Abstract
Vaccinating dogs against rabies is an effective means of reducing human rabies. We subjected 1327 clinically diagnosed human rabies death and mass dog vaccination (MDV) data during 2006–2018 to quantify the impacts of MDV on human rabies incidence in Bangladesh and a subset of rabies death data (422) for clinico-epidemiological analysis. A positive and increasing trend of MDV (p = 0.01 and tau = 0.71) and a negative and declining trend (p < 0.001 and tau = −0.88) of human rabies cases (Correlation coefficient: −0.82) have been observed. Among 422 deaths, the majority (78%) of the victims sought treatment from traditional healers, and 12% received post-exposure prophylaxis (PEP). The mean incubation period of rabies in cases with exposure sites on the head & neck (35 days) was shorter than the upper limb (mean = 64 days, p = 0.02) and lower limb (mean = 89 days, p < 0.01). MDV has been found to be effective for reducing human rabies cases in Bangladesh. Creating awareness among the animal bite victims to stop reliance on traditional healers rather seeking PEP, addressing the role of traditional healers through awareness education programme with respect to the treatment of dog bites, ensuring availability of PEP, and continuing to scale up MDV may help to prevent human rabies deaths.
Subject terms: Viral infection, Viral infection, Epidemiology, Epidemiology
Introduction
Rabies is a zoonotic viral disease responsible for the death of approximately 59,000 people worldwide with more than 3.7 million disability-adjusted life years lost annually1. Due to acute progressive encephalitis, rabies is almost always fatal once clinical signs appear. The disease occurs predominantly in impoverished communities, in both rural and urban areas, and has been recognized for over 4000 years2,3. Rabies is present across all continents, except Antarctica with more than 95% of human fatalities happening in the areas of Asia and Africa, and approximately 40% of cases in the population are aged below 15 years. Although all warm-blooded animals are susceptible to rabies, domestic dogs are the main cause of rabies virus transmission to humans in up to 99% of cases in rabies-endemic regions4. Most cases of rabies are caused by the bite of an infected dog. The effect of rabies virus (RABV) exposure depends on a number of factors, including the gravity of the wound, the anatomical site of the bite on the body, the viral quantity and variant (genotype) inoculated into the wound(s) and the timeliness of post-exposure prophylaxis (PEP)3. The clinical manifestation of human rabies can appear in any of two forms: the widely perceived furious (classical or encephalitic) form or the paralytic (or dumb) form5,6. Each case of rabies has its own diverse clinical characteristics, which may be related to different viral tropisms and neural sites, courses of neural spread, variable immune responses and/or potentially different pathological mechanisms7–9.
Globally, the WHO and the World Organisation for Animal Health, in collaboration with the UN Food and Agriculture Organization and Global Alliance for Rabies Control, also known as “United Against Rabies,” called for the elimination of dog-mediated human rabies by 2030 and are providing/have agreed to provide technical supports towards the elimination of dog mediated human rabies10,11.
Controlling rabies in dogs can significantly reduce human exposure12. Vaccinating domestic dogs against rabies is an effective means of controlling rabies in dogs by disrupting the transmission of RABV between dogs. If coverage of 70% can be achieved in annual pulse vaccination campaigns, the rabies incidence in dogs is likely to be dramatically reduced, and if this coverage is maintained over periods of several years, regional elimination is possible3,13. This rabies elimination approach has been demonstrated in various settings in Africa, Asia, Europe, and the Americas3,12,13.
Rabies remains endemic in Bangladesh and has high public health importance14. However, the number of deaths from human rabies has decreased by approximately 50 per cent in recent years, resulting from a combined government effort involving advocacy, communication and social mobilization (ACSM), modern treatment for animal bites, mass dog vaccination (MDV) and dog population management (DPM)15–17. While ACSM and animal bite management have been continuing, MDV was first piloted in Bangladesh in November 2011, covering a small municipality, namely, Cox’s Bazar18. Upon successful piloting, more comprehensive campaigns of MDV were scaled up throughout the country.
An evaluation of MDV against rabies and other factors affecting the advancement of the continuous rabies control programme is essential for rolling out fundamental improvements to future programme implementation19. In addition, knowledge regarding the trends and clinico-epidemiological features of human rabies is crucial to direct further research and to implement disease control measures9. Therefore, this study was aimed to understand the impacts of MDV and to describe the trends and clinico-epidemiological features of human rabies cases in Bangladesh.
Methods
Data source
We obtained data from the record books of patients with animal bites and rabies cases treated at the National Rabies Prevention and Control Center (NRPCC) of the Infectious Disease Hospital (IDH) in Dhaka, Bangladesh, from January 2006 through December 2018. IDH (NRPCC) is the primary referral centre for animal bites and rabies patients in Bangladesh, and hence, most cases of animal bites from different parts of the country come up here for free vaccination and treatment20. Other than NRPCC, there are 66 public District Rabies Prevention and Control Centers (DRPCCs) with at least one centre in each of the 64 districts that provide a free anti-rabies vaccine (ARV) and treatment for victims of dog bites16,21. However, all the rabies suspect cases have to be referred to IDH (NRPCC) from the DRPCCs and different areas of the country for further management and data recording. All rabies cases were diagnosed clinically and not by the laboratory test. This situation was inevitable due to the socio-cultural practices that the relatives did not permit brain tissue sampling for confirmation and poor laboratory diagnostic facilities in Bangladesh.
Data included demographic information regarding the patient’s origin, age, and sex. Clinical data contained but was not limited to the estimated date of animal exposure, bite site, type of exposure, incubation period (the time between the exposure and the manifestation of signs and symptoms), the presence of various prodromal clinical signs and signs of dysfunction in the central and autonomic nervous system, health-seeking behaviour following animal exposure, and PEP utilization by the animal bite victims.
The Communicable Disease Control Division (CDC) of the Directorate General of Health Services (DGHS) of Bangladesh was coordinating the MDV programme under the National Rabies Elimination Programme. They also provided dog rabies vaccines for conducting MDV. Along with DGHS, the Department of Livestock Services, the Local Government Division, Education sectors, and Non-Government Organizations or development organizations, all contributed in different ways to the MDV programme in Bangladesh. For data regarding MDV, we used the MDV database of the CDC, DGHS from November 2011 through December 2018. The dog population was estimated during the MDV campaign using the ‘Capture-mark-recapture’ method that consists of temporarily marking dogs, e.g., with a dye or distinctive collars22,23. We divided the dog population into three ownership types in this paper: (1) ‘stray dogs’ to be ownerless, seen in public areas and are not restricted to the house or property, therefore, have no health care and must forage for their own food, (2) ‘community dogs’, dogs without a particular owner that roamed in a particular community but lived on food mainly provided by the people of that community, and (3) ‘pet dogs’ that are fed regularly and have access to health care through their owners. The surveyors of the post MDV survey, have identified the dogs in this way during the campaign.
Data analysis
The MDV campaigns are usually conducted annually for particular areas and the duration of immunity persists for 1–3 years24. In this study, we considered that immunity against rabies in vaccinated dogs would persist for two years. The programme was implemented in different phases to complete vaccinating all the dogs of a district/area in a three year period. In the first phase, (Year 1) some areas/sub-districts of a district (typically a district of Bangladesh consists of 3–14 sub-districts) were selected and dogs of those areas/sub-districts were vaccinated. In the next phase (Year 2), the remaining areas/sub-districts of the same district were selected, and dogs were vaccinated. The areas/sub-districts selected each year was different until year 3. The number of dogs vaccinated in Year 1 and Year 2 were added together to identify the number of dogs vaccinated until the second year. We considered the possibility of multiple vaccinations of the same dog. While we acknowledge the limited potential for movement to a new area/sub-district, we ignored this minimal chance due to the lack of data to consider this scenario. We performed the Mann-Kendall trend test to identify the trend of human rabies cases over the period of 2006–2018, as well as the number of dogs vaccinated over the period of 2011 to 2018. Additionally, we conducted Sen’s slope test to identify the changes in human rabies cases annually25. Finally, we executed a correlation coefficient test to identify the relationship between human rabies cases (2006–2018) and the number of dogs being vaccinated against rabies (2011–2018). We hypothesized that bites closer to the brain would have a shorter incubation period and compared the incubation period of rabies in persons exposed to the head and neck with persons exposed to other parts of the body and thus tested a null hypothesis for this. We performed Student’s t-test to identify the difference between the incubation period of rabies in cases with exposure sites on the head & neck and in other organs and reported the p-value.
We generated a map to show the regional variation of rabies deaths and death rates (cases per 100,000 populations) in Bangladesh. District wise death rates are standardized to 2011 population estimates26. We created a heat map to demonstrate year-wise MDV and its coverage in different districts of Bangladesh and a box plot to show the incubation period according to the site of the exposure. Using descriptive statistics, we analyzed different clinical and epidemiological characteristics of rabies cases reported in the IDH, Dhaka, Bangladesh, from 2011 to 2015.
Results
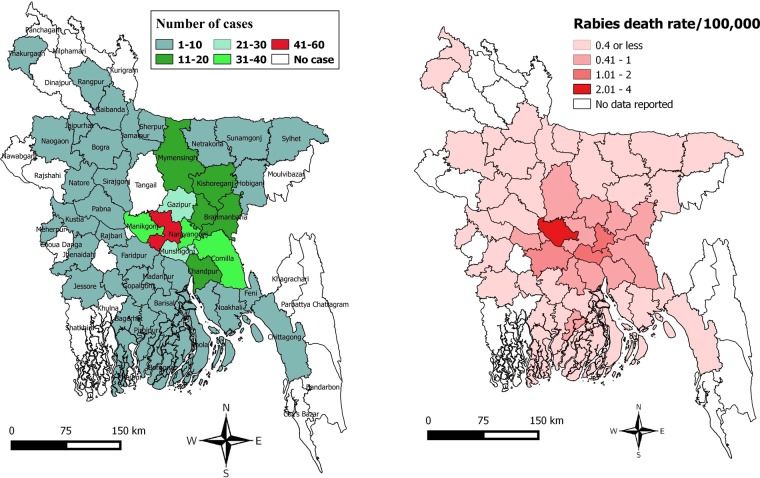
We analysed 1327 human rabies death data reported at the IDH, Bangladesh, from 2006 to 2018 to explore the trends of human rabies over time. A subset of those rabies case data (422 cases from 2011 to 2015) was reviewed for the clinico-epidemiological study. The highest number of cases was observed in the districts in the middle of the country (Fig. 1).
Figure 1.
Regional variation of rabies deaths and death rates in Bangladesh. (a) Distribution of human rabies cases from different districts of Bangladesh reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015 (b) Regional variation of rabies death rates (cases per 100,000 populations) in Bangladesh.
Socio-demographic characteristics of the deceased rabies victims
There were a high number of rabies deaths in males when compared to females (296 vs. 126). Almost half of the deceased rabies victims (n = 201, 47%) were under the age of 15 with a median age of 18.5 years (Figure S1). A total of 346 (82%) rabies victims came from rural areas, and 88% (370) of the cases died at home (Table 1).
Table 1.
Demographic and socioeconomic characteristics of deceased rabies victims reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015.
| Variables/categories | N = 422 n (%) |
|---|---|
| Gender-male | 296 (70) |
| Mean age (in years, range) | 26.5 (2–90) |
| Age (years) | |
| <15 | 201 (47) |
| 15–30 | 63 (15) |
| 30–45 | 67 (16) |
| 45–60 | 67 (16) |
| 60–90 | 24 (6) |
| Occupation | |
| Dependent (including children) | 282 (67) |
| Farming | 63 (15) |
| Business | 48 (11) |
| Day labour | 29 (7) |
| Residence | |
| Rural | 346 (82) |
| Urban | 76 (18) |
| Dog ownership | 10 (2) |
| Place of death | |
| In a health facility/hospital | 52 (12) |
| At home/other location | 370 (88) |
Characteristics of animals to which the deceased rabies victims were exposed
Dogs comprised the majority of exposure sources (n = 380, 90%) followed by cats (n = 24, 6%), jackals (n = 12, 3%) and mongooses (n = 6, 1%). Stray dogs contributed the majority of exposures (n = 390, 93%) while wild, pet and community animals made up 4% (n = 18), 2% (n = 10) and 1% (n = 4) of cases, respectively. Seventy-four per cent (n = 312) of the animal bite cases took place without any provocation by the victims. In almost all cases, the vaccination status of the attacking animals was unknown (n = 412, 98%) (Table S1).
Characteristics of animal exposure to the deceased victims
Animal bites accounted for most of the exposures (n = 399, 95%), whereas the remaining was scratch either by teeth (n = 13, 3%) or by claws (n = 10, 2%), and 56% of cases were attributed to a single bite (n = 223). The anatomical position of the bites involved mainly the lower limbs (n = 320, 76%) with the majority of the bites being category III (n = 399, 95%) (WHO category of exposure) (Table 2).
Table 2.
Characteristics of animal exposure to the deceased rabies victims reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015.
| Variables/categories | N = 422 n(%) |
|---|---|
| Nature of the exposure | |
| Bite | 399 (95) |
| Scratch by teeth | 13 (3) |
| Scratch by claws | 10 (2) |
| Anatomical site of exposure | |
| Trunk | 21 (5) |
| Head & neck | 42 (10) |
| Upper limb | 39 (9) |
| Lower limb | 320 (76) |
| Categories of contact | |
| Category II | 23 (5) |
| Category III | 399 (95) |
| Number of bites (N = 399) | |
| Single | 223 (56) |
| Multiple | 176 (44) |
Heath seeking behaviour following animal exposures
Seventy-eight per cent (n = 327) of the victims sought treatment from traditional healers (faith healers generally entrusted with minor medical illnesses). Only 12% (n = 51) received PEP, and among the patients who received PEP, 84% (n = 43) received an intradermal rabies vaccine (IDRV) [commercial tissue culture vaccines, Rabix-VC® (Incepta Pharmaceuticals, Ltd., Bangladesh)] but did not receive the complete vaccination series [WHO approved Thai Red Cross (TRC) ID regimen]. Only 6 (12%) patients received the complete vaccination series; however, they experienced an average of 5 days (range: 4–7 days) of treatment delay in receiving vaccines with no rabies immunoglobulin (RIG) (Table 3).
Table 3.
Heath seeking behaviour following animal exposures among the rabies victims reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015.
| Variables/categories | N = 422 n (%) |
|---|---|
| Treatment-seeking behaviours | |
| Received traditional treatment | 327 (78) |
| Rabies PEP received | 51 (12) |
| Consult with local doctors | 38 (9) |
| No measures taken | 6 (1) |
| History of proper wound washing | |
| No | 162 (39) |
| Yes | 127 (30) |
| Unknown | 133 (31) |
| Types of vaccine received, (N = 51)# | |
| Intradermal rabies vaccine (IDRV) | 43 (84) |
| IDRV + rabies immunoglobulin (RIG) | 2 (4) |
| Nerve tissue vaccine (NTV) | 6 (12) |
| Vaccination facility, (N = 51)# | |
| District Rabies Prevention and Control Centre (DRPCCs) | 16 (31) |
| Infectious Disease Hospital (IDH) | 1 (2) |
| Institute of Public Health (IPH) | 10 (20) |
| Municipality | 3 (6) |
| Pharmacy | 18 (35) |
| Sub-district hospital (Thana Health Complex) | 3 (6) |
| Course of vaccination, (N = 51)# | |
| Complete | 6 (12) |
| Incomplete | 45 (88) |
#Described the subset of the data, i,e, 51 patients that received rabies PEP after animal exposure.
The incubation period of rabies ranged from 6 days (lowest incubation period) to 1095 days (highest incubation period), with a median of 52 days (Fig. 2). The median incubation period of rabies was 30 days (range: 11–120 days) in cases with exposure sites on the head & neck; 38 days (range: 6–240 days) for the trunk; 45 days (range: 20–407 days) for the upper limbs; and 60 days (range: 15–1095 days) for the lower limbs. The mean incubation period of rabies in cases with exposure sites on the head and neck (35 days) was shorter than those for the upper limb (mean = 64 days, p = 0.02), lower limb (mean = 89 days, p < 0.01) and trunk (mean = 58 days, p = 0.053).
Figure 2.

The estimated incubation period (in days)* of rabies per anatomical location of animal bite wounds reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015. The bottom and top of the box indicate the first and third quartiles, respectively; the band inside the box is the median. The dots outside the box are individual outliers *Period from the time of exposure to the time of manifest signs and symptoms.
Rabies cases, according to the type of clinical manifestation
The clinical symptoms were recorded during the patient’s admission (Table 4). Almost all patients (n = 409, 97%) demonstrated hydrophobia upon admission. Aerophobia occurred in 84% (n = 353) of the patients, elicited using the fan test. Photophobia was noted in 10% (n = 42) of patients.
Table 4.
Rabies cases according to the type of clinical manifestation reported at the National Rabies Prevention and Control Centers (NRPCC) of the Infectious Disease Hospital (IDH), Dhaka, Bangladesh, 2011–2015.
| Variables/categories | N = 422 n(%) |
|---|---|
| Clinical manifestation | |
| Hydrophobia | 409 (97) |
| Aerophobia | 353 (84) |
| Fever | 86 (20) |
| Photophobia | 42 (10) |
| Hypersalivation | 29 (7) |
| Restlessness | 27(1.4) |
| Vomiting/nausea | 24 (6) |
| Itchiness of the bite site | 24 (6) |
| Lethargy | 14 (3) |
| Anxiety | 13 (3) |
| Unusual sensation | 12 (3) |
| Dysphagia | 10 (2) |
| Respiratory distress | 4 (<1) |
| Convulsion | 4 (<1) |
MDV, PEP, and trends of human rabies
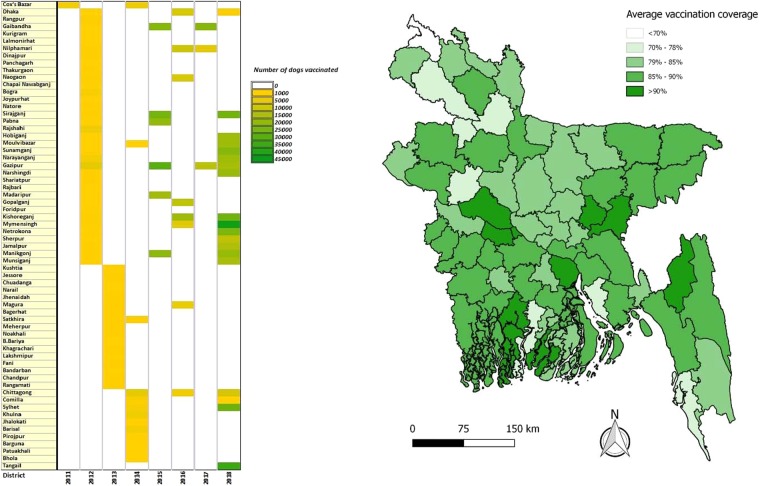
We analysed 8 years (2011–2018) of MDV data maintained at the DGHS of Bangladesh. During this period, MDV was scaled up for at least one round in all 64 district municipalities of the country, and of these, all areas of 23 selected districts were covered with MDV. Three rounds of MDV were scaled up in three areas, namely, Cox’s Bazar district municipality, Satkhira Sadar sub-district, and Sreepur sub-district (Figure S2) of Bangladesh. Under the MDV campaign, out of an estimated 871,765 dogs, an estimated 81% (95% CI: 80.88–81.04) have been vaccinated. From 2011 onward, an upward trend of MDV was found, and the highest number of dogs was vaccinated in 2018 (n = 365,316) (Fig. 3) (Tables S2, S3). From 2011 to 2015, a total of 942,378 animal bite cases managed at NRPCC and DRPCCs of Bangladesh with an average of 188475 cases managed each year (Table S4). We also found an increasing trend of ARV utilization and dog vaccination and a decreasing trend of human rabies cases in Bangladesh from 2011–2018 (Fig. 4) (Figure S3).
Figure 3.
Scaling up mass dog vaccination (MDV) in different districts of Bangladesh with vaccination coverage, 2011–2018. (a) Year-wise number of dogs vaccinated in different districts of Bangladesh. (b) Average vaccination coverage in different districts of Bangladesh.
Figure 4.
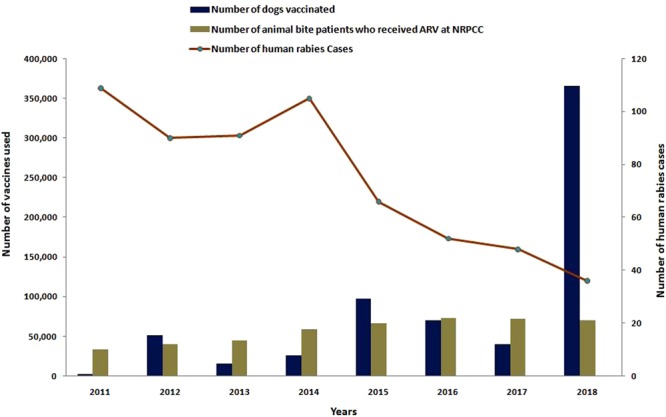
Impact of Mass Dog Vaccination (MDV) and Post-Exposure Prophylaxis (PEP) on human rabies incidence in Bangladesh, 2006–2018.
We identified a trend of declining human rabies cases in Bangladesh from 2006–2018 (p < 0.001 and tau = −0.88). Using Sen’s slope test, we found that over the 13-year period, the slope was 11.31 cases (95% CI: −9.00 to −14.00) (p < 0.001). On the other hand, we found a positive trend of dog population immunization from 2011–2018 (p = 0.01 and tau = 0.71). In Sen’s slope test, for dog population vaccination over a period of 8 years, the slope was 31,162 (95% CI: 11,192–67,634) per year (Fig. 4). The correlation coefficient between the number of human rabies cases and the number of dogs being vaccinated against rabies was −0.82 during the period of 2011 to 2018.
Discussion
Our study has identified several important clinico-epidemiological characteristics of human rabies cases and provides scientific information on the benefits of dog rabies vaccination on human rabies incidence that could be helpful for the structuring of suitable and successful dog mediated rabies control strategies in Bangladesh. In some instances, public health agencies around the developing world make substantial investments for identifying successful interventions to solve emerging public health problems but hardly ever pay enough attention to the known interventions. Our data showed that complex public health problems could be resolved with known interventions like MDV. This is a good example of how zoonotic diseases should be controlled with a true One Health approach. In addition to MDV, there are few other factors, such as raising awareness and access to PEP by the animal bite victims, that may explain changes in the detection of human rabies cases in Bangladesh. A key aim of the national rabies elimination plan of the government of Bangladesh (GOB) is dedicated to building awareness and focusing on the importance of prevention. To that end, the GOB has marked several events with ACSM like educational programmes in primary schools across the country and through a series of press conferences, seminars, and rallies15. Along with MDV, there are other two proven, effective interventions, namely, awareness and PEP, that have eliminated dog-mediated human rabies in Western Europe, North America, Japan, South Korea, and parts of Latin America, Africa and Asia27. During the eight-year period, we found that a total of 705,839 dogs have been vaccinated in Bangladesh, with >70% coverage achieved in almost all the areas where MDVs have scaled up. In view of the recommendations of the WHO, it is estimated that a country needs to vaccinate 70% of its total dog population for seven years in order to eliminate rabies from dogs28.
We identified a downward trend of human rabies cases in Bangladesh from 2006–2018 and an upward trend of dog immunization from 2011–2018. Over the 13-year period, annual cases reduced at a rate of approximately 12 per year, and over a period of 8 years, the number of vaccinated dogs increased at a rate of 3200 dogs per year. The time series for human rabies cases was available over a longer period (2006–2018) than that for MDV data (2011–2018). The trend of human rabies cases changed quite rapidly after MDV was introduced in Bangladesh in 2011. The combined impact of a mass awareness programme, PEP, and MDV has resulted in a consistent decrease in the annual incidence of rabies in Bangladesh17. Sri Lanka and Bhutan have also shown noticeable progress in their rabies elimination programme through awareness education and successful implementation of MDV29,30. In Bangladesh, all 64 districts now have at least one centre providing wound management and PEP to animal bite victims totalling more than 250,000 patients treated by trained nurses and physicians, incurring no out-of-pocket expenses to the patients. The expansion of MDV has reaffirmed how a multi-sectoral, One Health approach conjoining innovation, capacity-building and widespread implementation can lead to effective rabies elimination strategies17.
The epidemiological characteristics of human rabies cases found in this study were similar to those reported elsewhere in Bangladesh and neighbouring countries. Our study revealed that rabies victims were mostly males of less than 15 years of age, which is similar to some other studies in Bangladesh and neighbouring countries14,16,31. The bite locations were mostly on the patients’ lower limbs, with the majority of the bites being category III4. Similar results have been documented in other studies in developing countries31,32. However, bites on the head and neck were not infrequent (10%) in our study. In China33 and Tanzania34, bites on the head and neck region have made an important contribution to human rabies. Exposure of the upper body and limbs (head, neck, arm, or hand) to a rabid animal is likely to have more adverse consequences than the exposure of the lower parts of the body31. The median danger-of-death toll after exposure of the head, hand, trunk, and legs to a rabid animal was reported to be 45%, 28%, 5%, and 5%, respectively31,35. In our study, we found that 5% of the patients developed rabies due to scratches or abrasions without bleeding either by cats or by puppies; however, we were uncertain whether the patient’s attendants recalled the event correctly. Similar findings have also been documented in other studies in Bangladesh and India20,36. WHO recommends a rabies vaccine for category II bites that include minor scratches or abrasions without bleeding, and additional local RIG infiltration may be necessary36.
We found a difference in incubation times, depending on the sites of exposure. Similar findings have also documented in another study where the incubation period was shorter in cases with exposure sites on the head and neck than in cases with a wound on the trunk, upper or lower limbs37. The incubation period depends on a number of factors including the site and type of bite wound(s), virus inoculum and rapid treatment of wounds, viral strains, and host factors9,38,39. Our study identified three patients with unusual incubation time (two for 1095 and one 1080 days) which might be due to recall bias. In rabies endemic areas, there could have been recurrent exposures following the first bite. Some other studies have documented a much longer incubation time (27 years in the Philippines) than usual for rabies9,40. It may be difficult to diagnose rabies if the incubation period is longer, especially when the exposure history is missing41. We found that bites in the head and neck region seemed to have a shorter incubation time in contrast to that for bites in the lower extremity. Other studies have also demonstrated a similar phenomenon9,42.
Our study showed the manifestation of hydrophobia evoked upon admission in the vast majority of cases (97%). Hydrophobia can be seen in classic rabies in 50–80% of cases43. Despite the fact that there are no confirmatory laboratory diagnostic facilities for human rabies in Bangladesh, a history of an animal bites and evidence of hydrophobia along with the characteristics of a rapidly fatal encephalopathy, have enabled us to diagnose the cases as rabies. We also observed a high percentage of victims (84%) manifesting aerophobia, which is another hallmark feature of rabies. This finding is parallel to other studies conducted in Bangladesh44 and its’ neighbouring countries45.
Our analysis showed that the majority of the rabies victims primarily sought treatment from traditional healers following animal exposure, with no history of taking any PEP. Previous studies in Bangladesh revealed that a good number of animal bite victims first sought treatment from traditional healers instead of taking any modern PEP16. These sorts of health-seeking behaviour might be attributed to persevering myths and dogmas among the people of the community, poor socio-economic status, lack of proper education, lack of accessibility and affordability of PEP treatment in local government hospitals, distance to government hospitals from the victims’ residence, etc.16. The following measures should be taken to prevent the dog mediated human rabies: setting up awareness and education campaigns and implementing dog bite prevention strategies, especially targeting the children, encouraging and educating about responsible dog ownership and conceptualizing cultural differences that impact the societal role and value of dogs. The WHO recommends immediate wound washing along with vaccination following exposure to a suspected rabid animal, which would help to prevent nearly 100% of rabies deaths. However, in type III wounds, RIG must be required to be infiltrated in and around the wound(s)24.
PEP for rabies involves recurrent visits to the treatment centre by the bite victims to maintain a complete course within 28 days. Hence, emphasis must be placed on patient compliance to make the immunization appropriate46,47. Our study unveiled that among the patients who received PEP, only 12% followed the complete vaccination series (Thai Red Cross ID regimen), but they experienced a treatment delay in receiving PEP with no RIG. It is unfortunate that 6 people have died of rabies despite the completion of PEP. We have no information that revealed the exact cause of PEP failure in these cases. We, however, assume that this might be due to treatment delay or administering no RIG following exposed to suspected animal bites. While an estimated 10 million people receive rabies PEP every year after exposure to suspected animal bites, there are only sporadic reports of PEP failure exist. The various reasons for the probable or possible failure of rabies PEP include RIG not being used at all or incorrect administration, poor quality vaccine or RIG, the introduction of an exceptionally large viral load, virus injected directly into the nerve, unrecognized or unreported deviations from the WHO PEP protocol48. In Bhutan, 40% of rabies victims receive an incomplete vaccine course47. Approximately 33% of patients with dog bite cases in Thailand neglected to come for the last dose of the PEP series and failed to finish a complete series of PEP49. Experts have underscored the need for an improved PEP regimen requiring fewer hospital visits, helping to improve patient compliance in order to achieve PEP course completion and to minimize the victim’s burden of lost time and money50,51.
Stray dogs comprised the majority of exposures (90%), followed by stray cats (6%), jackals (3%), and mongooses (1%). A similar pattern was reported by other studies in Bangladesh and neighbouring countries16,20,52. We found, however, that cat, jackal and mongoose exposures were not properly addressed by the rabies victims in terms of seeking medical attention and PEP. This may be due to inadequate knowledge about the gravity of the disease and its host range. The vaccination status of the attacking animals (including dogs) was unknown in this study, indicating a higher number of free-roaming/stray dogs with no registration and vaccination records. Hence, along with continuing to scale up MDV, strategies to reduce stray dogs are essential; these measures have been documented to be a more advantageous, cost-effective, rational and ethical way to control rabies53,54.
The majority of the rabies patients died at home and only a fraction of them died in a health facility or hospital. The main underlying reason for this is that the socio-cultural practice in the country where the relatives of the rabies victims were not willing to stay in the hospital once they were informed about the grave prognosis of rabies. At that time, relatives urged the hospital management to discharge the patients from the IDH. This is the condition not only in Bangladesh but also in other developing countries, where taking a suspect/confirmed rabid patient to the hospital and bringing the dead body home is a burden for impoverished families14. Unfortunately, there are some countries where many rabies patients are turned away from hospitals and are only receiving terminal care from their families. Whenever possible, hospital care for patients with clinical rabies is advisable to reduce their suffering and ensure adequate, respectful palliative care24.
Our study had limitations. We have estimated the mortality rates based on the human rabies cases visited the public hospital facilities (NRPCC/DRPCCs). However, we are aware that we might have missed some cases, especially those who attended private hospital facilities or sought treatment from traditional healers and never visited the public hospital facilities. Thus the proportion we are reporting might be a fraction of total cases observed in Bangladesh. However, because of improvement in education and awareness, we believe only a few rabies deaths were missed by the government mainstream data-collecting centres. Identifying the occurrence of paralytic rabies was not possible, and it is not practical to diagnose this condition on clinical grounds. The present investigation was a retrospective study and there may have been recall bias as historical recollections depended almost entirely on the patients’ relatives. We, however, tried to mitigate this bias by calling some of the patients’ relatives directly. Despite these drawbacks, we believe that our investigation has revealed helpful information about rabies in Bangladesh and could be utilized in underdeveloped countries to control rabies.
Conclusion
Our study showed that mass dog vaccination (MDV) is one of the most important components of controlling human rabies in Bangladesh. Also, these data clearly spelt out that most deaths had occurred as a result of consultation with the traditional healers instead of seeking post-exposure prophylaxis (PEP) from the hospitals and also due to the incomplete treatment course. Poor health-seeking behaviour of the rabies victims indicates the necessity to improve such behaviour through advocacy, communication, and social mobilization. It is necessary to address the role of traditional healers through an awareness education programme with respect to the treatment of dog bites and rabies and discouraging animal bite victims from visiting them. Ensuring the affordability and availability of rabies PEP in all areas of Bangladesh, especially in local public hospitals, is also important. Proper vaccine delivery needs sufficient personnel training to ensure correct storage, reconstitution, and injection. Sharing local epidemiological knowledge of rabies in animals may assist clinicians in making the right choice in treating rabies with PEP. We recommend conducting a humane method of dog population management programme along with the promotion of dog ownership and the continuation of scaling up MDV throughout the country to eliminate dog-mediated human rabies in Bangladesh. In addition, establishing a laboratory for rabies diagnosis and introducing an active surveillance system is necessary to monitor and evaluate emerging patterns and trends of the disease in Bangladesh. Strengthening and encouraging multi-sectoral involvement through the One Health approach is necessary for the sustainability of the rabies elimination programme in Bangladesh.
Supplementary information
Acknowledgements
We wish to express our sincere gratitude and thanks to Dr. Dananjaya Karunaratna, World Animal Protection, London, UK and Dr. Omesh Kumar Bharti, State Institute of Health and Family Welfare, Shimla, India for their advice and help in documenting our work. We also thanks to Beth Ann Fiedler, PhD for her editing commentary and contributions to the final draft and appreciate the critical comments and suggestions made by anonymous reviewers to improve the manuscript.
Author contributions
Conceptualization, S.G., M.S.R., and N.H.; Methodology, S.G., M.S.R., M.A.H.K., and N.H.; Data Analysis, M.A.H.K., N.H., S.C. and S.G.; Field Investigation, M.K.I., S.M.U., M.R.A.S., A.A.J., A.S.P.M., S.M.E.A. and S.H.; Data Processing, M.A.H.K., and H.S.M.; Writing – Original Draft, S.G., M.S.R., S.C., M.A.H.K., R.B. and N.H.; Supervision, N.C.D., A.K.M.S., B.A., U.R.S. and S.T.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59109-w.
References
- 1.Hampson K, et al. Estimating the global burden of endemic canine rabies. PLoS neglected tropical diseases. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarantola A. Four thousand years of concepts relating to rabies in animals and humans, its prevention and its cure. Tropical Medicine and Infectious Disease. 2017;2:5. doi: 10.3390/tropicalmed2020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Rabies vaccines: WHO position paper. Weekly epidemiological record; (93) 201–220, http://www.who.int/wer (2018).
- 4.WHO fact sheet on rabies. Available at, http://www.who.int/news-room/fact-sheets/detail/rabies. (Accessed August 2018).
- 5.Rupprecht, C. E. et al. In: Plotkin, S. A., O. W., Offit PA, editors. Vaccines. 7th ed. Philadelphia: Elsevier Saunders.: 918–942 (2017).
- 6.Jackson AC. Human Rabies: a 2016 Update. Current infectious disease reports. 2016;18:38. doi: 10.1007/s11908-016-0540-y. [DOI] [PubMed] [Google Scholar]
- 7.Mitrabhakdi E, et al. Difference in neuropathogenetic mechanisms in human furious and paralytic rabies. Journal of the neurological sciences. 2005;238:3–10. doi: 10.1016/j.jns.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. The Lancet Neurology. 2002;1:101–109. doi: 10.1016/S1474-4422(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 9.Dimaano EM, Scholand SJ, Alera MTP, Belandres DB. Clinical and epidemiological features of human rabies cases in the Philippines: a review from 1987 to 2006. International Journal of Infectious Diseases. 2011;15:e495–e499. doi: 10.1016/j.ijid.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Khelef, N., WHO, D. E., WHO, M. C. & Nel, L. Aiming for elimination of dog-mediated human rabies cases by 2030. Veterinary Record (2016). [DOI] [PubMed]
- 11.Minghui R, Stone M, Semedo MH, Nel L. New global strategic plan to eliminate dog-mediated rabies by 2030. The Lancet Global Health. 2018;6:e828–e829. doi: 10.1016/S2214-109X(18)30302-4. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Rabies vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:309–20. [Google Scholar]
- 13.Zinsstag, J. et al. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proceedings of the National Academy of Sciences, pnas. 0904740106 (2009). [DOI] [PMC free article] [PubMed]
- 14.Hossain M, et al. Human rabies in rural Bangladesh. Epidemiology and infection. 2012;140:1964–1971. doi: 10.1017/S095026881100272X. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Bangladesh tackles rabies through mass dog vaccination. WHO, http://www.who.int/features/2014/bangladesh-tackles-rabies/en/ (2014).
- 16.Ghosh S, et al. Awareness of rabies and response to dog bites in a Bangladesh community. Veterinary Medicine and Science. 2016;2:161–169. doi: 10.1002/vms3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. The Rabies Elimination Program of Bangladesh. Neglected tropical diseases. Geneva: WHO 2017, http://www.who.int/neglected_diseases/news/Bangladesh-rabies-elimination-program/en/.
- 18.Mass Dog Vaccination in Bangladesh: The silent Revolution towards rabies elimination. Community News, Global Alliance for Rabies Control (GARC). 30 Apr 2014, https://rabiesalliance.org/resource/mass-dog-vaccination-bangladesh-silent-revolution-towards-rabies-elimination. (Accessed August 2018).
- 19.Pimburage R. M. S., Harischandra P. A. L., Gunatilake M., Jayasinhe D. N., Balasuriya A., Amunugama R. M. S. K. Erratum to ‘a cross-sectional survey on dog ecology and dog anti-rabies vaccination coverage in selected areas in Sri Lanka’ [S.L.Vet.J. 2017, 64:1(A): 1-9] Sri Lanka Veterinary Journal. 2017;64(2):17. doi: 10.4038/slvj.v64i2.24. [DOI] [Google Scholar]
- 20.Hossain M, et al. Five-year (January 2004–December 2008) surveillance on animal bite and rabies vaccine utilization in the Infectious Disease Hospital, Dhaka, Bangladesh. Vaccine. 2011;29:1036–1040. doi: 10.1016/j.vaccine.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Li, A. J. et al. Descriptive assessment of rabies post-exposure prophylaxis procurement, distribution, monitoring, and reporting in four Asian countries: Bangladesh, Bhutan, Cambodia, and Sri Lanka, 2017–2018. Vaccine (2018). [DOI] [PMC free article] [PubMed]
- 22.4, https://caninerabiesblueprint.org/IMG/pdf/crb_v4_-_dog_marking_methods.pdf (Canine Rabies Blueprint, London, 2017).
- 23.Tenzin T, Ahmed R, Debnath NC, Ahmed G, Yamage M. Free-roaming dog population estimation and status of the dog population management and rabies control program in Dhaka City, Bangladesh. PLoS neglected tropical diseases. 2015;9:e0003784. doi: 10.1371/journal.pntd.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Expert Consultation on Rabies, third report: WHO Technical Series Report No. 1012, Geneva, (ISBN 978-92-4-121021-8) (2018).
- 25.Sen PK. Estimates of the regression coefficient based on Kendall’s tau. Journal of the American statistical association. 1968;63:1379–1389. doi: 10.1080/01621459.1968.10480934. [DOI] [Google Scholar]
- 26.Statistics BB. Statistical Yearbook of Bangladesh. Statistics Division, M. o. P., Dhaka, Government of the People’s Republic of Bangladesh. (2018).
- 27.World Health Organization. Zero by 30: the Global Strategic Plan to end human deaths from dog-mediated rabies by 2030, https://apps.who.int/iris/bitstream/handle/10665/272756/9789241513838-eng.pdf (2018).
- 28.Wallace RM, Undurraga EA, Blanton JD, Cleaton J, Franka R. Elimination of dog-mediated human rabies deaths by 2030: needs assessment and alternatives for progress based on dog vaccination. Frontiers in Veterinary Science. 2017;4:9. doi: 10.3389/fvets.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matibag GC, et al. A pilot study on the usefulness of information and education campaign materials in enhancing the knowledge, attitude and practice on rabies in rural Sri Lanka. The journal of infection in developing countries. 2009;3:055–064. doi: 10.3855/jidc.106. [DOI] [PubMed] [Google Scholar]
- 30.Tenzin T, et al. Community-based study on knowledge, attitudes and perception of rabies in Gelephu, south-central Bhutan. Int Health. 2012;4:210–219. doi: 10.1016/j.inhe.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Tenzin, et al. Dog bites in humans and estimating human rabies mortality in rabies endemic areas of Bhutan. PLoS Negl Trop Dis. 2011;5:e1391. doi: 10.1371/journal.pntd.0001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudarshan M, et al. An epidemiological study of animal bites in India: results of a WHO sponsored national multi-centric rabies survey. Journal of Communicable Diseases. 2006;38:32. [PubMed] [Google Scholar]
- 33.Song M, et al. Epidemiological investigations of human rabies in China. BMC infectious diseases. 2009;9:210. doi: 10.1186/1471-2334-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampson K, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS biology. 2009;7:e1000053. doi: 10.1371/journal.pbio.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knobel DL, et al. Re-evaluating the burden of rabies in Africa and Asia. Bulletin of the World health Organization. 2005;83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 36.Bharti OmeshKumar, Chand Ramesh, Chauhan Anjali, Rao Rahul, Sharma Hamender, Phull Archana. “Scratches/Abrasions without bleeding” cause rabies: A 7 years rabies death review from medical college Shimla, Himachal Pradesh, India. Indian Journal of Community Medicine. 2017;42(4):248. doi: 10.4103/ijcm.IJCM_37_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi L, et al. Epidemiological characteristics and post-exposure prophylaxis of human rabies in Chongqing, China, 2007–2016. BMC infectious diseases. 2018;18:6. doi: 10.1186/s12879-017-2830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Susilawathi NM, et al. Epidemiological and clinical features of human rabies cases in Bali 2008–2010. BMC Infect Dis. 2012;12:81. doi: 10.1186/1471-2334-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson AC, Scott CA, Owen J, Weli SC, Rossiter JP. Therapy with minocycline aggravates experimental rabies in mice. Journal of virology. 2007;81:6248–6253. doi: 10.1128/JVI.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masci, J. R. & Wormser, G. P. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Edited by Gerald L. Mandell, John E. Bennett, and Raphael Dolin Philadelphia: Elsevier Churchill Livingstone, 2005. 3661 pp., illustrated.
- 41.Kienzle, T. E.: Rabies New York, NY: Chelsea House (2006).
- 42.Warrell D, Warrell M. Human rabies and its prevention: an overview. Reviews of infectious diseases. 1988;10:S726–S731. doi: 10.1093/clinids/10.Supplement_4.S726. [DOI] [PubMed] [Google Scholar]
- 43.Hemachudha T, et al. Pathophysiology of human paralytic rabies. Journal of neurovirology. 2005;11:93–100. doi: 10.1080/13550280590900409. [DOI] [PubMed] [Google Scholar]
- 44.Haque MS, Yeasmin T, Islam MM. Epidemiological characteristics of human rabies at Infectious Disease Hospital, Dhaka. Bangladesh Journal of Child Health. 2011;35:102–107. doi: 10.3329/bjch.v35i3.10521. [DOI] [Google Scholar]
- 45.Chowdhury J, Modak K. Report on rabies in West Bengal. Journal of the Indian Medical Association. 1983;81:69–74. [PubMed] [Google Scholar]
- 46.Madhusudana S, Anand NP, Shamsundar R. Economical multi-site intradermal regimen with purified chick embryo cell vaccine (Rabipur) prevents rabies in people bitten by confirmed rabid animals. International journal of infectious diseases. 2002;6:210–214. doi: 10.1016/S1201-9712(02)90113-X. [DOI] [PubMed] [Google Scholar]
- 47.Tenzin DNK, Ward MP. Human rabies post exposure prophylaxis in Bhutan, 2005-2008: trends and risk factors. Vaccine. 2011;29:4094–4101. doi: 10.1016/j.vaccine.2011.03.106. [DOI] [PubMed] [Google Scholar]
- 48.Wilde H, et al. Failure of postexposure treatment of rabies in children. Clinical infectious diseases. 1996;22:228–232. doi: 10.1093/clinids/22.2.228. [DOI] [PubMed] [Google Scholar]
- 49.Sriaroon C, et al. Common dilemmas in managing rabies exposed subjects. Travel medicine and infectious disease. 2005;3:1–7. doi: 10.1016/j.tmaid.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Dodet B. Report of the sixth AREB meeting, Manila, The Philippines, 10–12 November 2009. Vaccine. 2010;28:3265–3268. doi: 10.1016/j.vaccine.2010.02.093. [DOI] [PubMed] [Google Scholar]
- 51.Shantavasinkul P, et al. Postexposure rabies prophylaxis completed in 1 week: preliminary study. Clinical Infectious Diseases. 2010;50:56–60. doi: 10.1086/649211. [DOI] [PubMed] [Google Scholar]
- 52.Tumram, N. K., Bardale, R. V., Dixit, P. G. & Deshmukh, A. Y. Unusual association of diseases/symptoms: A fatal mongoose bite. BMJ case reports2012 (2012). [DOI] [PMC free article] [PubMed]
- 53.Lembo T, et al. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS neglected tropical diseases. 2010;4:e626. doi: 10.1371/journal.pntd.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cleaveland S, Kaare M, Knobel D, Laurenson MK. Canine vaccination—providing broader benefits for disease control. Veterinary microbiology. 2006;117:43–50. doi: 10.1016/j.vetmic.2006.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.