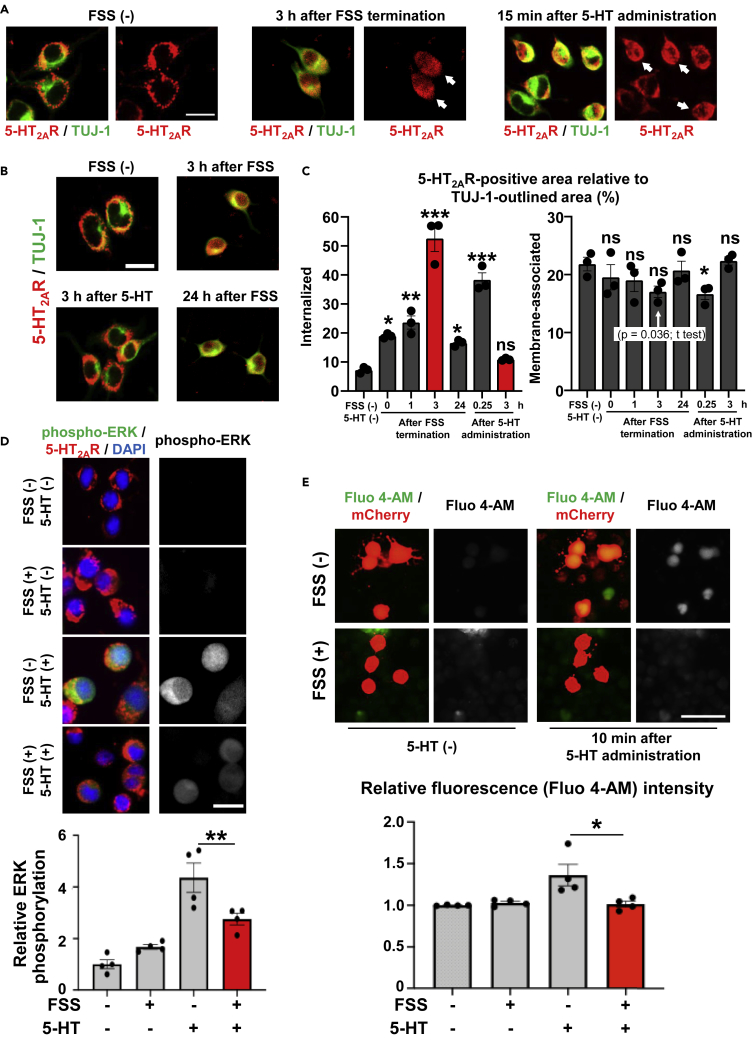
Figure 3.
Fluid Shear Stress (FSS) Internalizes 5-HT2A Receptor Expressed in Neuro2A Cells and Modulates Their Responses to 5-HT
(A) 5-HT2A receptor was internalized after FSS. Neuro2A cells grown in a poly-D-lysine-coated dish were subjected to pulsatile FSS (average 0.91 Pa, 0.5 Hz, 30 min) or treated with 5-HT (10 μM), fixed, and stained for 5-HT2A receptor (5-HT2AR; red). To define their soma outlines, co-immunostaining of TUJ-1 was conducted (green). Left, control; middle, 3 h after the termination of FSS; right, 15 min after 5-HT administration. Arrows point to cells with apparent 5-HT2A receptor internalization. Scale bar, 20 μm. Images are representative of three independent experiments with similar results.
(B and C) 5-HT2A receptor internalization lasted longer after FSS, when compared with after 5-HT administration. (B) Micrographic images of Neuro2A cells, with and without FSS application, stained for 5-HT2A receptor (5-HT2AR; red) and TUJ-1 (green). Scale bar, 20 μm. Images are representative of three independent experiments with similar results. (C) Quantification of internalized and membrane-associated 5-HT2A receptor-positive area relative to TUJ-1-outlined area in Neuro2A cells. Internalized and membrane-associated 5-HT2A receptor-positive immunosignals were defined as in Figure S2D and quantified as in Figure 1J (left chart: FSS, p < 0.0001 for ANOVA, p = 0.014 for 0 h, p = 0.0016 for 1 h, p < 0.0001 for 3 h, p = 0.049 for 24 h. 5-HT, p < 0.0001 for ANOVA, p < 0.0001 for 0.25 h, p = 0.20 for 3 h. Right chart: FSS, p = 0.36 for ANOVA, p = 0.73 for 0 h, p = 0.58 for 1 h, p = 0.19 for 3 h, p = 0.97 for 24 h. 5-HT, p = 0.010 for ANOVA, p = 0.016 for 0.25 h, p = 0.89 for 3 h, one-way ANOVA with post hoc Dunnett's test; 20 cells analyzed in each sample, n = 3 for each group).
(D) FSS alleviated 5-HT-induced ERK phosphorylation in neuronal cells. Neuro2A cells were either left unexposed or exposed to pulsatile FSS (average 0.91 Pa, 0.5 Hz, 30 min). Three hours after FSS termination, cells were treated with 5-HT (10 μM) for 15 min, fixed and stained for 5-HT2A receptor (5-HT2AR; red), phospho-ERK (green), and DAPI (blue). Top, micrographic images. Scale bar, 25 μm. Bottom, Quantification of anti-phospho-ERK immuno-intensity: signal intensity of anti-phospho-ERK immunostaining was quantified using “auto-threshold” of ImageJ, and immuno-intensity was calculated by referring the cumulated intensity values to the total positive signal area and scaled with the mean of the control sample (FSS-, 5-HT-) set at 1 (p = 0.0058, one-way ANOVA with post hoc Bonferroni test, 50 cells analyzed in each sample, n = 4 for each group).
(E) FSS attenuated 5-HT-induced increase in intracellular Ca2+ concentration. Neuro2A cells were either left unexposed or exposed to FSS (average 0.91 Pa, 0.5 Hz, 30 min). Three hours after the termination of FSS, cells were treated with 5-HT (10 μM) for 10 min and subjected to measurement of intracellular Ca2+ concentration using Fluo 4-AM as described in Methods. Top, micrographic images. Scale bar, 50 μm. Bottom, intracellular Ca2+ concentration represented as relative fluorescence intensity with the mean fluorescence value from cells before 5-HT administration set as 1 (p = 0.0138, one-way ANOVA with post hoc Bonferroni test; 50 cells analyzed in each sample, n = 4 for each group).
Data are represented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant. See also Figure S2.