Abstract
Purpose
This study evaluated the extent of bacterial contamination in water from dental unit waterlines (DUWLs).
Methodology
Water samples were collected (before flushing, 1 min post-flushing, and 3 min post-flushing) from 24 clinics (Group A: no disinfection, Group B: citric acid disinfectant) of a Government Dental College. Bacterial counts, identification, antibiotic sensitivity tests, determination of endotoxin levels, and scanning electron microscopy (to confirm the presence of biofilm) were performed.
Results
The most common opportunistic bacteria were P. aeruginosa (95%), S. aureus (58%), S. auricularis (49%), P. fluorescens (44%), and A. baumannii (20%). Approximately 50% of the bacterial isolates were resistant to two or more antibiotics. Flushing for 3 min did not reduce the contamination of water from Group A clinics which exceeded the recommendation of ≤500 CFU/ml. No bacterial growth was seen in Group B samples. Endotoxin levels were >5.00 endotoxin units (EU)/ml in Group A and ranged from 1.33 to 5.00 EU/ml in Group B clinics. Scanning electron microscopy images showed bacterial biofilms on the surfaces of the tubes.
Conclusions
DUWL contamination is a serious issue in dentistry, and the microbiological quality of the water must be monitored regularly. Further studies on endotoxin exposure and prevention are therefore necessary.
Keywords: Bacteria, Biofilm, Endotoxin, Dental unit waterlines, Flushing
1. Introduction
Bacterial contamination of DUWL water is a universal problem, and untreated DUWLs eventually produce an interface that results in biofilm formation (Franco et al., 2005). Biofilm formation may increase bacterial adhesion and protect bacteria from unfavorable conditions (Szymanska et al., 2008). The small diameter of DUWLs combined with periods of prolonged water stagnation provides the perfect ecological niche for biofilm development and the proliferation of microorganisms (Rice et al., 2006).
Previous studies have reported high bacterial counts in DUWLs, A diverse range of environmental bacteria, opportunistic bacteria, and bacteria commonly found in the oral cavity have been isolated from DUWLs, including Legionella pneumophila, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococci, Klebsiella pneumoniae (Szymanska et al., 2008, Coleman et al., 2009, Pawar et al., 2016). Moreover, gram-negative bacteria, can produce endotoxins which cause inflammation, fever, toxic shock, and possibly even asthma (American Dental Association (ADA), 1999).
Different strategies have been suggested to maintain water quality and control the transmission of infections, including flushing, independent water reservoir systems, sterilized water, microfiltration and the treatment of DUWLs with disinfectants such as hydrogen peroxide (Schel et al., 2006), chlorine dioxide (Bansal et al., 2011), sodium hypochlorite (Liaqat and Sabri, 2008).
Interestingly, no standard protocol currently exists to test the efficacy of the different disinfection methods used in dental clinics. Therefore, waterline contamination is still a major issue as DUWLs can be contaminated by the water supply and the bio-aerosols and skin flora of the patients (Szymanska et al., 2008). Although the risk of infection may not be high in healthy individuals, patients with weakened immune systems, toddlers, patients with chronic diseases, hospitalized patients, diabetics, and patients with heart disease and renal diseases are at a greater risk (Gobin et al., 2009). Thus, it is essential to identify the pathogenic bacteria in DUWLs (Kohn et al., 2003, Schel et al., 2006).
The Center for Disease Control (CDC) for dental healthcare indicated that the water used for routine dental treatment should meet the regulatory standards for drinking water (≤500 CFU/mL of bacteria) established by the Environmental Protection Agency (EPA) (Kohn et al., 2003). Therefore, the aim of the study was to assess the level of contamination in water from DUWLs with different flushing periods. In addition, biofilm presence, antimicrobial susceptibility, and endotoxin levels were investigated.
2. Materials and methods
This study was carried out from 14th May to 31st August 2017. A total of 432 samples were collected from 24 clinics (Group A: no disinfection, Group B: citric acid disinfectant) of a Government Dental College. Water samples were collected from various parts of the DUWLs such as the high-speed drill hand piece line (HP), air/water dental-syringe (WS), and oral rinse (OR). Sampling was performed before work (BW), and after work (AW). 500 ml of water was collected before flushing, 1 min after flushing, and 3 min after flushing. Samples were then immediately transported to the lab for processing.
2.1. Bacterial enumeration
To count the viable bacteria, 1 ml of each sample was added to molten Stander plate count (SPC) agar and incubated at 37 °C for 24. Bacterial colonies were then counted under 10–15× magnification.
2.2. Bacterial identification
100 ml of each water sample was filtered through 0.45 µm Millipore membrane unit (Fisher Scientific). Membranes were removed from the funnel and deposited on the surface of the following culture media: blood agar (BA), MacConkey agar (MAC), Xylose lysine deoxycholate agar (XLD), cetrimide agar, and mannitol salt agar (MSA). After incubation, bacteria were identified using colony characteristics, Gram staining, and biochemical tests.
2.3. Antibiotic susceptibility
Susceptibility to antibiotics was tested by using the disc diffusion method and the automated MicroScan system according to the manufacturer's guidelines. The antibiotics used were Cefoxitin, Gentamicin, Cefpodoxime, Rifampicin, Ciprofloxacin, Chloramphenicol, Azithromycin, Teicoplanin, Vancomycin, Mupirocin, Norfloxacin, Cefuroxime, Ceftazidime, Augmentin, Ampicillin. The zones of inhibition were interpreted according to Clinical and Laboratory Standards Institute guidelines (CLSI, 2017).
3. Determination of endotoxin concentration
Selected water samples were analyzed using the Limulus amoebocyte lysate (LAL) rapid endotoxin assay according to the manufacturer’s instructions. Water samples were collected in endotoxin- and pyrogen-free containers, filtered, and screened at 150 endotoxin units (EU)/ml by diluting the samples 1:600. Any positive reactions were diluted further to give a sensitivity of 300, 600, and 1200 EU/ml, whilst negative reactions were re-analyzed at a sensitivity of 25, 50, and 100 EU/ml. Samples were incubated at 37 °C for 60 min; positive reactions were recorded for samples containing a firm gel clot. The sensitivity of the test was >25 EU/ml.
3.1. Biofilm detection using Scanning Electron Microscopy (SEM)
Certain WS samples were selected for biofilm detection. A 15 mm length of each tube was cut into two pieces longitudinally, then were immediately placed in a cool box and transported to the laboratory. Next, the samples were fixed by immersion in 50% glutaraldehyde overnight at 4 °C, washed using phosphate buffers (pH = 7.2), exposed to 1% osmium tetroxide for 1 h at 4 °C, and dehydrated using ethanol. Prior to SEM (JEOL, JSM-6060LV), the samples were sputter coated with gold.
3.2. Statistical analysis
Data are expressed as the mean ± standard deviation (SD) of experiments. All assay values were derived from triplicate measurements, unless otherwise specified. The significance of multiple comparisons was determined by one-way analysis of variance (ANOVA), with Tukey’s post hoc multiple comparisons test when equal variance was assumed; otherwise the Games-Howell post hoc test was used. Statistical analyses were carried out using the SPSS statistical package and statistical significance was set at P ≤ 0.05.
4. Results
4.1. Bacterial enumeration
All sources tested from Group A units (HP, OR, and WS) showed significantly high levels of bacterial contamination, whilst no bacterial growth was seen in the water samples from Group B clinics (Fig. 1), with an average bacterial count of 2241.9, 2215.2, and 2469.5 CFU/ml in OR, WS, and HP, respectively. HP water had the highest level of bacterial contamination regardless of the collection time or flushing protocol (Fig. 1). HP bacterial counts varied between 37519 CFU/ml (BW and BF) and 25453.5 CFU/ml. The same pattern was observed for the WS and OR water in both BW and AW conditions.
Fig. 1.
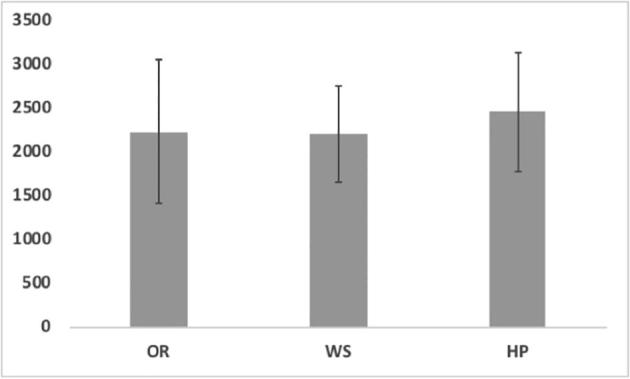
Average bacterial count in oral rinse (OR), air/water syringe (WS) and hand piece (HP) collected before working and 3 min after flushing.
For all water sources tested, bacterial counts were significantly reduced 3 min after flushing compared to before flushing. The average bacterial count of OR and WS samples was significantly reduced from 3272.26 and 2963.7 to 2241.9 and 2215.2 CFU/ml, respectively, 3 min after flushing. Interestingly, the bacterial count of HP samples was significantly (>0.01) reduced from 3376.3 to 2469.5 CFU/ml, 3 min after flushing (Fig. 2). AW samples of both WS and HP showed a significant reduction in bacterial contamination 3 min after flushing compared to AW samples before flushing (Fig. 2). The average bacterial count in WS and HP was significantly reduced from 2967.7 and 3192.3 to 2045.5 and 2283.61 CFU/ml, respectively, 3 min after flushing (Fig. 3).
Fig. 2.
Effect of flushing on bacterial counts in different water sources before flushing (BF), 1 min after flushing (AF) and 3 min AF.
Fig. 3.
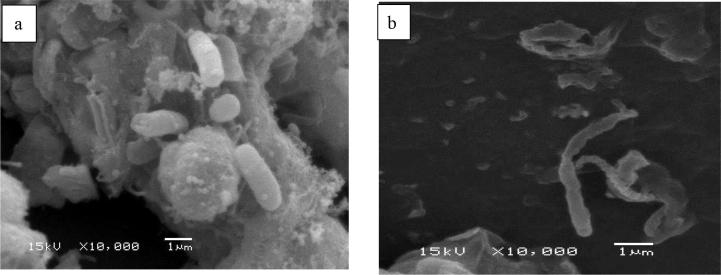
SEM image from Group A dental units revealed a bacterial biofilm covering the tube supplying water to the air-water syringe (a). SEM image from Group B units showed dispersed rod-shaped bacteria (b).
4.2. Identification of bacteria
Gram-positive rods of the genus Bacillus spp. were observed in 100% of the units sampled (Table 1). Five opportunistic bacterial species were isolated from the DUWLs, P. aeruginosa (95%), S. aureus (58%), S. auricularis (49%), P. fluorescens (44%), and A. baumannii (20%) (Table 1). However, HP, WS, and OR water samples had high percentages (>90%) of P. aeruginosa. 83% of S. aureus isolates were obtained from HP, 50% from WS and 42% from OR, whilst 63% of P. fluorescens were isolated from WS, 37% from OR, and 31% from HP, and S. auricularis was found in 63% of HP, 43% of OR, and 40% of WS samples. 30% of A. baumannii isolates were isolated from HP and 31% were present in WS.
Table 1.
Presence of opportunistic bacteria in water samples from DUWLs.
| Bacterial species | Collection Site [No. of isolated bacteria] (%) |
Total No. (%) | ||
|---|---|---|---|---|
| HP n = 66 |
WS n = 66 |
OR n = 66 |
||
| S. aureus | 55 (83%) | 33 (50%) | 28 (42%) | 116 (58.5%) |
| S. auricularis | 42 (63%) | 27 (40%) | 29 (43%) | 98 (49.4%) |
| P. aeruginosa | 62 (93%) | 65 (98%) | 62 (93%) | 189 (95.4%) |
| P. fluorescens | 21 (31%) | 42 (63%) | 25 (37%) | 88 (44.4%) |
| A. baumannii | 20 (30%) | 21 (31%) | 0 | 41 (20.7%) |
4.3. Antibiotic susceptibility
Approximately 50% of the bacterial isolates tested, P. aeruginosa, P. fluorescens, A. baumannii, S. aureus, S. auricularis were resistant to two or more antibiotics (Table 2). Our findings showed that 48% of P. aeruginosa isolates were resistant to Cefpodoxime. 69.4% of A. baumannii isolates were resistant to Ceftazidime, 53% of P. fluorescens isolates were resistant to Ceftazidime, 64.8% of S. auricularis isolates were resistant to Azithromycin, and 40% of S. aureus isolates were resistant to Cefoxitin.
Table 2.
Antibiotic susceptibility patterns of bacteria isolated from different water samples from Group A DUWLs.
| Bacteria | No. of Susceptibility Profiles (%) |
||
|---|---|---|---|
| S | R to One | MR | |
|
P. aeruginosa n = 54 |
20 (37%) | 3 (5.5%) | 31 (57.4%) |
|
P. fluorescens n = 54 |
11 (20.3%) | 11 (20.3%) | 32 (59.2%) |
|
A. baumannii n = 36 |
2 (5.5%) | 7 (19.5%) | 27 (75%) |
|
S. aureus n = 54 |
14 (25.9%) | 5 (9.2%) | 35 (64.8%) |
|
S. auricularis n = 54 |
8 (14.8%) | 14 (25.9%) | 32 (59.2%) |
|
Bacillus spp. n = 54 |
20 (37%) | 5 (9.2%) | 29 (53.7%) |
S = Sensitive to all tested antibiotics.
R to One = resistant to one antibiotic.
MR = multi-resistant to 2 antibiotics or more.
4.4. Determination of endotoxin concentration
As shown in Table 3, the measured endotoxin levels were >5.00 EU/ml in Group A, and varied from 1.33 EU/ml to 5.00 EU/ml in Group B. Most of the HP samples had high levels of endotoxin (>5.00 EU/ml in samples collected 3 min after flushing, with the remaining HP samples having an endotoxin level of 2.4 EU/ml. 50% of the WS and OR samples tested had endotoxin levels above 5.00 EU/ml.
Table 3.
Endotoxin levels in DUWLs 3 min after flushing.
| Sample Source | Number of Instruments Tested | Mean Endotoxin Levels |
|---|---|---|
| HP | 34 | >5.00 EU/ml |
| 6 | 2.4 EU/ml | |
| WS | 3 | >5.00 EU/ml |
| 3 | 1.33 EU/ml | |
| OR | 4 | >5.00 EU/ml |
| 4 | 1.35 EU/ml | |
4.5. Biofilm detection using Scanning Electron Microscopy (SEM)
The SEM images of the tubes taken from Group A dental units showed a dense, homogenous bacterial biofilm covering the surface of the tubes (Fig. 3a). Conversely, the SEM images of the tubes from the Group B dental units showed scattered rod-shaped bacteria (Fig. 3b).
5. Discussion
The effective control of potential infectious agents is one of the cornerstones of good clinical practice and governance, therefore this study was conducted to understand the extent of bacterial contamination in DUWLs. The water samples obtained from Group A clinics which had no disinfectant systems showed high levels of bacteria from the HP followed by the OR and WS. However, no bacterial growth was seen in the water samples from Group B clinics which used a descaling solution (citric acid treatment). According to the ADA, water from dental units should have no more than 200 CFU/ml of bacteria, with the ADA and the CDC concluding that the maximum contamination of dental treatment water should be limited to 500 CFU/ml (Lin et al., 2011).
In this study, the mean concentration of bacteria in BW HP water samples 3 min after flushing was 2469.5 CFU/ml, significantly exceeding the recommended values (200 CFU/ml). This agrees with previously studies which reported that the mean concentration of microorganisms in water samples was between 3.90 × 104 and 1.52 × 106 CFU/ml (Szymanska et al., 2008, Türetgen et al., 2009). Dahlén et al., showed that water in 75% of the DUWLs examined in Sweden were contaminated with bacteria at levels that did not meet the international requirements (Dahlén et al., 2009). It has also been reported that water from DUWLs in one of the cantons in Switzerland did not comply with the CDC recommendations (Barben et al., 2009).
Flushing DUWLs has been reported to be an important method of controlling microbial levels in DUWLS. Consistent to this, the bacterial contamination of WS and HP samples was significantly reduced 3 min after flushing compared to before flushing. However, the bacterial count 3 min after flushing was still higher than the recommended limit of 500 CFU/ml. Previous studies have reported similar outcomes (Agarwal et al., 2008, Singh et al., 2013, Fotedar and Ganju, 2015). Therefore, flushing does not play a significant role in disinfection, and the latest CDC recommendations suggest that flushing alone is not sufficient and other strategies are required to improve water quality in dental treatment (Kohn et al., 2003, Rice et al., 2006). However, it has been shown that while disinfectant treatments decreased the bacterial count, the biofilm persisted (Salam et al., 2017).
The presence of biofilms in DUWLs is one of the predominant factors leading to high bacterial load (Franco et al., 2005). Previous studies on disinfectant exposure and flushing have shown that flushing leads to just a 9.1% reduction in viable count and a 0.5% reduction in biofilm coverage. The use of chemical germicides has been recommended for the removal of biofilms in DUWLs. Moreover, chemical disinfectants should be safe, and therefore their corrosive and toxic properties need to be carefully examined as well as their effectiveness in DUWLs (Agarwal et al., 2008, Fotedar and Ganju, 2015, Salam et al., 2017).
The types of bacteria present in DUWLs were also evaluated. While the most predominant opportunistic organism was P. aeruginosa, the levels of S. aureus and S. auricularis were higher in HP samples. Research has indicated that P. aeruginosa is one of the most difficult to treat pathogens, and is commonly isolated in high percentages of DUWLs (James et al., 2015, Oliveira et al., 2008). A study conducted in Saudi Arabia showed that the most common bacteria in DUWLs were Bacillus spp. (29.6%) and Pseudomonas spp. (22.8%) (Al-Saif et al., 2007).
High concentrations of bacteria and the incidence bacterial endotoxins are the most important health risk factors as they can be transmitted through the water from dental units (Szymanska et al., 2008, Coleman et al., 2009). Previous reports revealed that Gram-negative bacteria account for the majority of bacteria identified from DUWLs, and the endotoxins they release significantly reduce water quality of DUWLs (Huntington et al., 2007, Szymańska and Sitkowska, 2013). Relatively high bacterial endotoxin levels, ranging from 480 to 1008 EU/ml, have been reported in DUWLs (Putnins et al., 2001). Interestingly, flushing waterlines for 5–10 min did not reduce endotoxin levels to zero. This study also indicated the presence of high levels of endotoxins in Group A DUWLs, and revealed a positive correlation between endotoxin levels and bacterial load. Although Group B units showed no bacterial growth due to the chemical disinfectant used, low levels of endotoxin were still present. Therefore, a more effective procedure for eliminating bacteria and their components is required. Further studies on the consequences of dental endotoxin exposure, and the means by which this exposure may be prevented, are thus warranted (Huntington et al., 2007).
In this study, the susceptibility patterns of the bacteria isolated from DUWLs showed that approximately 50% of the bacterial isolates were resistant to two or more antibiotics. The spread of antibiotic resistant and opportunistic bacteria could cause infections in dental clinic employees and patients with deficient immune mechanisms (Schiavano et al., 2017, Goldman and Green, 2008). Therefore practical methods for controlling microbial contamination are urgently needed (Oliveira et al., 2008). Taken together, it is clear that effective mechanisms are required to reduce microbial contamination in DUWLs and reduce the risk of cross-infection in general practice, especially for the immunocompromised individuals (Pawar et al., 2016, Szymanska et al., 2008).
6. Conclusions
This study highlights the need for regular monitoring of the microbiological quality of water in DUWLs, including the detection of opportunistic pathogens, the prevention of water stagnation, and the use of various procedures to reduce biofilm formation. An easy-to-use, cheap, and safe protocol for DUWL water disinfection is required, and further studies concerning endotoxin exposure and its prevention are necessary.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Saudi Arabia, for funding this work through the Undergraduate Research Support Program, (Project number: USRSP-17-89) for funding this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal D., Sunitha S., Reddy C.V. Awareness and estimation of bacterial contamination of dental unit waterlines in dental clinics and dental institutions in Mysore City, Karnataka. J. Indian Assoc. Public Health Dent. 2008;6:46–52. [Google Scholar]
- AlSaif K.M., Assery M., Nahas M.A. Microbial contamination of dental unit water systems in Saudi Arabia. Saudi Dent. J. 2007;19:110–114. [Google Scholar]
- American Dental Association (ADA) Council on Scientific Affairs Dental unit waterlines: approaching the year 2000. J. Am. Dent. Assoc. 1999;130:653–664. [PubMed] [Google Scholar]
- Bansal R., Puttaiah R., Harris R., Reddy A. Evaluation of two methods in controlling dental treatment water contamination. J. Contemp. Dent. Pract. 2011;12:73–83. doi: 10.5005/jp-journals-10024-1013. [DOI] [PubMed] [Google Scholar]
- Barben J., Kuehni C.E., Schmid J. Water quality in dental chair units. A random sample in the canton of St Gallen. Schweiz. Monatsschr. Zahnmed. 2009;119:976–985. [PubMed] [Google Scholar]
- Coleman D.C., O’Donnell M.J., Shore A.C., Russell R.J. Biofilm problems in dental unit water systems and its practical control. J. Appl. Microbiol. 2009;106:1424–1437. doi: 10.1111/j.1365-2672.2008.04100.x. [DOI] [PubMed] [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2017. Performance Standards for Antimicrobial Susceptibility Testing. 474 M100–S27. [Google Scholar]
- Dahlen G., Alenas-Jarl E., Hjort G. Water quality in water lines of dental units in the public dental health service in Goteborg, Sweden. Swed. Dent. J. 2009;33:161–172. [PubMed] [Google Scholar]
- Fotedar S., Ganju S. Microbial contamination of dental unit water lines in H.P. Government Dental College, Shimla. Saudi J. Dent. Res. 2015;6:129–132. [Google Scholar]
- Franco F.F.S., Spratt D., Leao C., Porter S.R. Biofilm formation and control in dental unit waterlines. Bioflims. 2005;2:9–17. [Google Scholar]
- Gobin I., Newton P.R., Hartland E.L., Newton H.J. Infections caused by non pneumophila species of Legionella. Rev. Med. Microbiol. 2009;20:1–11. [Google Scholar]
- Goldman E., Green L.H. second ed. CRC Press/Taylor & Francis Group; FL, USA: 2008. Practical Handbook of Microbiology. [Google Scholar]
- Huntington M.K., Williams J.F., Mackenzie C.D. Endotoxin contamination in the dental surgery. J. Med. Mirobiol. 2007;56:1230–1234. doi: 10.1099/jmm.0.47231-0. [DOI] [PubMed] [Google Scholar]
- James A., Shetty A., Hedge M.N., Bhandary S. Detection and quantification of microorganisms in dental unit waterlines. J. Dent. Med. Sci. 2015;14:8891. [Google Scholar]
- Kohn W.G., Collins A.S., Cleveland J.L., Harte J.A., Eklund K.J., Malvitz D.N. Guidelines for infection control in dental health-care settings—2003. MMWR Surveill. Sum. 2003;52:1–61. [PubMed] [Google Scholar]
- Liaqat I., Sabri A.N. Effect of biocides on biofilm bacteria from dental unit water lines. Curr. Microbiol. 2008;56:619–624. doi: 10.1007/s00284-008-9136-6. [DOI] [PubMed] [Google Scholar]
- Lin S.M., Svoboda K.K., Giletto A., Seibert J., Puttaiah R. Effects of hydrogen peroxide on dental unit biofilms and treatment water contamination. Eur. J. Dent. 2011;5:47. [PMC free article] [PubMed] [Google Scholar]
- Oliveira A.C.D., Maluta R.P., Stella A.E., Rigobelo E.C., Marin J.M., Ávila F.A.D. Isolation of Pseudomonas aeruginosa strains from dental office environments and units in Barretos, state of São Paulo, Brazil, and analysis of their susceptibility to antimicrobial drugs. Braz. J. Microbiol. 2008;39:579–584. doi: 10.1590/S1517-838220080003000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A., Garg S., Mehta S., Dang R. Breaking the chain of infection: dental unit water quality control. J. Clin. Diagn. Res. 2016;10:80–84. doi: 10.7860/JCDR/2016/19070.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnins E.E., DiGiovanni D., Bhullar A.S. Dental unit waterline contamination and its possible implications during periodontal surgery. J. Periodontol. 2001;72:393–400. doi: 10.1902/jop.2001.72.3.393. [DOI] [PubMed] [Google Scholar]
- Rice E.W., Rich W.K., Johnson C.D., Lye D.J. The role of flushing dental water lines for the removal of microbial contamination. Public Health Rep. 2006;121:270–274. doi: 10.1177/003335490612100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam N., Mulamoottil V.M., George B. Assessment of microbial contamination in dental-unit water lines: an analytical study. J. Indian Assoc. Public Health Dent. 2017;15:97–101. [Google Scholar]
- Schel A.J., Marsh P.D., Bradshaw D.J., Finney M., Fulford M.R., Frandsen E. Comparison of the efficacies of disinfectants to control microbial contamination in dental unit waterline systems in general dental practices across the European Union. Appl. Environ. Microbiol. 2006;72:1380–1387. doi: 10.1128/AEM.72.2.1380-1387.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavano G.F., Carloni E., Andreoni F., Magi S., Chironna M., Brandi G., Amagliani G. Prevalence and antibiotic resistance of Pseudomonas aeruginosa in water samples in central Italy and molecular characterization of oprD in imipenem resistant isolates. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0189172. e0189172 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Nagaraja C., Hungund S.A. A study of different modes of disinfection and their effect on bacterial load in dental unit waterlines. Eur. J. Gen. Dent. 2013;2:246–251. [Google Scholar]
- Szymanska J., Sitkowska J., Dutkiewicz J. Microbial contamination of dental unit waterlines. Ann. Agric. Environ. Med. 2008;15:173–179. [PubMed] [Google Scholar]
- Szymańska J., Sitkowska J. Bacterial contamination of dental unit waterlines. Environ. Monit. Assess. 2013;185:3603–3611. doi: 10.1007/s10661-012-2812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türetgen I., Göksay D., Cotuk A. Comparison of the microbial load of incoming and distal outlet waters from dental unit water systems in Istanbul. Environ. Monit. Assess. 2009;158:9–14. doi: 10.1007/s10661-008-0560-7. [DOI] [PubMed] [Google Scholar]