Abstract
Uncommon tropical fruits are emerging as raw-material for new food products with health benefits. This work aimed at formulating and processing microemulsions from pitanga (Eugenia uniflora) and buriti (Mauritia flexuosa) fruits, since they are very rich in carotenoids (particularly lycopene and β-carotene), in order to encapsulate and increase carotenoids’ bioaccessibility. Pitanga and buriti microemulsions were produced by applying a direct processing (high-speed homogenization at 15,000 rpm and ultrasound with 20 kHz probe at 40% amplitude) of the whole pulp together with surfactant (Tween 80 or Whey Protein Isolate at 2%) and corn oil (5%). All treatments (HSH—US for 0–4, 4–0, 4–4, 4–8 min–min) applied were able to increase the amount of carotenoid released. However, the processing also decreased the total amount of carotenoids in the whole pulp of studied fruits. The impact of processing during microemulsion production was not severe. The overall data suggest that the presence of surfactant and oil during processing may protect the carotenoids in fruits and microemulsions. Final recovery of total carotenoids, after passing the samples through a dynamic gastrointestinal system that simulates the human digestion, was higher for microemulsions than for whole pulps. High losses of total carotenoids in buriti and β-carotene and lycopene in pitanga occurred during jejunum and ileum phases. The present work confirms that it is possible to increase β-carotene and lycopene bioaccessibility from fruits by directly processing microemulsions (p < 0.01).
Keywords: Dynamic digestive system, Tropical fruits, Bioavailability, Beta-carotene, Lycopene, Structure design
Introduction
There is a growing demand for plant based products that promote health and well-being. Thereby, fruits represent the most popular option for new products due to their bioactive compounds composition (e.g. carotenoids). Carotenoids are very present in many common foods and their ingestion provides vitamin A, that offers benefits to consumers, such as protection for the eyes, improvement in vision health, avoidance of abortion, promotion of the immune system and reduction of the overall risk of chronic diseases (Saini et al. 2015).
The biodiversity of Brazilian native fruits can be a rich source of innovation and opportunities, growth of income, value-adding and scientific contribution. Pitanga (Eugenia uniflora) belongs to the botanic family of Myrtaceae, its trees grow on tropical and subtropical regions, and it is originally from Brazilian restinga. The fruit is very valued due to its strong red color, juicy pulp, unique aroma, sour and sweet taste. The northeastern region of Brazil has the largest production that is basically commercialized in natura or processed as fruit pulp. Pitanga has a very high concentration of carotenoids—mainly lycopene in concentration levels around 70 µg/g of fresh pulp (Porcu and Rodriguez-Amaya 2008). Buriti is a palm fruit from Arecaceae family, it naturally grows on amazônia, caatinga and cerrado biomes. Buriti fruit is very rich in lipids, around 20% of fresh pulp, which are extracted and commercialized as crude or refined oil. Usually, buriti oil is consumed in foods or applied in cosmetics due to its healing properties promoted by the high concentration of carotenoids, tocopherol and fatty acids. Probably buriti is the plant with the highest content of all-trans-β-carotene in Nature, reaching levels of 373 µg/g of whole pulp (de Rosso and Mercadante 2007).
The preserving techniques, processing, storage and final product matrix/formulation have a great impact on stability and bioaccessibility of carotenoids, and consequently on the intended health benefits (Failla et al. 2014). Carotenoid bioaccessibility is defined as the portion of carotenoids that are ready for being absorbed by intestinal cells after food digestion (Failla and Chitchumroonchokchai 2005). Once carotenoids are released from plant cells, they are subjected to structural modifications and degradation that can impair bioaccessibility. Therefore, processing implies changes in the food matrix that causes two main effects: on one hand carotenoids can be degraded losing their health effect but, on the other hand, bioaccessibility can be improved/impaired due to structural changes. Indeed, losses of bioactive compounds and/or increase in bioaccessibility due to processing are often reported (Pugliese et al. 2013; Failla et al. 2014; Anese et al. 2015).
Carotenoid bioaccessibility in foods is very low, but it is possible to strategically formulate and process them in order to increase their release, bioaccessibility and consequently their health benefits (Buggenhout et al. 2012). For instance, bioaccessibility of β-carotene, lutein and lycopene were augmented due to cooking processes such as frying (Berni et al. 2014), microemulsion addition (Li et al. 2017) or optimal combination of adequate process/formulation (Buggenhout et al. 2012), respectively. Several techniques combine structure design approaches and/or delivery systems engineering that aim at protecting, preserving, enhancing solubility, increasing bioaccessibility and bioactivity of lipophilic bioactive compounds. The most studied and applied strategy is the encapsulation of lipophilic bioactive compounds in emulsion-based systems at the micro and nano scales (McClements 2015).
Therefore, our approach attempts to manipulate structure and composition of the fruit matrix in order to encapsulate carotenoids leading to their higher retention and improved bioaccessibility. We produced a microemulsion-based product by directly processing fruit pulps of pitanga and buriti together with a surfactant that stabilized the whole system. Our hypothesis was that an adequate combination of process and formulation would transfer the carotenoids from the matrix to lipid microcapsules while protecting the carotenoids and increasing their bioaccessibility. The present paper focuses on carotenoid contents, retention, the efficiency of encapsulation and bioaccessibility throughout an in vitro dynamic digestive system. Data also show the effect of two different types of surfactants: Tween 80 (T80) and Whey Protein Isolate (WPI), and processing by high-speed homogenization (HSH) and ultrasounds (US) on carotenoid behavior over emulsion formation.
Materials and methods
Pitanga and buriti fruit pulp
Buriti pulp was commercialized by the co-op network Central do Cerrado, Brasilia, Distrito Federal, Brazil. Fruits were soaked in drinkable water, 3 parts of water to 1 part of fruit (v/v), during 12 h, and pulp was taken off by a crushing machine for pulps (Bonina®, 0,5 mm pore size). Pitanga pulp was acquired from the tropical fruits company Sítio do Bello, Paraibuna, São Paulo, Brazil. Red ripe pitanga was harvested, selected, sanitized and pulped at Sitio do Bello using a crushing machine for pulps. There was no water added and seeds were separated during the processing of pitanga. Pulps were freeze–dried, packed in vacuum-sealed bags and then shipped by plane to Braga, Portugal, where they were stored frozen at − 26 °C until the time of conducting the experiments. Dried samples of lyophilized pitanga and buriti pulp were re-suspended in distilled water until the final water content in whole pulp be standardized to 93% of wet weight. After this equalization, final natural occurring lipids contents in whole pulp were 0.1% and 3.64% respectively for pitanga and buriti.
Reagents
Commercial standards of β-carotene, lycopene and enzymes for the dynamic in vitro digestion were purchased from Sigma-Aldrich (St Louis, MO, USA). Organic solvents for extraction and HPLC analysis were chromatographic grade (Chromasolv™, Muskegon, MI, USA). Surfactant Tween80 (T80) was purchased from Panreac AppliChem (Germany) and Whey Protein Isolate (WPI) from Arla Foods (Denmark). Corn oil (Fula®, Sovena, Portugal) was used without further purification.
Microscopy
Brightfield images were analyzed using an epifluorescence microscope (Olympus BX51) coupled with a DP71 digital camera (Olympus Portugal SA, Porto, Portugal). All images were acquired using the Olympus cellSens soſtware. Fluorescence images were acquired using a Confocal Scanning Laser Microscope (CLSM) (Olympus BX61, Model FluoView 1000). Calcofluor white (Sigma-Aldrich, EUA) was used for staining the cellulose fibers and Nile Red (TCI, Tokyo, Japan) stain for detection of lipid droplets. According to Kilcrease et al. (2013) carotenoids have autofluorescence that indicate their location. Images were acquired with the program FV10-Ver4.1.1.5 (Olympus).
Microemulsion formulation and processing
For releasing the carotenoids from fruit chromoplasts, samples were submitted for processing by a high-speed homogenization (HSH) equipment (Ultra-Turrax® homogenizer, T 25, Ika-Werke, Germany) at 15,000 rpm during 0 min and 4 min combined with ultrasound (US) treatment with a 20 kHz probe (Vibra-Cell™, Sonics®, EUA), 40% amplitude during 0, 4 and 8 min. The samples were processed in 50 mL centrifuge tubes and kept inside ice-bath all the time to avoid overheating and carotenoid degradation. To assess treatment impact, total carotenoids, as well β-carotene and lycopene were analyzed in whole, processed, and in filtered pulp after processing (stainless steel sieves, particle size < 100 µm).
Microemulsion formulation was based on the work of Zhang et al. (2015) and Salvia-Trujillo and McClements (2016). Oil-in-water (O/W) microemulsions were produced by processing together fruit pulp, surfactant (T80 or WPI), corn oil and water at equal proportions for both fruits. Briefly, microemulsions were made by preparing surfactant solution (T80 or WPI) in distilled water and then mixing with whole pulp and corn oil. Final microemulsion product was composed of 5% corn oil, 91% distilled water, 2% of surfactant and 2% of pulp (in dry basis). Processing parameters for microemulsion and the selected one for the dynamic gastrointestinal system study were chosen based on previous data of total carotenoids, rheological behavior, and on microemulsion stability during storage. Mixtures were first processed by HSH (varying 15,000 rpm during 4 min) and immediately taken to US sonication (20 kHz probe, 40% amplitude, varying time). Both probes for HSH and US processing were placed at sample geometric center. Samples were kept all the time in ice inside a Styrofoam box to avoid heat and light.
Dynamic gastrointestinal system
A dynamic gastrointestinal system equipment and protocol, described in Pinheiro et al. (2013), was used to evaluate carotenoids stability to digestion and bioaccessibility when compared in natura samples and microemulsions. This model simulates the main events that occur during digestion—i.e. simulation of stomach, duodenum, jejunum and ileum digestions by separated reactors. The bioaccessible phase is separated by filtration through hollow-fibre devices (SpectrumLabs Minikros®, M20S-100-01P, USA). Gastric and intestinal secretions were freshly prepared and secreted inside the reactors by syringe pumps at pre-set flow rates (Pinheiro et al. 2013). The simulation of digestions was done in triplicate.
Carotenoid extraction for analysis
For carotenoid assessment aliquots were taken and had their carotenoid extracted and quantified using a small scale method based on Amorim-Carrilho et al. (2014) review, briefly described as follows. Aliquots (50–300 µL for initial and processed samples or 1–5 mL for digested samples) were vigorously mixed in the vortex for 1 min with 1.5 mL of acetone/ethanol/hexane (50/25/25 v/v/v) solution containing 0.01% of BHT. Distilled water was added until 2 mL of final volume and centrifuged for 3 min at 1000 rpm. Upper phase containing carotenoids were collected and then dried under nitrogen flow, resuspended in petroleum ether (for spectrophotometric measurement of total carotenoids) or in methanol/MTBE (50/50 v/v) for HPLC–DAD analysis of β-carotene and lycopene. To evaluate retention and release of carotenoids due to processing, samples were analyzed: initially, i.e. the control sample; in the HSH and US processed fruit pulp; and after filtering throughout 100 µm cutoff sieve, that estimates free carotenoids. Microemulsions were analyzed freshly and in the upper phase after centrifuging for 5 min at 8385 × g, that allowed to estimate efficiency of encapsulation, since un-encapsulated carotenoids are attached to cell fibers in pellets. Each step of dynamic digestion simulation had the carotenoids contents measured to evaluate stability of carotenoids to digestion and final bioaccessibility.
Carotenoid analysis
In a 96 well microplate reader (Synergy™ HT, Bio-tek®), 300 µL of extracts, as well as β-carotene standard curves, had absorbance measured at 450 nm for total carotenoids analysis. Chromatographic analysis of carotenoid is presented in Berni et al. (2014). Briefly, the HPLC–DAD system used was a Shimadzu® Nexera X2 (modules: degasser DGU 20A 5R, pump LC-30AD, autosampler SIL-30AC, oven CTO-20AC and detector DAD SPD-M20A) with a polymeric YMC™ C30 (150 mm × 4.6 mm, 5 µm particle size). The mobile phase was methanol and MTBE at 90:10 (v/v) to 40:60 in 60 min. Identification and quantification of β-carotene and lycopene was based on commercial standards. Chromatograms where taken at fixed wavelength of 450 nm for β-carotene and 470 nm for lycopene.
Statistics
Experimental data were analyzed for significant differences using ANOVA, means were compared using the Tukey test and significance levels (p < 0.01 or p < 0.05) are mentioned in the figures or text. Software used for the surface design, ANOVA and Tukey tests was Statistica 13© (Dell Inc.).
Results
Carotenoid release and impact of processing
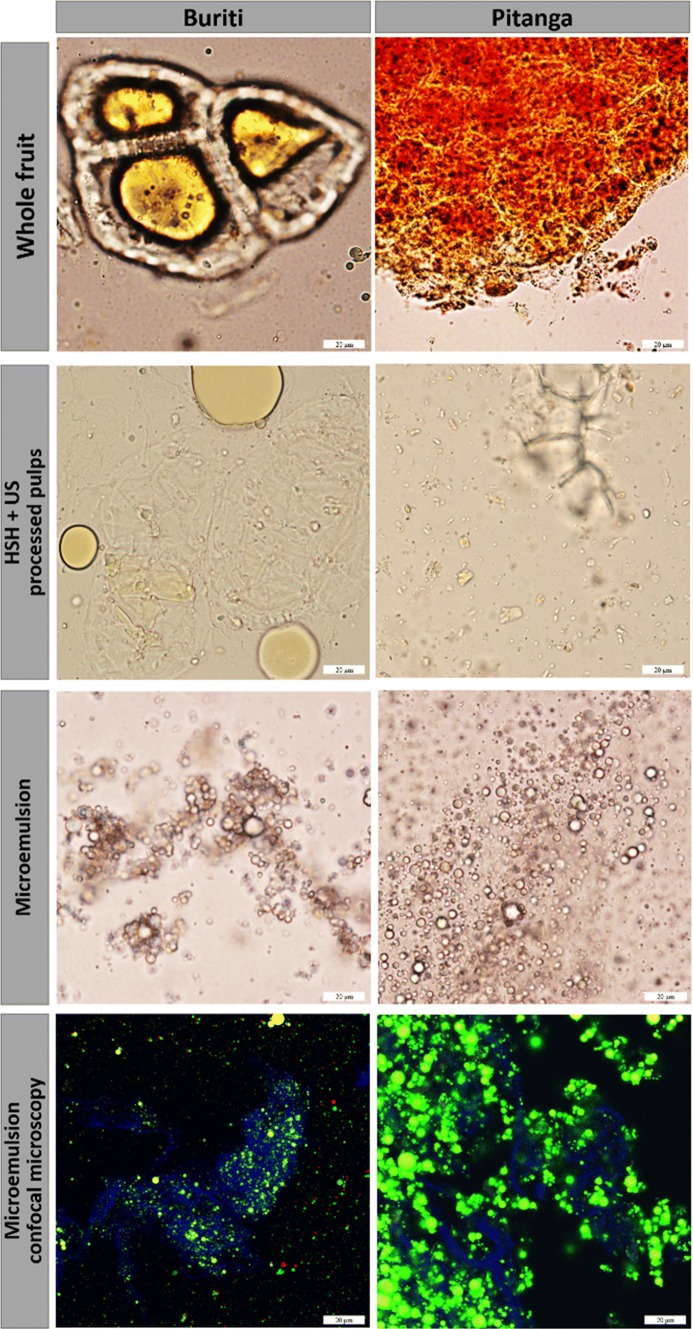
For the purpose of producing a microemulsion while transferring the carotenoids from pitanga and buriti to the oil droplets, the main obstacle is to release the carotenoids as much as possible. An experiment was carried out to test carotenoid release and establish the parameters for the microemulsion production. Results of total carotenoid release and retention after processing are presented in Fig. 1. The observed behavior is that all treatments applied are able to increase the ratio of free carotenoid/initial carotenoid, i.e. carotenoids’ release. However, at the same time processing decreases the total amount of carotenoids from studied fruits. All treatments tested, especially when combining HSH–US, were successfully capable to break cells and release its intracellular content as can be seen by empty cells and small fragments observed in Fig. 2. After testing different treatments, it was chosen to discard the HSH without US (T-U 4′–0′) process because the results of carotenoids release are unexpressive, and the microemulsion formation in further tests was not homogeneous. Images very similar to ours are reported in Anese et al. (2015) using US treatment at 24 kHz for releasing lycopene crystalloids from tomatoes.
Fig. 1.
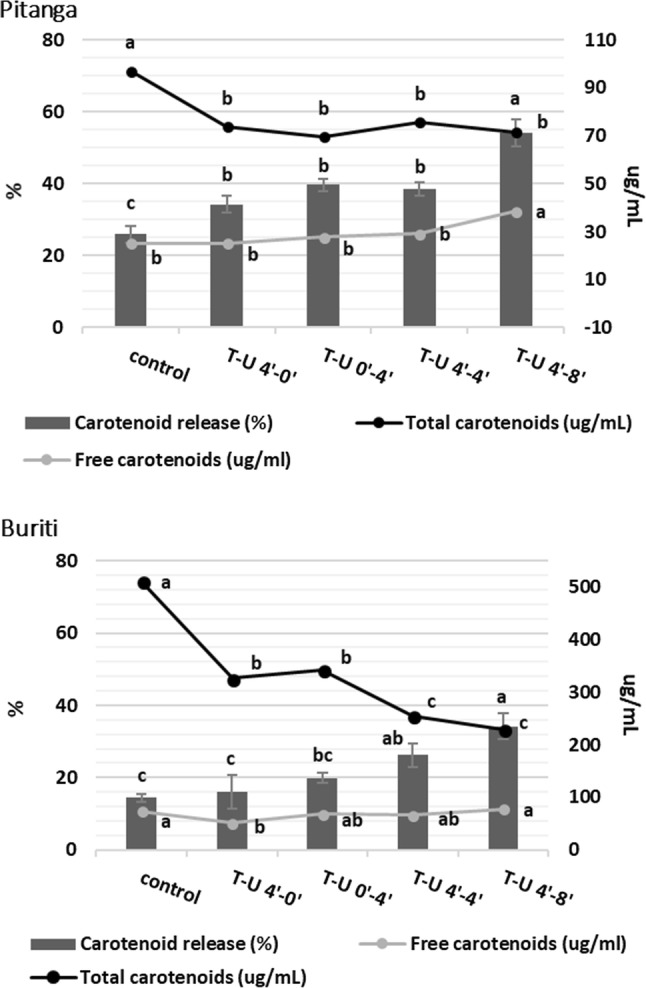
Evaluation of carotenoid release and impact of processing pitanga and buriti whole pulps by HSH and US. Results are total carotenoids determined spectrophotometrically. Acronyms meaning treatments HSH–US (T-U) and time of processing (min–min). Different letters for the same series represents significant differences between treatments (p < 0.05)
Fig. 2.
Microemulsion formation: carotenoid location in the cell; carotenoid release; formed microemulsion (T80, 4 min of HSH and 4 min of US); microemulsion (Nile red stained and carotenoid autofluorescence) interaction with fruit cellulose (calcofluor white stained)
Carotenoids in plants are stored inside chromoplasts that vary in shape and size related to their carotenoid composition (Kilcrease et al. 2013; Saini et al. 2015), and are stored in two main forms, lipid-dissolved or liquid-crystalline (Schweiggert et al. 2012). Images from light microscopy on Fig. 2 show these differences in buriti (lipid-dissolved) and in pitanga (liquid-crystalline), as well the carotenoid entrapment inside cellular structures. In some fruits like mango and papaya, β-carotene and xanthophylls are in the both states, lipid-dissolved and liquid-crystalline, together with crystalloid lycopene (not easily seen by light microscopy), all inside of globular chromoplasts (Schweiggert et al. 2012). This seems to be the case of pitanga that is rich in lycopene, β-carotene and xanthophylls (Porcu and Rodriguez-Amaya 2008). The entrapment of carotenoids is numerically perceived in Fig. 1 by the data of carotenoid release from control samples. Pitanga whole pulp has around 25% of its carotenoids released from cellular walls while buriti has only 15%. In the whole pulp, the free carotenoids exist due to pulp’s taking of, and are dispersed in the aqueous fraction inside lipid droplets or as crystalloids.
The processing goal was to get the highest content of free carotenoids. Only HSH (15,000 rpm, 4 min) followed by US (20 kHz, 40% amplitude, 8 min) in pitanga presented higher quantity of free carotenoids compared to control (Fig. 1). The extent of this increment is low (14 µg/mL) despite statistically significant. The food matrix has a protection rule on retention, but once carotenoids are released they are more subject of degradation and environmental stresses. Carail et al. (2015) investigated the kinetics of all-trans-β-carotene degradation due to US processing (20 kHz, same used in our work), and they demonstrated that degradation is high in the presence of water and oxygen, and have more influence of time of exposure than US intensity.
Microemulsion development
To produce the microemulsion it was applied a direct processing of the whole pulp together with surfactant (T80 or WPI at 2% of final weight) and corn oil (5% of final weight). Figure 2 shows the microemulsion formed due to surfactant action that stabilizes the system. Many works have demonstrated the efficacy of US to break cell walls and improve carotenoid extraction to oil. For example, Goula et al. (2017) were able to extract up to 93.8% of total carotenoids from pomegranate peel using US-assisted extraction with sunflower oil as extracting solvent, i.e. breaking cellular walls and transferring carotenoid to oil. Also, US is an effective tool for producing stable microemulsions of β-carotene in oil (de Paz et al. 2013; Kentish and Feng 2014).
CLSM images (Fig. 2) show the microstructure formed. The CLSM micrograph (Fig. 2) reveals this gel-like structure since the oil droplets are stained with Nile red, that combined with green autofluorescence from carotenoids, exhibits a green circle with a yellow interior. Calcofluor white stained the cellulose fibers from the fruit matrix, that is seen as a blue net binding the oil droplets and forms agglomerates dispersed in the aqueous phase. This outline of fluorescence is a strong evidence that confirms our hypothesis that through direct processing of the whole pulp, surfactant and oil it is possible to release, transfer and encapsulate carotenoids from fruits inside oil droplets. A study of the interaction between microemulsions made with different surfactants (sodium caseinate, Tween 80 or lactoferrin) with different levels of low methoxy pectin showed the occurrence of oil droplets aggregation, flocculation and gel-like structures by CLSM images, very similar to the ones presented in this paper (Zhang et al. 2015). This gel-forming capacity due to surfactant interaction with polysaccharides was also demonstrated in sodium caseinate oil-in-water emulsions with cellulose, exploring the cellulose for increasing the whole system stability (Hu et al. 2016). Micro- and nanoemulsions have been extensively explored as delivery systems for carotenoids such as lutein, lycopene, β-carotene and total carotenoids (Davidov-Pardo et al. 2016, McClements and Gumus 2016; Zhang et al. 2016; Salvia-Trujillo and McClements 2016; Liu et al. 2015). However, to the best of our knowledge, this is the first time that a microemulsion is produced by directly processing surfactant, oil and carotenoid-rich fruit pulp aiming at encapsulating carotenoids and increasing bioaccessibility of lycopene and β-carotene.
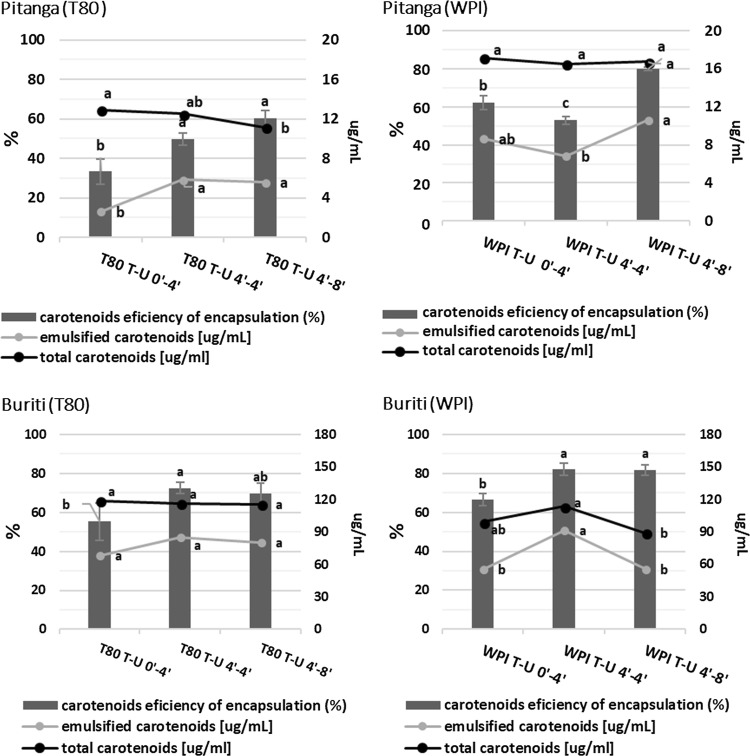
Tracking carotenoids over the microemulsion production
Figure 3 shows a slight degradation of total carotenoids, being significant only for pitanga WPI and buriti T80 microemulsions that were US processed for 8 min. The overall data of total carotenoids, when compared to previous retention study (Fig. 1), suggest that surfactant and oil presence during processing may protect total carotenoids, since the impact is much less noted. The energy input is probably caught by the emulsion formation process, by size reduction of the oil droplets and by the surfactant stabilization of the whole system avoiding the hydroxylation, isomerization and cleavage phenomena since the chemical degradation of these main forms of carotenoid is energy-dependent (Carail et al. 2015; Sun et al. 2010). Further, Carail et al. (2015) demonstrated that degradation caused by ultrasound needs that carotenoids have contact with oxygen and/or with hydroxyl radicals (OH·) and hydrogen peroxyl radicals (HO·2) formed due to sonolysis of water, effects that the surfactant cover may prevent.
Fig. 3.
Effect of treatment and surfactant on total carotenoid content and its encapsulation for pitanga and buriti fruits. Acronyms are T80 = tween 80, WPI = whey protein isolate, T = high speed homogenization and U = ultrasound. Different letters for the same series represent significant differences between treatments (p < 0.05)
Efficiency of encapsulation of US exclusive processing is lower than HSH combined with US in all cases, except for pitanga WPI microemulsion processed 4 min for HSH and US (Fig. 3). Surfactant effect is significant only for pitanga (p < 0.05), where WPI have a higher efficiency of encapsulation (Fig. 3). Regarding the amount of total carotenoid emulsified there are significant differences for pitanga T80, pitanga WPI and buriti WPI microemulsions (p < 0.05). These differences may be related to total carotenoid degradation, i.e. similar to demonstrated at Fig. 1, along with the carotenoid encapsulation some degradation also occurs. It is noteworthy that pitanga and buriti are very complex matrices and have a diverse profile of carotenoids (Azevedo-Meleiro and Rodriguez-Amaya 2004; de Rosso and Mercadante 2007). The containment of carotenoids into the oil droplet may not obey a balanced incorporation, so that interactions between carotenoids may play an important role. The encapsulation of carotenoids inside the oil droplets can be compared to micellarization during intestinal digestion. Many reports show greater micellarization of xanthophylls than carotenes in vegetables due to their degree of polarity (Petry and Mercadante 2017; Dube et al. 2018). Also, β-carotene, lycopene, α-carotene and lutein micellarization are affected differently by the addition of unsaturated fat (higher micellarization) or saturated fat (lower micellarization) (Mashurabad et al. 2017). Therefore, in the case of preferable incorporation of some carotenoids, the spectrophotometric method used to determine total carotenoids will mislead the interpretation of data, that is why we also used HPLC to follow singly β-carotene and lycopene (Fig. 4).
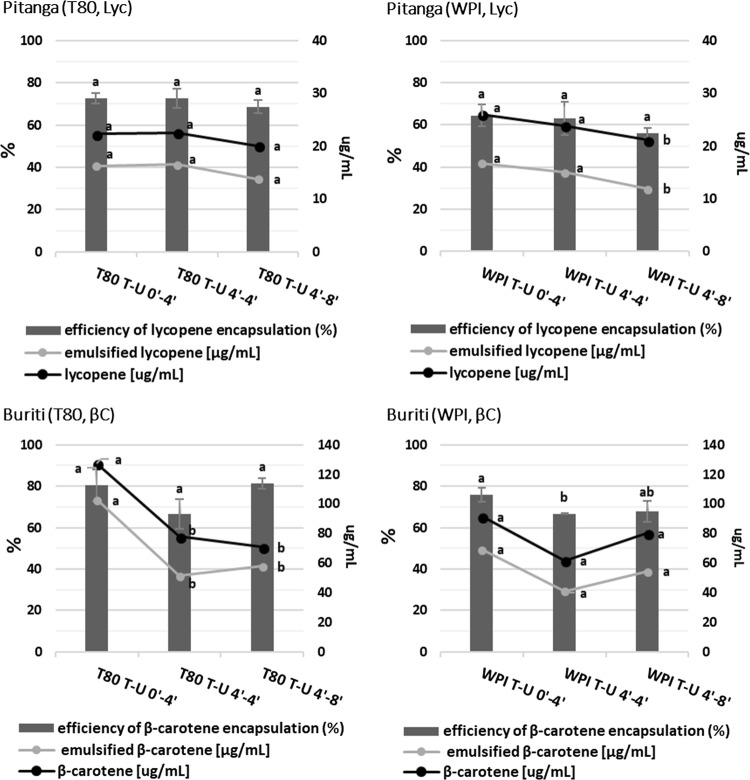
Fig. 4.
Effect of treatment and surfactant on pitanga’s lycopene (Lyc) and buriti’s β-carotene (βC) contents and its encapsulation for each fruit. Acronyms are T80 = tween 80, WPI = whey protein isolate, T = high speed homogenization and U = ultrasound. Different letters for the same series represent significant differences between treatments (p < 0.05)
There are no significant differences regarding efficiency of encapsulation of lycopene in pitanga due to treatment or surfactant used. Degradation of lycopene was light and it was significant only for WPI T-U 4′–8′. The same was observed regarding absolute amount of encapsulated lycopene. Total and encapsulated amounts of β-carotene from buriti decreased for T80 T-U 4′–8′ microemulsion. By general observation of Figs. 3 and 4, it is possible to observe that lycopene and β-carotene tendency of degradation is related to intensification of the processing, independently of fruit or surfactant used. Carotenes are very sensitive molecules and can easily degrade due to light, heat, energy input, presence of oxygen and acidity (Rodriguez-Amaya 2001). Since our preliminary study of processing the whole pulp showed great reduction for total carotenoids (Fig. 1), it was expected a great reduction of β-carotene and lycopene due to microemulsion preparation. However, the significant impact of processing to produce the microemulsion was not severe (Fig. 4). We attribute this to the protection effect of surfactant, as discussed above. Accordingly, Hejri et al. (2013) demonstrated that surfactants have a protective behavior regarding the effect of light in the stability of β-carotene in microemulsion during storage, and observed that this protection is surfactant-dependent. Lycopene microemulsions made with eight different surfactants, including T80, were processed by diverse methods and affected lycopene concentration only in higher-heat shorter-time processing and sterilization of 25% reduction (Amiri-Rigi and Abbasi 2017). These results show the importance of carotenoid stability during microemulsion formulations and how carotenes microemulsions present a relative high stability to processing.
Carotenoid recovery after dynamic simulation of the digestion in the gastrointestinal system
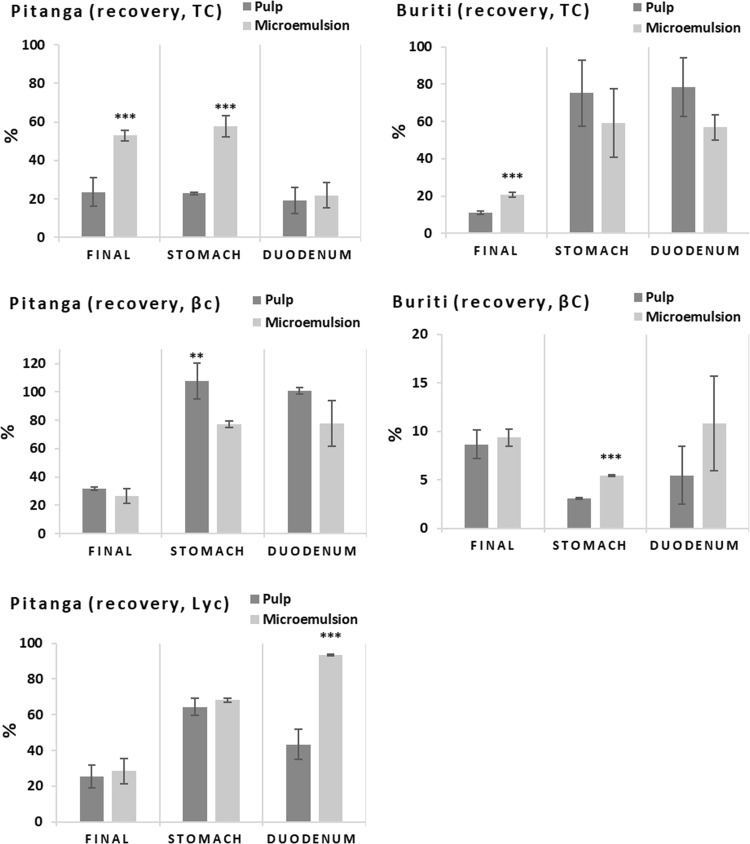
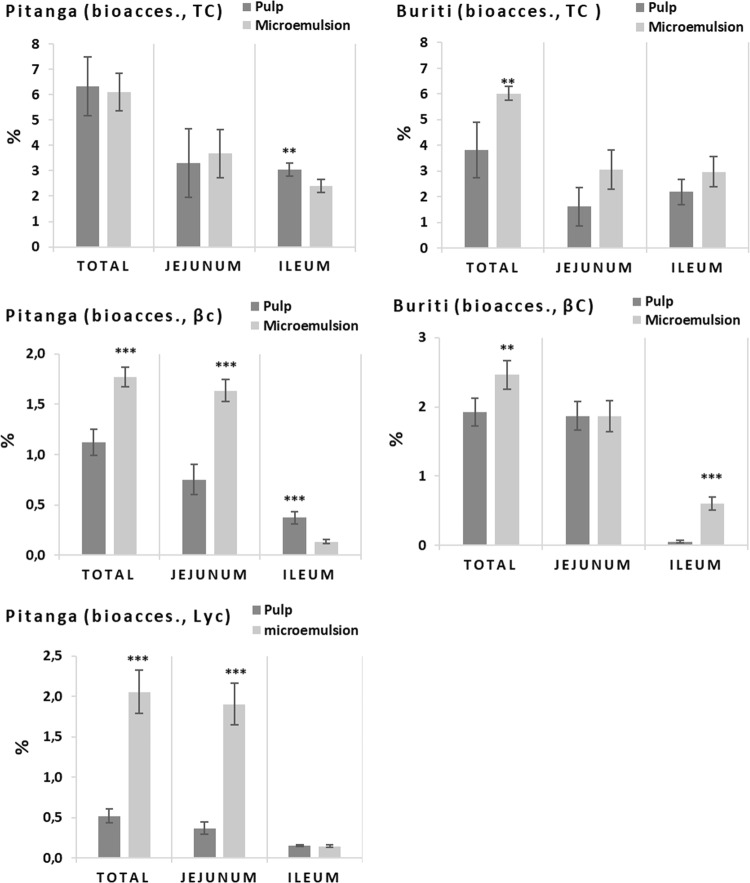
Degradation and losses of β-carotene, lutein and lycopene during static in vitro digestions, in the presence or not of digestive enzymes as well as dietary pro-oxidants, were minutely studied in the work of Kopec et al. (2017). They reported a decrease in the remaining β-carotene, lutein and lycopene of around 40%, 40% and 20%, respectively, after in vitro digestion with digestive enzymes without pro-oxidants. The degradation reached up to 80% of β-carotene in vitro digested with enzymes and presence of metmyoglobin (Kopec et al. 2017). Our results of total carotenoids, β-carotene and lycopene recovery from whole pulp and fruit microemulsions (T80 T-U 4′–4′) after digestion in dynamic gastrointestinal system are presented in the Fig. 5. Whereas our samples presented high instability of carotenoids during the digestion (ranging 8.7–53% of total recovery, Fig. 5) along with high variation of data, we chose to present statistics indicating highly significant (p < 0.01, ***) and significant (p < 0.05, **) differences.
Fig. 5.
Total carotenoids (TC), β-carotene (βC) and lycopene (Lyc) recovery after dynamic gastrointestinal digestion. Recovery of stomach and duodenum were determined from an aliquot collected at 90 min and 120 min respectively. Final recovery is the fraction between the amounts found in jejunal filtrate, ileum filtrate and the digesta residue (unfiltered) with the initial sample. Statistics are differences between whole pulp and microemulsion (***p < 0.01; **p < 0.05)
Final recovery of total carotenoids from microemulsions was higher than whole pulps. Pitanga microemulsions also presented a higher recovery of total carotenoids in stomach, i.e. after 90 min of digestion. Recovery was calculated individually for stomach and duodenum while final recovery is related to all digestive steps (stomach, duodenum, jejunum and ileum). Therefore, bigger losses of total carotenoids in buriti, β-carotene and lycopene in pitanga happened during jejunum and ileum phases. β-carotene from buriti had very low recovery, especially in the stomach that was 3.1% and 5.4%, respectively for pulp and microemulsion. Blanquet-Diot et al. (2009) reported recoveries for β-carotene from yellow and red tomatoes digested in the TNO gastrointestinal tract model (TIM) of ~ 20% in the stomach and ~ 6.5% in the duodenum.
Since the oil droplets’ size is an important factor in microemulsions, we tried to measure the particle size of microemulsions by light scattering diffraction (DLS), however reliable data could not be obtained for the lipid droplets in the presence of fruit matrix. The various size of fruit fibers, fragments and agglomerates (Fig. 2) dominated the light-scattering signal. For this reason, the DLS was used only during the digestions to follow samples charge (ζ-potential) over the stomach, duodenum, jejunal and ileal filtrates as well as the residual digesta. ζ-potential followed the expected during the digestion in the gastrointestinal simulator, keeping up with the pH from acidity in the stomach to alkalinity in the intestinal phases (Pinheiro et al. 2013).
The results obtained are not only due to β-carotene degradation, but also to substantial losses that happen inside the dynamic gastrointestinal system. It was observed that after the whole digestion, the stomacher bags changed to light yellow color (for buriti) and light red (for pitanga), especially in the stomach phase. Carotenes easily interact with plastic materials due to their hydrophobicity, mainly when they are dispersed in aqueous solutions (Rodriguez-Amaya 2001). For some buriti samples, the light yellow color appeared as a line in the stomacher bag coincident with the digesta surface, probably due to the oil that was released during the process and separated to the surface, allowing carotene to contact and adsorb at the stomacher bag surface in that particular place. In the case of pitanga, small red fibers were observed when cleaning the hollow-fibre device, indicating material losses entrapped in the system. These losses contribute to the low recovery found, and probably caused an underestimation of the bioaccessibility results despite the agreement of our findings with those of other authors (Kopec et al. 2017; Blanquet-Diot et al. 2009). Thus, the low recovery of carotenoids after dynamic gastrointestinal digestions may be caused not only by carotenoids degradation—i.e. oxidation, isomerization and breakdown due to pH, oxygen, enzymes and pro-oxidants—but also by the characteristics of the system used—such carotenoid adsorption to stomacher bags, entrapment of undigested fibers and photodegradation. Our data, in line with the available literature (Kopec et al. 2017; Blanquet-Diot et al. 2009), implicates that researchers need to increase the attention on the main role of carotenoid stability during human digestion and its simulation by in vitro digestions and gastrointestinal systems.
Carotenoid bioaccessibility determined by dynamic simulation of the digestion in the gastrointestinal system
Our hypothesis was that the direct processing of the whole pulp, surfactant and oil together would transfer the carotenoids, specially β-carotene and lycopene, to the oil droplets and increase their bioaccessibility. Carotenoids’ encapsulation into the oil droplets and the characterization of the microemulsion microstructure is reported elsewhere. The present paper confirms that it is possible to increase β-carotene and lycopene bioaccessibility (p < 0.01), and results are shown in Fig. 6. The microemulsion formulation and processing conditions chosen were able to improve the final bioaccessibility of total carotenoids from buriti, β-carotene from pitanga and buriti, and of lycopene from pitanga (Fig. 6). The final bioaccessibility is the sum of jejunal and ileal bioaccessibility. The microemulsion of pitanga presented better bioaccessibility of β-carotene and lycopene in the jejunum, while microemulsion of buriti had higher bioaccessibility of β-carotene in the ileum (Fig. 6). General bioaccessibility is higher in the jejunum (p < 0.05). Whole pulp was better than microemulsion in total carotenoids and β-carotene bioaccessibility from pitanga only in the ileal phase.
Fig. 6.
Total carotenoids (TC), β-carotene (βC) and lycopene (Lyc) bioaccessibility after dynamic gastrointestinal digestion. Bioaccessibility is the fraction between the amounts found in jejunal filtrate and ileal filtrate in relation to the amount in the initial sample. Statistics are differences between whole pulp and microemulsion (***p < 0.01; **p < 0.05)
Carotenoids’ bioaccessibility is very dependent of the fruit matrix, specially their deposition form in chromoplasts, and can vary widely. β-carotene bioaccessibility reported in Schweiggert et al. (2012), measured using a static in vitro digestion system, was approximately 0.5% in carrots, 3% in tomatoes, 5% in papaya and 10% in mango. Lycopene bioaccessibility reported in the same work was ~ 0.3% for tomato and papaya, and significantly increased to ~ 0.7% for tomato by the addition of 2.5% of sunflower oil (Schweiggert et al. 2012). Beyond the simple oil addition, excipient microemulsions were able to increase carotenoid bioavailability from vegetables, like total carotenoids from yellow peppers (Liu et al. 2015), total carotenoids (Li et al. 2017) and lycopene (Salvia-Trujillo and McClements 2016) from tomatoes, and α-carotene and β-carotene from carrots (Zhang et al. 2016). For example, for the excipient emulsions made with corn oil and WPI as surfactant, mixed with raw carrots, the final extent of α-carotene and β-carotene bioaccessibility was approximately 0.9, 0.8, 13 and 26% respectively for microemulsions made at 0, 2, 4 and 8% of corn oil (Zhang et al. 2016). Despite some similarity with our data, these works evaluated carotenoids’ bioaccessibility by static in vitro digestion systems and none prepared the microemulsion together with the raw carotenoid source like we did.
Despite the positive conclusions regarding microemulsion data, some of our results are below those presented in previous publications. We attribute this discrepancy primarily to differences of static/dynamic in vitro digestion models applied, and also to the low stability and carotenoids losses inside the dynamic gastrointestinal system. Previous research using the same dynamic gastrointestinal system equipment found ~ 15% bioaccessibility of β-carotene from lipid nanoparticles of cupuaçu (Theobroma grandiflorum) butter (Gomes et al. 2017). The authors demonstrated that their lipid nanoparticles were highly stable during storage and digestion, being that nanoparticles were very resistant to stomach acidity, releasing β-carotene only in the duodenum. Thus, they identified lower losses of β-carotene inside the system (e.g., residues adhered to the dynamic digestion model walls). The carotenoids degradation and losses may happen preferentially with the already released carotenoids from the cellular structures or from microemulsions/microcapsules. There are only a few works that evaluated bioaccessibility of carotenoids microemulsions using a dynamic gastrointestinal model, that represents a more realistic simulation of the human digestion process comparing to static models. Van Loo-Bouwman et al. (2014) reported 30 and 53% of β-carotene bioaccessibility from a mixed diet and an oil diet, respectively, measured by the TIM-1 equipment, but they did not compare results with other studies using the dynamic gastrointestinal model. Bioaccessibility of egg’s xanthophylls, zeaxanthin and lutein, were measured by the TIM-1 equipment, that presented a range of 20–40% of these compounds found in the ileal and jejunal filtrates (Nimalaratne et al. 2015). Note that carotenoids recovery in both studies were very high (69–105%) (Van Loo-Bouwman et al. 2014; Nimalaratne et al. 2015).
In summary, pitanga and buriti microemulsion processing and formulation established in the present work were able to increase carotenoids bioaccessibility. The improvement was higher for pitanga’s microemulsion, specially in the case of lycopene, that was 4 times higher than in the whole pulp.
Conclusion
The treatments and microemulsion formulations used in this work were able to: release carotenoids from pitanga and buriti fruit matrices; encapsulate the released carotenoids into oil droplets from fruit microemulsions; protect the carotenoids against degradation during processing; provide higher recovery of total carotenoids from microemulsion than from whole pulp after passing through a dynamic gastrointestinal system; and increase β-carotene and lycopene bioaccessibility in the microemulsions. The limitations are related to the sensitivity of the carotenoids to the many steps of the process, i.e. pulp extraction, drying and transportation, microemulsion fabrication, 5 h being digested, extractions in organic solvents and finally HPLC analysis. Nevertheless, reliable data were produced regarding carotenoid behavior during the pitanga and buriti microemulsions processing and its bioaccessibility. For the best of our knowledge, this is the only work where microemulsions are produced by directly processing surfactant, oil and fruit pulp rich in carotenoids, leading to a direct encapsulation of the carotenoids (specifically lycopene and β-carotene) and to an increase of their bioaccessibility. The overall bioaccessibility of carotenoids in complex systems including microemulsions still needs much research. Moreover, there is still a lack in research applying dynamic gastrointestinal models for studying carotenoids bioaccessibility in complex matrices. Our work contributes to the field of food structure design aiming at increasing carotenoids bioaccessibility.
Acknowledgements
This work was supported by the São Paulo Research Foundation—FAPESP through research funding [Grant #2015/15507-9] and Ph.D. scholarship for Paulo Berni [Grant #2014/15119-6] and a Research Internships Abroad (BEPE) support [Grant #2016/13355-0]. The author Ana C. Pinheiro is recipient of a fellowship from the Portuguese Foundation for Science and Technology (FCT) [Grant SFRH/BPD/101181/2014].
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paulo Berni, Email: paulo.berni@usp.br.
Ana Cristina Pinheiro, Email: anapinheiro@deb.uminh.pt.
Ana Isabel Bourbon, Email: isabelbourbon@gmail.com.
Maura Guimarães, Email: maurafrancisca@ceb.uminho.pt.
Solange G. Canniatti-Brazaca, Email: sgcbraza@usp.br
Antonio A. Vicente, Email: avicente@deb.uminho.pt
References
- Amiri-Rigi A, Abbasi S. Stability assessment of lycopene microemulsion prepared using tomato industrial waste against various processing conditions. J Sci Food Agric. 2017;97:4922–4928. doi: 10.1002/jsfa.8368. [DOI] [PubMed] [Google Scholar]
- Amorim-Carrilho KT, Cepeda A, Fente C, Regal P. Review of methods for analysis of carotenoids. Trends Analyt Chem. 2014;56:49–73. doi: 10.1016/j.trac.2013.12.011. [DOI] [Google Scholar]
- Anese M, Bot F, Panozzo A, Mirolo G, Lippe G. Effect of ultrasound treatment, oil addition and storage time on lycopene stability and in vitro bioaccessibility of tomato pulp. Food Chem. 2015;172:685–691. doi: 10.1016/j.foodchem.2014.09.140. [DOI] [PubMed] [Google Scholar]
- Azevedo-Meleiro C, Rodriguez-Amaya DB. Confirmation of the identity of the carotenoids of tropical fruits by HPLC-DAD and HPLC-MS. J Food Compos Anal. 2004;17:385–396. doi: 10.1016/j.jfca.2004.02.004. [DOI] [Google Scholar]
- Berni P, Chitchumroonchokchai C, Canniatti-Brazaca SG, de Moura FF, Failla ML. Impact of genotype and cooking style on the content, retention, and bioaccessibility of β-carotene in biofortified cassava (Manihot esculenta Crantz) conventionally bred in Brazil. J Agric Food Chem. 2014;62:6677–6686. doi: 10.1021/jf5018302. [DOI] [PubMed] [Google Scholar]
- Blanquet-Diot S, Soufi M, Rambeau M, Rock E, Alric M. Digestive stability of xanthophylls exceeds that of carotenes as studied in a dynamic in vitro gastrointestinal system. J Nutr. 2009;139:876–883. doi: 10.3945/jn.108.103655. [DOI] [PubMed] [Google Scholar]
- Buggenhout SV, Ahrne L, Alminger M, et al. Structural design of natural plant-based foods to promote nutritional quality. Trends Food Sci Technol. 2012;24:47–59. doi: 10.1016/j.tifs.2011.10.005. [DOI] [Google Scholar]
- Carail M, Fabiano-Tixier A, Meullemiestre A, Chemat F, Caris-Veyrat C. Effects of high power ultrasound on all-E-b-carotene, newlyformed compounds analysis by ultra-high-performance liquid chromatography–tandem mass spectrometry. Ultrason Sonochem. 2015;26:200–209. doi: 10.1016/j.ultsonch.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Davidov-Pardo G, Gumus CE, McClements DJ. Lutein-enriched emulsion-based delivery systems: influence of pH and temperature on physical and chemical stability. Food Chem. 2016;196:821–827. doi: 10.1016/j.foodchem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- de Paz E, Martín A, Mateos E, Cocero MJ. Production of water-soluble β-carotene micellar formulations by novel emulsion techniques. Chem EngProcess. 2013;74:90–96. [Google Scholar]
- de Rosso VV, Mercadante AZ. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J Agric Food Chem. 2007;55:5062–5072. doi: 10.1021/jf0705421. [DOI] [PubMed] [Google Scholar]
- Dube N, Mashurabad PC, Hossain F, et al. β-Carotene bioaccessibility from biofortified maize (Zea mays) is related to its density and is negatively influenced by lutein and zeaxanthin. Food Funct. 2018;9:379–388. doi: 10.1039/C7FO01034F. [DOI] [PubMed] [Google Scholar]
- Failla ML, Chitchumroonchokchai C (2005) In vitro models as tools for screening the relative bioavailabilities of provitamin A carotenoids in foods. Harvestplus, Technical monograph 3
- Failla ML, Chitchumroonchokchai C, Ferruzzi M, et al. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct. 2014;5(6):1101–1112. doi: 10.1039/C3FO60599J. [DOI] [PubMed] [Google Scholar]
- Gomes GVL, Sola MR, Marostegan LFP, et al. Physico-chemical stability and in vitro digestibility of beta-carotene loaded lipid nanoparticles of cupuacu butter (Theobroma grandiflorum) produced by the phase inversion temperature (PIT) method. J Food Eng. 2017;192:93–102. doi: 10.1016/j.jfoodeng.2016.08.001. [DOI] [Google Scholar]
- Goula AM, Ververi M, Adamopoulou A, Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason Sonochem. 2017;34:821–830. doi: 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Hejri A, Gharanjig K, Khosravi A, Hejazi M. Effect of surfactants on kinetics of β-carotene photodegradation in emulsions. Chem Eng Commun. 2013;200(3):437–447. doi: 10.1080/00986445.2012.712581. [DOI] [Google Scholar]
- Hu H, Xing L, Hu Y, et al. Effects of regenerated cellulose on oil-in-water emulsions stabilized by sodium caseinate. Food Hydrocoll. 2016;52:38–46. doi: 10.1016/j.foodhyd.2015.06.019. [DOI] [Google Scholar]
- Kentish S, Feng H. Applications of power ultrasound in food processing. Annu Rev Food Sci Technol. 2014;5:263–284. doi: 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- Kilcrease J, Collins AM, Richins RD, Timlin JA, O’Connell MA. Multiple microscopic approaches demonstrate linkage between chromoplast architecture and carotenoid composition in diverse capsicum annum fruit. Plant J. 2013;76(6):1074–1083. doi: 10.1111/tpj.12351. [DOI] [PubMed] [Google Scholar]
- Kopec RE, Gleize B, Borel P, Desmarchelierd C, Caris-Veyrat C. Are lutein, lycopene, and β-carotene lost through the digestive process? Food Funct. 2017;8:1494–1503. doi: 10.1039/C7FO00021A. [DOI] [PubMed] [Google Scholar]
- Li Q, Li T, Liu C, et al. Enhancement of carotenoid bioaccessibility from tomatoes using excipient emulsions: influence of particle size. Food Biophys. 2017 doi: 10.1007/s11483-017-9474-7. [DOI] [Google Scholar]
- Liu X, Bi J, Xiao H, McClements DJ. Increasing carotenoid bioaccessibility from yellow peppers using excipient emulsions: impact of lipid type and thermal processing. J Agric Food Chem. 2015;63:8534–8543. doi: 10.1021/acs.jafc.5b04217. [DOI] [PubMed] [Google Scholar]
- Mashurabad PC, Palika R, Jyrwa YW, Bhaskarachary K, Pullakhandam R. Dietary fat composition, food matrix and relative polarity modulate the micellarization and intestinal uptake of carotenoids from vegetables and fruits. J Food Sci Technol. 2017;54(2):333–341. doi: 10.1007/s13197-016-2466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements DJ. Nanoscale nutrient delivery systems for food applications: improving bioactive dispersibility, stability, and bioavailability. J Food Sci. 2015 doi: 10.1111/1750-3841.12919. [DOI] [PubMed] [Google Scholar]
- McClements DJ, Gumus CE. Natural emulsifiers—biosurfactants, phospholipids, biopolymers, and colloidal particles: molecular and physicochemical basis of functional performance. Adv Colloid Interface Sci. 2016;234:3–26. doi: 10.1016/j.cis.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Nimalaratne C, Savard P, Gauthier SF, Schieber A, Wu J. Bioaccessibility and digestive stability of carotenoids in cooked eggs studied using a dynamic in vitro gastrointestinal model. J Agric Food Chem. 2015;63:2956–2962. doi: 10.1021/jf505615w. [DOI] [PubMed] [Google Scholar]
- Petry FC, Mercadante AZ. Impact of in vitro digestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017;8(11):3951–3963. doi: 10.1039/C7FO01075C. [DOI] [PubMed] [Google Scholar]
- Pinheiro AC, Lad M, Silva HD, et al. Unravelling the behaviour of curcumin nanoemulsions during in vitro digestion: effect of the surface charge. Soft Matter. 2013;9:3147. doi: 10.1039/c3sm27527b. [DOI] [Google Scholar]
- Porcu MO, Rodriguez-Amaya DB. Variation in the carotenoid composition of the lycopene-rich Brazilian fruit Eugenia uniflora L. Plant Food Hum Nutr. 2008;63:195–199. doi: 10.1007/s11130-008-0085-9. [DOI] [PubMed] [Google Scholar]
- Pugliese A, O’Callaghan Y, Tundis R, et al. In vitro investigation of the bioaccessibility of carotenoids from raw, frozen and boiled red chili peppers (Capsicum annuum) Eur J Nutr. 2013;53:501–510. doi: 10.1007/s00394-013-0555-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. Washington, D.C.: ILSI Press; 2001. [Google Scholar]
- Saini RK, Nile SH, Park SW. Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res Int. 2015;76:735–750. doi: 10.1016/j.foodres.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L, McClements DJ. Enhancement of lycopene bioaccessibility from tomato juice using excipient emulsions: influence of lipid droplet size. Food Chem. 2016;210:295–304. doi: 10.1016/j.foodchem.2016.04.125. [DOI] [PubMed] [Google Scholar]
- Schweiggert RM, Mezger D, Schimpf F, Steingass CB, Carle R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012;135:2736–2742. doi: 10.1016/j.foodchem.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Sun Y, Mab G, Ye X, Kakuda Y, Meng R. Stability of all-trans-b-carotene under ultrasound treatment in a model system: effects of different factors, kinetics and newly formed compounds. Ultrason Sonochem. 2010;17:654–661. doi: 10.1016/j.ultsonch.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Van Loo-Bouwman CA, Naber THJ, Minekus M, et al. Food matrix effects on bioaccessibility of β-carotene can be measured in an in vitro gastrointestinal model. J Agric Food Chem. 2014;62:950–955. doi: 10.1021/jf403312v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang H, Decker EA, McClements DJ. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin) Food Hydrocoll. 2015;45:175–185. doi: 10.1016/j.foodhyd.2014.11.020. [DOI] [Google Scholar]
- Zhang R, Zhang Z, Zou L. Impact of lipid content on the ability of excipient emulsions to increase carotenoid bioaccessibility from natural sources (raw and cooked carrots) Food Biophys. 2016;11:71–80. doi: 10.1007/s11483-015-9418-z. [DOI] [Google Scholar]