Abstract
Background
There is growing interest in Internet-delivered cognitive behavioural therapy (ICBT) as an alternative to face-to-face therapy for anxiety and depression because it reduces barriers to accessing traditional treatment (e.g., travel distance, cost, stigma). Extensive research has demonstrated that ICBT is an effective treatment for anxiety and depression and that it produces effect sizes comparable to medication and face-to-face therapy. In routine practice, however, ICBT patients commonly receive simultaneous pharmacological treatment, and few studies have examined how medication affects patient outcomes.
Objective
The objective of this study was to explore whether use of psychotropic medications predicts outcomes or adherence among patients receiving ICBT for depression and anxiety in a large community sample.
Methods
This study used data from 1201 patients who received an 8-week course of ICBT for anxiety and depression that included weekly therapist support as part of routine care. Patients reported medication usage and completed measures of depression and anxiety before treatment, after treatment, and at three-month follow-up.
Results
60% of patients at pre-treatment reported regularly taking psychotropic medication. Common classes of medication reported included: (i) selective serotonin reuptake inhibitors (34%); (ii) anxiolytics (15%); (iii) serotonin and norepinephrine reuptake inhibitors (14%); (iv) antipsychotics (8%); and (v) norepinephrine-dopamine reuptake inhibitors (7%). At post-treatment and three-month follow-up, overall medication usage reduced slightly to 55%, with the greatest reduction seen in anxiolytics. Logistic regression revealed that none of the classes of medication commonly reported at pre-treatment were associated with study completion rates. A recursive partitioning algorithm found that usage of tetracyclic medication was related to smaller pre-to-post reductions in anxiety symptoms and did not identify any medication types that were related to differences in depressive symptom change. Patients on medication tended to report higher levels of anxiety symptoms at intake and experienced somewhat more modest symptom reductions than patients not taking medications; nevertheless, they still experienced large reductions in depression and anxiety over the course of treatment.
Conclusions
These results show that medication usage is very common in a diverse community sample of patients seeking ICBT for anxiety and depression. Patients reporting medication usage at intake are likely to benefit from treatment approximately as much as patients not taking medication. These results support the continued referral of patients receiving psychotropic medication to ICBT programs for anxiety and depression. Program designers might also consider providing information about the common medications (SSRIs, SNRIs, anxiolytics) used by this population alongside CBT materials.
Keywords: Internet, Cognitive behavior therapy, Anxiety, Depression, Pharmacotherapy
Highlights
-
•
Internet-delivered CBT reduced self-reported symptoms of anxiety and depression.
-
•
60% of patients reported taking psychotropic medication at pre-treatment.
-
•
The most common medications were SSRIs (34%), anxiolytics (15%) and SNRIs (14%).
-
•
Patients taking medication had similar completion rates to those not on medication.
-
•
Patients taking medication reported similar reductions in symptoms over therapy.
1. Introduction
Anxiety and depression are common and disabling conditions that produce significant strain on health care systems and incur significant economic costs on society (Vos et al., 2015). Psychotropic medication and psychotherapy are two common and efficacious treatment options for patients with anxiety and depression (Cuijpers, 2017; Cuijpers et al., 2013). Extensive research has shown that cognitive behavioural therapy can produce significant reductions in symptoms in patients, and Internet-delivered Cognitive Behaviour Therapy (ICBT) has emerged as an efficient method for delivering therapy to anxious and depressed individuals (Andersson et al., 2019). Transdiagnostic ICBT (TD-ICBT) programs teach patients skills for managing both anxiety and depression. TD-ICBT is increasingly being deployed in routine care as a cost-effective option, often within a stepped-care model for patients with mild to moderate symptoms and lower risk of suicidality (Titov et al., 2018).
Concurrent medication use is reported by large proportions of ICBT users in routine care settings in Norway (62%), Canada (58%), Australia (37%), and Denmark (25%; Titov et al., 2018). Lindner et al. (2015) found that current or past use of psychotropic medication was reported by approximately 50% of people seeking ICBT across various recruitment sources and by 64% of people recruited from clinical settings. Despite the high frequency of medication usage in some countries, few studies have investigated whether concurrent medication usage affects outcomes for patients receiving TD-ICBT, and the research that does exist has often simplified medication usage to a binary variable (i.e., patients are either on medication or not), which is problematic given that mental health medications are known to have a wide range of targets, effects, and side-effects. The limited research in this area has also yielded mixed findings. One group found that a history of psychotropic medication use was related to worse outcomes in ICBT (Alaoui et al., 2016). Another found that concurrent use of psychotropic medications was related to fewer modules started in disorder-specific ICBT programs for anxiety and depression (e.g., Hadjistavropoulos et al., 2016). However, one group found that concurrent use of antidepressant medication was related to more favorable outcomes in ICBT (Cientanni et al., 2019). Consistent with the concept that psychotherapy plus pharmacotherapy may produce an additive effective, trials of face-to-face psychotherapy programs have shown that pharmacotherapy can be augmented by CBT to improve patient outcomes (e.g., Wiles et al., 2013; Trial: ISRCTN38231611). However, no similar trials have investigated how concurrent medication usage affects outcomes in the unique TD-ICBT population, which may differ from other clinical samples in many respects, including severity.
The purpose of the present study was to perform a retrospective review of a large sample of patient case records from a publicly-funded TD-ICBT program to better understand: (a) what the most common classes of medications being used by this population are; and (b) how medication usage is related to treatment outcome. We expected to find that SSRI medications in particular are common among TD-ICBT patients and hypothesized that individuals reporting medication use would exhibit more severe symptoms than those not reporting medication use. We have two a priori reasons to believe that medication usage may be related to treatment outcome. First, there are demonstrated individual differences in beliefs about the acceptability of psychotropic medication and psychotherapy, including research showing that depressed individuals prefer psychotropic medications over therapy (Wagner et al., 2005); this is important because patient perceptions of ICBT as a credible treatment option are related to greater symptom improvement in patients with depression (Alaoui et al., 2016). Second, the patient's prescription also represents the product of a physician's diagnostic process, and we can therefore expect to observe systematic differences in symptoms between individuals using different medications—for example, physicians prescribe anxiolytic medications to relieve different symptoms than when they prescribe tetracyclic antidepressants. This exploratory study therefore examined whether any specific classes of medications were linked to dropout rates or symptom trajectories. The present study extends the existing research in this area by considering the effects of different classes of medication (i.e., rather than operationalizing medication usage as a single binary variable) and was designed to inform implementation of TD-ICBT in routine care and highlight areas of future research.
2. Methods
2.1. Participants
The present study consisted of a review of past patient records and a series of novel analyses. The data discussed herein were initially collated and analyzed by Edmonds et al., 2018. Participants in this study came from a publicly-funded ICBT clinic called the Online Therapy Unit, which offers free ICBT to residents from across the province of Saskatchewan, Canada. Specifically, participants included in this study were screened for and enrolled in The Wellbeing Course, which is a transdiagnostic, therapist-guided ICBT intervention for depression and anxiety (Titov et al., 2011). It includes five core lessons offered over eight weeks; additional resources are available for review depending on patient preferences. A total of 1201 participants received TD-ICBT between October 2014 and June 2017 and were used in this study. The screening process included a battery of questionnaires, which prospective participants completed online, and an additional series of questions administered during a telephone interview. To be eligible for inclusion, participants were required to (a) be at least 18 years old, (b) be residents of Saskatchewan, (c) self-reporting symptoms of anxiety and/or depression, (d) have access to the Internet and a reasonable level of comfort using it, and (e) provide consent and an emergency medical contact. Prospective participants were excluded if they were (a) deemed to be at high risk of suicide, (b) seeking treatment primarily for a mental health problem besides anxiety or depression, (c) concurrently receiving face-to-face psychotherapy, or (d) no longer interested in receiving treatment at the end of the eligibility screening process.
2.2. Materials
The materials used in this study included a two-stage eligibility screening process (as described above), the Patient Health Questionnaire 9-item scale (PHQ-9; Kroenke et al., 2001), the Generalized Anxiety Disorder 7-item scale (GAD-7; Spitzer et al., 2006) and The Wellbeing Course. The PHQ-9 measures the frequency with which respondents experience various symptoms of depression. Answers range on a four-point Likert scale from 0 (not at all) to 3 (nearly every day), for a minimum possible score of 0 and a maximum possible score of 27 across the scale's 9 items. Prior research suggests that a score of ten or greater indicates a likelihood that an individual meets diagnostic criteria for major depressive disorder (Manea et al., 2012). The PHQ-9 has demonstrated good validity and reliability (Kroenke et al., 2010) and high internal reliability among the present study's sample (Cronbach's α = 0.84–0.90).
Likewise, the GAD-7 measures the frequency with which respondents experience certain, common symptoms of generalized anxiety disorder. As with the PHQ-9, answers range on a four-point Likert scale from 0 (not at all) to 3 (nearly every day). Across the scale's seven items, a respondent's score can vary from a minimum of 0 to a maximum of 21, with a score of 10 or greater indicating that an individual likely meets diagnostic criteria for generalized anxiety disorder (Spitzer et al., 2006). Research has found the GAD-7 to be both valid and reliable (Bandelow and Brasser, 2009), and the scale demonstrated high internal reliability in the present study (Cronbach's α = 0.87–0.90).
Adherence in the present study was measured by whether or not each participant reviewed all five lessons of the The Wellbeing Course. Notably, several measures not germane to the purposes of the present study were employed as well and are described in Edmonds et al., 2018.
2.3. Procedure
Prospective participants of Edmonds et al., 2018 study first completed a battery of eligibility questionnaires online. Those who were deemed eligible then underwent an additional screening interview by telephone. After consenting to participate in the study, eligible participants began using The Wellbeing Course. Therapists contacted participants once each week by telephone or through a secure messaging system throughout the eight-week course. In addition to completing the PHQ-9 and GAD-7 during assessment, participants completed both scales before lessons 2, 3, 4, and 5, and again at post-treatment and three-month follow-up.
Additionally, during their initial assessment, at post-treatment, and at three-month follow-up, participants were asked to identify the names and dosages of any medications they were currently taking. During the present study, two coders (a psychiatric nurse and a doctoral candidate in clinical psychology) then independently classified each medication and compared classifications to identify any possible coding errors. Each medication was classified as a selective serotonin reuptake inhibitor (SSRI), serotonin and norepinephrine reuptake inhibitor (SNRI), tetracyclic, tricyclic antidepressant (TCA), monoamine oxidase inhibitor (MAOI), norepinephrine-dopamine reuptake inhibitor (NDRI), antipsychotic, anxiolytic, other mental health medication, or other non-mental health medication.
2.4. Statistical analysis
Descriptive statistics were calculated to summarize the demographic characteristics and pre-treatment symptoms of those who were taking medications during assessment, of those who were not, and of all participants regardless of medication usage. We conducted independent samples t-tests to determine whether medication users and nonusers differed in age and pre-treatment symptoms and a series of chi-square analyses to determine whether users and nonusers differed across demographic variables. We also examined the percentage of participants who reported using each class of medication at pre-treatment, post-treatment, and three-month follow-up.
A series of multiple regression analyses were then conducted to determine whether usage of different medications predicted post-treatment symptoms of depression and anxiety. In half of these regressions, pre-treatment GAD-7 scores were input as a predictor in a first block, pre-treatment usage of each category of medication was input as a predictor in a second block, and post-treatment GAD-7 scores were input as the dependent variable. In parallel, the other half of the regressions included pre-treatment PHQ-9 scores in the first block, pre-treatment usage of all medication types in the second block, and post-treatment PHQ-9 scores as the dependent variable. Each of these regression analyses was then repeated using three-month follow-up scores instead of post-treatment scores as the dependent variable.
Next, we conducted a series of logistic regression analyses to determine whether medication usage predicted adherence. Each logistic regression simply included medication usage as a predictor and program completion as an outcome. As with the multiple regressions discussed above, each category of medication was included as a predictor in a separate regression model.
We repeated these multiple regression and logistic regression analyses for subgroups of participants whose pre-treatment symptom scores exceeded clinical cutoffs. Specifically, for participants with pre-treatment GAD-7 scores of 10 or greater, we conducted a series of multiple regressions to predict post-treatment GAD-7 scores and a series of logistic regressions to predict adherence. Likewise, for participants with pre-treatment PHQ-9 scores of 10 or greater, we conducted multiple regressions to predict post-treatment PHQ-9 scores and logistic regressions to predict adherence. These subgroup analyses were conducted using the same procedures as our primary regression analyses, as described above.
To further identify subgroups of medication users who might experience significantly different symptom outcomes, we also analyzed patient data using a recursive partitioning algorithm known as classification and regression trees (CART). CART is a commonly used technique for predicting patient outcomes in medical studies (Lemon et al., 2003). Although standard regression models are useful for determining the average effect of a predictor variable on an outcome variable, regression models can fail to identify important subgroups that are related to outcomes. Furthermore, when multiple predictor variables are potentially related to an outcome, selecting which variables to include in a regression model and interpreting the results can be complex. By contrast, CART is a nonparametric method specifically designed to identify subgroups in a population that are related to the outcome variable and is also well-suited to investigating the effect of multiple potential predictor variables. We therefore used the package rpart within the R statistical environment to create three clinical decision trees for predicting GAD-7 scores, PHQ-9 scores, and course completion (Therneau and Atkinson, 2015).
Finally, we conducted a series of paired samples t-tests to determine whether the intervention was effective for participants who began treatment using any medication, using no medication, or using medication belonging to each of the specific categories discussed above. For each of these groups, we compared participants' pre-treatment and post-treatment scores on the GAD-7 and PHQ-9. We conducted a second series of paired samples that was identical to the first in all respects except that we compared participants' pre-treatment and three-month follow-up scores. Our rationale for conducting these analyses was that they would address the intervention's efficacy among those who began treatment using medication independent of its efficacy among those who did not.
3. Results
3.1. Participant characteristics
Participants had a mean age of 37.92. The majority (71%) were female, and the overwhelming majority (90%) were white. Approximately half (45%) of the participants lived in large urban centers, while the other half (55%) lived in rural Saskatchewan. A substantial minority (36%) held university degrees. A slight majority of participants (63%) were in committed romantic relationships at assessment, and a considerable number (17%) were unemployed or on disability. Participants had mean pre-treatment GAD-7 scores of 11.95 (SD 5.19) and PHQ-9 scores of 12.24 (SD 5.88). Those who reported taking medications at assessment, as compared to those who did not, had higher PHQ-9 scores and were older, more likely to be white, less likely to hold university degrees, and more likely to be unemployed or on disability, all Ps < 0.05. See Table 1 below for details.
Table 1.
Participant characteristics.
| All participants (N = 1201) | Participants on medication (n = 723) | Participants not on medication (n = 478) | Significance of differencea | |
|---|---|---|---|---|
| Age, mean (SD) | 37.92 (12.84) | 38.98 (12.77) | 36.33 (12.79) | t(1199) = −3.51, p < .001 |
| Female, n (%)b | 845 (70.7) | 505 (69.8) | 340 (71.4) | χ2(1, N = 1195) = 0.20, p = .658 |
| White, n (%) | 1071 (89.8) | 658 (91.6) | 413 (87.1) | χ2(1, N = 1201) = 6.33, p = .012 |
| Urban, n (%) | 536 (44.6) | 322 (44.5) | 214 (44.8) | χ2(1, N = 1201) = 0.006, p = .94 |
| University degree, n (%) | 430 (35.8) | 239 (33.1) | 191 (40.0) | χ2(1, N = 1201) = 5.96, p = .015 |
| Relationship, n (%) | 745 (62.5) | 448 (62) | 297 (62.7) | χ2(1, N = 1192) = 0.008, p = .93 |
| Unemployed, n (%) | 102 (8.5) | 71 (9.8) | 31 (6.5) | χ2(1, N = 1201) = 4.02, p = .04 |
| Unemployed or disability, n (%) | 208 (17.3) | 155 (21.4) | 53 (11.1) | χ2(1, N = 1201) = 21.53, p < .001 |
| Pre-treatment GAD-7 scores, mean (SD) | 11.95 (5.19) | 12.11 (5.17) | 11.69 (5.22) | t(1181) = −1.37, p = .17 |
| Pre-treatment PHQ-9 scores, mean (SD) | 12.24 (5.88) | 13.10 (5.87) | 10.91 (5.64) | t(1182) = −6.37, p < .001 |
categorical variables.
The p-values displayed in this column were derived from t-tests for continuous variables and chi-square tests.
As many as 19 participants did not respond to certain items. Percentages in this table represent percentages of responses rather than percentages of participants overall.
3.2. Medication usage
At assessment, 723 out of 1201 (60%) participants reported taking psychotropic medications. At post-treatment, 460 of the 836 (55%) participants who had completed the intervention reported taking psychotropic medications, indicating a slight decrease in medication usage over the course of the intervention. The number of participants who reported taking medications belonging to each class of medications at assessment is displayed in Table 2 below.
Table 2.
Number of participants using each medication and mean symptom change.
| Participants at pre-treatment, n | Participants who completed post-treatment questionnaires, n (%)a | Mean difference on GAD-7 | Mean difference on PHQ-9 | |
|---|---|---|---|---|
| Total sample | 1201 | 836 (69.6) | −5.98 (5.14) | −5.73 (5.08) |
| Not on medication | 478 | 326 (68.2) | −6.27 (5.05) | −5.29 (4.75) |
| On any medication | 723 | 510 (70.5) | −5.80 (5.19) | −6.03 (5.26) |
| On SSRIs | 410 | 288 (70.2) | −6.08 (5.13) | −6.34 (5.18) |
| On anxiolytics | 180 | 128 (71.1) | −6.73 (5.05) | −6.37 (5.21) |
| On SNRIs | 164 | 110 (67.1) | −5.69 (4.96) | −6.22 (5.38) |
| On antipsychotics | 101 | 67 (66.3) | −5.01 (5.31) | −5.03 (5.25) |
| On NDRIs | 88 | 66 (75) | −5.26 (5.36) | −5.41 (5.37) |
| On tetracyclics | 72 | 48 (66.7) | −3.25 (4.68) | −5.35 (4.31) |
| On TCAs | 16 | 15 (93.8) | −5.07 (5.01) | −3.87 (4.34) |
| On MAOIs | 1 | 1 (100) | 3.00 | 0.00 |
| On other mental health medications | 52 | 36 (69.2) | −4.25 (5.72) | −5.11 (6.47) |
| On other non-mental health medications | 83 | 62 (74.7) | −5.63 (5.04) | −6.52 (5.85) |
This column displays, for each medication, the number and percentage of participants who began treatment on that medication and completed post-treatment questionnaires, rather than the absolute number of participants on that medication at post-treatment.
3.3. Adherence
Of the 1201 participants who began The Wellbeing Course, 880 accessed all five lesson (73%), and 836 (70%) completed pre and post-treatment measures. Logistic regression analyses indicated that pre-treatment usage of any medication and usage of medications belonging to any specific class did not significantly predict completion, all Ps > 0.06. Subgroup analyses for participants with GAD-7 scores of 10 or greater and participants with PHQ-9 scores of 10 or greater also showed that completion was not predicted by pre-treatment use of any medication or medication belonging to any class, all Ps > 0.06. Descriptively, across classes of medications that participants reported using at assessment, completion rates ranged from 66% to 100%.
3.4. Symptom change
Regression analyses of anxiety symptoms showed that after accounting for pre-treatment GAD-7 scores, the use of any medication predicted higher post-treatment GAD-7 scores (beta coefficient = 0.73 points, 95% CI 0.13 to 1.33, P = .02). Further regression analyses using each medication class showed that higher post-treatment GAD-7 scores were predicted—again, after accounting for pre-treatment GAD-7 scores—by pre-treatment use of tetracyclics (beta coefficient = 2.41 points, 95% CI 1.16 to 3.66, P < .001), antipsychotics (beta coefficient = 1.53 points, 95% CI 0.46 to 2.60, P = .005), and other mental health medications (beta coefficient = 2.33 points, 95% CI 0.90 to 3.76, P = .001). Subgroup analyses revealed an identical pattern of findings—based on the direction and statistical significance of regression coefficients—among participants with pre-treatment GAD-7 scores of 10 or greater.
Regression analyses of depression symptoms showed that after accounting for pre-treatment PHQ-9 symptoms, higher post-treatment PHQ-9 symptoms were predicted by pre-treatment use of TCAs (beta coefficient = 2.60 points, 95% CI 0.34 to 4.85, P = .02), NDRIs (beta coefficient = 1.20 points, 95% CI 0.09 to 2.31, P = .03), antipsychotics (beta coefficient = 1.34 points, 95% CI 0.24 to 2.44, P = .02), and other mental health medications (beta coefficient = 1.87 points, 95% CI 0.39 to 3.35, P = .01). A similar pattern of findings was observed among participants with pre-treatment PHQ-9 scores of 10 or greater, except that post-treatment PHQ-9 scores were only statistically significantly predicted by pre-treatment use of TCAs and antipsychotics. Further regression analyses showed that higher GAD-7 symptoms at three-month follow-up were predicted by pre-treatment use of MAOIs (beta coefficient = 8.45 points, 95% CI 0.39 to 16.5, P = .04), antipsychotics (beta coefficient = 1.57 points, 95% CI 0.14 to 3.01, P = .03), and other non-mental health medications (beta coefficient = 1.63 points, 95% CI 0.09 to 3.18, P = .04). Higher PHQ-9 symptoms at three-month follow-up were predicted by pre-treatment use of non-mental health medications (beta coefficient = 1.74 points, 95% CI 0.09 to 3.38, P = .04).
Although regression revealed small effects of some medications on symptom change and dropout, applying the CART algorithm revealed no significant predictors of PHQ-9 change scores or completion rates. When analyzing GAD-7 scores using the CART method, however, tetracyclic usage at pre-treatment was found to be related to a smaller improvement over the course of treatment. Among the 790 participants who completed the post-treatment GAD-7 measure, the 49 participants who reported pre-treatment tetracyclic use experienced a mean reduction of 6.1 points on the GAD-7, while the remaining 741 participants experienced a mean reduction of 7.5 points. Notably, the same analyses were run again including only the patients who scored above the clinical cutoff score of 10 or above on the PHQ-9 and GAD-7, and the overall results were the same.
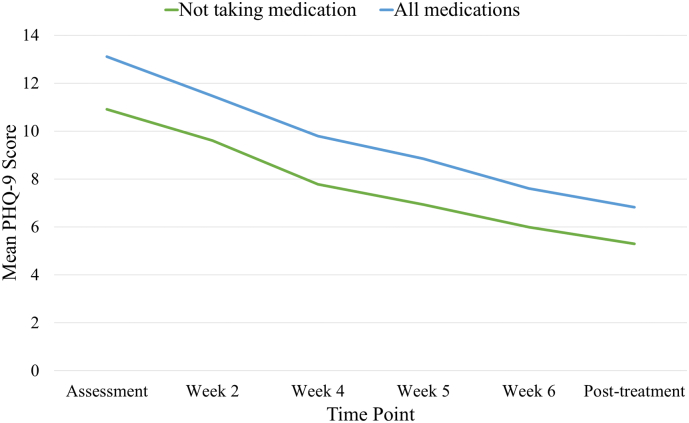
Descriptively, mean symptom change scores for participants who were taking medications at assessment were −5.80 (SD 5.19) on the GAD-7 and −6.03 (SD 5.26) on the PHQ-9. By comparison, participants who were not taking any medications at assessment experienced symptom changes of −6.27 (SD 5.05) and −5.29 (4.75) on the GAD-7 and PHQ-9, respectively. The mean pre-treatment-to-post-treatment GAD-7 and PHQ-9 difference scores for participants taking medications of each class at assessment are displayed below in Table 2. Symptom change over time for participants who reported pre-treatment medication use and for participants who did not is shown in Fig. 1, Fig. 2 for GAD-7 and PHQ-9 scores, respectively. A series of paired samples t-tests showed that participants using no medication, any medication, or any specific category of medication (excluding MAOIs, which only 1 participant reported using pre-treatment) experienced significant pre-post reductions in GAD-7 and PHQ-9 scores, all Ps < 0.01. An identical pattern of results (again, excluding MAOIs) was found for paired samples t-tests comparing pre-treatment and three-month follow-up scores, all Ps < 0.01.
Fig. 1.
GAD-7 score changes by pre-treatment medication use.
Fig. 2.
PHQ-9 score changes by pre-treatment medication use.
4. Discussion
This study reviewed patient case records from 1201 patients who received TD-ICBT for anxiety and depression through a publicly-funded free ICBT serving residents of the Canadian province of Saskatchewan. Concurrent medication usage was found to be very common in this population (60%), with SSRI, SNRI, and anxiolytics being the most commonly prescribed medications. A small pre-to-post reduction in medication usage (from 60 to 55%) was mainly attributable to a reduction in use of anxiolytics and remained at three-month follow-up. The most common medications used that were classified as anxiolytics were the benzodiazepines lorazepam and alprazolam, which are typically prescribed for short-term relief of anxiety. It is therefore plausible that the reported reduction in medication usage is the result of the patient acquiring other skills for coping with anxiety attacks, such as the controlled breathing and thought challenging exercises taught in TD-ICBT. It is also possible that patients lowered medications use as a result of experiencing symptom improvement related to graded exposure for anxiety/panic during the course. If anxiolytic medication interfered with treatment progress, the results of the present study may underrepresent the extent to which it did so, because anxiolytic medication usage reduced slightly during treatment. Further research into concurrent anxiolytic medication among ICBT patients should be conducted and researchers should also consider asking patients whether they consider reducing medication usage a goal of therapy.
This study investigated how concurrent medication usage among TD-ICBT patients was related to course completion rates and symptom change. No difference in course completion rates was found between patients who were or were not on medication and no specific class of medication significantly predicted risk of dropping out of the TD-ICBT course before completion. Both patients on medication and those not on medication reported significant reductions in mean symptom scores from pre-treatment to post-treatment; however, this study detected some noteworthy differences in patient symptom trajectories between those on medication and those not on medication.
Consistent with the hypothesis that TD-ICBT patients receiving concurrent pharmacological treatment are a population with somewhat more severe symptoms, patients on medication reported somewhat higher mean symptom scores at pre-treatment and post-treatment. Given the difference in pre-treatment scores between those who were on and those who were not on medication, it was important to account for pre-treatment severity in our analyses, as previous research has shown that higher pre-treatment scores are related to greater symptom reductions among TD-ICBT patients (Edmonds et al., 2018). After accounting for pre-treatment scores, regression models revealed some significant relationships between medication usage and post-treatment scores. The regression models indicated that the use of any psychotropic medication at pre-treatment predicted smaller reductions in anxiety symptoms, but use of any psychotropic medication usage did not predict changes in depression symptoms. To further investigate the effects of each individual class of medications on patient symptoms, we conducted further regressions and found that smaller improvements in anxiety were related to pre-treatment usage of tetracyclic, antipsychotic, or other mental health medications, while smaller improvements in depression symptoms were associate with pre-treatment usage of antipsychotic, TCA, NDRI other mental health medication usage. To help decide which classes of medications might be most important in terms of predicting symptom trajectories, we also applied the CART approach to predict symptom change scores. Consistent with the regression results, CART analyses showed that tetracyclic usage was related to smaller improvements in anxiety symptoms, but no other medications were found to be significantly related to symptom change using the CART technique.
Possible explanations for the finding that TD-ICBT patients taking medication improve somewhat less include the possibility that medication has interfered with the treatment process or that individuals on medication represent a population with somewhat more treatment resistant symptoms. Although this finding highlights the need for further research on how medication affects treatment outcomes, it should again be noted that all medication groups reported large reductions in mean symptom scores and our results support the continued referral of patients receiving psychotropic medications to TD-ICBT treatment.
Although patients on tetracyclic medications reported smaller reductions in anxiety symptoms compared to patients not taking tetracyclics, they still reported benefiting overall compared to pretreatment, and they still reported reductions in depression symptoms similar to other medication users. Mirtazipine was the most common tetracyclic medication reported. Notably, 46% of patients who reported taking tetracyclics at screening were also taking an SSRI medication. Many treatment guidelines suggest prescription of an SSRI as the first line pharmacological option for depression and anxiety (National Collaborating Centre for Mental Health, 2009), so it may be the case that patients taking tetracyclics at admission to TD-ICBT are longer-term patients who have tried many different treatments and more modest reductions would be expected in this group. Overall, the findings of this study suggest that medication users also benefit from TD-ICBT, with the caveat that they, and tetracyclic users in particular, may experience somewhat smaller improvements in symptoms than other TD-ICBT patients.
4.1. Limitations
An important limitation of this study is that the results may not generalize to other populations or other ICBT interventions. The context in which the TD-ICBT program used in this study is offered could have affected results in a couple of different ways. The TD-ICBT program is offered free of charge with support by the government of Saskatchewan; family physicians and mental health providers are informed of this service and encouraged to refer patients to this service. It is possible that the free program may differentially attract low-income individuals who do not have insurance coverage and the results of this study may therefore not generalize well in other populations. Additionally, while patients learn about ICBT from diverse sources, all users of ICBT must initiate the treatment process and therefore may be more likely to benefit than the wider population due to their beliefs about ICBT. Notably, patients in this study had moderate symptom severity and results may be different in more severe populations. Medication may also have different effects on participants of disorder-specific ICBT programs for anxiety and depression, or ICBT programs for other disorders.
The medication usage data collected during this study is limited to three time points and due to the retrospective nature of this study we did not have access to more extensive histories of medication usage and symptoms from patients. The nature of the primary symptom measures used (i.e., GAD-7 and PHQ-9) also imposes some limitations on our conclusions because these measures ask patients about the frequency of symptoms but not symptom severity. Furthermore, analyzing patient symptom data with many regressions produces a high family-wise error rate which could have influenced our results. Finally, we were only able to investigate the effects of medications that were commonly used in this population and the possibility remains that some rarer medications could have larger impacts on TD-ICBT outcomes. A future study with a larger sample would allow for analysis of the less common medication classes and greater power to look for small effect sizes.
4.2. Implications
Given how common medication usage is in this population, designers of future TD-ICBT programs should consider including information about common medications and the role they may play in a patient's experience of anxiety and depression. Additionally, e-therapists working for TD-ICBT programs may wish to educate themselves on the most commonly used classes of medication in order to include medication usage in case conceptualizations and account for the potential effects and side-effects of medications that patients are likely to experience as a result of simultaneous pharmacotherapy. Finally, health care practitioners considering referring a patient for TD-ICBT to treat anxiety and depression should be aware that on average, patients receiving simultaneous pharmacotherapy are likely to benefit from TD-ICBT.
Declaration of competing interest
Titov and Dear are authors of the Wellbeing Course but received no financial benefit from its use in this study. The other authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgments
The authors would like to thank Annemieke Kidd for her assistance in classifying participants' reported medications. Author ME received funding to support this work from the Canadian Institutes of Health Research (reference #412347). HM received funding from the Social Sciences and Humanities Research Council of Canada and the University of Regina. HH was funded by the Canadian Institutes of Health Research (reference # 293379 & 152917), Saskatchewan Health Research Foundation, Rx & D Health Research Foundation and Saskatchewan Ministry of Health. Funders had no involvement in the study design, collection, analysis, or interpretation of the data. N.T. and B.F.D. are funded by the Australian Government to operate the national MindSpot Clinic.
Abbreviations
- CBT
cognitive behavioural therapy
- GAD-7
Generalized Anxiety Disorder 7-item scale
- ICBT
Internet-delivered cognitive behavioural therapy
- MAOI
monoamine oxidase inhibitor
- NDRI
norepinephrine–dopamine reuptake inhibitor
- PHQ-9
Patient Health Questionnaire 9-item scale
- SNRI
serotonin-norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- TCA
tricyclic antidepressant
- TD-ICBT
transdiagnostic Internet-delivered cognitive behavioural therapy
References
- Alaoui S.E., Ljótsson B., Hedman E., Svanborg C., Kaldo V., Lindefors N. Predicting outcome in internet-based cognitive behaviour therapy for major depression: a large cohort study of adult patients in routine psychiatric care. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson G., Carlbring P., Titov N., Lindefors N. Internet interventions for adults with anxiety and mood disorders: a narrative umbrella review of recent meta-analyses. Can. J. Psychiatr. 2019, July 1;64:465–470. doi: 10.1177/0706743719839381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B., Brasser M. Clinical suitability of GAD-7 scale compared to hospital anxiety and depression scale-A for monitoring treatment effects in generalized anxiety disorder. Eur. Neuropsychopharmacol. 2009;19(3):604–605. [Google Scholar]
- Cientanni F., Power K., Wright C., Sani F., Reilly D., Blake M.L., Clark S. Psychosocial, psychopharmacological and demographic predictors of changes in psychological distress over a course of computerised cognitive behavioural therapy (cCBT) Internet Interv. 2019;17 doi: 10.1016/j.invent.2019.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P. Four decades of outcome research on psychotherapies for adult depression: an overview of a series of meta-analyses. Can. Psychol. 2017;58:7–19. [Google Scholar]
- Cuijpers P., Sijbrandij M., Koole S.L., Andersson G., Beekman A.T., Reynolds C.F. The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: a meta-analysis of direct comparisons. World Psychiatry. 2013;12(2):137–148. doi: 10.1002/wps.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Hadjistavropoulos H.D., Schneider L.H., Dear B.F., Titov N. Who benefits most from therapist-assisted internet-delivered cognitive behaviour therapy in clinical practice? Predictors of symptom change and dropout. Journal of Anxiety Disorders. 2018;54:24–32. doi: 10.1016/j.janxdis.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos H.D., Pugh N.E., Hesser H., Andersson G. Predicting response to therapist-assisted internet-delivered cognitive behavior therapy for depression or anxiety within an open dissemination trial. Behav. Ther. 2016;47(2):155–165. doi: 10.1016/j.beth.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B.W., Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen. Hosp. Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Lemon S.C., Roy J., Clark M.A., Friedmann P.D., Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann. Behav. Med. 2003;26(3):172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- Lindner P., Nyström M.B.T., Hassmén P., Andersson G., Carlbring P. Who seeks ICBT for depression and how do they get there? Effects of recruitment source on patient demographics and clinical characteristics. Internet Interv. 2015;2(2):221–225. [Google Scholar]
- Manea L., Gilbody S., McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health . NICE guidelines [CG90] 2009. Depression: The NICE Guideline on the Management of Depression in adults.http://www.ncbi.nlm.nih.gov/books/NBK55364/ Retrieved from. [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Therneau T.M., Atkinson E.J. An introduction to recursive partitioning using the RPART routines. Mayo Clinic Division of Biostatistics. 2015 https://cran.r-project.org/web/packages/rpart/vignettes/longintro.pdf [Google Scholar]
- Titov N., Dear B.F., Schwencke G., Andrews G., Johnston L., Craske M.G., McEvoy P. Transdiagnostic internet treatment for anxiety and depression: a randomised controlled trial. Behav. Res. Ther. 2011;49(8):441–452. doi: 10.1016/j.brat.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Titov N., Dear B., Nielssen O., Staples L., Hadjistavropoulos H., Nugent M., Kaldo V. ICBT in routine care: a descriptive analysis of successful clinics in five countries. Internet Interv. 2018;13:108–115. doi: 10.1016/j.invent.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T., Barber R.M., Bell B., Bertozzi-Villa A., Biryukov S., Bolliger I., Murray C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.W., Bystritsky A., Russo J.E., Craske M.G., Sherbourne C.D., Stein M.B., Roy-Byrne P.P. Beliefs about psychotropic medication and psychotherapy among primary care patients with anxiety disorders. Depression and Anxiety. 2005;21(3):99–105. doi: 10.1002/da.20067. [DOI] [PubMed] [Google Scholar]
- Wiles N., Thomas L., Abel A., Ridgway N., Turner N., Campbell J., Lewis G. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet. 2013;381(9864):375–384. doi: 10.1016/S0140-6736(12)61552-9. [DOI] [PubMed] [Google Scholar]