Abstract
An increase in inulin and plant-protein based nutraceutical demand ultimately puts pressure on available resources. Therefore, there is a need to prospect for supplementary feedstocks and sustainable ways to exploit them. The aim of this study was to explore the technical feasibility of sequential extraction of inulin and protein from Jerusalem artichoke tubers and understand the interrelationships between processes and product functional properties. The response surface methodology was used to determine the optimal parameters for sequential extraction. Protein functional properties analysis was done to identify the effects of the extraction process. The extraction approach adopted in this study was preceded by mechanical pressing of the tuber to yield a protein-rich juice. However, only 40.8% of the protein was recovered from the juice, therefore a subsequent solvent extraction step followed to extract the residual protein and inulin retained in the solids. Selective extraction was achieved when protein was solubilised in the first step of solvent extraction. The overall protein and inulin yields from pressing and both sequential extraction steps were 71.88 and 67.6%, respectively. The inulin yields were substantially higher than the maximum overall yields when inulin extraction, from the pressed tuber, was performed first thus improving yields from 57.3 to 67.6%. Consequently, mechanical pressing improved the overall protein yield. Sequential extraction resulted in an inulin extract with minimal protein contamination compared to the conventional method. Therefore, sequential extraction was efficient in yielding extracts with reduced impurities and good functional properties.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04110-z) contains supplementary material, which is available to authorized users.
Keywords: Mechanical pressing, Nutraceuticals, Protein, Inulin, Biorefinery
Introduction
Plant proteins and polysaccharides are becoming a more significant part of human nutritional diet due to their perceived health benefits (Singh et al. 2008; Franck 2002). Plant proteins are an alternative to animal protein because crop production is associated with lesser greenhouse gases emissions compared to animal farming. Proteins and polysaccharides consumed for their perceived health benefits beyond primary nutritional and dietary benefits, are known as nutraceuticals (Palthur et al. 2010). Nutraceuticals have been reported to reduce the risk of cancer, cardiovascular diseases, and have immuno-modulatory functions (Singh et al. 2008).
Nutraceuticals are consumed as dietary supplements or conventional food product (Palthur et al. 2010). Furthermore, nutraceuticals are used as ingredients in the processing of conventional foods, such as salad dressings, desserts and beverages, due to their low cholesterol and calorie content (Singh et al. 2008). Nutraceuticals also enhance the texture, viscosity, water activity, emulsion, heat stability, and mouthfeel of food products (Singh et al. 2008; Franck 2002).
Inulin is a popular polysaccharide-based nutraceutical, composed of β (2 → 1) linked d-fructose and found in a range of plants belonging to the family Compositae such as chicory, Jerusalem artichoke (JA) and dandelion (Apolinário et al. 2014). Chicory is currently the major feedstock crop for industrial inulin production (Zarroug et al. 2016). JA tubers are also rich in inulin, accounting for approximately 80% w/w of the total tuber dry mass (DM). JA tubers are thus, a potential supplementary source of inulin and fructooligosaccharides (Gunnarsson et al. 2014).
JA is a herbaceous perennial, plant composed of the stalk and flower, and tubers that develop underground (Yang et al. 2015). Although it is native to the temperate North America, its cultivation was adopted in Europe around the seventeenth century (Gunnarsson et al. 2014). Currently the plant is widely cultivated in China, with uses such as a vegetable for human consumption due the health benefits and soil rehabilitation since it is a highly salt intolerant plant (Xiao et al. 2011). Protein accumulation in the tubers has resulted in the use of JA tubers as animal feed (Alla et al. 2014). The plant has however, gained interest as a rich source of inulin. JA is agronomically attractive since it can be cultivated under a range of microclimatic conditions, exhibits good agronomic traits, requires minimal input cultivation, and produces high yields (Yang et al. 2015).
Hot-water extraction is a commonly used method to extract inulin from tubers, followed by inulin purification for use in powder and syrup form or hydrolysis into fructooligosaccharide (FOS) (Franck 2002). Purification involves the removal of impurities, largely composed of water-soluble proteins (Li et al. 2012), that affect the functional properties of inulin. In addition, protein accounts for between 7 and 15% w/w of the tuber DM (Bekers et al. 2008; Gunnarsson et al. 2014). Therefore, there is a need to explore the potential of an integrated strategy for inulin and protein co-production to minimise protein contamination, purification cost and waste production.
Although the co-production of inulin and protein from JA tubers has a potential to enhance the sustainability of the crop as a biorefinery feedstock, a potential loss in the yield and quality of the products is inevitable due to co-extraction during sequential extraction. Therefore, sequential extraction will only be valuable if it achieves selective extraction of one component over the other by manipulating the differences in the biochemical structure of protein and inulin that impact on solubility. The overlap in inulin and protein solubility will significantly compromise selectivity during extraction (Li et al. 2012). The higher temperatures optimal for inulin extraction will lead to severe protein co-extraction thereby compromising selectivity (Lingyun et al. 2007). Moreover, inulin co-extraction may occur under mild temperatures favourable for protein solubility (Mizubuti et al. 2000). Short chain inulin oligomers (DP 2-9) have been reported to be relatively soluble at temperatures around 25 °C compared to long chain polymers (DP 10-60) which are soluble at temperatures above 60 °C (Wada et al. 2005).
Therefore, an integrated strategy for the co-production of inulin and protein will justify the commercial and sustainable cultivation of JA for use as a biorefinery feedstock. Furthermore, an integrated extraction process has the potential to yield superior quality products. Thus, the aim of this study was to use the response surface methodology to determine the best sequential extraction parameters and functional properties analysis to identify and understand interrelationships between processing sequence or conditions and product properties.
Materials and methods
Tuber feedstock
JA tubers were obtained from the Glen Agricultural College, Free State, South Africa. Fresh tubers were harvested in the late winter, subsampled for chemical composition analysis and subsequently stored at − 18 °C. Fresh tubers (80% moisture content) for chemical composition analysis were sliced and dried, in a Scientific® Series 200 oven at 40 °C for 60 h, to constant dry weight with a moisture content of 15%. The moisture content was determined according to the National Renewable Energy Laboratory (NREL) method (Sluiter et al. 2008). Dried tubers were crushed (6 mm sieve) and milled (2 mm sieve) at 2000 rpm using a Retsch® ZM 200 labscale mill (Retsch®, Haan, Germany) and fractionated with a Retsch® shaker sieve at 70 amplitudes for 10 min. The fraction collected in the 425 mm sieve was used for the chemical composition analysis.
The tuber mash used in the extraction process was prepared from the frozen-tuber stock in two different methods. The tuber mash used in preliminary screening extraction experiments was prepared by washing the frozen tubers and rasping with a hand-grater. The tubers used in the sequential extraction experiments were prepared by manually pressing the tuber mash and solid residue subsequently used in the optimization experiments. The press-juice was used to determine the inulin and protein concentration. The inulin and protein yields from the juice were determined as a function of the tuber-mash dry weight, the inulin and protein content as described in the analysis section.
Analysis
Inulin determination
The inulin content of the tubers and extraction solid residues were determined as a function of free and total sugar (fructose) content. The free sugar content was determined by dispersing 200 mg dry raw-tuber granules or dried extraction residue in 50 ml distilled water and incubating at 25 °C for 15 min in a shaking water bath. Total sugars, for inulin content measurement, were determined through acid hydrolysis of the tuber granules or extraction residue in 0.2% v/v H2SO4 (Sigma®, Saint Lois, USA) for 60 min (Gunnarsson et al. 2014). Sugars were analysed using high-performance liquid chromatography (HPLC) with a Dionex (Dionex, California, USA) 3000 System equipped with a Grace® (Hichrom, Berkshire, UK) Prevail Carbohydrate ES Column (250 × 4.6 mm) and a Varian® evaporative light scattering detector. The HPLC operating conditions such as the injection volume, column temperature, flow rate and gradient mobile phase were 10 µL, 30 °C, 0.6 ml/min and acetonitrile, respectively. Inulin was defined as non-monomeric polyfructan composed of at least two fructan monomers (F2). Total inulin content was calculated as follows:
| 1 |
where F0 and Ft is the amount (g) of fructose before and after acid hydrolysis, respectively. The total solids, ash and structural sugars content of the non-extracted tubers were determined according to the standard NREL procedures (Sluiter et al. 2005, 2008, 2011). Structural sugars (xylose, glucose and cellobiose) were analysed with HPLC, after acid hydrolysis in 72% H2SO4 (Sluiter et al. 2011). HPLC analysis was performed with an injection volume of 30 µl in a Thermo separation (Spectralab Scientific Incorporated, Toronto, Canada) Spectra System, equipped with a Shodex refractive index detector operated at 45 °C. The system was also equipped with an Aminex HPX-87H Ion Exclusion Column with a Cation-H cartridge (Biorad®, Johannesburg, RSA) and operated at a flow rate of 0.6 ml/min, column temperature of 65 °C, an isocratic mobile phase.
Crude protein from raw tubers and extraction residues was determined through the Dumas method. The DUMAS analysis was done according to the Association of Official Analytical Chemist’s Standard Techniques (AOAC 2002) in a LECO (Leco Corporation, Saint Joseph, USA) FP 528 system calibrated with ALFALFA with a Nitrogen content of 3.38%. A conversion factor of 6.25 was used for the determination of the crude protein content (Gunnarsson et al. 2014). The concentration of soluble protein from the extracts was determined using the bicinchoninic acid (BCA) assay (ThermoFisher Scientific, Rockford, USA).
Protein solubility
Protein solubility was determined according the method of Stone et al. (2015). A 0.01% w/v protein solution was prepared in 0.1 N NaCl pH 7, mixed at 50 °C for an hour and then centrifuged. The protein content in the supernatant was determined using the BCA assay. Protein solubility was expressed as the amount of solubilised protein relative to the total amount of protein.
Emulsification properties
A five ml protein solution (1%) in distilled water at pH 7 was mixed with an equal volume of canola oil and vortexed briefly. The mixture was centrifuged, and emulsion activity was calculated as the ratio of the height of the emulsion layer to the total height of the liquid. Emulsion stability was determined by transferring the emulsion to another test tube and incubating in water bath at 70 °C. Emulsion stability was calculated as described for the emulsion activity (Lin and Zayas 1987).
Foaming properties
Aqueous solutions of the proteins in a 50 ml measuring cylinder were sparged with compressed air for 10 s and the height of the foam measured at 10 min interval for 30 min. The height of the foam at T0 was the foaming capacity and the forming stability (FS) was calculated from the equation below:
| 2 |
where V0 is the volume of the foam in ml at T0 and V is the change in volume of the foam over time (Kato et al. 1983).
Water retention
The protein water retention capacity was determined according to the method of Stone et al. (2015). Briefly, 0.5 g of the protein was suspended in 5 g of water. The mixture was vortexed for 10 s at 5 min intervals for 30 min, and subsequently centrifuged. The supernatant was discarded, and weight of the pellet was measured. Water retention was calculated by dividing the weight gained by the original weight of protein.
Preliminary screening
Tuber mash was extracted in a 250 ml Erlenmeyer flask at a temperature of 50 °C, pH 7 for 30 min in a shaking water bath at 100 rpm, to evaluate the effect of the solids loading on inulin and protein yield. The extract was centrifuged at 5000 rpm for 15 min and liquid fraction used for inulin and protein content determination through HPLC and the BCA assay, respectively. The inulin content was determined with HPLC after acid hydrolysis in 0.2% v/v H2SO4 and subsequently determined according to Eq. 1. The inulin and protein yields were calculated as follows:
| 3 |
where and was the total inulin or protein content (g) in the liquid fraction and in the raw material, respectively.
Sequential extraction from the pulp
A two-step approach was used for sequential protein and inulin extraction from the pressed tuber. The two extraction sequences were compared to each other to determine the sequence that achieved an acceptable level of selectivity by minimizing co-extraction. Inulin and protein extractions were performed in 250 ml Erlenmeyer flasks by dispersing 5 g and 7.5 g (DM basis) of the raw material, respectively, in distilled water to a final volume of 50 ml. The pH of the mixture was adjusted with 1 M H2SO4 or 0.01 M NaOH (Sigma®) and water bath temperature set, according to the central composite design (CCD). Extraction was performed for 60 min at 100 rpm. The extracts were centrifuged at 5000 rpm for 15 min, and the liquid fraction was used for inulin and protein content determination. The extraction and co-extraction yields were subsequently determined according to Eq. 3. The molecular weight (MW) and degree of polymerisation (DP) of the inulin extracts were determined through size exclusion chromatography with a Dionex® (Dionex, California, USA) Ultimate 3000 HPLC System equipped with a PSS (GmbH, Mainz, Germany) Suprema 10 µm column set (2 × 3000 Å and 1 × 30 Å) and PSS Pullon standards (315 Daltons-805 kDa). The operating conditions such as injection volume, column temperature, flow rate and mobile phase were 10 µL, 70 °C, 1 ml/min and 0.125 M ammonium acetate, respectively.
Experimental design: protein and inulin sequential extraction
A 23 full factorial design (ESM_2: Table S2) with 11 runs (three center points) was used to determine significant factors for protein and inulin extraction from the pressed tuber. The independent variables were pH (3, 5, 7), temperature (25, 42.5, 60 °C) and solids loading (3, 4.5, 6% w/v on DM basis) whereas the response variables were inulin and protein yield. A five-coded levels CCD was subsequently used to optimize the parameters for selective extraction at each step of the sequential extraction process. The first scenario (S1) involved protein and inulin extraction in the first and second step, respectively, while the sequence of extraction was reversed in Scenario 2 (S2). Therefore, the solid residue obtained from the first extraction step was subsequently used as the raw material in the second step to extract the retained product. Each experiment had 13 runs (five center runs). The independent variables for this experiment were pH and temperature. The temperature (56, 60, 70, 80, 84 °C) and pH (6.3, 6.5, 7, 7.5, 7.7) range for the inulin extraction step were used since several studies have reported an optimum temperature of approximately 70 °C and pH 7 (Lingyun et al. 2007). The temperature range (14, 25, 52.5, 80, 92 °C) for the protein extraction step was chosen to achieve selectivity since specific proteins have been reported to be soluble at room temperature (Lai et al. 2013) while inulin oligomers soluble at temperatures below 50 °C (Wada et al. 2005).
Multiple responses optimization was used to simultaneously optimize the extraction and co-extraction yields in each extraction step. The general formula for the response is shown in the equation below:
| 4 |
where yi is the ith response variable, xi is the ith input parameter, n is the number of input parameters and β0, βi, βii, βij are the fixed response, linear, quadratic and cross products coefficients, respectively. The general function optimization method of the Derringer’s desirability function was used to search a set of optimal conditions that improve the overall desirability and simultaneously maximize the extraction yield and minimize co-extraction yield. Statistica® (Statsoft Inc., Tulsa, USA) was used for the experimental design and statistical analysis, and all the analysis were performed at 5% level of significance.
Statistical analysis for protein functional properties
Protein functional properties experiments were done in triplicates and analysis of variance (ANOVA) was used to determine the differences in the properties between the juice and solvent extracted protein. ANOVA was performed at 5% level of significance.
Results and discussion
Chemical composition of tubers
The inulin and protein content of the tubers was 71.0% and 7.5% w/w (ESM_2: Table S1) of the tuber DM, respectively. The inulin content is comparable to chicory, currently the major feedstock for industrial inulin production (Zarroug et al. 2016). Thus, JA is a potential, additional feedstock for inulin production and biorefinery development. The content of the structural sugars was relatively low, making up to 12% of the tuber composition (ESM_2: Table S1), compared to inulin. Structural sugars are highly recalcitrant and not readily soluble in water, thus a bulk of this will be retained in the solid residue. Therefore, the presence of structural sugars in the tuber was unlikely to compromise selective extraction as well as the quality of inulin and protein extracts.
Protein and inulin recovery from the press-juice
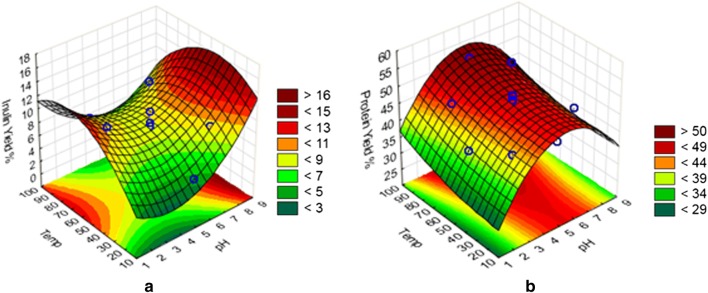
Preliminary screening data demonstrated that protein was readily available in the tuber juice, which could be obtained through pulping the tubers and mechanical pressing, similar to solvent-free protein separation from potato pulp (Bartova and Bárta 2009). The protein yield from the juice obtained through pressing, represented 40.8% of the tuber protein, while the inulin yield was relatively low at 2.3% (ESM_2: Fig. S1), demonstrating that pressing was selective for protein extraction. The low inulin co-extraction yield from the juice was expected since inulin extraction is commonly done through hot-water method (Lingyun et al. 2007). A subsequent solvent-based (water) extraction step was, therefore, necessary for selectively recovering the residual protein and inulin from the pressed tuber.
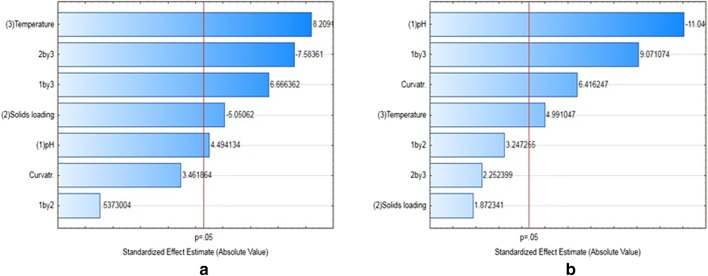
Significant process conditions for inulin and protein extraction
A full factorial design (ESM_2: Table S2) was used to determine the significant factors for inulin and protein extraction from the pressed tuber. Inulin and protein yields were both significantly affected by temperature and pH, while the solids loading affected the inulin yields alone (Fig. 1). Therefore, a face-centred CCD was selected for the optimization of temperature and pH as key process parameters for selective and sequential extraction of protein and inulin. The solids loading for the protein extraction step was selected as 15% w/v, higher than the 10% w/v for the inulin extraction step, to maximise selectivity for protein extraction, rather than inulin, since inulin yields were inversely related to the solids loading (Fig. 1). A solids loading of 15% w/v was utilised for protein extraction step since screening experiments showed a significant decrease in the protein yield when the solids loading was increased beyond 15% w/v. The solids loading for the inulin extraction step was fixed at 10% w/v since high viscosity at high solids loading causes insufficient mixing (Fan et al. 2003).
Fig. 1.
Standardized Pareto chart to estimate the significant factors for inulin (a) and protein (b) extraction from JA residue after pressing
Optimisation of process parameters for selective extraction of protein and inulin from tubers
Model fitting
The adequacy of the response surface methodology (RSM) model coefficients, for inulin and protein extraction and co-extraction, was evaluated using ANOVA of the response variables, and is summarized in Table 1. The model validity was assessed by calculating the coefficient of determination (R2), adjusted R2 and the lack of fit (Table 1). The adjusted R2 values for the models were in the range of 0.7–0.9 (Table 1), thereby showing that at least 70% of the variation in the extraction and co-extraction yields could be explained by second-order polynomial models in relation to pH and temperature.
Table 1.
Analysis of variance for the CCD models for sequential extraction and coefficients for the predictive models of the inulin and protein extraction/co-extraction yields. ANOVA was determined with a 95% confidence level
| S1a | S2a | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein extraction | Inulin extraction | Inulin extraction | ||||||||||||||
| Protein yield | Inulin co-extraction yield | Inulin yield | Inulin yield | Protein co-extraction yield | ||||||||||||
| Source | D.f.b | Co-efficient | Sum of squares | P-value | Co-efficient | Sum of squares | P-value | Co-efficient | Sum of squares | P-value | Co-efficient | Sum of squares | P-value | Co-efficient | Sum of squares | P-value |
| Linear | ||||||||||||||||
| Temp (T) | 1 | 0.01 | 31.14 | 0.019 | 0.35 | 4.24 | 0.127 | 4.44 | 50.96 | 0.009 | 5.11 | 63.25 | 0.004 | 5.35 | 132.41 | 0.005 |
| pH (P) | 1 | 10.34 | 16.51 | 0.051 | − 2 | 6.67 | 0.086 | 3.65 | 25.08 | 0.03 | 3.25 | 33.95 | 0.014 | − 24 | 2.38 | 0.51 |
| Quadratic | ||||||||||||||||
| T2 | 1 | 0. 9 | 3.56 | 0.271 | − 0.002 | 22.35 | 0.014 | − 0.33 | 63.66 | 0.006 | − 0.04 | 87.05 | 0.002 | 0.05 | 172.09 | 0.003 |
| P2 | 1 | − 0.94 | 98.64 | 0.002 | − 0.33 | 11.89 | 0.039 | 3.99 | 12.66 | 0.077 | 4.05 | 7.315 | 0.13 | 0.599 | 40.03 | 0.04 |
| Interaction | ||||||||||||||||
| TP | 1 | − 0.004 | 0.19 | 0.78 | − 0.02 | 2.89 | 0.21 | 0.04 | 0.125 | 0.82 | 0.1 | 1 | 0.517 | − 0.96 | 92.16 | 0.01 |
| Regression | 1 | 150.06 | 0.001 | 48.59 | 0.01 | 151.3 | 0.01 | 439.06 | 0.01 | 192.39 | 0.01 | |||||
| Residual error | 1 | 32.78 | 11.81 | 24.4 | 20.98 | 32.79 | ||||||||||
| Lack of fit | 3 | 24.05 | 0.12 | 6.61 | 0.3 | 16.32 | 0.208 | 27.97 | 0.084 | 24.05 | 0.09 | |||||
| Pure error | 4 | 8.73 | 5.21 | 9.06 | 7.96 | 8.73 | ||||||||||
| Model | 12 | 189.59 | 65.33 | 171.28 | 236.56 | 440.7 | ||||||||||
| R2 | 0.82 | 0.81 | 0.85 | 0.95 | 0.82 | |||||||||||
| Adjusted R2 | 0.7 | 0.69 | 0.74 | 0.92 | 0.7 | |||||||||||
aS1 and S2 is scenario 1 (protein extraction and inulin extraction first and second, respectively) and scenario 2 (inulin extraction first) for the sequential extraction, respectively
bd.f. is the degrees of freedom
Inulin extraction
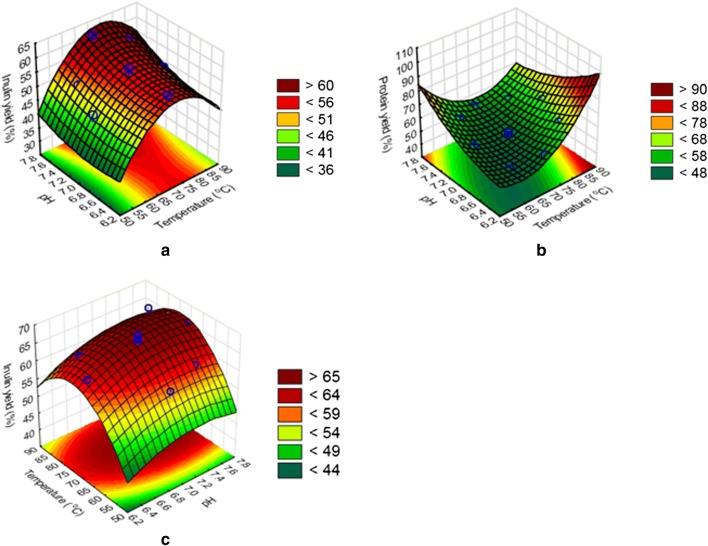
Inulin extraction from the protein extraction residues (S1) was compared to inulin extraction from pressed tuber (S2) to determine the process with better selectivity. The observed inulin yields from S1 and S2 were in the range of 55–68.5% (Fig. 2c) and 46–62% (Fig. 2a), respectively. The yields are superior compared to those reported in literature (14.42–21.69 g/100 g of tuber) for JA (Li et al. 2012; Bekers et al. 2008). Furthermore, the yields were obtained at solids loading four times higher (10% w/v of dry matter) than reported in literature (Li et al. 2012; Bekers et al. 2008). Interestingly, the maximum inulin yield in this study (67.6%) was more than the inulin yield (51.2%) from chicory roots, at a solids loading of 2.5% w/v DM using the conventional hot-water extraction method (Tewari et al. 2015),
Fig. 2.
Response surface plot showing inulin extraction (a) and protein co-extraction (b) from the pressed tuber and inulin extraction from the protein extraction residue (c), as a function of pH and temperature
In contrast the high inulin yields (27.6–38.7 g/100 g of pressed tuber) demonstrated at a higher solids loading, could be attributed to mechanical pressing before sequential extraction, which reduced the moisture content of the pressed tuber, enabling sufficient mixing and mass transfer (Lingyun et al. 2007). The removal of proteins before solvent extraction could have significantly contributed to enhanced mass transfer by minimising the viscosity of the solution. Severe protein co-extraction in the range of 47.1–65.8% (corresponding to 2.2–3.6 g/L), occurred when inulin was extracted from the pressed tuber (Fig. 2b). The severity of the co-extraction increased with an increase in temperature (Fig. 2b) and this could be attributed to enhanced protein solubility (Karazhiyan et al. 2011). In contrast, protein co-extraction from S2 substantially lower than in S1, with protein concentrations of 0.3–0.6 g/L, primarily due to low protein content of these residues. Protein extraction in the first step of sequential extraction was also important in minimising the amount of protein impurities associated with inulin extraction (Li et al. 2012). Although inulin extraction yields in S2 were comparable to S1, the unacceptable high protein co-extraction yield (Fig. 2b) indicated that inulin extraction conditions were not sufficiently selective for inulin. The low protein co-extraction yield paves way for a cost-effective method for inulin downstream processing since there is a potential to eliminate the deprotonation step prior to chromatograph purification (Apolinário et al. 2014).
Inulin extraction data was fitted using quadratic models to describe the effects of temperature (T) and pH (P) on inulin extraction and protein co-extraction yields. The estimated regression models summarising the significant factors are represented below:
| 5 |
| 6 |
| 7 |
Regression analysis showed that temperature had the most impact on inulin extraction yields as demonstrated by the size of the temperature regression co-efficient (4.44) relative to pH (Eqs. 5, 6 and 7). The negative sign on the coefficient of the quadratic terms of temperature (Eqs. 5 and 6) shows that a further increase in temperature resulted in a decline in the inulin yield. The surface plots (Fig. 2a, c) show a similar relationship between inulin extraction yields and the temperature The increase in the yield could be attributed to improved mass transfer and inulin solubility as the temperature increased (Koutinas et al. 2013). A reduction in the inulin yield when the temperature exceeded a certain level has been reported in literature and is due to inulin degradation into monomers (Lingyun et al. 2007; Lai et al. 2013).
Protein extraction
The protein extraction step was performed from the pressed tuber and not performed from the solid residues after inulin extraction, due to the severe protein co-extraction from S2 (Fig. 2b). The latter process scenario therefore did not meet the requirements of selective inulin and protein extraction. Selective protein extraction was achieved in S1 by performing the extraction at the maximum-feasible solids loadings, (15% w/v; higher than 10% w/v used in the inulin extraction steps), to minimize inulin co-extraction. The optimum temperature and pH for S1 was determined, to simultaneously maximize the protein yield while minimizing the inulin co-extraction yield. The results showed that protein extraction yields were substantially higher compared to the inulin co-extraction yields, ranging between 40 and 52.5% (Fig. 3a) and 4–11.8% (Fig. 3b), respectively. Residual inulin in the solid extraction residue was relatively high, ranging between 47.1 and 52.4 g/100 g, corresponding to 74.7–83.2% of inulin from the raw material, thereby confirming that inulin co-extraction was minimal. Protein extraction from the tuber pulp at high solids loading, therefore, demonstrated acceptable selectivity for protein, while retaining inulin in the insoluble form in the solids, for subsequent extraction.
Fig. 3.
Response surface plots showing protein extraction (a) and inulin co-extraction (b) yields from the pressed tuber as a function of pH and temperature
Lower inulin co-extraction yields (4–11.8%) from the protein extraction step (Fig. 3b), were demonstrated in comparison to the higher inulin yields (46–67.6%) from the inulin extraction steps (Fig. 2a, c). This was consequently due to differing solids loading between the two process steps. The negative effects of higher solids loading on the inulin extraction yield was observed with the tuber mash and pressed tuber (Fig. 1) and could be attributed to mass transfer limitations as the solids loading increases (Koutinas et al. 2013). Zarroug et al. (2016) reported that a lower water to raw-material ratio decreases the concentration gradient between the plant cell interior and surrounding solvent, thereby reducing inulin diffusion and solubility.
The effects of temperature and pH on the protein extraction and inulin co-extraction yields from the pressed tuber were illustrated by fitting regression models shown below:
| 8 |
| 9 |
Equation 7 and 8 shows that pH (p value = 0.003) had a greater effect on protein yields than temperature (0.009) and this was further confirmed by the steeper slope for pH against protein yield (Fig. 3a). The negative sign on the coefficient of the quadratic term of pH illustrated that a change of pH from acidic to mild-acidic (pH 3–5) increased the protein extraction yield. Moreover a change towards pH 7 resulted in a decrease in the yield as illustrated by the response surface plot (Fig. 3a). The plot shows that the protein yield reached a maximum at mild acidic conditions (pH 5). Therefore, mild acidic conditions provided sufficient charged ions to change the protein’s net charge, resulting in repulsion of the protein molecules and consequently promoting solubility. Although protein extraction above room temperature was shown to increase the protein extraction yield, it also resulted in a simultaneous increase in the inulin co-extraction yields (Fig. 3b). Furthermore, temperature (P-value = 0.005) had the most impact on inulin co-extraction during protein extraction from the pressed tuber, this observation is similar to that observed in the inulin extraction steps (Fig. 2a and c). The simultaneous increase in the protein extraction and inulin co-extraction yields relative to temperature were a result of improved mass transfer due to enhanced solvent diffusion, improved inulin and enhanced protein solubility (Koutinas et al. 2013; Karazhiyan et al. 2011). Therefore, a compromise had to be achieved to simultaneously maximise protein extraction yield and minimise inulin co-extraction.
Simultaneous optimisation and model validation
The Derringer’s desirability function was used to simultaneously optimize extraction and co-extraction yields from the sequential extraction steps. The optimal conditions to simultaneously maximize protein extraction and minimize inulin co-extraction in the first extraction step of S1 were pH 5 and temperature of 25 °C, with protein extraction and inulin co-extraction yields of 46.98 and 6.3%, respectively (Table 2). The maximum inulin extraction yield in the second step was predicted to be 67.6% at a temperature of 74.4 °C and pH 6.4. It is noteworthy to state that the concentration of the co-extracted protein from the nulin extraction step of S1 was only 0.5 g/L. The optimal inulin extraction yield from the pressed tuber (S2) was 57.3% at a temperature of 70.0 °C and pH 7.3. Moreover, the concentration of protein co-extracted under these conditions was 1.5 g/L, equivalent to a protein co-extraction yield of 50.45%. Proteins are surface active molecules and exist in tertiary structures that are stabilised by electrostatic and hydrophobic interactions which are easily disrupted when temperature increases. Proteins have been reported to unfold rapidly above 60 °C, exposing hydrophilic amino acids which ultimately form hydrogen bonds with water and thus making proteins highly soluble (Mitra et al. 2007). Polysaccharides have been reported to form soluble complexes with proteins at pH above protein’s isoelectric point where the protein assumes a cationic charge (Cooper et al. 2005). On the other hand, inulin possesses differential solubility, relative to temperature, since it is composed of oligomers of different degrees of polymerisation (Wada et al. 2005). For instance, FOS, which is composed of low molecular weight fructans with a degree of polymerization that is less than 10 fructose monomers, is soluble at room temperature (Wada et al. 2005). Thus, overcoming the effects of these complex interactions between proteins and polysaccharides on solubility, was critical in achieving selective extraction. Selective protein extraction in this study was best achieved at room temperature (25 °C) and pH 5 while severe protein co-extraction was observed during inulin extraction at pH 7 and temperatures above 60 °C.
Table 2.
Protein and inulin yields from the predicted models and confirmatory tests. Inulin and protein recoveries were determined as % yield based on the inulin and protein content, respectively, in tuber. Statistical analysis was done with a 95% confidence level
| Optimum conditions | Protein recoveries % | Inulin recoveries % | ||||
|---|---|---|---|---|---|---|
| Temp °C | PH | Predicted | Confirmatorya | Predicted | Confirmatorya | |
| Protein extraction S1 | 25 | 5 | 46.98 | 47.26 ± 2.30 | 6.29 | 9.02 ± 0.78 |
| Inulin extraction S1 | 74.4 | 6.4 | 47.65 | 52.39 ± 3.15 | 67.6 | 69.44 ± 4.12 |
| Inulin extraction S2 | 70 | 7.2 | 50.45 | 54.15 ± 2.89 | 57.29 | 56.84 ± 3.21 |
aData represent mean values ± standard deviation for experimental data done in triplicates
Experimental tests were performed to verify the validity and adequacy of the predicted models for inulin and protein extraction for the sequential extraction process. The tests were performed under optimal conditions (Table 2) as predicted by the simplex method of the Derringer’s desirability function. Three experimental replicates were used, and the experimental values were compared to the predicted values. The observed inulin and protein recoveries from the confirmatory tests were within the 95% confidence interval of the predicted inulin and protein recoveries from the various steps of sequential extraction. These results proved that the models can be used to determine process conditions for the selective and sequential extraction of protein and inulin from the JA tuber pulp obtained after pressing the tuber mash. The molecular weight distribution of the inulin extracts from S1 and S2 was in the range of 397–15,103 Daltons (equivalent to DP 2-80) and the average molecular weight was 2521 Daltons (DPav of ~ 16). Therefore, the inulin extract will have a wider range of applications, such as fat replacements, sweeteners and prebiotics, with or without hydrolysis into fructose monomers or oligomers (Franck 2002). The freeze-dried inulin powder from S1 contained 63.0% inulin, 8.0% free-sugars and 3.0% protein. Moreover, the protein powder from the juice contained 67.0% protein and 28.0% inulin while the solvent extracted protein contained 56% protein and 39.0% inulin.
Protein functional properties
Protein functional properties are crucial for their application as ingredients in food processing thus, making the extraction process an important step to preserve the properties for subsequent uses. Comparison of the functional properties between proteins from the tuber juice and solvent extraction demonstrated that the extraction method significantly impacted the functional properties (Table 3). Protein recovered from the juice had superior solubility than solvent extracted protein. The higher solubility of the former is potentially a result of the native nature of the protein, since no chemicals were applied to extract the protein from the tuber. In contrast, the mild acidic conditions used for solvent extraction are known to affect protein surface characteristics that promote protein–protein interaction and consequently insolubility (Can Karaca et al. 2011).
Table 3.
Functional properties of protein from the press juice and solvent extraction step. Statistical analysis was done with a 95% confidence level
| Property | Juicea | Solvent extracteda | P-value |
|---|---|---|---|
| Solubility (%) | 68.2 ± 2.15 | 61.9 ± 3.12 | 0.005 |
| Water retention (g/g) | 3.18 ± 0.08 | 6.79 ± 1.01 | 0.000016 |
| Emulsion capacity (%) | 83.2 ± 5.21 | 91.2 ± 4.02 | 0.0001 |
| Emulsion stability (%) | 99.5 ± 4.51 | 98.4 ± 3.86 | 0.014 |
| Foaming capacity (%) | 151 ± 7.71 | 149 ± 7.02 | 0.04 |
| Foaming stability (%) | 99.2 ± 6.23 | 99.4 ± 5.71 | 0.34 |
aData represent the mean value ± standard deviation of 3 measurements
The water holding capacity of solvent extracted proteins (6.8 g/g) was significantly higher than juice derived protein (3.2 g/g). Protein solubility has been reported to be related to the water absorption capacity with less soluble protein having the capacity to bind to water due to the abundance of hydrophobic groups on the surface (Stone et al. 2015). Although the emulsion capacity and stability of both protein forms were high (Table 3), the properties for solvent extracted proteins were significantly higher than juice obtained proteins. Conversely, the method of extraction did not have an impact on the foaming capacity and stability (Table 3).
The excellent functional properties demonstrated by JA tuber proteins makes them a potential ingredient for application in the food and beverage industries within a wide range of products. The proteins showed high emulsification and solubility properties that are essential for application as emulsifiers or stabilisers in meats, burgers and sausages. The good foaming properties are essential for potential application in ice-cream, spread and salad dressing.
Conclusion
An integrated strategy for protein and inulin extraction was proven to yield products with minimal impurities. The preferred extraction process started with pressing of the tuber mash to obtain a protein-rich juice resulting in a protein yield of 40.7% and an inulin loss of 2.3%. Selective inulin and protein extraction during the subsequent solvent extraction step was achieved through manipulation of the process parameters to maximize the yield of one product while minimizing the co-extraction of the product to be extracted at a later stage. Sequential extraction resulted in a further protein yield of 47.6% and inulin yield of 67.6%, from their respective extraction steps. An overall protein balance indicated that 71.8% of protein was extracted into the soluble product streams, while 17.1% was found as a co-extracted product in an inulin-stream. On the other hand, 58.4% of inulin was extracted into soluble product stream, while 11.8% was found as co-extracted product in a protein-stream. An inulin extract with a lower protein contamination was obtained, compared to inulin obtained through conventional method. The low protein content thus eliminates the need for pre-processing steps during downstream purification and therefore has the potential to lower production costs. Moreover, mechanical pressing was an important pre-extraction step that enabled selective protein isolation from the juice and improved the overall yield of protein. Pressing also enabled inulin extraction at high solids loading compared to the conventional method. Interestingly, the proteins showed an overall good functional properties with potential applications in the food and/or nutraceutical industry. This study successfully generated data that is important in designing downstream processes for the inulin and protein extracts as well as for techno-economic studies to assess the economic viability of a Jerusalem artichoke-based biorefinery.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to the National Research Foundation for the financial support and the Glen Agricultural College, Bloemfontein, Free State Province, for kindly donating the JA tubers.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eugéne van Rensburg, Email: eugenevrb@sun.ac.za.
Johann F. Görgens, Email: jgorgens@sun.ac.za
References
- Alla NA, Domokos-Szabolcsy E, El-Ramady H, Hodossi S, Fári M, Ragab M, Taha H. Jerusalem artichoke (Helianthus tuberosus L.): a review of in vivo and in vitro propagation. Int J Hortic Sci Technol. 2014;20:131–136. [Google Scholar]
- AOAC International (2002) Official methods of analysis (17th edition), Arlington, Virginia, USA: Association of Official Analytical Chemists Inc. Protein (Crude) in animal feed and pet food. AOAC official method
- Apolinário AC, de Lima Damasceno BP, de Macedo BME, Converti PA, de Silva JA. Inulin-type fructans: a review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym. 2014;101:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- Bartova V, Bárta JAN. Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride. J Agric Food Chem. 2009;19:9028–9034. doi: 10.1021/jf900897b. [DOI] [PubMed] [Google Scholar]
- Bekers M, Grube M, Upite D, Kaminska E, Danilevich A, Viesturs U. Inulin syrup from dried Jerusalem artichoke. Proc Latv Univ Agric. 2008;21:116–121. [Google Scholar]
- Can Karaca A, Low N, Nickerson M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int. 2011;44:2742–2750. doi: 10.1016/j.foodres.2011.06.012. [DOI] [Google Scholar]
- Cooper CL, Dubin PL, Kayitmazer AB, Turksen S. Polyelectrolyte-protein complexes. Curr Opin Colloid Interface Sci. 2005;10(1–2):52–78. doi: 10.1016/j.cocis.2005.05.007. [DOI] [Google Scholar]
- Fan ZL, South C, Lyford K, Munsie J, van Walsum P, Lynd LR. Conversion of paper sludge to ethanol in a semicontinuous solids-fed reactor. Bioproc Biosyst Eng. 2003;26:93–101. doi: 10.1007/s00449-003-0337-x. [DOI] [PubMed] [Google Scholar]
- Franck A. Technological functionality of inulin and oligofructose. Br J Nutr. 2002;87:87–91. doi: 10.1079/BJNBJN/2002550. [DOI] [PubMed] [Google Scholar]
- Gunnarsson IB, Svensson SE, Johansson E, Karakashev D, Angelidaki I. Potential of Jerusalem artichoke (Helianthus tuberosus L.) as a biorefinery crop. Ind Crops Prod. 2014;56:231–240. doi: 10.1016/j.indcrop.2014.03.010. [DOI] [Google Scholar]
- Karazhiyan H, Razavi SMA, Phillips GO. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011;25:915–920. doi: 10.1016/j.foodhyd.2010.08.022. [DOI] [Google Scholar]
- Kato A, Takahashi A, Matsudomi M, Kobayashi K. Determination of foaming properties of proteins by conductivity measurements. J Food Sci. 1983;48:62–65. doi: 10.1111/j.1365-2621.1983.tb14788.x. [DOI] [Google Scholar]
- Koutinas AA, Garcia IL, Kopsahelis N, Papanikolaou S, Webb C, Villar MA, Lopez JA. Production of fermentation feedstock from Jerusalem artichoke tubers and its potential for polyhydroxybutyrate Synthesis. Waste Biomass Valoriz. 2013;4:359–370. doi: 10.1007/s12649-012-9154-2. [DOI] [Google Scholar]
- Lai YP, Mondor M, Moresoli C, Drolet H, Gros-Louis M, Ippersiel D, Lamarche F, Arcand Y. Production of soy protein isolates with low phytic acid content by membrane technologies: impact of the extraction and ultrafiltration/diafiltration conditions. J Food Eng. 2013;114:221–227. doi: 10.1016/j.jfoodeng.2012.08.012. [DOI] [Google Scholar]
- Li H, Zhu H, Qiao J, Du J, Zhang H. Optimization of the main liming process for inulin crude extract from Jerusalem artichoke tubers. Front Chem Sci Eng. 2012;6:348–355. doi: 10.1007/s11705-012-1295-0. [DOI] [Google Scholar]
- Lin CF, Zayas JF. Protein, solubility, emulsifying stability and capacity of two defatted corn germ proteins. J Food Sci. 1987;52:1615–1619. doi: 10.1111/j.1365-2621.1987.tb05890.x. [DOI] [Google Scholar]
- Lingyun W, Jianhua W, Xiaodong Z, Da T, Yalin Y, Chenggang C, Tianhua F, Fan Z. Studies on the extracting technical conditions of inulin from Jerusalem artichoke tubers. J Food Eng. 2007;79:1087–1093. doi: 10.1016/j.jfoodeng.2006.03.028. [DOI] [Google Scholar]
- Mitra RK, Sinha SS, Pal SK. Hydration in protein folding: thermal unfolding/refolding of human serum albumin. Langmuir. 2007;23:10224–10229. doi: 10.1021/la7014447. [DOI] [PubMed] [Google Scholar]
- Mizubuti IY, Junor OB, Souza OWB, da Silva RSD, Ida EI. Response surface methodology for extraction optimization of pigeon pea protein. Food Chem. 2000;70:259–265. doi: 10.1016/S0308-8146(00)00078-9. [DOI] [Google Scholar]
- Palthur MP, Palthur SS, Chitta SK. Nutraceuticals: a conceptual definition. Int J Pharm Pharm Sci. 2010;3:19–27. [Google Scholar]
- Singh P, Kumar R, Sabapathy SN, Bawa AS. Functional and edible uses of soy protein products. Compr Rev Food Sci Food Saf. 2008;7:14–28. doi: 10.1111/j.1541-4337.2007.00025.x. [DOI] [Google Scholar]
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2005) Determination of Ash in Biomass. National Renewable Energy Laboratory, technical report. NREL/TP-510-42622
- Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C, Sluiter J, Templeton D, Wolfe J (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples. National Renewable Energy Laboratory, technical report. NREL/TP-510-42621
- Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2011) Determination of structural carbohydrates and lignin in biomass. National Renewable Energy Laboratory, technical report. NREL/TP-510-42618
- Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int. 2015;76:31–38. doi: 10.1016/j.foodres.2014.11.017. [DOI] [Google Scholar]
- Tewari S, Ramalakshmi K, Methre L, Mohan Rao LJ. Microwave-assisted extraction of inulin from chicory roots using response surface methodology. J Nutr Food Sci. 2015;5:342. doi: 10.4172/2155-9600.1000342. [DOI] [Google Scholar]
- Wada T, Suqatani J, Terada E, Ohquchim M, Miwa M. Physicochemical characterization and biological effects of inulin enzymatically synthesized from sucrose. J Agric Food Chem. 2005;53:1246–1253. doi: 10.1021/jf048711u. [DOI] [PubMed] [Google Scholar]
- Xiao YM, Li HZ, Hong BS, Gang X, Feng Z, Fu TN, Brestic M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J Med Plants Res. 2011;5:1272–1279. [Google Scholar]
- Yang L, He QS, Corscadden K, Udeningwe CC. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol Rep. 2015;5:77–88. doi: 10.1016/j.btre.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarroug Y, Abdelkarim A, Dorra ST, Hamdaoui G, Felah MEL, Hassouna M. Biochemical characterization of Tunisian Cichorium Intybus L. roots and optimization of ultrasonic inulin extraction. Mediterr J Chem. 2016;6:674–685. doi: 10.13171/mjc61/01611042220-zarroug. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.