Abstract
Arc-welders’ pneumoconiosis (AWP) is an occupational lung disease and has nonspecific symptoms typically with the patterns of centrilobular and/or branching opacities on chest high-resolution computed tomography (HRCT) which are similar to those of hypersensitivity pneumonitis (HP) and/or respiratory tract infections. Therefore, the differential diagnosis is often difficult if they are not suspected.
We report a case of AWP which was initially suspected to be pulmonary tuberculosis because of the chest HRCT findings: centrilobular opacities distributed predominantly on the right lobe. On detailed review of the work history, however, the patient was found to be involved in welding. Prussian blue staining of the lung tissues and the bronchoalveolar lavage fluid (BALF) ferritin analysis were useful for the final diagnosis and the appropriate treatment for AWP. The atypical lymphocytosis in BALF in this case suggested the involvement of HP in the pathogenesis due to the occupational sensitization to causal antigens.
To the best of our knowledge, this is the first case report of AWP showing features of HP. AWP should be noted even in patients with the typical patterns of centrilobular opacities on chest HRCT. Medical history, iron staining of lung tissues, and the BALF ferritin analysis would be useful for the diagnosis of these patients. The BALF findings are sometimes indeterminate for the diagnosis because the occupational sensitization to causal antigens might be involved in some cases of AWP.
Keywords: Hemosiderin-laden macrophage, Hypersensitivity pneumonitis, Iron oxide, Occupational lung disease, Welding
1. Introduction
Arc-welders’ pneumoconiosis (AWP) is one of the major pneumoconioses and is caused by chronic inhalation of welding fumes [1]. Inhaled welding fumes, the major component of which is iron oxide, are deposited in the lungs and induce pulmonary dysfunction [2]. AWP is reversible if the exposure is limited [3]. Therefore, early diagnosis is important because an overload of iron fumes can trigger irreversible fibrosis [3]. However, the differential diagnosis is sometimes challenging because the major respiratory symptoms, which include chronic cough and shortness of breath, are nonspecific. Moreover, high-resolution computed tomography (HRCT) findings of AWP, typically with centrilobular nodules/ground-glass opacities and/or branching opacities, are also nonspecific and sometimes difficult to differentiate from respiratory tract infections particularly when the opacities are focally observed in lungs [4].
We report a case of AWP with features of hypersensitivity pneumonitis (HP) that was initially misdiagnosed as tuberculosis based on the respiratory symptoms and the HRCT findings. Once diagnosed as AWP, the patient was successfully treated by removal from his workplace.
2. Case presentation
A 40-year-old man presented with a 6-week history of exertional dyspnea and cough. He was referred to our hospital because his former doctor suspected tuberculosis based on the chest HRCT findings. The patient had worked as a construction worker for 18 years. He had a smoking history of 23 pack-years, and the symptoms were not improved 6 weeks after stopping smoking. He had had allergic rhinitis for 12 years. He was not prescribed any drugs.
On physical examination, percussion and auscultation over both lungs were normal, his legs were normal, his temperature was 36.1 °C, his heart rate was 86 beats/min, blood pressure was 137/87 mmHg, and respiratory rate was 27/min. His peripheral oxygen saturation was 91% on room air. Blood examination findings were as follows: total leukocyte count, 3,150/μL; lactate dehydrogenase, 394 U/L; C-reactive protein, 4.48 mg/dL; and Krebs von den Lungen-6, 435.2 U/mL. Except for the patient's anti-nuclear antibody titer (40 times), the immunological workup was normal. Sputum and blood cultures were negative for pathogens.
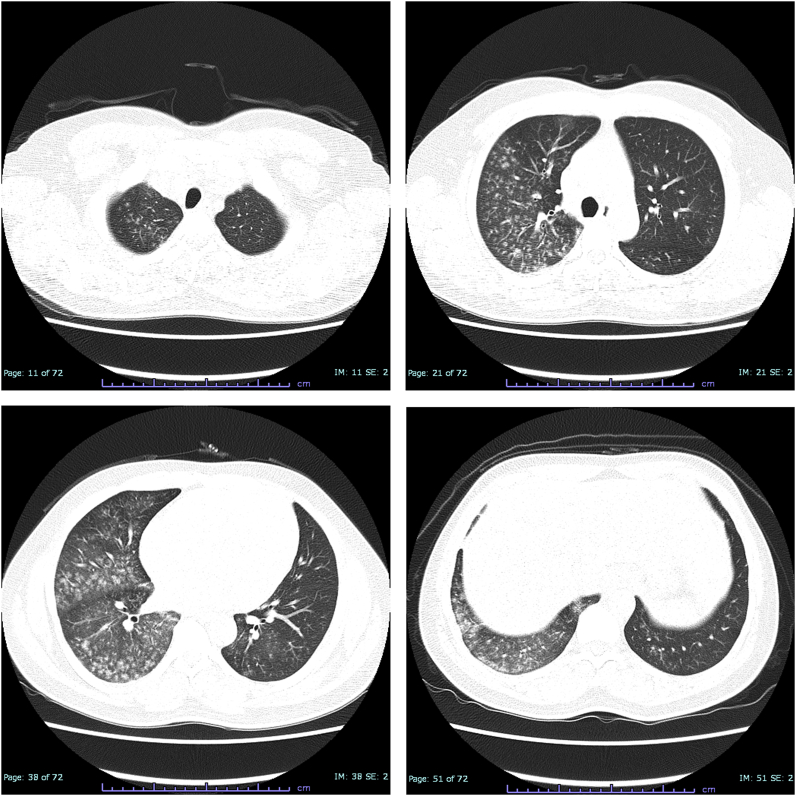
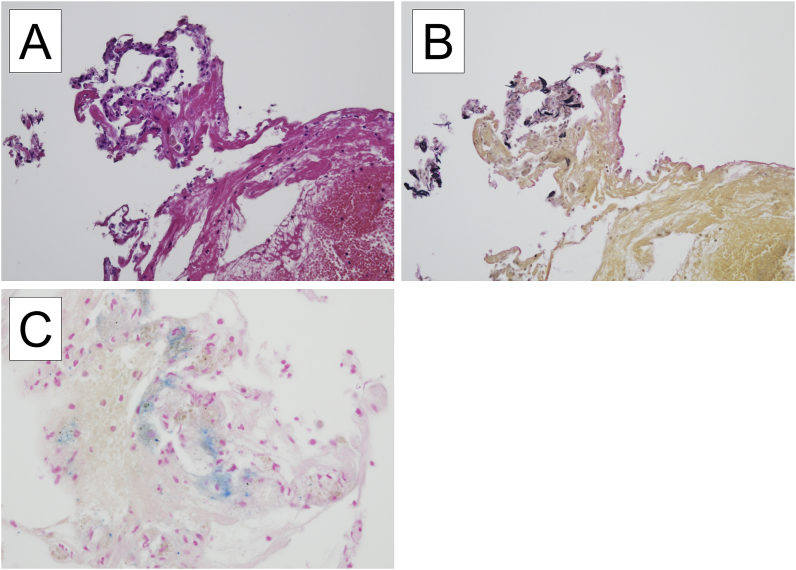
Chest HRCT showed centrilobular lung opacities that were distributed predominantly in the right lung (Fig. 1). Pulmonary function tests (PFTs) showed vital capacity (VC) of 2.48L, VC % predicted of 53.6%, forced vital capacity (FVC) of 2.44L, forced expiratory volume in 1 second (FEV1) of 1.57L, and FEV1/FVC ratio of 64.34%, suggesting the mixed pattern of ventilatory impairment. Bronchoalveolar lavage fluid (BALF) cultures were negative for bacteria, viruses, and fungi. BALF analyses showed a total cell count of 69.94 × 104/mL, with 61.3% lymphocytes and 37.5% macrophages, and a CD4+/CD8+ T-lymphocyte ratio of 0.81, and no findings of alveolar hemorrhage. Hematoxylin-eosin (HE) staining and Elastica van Gieson (EvG) staining of the transbronchial lung biopsy specimen showed alveolitis but no findings of malignancy, granuloma, or fibrosis (Fig. 2A and B).
Fig. 1.
Chest high-resolution computed tomography on admission.
At the first visit, centrilobular opacities are seen in the bilateral lung fields, though they are distributed predominantly in the right lung field.
Fig. 2.
Histological findings of lung biopsy specimens taken at bronchoscopy.
Although hematoxylin-eosin staining (2A) and Elastica van Gieson staining (2B) fail to show the iron accumulation, macrophages containing cytoplasmic iron pigment are identified by the Prussian Blue staining (2C). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The patient could not be clinically diagnosed, however, on detailed review of the work history, he was found to be involved in welding. Moreover, he developed a cough when he forgot to wear a dust-proof mask, suggesting the involvement of welding fume inhalation in the pathogenesis. Of note, additional BALF analyses showed a high ferritin level of 1,700 ng/mL and Prussian Blue staining specifically detected hemosiderin-laden macrophages in the lung tissues (Fig. 2C), although other staining failed (Fig. 2A and B).
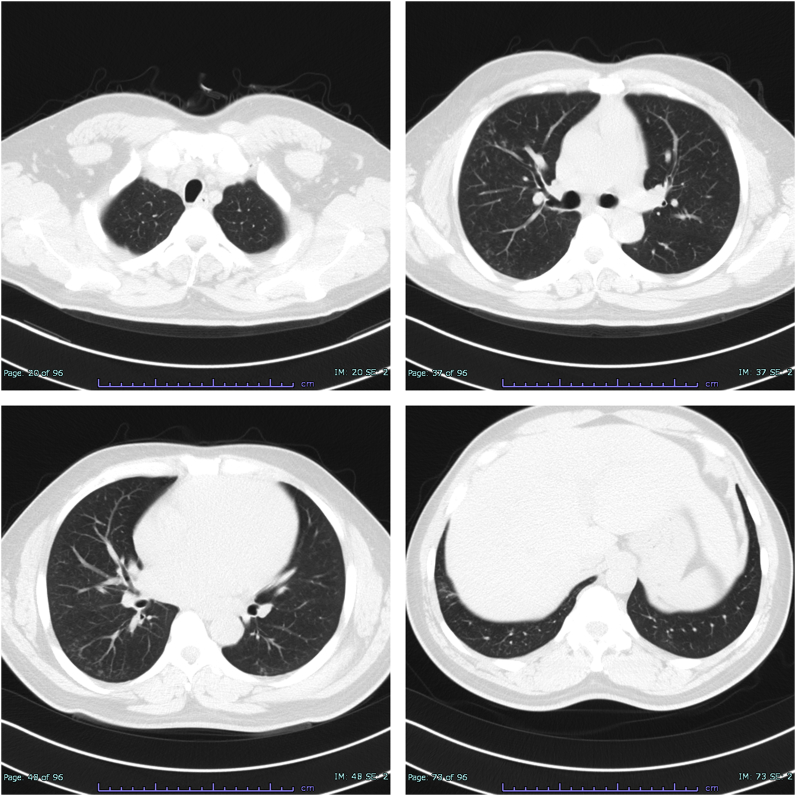
The patient was diagnosed with AWP based on the clinical and histological evidence of welding fume exposure to the lungs. Due to the lymphocytosis in BALF and the centrilobular opacities on HRCT, HP in part might have been involved in the pathogenesis. Given the patient's work history and the clinical course, the patient was suspected to have been sensitized to causal antigens including welding fumes. Accordingly, the patient was removed from his workplace. The opacities and the clinical symptoms improved significantly without recurrence 7 weeks after the isolation from welding fumes (Fig. 3). FVC drastically increased to 3.51L, which was 48.3% of increase from the admission. Inhalation challenge test was not conducted because the patient declined further testing.
Fig. 3.
Chest high-resolution computed tomography after removal from the patient's workplace.
Although ground-glass opacities remain in the peripheral lung field, centrilobular opacities have almost disappeared after the patient was kept away from his workplace for 7 weeks.
3. Discussion
The present case highlights two major clinical issues regarding the differential diagnosis of AWP. First, AWP should always be considered in the differential diagnosis of centrilobular/branching opacities regardless of their distribution patterns. A focal distribution pattern of lung opacities in AWP patients has rarely been reported, probably because welding fume particles are fine enough to be distributed diffusely [2,5]. Although no mechanism has been elucidated regarding the focal distribution pattern on HRCT, one possible explanation of the right lung-dominant distribution pattern in this case would be that the relatively poor lymphatic flow in the right lung limits washout of the deposited iron particles and the alveolar macrophages with iron contents [6]. However, little is known about the dynamics of alveolar macrophages in the lungs, and further investigation is needed to elucidate the distribution pattern of lung opacities in AWP. The present case showed the difficulty in the differential diagnosis of AWP when the lung opacities on HRCT are distributed focally and resemble those of respiratory tract infections. Thus, taking a detailed work history and iron staining of lung specimens are useful for the differential diagnosis of AWP, given the failure to identify hemosiderin-laden macrophages by routine HE and EvG staining.
Second, lymphocytosis in the BALF suggests the possibility of overlapping AWP and HP in this case. Regarding ordinary AWP, centrilobular lung opacities are observed on HRCT and disappear only after several years of the exposure avoidance [7]. Moreover, the immunological involvement has never been elucidated and the BALF cell analysis has been reported to be normal [8,9]. However, previous articles reported that inhaled metal fumes can also cause asthma, allergic rhinitis, and HP [2,10]. In cases with HP, centrilobular lung opacities are also observed on HRCT and BALF show lymphocytosis [11]. In addition, lymphocytosis over 50% in BALF strongly supports the diagnosis of HP [12].
In the present case, the diagnosis of AWP was confirmed based on the diagnostic criteria that requires an identification of exposure history to welding fumes, a chest radiographic finding, and a confirmation of iron accumulation in lungs by staining iron particles in the specimens [5,13]. Moreover, the ferritin level of BALF that was extremely high compared with previous AWP cases (95–580 ng/mL) supported the diagnosis (8). In addition, the striking lymphocytosis in BALF and the centrilobular lung opacities met the definition of probable HP, although the lung specimens did not show granuloma and the causal occupational antigens could not be determined [12]. In most cases of occupational HP, causal antigen remains uncertain because of the complexed occupational environment and the absence of definite diagnostic criteria [10]. In this case, despite the patient declining the inhalation challenge test, the association of welding fumes was suspected because the patient had cough after the accidental inhalation of welding fumes [14]. Moreover, as an alternative to inhalation challenge test, the antigen avoidance test and the improvement in PFTs including the increase in FVC after removal from the workplace supported the diagnosis of occupational HP [10,15]. Therefore, welding fumes might have been associated with the pathogenesis although the causal antigen could not be determined. This case suggests that the BALF cell analysis is indeterminate for the diagnosis of AWP, because the occupational sensitization to causal antigens might affect the BALF cell analysis.
In conclusion, we reported a case of AWP with features of HP that was difficult to differentiate from respiratory tract infections. AWP should always be considered even in patients with the atypical patterns of centrilobular lung opacities to prevent diagnostic delay and the subsequent irreversible fibrotic changes due to continued environmental exposure. Taking a detailed work history, iron staining of lung tissues, and measurement of ferritin levels in BALF would be useful for the diagnosis. The BALF cell analysis is indeterminate for the diagnosis because the occupational sensitization to causal antigens can trigger lymphocytosis.
Statement of ethics
Written consent for publication was obtained from the patient.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Authors’ contributions
All authors were responsible for the diagnosis, treatment, and care of the patient. YY and KT wrote the original draft of the manuscript. YY and KU obtained the images. TM, KF, and HK supervised the manuscript. All authors edited and finalized the final version.
Declaration of competing interest
The authors declare that there are no conflicts of interest associated with this manuscript.
Availability of data and materials
All necessary data are included in this published article.
Acknowledgement
None.
References
- 1.Doig A.T., McLaughlin A.I.G. X-ray appearances of the lungs of electric arc welders. Lancet. 1936;227:771–774. [Google Scholar]
- 2.Antonini J.M. Health effects of welding. Crit. Rev. Toxicol. 2003;33:61–103. doi: 10.1080/713611032. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove M.P. Pulmonary fibrosis and exposure to steel welding fume. Occup. Med. 2015;65:706–712. doi: 10.1093/occmed/kqv093. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M., Nitta N., Kishimoto T., Ohtsuka Y., Honda S., Ashizawa K. Computed tomography findings of arc-welders’ pneumoconiosis: comparison with silicosis. Eur. J. Radiol. 2018;107:98–104. doi: 10.1016/j.ejrad.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 5.Oh S.J., Hwang K.E., Jeong E.T., Kim H.R. A case of pulmonary siderosis misdiagnosed as pneumonia. Respir. Med. Case Rep. 2018;25:58–60. doi: 10.1016/j.rmcr.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim K.I., Kim C.W., Lee M.K., Lee K.S., Park C.K., Choi S.J. Imaging of occupational lung disease. Radiographics. 2001;21:1371–1391. doi: 10.1148/radiographics.21.6.g01nv011371. [DOI] [PubMed] [Google Scholar]
- 7.Doig A.T., McLaughlin A.I.G. Clearing of X-ray shadows in welders' siderosis. Lancet. 1948;1:789–791. doi: 10.1016/s0140-6736(48)90858-7. [DOI] [PubMed] [Google Scholar]
- 8.Yoshii C., Matsuyama T., Takazawa A., Ito T., Yatera K., Hayashi T. Welder's pneumoconiosis: diagnostic usefulness of high-resolution computed tomography and ferritin determinations in bronchoalveolar lavage fluid. Intern. Med. 2002;41:1111–1117. doi: 10.2169/internalmedicine.41.1111. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro C.R., Jones J.C., Alfaro T., Ferreira A.J. Bronchoalveolar lavage in occupational lung diseases. Semin. Respir. Crit. Care Med. 2007;28:504–513. doi: 10.1055/s-2007-991523. [DOI] [PubMed] [Google Scholar]
- 10.Quirce S., Vandenplas O., Campo P., Cruz M.J., de Blay F., Koschel D. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy. 2016;71:765–779. doi: 10.1111/all.12866. [DOI] [PubMed] [Google Scholar]
- 11.Vasakova M., Morell F., Walsh S., Leslie K., Raghu G. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am. J. Respir. Crit. Care Med. 2017;196:680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- 12.Meyer K.C., Raghu G., Baughman R.P., Brown K.K., Costabel U., du Bois R.M. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 13.Kusaka Y., Sato K., Suganuma N., Hosoda Y. Metal-induced lung disease: lessons from Japan's experience. J. Occup. Health. 2001;43:1–23. [Google Scholar]
- 14.Lacasse Y., Selman M., Costabel U., Dalphin J.C., Ando M., Morell F. Clinical diagnosis of hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2003;168:952–958. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui T., Miyazaki Y., Okamoto T., Tateishi T., Furusawa H., Tsuchiya K. Antigen avoidance tests for diagnosis of chronic hypersensitivity pneumonitis. Respir. Investig. 2015;53:217–224. doi: 10.1016/j.resinv.2015.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All necessary data are included in this published article.