Abstract
If dental caries (or tooth decay) progresses without intervention, the infection will advance through the dentine leading to severe pulpal inflammation (irreversible pulpitis) and pulp death. The current management of irreversible pulpits is generally root-canal-treatment (RCT), a destructive, expensive, and often unnecessary procedure, as removal of the injurious stimulus alone creates an environment in which pulp regeneration may be possible. Current dental-restorative-materials stimulate repair non-specifically and have practical limitations; as a result, opportunities exist for the development of novel therapeutic strategies to regenerate the damaged dentine-pulp complex. Recently, epigenetic modification of DNA-associated histone ‘tails’ has been demonstrated to regulate the self-renewal and differentiation potential of dental-stem-cell (DSC) populations central to regenerative endodontic treatments. As a result, the activities of histone deacetylases (HDAC) are being recognised as important regulators of mineralisation in both tooth development and dental-pulp-repair processes, with HDAC-inhibition (HDACi) promoting pulp cell mineralisation in vitro and in vivo. Low concentration HDACi-application can promote de-differentiation of DSC populations and conversely, increase differentiation and accelerate mineralisation in DSC populations. Therapeutically, various HDACi solutions can release bioactive dentine-matrix-components (DMCs) from the tooth’s extracellular matrix; solubilised DMCs are rich in growth factors and can stimulate regenerative processes such as angiogenesis, neurogenesis, and mineralisation. The aim of this mini-review is to discuss the role of histone-acetylation in the regulation of DSC populations, while highlighting the importance of HDAC in tooth development and dental pulp regenerative-mineralisation processes, before considering the potential therapeutic application of HDACi in targeted biomaterials to the damaged pulp to stimulate regeneration.
Keywords: histone deacetylases, dentinogenesis, regenerative endodontics, dental pulp, acetylation, histone acetyltransferases
Introduction
Dental caries (decay) is the most prevalent global non-communicable disease (WHO, 2017). The caries process initiates with a microbial biofilm forming on the tooth surface, which ‘fuelled’ by a dietary source of fermentable carbohydrates, ecologically shifts the plaque to an acidogenic flora, breaking down the hard tooth tissues of enamel and dentine (Nyvad et al., 2013). If the carious lesion progresses without remedial treatment, the pulp tissue in the centre of the tooth will become progressively infected and inflamed (Mjor and Tronstad, 1972; Mjor and Tronstad, 1974). The pulpal inflammation (pulpitis) provokes a robust defensive reaction with new dentine produced by the pulp’s secretory cells, the odontoblasts, locally beneath the caries in a process called reactionary dentinogenesis (Smith, 2002). If the advancing caries continues until the bacteria invade the pulp tissue, odontoblast death will occur, prior to more widespread pulpal necrosis. Traditional treatment for pulp necrosis is root-canal-treatment (RCT) ( Table 1 ), which effectively removes all pulp tissue; however, this is a very destructive and empirical approach. The absence of vital pulp tissue has other consequences, including removal of the tooth’s developmental, reparative, and immune capacity as well as loss of the pulps proprioceptive sensors, accompanied by a significantly greater risk of fracture and tooth loss (Paphangkorakit and Osborn, 1998; Smith, 2002). The pulp; however, has considerable potential to regenerate if the insult is removed and the tooth effectively restored during vital-pulp-treatment (VPT) (Mjor and Tronstad, 1974). The damaged odontoblast layer can regenerate in a stem-cell (SC) led process, in which stem/progenitor cells cyto-differentiate under the influence of bioactive molecules released from the damaged dentine and pulp cells (Lesot et al., 1994; Smith et al., 2016; Neves et al., 2017). Unfortunately, current therapies, which aim to maintain and regenerate the pulp in VPT, are limited by low-quality hard-tissue formation and non-specific responses (Nair et al., 2008; Sangwan et al., 2013). As a result, there is significant interest in developing scientific understanding of the mechanisms that control dental SC (DSC) fate as well as identifying potential therapeutic targets to promote more effective tissue regenerative processes.
Table 1.
A list of abbreviations and definitions used in the text and figures.
| Abbreviations | Definition |
|---|---|
| BDNF | Brain-derived neurotrophic factor |
| BMP | Bone morphogenetic protein |
| DFPC | Dental follicle progenitor cell |
| DMC | Dentine matrix component |
| DMP | Dentin matrix acidic phosphoprotein 1 |
| DPC | Dental pulp cell |
| DPSC | Dental pulp stem cell |
| DSC | Dental stem cell |
| DSPP | Dentin sialophosphoprotein |
| ESC | Embryonic stem cell |
| FDA | US Food and Drug Administration |
| GDF-15 | Growth/differentiation factor 15 |
| GF | Growth factor |
| GNAT | GCN5-related N-acetyltransferases |
| HAT | Histone acetyl transferase |
| HDAC | Histone deacetylase |
| HDACi | Histone deacetylase inhibitor |
| LMK-235 | N-[[6-(hydroxyamino)-6-oxohexyl]oxy]-3,5-dimethyl-benzamide |
| MMP | Matrix metalloproteinase |
| MYST | MOZ, YBF2/SAS3, SAS2, and TIP60 |
| PDLC | Periodontal ligament cell |
| RCT | Root canal treatment |
| SAHA | Suberoylanilide hydroxamic acid |
| SC | Stem cell |
| TGF | Transforming growth factor |
| TSA | Trichostatin A |
| VPA | Valproic acid |
| VPT | Vital pulp treatment |
Epigenetic modulations, DNA-methylation and histone modifications, are important regulators of DSC fate (Gopinathan et al., 2013), with histone acetylation being identified as an important regulator of bone, periodontal ligament, and dental pulp mineralisation processes as well as being a target for therapeutic inhibition (Duncan et al., 2016; Huynh et al., 2016; Ricarte et al., 2016; Cantley et al., 2017). The acetylation of DNA-associated histone (and non-histone) proteins is controlled by the enzymes histone-deacetylases (HDACs) and histone-acetyl-transferases (HATs), which alter chromatin architecture in response to cellular needs, regulating transcription (Kouzarides, 2007). HATs or lysine acetyltransferases, are bi-substrate enzymes, which are generally divided into categories of which the GCN5-Related N-Acetyltransferases (GNAT) and MYST families the largest, although others such as CBP/p300 may also be functionally important (Lalonde et al., 2014). HATs are further classified by their nuclear or cytoplasmic distribution (Richman et al., 1988) and have been implicated in a range of inflammatory diseases (e.g. asthma) and cancer (Ito et al., 2002; Yang, 2004). To date, HATs have not been the focus of the same level of attention as HDACs in regenerative medical or dental research and although HAT inhibitors are available, in vitro performance has not been replicated therapeutically (Wu et al., 2009; Lasko et al., 2017). This has been attributed to the difficulty in designing effective HAT inhibitors, as they influence a range other cellular substrates and operate as part of multi-function complexes (Wapenaar and Dekker, 2016).
There are eighteen human HDAC enzymes categorised into four separate classes, with classes I, II, and IV containing zinc-dependent enzymes (Seto and Yoshida, 2014). Class I HDACs demonstrate ubiquitous expression, while class II show tissue-specific expression and cellular localisations (Montgomery et al., 2007). The importance of class II HDAC expression in mineralising tissues has been demonstrated in bone (Ricarte et al., 2016) and teeth (Klinz et al., 2012), with the individual isoforms, -6 (Westendorf et al., 2002), -5, and -4 (Nakatani et al., 2018), highlighted as being important cellular mediators which regulate osteoblast differentiation. HDACs’ roles in the regulation of mineralisation and developmental cellular processes (Gordon et al., 2015), also make them attractive therapeutic targets for pharmacological inhibition (Richon et al., 1996). Several HDAC inhibitors (HDACis), including trichostatin A (TSA), valproic acid (VPA), and suberoylanilide hydroxamic acid (SAHA), have been shown to have clinical application in a range of diseases including cancer and inflammatory and neurodegenerative disorders (Bolden et al., 2006; Das Gupta et al., 2016; Naftelberg et al., 2017). The medical and dental literature also reports that HDACis are associated with anti-inflammatory effects, pro-mineralisation, increased SC differentiation, and overall improved regenerative responses (Halili et al., 2009; Xu et al., 2009; Wang et al., 2010; Duncan et al., 2013; Luo et al., 2018). Consequently, HDACis have the potential to enhance dentine regenerative processes in VPT by directly influencing DSC populations (Duncan et al., 2012; Luo et al., 2018) and indirectly, by inducing the solubilisation of dentine matrix components (DMCs) rich in growth factors (GFs) and other bioactive molecules (Smith et al., 2016; Duncan et al., 2017). An emerging role for HDACs in tooth development and regeneration presents an opportunity for HDACi use in novel dental regenerative materials.
The following section of this mini-review is to discuss specifically the role of histone-acetylation in the regulation of DSC populations, while highlighting the importance of HDAC in tooth development (primary dentinogenesis) and dental pulp regenerative-mineralisation processes (tertiary dentinogenesis). Finally, the therapeutic regenerative potential of a topically applied HDACi as part of next-generation dental biomaterials to regenerate the damaged pulp is considered.
Review
The Need to Regenerate Dental Pulp Tissue
The tooth consists of the outermost enamel and inner dentine, which surround a centrally-placed connective tissue called the pulp. Enamel is a highly mineralised tissue produced by the ameloblast cell during tooth development; however, after eruption, enamel has no cellular capacity to continue development, repair, or regenerate. Dentine is formed by the secretory odontoblast cells, which reside at the interface between dentine and pulp, linking the two tissues in a structure that is known as the dentine-pulp-complex (Pashley, 1996). Primary dentine forms during tooth development; however, unlike enamel, secondary dentine continues to form throughout the life of the tooth and furthermore the tooth can repair damaged tissue by forming tertiary dentine in response to injurious stimuli, including caries or tooth wear (Lesot et al., 1994; Smith, 2002). There are two types of tertiary dentine, with reactionary dentine formed in response to mild to moderate irritation due to the upregulation of existing primary odontoblast activity and reparative dentine generated when severe irritation leads to odontoblast death followed by the regeneration of a new layer of odontoblast-like cells from SCs (Lesot et al., 1994).
The origin of the progenitor cells in reparative dentinogenesis is mesenchymal (Simon and Smith, 2014). Attributed to SC populations within the pulp (e.g. dental-pulp-SCs [DPSCs]) (Smith and Lesot, 2001), SCs migrating from outside the tooth (Feng et al., 2011; Frozoni et al., 2012) or undifferentiated mesenchymal cells from cell-rich and central pulp perivascular regions (e.g. pericytes) (Fitzgerald et al., 1990; Machado et al., 2016). DPSCs, reportedly account for between 1 and 5% of total permanent pulpal cells (Gronthos et al., 2000) and reside in perivascular areas potentially enabling their mobilisation to wound sites (Shi and Gronthos, 2003; Crisan et al., 2008; Casagrande et al., 2011). The dentine stores a plethora of bioactive DMCs including GFs, chemokines, bioactive-proteins, tissue proteases, and other mobilisation factors, which are released by the caries process and orchestrate healing contributing to regenerative process in the tooth (Smith, 2003; Smith et al., 2016; Duncan et al., 2017; Tomson et al., 2017). Certain dental materials exhibit the ability to solubilise DMCs and influence the quality of the new mineral tissue formed, with the outcome of VPT dependent on the dental biomaterial placed in contact with the pulp (Nair et al., 2008). Notably, calcium-silicate materials, such as mineral-trioxide-aggregate have now superseded calcium hydroxide (Bjorndal et al., 2017) as the VPT material of choice (Hilton et al., 2013). However, all current materials are limited by low-quality tertiary dentine formation, non-specific actions, and the absence of targeted components focused on tissue regenerative strategies (Duncan et al., 2011).
Regeneration processes within the dentine-pulp-complex require the presence of vital pulp tissue; however, if the inflammatory process is allowed to continue without treatment, pulp necrosis results. Regenerative endodontic efforts to avoid RCT and ‘regrow’ the dental pulp using either a SC-based (Iohara et al., 2011) or cell-homing (Shimizu et al., 2012) technique have demonstrated that pulpal regeneration is possible. DPSCs can be transplanted in vivo with a scaffold to form a new physiologically functioning pulp tissue (Nakashima et al., 2017) and although, development is hampered by expense, risk of immune-rejection, ethics, and other regulatory issues (Kim et al., 2013) these therapies have proceeded to clinical trial stage (Nakashima and Iohara, 2017). In an alternative revitalisation procedure, a decellularised or synthetic scaffold containing bioactive molecules such as GFs, pharmacological inhibitors, and mobilisation factors is placed into the root canal and endogenous SCs are ‘homed’ into the space before undergoing differentiation (Galler, 2016). Although, revitalisation can successfully develop a biological pulp replacement, current protocols do not specifically regenerate the odontoblast layer or indeed enable further tooth root growth, which may be necessary in under developed teeth (Shimizu et al., 2013; Eramo et al., 2018).
There is significant need to develop regenerative endodontic techniques by developing our understanding of the epigenetic processes, which control the fate and the odontogenic potential of various SC populations (Gopinathan et al., 2013; Ching et al., 2017). Histone acetylation is an obvious focus, playing a critical role in a wide range of biological processes including inflammation, mineralised tissue formation, and SC regulation, (Schroeder and Westendorf, 2005; Shuttleworth et al., 2010; Jamaladdin et al., 2014) and can be targeted by HDACi, potentially benefiting the regenerative response within VPT (de Boer et al., 2006; Duncan et al., 2016).
Histone Acetylation Regulation of Regenerative Mineralisation Processes
The nucleosome consists of tightly-coiled DNA, wrapped around a histone core. The core contains an octamer of histone proteins (H2A, H2B, H3, and H4), each with a positively charged N-terminal tail (Biswas et al., 2011). These tails extend from the core structure, facilitating post-translational modification by acetylation, methylation, phosphorylation, ubiquitination, and SUMOylation (Zhang and Reinberg, 2001). Histone acetylation generates an architecturally open chromatin structure, which is transcriptionally active, while deacetylation tightens the DNA-histone association and represses gene expression (Verdone et al., 2005). The enzymes, HAT and HDAC, mediate these processes. Histone modifications, in contrast to DNA-methylation, are highly labile, presenting attractive targets for therapeutic intervention (Kelly et al., 2010).
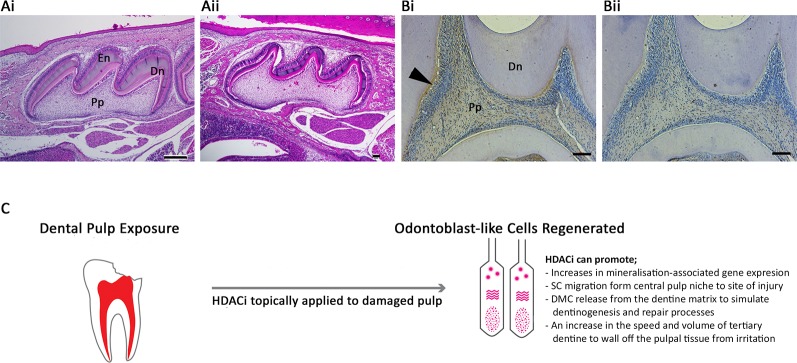
Notably altered HDAC expression occurs during osteogenesis (Westendorf et al., 2002; Schroeder et al., 2004), dentinogenesis (Klinz et al., 2012), and cementogenesis (Huynh et al., 2016) in a tissue-specific manner. Class I and II HDAC expression analysed in human tooth periodontal ligament cell (PDLC) cultures demonstrated that all of the five HDACs studied (HDAC-1 to -4 and -6) were highly expressed, although HDAC3 was downregulated during osteogenic differentiation (Huynh et al., 2016). Furthermore, a dental pulp study analysing extracted adult human molar teeth demonstrated that HDAC-2 and -9 were expressed in DPC, and exhibited a relatively strong expression in odontoblasts, while HDAC-1, -3, and -4 were relatively weakly expressed within the pulp tissue (Klinz et al., 2012). In the developing tooth, the role of histone methylation and demethylation has been studied (Zheng et al., 2014; Yi et al., 2016); however, currently little is known about the influence of acetylation in this process. Several studies have investigated the importance of HDACs in pulpal mineralisation processes and odontoblast differentiation in vitro (Duncan et al., 2012; Duncan et al., 2013; Paino et al., 2014), but further work is required to understand HDACs role during tooth development in vivo. Deletion of HDAC-4 in mice inhibited bone resorption and reduced thickness and cortical bone mass (Nakatani et al., 2016), and had the additional effect of inhibiting MMP-13 and Sost/sclerostin expression (Nakatani et al., 2018). Dentally, a mouse model of HDAC-4 KO demonstrated altered mineralisation in the roots of developing teeth (Ono et al., 2016) and the volume of enamel and dentine ( Figure 1A ). Other histological work has highlighted strong expression of another class II HDAC, -5, in the odontoblasts of developing teeth ( Figure 1B ). Supplementing DPSC cultures with HDACi has also indicated the importance of HDAC-3 downregulation during odontoblast differentiation (Jin et al., 2013), while HDAC-2 silencing in DPSCs promoted matrix mineralisation and related gene expression (Paino et al., 2014).
Figure 1.
Histone acetylation as a potential therapeutic target within the dentine-pulp complex. (A) Morphological comparison of post-natal day 10 maxillary first molar teeth of (Ai) WT and (Aii) HDAC4−/− mice using haematoxylin and eosin staining of sagittal sections highlighting differences in the volume of dentine and enamel deposited in the crown of the tooth. (Bi) Immunohistochemical analysis demonstrating HDAC-5 expression was evident in the odontoblasts (arrow), predentine layer, and pulp of WT adult first molar teeth in rats compared with (Bii) negative control. Dn = mineralised dentine; En = enamel; Pp = pulp tissue. Scale bars = (Ai) 250 μm, (Aii) 10 μm (original magnification x4), (Bi-ii) 50 μm (original magnification x10) (Duncan, 2017) (C) Schematic illustration of the potential of HDACi to be applied topically to damaged pulp tissue in a dental procedure to promote regenerative responses in VPT. Odontoblast-like cells are a replacement secretory cell after the death of primary odontoblast cells, which have been lost during the traumatic or carious insult. The differentiation of this cell type is crucial to the regeneration of dentine and mineralised tissue within the dentine-pulp complex. HDACi have been shown to augment several cellular processes central to this regenerative process, including increasing odontogenic gene expression, stimulating stem cell migration, promoting the release of bioactive dentine matrix components and accelerating mineralisation. SC, stem cell; DMC, dentine matrix component.
HDAC and HAT activity preserves the self-renewal capabilities of mesenchymal SCs (Romagnani et al., 2007; Lee et al., 2009; Jamaladdin et al., 2014) by maintaining expression of key pluripotent transcription factors, which are required to enable an open chromatin structure characteristic of embryonic SC (ESC) populations (Jamaladdin et al., 2014). Dental pulp tissue in adult teeth contains a characterised post-natal SC population of DPSCs (Gronthos et al., 2000) and as a result, modulators of SC behaviour have attracted significant interest in dentistry with suggestions that dental developmental anomalies, including dentine dysplasia and dentinogenesis imperfecta, may be related to dysregulated epigenetic modifications present during odontoblast differentiation (Sun et al., 2015). Epigenetic modifications and related differentiation profiles of two dental SC populations, DPSCs and dental follicle progenitor cells (DFPCs), were compared via the analysis of odontogenic gene expression including dentine sialophosphoprotein (DSPP) and dentin matrix acidic phosphoprotein 1 (DMP-1) (Gopinathan et al., 2013). Transcript levels were epigenetically-suppressed in DFPCs, while osteogenic stimulation in vitro demonstrated significant mineralisation increases only in DPSCs (Gopinathan et al., 2013). Notably, a highly dynamic histone modification response was demonstrated in mineralising DFPCs, but not in DPSCs, with the latter also expressing relatively high levels of the pluripotency-associated transcripts, Oct4 and Nanog. It was concluded that these two neural crest-derived SC populations were distinguished by epigenetic repression of dentinogenic genes with dynamic histone enrichment in DFPCs during mineralisation. This study highlighted the potential important role of epigenetic control in odontoblasts.
HDAC role in modulation of immune and inflammatory responses are also emerging (Leoni et al., 2002; Shanmugam and Sethi, 2013; Das Gupta et al., 2016), as well as, their role in angiogenesis (Mahpatra et al., 2010; Tsou et al., 2016) and neurogenesis (Cho and Cavalli, 2014), which are critical to the promotion of regenerative processes in the dental pulp. Together, these studies highlight that HDACs are involved in range of cellular events associated with the regeneration of dentine-pulp complex, suggesting their potential roles as therapeutic targets for VPT.
HDACi in Regenerative Endodontic Therapies
HDACis chemically include short-chain fatty acids, hydroxamic acids, cyclic peptides, and benzamides (Dokmanovic et al., 2007; Marks and Xu, 2009; Yusoff et al., 2019). VPA is a short-chain fatty acid that weakly inhibits class I and IIa HDACs, while the common hydroxamic-acid-based HDACis target classes I and II HDACs. HDACi are prime discovery targets for introduction into clinical trials including SAHA (Richon et al., 1998), also known as Vorinostat, being the first HDACi to obtain US FDA-approval in 2006 for treatment of lymphoma (Grant et al., 2007). Although HDACs are critical to the control of transcription, less than 5% of expressed genes are altered by low-dose HDACi in primary DPC cultures (Duncan et al., 2016).
Pharmacological inhibition of HDACs can modulate dental-derived SC populations and promote odontoblast-like cell differentiation and mineralised tissue formation (Kwon et al., 2012; Duncan et al., 2013; Paino et al., 2014). Application of pan-HDACi, TSA, VPA, and SAHA, to rodent and human DPSC cultures enhanced mineralisation, accompanied by an upregulation of genes associated with odontoblast differentiation and mineralisation, including TGF-β1, bone morphogenic proteins (BMPs), DMP, and DSPP (Duncan et al., 2012; Duncan et al., 2013; Paino et al., 2014; Duncan et al., 2016). In contrast to the general upregulation of mineralisation-associated transcripts, the expression of the bone metabolism marker osteocalcin was reduced, a result attributed to the use of VPA (Jin et al., 2013; Paino et al., 2014). HDACis reduced cell proliferation and viability at relatively high doses, but at lower doses did not show cytotoxic or anti-proliferative effects (Duncan et al., 2013; Paino et al., 2014; Duncan et al., 2017). SAHA was also shown to promote other reparative processes in DPC populations, including cell migration (Duncan et al., 2016; Luo et al., 2018) and cell adhesion (Luo et al., 2018). In addition to the direct regulation of SCs, HDACis also induce bioactive DMC release from dentine (Duncan et al., 2017). Bioactive molecules ‘fossilised’ within the dentine matrix (Cassidy et al., 1997; Smith, 2003; Grando Mattuella et al., 2007), can be released by caries, trauma, or by dental materials (Graham et al., 2006; Tomson et al., 2007). Released DMCs regulate the cyto-differentiation of progenitor cells and subsequent reparative dentine formation with bioactive components including BMPs and other GFs (Smith et al., 2016). Three HDACis, SAHA, TSA, and VPA, extracted a range of GFs from dentine, less efficiently than the well-characterised extractant EDTA for certain GFs (e.g. TGF-β1), but more effectively for others (e.g. Growth/differentiation factor 15 [GDF-15], Brain-derived neurotrophic-factor [BDNF]), while interestingly each HDACi exhibited a different extraction profile (Duncan et al., 2017). Furthermore, an in vivo study analysed the development of the dentine-pulp complex after systemic injection of TSA into prenatal mice and highlighted an increase in odontoblast numbers and dentine thickness compared with control specimens (Jin et al., 2013).
Currently, most research in medicine and dentistry employs pan-inhibitors; however, isoform-specific HDACis have been developed (Khan et al., 2008; Muraglia et al., 2008). It is proposed that isoform selectivity will counteract the multiple, often opposing cellular effects of HDACs (Balasubramanian et al., 2009) and reflect tissue-dependent expression of class II HDAC enzymes in particular (Verdin et al., 2003). For example, LMK-235 selectively inhibits HDAC-4 and -5 and was reported to upregulate odontoblast differentiation from human DPSCs (Liu et al., 2018). From a therapeutic perspective, low-dose short-duration HDACi application promotes DPC regenerative processes highlighting an opportunity for its use in next-generation VPT biomaterials (Duncan et al., 2016). Ethical, regulatory, and cost-effectiveness appraisal will need to be considered and material science aspects developed in order to create a controlled delivery-mechanism for the pulp. Notably, dental biomaterials containing antibiotics are commercially available (Imazato et al., 2007; Kamocki et al., 2015). Certainly, the low-dose, topical route of administration in dentistry should reduce the likelihood of systemic side effects such as fatigue, nausea, vomiting, diarrhoea, and thrombocytopenia (Subramanian et al., 2010), which have been reported following systemic-administration of HDACis at high-dose and frequency for cancer therapy.
Conclusion
A range of HDACs are expressed in the dentine-pulp complex and pharmacologically targeting them promotes a range of regenerative processes in DPC populations. Acetylation is central to orchestrating the differentiation and de-differentiation potential of DPSCs and understanding the intricacies of this control is crucial to enable pulpal regenerative responses as well as for designing novel therapeutic solutions. Further translational research is required to address clinical application and safety concerns in combination with scientific research to understand the mechanisms of epigenetic regulation of DPSC populations.
Author Contributions
YY searched the literature, wrote and edited the manuscript. PC and AS provided guidance and edited the manuscript. ES and YK provided guidance, contributed to the figures and edited the manuscript. HD planned, provided guidance, wrote sections, contributed to the figures and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
ES work contained within this review was supported by National Institutes of Dental and Craniofacial Research grant R01-DE025885.
References
- Balasubramanian S., Verner E., Buggy J. J. (2009). Isoform-specific histone deacetylase inhibitors: the next step? Cancer Lett. 280 (2), 211–221. 10.1016/j.canlet.2009.02.013 [DOI] [PubMed] [Google Scholar]
- Biswas M., Voltz K., Smith J. C., Langowski J. (2011). Role of histone tails in structural stability of the nucleosome. PloS Comput. Biol. 7 (12), e1002279. 10.1371/journal.pcbi.1002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndal L., Fransson H., Bruun G., Markvart M., Kjaeldgaard M., Nasman P., et al. (2017). Randomized clinical trials on deep carious lesions: 5-year follow-up. J. Dent. Res. 96 (7), 747–753. 10.1177/0022034517702620 [DOI] [PubMed] [Google Scholar]
- Bolden J. E., Peart M. J., Johnstone R. W. (2006). Anticancer activities of histone deacetylase inhibitors. Nat. Rev. Drug Discovery 5 (9), 769–784. 10.1038/nrd2133 [DOI] [PubMed] [Google Scholar]
- Cantley M. D., Zannettino A. C. W., Bartold P. M., Fairlie D. P., Haynes D. R. (2017). Histone deacetylases (HDAC) in physiological and pathological bone remodelling. Bone 95, 162–174. 10.1016/j.bone.2016.11.028 [DOI] [PubMed] [Google Scholar]
- Casagrande L., Cordeiro M. M., Nor S. A., Nor J. E. (2011). Dental pulp stem cells in regenerative dentistry. Odontology 99 (1), 1–7. 10.1007/s10266-010-0154-z [DOI] [PubMed] [Google Scholar]
- Cassidy N., Fahey M., Prime S. S., Smith A. J. (1997). Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch. Biol. 42 (3), 219–223. 10.1016/S0003-9969(96)00115-X [DOI] [PubMed] [Google Scholar]
- Ching H. S., Luddin N., Rahman I. A., Ponnuraj K. T. (2017). Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review. Curr. Stem Cell Res. Ther. 12 (1), 71–79. 10.2174/1574888X11666160815095733 [DOI] [PubMed] [Google Scholar]
- Cho Y., Cavalli V. (2014). HDAC signaling in neuronal development and axon regeneration. Curr. Opin. Neurobiol. 27, 118–126. 10.1016/j.conb.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C. W., Corselli M., Park T. S., et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3 (3), 301–313. 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Das Gupta K., Shakespear M. R., Iyer A., Fairlie D. P., Sweet M. J. (2016). Histone deacetylases in monocyte/macrophage development, activation and metabolism: refining HDAC targets for inflammatory and infectious diseases. Clin. Transl. Immunol. 5 (1), e62. 10.1038/cti.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J., Licht R., Bongers M., van der Klundert T., Arends R., van Blitterswijk C. (2006). Inhibition of histone acetylation as a tool in bone tissue engineering. Tissue Eng. 12 (10), 2927–2937. 10.1089/ten.2006.12.2927 [DOI] [PubMed] [Google Scholar]
- Dokmanovic M., Clarke C., Marks P. A. (2007). Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 5 (10), 981–989. 10.1158/1541-7786.MCR-07-0324 [DOI] [PubMed] [Google Scholar]
- Duncan H. F., Smith A. J., Fleming G. J., Cooper P. R. (2011). HDACi: cellular effects, opportunities for restorative dentistry. J. Dent. Res. 90 (12), 1377–1388. 10.1177/0022034511406919 [DOI] [PubMed] [Google Scholar]
- Duncan H. F., Smith A. J., Fleming G. J., Cooper P. R. (2012). Histone deacetylase inhibitors induced differentiation and accelerated mineralization of pulp-derived cells. J. Endod. 38 (3), 339–345. 10.1016/j.joen.2011.12.014 [DOI] [PubMed] [Google Scholar]
- Duncan H. F., Smith A. J., Fleming G. J., Cooper P. R. (2013). Histone deacetylase inhibitors epigenetically promote reparative events in primary dental pulp cells. Exp. Cell Res. 319 (10), 1534–1543. 10.1016/j.yexcr.2013.02.022 [DOI] [PubMed] [Google Scholar]
- Duncan H. F., Smith A. J., Fleming G. J., Partridge N. C., Shimizu E., Moran G. P., et al. (2016). The histone-deacetylase-inhibitor suberoylanilide hydroxamic acid promotes dental pulp repair mechanisms through modulation of matrix metalloproteinase-13 activity. J. Cell Physiol. 231 (4), 798–816. 10.1002/jcp.25128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan H. F., Smith A. J., Fleming G. J., Reid C., Smith G., Cooper P. R. (2017). Release of bio-active dentine extracellular matrix components by histone deacetylase inhibitors (HDACi). Int. Endod. J. 50 (1), 24–38. 10.1111/iej.12588 [DOI] [PubMed] [Google Scholar]
- Duncan H. F. (2017). Epigenetic Approaches to the role of histone deacetylase inhibitors (HDACi) in promoting dental pulp cell repair in vitro [PhD thesis] (Birmingham (UK): University of Birmingham; ). [Google Scholar]
- Eramo S., Natali A., Pinna R., Milia E. (2018). Dental pulp regeneration via cell homing. Int. Endod. J. 51 (4), 405–419. 10.1111/iej.12868 [DOI] [PubMed] [Google Scholar]
- Feng J., Mantesso A., De Bari C., Nishiyama A., Sharpe P. T. (2011). Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc. Natl. Acad. Sci. U. S. A 108 (16), 6503–6508. 10.1073/pnas.1015449108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M., Chiego D. J., Jr., Heys D. R. (1990). Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch. Biol. 35 (9), 707–715. 10.1016/0003-9969(90)90093-P [DOI] [PubMed] [Google Scholar]
- Frozoni M., Zaia A. A., Line S. R., Mina M. (2012). Analysis of the contribution of nonresident progenitor cells and hematopoietic cells to reparative dentinogenesis using parabiosis model in mice. J. Endod. 38 (9), 1214–1219. 10.1016/j.joen.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler K. M. (2016). Clinical procedures for revitalization: current knowledge and considerations. Int. Endod. J. 49 (10), 926–936. 10.1111/iej.12606 [DOI] [PubMed] [Google Scholar]
- Gopinathan G., Kolokythas A., Luan X., Diekwisch T. G. (2013). Epigenetic marks define the lineage and differentiation potential of two distinct neural crest-derived intermediate odontogenic progenitor populations. Stem Cells Dev. 22 (12), 1763–1778. 10.1089/scd.2012.0711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. A. R., Stein J. L., Westendorf J. J., van Wijnen A. J. (2015). Chromatin modifiers and histone modifications in bone formation, regeneration, and therapeutic intervention for bone-related disease. Bone 81, 739–745. 10.1016/j.bone.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L., Cooper P. R., Cassidy N., Nor J. E., Sloan A. J., Smith A. J. (2006). The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 27 (14), 2865–2873. 10.1016/j.biomaterials.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Grando Mattuella L., Westphalen Bento L., de Figueiredo J. A., Nor J. E., de Araujo F. B., Fossati A. C. (2007). Vascular endothelial growth factor and its relationship with the dental pulp. J. Endod. 33 (5), 524–530. 10.1016/j.joen.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Grant S., Easley C., Kirkpatrick P. (2007). Vorinostat. Nat. Rev. Drug Discovery 6 (1), 21–22. 10.1038/nrd2227 [DOI] [PubMed] [Google Scholar]
- Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A 97 (25), 13625–13630. 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halili M. A., Andrews M. R., Sweet M. J., Fairlie D. P. (2009). Histone deacetylase inhibitors in inflammatory disease. Curr. Top. Med. Chem. 9 (3), 309–319. 10.2174/156802609788085250 [DOI] [PubMed] [Google Scholar]
- Hilton T. J., Ferracane J. L., Mancl L., Northwest Practice-based Research Collaborative in Evidence-based, D (2013). Comparison of CaOH with MTA for direct pulp capping: a PBRN randomized clinical trial. J. Dent. Res. 92 (7 Suppl), 16S–22S. 10.1177/0022034513484336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh N. C., Everts V., Pavasant P., Ampornaramveth R. S. (2016). Inhibition of histone deacetylases enhances the osteogenic differentiation of human periodontal ligament cells. J. Cell Biochem. 117 (6), 1384–1395. 10.1002/jcb.25429 [DOI] [PubMed] [Google Scholar]
- Imazato S., Tay F. R., Kaneshiro A. V., Takahashi Y., Ebisu S. (2007). An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater. 23 (2), 170–176. 10.1016/j.dental.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Iohara K., Imabayashi K., Ishizaka R., Watanabe A., Nabekura J., Ito M., et al. (2011). Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 17 (15-16), 1911–1920. 10.1089/ten.tea.2010.0615 [DOI] [PubMed] [Google Scholar]
- Ito K., Caramori G., Lim S., Oates T., Chung K. F., Barnes P. J., et al. (2002). Expression and activity of histone deacetylases in human asthmatic airways. Am. J. Respir. Crit. Care Med. 166, 392–396. 10.1073/pnas.1321330111 [DOI] [PubMed] [Google Scholar]
- Jamaladdin S., Kelly R. D., O'Regan L., Dovey O. M., Hodson G. E., Millard C. J., et al. (2014). Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 111 (27), 9840–9845. 10.1073/pnas.1321330111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Park J. Y., Choi H., Choung P. H. (2013). HDAC inhibitor trichostatin A promotes proliferation and odontoblast differentiation of human dental pulp stem cells. Tissue Eng. Part A 19 (5-6), 613–624. 10.1089/ten.tea.2012.0163 [DOI] [PubMed] [Google Scholar]
- Kamocki K., Nor J. E., Bottino M. C. (2015). Effects of ciprofloxacin-containing antimicrobial scaffolds on dental pulp stem cell viability-In vitro studies. Arch. Biol. 60 (8), 1131–1137. 10.1016/j.archoralbio.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. K., De Carvalho D. D., Jones P. A. (2010). Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 28 (10), 1069–1078. 10.1038/nbt.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Jeffers M., Kumar S., Hackett C., Boldog F., Khramtsov N., et al. (2008). Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem. J. 409 (2), 581–589. 10.1042/BJ20070779 [DOI] [PubMed] [Google Scholar]
- Kim S. G., Zheng Y., Zhou J., Chen M., Embree M. C., Song K., et al. (2013). Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod. Topics 28 (1), 106–117. 10.1111/etp.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinz F. J., Korkmaz Y., Bloch W., Raab W. H., Addicks K. (2012). Histone deacetylases 2 and 9 are coexpressed and nuclear localized in human molar odontoblasts in vivo. Histochem. Cell Biol. 137 (5), 697–702. 10.1007/s00418-012-0920-9 [DOI] [PubMed] [Google Scholar]
- Kouzarides T. (2007). Chromatin modifications and their function. Cell 128 (4), 693–705. 10.1016/j.cell.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Kwon A., Park H. J., Baek K., Lee H. L., Park J. C., Woo K. M., et al. (2012). Suberoylanilide hydroxamic acid enhances odontoblast differentiation. J. Dent. Res. 91 (5), 506–512. 10.1177/0022034512443367 [DOI] [PubMed] [Google Scholar]
- Lalonde M. E., Cheng X., Côté J. (2014). Histone target selection within chromatin: an exemplary case of teamwork. Genes Dev. 28 (10), 1029–1041. 10.1101/gad.236331.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasko L. M., Jakob C. G., Edalji R. P., Qiu W., Montgomery D., Digiammarino E. L. (2017). Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550, 128–132. 10.1038/nature24028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Park J. R., Seo M. S., Roh K. H., Park S. B., Hwang J. W., et al. (2009). Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 42 (6), 711–720. 10.1111/j.1365-2184.2009.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni F., Zaliani A., Bertolini G., Porro G., Pagani P., Pozzi P., et al. (2002). The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl. Acad. Sci. U. S. A 99 (5), 2995–3000. 10.1073/pnas.052702999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesot H., Smith A. J., Tziafas D., Begue-Kirn C., Cassidy N., Ruch J. V. (1994). Biologically active molecules and dental tissue repair: a comparative view of reactionary and reparative dentinogenesis with the induction of odontoblast differentiation in vitro. [Google Scholar]
- Liu Z., Chen T., Han Q., Chen M., You J., Fang F., et al. (2018). HDAC inhibitor LMK235 promotes the odontoblast differentiation of dental pulp cells. Mol. Med. Rep. 17 (1), 1445–1452. 10.3892/mmr.2017.8055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., Wang Z., He X., Liu N., Liu B., Sun L., et al. (2018). Effects of histone deacetylase inhibitors on regenerative cell responses in human dental pulp cells. Int. Endod. J. 51 (7), 767–778. 10.1111/iej.12779 [DOI] [PubMed] [Google Scholar]
- Machado C. V., Passos S. T., Campos T. M., Bernardi L., Vilas-Boas D. S., Nor J. E., et al. (2016). The dental pulp stem cell niche based on aldehyde dehydrogenase 1 expression. Int. Endod. J. 49 (8), 755–763. 10.1111/iej.12511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahpatra S., Firpo M. T., Bacanamwo M. (2010). Inhibition of DNA methyltransferases and histone deacetylases induces bone marrow-derived multipotent adult progenitor cells to differentiate into endothelial cells. Ethn Dis. 20 (1 Suppl 1), S1–60-64. [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Xu W. S. (2009). Histone deacetylase inhibitors: potential in cancer therapy. J. Cell Biochem. 107 (4), 600–608. 10.1002/jcb.22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjor I. A., Tronstad L. (1972). Experimentally induced pulpitis. Surg. Med. Pathol. 34 (1), 102–108. 10.1016/0030-4220(72)90278-2 [DOI] [PubMed] [Google Scholar]
- Mjor I. A., Tronstad L. (1974). The healing of experimentally induced pulpitis. Surg. Med. Pathol. 38 (1), 115–121. 10.1016/0030-4220(74)90322-3 [DOI] [PubMed] [Google Scholar]
- Montgomery R. L., Davis C. A., Potthoff M. J., Haberland M., Fielitz J., Qi X., et al. (2007). Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 21 (14), 1790–1802. 10.1101/gad.1563807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraglia E., Altamura S., Branca D., Cecchetti O., Ferrigno F., Orsale M. V., et al. (2008). 2-Trifluoroacetylthiophene oxadiazoles as potent and selective class II human histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 18 (23), 6083–6087. 10.1016/j.bmcl.2008.09.076 [DOI] [PubMed] [Google Scholar]
- Naftelberg S., Ast G., Perlson E. (2017). Phosphatidylserine improves axonal transport by inhibition of HDAC and has potential in treatment of neurodegenerative diseases. Neural Regener. Res. 12 (4), 534–537. 10.4103/1673-5374.205082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P. N., Duncan H. F., Pitt Ford T. R., Luder H. U. (2008). Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int. Endod. J. 41 (2), 128–150. 10.1111/j.1365-2591.2007.01329.x [DOI] [PubMed] [Google Scholar]
- Nakashima M., Iohara K. (2017). Recent progress in translation from bench to a pilot clinical study on total pulp regeneration. J. Endod. 43 (9S), S82–S86. 10.1016/j.joen.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Nakashima M., Iohara K., Murakami M., Nakamura H., Sato Y., Ariji Y., et al. (2017). Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res. Ther. 8 (1), 61. 10.1186/s13287-017-0506-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T., Chen T., Partridge N. C. (2016). MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton. Bone. 90, 142–151. 10.1016/j.bone.2016.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T., Chen T., Johnson J., Westendorf J. J., Partridge N. C. (2018). The deletion of Hdac4 in mouse osteoblasts influences both catabolic and anabolic effects in bone. J. Bone Miner Res. 33 (7), 1362–1375. 10.1002/jbmr.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves V. C., Babb R., Chandrasekaran D., Sharpe P. T. (2017). Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci. Rep. 7, 39654. 10.1038/srep39654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyvad B., Crielaard W., Mira A., Takahashi N., Beighton D. (2013). Dental caries from a molecular microbiological perspective. Caries Res. 47 (2), 89–102. 10.1159/000345367 [DOI] [PubMed] [Google Scholar]
- Ono W., Sakagami N., Nishimori S., Ono N., Kronenberg H. M. (2016). Parathyroid hormone receptor signalling in osterix-expressing mesenchymal progenitors is essential for tooth root formation. Nat. Commun. 7, 11277. 10.1038/ncomms11277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paino F., La Noce M., Tirino V., Naddeo P., Desiderio V., Pirozzi G., et al. (2014). Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: evidence for HDAC2 involvement. Stem Cells 32 (1), 279–289. 10.1002/stem.1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paphangkorakit J., Osborn J. W. (1998). Discrimination of hardness by human teeth apparently not involving periodontal receptors. Arch. Biol. 43 (1), 1–7. 10.1016/S0003-9969(97)00090-3 [DOI] [PubMed] [Google Scholar]
- Pashley D. H. (1996). Dynamics of the pulpo-dentin complex. Crit. Rev. Biol. Med. 7 (2), 104–133. 10.1177/10454411960070020101 [DOI] [PubMed] [Google Scholar]
- Ricarte F., Nakatani T., Partridge N. (2016). PTH signaling and epigenetic control of bone remodeling. Curr. Mol. Biol. Rep. 2 (1), 55–61. 10.1007/s40610-016-0033-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman R., Chicoine L. G., Collini M. P., Cook R. G., Allis C. D. (1988). Micronuclei and the cytoplasm of growing Tetrahymena contain a histone acetylase activity which is highly specific for free histone H4. J. Cell Biol. 106 (4), 1017–1026. 10.1083/jcb.106.4.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V. M., Webb Y., Merger R., Sheppard T., Jursic B., Ngo L., et al. (1996). Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc. Natl. Acad. Sci. U. S. A 93 (12), 5705–5708. 10.1073/pnas.93.12.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richon V. M., Emiliani S., Verdin E., Webb Y., Breslow R., Rifkind R. A., et al. (1998). A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc. Natl. Acad. Sci. U. S. A 95 (6), 3003–3007. 10.1073/pnas.95.6.3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani P., Lasagni L., Mazzinghi B., Lazzeri E., Romagnani S. (2007). Pharmacological modulation of stem cell function. Curr. Med. Chem. 14 (10), 1129–1139. 10.2174/092986707780362880 [DOI] [PubMed] [Google Scholar]
- Sangwan P., Sangwan A., Duhan J., Rohilla A. (2013). Tertiary dentinogenesis with calcium hydroxide: a review of proposed mechanisms. Int. Endod. J. 46 (1), 3–19. 10.1111/j.1365-2591.2012.02101.x [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., Westendorf J. J. (2005). Histone deacetylase inhibitors promote osteoblast maturation. J. Bone Miner Res. 20 (12), 2254–2263. 10.1359/JBMR.050813 [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., Kahler R. A., Li X., Westendorf J. J. (2004). Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J. Biol. Chem. 279 (40), 41998–42007. 10.1074/jbc.M403702200 [DOI] [PubMed] [Google Scholar]
- Seto E., Yoshida M. (2014). Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 6 (4), a018713. 10.1101/cshperspect.a018713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam M. K., Sethi G. (2013). Role of epigenetics in inflammation-associated diseases. Subcell Biochem. 61, 627–657. 10.1007/978-94-007-4525-4_27 [DOI] [PubMed] [Google Scholar]
- Shi S., Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner Res. 18 (4), 696–704. 10.1359/jbmr.2003.18.4.696 [DOI] [PubMed] [Google Scholar]
- Shimizu E., Jong G., Partridge N., Rosenberg P. A., Lin L. M. (2012). Histologic observation of a human immature permanent tooth with irreversible pulpitis after revascularization/regeneration procedure. J. Endod. 38 (9), 1293–1297. 10.1016/j.joen.2012.06.017 [DOI] [PubMed] [Google Scholar]
- Shimizu E., Ricucci D., Albert J., Alobaid A. S., Gibbs J. L., Huang G. T., et al. (2013). Clinical, radiographic, and histological observation of a human immature permanent tooth with chronic apical abscess after revitalization treatment. J. Endod. 39 (8), 1078–1083. 10.1016/j.joen.2013.04.032 [DOI] [PubMed] [Google Scholar]
- Shuttleworth S. J., Bailey S. G., Townsend P. A. (2010). Histone Deacetylase inhibitors: new promise in the treatment of immune and inflammatory diseases. Curr. Drug Targets 11 (11), 1430–1438. 10.2174/1389450111009011430 [DOI] [PubMed] [Google Scholar]
- Simon S., Smith A. J. (2014). Regenerative endodontics. Br. Dent. J. 216 (6), E13. 10.1038/sj.bdj.2014.243 [DOI] [PubMed] [Google Scholar]
- Smith A. J., Lesot H. (2001). Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit. Rev. Biol. Med. 12 (5), 425–437. 10.1177/10454411010120050501 [DOI] [PubMed] [Google Scholar]
- Smith A. J., Duncan H. F., Diogenes A., Simon S., Cooper P. R. (2016). Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J. Endod. 42 (1), 47–56. 10.1016/j.joen.2015.10.019 [DOI] [PubMed] [Google Scholar]
- Smith A. J. (2002). Pulpal responses to caries and dental repair. Caries Res. 36 (4), 223–232. 10.1159/000063930 [DOI] [PubMed] [Google Scholar]
- Smith A. J. (2003). Vitality of the dentin-pulp complex in health and disease: growth factors as key mediators. J. Dent. Educ. 67 (6), 678–689. [PubMed] [Google Scholar]
- Subramanian S., Bates S. E., Wright J. J., Espinoza-Delgado I., Piekarz R. L. (2010). Clinical toxicities of histone deacetylase inhibitors. Pharmaceut. (Basel) 3 (9), 2751–2767. 10.3390/ph3092751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Liu H., Chen Z. (2015). The fine tuning role of microRNA-RNA interaction in odontoblast differentiation and disease. Dis. 21 (2), 142–148. 10.1111/odi.12237 [DOI] [PubMed] [Google Scholar]
- Tomson P. L., Grover L. M., Lumley P. J., Sloan A. J., Smith A. J., Cooper P. R. (2007). Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 35 (8), 636–642. 10.1016/j.jdent.2007.04.008 [DOI] [PubMed] [Google Scholar]
- Tomson P. L., Lumley P. J., Smith A. J., Cooper P. R. (2017). Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int. Endod. J. 50 (3), 281–292. 10.1111/iej.12624 [DOI] [PubMed] [Google Scholar]
- Tsou P. S., Wren J. D., Amin M. A., Schiopu E., Fox D. A., Khanna D., et al. (2016). Histone Deacetylase 5 is overexpressed in scleroderma endothelial cells and impairs angiogenesis via repression of proangiogenic factors. Arthritis Rheumatol. 68 (12), 2975–2985. 10.1002/art.39828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E., Dequiedt F., Kasler H. G. (2003). Class II histone deacetylases: versatile regulators. Trends Genet. 19 (5), 286–293. 10.1016/S0168-9525(03)00073-8 [DOI] [PubMed] [Google Scholar]
- Verdone L., Caserta M., Di Mauro E. (2005). Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 83 (3), 344–353. 10.1139/o05-041 [DOI] [PubMed] [Google Scholar]
- Wang G., Badylak S. F., Heber-Katz E., Braunhut S. J., Gudas L. J. (2010). The effects of DNA methyltransferase inhibitors and histone deacetylase inhibitors on digit regeneration in mice. Regener. Med. 5 (2), 201–220. 10.2217/rme.09.91 [DOI] [PubMed] [Google Scholar]
- Wapenaar H., Dekker F. J. (2016). Histone acetyltransferases: challenges in targeting bi-substrate enzymes. Clin. Epigenet. 8, 59. 10.1186/s13148-016-0225-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J. J., Zaidi S. K., Cascino J. E., Kahler R., van Wijnen A. J., Lian J. B., et al. (2002). Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol. Cell Biol. 22 (22), 7982–7992. 10.1128/MCB.22.22.7982-7992.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017). Sugars and Dental Caries. Geneva, Switzerland: WHO; publication no: WHO/NMH/NHD/17.12 [WWW document]. [Google Scholar]
- Wu J., Xie N., Wu Z., Zhang Y., Zheng Y. G. (2009). Bisubstrate inhibitors of the MYST HATs Esa1 and Tip60. Bioorg. Med. Chem. 17, 1381–1386. 10.1016/j.bmc.2008.12.014 [DOI] [PubMed] [Google Scholar]
- Xu Y., Hammerick K. E., James A. W., Carre A. L., Leucht P., Giaccia A. J., et al. (2009). Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng. Part A 15 (12), 3697–3707. 10.1089/ten.tea.2009.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. (2004). The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32 (3), 959–976. 10.1093/nar/gkh252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q., Cao Y., Liu O. S., Lu Y. Q., Wang J. S., Wang S. L., et al. (2016). Spatial and temporal expression of histone demethylase, Kdm2a, during murine molar development. Biotech. Histochem. 91 (2), 137–144. 10.3109/10520295.2015.1106586 [DOI] [PubMed] [Google Scholar]
- Yusoff S. I., Roman M., Lai F. Y., Eagle-Hemming B., Murphy G. J., Kumar T., et al. (2019). Systematic review and meta-analysis of experimental studies evaluating the organ protective effects of histone deacetylase inhibitors. Transl. Res. 205, 1–16. 10.1016/j.trsl.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Reinberg D. (2001). Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 15 (18), 2343–2360. 10.1101/gad.927301 [DOI] [PubMed] [Google Scholar]
- Zheng L. W., Zhang B. P., Xu R. S., Xu X., Ye L., Zhou X. D. (2014). Bivalent histone modifications during tooth development. Int. J. Sci. 6 (4), 205–211. 10.1038/ijos.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]