Abstract
Reintegration of grape stem, a by-product from wine production, into the food chain is of high interest from an economic and environmental perspective. Therefore, an investigation of stems was undertaken and is described here. It is known that quality of stems is of high variability. In this study the stems from four grapevine varieties (Syrah, Cabernet Sauvignon, Merlot and Chasselas) cultivated in Switzerland were treated in following ways: drying, cutting and separation into fractions based on particle size. All fractions were then characterised for their phenolic compounds content. It was found that Chasselas fractions contained most phenolic compounds. The addition of grape stems of the four different varieties allowed reduction of the protein content of a model wine. The extent of protein precipitation was highly correlated with the amount of phenolic compounds in stems added. Among the examined varieties, Chasselas brought most promising results, with the high reduction of the protein at low level of stem addition.
Keywords: Grape stems, Chasselas, Model wine, Phenolic compounds, Haze
Introduction
Grapevine is cultivated in very large quantities, mainly for wine production. The winemaking process generates huge amounts of solid waste, which accounts for more than 30% of the grapes used for wine production. It was estimated that the residues derived from the wine industry exceeded 15 million t in 2009 (González-Centeno et al. 2012). Efforts are made to find ways of utilisation of this by-product in order to diminish its environmental impact. Up to now the most common way of by-product exploitation is the production of compost and energy. Before utilisation of stems as compost, polyphenols have to be removed due to their phytotoxic and antimicrobial effects. Alternative ways to valorise stems are still under investigation. Grape stems were used as biosorbents as they have the ability to bind and concentrate heavy metals from very dilute aqueous solutions (Villaescusa et al. 2004).
Stems are a rich source of dietary fibre (60–90% of total dry matter) and bioactive compounds, which reach up to 5.8% of the dry matter (Barros et al. 2015). Flavanols constitute the most abundant phenolic class in grape stems, and as general trend, their content is up to 30-folds higher than flavonols, phenolic acid, or stilbenes (Barros et al. 2015). The content of the individual phenolic in grape stems from Spain (González-Centeno et al. 2012), Greece (Anastasiadi et al. 2012) and Italy (Spatafora et al. 2013) have been shown to be highly dependent on the variety and growing conditions. In grape stems of varieties grown in Italy gallic acid, procyanidin B1, catechin, quercetin 3-O-glucoside, quercetin 3-O-glucuronide, (E)-resveratrol, ε-viniferin were identified with procyanidin B1 and catechin as the most abundant (Spatafora et al. 2013).
The reintegration of wine stems into the food chain is of high economic and environmental interest. So far the attempts to produce antioxidant or dietary fibre concentrates for food supplementation have been made. Stems from ‘Sousão’, a red grape variety from Portugal were used for production of liqueur (Barros et al. 2016). The product obtained was rich in oligomeric flavanols, mainly dimers and trimers. Grape stems are cheap and easily available in vineyards. They might be used directly or after fractioning, in order to enrich valuable components and thereby improve efficiency of resource utilisation (Pujol et al. 2013). It was postulated that heterogeneous materials from biomass need to be shred into small particles before processing (Miranda et al. 2012).
In order to avoid transport costs of stems, oenological applications seem to be most preferable. The results of an interesting trial were published recently reporting the utilisation of grape by-products i.e. seeds and stems for colour protection of red wine (Pascual et al. 2016). Proanthocyanidins present in stems are considered to be very bitter and astringent. However, in the cited study wines with stems added are more astringent but not bitter. A greater degree of polymerisation and a higher degree of galloylation of proanthocyanidins lead to perception of astringency. Despite the risk of stemmy flavour, retaining the stems during wine maceration can have a beneficial effect on colour extraction, while increasing polyphenols content and improving aging ability. It is already applied in production of some wines in France: Châteauneuf du-Pape (Côtes du Rhône), Pinot noir (Burgundy) and Médoc region (Bordeaux) or in Italy (Suriano et al. 2015; Pascual et al. 2016). The incorporation of stems to wine during the fermentation might also protect from oxygen and therefore enables lowering doses of sulphur dioxide. The antioxidant and antimicrobial activity and the olfactometry profile of the grape stem extract have been tested as a potential alternative to SO2 in wine-making (Ruiz-Moreno et al. 2015). Promising results were obtained for extract from stems of Syrah variety cultivated in Spain. Additionally, a reduction in SO2 might be advantageous to wine quality in terms of a recent report indicating participation of SO2 in aggregates making, a factor triggering protein haze formation and instability of wine (Chagas et al. 2016).
The phenomenon of white wine instability is of extreme complexity and no concise theory exists yet, despite of intense efforts made for its understanding. Formation of protein haze results in wine turbidity (Sauvage et al. 2010). Although it has limited effects on the olfactory and gustatory properties, turbid wines are not attractive and thus consumer acceptance is significantly reduced (Batista et al. 2009). White wine contains between 10 and 500 mg/l protein depending on variety (Ferreira et al. 2001). Wine proteins are derived from yeast fermentation and grape pulp (Batista et al. 2009). The most prominent proteins found in wine are chitinases and thaumatin-like proteins. In instable wines, proteins unfold, aggregate and form cross-links (Van Sluyter et al. 2015). Studies have shown that formation of protein haze is not predictable from its protein content. This fact leads to the conclusion that either only part of the proteins are responsible for instability or some non-protein factors contribute also to haze formation (Batista et al. 2009). Several other factors have been proposed to influence wine stability such as polyphenols, sulphate, polysaccharides, wine pH, organic acids, ethanol content and metal ions (Lambri et al. 2012; Van Sluyter et al. 2015).
Wine fining with bentonite is a commercially applied method to prevent protein haze formation. Bentonite is a montmorillonite clay that interacts with positively charged wine proteins and other charged substances in wine (Lambri et al. 2012). However, the utilization of bentonite also has some disadvantages. Bentonite treatment is reported to be responsible for loss of colour, flavour and texture compounds (Waters et al. 2005). Another highly economic problem is the loss of wine volume. Furthermore, bentonite cannot be reused and its handling and disposal causes additional costs. Therefore, studies are conducted to develop alternative methods to replace bentonite fining but so far yield only limited success (Waters et al. 2005; Van Sluyter et al. 2015). Such alternatives should be effective, preferably less expensive than bentonite and not lead to any sensorial change.
In the present study, the ability of grape stems to remove unstable proteins in wine by precipitation has been assessed. The feasibility of this approach was first tested with stems of different varieties in a model wine solution.
Materials and methods
Reagents
All reagents used were of at least analytical grade. Folin–Ciocalteu’s phenol reagent, gallic acid, (+)–catechin, rutin, vanillic acid, caffeic acid, chlorogenic acid, syringic acid, p-coumaric acid, ferulic acid, quercetin, protocatechuic acid, gentisic acid, p-hydroxybenzoic acid, sinapic acid, tyrosol, bovine serum albumin fraction V (BSA), Coomassie Brilliant Blue G 250 and tartaric acid were obtained from Sigma-Aldrich (Switzerland). Phosphoric acid (80% w/w) and sodium carbonate were bought from Acros Organics (Belgium). (−)-Epicatechin, caftaric acid and scopoletin were purchased from Aktin Chemicals (China). Ethanol absolute was obtained from Alcosuisse (Switzerland). Sodium hydroxide (30%) was bought from Cochimy (Switzerland). Acetonitrile was purchased from Macron Fine Chemicals (Poland) and formic acid from Merck (Germany). Bentonite MIRACOL was acquired from Martin Vialatte (France). The deionised water was obtained with a Milli-Q system (Merck-Millipore, Germany).
Materials
Vitis vinifera of four varieties i.e. Chasselas (white), Syrah (red), Merlot (red) and Cabernet Sauvignon (red) was grown in the experimental vineyards of School of Viticulture and Oenology in Changins (Switzerland) in 2015. Grapes were harvested at the optimum ripening stage for each variety i.e. between 22nd September and 18th October. The climatic conditions of the growing period i.e. March–October consisted of average monthly temperature ranging from 7.8 °C (March) to 24.1 °C (July), and average monthly humidity ranging from 52.7% (July) to 84.4% (October). Grape stems were separated from the grapes directly after harvesting, freezed and stored until used. Before analysis, the stems were dried with dry air in a drying tower (Niro atomizer, Copenhagen, Denmark) during 12–14 h with dry air provided by the air dryer (Krüger, Walgerholm, Denmark). The temperature of the material during drying did not exceed 40 °C and the target water content was 5%. Dry stems were cut (Cut-O-Mat H10, HUG, Emmenbrücke, Switzerland) and sieved through laboratory sieves in order to obtain six fractions with different particle size: ≤ 0.25, 0.25–0.7, 0.7–1.0, 1.0–2.0, 2.0–2.5, 2.5–4.0 and > 4.0 mm for each variety.
A model wine was prepared by adding tartaric acid (5 g/l), absolute ethanol (15%) to deionised water and adjusting the pH to 3.5 with sodium hydroxide (Fontoin et al. 2008). In order to mimic protein content in wine, 200 mg/l or 500 mg/l of BSA were added to the model wine solution. BSA has a molecular weight of 66 kDa and an isoelectric point of 4.3, which are in the range of wine proteins. It is considered to provide conservative estimates regarding wine protein diffusion, because it is larger than most wine proteins (Blade and Boulton 1988).
Grape stem characteristics
Determination of dry matter content (DM)
The DM content of the stems was determined in duplicate with a halogen-balance (Mettler Toledo HG53 Moisture Analyzer, Switzerland). The loss of mass at 110 °C was determined gravimetrically (in %).
Extraction of polyphenols
For polyphenols extraction, 1 g of stems were mixed with 8 ml of deionised water and placed for 10 min in the ultrasonic bath (working frequency 35 kHz, VWR, Dietikon, Switzerland). The mixture was centrifuged using an Eppendorf Centrifuge 5810 (Germany), supernatant collected and residue extracted twice more. The supernatants were combined and adjusted to 25 ml. The extraction was performed in triplicate. The extracts were used for total polyphenol content (TCP) and individual polyphenols determination.
Total polyphenols content (TPC) with Folin–Ciocalteu reagent
Absorbance measurements were performed using an Infinite M200 pro microplate reader (Tecan, Switzerland). TPC was analysed with Folin–Ciocalteu assay as described by Horszwald and Andlauer (2011) with some modifications. Briefly, 25 µl of the sample (or standard or water as blank) was filled into a well of the 96-well microplate (Nunc A/S, Ruskilde, Denmark). To each well 250 µl of diluted Folin–Ciocalteu reagent (2 + 30 with water) was automatically added with the injector. After 10 min of incubation at room temperature, 25 µl of sodium carbonate solution (5% in water) was added to the wells. The plate was incubated for 20 min and absorbance was measured at 755 nm. As standards, solutions of gallic acid in the concentration range of 50–500 mg/l in water were prepared. The results are indicated as mg gallic acid equivalent per g of DM.
Individual polyphenols content by HPLC
An Agilent 1220 Infinity LC (Switzerland) with a 100 × 2.1 mm Kinetex® 2.6 µm EVO C18 100 Å column (Phenomenex, Switzerland) was used for chromatographic separation. The method applied was previously described (Heeger et al. 2017). The detection was performed with a diode array detector at 260 nm, 280 nm, 320 nm and 340 nm. The identification was performed via comparison of retention time and UV-spectrum with those of standard compounds. Before HPLC analysis samples were filtered with Exapure 0.45 µm filter (Switzerland). Elution was performed with a gradient of 1% aqueous formic acid (eluent A) and acetonitrile containing 1% formic acid (eluent B) delivered at 0.3 ml/min. The separation started with 100% A for 2 min, 2–25 min B was increasing to 10%, 25–26 min B was kept at 10%, 26–30 min B increased from 10 to 60%, then B was kept for 5 min at 60%. From 35 to 35.1 min A was set to 100%. For analysis, 1 µl of sample was injected onto the column. The quantification was made via external calibration with the following substances used as standards: caftaric acid, gallic acid, rutin, tyrosol, (+)-catechin.
Effect of stems addition on protein and polyphenols content in model wine
Grape stems fractions with particle size between 1.0 and 2.0 mm of each variety were weighed into a falcon tube (20–220 mg) and 10 ml of model wine or a model wine solution with BSA were added. The model wine and stems were mixed using a rotational shaking machine (Labnet Labroller II, Switzerland) at 30 rpm for 60 s and then left to rest at room temperature. After that the samples were centrifuged with an Eppendorf Centrifuge 5810 (Germany) and the supernatant was taken to analyse protein and TPC content.
In order to investigate the dose dependence of protein precipitation, samples were left for 1 h to rest.
For investigation of time dependence, 40 ml of model wine were treated with the grape stems and 400 µl are sampled and centrifuged after 15 min, 30 min, 1 h, 2 h and 4 h, each.
The precipitation dependence on particle size was tested for Chasselas stems. For this, model wine was treated with 8 mg/ml of each fraction for 1 h.
All experiments were performed in triplicate with model wine containing BSA and in duplicate with model wine without BSA.
Protein content by Bradford method
Protein content of the model wine was determined with the Bradford method as described by Ernst and Zor (2010) with some modifications for interference of phenolic compounds (unpublished results). Briefly, 50 µl of sample and 200 µl of reagent were filled into a microplate well. Absorbance at 595 nm as well as 450 nm and 720 nm were measured after incubation at room temperature for 10 min. Solutions of 5 mg/l to 500 mg/l of BSA in 15% ethanol were prepared as standards. Each extract was analysed in triplicate.
Statistical analysis
The results are presented as mean ± standard deviation for at least three individual analysis. The significance of difference was analysed using a one way ANOVA and a post hoc Tukey–Kramer test (Granato et al. 2014). The differences were considered significant at p < 0.05.
Results and discussion
Characteristics of stems of four grapevine varieties
Appearance of individual fractions
A drying process was applied to prevent stem spoilage. In order to obtain unified material, the stems of each variety were cut and sieved into fractions differing in particle size. Figure 1 depicts the original dried stems of Chasselas variety and the seven fractions thereof. The obtained grape stem fractions were considerably diverse in terms of colour and shape: the fraction of > 4 mm contained brown cylindrical structures, the medium fractions were formed by light brown fibrous material, and the two finest fractions contained dark brown powder. This different shape of the particles constituting individual fractions indicates that they contain different morphological parts of the plant. Fractionation performed was thus not only a separation according to size. Large concentration of pedicels was noted in fractions 2.0–2.5 mm and 1.0–2.0 mm depicted in Fig. 1c, d, respectively.
Fig. 1.

Stems of Chasselas variety (a) and its seven fractions: > 4.0 mm (b), 2.5–4.0 mm (c), 2.0–2.5 mm (d), 1.0–2.0 mm (e), 0.7–1.0 mm (f), 0.25–0.7 mm (g) and ≤ 0.25 mm (h)
Content of polyphenols
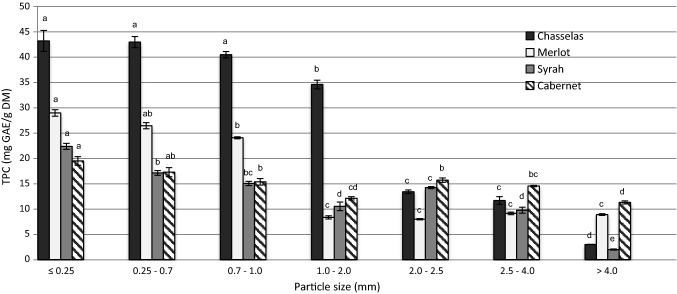
The TPC differed significantly between fractions of varied particle size within one variety as well as between varieties within one particle size (Fig. 2). The highest content was noted for four smallest fractions of Chasselas stems (< 2 mm) for which TPC amounted to more than 30 mg GAE/g DM. These four size fractions of Chasselas stems were considerably richer in phenolic compounds than fractions of Merlot, Syrah and Cabernet Sauvignon stems, independently from their size. The trend of the smaller the particle sizes higher the TPC extractability was noted for Chasselas stem fractions. The smallest differences between TPC of different fractions within one variety were observed for Cabernet Sauvignon.
Fig. 2.
Total phenolic compounds content (TPC) in stem fractions of four varieties. Within one variety, the bars denoted with different letters are significantly different at p < 0.05
The characterisation of different fractions of wine stems has limited literature reporting (Pujol et al. 2013). Focus is given mostly on fibre components; however, their authors also presented TPC analyses. The values reported are compatible to those obtained in our study for Syrah and Cabernet Sauvignon fractions.
Four phenolic compounds, gallic acid, caftaric acid, catechin and rutin were identified in all stem fractions analysed (Fig. 3). Caftaric acid and rutin were the predominant compounds in Merlot and Cabernet Sauvignon stem fractions whereas caftaric acid and catechin in Chasselas fractions. In the case of Syrah stem fractions caftaric acid was the most abundant compound. In general, Chasselas fractions were richest in phenolic compounds among all varieties analysed. It was reflected in highest quantity of sum of phenolic compounds identified ranging between 2.36 and 8.40 mg/g DM while for three other varieties it did not exceed 4 mg/g DM for the richest fraction. The content of caftaric acid in all the Chasselas fractions was twice as high as in those of other varieties whereas the content of catechin was nearly three times higher. Stem fractions of Merlot, Syrah and Cabernet Sauvignon fractions showed much smaller differences in individual phenolic compounds between different varieties for the fractions of the same particle size as well as between fractions of different size within one variety. Comparison of the results presented in Figs. 2 and 3 indicates that other phenolics than those identified in this study might also contribute to the TPC. We suppose the presence of oligo- and polymers of flavanols. The results of individual phenolic compounds content confirmed that lower size fractions had higher extractability of phenolic compounds for all varieties. It has been shown that shredding helps to access phenolic compounds during extraction.
Fig. 3.
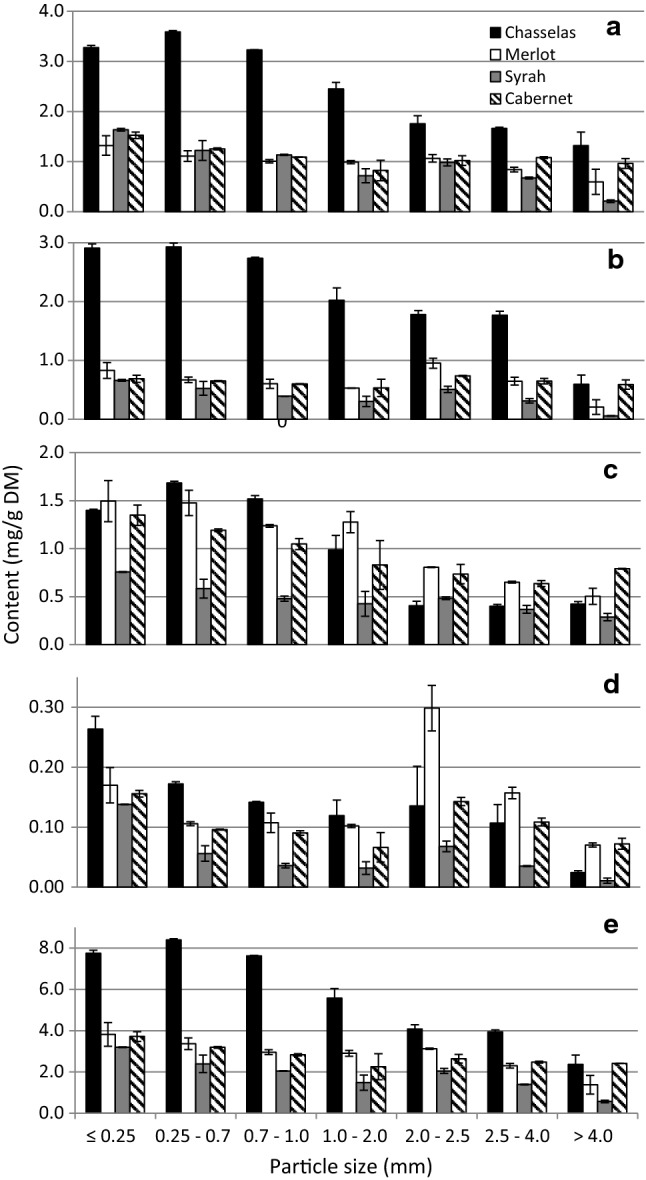
Content of caftaric acid (a), catechin (b), rutin (c), gallic acid (d) and sum of individual phenolic compounds (e) in fractions of stems from four grape varieties. Results are expressed as mean ± SD. Results within one variety and one compound annotated with different letters are significantly different at p < 0.05
Stems from different grape varieties were previously analysed in view of their utilisation as a source of phenolic compounds. González-Centeno et al. analysed stems from 10 different wine varieties cultivated in Spain (González-Centeno et al. 2012). Stems from Callet, Syrah, Premsal Blanc, Parellada, and Manto Negro varieties yielded the highest total phenolic and total proanthocyanidin contents and showed the greatest antioxidant capacities, whereas Chardonnay and Merlot stems presented the lowest values. The values obtained in the cited study were significantly higher than in our study due to application of Accelerated Solvent Extraction System. Stems obtained from white (Asyrtiko, Athiri and Aidani) and red cultivars (Mandilaria, Mavrotragano and Voidomatis) of Vitis vinifera cultivated in the Greek islands showed high differences in the content of phenolic compounds (Anastasiadi et al. 2012). The predominant compounds were catechin and gallic acid, and the highest content was noted for stems of one of white varieties—Asyrtiko amounting to 1.86 and 0.45 mg/g DM, respectively. The content of catechin was much higher in low size fractions of Chasselas stems analysed in our study.
Effectiveness of grape stem fraction for protein precipitation in model wine
The stem fractions of 1.0–2.0 mm particle size were selected for the study on the ability of stems to remove proteins from model wine. This fraction was characterised by the most unified appearance, which may reduce the variability of results due to biological heterogeneity of material.
BSA has been commonly applied for modelling wine proteins (Blade and Boulton 1988; Achaerandio et al. 2001; Sommer et al. 2016). The isoelectric point of BSA is 4.7, which is also within the range of pI of most of the wine proteins (4.0–5.0) (Dufrechou et al. 2012). Most of the protein in wine comprise of fractions with a molecular weight of 20–30 kDa and 60–70 kDa, whereas molecular weight of BSA is 66 kDa. BSA is thus a good model for wine protein stability studies (Harbertson et al. 2003; Sommer et al. 2016).
The effect of different treatment time was investigated (Fig. 4). Most of the reduction in protein content was observed within the first hour, after that there was only a small further reduction. Total polyphenols content remained almost constant between 15 min and 4 h exposure time (data not shown). Based on these results, it was decided that 1 h exposure time was appropriate for further experiments.
Fig. 4.
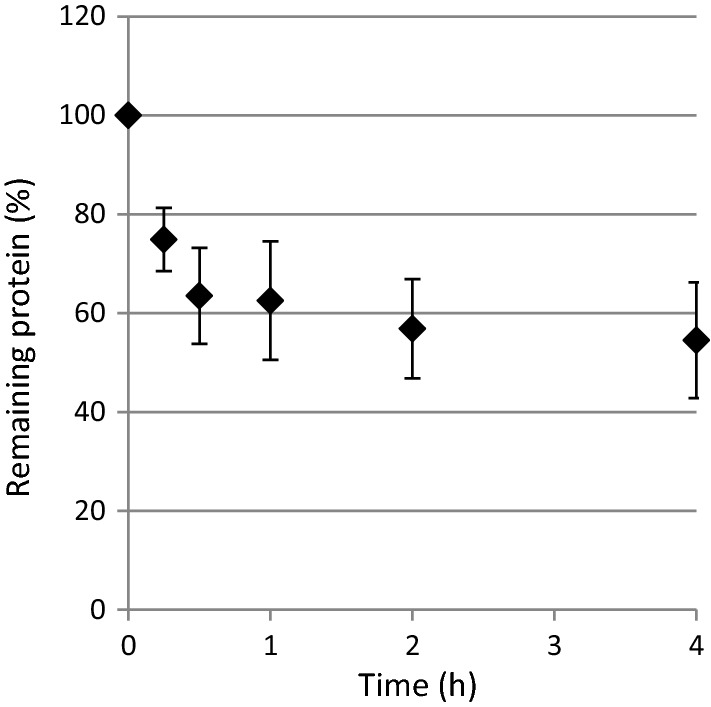
Effect of duration of the treatment with Chasselas stems fraction (1.0–2.0 mm, 8 mg/ml) on the content of protein remaining in the model wine containing BSA (500 mg/l)
The model wine was treated with different doses of grape stems of four different varieties (fraction of 1.0–2.0 mm). At a certain point, grape stems addition seemed to promote the formation of a white precipitate. After resting time at room temperature, the precipitate started to sediment. The amount of proteins remaining in the model wine diminished with increasing dose of grape stems (Fig. 5). The clear differences between varieties were noted. Comparing with other varieties, the stem fraction of Syrah had lower capacity to precipitate proteins of model wine. The addition of 10 mg of stems per ml of model wine precipitated all proteins in the case of all the stems apart from the Syrah fraction. For all examined varieties, the standard deviation was high when big amounts of protein were precipitated. This is probably due to the heterogeneity of stems fraction. However, the nature of interactions between polyphenols and protein are rather complex. The formed precipitate also interacts with still soluble protein so that once the protein precipitation has started the formation of new precipitate might be promoted.
Fig. 5.
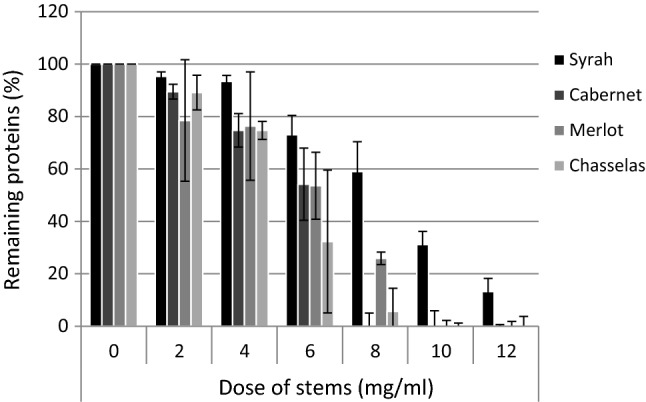
The percentage of proteins remaining in model wine after treating with different doses of grape stems (fraction of 1.0–2.0 mm) for 1 h. Results are expressed as mean of three individual treatments ± SD
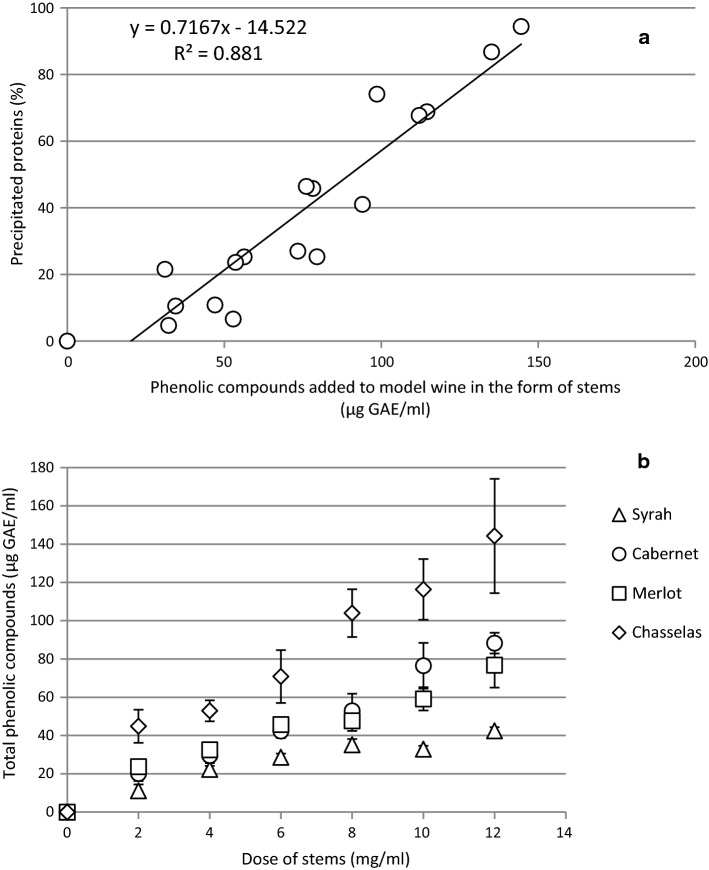
The extent of protein precipitation could be well correlated (R2 = 0.881) with the TPC introduced with stems into model wine (Fig. 6a). Some of the polyphenols introduced remains in the solution. Figure 6b shows that TPC in model wine containing proteins after treatment with stems increased linearly with the dose of stems with R2 = 0.988, 0.976, 0.959, 0.922 for Cabernet, Chasselas, Merlot and Syrah, respectively. Comparing the total polyphenol contents for the same dose of stems, Chasselas had the highest content, followed by Cabernet and Merlot. Considerably lower content was noted when Syrah stems were used. The amount of extracted total polyphenols is rather high considering that white wine contains between 100 and 400 mg/l (Mitić et al. 2010). A colour change of the model wine was visible when high doses of stems were used. For a Syrah grape stem extract it was reported that the majority of identified odorant compounds are substances that have already been detected in wine before (Ruiz-Moreno et al. 2015). It is speculated that the addition of phenolic compounds of stem origin into white wine might have an antioxidant effect. Therefore, it might allow diminishing addition of SO2 in wine-making process. It would be of great importance especially for the consumers sensitive to SO2.
Fig. 6.
Treatment of model wine with different amounts of grape stems of four varieties (fraction of 1.0–2.0 mm) for 1 h a protein precipitation in a function of the amounts of phenolic compounds added to model wine in the form of stems and. b Total polyphenols content in model wine after protein precipitation with stems. Results are expressed as gallic acid equivalents (GAE)
White wines contain relatively large insoluble proteins which slowly precipitate from solution. Most white wines lack sufficient tannins to cause initial protein precipitation. Nevertheless, tannins purified from Pinot Grigio wine have been shown to precipitate different wine protein fractions of the same wine with the extent of turbidity being dependent on the protein fraction (Marangon et al. 2010).
The complexity of protein-polyphenols interactions remains to be elucidated in order to find its practical application. The conformational mobility of phenolic molecules appears to be essential for protein binding and in particular interaction involves stacking of the planar proline cycle in proteins with the phenolic ring (Zhang et al. 2014). Interactions occur in successive stages, forming first aggregates, leading downstream to precipitation. Haze-forming polyphenols have at least two binding groups, each of which has at least two hydroxyl groups on the same aromatic ring. The protein/polyphenol ratio has a strong influence on the amount of haze formed; the largest amount occurs when the numbers of polyphenol and protein binding sites are nearly equal (Siebert 1999).
Conclusion
In the present study the hypothesis that grape stems could represent a valuable agent to remove unstable proteins in wine by protein precipitation, has been verified. The study demonstrates that the addition of grape stems fractions into model wine reduces the its protein content. Chasselas stems, rich in polyphenols showed most promising results in terms of their possible utilisation for reducing the protein content in unstable wine. The extent of protein precipitation was highly correlated with the amount of phenolic compounds added in the form of stems.
Further research might be of interest to valorise grape stems in order to achieve wine stabilization. Once a procedure has been successfully developed, sensory tests with the wine treated with stems will definitely need to be performed in order to investigate if and how the grape stems influence the sensorial profile of the wine.
Grape stems utilisation might also allow diminishing addition of SO2 in wine-making process. It might be advantageous to wine quality also regarding a recent report indicating participation of SO2 in aggregates making and therefore instability of wine.
Acknowledgements
The study was financially supported by the University of Applied Sciences and Arts Western Switzerland within the thematic research program HealthFood®. The excellent technical assistance of Kevin Pacios is highly appreciated. We extend our gratitude to Dr. Wolfram Brück for the linguistic revision of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achaerandio I, Pachova V, Güell C, López F. Protein adsorption by bentonite in a white wine model solution: effect of protein molecular weight and ethanol concentration. Am J Enol Vitic. 2001;52:122–126. [Google Scholar]
- Anastasiadi M, Pratsinis H, Kletsas D, et al. Grape stem extracts: polyphenolic content and assessment of their in vitro antioxidant properties. LWT Food Sci Technol. 2012;48:316–322. doi: 10.1016/j.lwt.2012.04.006. [DOI] [Google Scholar]
- Barros A, Gironés-Vilaplana A, Texeira A, et al. Grape stems as a source of bioactive compounds: application towards added-value commodities and significance for human health. Phytochem Rev. 2015;14:921–931. doi: 10.1007/s11101-015-9421-5. [DOI] [Google Scholar]
- Barros A, Gouvinhas I, Machado N, et al. New grape stems-based liqueur: physicochemical and phytochemical evaluation. Food Chem. 2016;190:896–903. doi: 10.1016/j.foodchem.2015.06.047. [DOI] [PubMed] [Google Scholar]
- Batista L, Monteiro S, Loureiro VB, et al. The complexity of protein haze formation in wines. Food Chem. 2009;112:169–177. doi: 10.1016/j.foodchem.2008.05.070. [DOI] [Google Scholar]
- Blade WH, Boulton R. Adsorption of protein by bentonite in a model wine solution. Am J Enol Vitic. 1988;39:193–199. [Google Scholar]
- Chagas R, Ferreira LM, Laia CAT, et al. The challenging SO2-mediated chemical build-up of protein aggregates in wines. Food Chem. 2016;192:460–469. doi: 10.1016/j.foodchem.2015.07.052. [DOI] [PubMed] [Google Scholar]
- Dufrechou M, Poncet-Legrand C, Sauvage F-X, Vernhet A. Stability of white wine proteins: combined effect of pH, ionic strength, and temperature on their aggregation. J Agric Food Chem. 2012;60:1308–1319. doi: 10.1021/jf204048j. [DOI] [PubMed] [Google Scholar]
- Ernst O, Zor T. Linearization of the bradford protein assay. J Vis Exp. 2010 doi: 10.3791/1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RB, Piçarra-Pereira MA, Monteiro S, et al. The wine proteins. Trends Food Sci Technol. 2001;12:230–239. doi: 10.1016/S0924-2244(01)00080-2. [DOI] [Google Scholar]
- Fontoin H, Saucier C, Teissedre P-L, Glories Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual Prefer. 2008;19:286–291. doi: 10.1016/j.foodqual.2007.08.004. [DOI] [Google Scholar]
- González-Centeno MR, Jourdes M, Femenia A, et al. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.) J Agric Food Chem. 2012;60:11850–11858. doi: 10.1021/jf303047k. [DOI] [PubMed] [Google Scholar]
- Granato D, de Araújo Calado VM, Jarvis B. Observations on the use of statistical methods in food science and technology. Food Res Int. 2014;55:137–149. doi: 10.1016/j.foodres.2013.10.024. [DOI] [Google Scholar]
- Harbertson JF, Picciotto EA, Adams DO. Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am J Enol Vitic. 2003;54:301. [Google Scholar]
- Heeger A, Kosińska-Cagnazzo A, Cantergiani E, Andlauer W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017;221:969–975. doi: 10.1016/j.foodchem.2016.11.067. [DOI] [PubMed] [Google Scholar]
- Horszwald A, Andlauer W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J Berry Res. 2011;1:189–199. doi: 10.3233/JBR-2011-020. [DOI] [Google Scholar]
- Lambri M, Dordoni R, Giribaldi M, et al. Heat-unstable protein removal by different bentonite labels in white wines. LWT Food Sci Technol. 2012;46:460–467. doi: 10.1016/j.lwt.2011.11.022. [DOI] [Google Scholar]
- Marangon M, Vincenzi S, Lucchetta M, Curioni A. Heating and reduction affect the reaction with tannins of wine protein fractions differing in hydrophobicity. Anal Chim Acta. 2010;660:110–118. doi: 10.1016/j.aca.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Miranda I, Gominho J, Mirra I, Pereira H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind Crops Prod. 2012;36:395–400. doi: 10.1016/j.indcrop.2011.10.035. [DOI] [Google Scholar]
- Mitić MN, Obradović MV, Grahovac ZB, Pavlović AN. Antioxidant capacities and phenolic levels of different varieties of Serbian white wines. Molecules. 2010;15:2016–2027. doi: 10.3390/molecules15032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, González-Royo E, Gil M, et al. Influence of grape seeds and stems on wine composition and astringency. J Agric Food Chem. 2016;64:6555–6566. doi: 10.1021/acs.jafc.6b01806. [DOI] [PubMed] [Google Scholar]
- Pujol D, Liu C, Fiol N, et al. Chemical characterization of different granulometric fractions of grape stalks waste. Ind Crops Prod. 2013;50:494–500. doi: 10.1016/j.indcrop.2013.07.051. [DOI] [Google Scholar]
- Ruiz-Moreno MJ, Raposo R, Cayuela JM, et al. Valorization of grape stems. Ind Crops Prod. 2015;63:152–157. doi: 10.1016/j.indcrop.2014.10.016. [DOI] [Google Scholar]
- Sauvage F-X, Bach B, Moutounet M, Vernhet A. Proteins in white wines: thermo-sensitivity and differential adsorbtion by bentonite. Food Chem. 2010;118:26–34. doi: 10.1016/j.foodchem.2009.02.080. [DOI] [Google Scholar]
- Siebert KJ. Effects of protein–polyphenol interactions on beverage haze, stabilization, and analysis. J Agric Food Chem. 1999;47:353–362. doi: 10.1021/jf980703o. [DOI] [PubMed] [Google Scholar]
- Sommer S, Dickescheid C, Harbertson JF, et al. Rationale for haze formation after carboxymethyl cellulose (CMC) addition to red wine. J Agric Food Chem. 2016;64:6879–6887. doi: 10.1021/acs.jafc.6b02479. [DOI] [PubMed] [Google Scholar]
- Spatafora C, Barbagallo E, Amico V, Tringali C. Grape stems from Sicilian Vitis vinifera cultivars as a source of polyphenol-enriched fractions with enhanced antioxidant activity. LWT Food Sci Technol. 2013;54:542–548. doi: 10.1016/j.lwt.2013.06.007. [DOI] [Google Scholar]
- Suriano S, Alba V, Tarricone L, Di Gennaro D. Maceration with stems contact fermentation: effect on proanthocyanidins compounds and color in Primitivo red wines. Food Chem. 2015;177:382–389. doi: 10.1016/j.foodchem.2015.01.063. [DOI] [PubMed] [Google Scholar]
- Van Sluyter SC, McRae JM, Falconer RJ, et al. Wine protein haze: mechanisms of formation and advances in prevention. J Agric Food Chem. 2015 doi: 10.1021/acs.jafc.5b00047. [DOI] [PubMed] [Google Scholar]
- Villaescusa I, Fiol N, Martínez M, et al. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004;38:992–1002. doi: 10.1016/j.watres.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Waters Alexander G, Muhlack R, et al. Preventing protein haze in bottled white wine. Aust J Grape Wine Res. 2005;11:215–225. doi: 10.1111/j.1755-0238.2005.tb00289.x. [DOI] [Google Scholar]
- Zhang H, Yu D, Sun J, et al. Interaction of plant phenols with food macronutrients: characterisation and nutritional–physiological consequences. Nutr Res Rev. 2014;27:1–15. doi: 10.1017/S095442241300019X. [DOI] [PubMed] [Google Scholar]