Abstract
Temperature-dependency on cell membrane injury and inactivation of Saccharomyces pastorianus by low-pressure carbon dioxide microbubbles (MBCO2) was investigated. The number of surviving S. pastorianus cells after MBCO2 treatment detected with yeast and mould agar (YMA, an optimum agar) was higher than that with YMA adding 2.5 g/L sodium chloride and yeast nitrogen base agar (a minimum agar). However, the decrease of the surviving number by thermal treatment was not changed among above agars used. The fluorescence polarization (FP), which indicated the phase transition of the membrane of S. pastorianus cells treated with MBCO2 increased with increasing temperature. The activity of the alkaline phosphatase (AP), a periplasmic enzyme, in S. pastorianus cells after MBCO2 and thermal treatments increased with the FP but was reduced by further increasing temperature. The FP and AP activities after MBCO2 treatment increased at a temperature lower than the temperature of the thermal treatment. In addition, intracellular pH of S. pastorianus decreased by the MBCO2 treatment at lower temperature with increasing pressure. Therefore, it was revealed that phase transition of the cell membrane and inactivation of S. pastorianus was caused by MBCO2 treatment at lower temperature than thermal treatment and that the effect was induced by the dissolved CO2 and increased with increasing pressure.
Keywords: Cell membrane injury, Inactivation, Intracellular pH, Low-pressure carbon dioxide microbubbles, Saccharomyces pastorianus
Introduction
Several non-thermal sterilization techniques such as Barba et al. (2017), Guimarāes et al. (2018) have been reported until now. Pressurized carbon dioxide (CO2) is one of non-thermal sterilization techniques and has been widely studied (Amaral et al. 2017; Garcia-Gonzalez et al. 2007; Silva et al. 2018). Silva et al. (2018) evaluated the influence of CO2 volume ratio on the inactivation of Lactobacillus casei by pressurized CO2 using a two-level plackett–burman design and a box-behnken design. The mechanism on the microbial inactivation by pressurized CO2 has not been completely understood yet. However, Howladar et al. (2017) indicated that pressurized CO2 triggered cell disruption or cell lysing of Rhodotorula glutinis. Liao et al. (2010) discussed that the rigidity increase and the fluidity decrease of the cell membrane were caused by the dissolution of CO2 of the lipid bilayer of the cell membrane as a result of pressurized CO2 treatment. Li et al. (2012) observed morphological changes of Saccharomyces cerevisiae cells treated with pressurized CO2 by electron microscopy and confirmed the fluidity decrease and the permeability increase of the cell membrane by fluorescence analysis. Tamburini et al. (2014) has proposed that the change of the cell membrane permeability is the first event on the inactivation of Escherichia coli by pressurized CO2. The correlation between the cell membrane permeability and inactivation of S. cerevisiae and R. mucilaginosa by pressurized CO2 has been furthermore reported (Li et al. 2013; Spilimbergo et al. 2010a). On the other hand, it was estimated that a key factor in microbial inactivation by pressurized CO2 was the lowering of intracellular pH (pHin) (Spilimbergo et al. 2010b). Intracellular acidification of E. coli and S. cerevisiae by pressurized CO2 has been recognized (Watanabe et al. 2005). Giulitti et al. (2011) additionally demonstrated that both pHin-lowering and membrane permeability increase were important factors on microbial inactivation by pressurized CO2.
In recent years, extremely minute bubbles with diameters of 50 μm or less, known as microbubbles (MB), have gained attention in food science and agriculture. Compared to millibubbles that are normally generated using air pumps, MB rise more slowly, have extremely high solubility because they compress, dissolve and disappear in water (Takahashi et al. 2007a, b; Li et al. 2009a, b). Therefore, we designed an equipment in which MBCO2 was fed into liquid samples at pressure, and reported that yeast in unfiltered beer and hiochi bacteria in unpasteurized sake were inactivated by the MBCO2 (Kobayashi et al. 2014; Kobayashi and Odake 2015). In the inactivation mechanism of S. pastorianus by MBCO2, it was proposed that the inactivation occurred due to the denaturation of the intracellular components by multiple factors such as the temperature increase, the pHin-lowering and the action of CO2 molecules (Kobayashi and Odake 2018). However, these actions have not been verified yet.
The following agars have been used for detecting bacteria injured by sterilization treatment; an agar adding sodium chloride to an optimum agar (live bacteria that injured cell membrane cannot grow) and a minimum agar (live bacteria that induced a lack of permselectivity and complex auxotrophy by injuring cell membrane cannot grow) (Straka and Stokes 1959; Hurst et al. 1973). Furthermore, the fluorescence polarization (FP) has been used for measuring phase transition of the cell membrane (Liao et al. 2010), and it has been reported that alkaline phosphatase (AP), a periplasmic enzyme (Nikerson et al. 1948), activates in bacterial cells with changing the membrane fluidity by thermal treatment (Katsui et al. 1982; Tsuchido et al. 1985). In this study, to clarify the temperature-dependency on the inactivation and the phase transition of cell membrane of S. pastorianus by MBCO2, the incubation test with above agars, and the measurements of FP, AP activity and pHin were performed.
Materials and methods
Preparation of Saccharomyces pastorianus suspension
As with our previous report (Kobayashi and Odake 2017), the S. pastorianus suspension was prepared by addition of S. pastorianus cells (NBRC11027) in 10 L of physiological saline (PS) containing 5% ethanol concentration (approximately 1.0 × 106 CFU/mL).
The MBCO2 equipment and the treatment procedure
The MBCO2 treatment was performed using the same equipment as in a previous report (Kobayashi and Odake 2017) and at the following conditions: the temperature, pressure and exposure time in the mixing vessel were set to 10 °C, 1 or 2 MPa, and 5 min, respectively, and the temperature, pressure and exposure time in the heating coil were set to 29–43 °C, 4 MPa, and 1 min, respectively. The temperature in the heating coil increased until a 6-log reduction was achieved.
Thermal treatment
The thermal treatment was performed at 29–51 °C for 1 min using only the heating coil of MBCO2 equipment. The temperature during the thermal treatment increased until a 6-log reduction was achieved.
Measurement of the number of the surviving S. pastorianus cells
The number of surviving S. pastorianus cells was measured as follows: 1 mL of a sample or diluted sample was incubated with yeast and mould agar (YMA, Difco, Becton-Dickinson, Flanklin Lakes, NJ, an optimum agar), YMA adding 2.5 g/L sodium chloride (YMAS) and yeast nitrogen base agar (YNBA, Difco, a minimum agar) plates, respectively. The sodium chloride concentration of YMAS was decided to 2.5 g/L which was the maximum concentration that injured S. pastorianus cells can grow (data not shown). After incubating the plates at 25 °C for 48 h, the plates with 30–300 CFU were chosen, and the colonies were counted. If there were low numbers of viable cells, the colonies on the plates from the undiluted sample were counted (Kobayashi and Odake 2018). The detection limit was 1 CFU/mL.
Measurement of fluorescence polarization (FP) of S. pastorianus
The FP of S. pastorianus cells was measured by reference to the method of Liao et al. (2010). Forty microliters of 2.0 × 10−3 mol/L 1,6-diphenyl-1,3,5-hexatriene (DPH, Sigma-Aldrich Co., St. Louis, USA) solution (in tetrahydrofuran) was added to 10 mL of S. pastorianus suspension (1.0 × 109 CFU/mL). The mixture was incubated in a water bath at 30 °C for 1 h, washed twice by centrifugation (5 °C, 10,000×g, 30 min) with PS and re-suspended in 10 L of PS containing 5% ethanol concentration. The suspension was subjected to MBCO2 treatment and an aliquot of the treated suspension was measured with a fluorescence spectrophotometer (RF-5300PC, Shimadzu Co., Kyoto, Japan) at an excitation wavelength of 358 nm and an emission wavelength of 410 nm. The results were expressed as a relative ratio of FP calculated by the following equation:
| 1 |
Measurement of alkaline phosphatase (AP) activity of S. pastorianus
Briefly, 750 μL of the S. pastorianus suspension, concentrated tenfold by centrifugation (5 °C, 10,000×g, 30 min), was mixed with 750 μL of 7.0 × 10−3 mol/L p-nitrophenolphosphate solution (in 1.0 × 10−3 mol/L glycine buffer, pH 10.5) and incubated at 37 °C for 4 h. Then, 1.5 mL of 1 mol/L sodium hydroxide solution was added to the solution to stop the reaction and the absorbance at 405 nm was measured with a spectrophotometer (U-5100, Hitachi High-Tech Science Co., Tokyo, Japan). The results were expressed as a relative ratio of AP activity calculated by the following equation:
| 2 |
Measurement of intracellular pH of S. pastorianus
The intracellular pH (pHin) of S. pastorianus was measured with 5(6)-carboxyfluorescein diacetate succinimidyl ester (Dojindo Laboratories, Kumamoto, Japan) and a fluorescence spectrophotometer (RF-5300PC, Shimadzu Co.) according to the report of Kobayashi and Odake (2017).
Statistical analysis
All experiments were performed in triplicate. The data represent the means with standard errors of the results of the triplicate experiments. The significant differences were evaluated by the Tukey–Kramer test (p < 0.05).
Results and discussion
Inactivation efficiency
The inactivation of S. pastorianus by MBCO2 and thermal treatments is shown in Fig. 1. The effect of MBCO2 on the inactivation of S. pastorianus cells was significantly higher than that of thermal treatment and the number of surviving S. pastorianus cells decreased with increasing pressure in the mixing vessel or temperature in the heating coil of MBCO2, because a 6-log reduction was achieved at temperatures in the heating coil of 51, 43 and 37 °C at thermal treatment and MBCO2 at 1 and 2 MPa of pressure in the mixing vessel, respectively. The results showed the same tendency as in the previous reports (Garcia-Gonzalez et al. 2007; Kobayashi and Odake 2017, 2018). The number of surviving S. pastorianus cells treated by MBCO2 with the mixing vessel at 2 MPa decreased from the temperature in the heating coil of 33 and 35 °C by measurement with both YMAS and YNBA, and with YMA, respectively. In addition, inactivation of S. pastorianus by MBCO2 with the mixing vessel at 1 MPa occurred from the temperature in the heating coil of 39 and 41 °C by measurement with both YMAS and YNBA, and with YMA, respectively. Conversely, the number of surviving S. pastorianus cells after thermal treatment decreased from the temperature in the heating coil of 41 and 43 °C by measurement with YNBA, and with both YMAS and YMA, respectively. Previously, it was documented that the membrane damage of S. pastorianus cells was induced from the temperature more than 40 °C (Kobayashi and Odake 2017, 2018). Hong and Pyun (2001) noted that the number of surviving Lactobacillus plantarum cells treated with pressurized CO2 at 30 °C and 5 MPa measured with de Man Rogosa and Sharpe (MRS) agar was higher than that with MRS agar adding 3 g/L sodium chloride and more than 90% survivors has been injuring. In addition, Wu et al. (2007) measured the number of surviving E. coli cells treated by pressurized CO2 at 35 °C and 7.8 MPa by agar adding sodium chloride and suggested that more than 90% survivors on agar without sodium chloride were subjected to injury on the cell membrane. Therefore, it was confirmed that most of S. pastorianus cells was injured by MBCO2 at a temperature less than 40 °C and by thermal treatment at a temperature more than 40 °C.
Fig. 1.
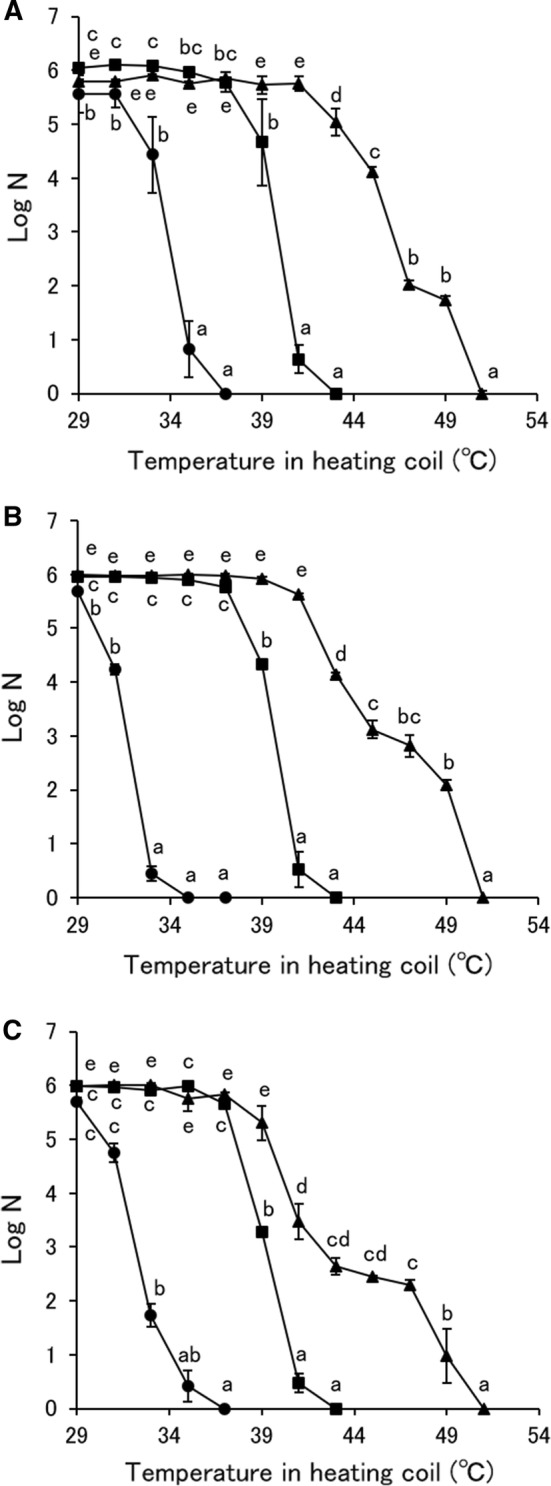
Inactivation of S. pastorianus cells by MBCO2 and thermal treatments. A Measurement by YMA,B measurement by YMAS,C measurement by YNBA. Triangle: thermal treatment, square: MBCO2 treatment with the mixing vessel pressure at 1 MPa, circle: MBCO2 treatment with the mixing vessel pressure at 2 MPa. Different letters indicate significant differences in each treatment by the Tukey–Kramer method (p < 0.05)
Fluorescence polarization (FP)
The FP of S. pastorianus cells after MBCO2 and thermal treatments is shown in Fig. 2. The FP of S. pastorianus cells treated by MBCO2 increased with increasing the temperature in the heating coil, equally at pressure in the mixing vessel of 1 and 2 MPa. On the other hand, the FP of S. pastorianus cells increased significantly from 41 °C by thermal treatment. The gel-fluid phase transition of aqueous dipalmitoylphosphatidylcholine liposomes occurred at 42 °C (Bothun et al. 2005). Temperature increase will decrease the physical stability of the membrane, adopting a crystalline liquid appearance (Barba et al. 2017). Therefore, MBCO2 caused the phase transition of the cell membrane of S. pastorianus. Liao et al. (2010) and Li et al. (2013) recognized that pressurized CO2 increased FP of E. coli and S. cerevisiae cells measured with DPH, indicating the ridigity increase and the fluidity decrease of the cell membrane. The change in FP induced by MBCO2 with the mixing vessel at 2 MPa agreed with the number of surviving S. pastorianus cells measured with YMAS and YNBA but not at 1 MPa. Therefore, it was revealed that membrane fluidity of S. pastorianus cells was influenced by thermal treatment at a temperature higher than 40 °C but became sensitive to less than 40 °C by using the MBCO2.
Fig. 2.
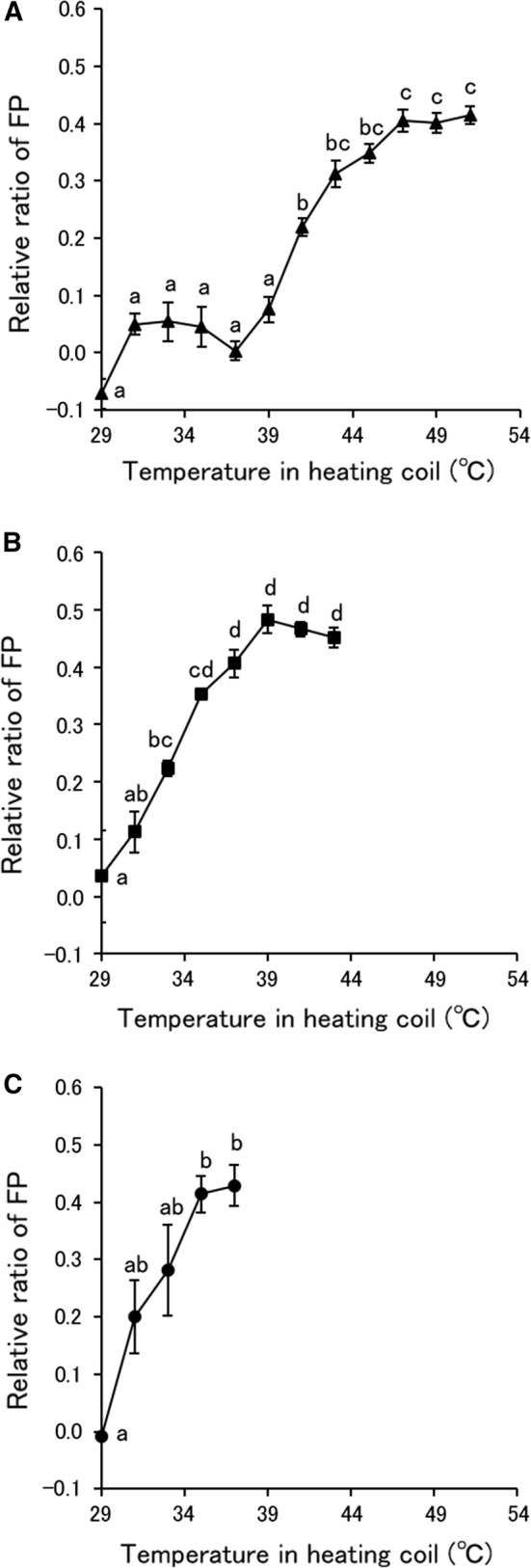
Fluorescence polarization (FP) of S. pastorianus cells treated by MBCO2 and thermal treatments. A Thermal treatment, B MBCO2 treatment with the mixing vessel pressure at 1 MPa, C MBCO2 treatment with the mixing vessel pressure at 2 MPa. Different letters indicate significant differences in each treatment by the Tukey–Kramer method (p < 0.05)
Alkaline phosphatase (AP) activity
The AP activity of S. pastorianus cells after MBCO2 and thermal treatments is shown in Fig. 3. The AP activity of S. pastorianus cells increased gradually from 39 °C by thermal treatment, reached a maximum at 47 °C and then decreased drastically with further increasing temperature. On the other hand, the AP activity of S. pastorianus cells treated by MBCO2 with the mixing vessel at 1 MPa increased with increasing temperature in the heating coil, peaked at 39 °C and then decreased with further increasing temperature. Conversely, AP activity of S. pastorianus cells treated by MBCO2 with the mixing vessel at 2 MPa showed a slight increase at the temperature in the heating coil of 31 °C, and significantly decreased by further increasing temperature. Therefore, it was recognized that the AP activity of S. pastorianus cells increased by MBCO2 and thermal treatments correlated highly with the FP but decreased by further increasing temperature. The AP activity of S. pastorianus cells after MBCO2 and thermal treatments might not be associated with the inactivation efficiency, because the AP activity reached a maximum when the survivors decreased. The correlativity between inactivation of AP and bacteria by pressurized CO2 has been previously reported (Li et al. 2012; Bertoloni et al. 2006). Bertoloni et al. (2006) also demonstrated that the release of AP from E. coli caused by pressurized CO2 led to the reduction in the enzyme activity. In contrast, Kim et al. (2007) reported that the AP of E. coli was activated by pressurized CO2. The AP of S. pastorianus may be activated due to the disengagement from the periplasm (Nikerson et al. 1948), associated with the change in the cell membrane fluidity by pasteurization (Tsuchido et al. 1985). It was also considered that inactivation of AP by MBCO2 with the mixing vessel at 2 MPa could be achieved at a lower temperature than that at 1 MPa because of the greater effect on enzyme inactivation with higher pressure.
Fig. 3.
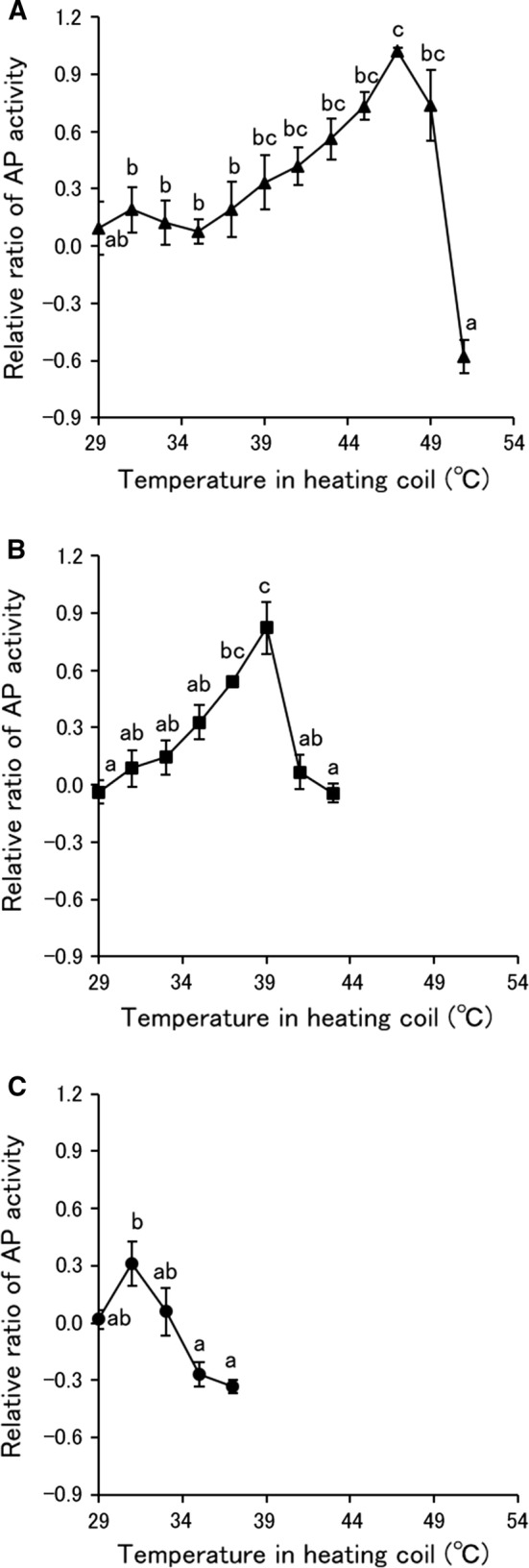
Relative ratio of alkaline phosphatase (AP) activity of S. pastorianus cells treated by MBCO2 and thermal treatments. A Thermal treatment, B MBCO2 treatment with the mixing vessel pressure at 1 MPa, C MBCO2 treatment with the mixing vessel pressure at 2 MPa. Different letters indicate significant differences in each treatment by the Tukey–Kramer method (p < 0.05)
Intracellular pH (pHin)
The pHin of S. pastorianus treated with MBCO2 is shown in Fig. 4. The pHin of S. pastorianus treated by MBCO2 with the mixing vessel at 1 and 2 MPa decreased significantly from the temperature in the heating coil of 43 and 33 °C, respectively. In the MBCO2 with the mixing vessel at 2 MPa, the pHin-lowering was consistent with the decrease in the survivors of S. pastorianus measured with YMA. Therefore, this result sustained the previous statement that inactivation of S. pastorianus by MBCO2 at 40 °C might be due to intracellular acidification (Kobayashi and Odake 2017), or it was considered that the pHin-lowering resulted from the penetration of dissolved CO2 or extracellular H+ due to the change in cell membrane fluidity associated with the death of S. pastorianus by MBCO2. Conversely, the pHin-lowering was a main factor of the inactivation of S. pastorianus by MBCO2 with the mixing vessel at 1 MPa and was caused due to only the temperature increase to more than 40 °C according to a previous report (Kobayashi and Odake 2018), because the temperature in the heating coil for inactivating S. pastorianus was lower than that for lowering the pHin. The relationship between pHin and temperature increase on the microbial inactivation by pressurized CO2 have been never reported besides our studies. Watanabe et al. (2005) also indicated the penetration of extracellular H+ into S. cerevisiae cells by pressurized CO2. Therefore, the marked injury of the cell membrane of S. pastorianus induced by MBCO2 with the mixing vessel at 2 MPa allowed extracellular H+ to penetrate inside the cells but at 1 MPa was not as severe as to cause the penetration of extracellular H+.
Fig. 4.
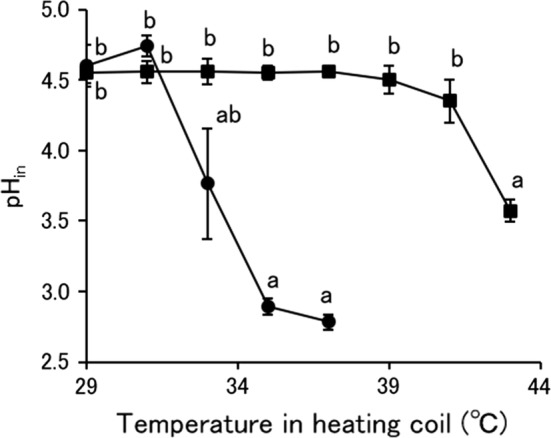
Intracellular pH (pHin) of S. pastorianus treated by MBCO2. Square: MBCO2 treatment with the mixing vessel pressure at 1 MPa, circle: MBCO2 treatment with the mixing vessel pressure at 2 MPa. Different letters indicate significant differences in each treatment by the Tukey–Kramer method (p < 0.05)
Conclusion
In this study, the change of the cell membrane fluidity of S. pastorianus by MBCO2 was revealed to be caused at lower temperature than thermal treatment and considered to be due to the dissolved CO2. It suggested that the extent differed between the pressures in the mixing vessel at 1 and 2 MPa, because the penetration of extracellular H+ inside the cells was induced by MBCO2 with the mixing vessel at 2 MPa but not 1 MPa.
Acknowledgements
We thank Riho Takanashi and Yuhei Kameda of the Faculty of Applied Life Science, Nippon Veterinary and Life Science University (Musashino, Japan) for experimental assistance. A part of this study was financially supported by Mishima Kaiun Memorial Foundation (Tokyo, Japan) in 2016.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fumiyuki Kobayashi, Email: fkoba@nvlu.ac.jp.
Sachiko Odake, Email: odake@nvlu.ac.jp.
References
- Amaral GV, Silva EK, Cavalcanti RN, Cappato LP, Guimaraes JT, Alvarenga VO, Esmerino EA, Portela JB, Sant’Ana AS, Freitas MQ, Silva MC, Raices RSL, Meireles MAA, Cruz AG. Dairy processing using supercritical carbon dioxide technology: theoretical fundamentals, quality and safety aspects. Trends Food Sci Technol. 2017;64:94–101. doi: 10.1016/j.tifs.2017.04.004. [DOI] [Google Scholar]
- Barba FJ, Koubaa M, Prado-Silva L, Orlien V, Sant’Ana AS. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: a review. Trends Food Sci Technol. 2017;66:20–35. doi: 10.1016/j.tifs.2017.05.011. [DOI] [Google Scholar]
- Bertoloni G, Bertucco A, de Cian V, Parton T. A study on the inactivation of micro-organisms and enzymes by high pressure CO2. Biotechnol Bioeng. 2006;95:155–160. doi: 10.1002/bit.21006. [DOI] [PubMed] [Google Scholar]
- Bothun GD, Knutson BL, Strobel HJ, Nokes SE. Liposome fluidization and melting point depression by pressurized CO2 determined by fluorescence anisotropy. Langmuir. 2005;21:530–536. doi: 10.1021/la0496542. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez L, Geeraerd AH, Spilimbergo S, Elst K, Van Ginneken L, Debevere L, Van Impe JF, Devlieghere F. High pressure carbon dioxide inactivation of microorganisms in foods: the past, the present and the future. Int J Food Microbiol. 2007;117:1–28. doi: 10.1016/j.ijfoodmicro.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Giulitti S, Cinquemani C, Quaranta A, Spilimbergo S. Real time intracellular pH dynamics in Listeria innocua under CO2 and N2O pressure. J Supercrit Fluids. 2011;58:385–390. doi: 10.1016/j.supflu.2011.07.012. [DOI] [Google Scholar]
- Guimarāes JT, Silva EK, de Freitas MQ, Meireles MAA, de Cruz AG. Non-thermal emerging technologies and their effects on the functional properties of dairy products. Curr Opin Food Sci. 2018;22:62–66. doi: 10.1016/j.cofs.2018.01.015. [DOI] [Google Scholar]
- Hong SI, Pyun YR. Membrane damage and enzyme inactivation of Lactobacillus plantarum by high pressure CO2 treatment. Int J Food Microbiol. 2001;63:19–28. doi: 10.1016/S0168-1605(00)00393-7. [DOI] [PubMed] [Google Scholar]
- Howladar MS, French WT, Shields-Menard SA, Amirsadeghi M, Green M, Rai N. Microbial cell disruption for improving lipid recovery using pressurized CO2: role of CO2 solubility in cell suspension, sugar broth, and spent media. Biotechnol Prog. 2017;33:737–748. doi: 10.1002/btpr.2471. [DOI] [PubMed] [Google Scholar]
- Hurst A, Hughes A, Beare-Rogers JL, Collins-Thompson DL. Physiological studies on the recovery of salt tolerance by Staphylococcus aureus after sublethal heating. J Bacteriol. 1973;116:901–907. doi: 10.1128/JB.116.2.901-907.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsui N, Tsuchido T, Hiramatsu R, Fujikawa S, Takano M, Shibasaki I. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J Bacteriol. 1982;151:1523–1531. doi: 10.1128/JB.151.3.1523-1531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Rhee MS, Kim BC, Kim KH. Modeling the inactivation of Escherichia coli O157:H7 and generic Escherichia coli by supercritical carbon dioxide. Int J Food Microbiol. 2007;118:52–61. doi: 10.1016/j.ijfoodmicro.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Odake S. Quality evaluation of unfiltered beer as affected by inactivated yeast using two-stage system of low pressure carbon dioxide microbubbles. Food Bioprocess Technol. 2015;8:1690–1698. doi: 10.1007/s11947-015-1530-z. [DOI] [Google Scholar]
- Kobayashi F, Odake S. Intracellular acidification and change of cellular membrane fluidity of Saccharomyces pastorianus by low pressure CO2 microbubbles. Food Cont. 2017;71:360–370. doi: 10.1016/j.foodcont.2016.07.023. [DOI] [Google Scholar]
- Kobayashi F, Odake S. The relationship between intracellular acidification and inactivation of Saccharomyces pastorianus by a two-stage system with pressurized carbon dioxide microbubbles. Biochem Eng J. 2018;134:88–93. doi: 10.1016/j.bej.2018.03.011. [DOI] [Google Scholar]
- Kobayashi F, Ikeura H, Odake S, Sakurai H. Quality evaluation of sake treated with a two-stage system of low pressure carbon dioxide microbubbles. J Agric Food Chem. 2014;62:11722–11729. doi: 10.1021/jf5038618. [DOI] [PubMed] [Google Scholar]
- Li P, Takahashi M, Chiba K. Enhanced free-radical generation by shrinking microbubbles using a copper catalyst. Chemosphere. 2009;77:1157–1160. doi: 10.1016/j.chemosphere.2009.07.062. [DOI] [PubMed] [Google Scholar]
- Li P, Takahashi M, Chiba K. Degradation of phenol by the collapse of microbubbles. Chemosphere. 2009;77:1371–1375. doi: 10.1016/j.chemosphere.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Li H, Deng L, Chen Y, Liao X. Inactivation, morphology, interior structure and enzymatic activity of high pressure CO2-treated Saccharomyces cerevisiae. Innov Food Sci Emerg Technol. 2012;14:99–106. doi: 10.1016/j.ifset.2011.11.009. [DOI] [Google Scholar]
- Li J, Wang A, Zhu F, Xu R, Hu S. Membrane damage induced by supercritical carbon dioxide in Rhodotorula mucilaginosa. Ind J Microbiol. 2013;53:352–358. doi: 10.1007/s12088-013-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Zhang F, Liao X, Hu X, Chen Y, Deng L. Analysis of Escherichia coli cell damage induced by HPCD using microscopies and fluorescent staining. Int J Food Microbiol. 2010;144:169–176. doi: 10.1016/j.ijfoodmicro.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Nikerson WJ, Krugelis EJ, Andresen N. Localization of alkaline phosphatase in yeast. Nature. 1948;162:192–193. doi: 10.1038/162192a0. [DOI] [PubMed] [Google Scholar]
- Silva EK, Alvarenga VO, Bargas MA, Sant’Ana AS, Meireles MAA. Non-thermal microbial inactivation by using supercritical carbon dioxide: synergic effect of process parameters. J Supercrit Fluids. 2018;139:97–104. doi: 10.1016/j.supflu.2018.05.013. [DOI] [Google Scholar]
- Spilimbergo S, Foladori P, Mantoan D, Ziglio G, Della Mea G. High-pressure CO2 inactivation and induced damage on Saccharomyces cerevisiae evaluated by flow cytometry. Process Biochem. 2010;45:647–654. doi: 10.1016/j.procbio.2009.12.013. [DOI] [Google Scholar]
- Spilimbergo S, Quaranta A, Garcia-Gonzalez L, Contrini C, Cinquemani C, Van Ginneken L. Intracellular pH measurement during high-pressure CO2 pasteurization evaluated by cell fluorescent staining. J Supercrit Fluids. 2010;53:185–191. doi: 10.1016/j.supflu.2010.03.004. [DOI] [Google Scholar]
- Straka RP, Stokes JL. Metabolic injury to bacteria at low temperature. J Bacteriol. 1959;78:181–185. doi: 10.1128/JB.78.2.181-185.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Chiba K, Li P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J Phys Chem B. 2007;111:1343–1347. doi: 10.1021/jp0669254. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Chiba K, Li P. Formation of hydroxyl radicals by collapsing ozone microbubbles under strongly acidic conditions. J. Phys Chem B. 2007;111:11443–11446. doi: 10.1021/jp074727m. [DOI] [PubMed] [Google Scholar]
- Tamburini S, Anesi A, Ferrentino G, Spilimbergo S, Guella G, Jousson O. Supercritical CO2 induced marked changes in membrane phospholipids composition in Escherichia coli K12. J Membr Biol. 2014;247:469–477. doi: 10.1007/s00232-014-9653-0. [DOI] [PubMed] [Google Scholar]
- Tsuchido T, Katsui N, Takeuchi A, Takano M, Shibasaki I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl Environ Microbiol. 1985;50:298–303. doi: 10.1128/AEM.50.2.298-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Furukawa S, Kitamoto K, Takatsuki A, Hirata R, Ogihara H, Yamasaki M. Vacuolar H+-ATPase and plasma membrane H+-ATPase contribute to the tolerance against high-pressure carbon dioxide treatment in Saccharomyces cerevisiae. Int J Food Microbiol. 2005;105:131–137. doi: 10.1016/j.ijfoodmicro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yao SJ, Guan YX. Inactivation of microoganisms in carbon dioxide at elevated pressure and ambient temperature. Ind Eng Chem Res. 2007;46:6345–6352. doi: 10.1021/ie0702330. [DOI] [Google Scholar]


