonyl glucoside, cyanidin-3-O-6”acetyl glucoside, peonidin-3-O-6”-acetyl glucoside, and pelargonidin-3-O-6”-acetylglucoside), and 1 pyranoanthocyanin (malvidin-3-O-glucoside pyruvic acid).
In all the above-mentioned cases, either the scope of metabolite identification was limited to specific objectives, or the technique which was used had a limited targeted approach. These deterred the possibility of establishing a comprehensive phenolic profile of phalsa. Despite the rising popularity of high resolution LC–MS technique and its ability to identify compounds based on accurate mass, elemental composition, and isotopic pattern for screening of secondary metabolites, scarcely any study exists so far on the phenolic profiling of this fruit. Given this gap of knowledge, we developed a high resolution accurate mass based screening method for the identification of various classes of polyphenols in the extracts of phalsa.
Materials and methods
Plant materials
The freshly harvested fruits of phalsa were collected from a local fruit grower (Varanasi, Uttar Pradesh, India), and preserved in ice until the LC–MS characterization.
Chemicals and apparatus
Methanol (LC–MS grade), and formic acid (88%) were supplied by J.T. Baker (NJ, USA). HPLC grade water (with resistivity of 18.2 mΩ) was obtained through a CASCADA water purification system (Pall Corporation, Bengaluru, India). Hydrophilic lipophilic balanced (HLB) cartridge (200 mg, 6 cc) was obtained from Waters Corporation (Bengaluru). Polyphenol standards were purchased from Sigma-Aldrich (Bengaluru).
Characterization of phenolic compounds
Sample preparation
The seeds were manually separated from 100 g fruit, and the fruit pulp was thoroughly comminuted. The homogenized sample (5 g) was drawn in a 50 mL amber-colored polypropylene centrifuge tube with a quality control standard (forchlorfenuron, 1 mg/L), and immediately extracted with methanol (20 mL, 1% formic acid) by vortexing at 2000 rpm for 2 min in the dark, followed by centrifugation at 10,000 rpm for 10 min at 10 °C. The supernatant (5 mL) was diluted with acidified water (20 mL), and passed through the HLB cartridge that was preconditioned with acidified water and methanol (10 mL each). The cartridge was washed with 5 ml water, and eluted with 5 mL acidified methanol. This extract was diluted with acidified water, filtered through 0.2 μm polytetrafluoroethylene (PTFE) membrane, and finally injected (5 μL) into the LC–QToF–MS.
LC–QToF–MS conditions
The analysis was performed on an Acquity Ultra-high Performance Liquid Chromatograph (UPLC), coupled to a QToF-MS (Synapt G2 HDMS, Waters Corporation, Manchester, UK), and operated with electrospray ionization (ESI) at the mass resolution of 20,000. The chromatographic separation was performed on an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.8 μm, Waters India Pvt. Ltd. (Bengaluru) at 35 °C. The mobile phase consisted of A: methanol: water (10:90, v/v) and B: methanol: water (90:10, v/v) with 0.1% formic acid in both phases. A gradient program was used with 0.4 mL/min flow rate, with 0–0.5 min/90% phase A, 4.5 min/50% A, 4.5–8 min/50–2%A, 8–11 min/2% A, 11–11.5 min/2–90% A, and 12–15 min/90% A.
The ESI source parameters were set as follows: capillary 3.5 kV, sampling cone 30 V, extraction cone 5 V, ion source temperature 120 °C, desolvation temperature 500 °C, desolvation gas flow 1000 L/h, and cone gas flow 50 L/h. The mass spectrometer was calibrated with 0.5 mM of sodium formate. With a flow rate of 10 μL/min at the concentration of 2 μg/mL at an interval of 20 s, the lock spray and the reference mass leucine enkephalin (m/z 556.2771 in positive polarity) was used for the mass correction. The system was controlled by MassLynx 4.1 software. The data acquisition was performed in the MSe mode, involving a quick switching from a low energy scan at 4 V (full scan MS) to a high energy scan (10–60 V ramping) during a single LC run. The low-CE experiments provided information about the full scan with intact molecular ion, e.g., M+, [M + H]+, while the high collision energy scan offered the product ion spectra information.
Data analysis
The LC–MS data files (n = 6) were processed by the UNIFI software (version 1.7, Waters Corporation) with a screening solution workflow. Each data file was processed against a database of polyphenols, comprising the parent molecules and their derivatives. This in-house developed database had over 1400 compound entries, and the relevant compound-specific information (chemical structure, molecular formula, molecular mass) from various web-based resources (e.g., Chemspider), and published research papers. The limit of mass errors was set at 5 ppm for the precursor, and also for one or more of the product ions. The identified non-anthocyanins and anthocyanin derivatives were quantified relative to the peak areas of reference standards, viz. quercetin and pelargonidin-3-O-glucoside, respectively. Finally, the distribution (%) of the identified classes of polyphenols was established
Results and discussion
Identification of phenolic compounds
The tentatively identified phenolic compounds are presented in Table 1. The total ion chromatogram of the phalsa extract acquired in the positive ionization mode is shown in Supplementary Fig. 1. The chemical class-wise details are presented below.
Table 1.
Identification of phenolic compounds in phalsa (Grewia asiatica) by high resolution accurate mass analysis
| Sr. no. | Compound name | Molecular formula | Expected mass (m/z) | Observed mass (m/z) | Mass error (ppm) | RT (min) | Detector response | Fragment ions (relative intensity, %) | Relative conc. (µg/g) |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | |||||||||
| 1. | p-coumaroyl glycolic acid | C11H10O5 | 222.0528 | 223.0593 [M + H]+ | − 3.68 | 5.62 | 2565 | 223.0594 (100%), 162.0302 (35%) | 0.58 |
| 2. | 5-Caffeoylquinic acid | C16H18O9 | 354.0951 | 377.0833 [M + Na]+ | − 2.55 | 2.56 | 1123 | 163.0378 (100%), 135.0427 (40%), 153.0174 (40%), 89.0376 (30%), 252.0721 (20%) | 0.25 |
| Flavones [M + H]+ | |||||||||
| 3. | Apigenin-6-C-galactoside-8-C-arabinoside | C26H28O14 | 564.1479 | 565.1566 [M + H]+ | 2.48 | 5.37 | 3166 | 565.1565 (100%), 303.0498 (50%), 409.0922 (40%), 325.0711 (40%) | 0.71 |
| 4. | Apigenin-7-O-apiosyl-glucoside | C26H28O14 | 564.1479 | 565.1565 [M + H]+ | 2.33 | 4.5 | 1455 | 271.0593 (100%), 565.1565 (35%), 147.0435 (20%), 403.1011 (15%) | 0.33 |
| 5. | Isovitexin | C21H20O10 | 432.1056 | 433.1130 [M + H]+ | 0.16 | 5.99 | 1467 | 283.0599(100%), 313.0707 (80%), 123.0788 (45%), 433.1132 (35%) | 0.33 |
| 6a. | Luteolin-4’-glucoside | C21H20O11 | 448.1006 | 449.1073 [M + H]+ | − 1.15 | 4.26 | 1245 | 287.0544 (100%), 419.0972 (10%), 449.1073 (5%) | 0.28 |
| 6b. | Luteolin-4’-glucoside | C21H20O11 | 448.1006 | 449.1081 [M + H]+ | 0.67 | 4.55 | 1820 | 287.0546 (100%), 137.0222 (5%), 209.0435 (5%), 449.1073 (5%) | 0.41 |
| 7a. | Luteolin-7-O-(2-apiosyl-6-malonyl)-glucoside | C29H30O18 | 666.1432 | 667.1522 [M + H]+ | 2.55 | 4.78 | 89,299 | 287.0548 (100%), 667.1522 (40%), 535.1092 (25%) | 20.09 |
| 7b. | Luteolin-7-O-(2-apiosyl-6-malonyl)-glucoside | C29H30O18 | 666.1432 | 667.1522 [M + H]+ | 2.52 | 4.57 | 45,176 | 287.0548 (100%), 667.1522 (40%), 535.1092 (25%) | 10.16 |
| 8. | 6-Hydroxyluteolin | C15H10O7 | 302.0427 | 303.0499 [M + H]+ | − 0.21 | 6.39 | 1025 | 127.0371 (100%), 302.0418 (95%), 285.0385 (50%) | 0.23 |
| 9a. | 6-Methoxyluteolin/Nepetin | C16H12O7 | 316.0583 | 317.0659 [M + H]+ | 1.14 | 6.18 | 2656 | 317.0663 (100%), 302.0422 (20%), 273.0395 (10%) | 0.60 |
| 9b. | 6-Methoxyluteolin/Nepetin | C16H12O7 | 316.0583 | 317.0652 [M + H]+ | − 1.25 | 6.72 | 2198 | 317.0652 (100%), 302.0410 (15%), 153.0172 (10%), 127.0359 (10%) | 0.49 |
| Flavanones [M + H]+ | |||||||||
| 10. | Narirutin | C27H32O14 | 580.1792 | 581.1883 [M + H]+ | 3.15 | 6.06 | 460 | 107.0481 (100%), 563.1754 (80%), 143.0846 (50%), 545.1674 (40%) | 0.10 |
| 11. | Hesperetin-3’-O-glucuronide | C22H22O12 | 478.1111 | 479.1193 [M + H]+ | 1.85 | 6.56 | 2849 | 317.0654 (100%), 153.0172 (25%), 302.0421 (20%), 285.0389 (5%) | 0.64 |
| Isoflavonoids [M + H]+ | |||||||||
| 12. | Dihydrodaidzein-7-O-glucuronide | C21H20O10 | 432.1056 | 433.1131 [M + H]+ | 0.3 | 5.8 | 735 | 303.0493 (100%), 339.0520 (25%), 287.0545 (25%), 105.0688 (20%) | 0.17 |
| 13. | 6,7,3’,4’-Tetrahydroxyisoflavone | C15H10O6 | 286.0477 | 287.0544 [M + H]+ | − 2.27 | 4.21 | 528 | 137.0222 (100%), 153.0167 (35%), 109.0275 (15%), 177.0169 (10%) | 0.12 |
| 14a. | 5,7,8,3’,4’-Pentahydroxyisoflavone | C15H10O7 | 302.0427 | 303.0493 [M + H]+ | − 2.07 | 5.77 | 2272 | 303.0493 (100%), 287.0545 (20%), 127.0374 (15%) | 0.51 |
| 14b. | 5,7,8,3’,4’-Pentahydroxyisoflavone | C15H10O7 | 302.0427 | 303.0500 [M + H]+ | 0.11 | 6.50 | 966 | 287.0545 (100%), 303.0496 (40%), 137.0585 (25%) | 0.22 |
| Flavanols [M + H]+ | |||||||||
| 15. | Catechin (L-) | C15H14O6 | 290.079 | 291.0862 [M + H]+ | − 0.56 | 4.63 | 635 | 139.0377 (100%), 123.0428(70%) | 0.14 |
| 16. | Epigallocatechin (-) | C15H14O7 | 306.074 | 307.0803 [M + H]+ | − 3.01 | 3.67 | 1012 | 137.0224 (100%) | 0.23 |
| 17. | (-)-Epigallocatechin-7-O-glucuronide | C21H22O13 | 482.106 | 483.1141 [M + H]+ | 1.65 | 2.68 | 721 | 153.0167 (100%), 321.0598 (30%) | 0.16 |
| Dihydroflavonols [M + H]+ | |||||||||
| 18a. | Dihydroquercetin | C15H12O7 | 304.0583 | 305.0649 [M + H]+ | − 2.31 | 4.18 | 4589 | 137.0222 (100%), 153.0167 (35%), 305.0622 (35%) 195.0275 (20%), 109.0275 (10%) | 1.03 |
| 18b. | Dihydroquercetin | C15H12O7 | 304.0583 | 305.0650 [M + H]+ | − 1.91 | 4.00 | 1682 | 137.0222 (100%) | 0.38 |
| 18c. | Dihydroquercetin | C15H12O7 | 304.0583 | 305.0650 [M + H]+ | − 1.96 | 3.66 | 445 | 139.0377 (100%), 305.0564 (80%) | 0.10 |
| 19. | Dihydroquercetin-3-O-hexoside | C21H22O12 | 466.1111 | 467.1180 [M + H]+ | − 0.82 | 4.18 | 605 | 137.0222 (100%), 153.0167 (40%), 259.1176 (40%), 305.0622 (35%), 195.0276 (30%) | 0.14 |
| Flavonols [M + H]+ | |||||||||
| 20. | Kaempferol | C15H10O6 | 286.0477 | 287.0550 [M + H]+ | 0.09 | 6.14 | 3863 | 287.05514 (100%), 153.0171 (50%), 229.0489 (25%) | 0.87 |
| 21. | Kaempferol-3-O-glucoside | C21H20O11 | 448.1006 | 449.1081 [M + H]+ | 0.59 | 6.52 | 625 | 287.0545 (100%), 153.0172 (50%), 347.0754 (45%), 303.0496 (35%), 137.0585 (25%), 201.0516 (15%) | 0.14 |
| 22. | Kaempferol-3-O-xylosyl-glucoside | C26H28O15 | 580.1428 | 603.1342 [M + H]+ | 3.57 | 6.14 | 432 | 303.0499 (100%), 287.0551(35%), 153.0171 (20%), 229.0489 (10%) | 0.10 |
| 23. | Kaempferol-3-O-galactoside-7-O-rhamnoside | C27H30O15 | 594.1585 | 595.1676 [M + H]+ | 3.16 | 4.93 | 9741 | 595.1675 (100%), 325.0701 (55%), 457.1138 (50%), 577.1570 (45%), 295.0595 (40%), 150.0769 (20%) | 2.19 |
| 24. | Kaempferol-3-O-ß-D-glucorhamnoside | C26H26O16 | 594.1221 | 595.1299 [M + H]+ | 0.98 | 6.72 | 355 | 317.0652 (100%), 127.0369 (10%), 252.0928 (10%), 565.1194 (10%) | 0.08 |
| 25a. | Methylgalangin | C15H10O6 | 286.0477 | 287.0548 [M + H]+ | − 0.8 | 5.11 | 619 | 287.0548 (100%), 209.0262 (15%), 194.0029 (5%) | 0.14 |
| 25b. | Methylgalangin | C15H10O6 | 286.0477 | 287.0546 [M + H]+ | − 1.31 | 6.51 | 2803 | 287.05453 (100%), 137.0585 (25%), 213.0515 (8%) | 0.63 |
| 26a. | Myricetin | C15H10O8 | 318.0376 | 319.0450 [M + H]+ | 0.61 | 5.55 | 21,671 | 319.04493 (100%), 153.90175 (20%), 179.03371 (5%), 165.0178 (5%), 301.0345 (5%) | 4.87 |
| 26b. | Myricetin | C15H10O8 | 318.0376 | 319.0448 [M + H]+ | − 0.1 | 5.86 | 4384 | 319.0447 (100%), 153.0166 (25%) | 0.99 |
| 27. | Myricetin-3-O-arabinoside | C20H18O12 | 450.0798 | 451.0873 [M + H]+ | 0.41 | 5.85 | 497 | 319.0447 (10%), 153.0166 (25%), 273.0378 (10%) | 0.11 |
| 28. | Myricetin-3-O-rhamnoside | C21H20O12 | 464.0955 | 465.1041 [M + H]+ | 2.79 | 6.13 | 3316 | 303.0499 (100%), 287.0551 (30%), 153.0171 (20%), 201.0531 (5%) | 0.75 |
| 29. | Myricetin-3-O-galactoside | C21H20O13 | 480.0904 | 481.0989 [M + H]+ | 2.48 | 5.55 | 4146 | 319.0449 (100%), 153.0175 (20%), 481.0996 (5%) | 0.93 |
| 30. | Morin | C15H10O7 | 302.0427 | 303.0501 [M + H]+ | 0.59 | 6.13 | 18,883 | 303.0499 (100%), 287.0551 (35%), 153.0171 (20%), 247.0586 (5%) | 4.25 |
| 31. | Quercetin | C15H10O7 | 302.0427 | 303.0496 [M + H]+ | − 1.06 | 4.43 | 1936 | 303.0485 (100%), 153.0170 (10%), 247.0587 (5%) | 0.44 |
| 32a. | Quercetin-3-O-xyloside | C20H18O11 | 434.0849 | 435.0910 [M + H]+ | − 2.72 | 4.08 | 720 | 303.0492 (100%), 287.0541 (50%), 435.0918 (10%), 153.0170 (5%) | 0.16 |
| 32b. | Quercetin-3-O-xyloside | C20H18O11 | 434.0849 | 435.0922 [M + H]+ | 0.05 | 4.43 | 22,536 | 303.0495 (100%), 435.0921 (8%), 153.0170 (5%) | 5.07 |
| 33a. | Quercetin-7-O-glucoside | C21H20O12 | 464.0955 | 465.1019 [M + H]+ | − 1.88 | 4.08 | 453 | 303.0492 (100%), 287.0541 (50%), 435.0918 (10%), 419.0971 (5%) | 0.10 |
| 33b. | Quercetin-7-O-glucoside | C21H20O12 | 464.0955 | 465.1031 [M + H]+ | 0.74 | 4.3 | 4319 | 287.0544 (100%), 419.0972 (10%), 147.0435 (5%) | 0.97 |
| 33c. | Quercetin-7-O-glucoside | C21H20O12 | 464.0955 | 465.1026 [M + H]+ | − 0.25 | 5.77 | 475 | 303.0493 (100%), 339.0520 (25%), 287.0545 (25%), 105.0688 (15%) | 0.11 |
| 34. | Quercetin-4’-O-glucoside | C22H22O12 | 478.1111 | 479.1198 [M + H]+ | 2.82 | 6.19 | 520 | 317.0663 (100%), 333.0608 (40%), 302.0422(15%), 245.0447 (15%) | 0.12 |
| 35a. | Quercetin-3-O-(6”-malonyl-glucoside) | C24H22O15 | 550.0959 | 551.1051 [M + H]+ | 3.59 | 6.25 | 1374 | 303.0499 (100%), 209.0253 (10%), 273.0762 (8%), 551.10436 (5%) | 0.31 |
| 35b. | Quercetin-3-O-(6”-malonyl-glucoside) | C24H22O15 | 550.0959 | 551.1040 [M + H]+ | 1.46 | 4.39 | 1141 | 507.1134 (100%), 325.0323 (70%), 173.0216 (55%), 179.0320 (50%) | 0.26 |
| 36. | Quercetin-3-O-glucosyl-xyloside | C26H28O16 | 596.1377 | 597.1451 [M + H]+ | 0.21 | 4.08 | 153,077 | 303.0492(100%), 287.0541(45%), 597.1451 (30%), 435.0918 (10%) | 34.43 |
| 37. | Quercetin-3-O-galactoside-7-O-rhamnoside | C27H30O16 | 610.1534 | 611.1633 [M + H]+ | 4.38 | 6.19 | 607 | 317.0663 (100%), 333.0608 (40%), 302.0422 (20%), 531.1452 (10%) | 0.14 |
| 38. | Quercetin-3-O-(6”-malonyl-glucoside)-7-O-glucoside | C30H32O20 | 712.1487 | 713.1575 [M + H]+ | 2.2 | 4.3 | 4089 | 287.0544 (100%), 581.1508 (35%), 713.1567 (5%) | 0.92 |
| 39. | Rhamnetin | C16H12O7 | 316.0583 | 317.0653 [M + H]+ | − 0.97 | 6.56 | 12,923 | 317.0654 (100%), 302.0421 (20%), 153.0172 (25%), 121.0274 (5%) | 2.91 |
| 40. | Isorhamnetin-3-O-pentaside-7-O-glucoside | C27H30O16 | 610.1534 | 611.1609 [M + H]+ | 0.34 | 4.08 | 5451 | 303.0492 (100%), 287.0541 (45%), 597.1451 (35%), 435.0918 (8%) | 1.23 |
| 41. | 5,4’-Dihydroxy-3,3’-dimethoxy-6:7-methylenedioxyflavone 4’-O-glucuronide | C24H22O14 | 534.101 | 535.1092 [M + H]+ | 1.77 | 6.64 | 615 | 287.0543 (100%), 141.0686 (40%), 183.1146 (35%), 129.0681 (30%), 229.1202 (25%) | 0.14 |
| Anthocyanins [M]+ | |||||||||
| 42a. | Cyanidin-3-O-arabinoside | C20H19O10 | 419.0978 | 419.0970 [M]+ | − 0.63 | 4.27 | 1422 | 287.0544 (100%), 419.0972 (15%), 259.0595 (5%) | 0.72 |
| 42b. | Cyanidin-3-O-arabinoside | C20H19O10 | 419.0978 | 419.0982 [M]+ | 2.12 | 5.11 | 4494 | 287.0548 (100%), 419.0976 (20%), 209.0262 (10%), 263.0719 (5%) | 2.29 |
| 43. | Cyanidin-3-O-sambubioside | C26H29O15 | 581.1506 | 581.1500 [M]+ | − 0.08 | 4.08 | 54,343 | 303.0492 (100%), 287.0541 (50%), 435.0918 (10%), 465.1026 (10%) | 27.67 |
| 44. | Cyanidin-3-O-(6’’-malonyl-3’’-glucosyl-glucoside) | C30H33O19 | 697.1616 | 697.1624 [M]+ | 1.96 | 4.51 | 1981 | 271.0593 (100%), 209.0435 (45%), 393.0798 (45%), 565.0156 (30%), 147.0435 (20%) | 1.0 1 |
| 45. | Delphinidin-3-O-arabinoside | C20H19O11 | 435.0927 | 435.0928 [M]+ | 1.36 | 4.72 | 12,777 | 419.09752 (100%), 435.0925 (80%), 257.0436 (40%) | 6.5 1 |
| 46. | Delphinidin-3-O-sambubioside | C26H29O16 | 597.1456 | 597.1467 [M]+ | 2.83 | 1.51 | 1579 | 303.0493 (100%), 163.0376 (25%), 551.1011 (5%) | 0.80 |
| 47a. | Petunidin | C16H13O7 | 317.0661 | 317.0652 [M]+ | − 1.13 | 6.41 | 1172 | 317.0651 (100%), 302.0418 (10%), 127.0371 (10%) | 0.60 |
| 47b. | Petunidin | C16H13O7 | 317.0661 | 317.0649 [M]+ | − 2.09 | 6.89 | 781 | 317.0651 (100%), 287.0546 (70%), 302.0418 (20%), 163.0373 (55%) | 0.40 |
| Other polyphenols [M + H]+ | |||||||||
| 48. | Umbelliferone | C9H6O3 | 162.0317 | 163.0383 [M + H]+ | − 4.07 | 5.01 | 429 | 163.0372 (100%), 147.0434 (85%) | 0.10 |
| 49. | Salvianolic acid D | C20H18O10 | 418.09 | 419.0979 [M + H]+ | 1.46 | 4.7 | 3719 | 257.0437 (100%), 229.0489 (85%), 177.0665 (50%), 115.0535 (50%) | 0.84 |
| 50. | 7-Hydroxyflavan | C15H14O2 | 226.0994 | 227.1056 [M + H]+ | − 4.54 | 6.68 | 460 | 227.1058 (100%), 109.0270 (50%) | 0.10 |
Hydroxycinnamic acid
Based on its parent ion at m/z of 223.0593 [M + H]+, and the fragment at m/z of 162.0302, p-coumaroyl glycolic acid (No. 1, Table 1) was tentatively identified for the first time in phalsa. 5-O-caffeoylquinic acid (No. 2), also earlier reported in phalsa (Sharma and Gupta 2013), was characterized depending on its parent ion at m/z of 377.0833 as the sodium adduct [M + Na]+, which produced two characteristic fragment ions at 163.0378, and 135.0427 Da on collision induced dissociation. Previously, 5-caffeoylquinic acid showed a strong cancer chemopreventive property (Jin et al. 2005). Later, the same compound was also reported to suppress P-selectin expression on platelets through inhibition of the cyclooxygenase (COX) enzymes, which indicated its beneficial effects on cardiovascular diseases (Park 2009).
Flavones
The tentatively identified flavones in the study included 3 apigenin glycosides, 4 luteolin glycosides, 1 hydroxyluteolin, and 2 methoxyluteolin. Two flavones had acylations with an aliphatic acid (malonic acid) at the 7th position of the A-ring. The compounds were identified based on certain criteria. For example, the compounds (No. 6, and 7, Table 1) were the isomers of luteolin-4’-glucoside, which were eluted at 4.26 min, and 4.55 min. Both these compounds had [M + H]+ at 449.1073 with a fragment at m/z 287.0544 (M + H+-162.0529). The loss of 162 Da corresponds to one glucosyl unit attached to the flavone moiety. The presence of the fragment m/z 287 confirmed the identity of the aglycone unit as luteolin. Luteolin aglycone was earlier reported in phalsa by Taskeen et al. (2010), although the glycoside forms of luteolin are reported through our study for the first time. Apigenin and luteolin glycosides were formerly found in other plants from the Tiliaceae family, e.g., Corchorus depressus (Khan et al. 1991), and Triumfetta procumbens (Iwashina and Kokubugata 2012).
In our study, we identified apigenin glycosides, hydroxyluteolin, and methoxyluteolin for the first time. The proportion of the glycosides of luteolin (No. 6–9, Table 1) was the highest among the flavones. Earlier, luteolin not only showed anti-inflammatory and antiallergic effects (Ueda et al. 2002), but also anti-carcinogenic effects against various human cancers, namely colorectal, prostate (Fang et al. 2007), and others.
The relatively minor flavones are also anticipated to possess various biological properties. For example, isovitexin (No. 5, Table 1) might suppress the lipopolysaccharide-mediated inducible nitric oxide synthase activity through inhibition of the transcription factor NF-kappa B in mouse macrophages (Lin et al. 2005). Previous researches also mentioned apigenin to have a protective affect against radiation-induced chromosomal damage in human lymphocytes (Rithidech et al. 2005). From the above, it can be inferred that the phalsa can be utilized as a potential functional food in managing multiple diseases.
Flavonols
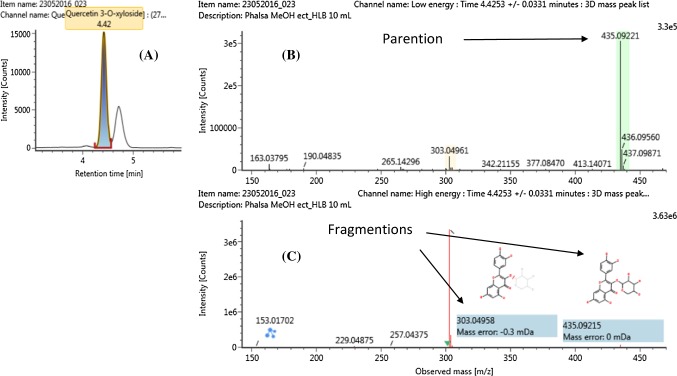
Several flavonols were also tentatively identified based on their precursor mass, and characteristic fragments. For example, the compound No. 32 (RT = 4.42 min) was assigned as quercetin-3-O-xyloside [M + H]+, based on the observed accurate mass [M + H+= 435.0922 Da], elemental composition [C20H18O11, with a mass error of 0.05 ppm] (Fig. 1.), and characteristic fragment ions at m/z of 303.0495 Da [M + H+-132.0427], and 153.01702 Da [M+-282.0751]. The loss of 132 Da corresponded to the elimination of 1 glucosyl, and 1 xylosyl unit, respectively.
Fig. 1.
Extracted ion signal (a), low energy spectrum (b) and high energy spectrum (c) of Quercetin-3-O-xyloside [M + H]+
Herein, 28 tentatively identified flavonols included 5 kaempferol and its glycosides, 5 myricetin and its glycoside, 12 quercetin and its glycosides, 1 rhamnetin, 1 isorhamnetin glycoside, 2 methyl galangin, 1 morin, and 5,4’-Dihydroxy-3,3’-dimethoxy-6:7-methylenedioxyflavone-4’-O-glucuronide. Among them, 2 were acylated glycosides (No. 35 and 38), and the rest 25 were non-acylated glycosides. The acylated flavonols could be identified based on the increase in the mass of the corresponding precursor ions. They were acylated singly with malonic acid at the 3rd position of the C ring. Except quercetin, myricetin, and rhamnetin aglycone (Taskeen et al. 2010; Sharma and Gupta 2013), all others are reported here for the first time. Among the identified flavonols, the presence of quercetin-3-O-6”-malonylglucoside (No. 35a, 35b) was found in the leaves of Corchorus olitorius, which is from the same family as phalsa (Azuma et al. 1999).
Various kinds of quercetin, and kaempferol glycosides were also noted in the genus Triumfetta from the Tiliaceae family (Iwashina and Kokubugata 2012). In our study, the concentration of quercetin, and its glycosides (No. 32–38) was the highest, followed by myricetin (No. 26–29), and kaempferol (No. 21–24). Previous works showed multiple health benefits of these compounds. Quercetin, and kaempferol improved the glucose uptake of the 3T3-L1 cells (of mice), suggesting their antidiabetic potentials (Fang et al. 2008). Some studies also demonstrated the anti-inflammatory properties of kaempferol, quercetin (Hämäläinen et al. 2007), and myricetin (Wang et al. 2010). Besides, kaempferol-3-O-glucoside showed hepatoprotective activities (Wang et al. 2015). Kaempferol (Zhou et al. 2015), was found to be cardioprotective. Quercetin-3-O-xyloside (a minor flavonol identified in phalsa, No. 32a, 32b) showed immunostimulating activity in murine macrophages via activation of the ASK1/MAPK/NF-κB signaling pathway (Lee et al. 2016). Thus, phalsa could be promoted as a functional food for the management of diabetes, and also improvement of immunity, and the cardiovascular and hepatic health.
Anthocyanins
We noted the following anthocyanins in phalsa: 4 cyanidin glycosides, 2 delphinidin glycosides, and 2 petunidin aglycones. Out of them, only one compound (No. 44) was in acylated form with malonic acid moiety linked at the C3 position of the flavylium ring. The compounds (No. 42a, and 42b) could be the isomers of cyanidin-3-O-arabinoside, which were identified on the basis of the parent ion of 419.0970 [M] +, and the fragment at 287.0544 [M+-132.0426]. Similarly, the compounds (No. 43, and 44) were identified as cyanidin-3-O-sambubioside, and cyanidin-3-O-(6’’-malonyl-3’’-glucosyl-glucoside), respectively. Based on the fragment mass, the compounds (No. 45, and 46) were tentatively identified as delphinidin-3-O-arabinoside, and delphinidin-3-O-sambubioside, respectively. Because the compounds (No. 47a, and 47b) yielded a fragment at m/z 317.0652 (petunidin aglycone), they were identified as the isomers of petunidin.
Earlier, Agarwal and Mishra (1979) found the presence of pelargonidin-3, 5-diglucoside in phalsa. Elsewhere, Khurdiya and Anand (1981) reported delphinidin-3-glucoside, and cyanidin-3-glucoside as the main pigments in phalsa fruits. The compounds, such as cyanidin sambubioside, and delphinidin sambubioside, were reported in rossele, which is genetically a close relative of phalsa (Ojeda et al., 2010). We also found cyanidin-3-O-sambubioside (No. 43), and delphinidin-3-O-sambubioside (No. 46), which were earlier reported to inhibit the blood pressure controlling angiotensin-converting enzyme (Ojeda et al. 2010).
Other compounds
In addition to the aforesaid compounds, 2 flavanones, 4 dihydroflavonols, 4 isoflavonoids, 3 flavanols, and 3 other phenolic compounds were tentatively identified. Except catechin (Sharma and Gupta 2013), none has appeared in the literature. Known for its multiple health benefitting properties, catechin along with nuercetin attenuates inflammation in adipose tissue of fructose-fed rat (Vazquez Prieto et al. 2015).
Relative distribution of phenolic compounds
The relative distribution of the major classes of phenolic compounds is presented in Fig. 2. The flavonols representing ~ 44.36% was the predominant group, which included quercetin-3-O-glucosyl-xyloside (~ 54.36%). Only ~ 2.3% existed in the acylated form.
Fig. 2.
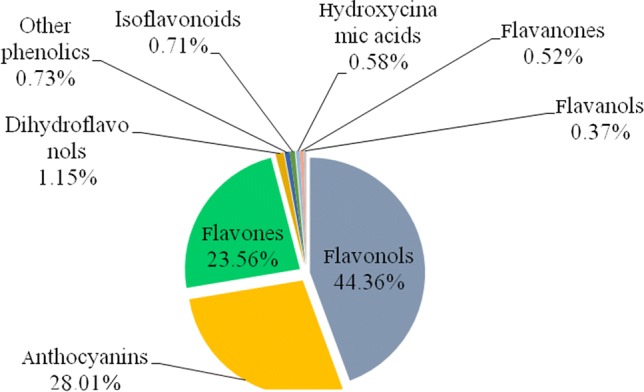
Relative distribution of phenolic compounds in phalsa fruit
The anthocyanins accounted for 28% of the total phenolics. Among all, cyanidins (79.1%), and delphinidins (18.3%) were the most prevalent groups. Petunidins accounted for 2.48% of the total anthocyanins. Cyanidin-3-O-sambubioside (No. 43) was the most predominant anthocyanin (69.2%). 2.52% of the anthocyanins were acylated.
Here, the flavones accounted for 23.56% of the total phenolics. Isomers of the luteolin-7-O-(2-apiosyl-6-malonyl)-glucoside were the predominant flavones (89.94%). The other phenolics constituted 4.82% of the total phenolics.
Several researchers stated that phalsa is an excellent source of flavonols, flavones, and anthocyanins. Besides the nutritional importance, they were also known to have played crucial roles in the plant defense mechanism, maintaining stress homeostasis, and pollination (Cheynier et al. 2013). Thus, the extract of phalsa fruits might have the potentiality of controlling plant pathogens, and can be used in integrated plant disease management.
Similar to the study conducted by Lancaster et al. (1994), the ratio of flavonol to anthocyanin here was approximately 1.6:1, indicating the possibilities of co-pigmentation of anthocyanins in phalsa. Although a small proportion of the anthocyanins were presented in the acylated form, a higher flavonol to anthocyanin ratio would have provided a better stability to the pigments.
Conclusion
The LC–QToF–MS technique adopted provided a satisfactory tentative characterization of the phytochemical constituents, and reported 50 compounds in phalsa on the basis of accurate mass measurement. These compounds included 21 flavonols, 2 dihydroflavonols, 7 flavones, 3 flavanols, 6 anthocyanins, 3 isoflavonoids, 2 phenolic acids, 2 flavanones, and 4 other phenolics. As the method allowed the detection of new compounds, it might serve as a useful approach for characterizing metabolites of lesser known plant-based foods. Based on our findings, we anticipate this underutilized crop could be a fruit of choice in developing nutraceutical products and functional foods, and thus, provide nutritional security in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflicts of interest
The authors declare that they do not have any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal S, Mishra K. New flavonoids from the Grewia asiatica. J Ind Chem Soc. 1979;56(6):649. [Google Scholar]
- Asghar MN, Khan IU, Sherin L, Ashfaq M. Evaluation of antioxidant activity of Grewia asiatica berry using 2,2-azinobis-(3-ethylbenzoline-6-sulphonic acid) and N, N-dimethyl-p-phenylenediamine radical cations decolourazation assays. Asian J Chem. 2008;20:5123–5132. [Google Scholar]
- Azuma K, Masayoshi N, Masaji K, Katsunari I, Yuichi Y, Katsunari K, Yuji Y, Hidekazu I, Hisao H. Phenolic antioxidants from the leaves of Corchorus olitorius L. J Agric Food Chem. 1999;47:3963–3966. doi: 10.1021/jf990347p. [DOI] [PubMed] [Google Scholar]
- Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem. 2013;72:1–20. doi: 10.1016/j.plaphy.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Das D, Mitra A, Datta D, Saha A, Hazra J. Evaluation of antipyretic and analgesic activity of parusaka (Grewia asiatica Linn.): an indigenous Indian plant. Int J Res Ayur Pharm. 2012;3:519–524. [Google Scholar]
- Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–622. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat Inflamm. 2007 doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashina T, Kokubugata G. Flavone and flavonol glycosides from the leaves of Triumfetta procumbens in Ryukyu Islands. Bull Natl Mus Nat Sci. 2012;38:63–67. [Google Scholar]
- Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Khan MSY, Javed K, Khan MH, Shamsi MA, Siddiqui AA. α-amyrin derivatives from Corchorus depressus. Phytochem. 1991;30:1989–1992. doi: 10.1016/0031-9422(91)85053-3. [DOI] [Google Scholar]
- Khan RS, Asghar W, Khalid N, Nazir W, Farooq M, Ahmed I, Syed QA. Phalsa (Grewia asiatica L) fruit berry a promising functional food ingredient: a comprehensive review. J Berry Res. 2019;9(2):179–193. doi: 10.3233/JBR-180332. [DOI] [Google Scholar]
- Khattab HA, El-Shitany NA, Abdallah IZ, Yousef FM, Alkreathy HM. Antihyperglycemic potential of Grewia asiatica fruit extract against streptozotocin-induced hyperglycemia in rats: anti-inflammatory and antioxidant mechanisms. Oxid Med Cell Longev. 2015;2015:549743–549750. doi: 10.1155/2015/549743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurdiya DS, Anand JC. Anthocyanin derivatives from the fruits of Grewia asiatica. J Food Sci Technol. 1981;18:112–114. [Google Scholar]
- Kirtikar KR, Basu BD. Indian medicinal plants. 2. Allahabad: Lalit Mohan Basu; 1989. [Google Scholar]
- Lancaster JE, Grant JE, Lister CE, Taylor MC. Skin color in apples—influence of copigmentation and plastid pigments on shade and darkness of red color in five genotypes. J Am Soc Hortic Sci. 1994;119:63–69. doi: 10.21273/JASHS.119.1.63. [DOI] [Google Scholar]
- Lee J, Choi JW, Sohng JK, Pandey RP, Park YI. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-κB signaling pathway. Int Immunopharmacol. 2016;31:88–97. doi: 10.1016/j.intimp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Lim TK. Grewia asiatica. In: Lim TK, editor. Edible medicinal and non medicinal plants. Netherlands: Springer Inc.; 2012. pp. 184–188. [Google Scholar]
- Lin CM, Huang ST, Liang YC, Lin MS, Shih CM, Chang YC, Chen TY, Chen CT. Isovitexin suppresses lipopolysaccharide-mediated inducible nitric oxide synthase through inhibition of NF-kappa B in mouse macrophages. Planta Med. 2005;71:748–753. doi: 10.1055/s-2005-871287. [DOI] [PubMed] [Google Scholar]
- Marya B, Dattani KH, Patel DD, Patel PD, Patel D, Suthar MP, Patel VP, Bothara SB. In vitro cytotoxicity evaluation of aqueous fruit and leaf extracts of Grewia asiatica using MTT assay. Pharm Chem. 2011;3:282–287. [Google Scholar]
- Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello J, Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- Park JB. 5-Caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J Nutr Biochem. 2009;20:800–805. doi: 10.1016/j.jnutbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Paul S. Pharmacological actions and potential uses of Grewia asiatica: a review. Int J App Res. 2015;1(9):222–228. [Google Scholar]
- Rithidech KN, Tungjai M, Whorton EB. Protective effect of apigenin on radiation-induced chromosomal damage in human lymphocytes. Mutat Res, Genet Toxicol Environ Mutagen. 2005;585:96–104. doi: 10.1016/j.mrgentox.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Sharma A, Gupta P. Evaluation of antioxidant activity and validated method for analysis of polyphenols from non-edible parts of Indian tropical fruits by using microwave assisted extraction and LC–MS/MS. Int J Pharm Biol Sci. 2013;4:227–241. [Google Scholar]
- Sharma KV, Sisodia R. Evaluation of the free radical scavenging activity and radioprotective efficacy of Grewia asiatica fruit. J Radiol Prot. 2009;29:429. doi: 10.1088/0952-4746/29/3/007. [DOI] [PubMed] [Google Scholar]
- Sharma KV, Sisodia R. Hepatoprotective efficacy of Grewia asiatica fruit against oxidative stress in Swiss albino mice. Iran J Radiat Res. 2010;8:75–85. [Google Scholar]
- Siddiqi R, Naz S, Ahmad S, Sayeed SA. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int J Food Sci Technol. 2011;46:250–256. doi: 10.1111/j.1365-2621.2010.02480.x. [DOI] [Google Scholar]
- Talpur MK, Talpur FN, Balouch A, Nizamani SM, Surhio MA. Analysis and characterization of anthocyanin from phalsa (Grewia asiatica) MOJ Food Process Technol. 2017;5(3):00127. doi: 10.15406/mojfpt.2017.05.00127. [DOI] [Google Scholar]
- Taskeen A, Naeem I, Bakhtawar S, Mehmood T. A comparative study of flavonoids in fruits and vegetables with their products using reverse phase high performance liquid chromatography (RP-HPLC) Electron J Environ Agric Food Chem. 2010;9:1372–1377. [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25:1197–1202. doi: 10.1248/bpb.25.1197. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prieto MA, Bettaieb A, Rodriguez Lanzi C, Soto VC, Perdicaro DJ, Galmarini CR, Haj FG, Miatello RM, Oteiza PI. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes. Mol Nutr Food Res. 2015;59:622–633. doi: 10.1002/mnfr.201400631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Tong Y, Lu S, Yang R, Liao X, Xu YF, Li X. Anti-inflammatory activity of myricetin isolated from Myrica rubra Sieb. et Zucc. leaves. Planta Med. 2010;76:1492–1496. doi: 10.1055/s-0030-1249780. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang C, Zhang H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J Food Drug Anal. 2015;23:310–317. doi: 10.1016/j.jfda.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Ren H, Han J, Wang W, Zheng Q, Wang D. Protective effects of kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of glycogen synthase kinase-3. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/481405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.