Highlights
-
•
TLE patients have older brain ages both functionally and structurally.
-
•
Functional brain age is a better predictor of cognitive abilites in TLE patients.
-
•
Weaker bilateral frontal/temporal connections affect functional brain aging in TLE.
-
•
Functional age mediates some of chronological age-fluid cognition relationships.
-
•
Accelerated brain aging in TLE affected by both seizure burden and polytherapy.
Keywords: Temporal lobe epilepsy (TLE), Accelerated brain aging, Cognitive aging, Age prediction, Functional connectivity
Abstract
The association of epilepsy with structural brain changes and cognitive abnormalities in midlife has raised concern regarding the possibility of future accelerated brain and cognitive aging and increased risk of later life neurocognitive disorders. To address this issue we examined age-related processes in both structural and functional neuroimaging among individuals with temporal lobe epilepsy (TLE, N = 104) who were participants in the Epilepsy Connectome Project (ECP). Support vector regression (SVR) models were trained from 151 healthy controls and used to predict TLE patients’ brain ages. It was found that TLE patients on average have both older structural (+6.6 years) and functional (+8.3 years) brain ages compared to healthy controls. Accelerated functional brain age (functional – chronological age) was mildly correlated (corrected P = 0.07) with complex partial seizure frequency and the number of anti-epileptic drug intake. Functional brain age was a significant correlate of declining cognition (fluid abilities) and partially mediated chronological age-fluid cognition relationships. Chronological age was the only positive predictor of crystallized cognition. Accelerated aging is evident not only in the structural brains of patients with TLE, but also in their functional brains. Understanding the causes of accelerated brain aging in TLE will be clinically important in order to potentially prevent or mitigate their cognitive deficits.
1. Introduction
Chronic temporal lobe epilepsy (TLE) is associated with abnormalities in cognition, brain structure and brain connectivity in midlife (Baxendale and Thompson, 2016; Helmstaedter and Witt, 2012; Hermann et al., 2017; Tavakol et al., 2019), findings that have raised concern regarding the future course of cognitive and brain aging and the risk of cognitive disorders of aging including dementia (Breteler et al., 1991). While different models of cognitive aging in epilepsy have been proposed (progressive decline, accelerated aging [two hit model], stable non-progressive abnormality) (Sen et al., 2018), consensus has yet to be achieved. Importantly, all models predict significantly more impaired cognition in aging individuals with chronic epilepsy compared to controls (Baxendale et al., 2010; Breuer et al., 2016; Helmstaedter and Elger, 2009). Similarly, cross-sectional modeling of structural brain aging has suggested greater abnormality in chronic epilepsy compared to controls with advancing age (Caciagli et al., 2017; Dabbs et al., 2012).
In a novel approach, Pardoe et al. (2017) trained a machine learning regression model using T1-weighted structural MRI scans of 2001 healthy controls to predict their chronological ages. They then used the model to predict the ages of 94 medically refractory focal epilepsy patients and showed that these patients had structural brains that were on average 4.5 years older than the healthy controls. Sone et al. (2019) recently reported findings from a similar study examining different types of epilepsy including TLE using T1 images, and found the same trend of accelerated aging (10.9 years older for TLE patients with inter-ictal psychosis, and 5.3 years without).
There are many paths of exploration from these studies that can be considered. First, will the functional brains of epilepsy patients similarly show accelerated brain aging (or premature brain aging in Pardoe et al., 2017)? Accelerated brain aging in epilepsy has been investigated mainly in the structural brain. While many studies have reported changes in the functional connectivity of epilepsy patients (Constable et al., 2013; Tracy and Doucet, 2015), whether the changes resemble accelerated aging is unknown.
Second, what factors are associated with age accelerated structural and functional brains? Possibilities include clinical seizure characteristics (e.g., age of onset, seizure frequency), treatment factors (e.g., number or type of anti-epileptic drug [AED] use), and of course demographic characteristics. Previous studies have reported that brain volume reductions in epilepsy may be independent of or only weakly related to seizure activity (Alvim et al., 2016) and potentially more related to AED use (Pardoe et al., 2013). Pardoe et al. (2017) and Sone et al. (2019) in their secondary analyses briefly reported that increased brain age difference (or brain-PAD: predicted age – chronological age in Sone et al., 2019) in epilepsy was associated with earlier age of onset, but not with epilepsy duration nor AED use. More systematic search of potential correlates of accelerated brain aging is desired.
Third, is accelerated brain aging in epilepsy directly related to cognitive status and cognitive decline over time? Are brain ages better predictors of cognitive performance than the patients’ chronological ages? Cognitive aging and its core dimensions (crystallized and fluid cognitive abilities) in epilepsy have yet to be examined in relation to potential age-accelerated alterations in functional connectivity patterns and brain structure. Whether they have explanatory power beyond chronological age remains to be determined.
TLE is the most common form of adult epilepsy and the largest group among those with medically refractory seizures (Tellez-Zenteno and Hernandez-Ronquillo, 2012). Through the Epilepsy Connectome Project (ECP) (Cook et al., 2018; Hwang et al., 2019) we were able to focus on a more homogenous group of patients than examined previously. The ECP dataset provided a broader set of imaging, clinical, and cognitive metrics to address the main aim of the study, which was to characterize age-related brain structure and connectivity abnormalities and their clinical significance in people with TLE.
2. Materials and methods
2.1. Participants
Participants included 104 TLE patients (mean age = 40.4 ± 11.8 years, range = 19 – 60 years, 64 females) and 151 healthy controls (mean age = 53.7 ± 19.4 years, range = 18 – 89 years, 88 females). All TLE patients were from the ECP. 57 controls were from the ECP, and additionally 94 controls who matched the ECP criteria were drawn from a related Alzheimer's Disease Connectome Project (ADCP) (Hwang et al., 2018). The use of healthy controls from the two projects allowed investigation of participants with a wider age range than provided by either project alone, without compromising scanner, site or protocol variabilities. Both studies are two-site research projects involving the Medical College of Wisconsin (MCW) and the University of Wisconsin-Madison (UW-Madison). Both studies were reviewed and approved by the IRB (Institutional Review Board) at MCW and all participants provided written informed consent. 42 TLE patients and 51 controls were scanned at MCW, while 62 TLE patients and 100 controls were scanned at UW-Madison. The recruitment and data collection took place between March of 2016 and December of 2018.
TLE patients were enrolled in the ECP if they were between the ages of 18 and 60, demonstrated estimated full-scale IQ (Intelligence Quotient) at or above 70, spoke English fluently, and had no medical contraindications to MRI. They had a diagnosis of TLE supported by two or more of the following: 1) described or observed clinical semiology consistent with seizures of temporal lobe origin, 2) EEG evidence of either Temporal Intermittent Rhythmic Delta Activity or temporal lobe epileptiform discharges, 3) temporal lobe onset of seizures captured on video EEG monitoring study, or 4) MRI evidence of mesial temporal sclerosis or hippocampal atrophy. Both high resolution T1- and T2-weighted MRI sequences were used to evaluate the medial temporal lobes and hippocampi by a neuroradiologist to determine if there were alterations in signal intensity, laminar structure, and volume of the hippocampi that would be consistent with the diagnosis of hippocampal sclerosis. Patients with any of the following were excluded: 1) Presence of any lesions other than mesial temporal sclerosis causative for seizures on 3 Tesla MRI, 2) an active infectious/autoimmune/inflammatory etiology of seizures, either suspected by treating clinician or documented through laboratory testing or response to immunosuppressive therapy. 104 TLE patients in the study represented a combination of pharmaco-resistant and well-controlled patients (45% reported having at least one seizure during the past year). Supplementary Table 1 provides clinical and demographic information for the TLE participants.
Healthy controls of ages between 18 and 60 were enrolled in ECP, and between 55 and 90 in ADCP. Exclusion criteria common to both Connectome projects for healthy controls included brain injury or illness, major psychiatric condition (major depression, bipolar disorder, or schizophrenia), or medical contraindications to MRI.
2.2. Data acquisition
MRI was performed on 3T GE (General Electric) 750 scanners at both institutions. T1-weighted structural images were acquired using MPRAGE (magnetization prepared gradient echo sequence, TR/TE = 604 ms/2.516 ms, TI = 1060.0 ms, flip angle = 8°, FOV = 25.6 cm, 0.8 mm isotropic). Resting-state functional MRI (rs-fMRI) images were acquired using whole-brain SMS (simultaneous multi-slice) imaging (Moeller et al., 2010) (8 bands, 72 slices, TR/TE = 802 ms/33.5 ms, flip angle = 50°, matrix = 104 × 104, FOV = 20.8 cm, voxel size 2.0 mm isotropic) and a Nova 32-channel receive head coil. The participants were asked to fixate on a white cross at the center of a black screen during the scans for better reliability (Patriat et al., 2013). There was minimal loss of rs-fMRI image data only in healthy controls due to technical or scheduling issues (N = 10).
2.3. Data processing
MRI images were processed using the Human Connectome Project (HCP) minimal processing pipelines (Glasser et al., 2013) which is primarily based on FreeSurfer (Dale et al., 1999) and FSL (Functional MRI of the brain Software Library) (Jenkinson et al., 2012). In brief, the function of this pipeline is to nonlinearly register T1-weighted images to the MNI (Montreal Neurological Institute) space, segment the volume into predefined structures, reconstruct white and pial cortical surfaces, and perform FreeSurfer's standard folding-based surface registration to a surface atlas (the “fsaverage” template). The functional portion of the pipelines removes nonlinear spatial distortions in the rs-fMRI images using spin echo unwarping maps, realigns volumes to compensate for subject motion, registers to the structural images, reduces the bias field, normalizes the 4D image to a global mean, masks the data with the final brain mask and maps the voxels within the cortical gray matter ribbon onto the native cortical surface space. More details on the HCP processing pipelines can be found in Glasser et al. (2013)
254 structural features generated by FreeSurfer's standard reconstruction (recon-all) were extracted from the T1-weighted images, including cortical thicknesses, surface areas, volumes and also subcortical and global volumes. Surface areas and volumes were divided by the total surface area and total gray matter volume respectively to normalize for brain size. Then the structural features were normalized through z-score transform.
Additional pre-processing was performed on the rs-fMRI images using AFNI (Analysis of Functional Neuro-Images) (Cox, 1996). This included motion regression using 12 motion parameters, regression-based removal of signal changes in the white matter, CSF, global signal, and band-pass filtering (0.01–0.1 Hz). There are trade-offs of regressing out the global signal from the raw signals, such as potential false negative correlations (Murphy and Fox, 2017). Therefore, another machine learning model was trained without the global signal regression to confirm whether similar results were obtained.
Using the Connectome Workbench (version 1.1.1) (Marcus et al., 2013), time-series data from four 5-min rs-fMRI scans acquired in a single session were concatenated. 360 time-series from Glasser Parcellation (Glasser et al., 2016) plus 19 FreeSurfer subcortical regions (Fischl et al., 2002) were extracted per subject. MATLAB (Matrix Laboratory) R2018a was used to calculate pairwise Pearson correlations between 379 timeseries for generating connectivity matrices and also for most visualization in this study.
A subset of connectivity features were found to be affected by the subject motion in the scanner (absolute and relative mean root-mean-squared [RMS] motion) (Marcus et al., 2013). Therefore, absolute mean RMS motion was linearly regressed out from features that showed significant correlation (raw P < 0.05), first separately for healthy controls and then for TLE patients, by combining the two groups. Without this regression, the accelerated functional brain ages were significantly correlated with motion (P < 0.01), while regressing it out from the entire matrices resulted in the opposite correlation (P < 0.05). Supplementary Figure 1 shows the results without motion correction.
2.4. Support Vector Regression (SVR)
Two age-prediction Support Vector Regression (SVR) models (Amoroso et al., 2019; Smola and Scholkopf, 2004) were built in Python using the scikit-learn library (Pedregosa et al., 2011): with structural and functional (resting-state correlation matrices) features from the healthy controls. A linear kernel was used with no feature selection. First, the SVR models were trained and tested on the healthy controls using 10-fold cross validation. A linear correction that was suggested by Le et al. (2018) was applied to remove known systematic bias caused by regression dilution and regression towards the mean (old subjects predicted young, and vice versa) (Liang et al., 2019). The accuracy of the models were quantified using the correlation between chronological age and predicted, the amount of variance in age explained by the model (R2), the mean absolute error (MAE) and the root mean squared error (RMSE).
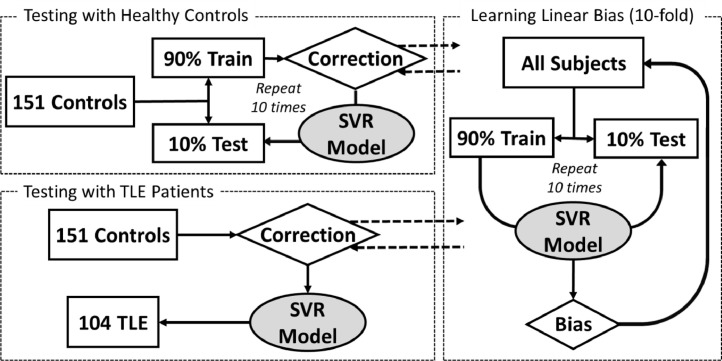
The final models were trained with the entire healthy control dataset and applied on the TLE patients. The predicted ages (brain ages) were compared to the chronological ages. Accelerated ages (brain – chronological ages) were calculated. The entire training and testing process is summarized in Fig. 1.
Fig. 1.
This diagram summarizes the process of support vector regression (SVR) model training and testing procedure. 10-fold cross validation on the healthy controls were first performed (left top), and then separately the testing on the temporal lobe epilepsy (TLE) patients (left bottom). Linear correction suggested by Le et al. (2018) was preformed to remove systematic bias caused by regression dilution and regression towards the mean.
2.5. Clinical information
The accelerated structural and functional ages of TLE patients were correlated with selected clinical epilepsy characteristics (age of onset and duration of epilepsy, frequency of complex partial and generalized seizures), AED treatment (number and duration of medications), and demographic characteristics (chronological age, sex, education). Spearman correlation was used for all variables except sex, as they were not normally distributed (Shapiro and Wilk, 1965). Point-biserial correlation was used for sex, as this variable was dichotomous.
2.6. Cognitive tests
TLE patients were administered subtests from the National Institutes of Health Toolbox Cognition Battery (NIHTB-CB) (Gershon et al., 2013; Weintraub et al., 2013). The seven administered tests fall into four cognitive domains, listed in Table 1. The four domains tested (and related tests) included broad executive function (Flanker Inhibitory Control and Attention, Dimensional Change Card Sort, List Sorting Working Memory) (Zelazo et al., 2013), episodic memory (Picture Sequence Memory), processing speed (Pattern Comparison Processing Speed), and language (Picture Vocabulary, Oral Reading Recognition). These seven subtests belong to two core dimensions of cognition (crystallized and fluid) (Akshoomoff et al., 2013). The two language subtests – Picture Vocabulary and Oral Reading Recognition – measure aspects of crystallized cognition, whereas the rest of the tests assess fluid cognition.
Table 1.
This table summarizes the correlation results between the three brain ages of the temporal lobe epilepsy (TLE) patients and their cognitive test scores. False discovery rate (FDR) correction was made on the P-values within each age measure and cognition type. Overall, fluid cognition was well associated with both chronological and functional ages. Chronological age was the best predictor among the three age measures of Picture Vocabulary (Z > 2.1, P < 0.05).
| Cognition Type | NIHTB-CB Measure | Subdomain | Chronological Age Correlation | Structural Age Correlation | Functional Age Correlation | |||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |||
| Fluid | Flanker Inhibitory Control and Attention |
Executive Function |
−0.174 | 0.091 | −0.094 | 0.605 | −0.172 | 0.088 |
| Dimensional Change Card Sort | −0.239 | 0.028* | −0.070 | 0.608 | −0.214 | 0.041* | ||
| List Sorting Working Memory | −0.171 | 0.091 | −0.033 | 0.748 | −0.221 | 0.041* | ||
| Picture Sequence Memory | Episodic Memory | −0.290 | 0.008* | −0.254 | 0.055 | −0.335 | 0.005* | |
| Pattern Comparison Processing Speed | Processing Speed | −0.313 | 0.008* | −0.092 | 0.605 | −0.231 | 0.041* | |
| Crystallized | Picture Vocabulary | Language | 0.293 | 0.006* | 0.094 | 0.508 | 0.154 | 0.180 |
| Oral Reading Recognition | 0.143 | 0.156 | 0.067 | 0.508 | 0.135 | 0.180 | ||
corrected P < 0.05. NIHTB-CB for National Institutes of Health Toolbox Cognition Battery.
The age-uncorrected standardized scores were correlated with chronological and brain ages (structural and functional) using Pearson correlation. There was minimal loss of cognitive data due to technical, seizure or scheduling issues (N = 5).
2.7. Mediation analysis
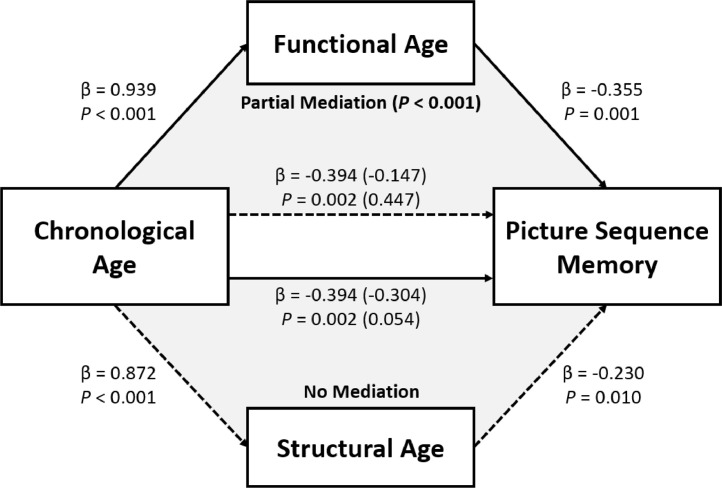
In order to investigate whether the brain ages mediate the association between chronological age and cognitive abilities in our TLE population, mediation analyses (Baron and Kenny, 1986) were performed in Python using the statsmodels library (Seabold and Perktold, 2010). Bootstrapping (5000 iterations) was performed for significance testing (Preacher et al., 2007). Fig. 6 shows the diagram of this analysis.
Fig. 6.
This diagram shows the results from the mediation analysis for Picture Sequence Memory test. The independent variable was the chronological age of the TLE patients. The mediator was either their functional or structural brain age. Functional age partially mediated (P < 0.001) the association between chronological age and the test score (top triangle), whereas structural age did not (bottom triangle). Numbers in parentheses are results after the mediator was introduced.
2.8. Data availability
Efforts are ongoing to release raw DICOM data from the ECP through the CCF (Connectome Coordination Facility, www.humanconnectome.org/software/connectomedb) (Hodge et al., 2016) at Washington University in St. Louis, by the end of 2019.
3. Results
3.1. Testing on healthy controls
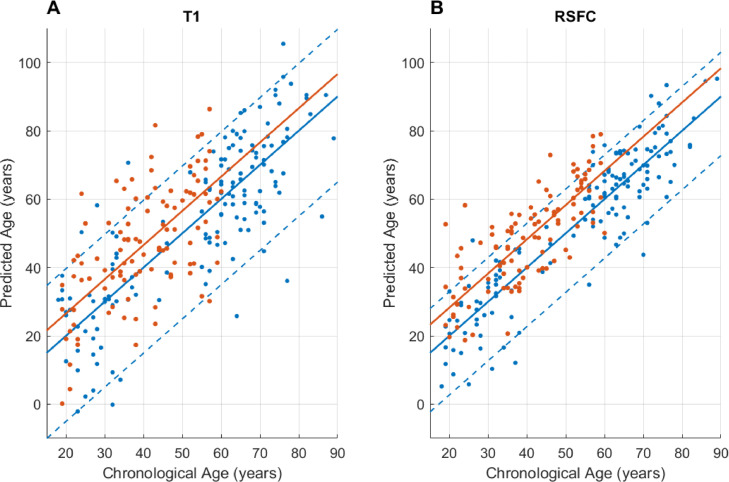
Cross validation showed that the ages of older validation subjects were systematically predicted younger, and vice versa, most likely due to regression dilution and regression toward the mean (Le et al., 2018; Liang et al., 2019). A linear correction that was suggested by Le et al. (2018) was applied, and then the final model was applied on the testing subjects (Fig. 1). The final prediction results of healthy controls are visualized in Fig. 4 (r = 0.82, R2 = 0.67, MAE = 10.7, RMSE = 13.65 for structural, r = 0.91, R2 = 0.83, MAE = 6.94, RMSE = 8.86 for functional model). The variance was significantly larger (P < 0.001, two-sample F-test for equal variances) in the accelerated structural ages compared to the functional ages.
Fig. 4.
These scatter plots show the support vector regression (SVR) age prediction results of both healthy controls (blue) and temporal lobe epilepsy (TLE) patients (orange): (A) with structural, and (B) functional features. The dotted lines indicate the 5th and the 95th percentiles of the cross validation results.
3.2. TLE age prediction
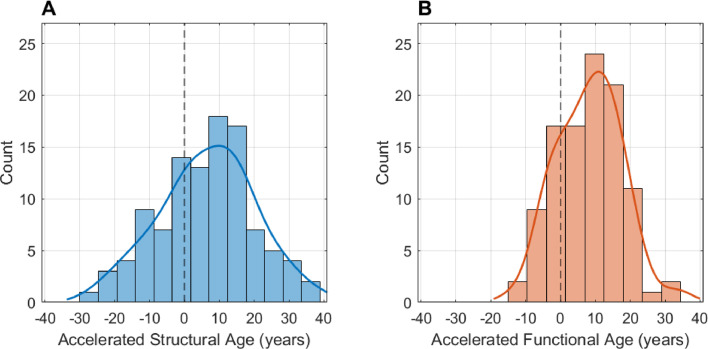
Fig. 2 shows the histograms of the accelerated brain ages of the TLE patients. The final SVR model with the linear correction predicted their structural brain ages to be on average 6.6 years older than their chronological ages (P < 0.001, paired t-test). Their structural brain ages were significantly older than those of the healthy controls (P < 0.001, unpaired t-test). The accelerated structural ages (structural brain age – chronological age) ranged from −27 (brain age younger than chronological age) to +39 years (brain age older than chronological age), with the standard deviation of 13.7 years, which was the same as in healthy controls. There was no specific structural feature whose value was significantly associated with the accelerated structural ages (Spearman correlation).
Fig. 2.
These histograms show the accelerated brain ages of 104 temporal lobe epilepsy (TLE) patients: (A) with structural, and (B) functional features. Accelerated aging in TLE was observed both in the structural (6.6 ± 13.7 years) and functional brains (8.3 ± 9.2 years). .
The final SVR model predicted the functional brain ages of the TLE patients to be on average 8.3 years older than their chronological ages (P < 0.001, paired t-test). Without the global signal regression of the raw signals, a similar results were found with the TLE patients’ functional brain ages predicted to be on average 5.1 years older than their chronological ages (P < 0.001, paired t-test). Their functional brain ages were significantly older than those of the healthy controls (P < 0.001, unpaired t-test). The accelerated functional ages ranged from −14 to +34 years with the standard deviation of 9.2 years, which was similar to 8.9 years in healthy controls. They were not significantly associated with the absolute/relative mean RMS motion (P’s > 0.6, r’s < 0.05). The variance was significantly larger (P < 0.001, two-sample F-test for equal variances) in the accelerated structural ages compared to the functional ages.
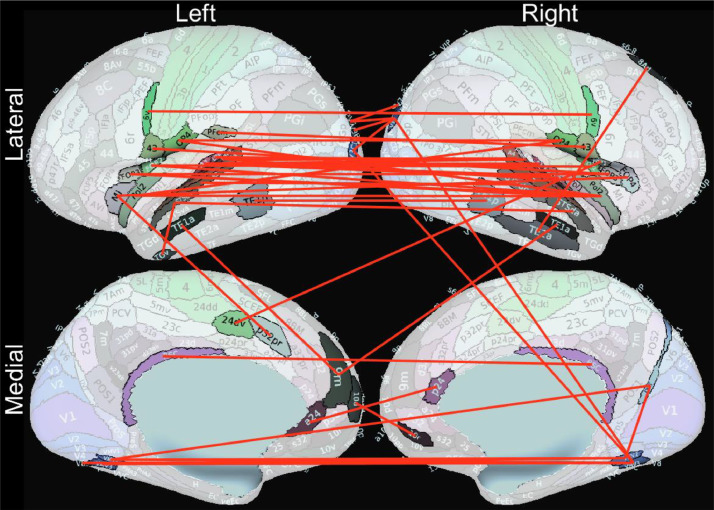
8341 out of 71,631 connectivity features were significantly associated (corrected P-values < 0.05, Spearman correlation) with the accelerated functional ages, with the top 48 features (ρ’s < −0.53) all showing negative correlation (weaker connection associated with more accelerated functional age). Most of these 48 connections were bilateral temporal or frontal lobe connections (Fig. 3). Supplementary Figure 2 shows the matrix representation of the connections based on the correlation values (Spearman ρ).
Fig. 3.
These 48 resting-state functional connections are most significantly associated with accelerated functional brain aging (corrected P-values < 0.0001, ρ’s < −0.53). Weaker correlations in these connections are associated with more accelerated functional brain aging.
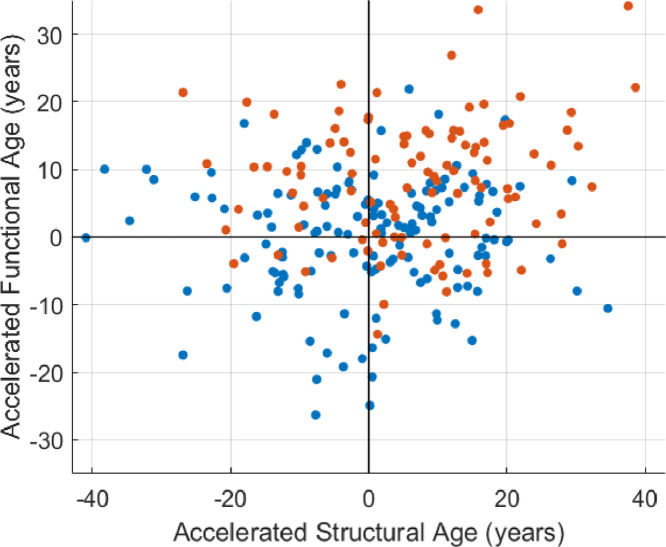
Fig. 4 shows the age prediction results. The brain aging effect in TLE was found in all age groups. The 5th and 95th percentiles of the cross-validation (healthy control) results were marked. 17 TLE patients (16%) showed structural brain ages greater than the 95th percentile (>19.7 years of acceleration), and 34 patients (33%) showed functional brain ages greater than the 95th percentile (>12.9 years of acceleration), with seven patients who overlapped. There was no significant correlation between the two accelerated ages (r < 0.01, P = 0.94 in healthy controls, r = 0.14, P = 0.15 in TLE patients) (Fig. 5).
Fig. 5.
This scatter plot shows the relationship between two accelerated ages. No statistically significant relationship was found in neither healthy controls (blue, r < 0.01, P = 0.94, Pearson correlation), nor TLE patients (orange, r = 0.14, P = 0.15). .
3.3. Correlation between ages
In healthy controls, structural (r = 0.82) and functional (r = 0.91) brain ages were highly correlated with chronological age, and also were inter-correlated (r = 0.74). The three ages were still correlated in TLE (r = 0.60, r = 0.77, r = 0.53 correspondingly), but to a significantly lesser degree compared to healthy controls (P’s < 0.01, z = 3.54, z = 3.87, z = 2.75).
3.4. Clinical correlates
Out of 104 TLE patients that were examined, 74 reported having had complex partial seizures (49 currently) and 62 reported secondary generalized seizures (22 currently). After correcting the P-values for multiple comparisons with Benjamini-Hochberg false discovery rate (FDR) correction (Benjamini and Hochberg, 1995), only trend-to-significant relationships were found between the functional accelerated ages of the TLE patients and their complex partial seizure frequency (P = 0.07) and AED count (P = 0.07). Patients who reported having at least one seizure during the past year were taking a greater number of AEDs (P < 0.01) compared to those who were seizure-free the past year, although there were no significant differences in the accelerated brain ages between the two groups. These results are summarized in Supplementary Table 2 with their raw P-values.
3.5. Cognitive correlates
Table 1 shows the correlation results between the three ages of TLE patients and their cognitive test scores. The FDR multiple comparison correction was performed on the P-values within each age measure and cognition type.
Chronological age was significantly associated (corrected P < 0.05) with four of seven tests, with trends (corrected P < 0.1) seen for two others. Structural age was not significantly associated with any test. Functional age was significantly associated with four of seven tests, with trends seen for one other: all fluid cognitive tests. Brain ages were not significantly associated with the crystallized subtests. Chronological age was significantly more associated with Picture Vocabulary than the brain ages (Z = 2.31, P = 0.02 for structural, Z = 2.13, P = 0.03 for functional age, Steiger's Z-test).
Three of seven tests (Dimensional Change Card Sort, Picture Sequence Memory, Pattern Comparison Processing Speed) were significantly associated with both chronological and functional age measures. Subsequent mediation analyses addressed the question of whether structural or functional brain age mediated the association between chronological age and these cognitive scores. Structural age was never a significant mediator while functional age partially mediated the relationship between chronological age and performance on three tests: Picture Sequence Memory (P < 0.001), Dimensional Change Card Sort (P = 0.004) and Flanker Inhibitory Control and Attention (P = 0.03) (Fig. 6).
4. Discussion
4.1. Accelerated brain aging in TLE
The results of this investigation demonstrated an accelerated brain aging effect in TLE using both structural and functional imaging. The SVR models predicted the structural brain ages of TLE patients to be an average of 6.6 years older, and the functional brain ages to be 8.3 years older than their chronological ages. 16% of the patients showed structural brain ages greater than the 95th percentile (>19.7 years of acceleration) of the healthy control sample, and 33% showed functional brain ages greater than the 95th percentile (>12.9 years of acceleration).
Accelerated aging is evident not only in the structural brains of patients with TLE, but also in their functional brains. This confirms and expands prior findings, here in a TLE group. Pardoe et al. (2017) and Sone et al. (2019) trained their regression models with a larger number of healthy control data (N = 2001 and 1196 respectively). Although the present study comparably lacks power in the trained age regression models (N = 151), the parameters and qualities of MRI images here are more controlled and the comparisons between the structural and functional brain ages provide novel insights into different dimensions of the brain aging effect in TLE.
While the overall structural and functional brain ages are indeed accelerated compared to chronological age (Fig. 2), inspection of the age discrepancy plots (Fig. 4) shows that this accelerated aging effect is evident across the chronological age range of the TLE participants examined here. We did not observe increased accelerated brain aging in the older compared to younger TLE participants, nor in participants with longer history of seizures compared to shorter (Supplementary Table 2).
It is worth noting that the correlations among the chronological and the two brain ages were significantly weaker in TLE patients compared to healthy controls (P’s < 0.01), suggesting a detectable dissociation of brain ages from chronological age. Weintraub et al. (2013) reported correlations between chronological age and NIH-TB cognition scores in healthy controls (N > 230), and observed significantly stronger negative correlations in fluid cognitive abilities (P’s < 0.001, −0.46 > r's > −0.65) compared to those seen in the TLE patients in our study (P’s < 0.03, Z> 2.2). Together with the finding that the functional age mediated the relationship between chronological age and cognition in TLE patients, this leads us to conclude that judgment of cognitive abilities in the TLE patients based on their chronological ages may be less predictable compared to healthy controls.
There were significantly smaller variances (P < 0.001) in the predicted accelerated functional brain ages compared to the structural ages, both from the healthy control and TLE groups (Figs. 2 and 4), although the opposite was expected given the increased complexity of the model (71,631 dimensions in functional, compared to 254 in structural). This suggests that the functional brain age calculated from resting-state functional connectivity is a more stable measure of brain age.
4.2. Clinical correlates
It was hypothesized that accelerated brain aging in TLE was related to either or both the clinical features of the epilepsy and AED use. Accelerated functional brain age was correlated with both complex partial seizure frequency (corrected P = 0.07) and the number of AEDs (corrected P = 0.07), suggesting that the accelerated functional brain aging in TLE patients may be related to both seizure burden and related polytherapy. It may also be argued that the association of the number of AEDs may not be directly related to AED effects, but from more severe epilepsy leading to more intensive AED therapy.
Results from this study confirm those from Pardoe et al. (2017) and Sone et al. (2019) which reported that there was no significant relationship between epilepsy duration and the accelerated brain age. However, the relationship between age of seizure onset and the accelerated brain age in our TLE population was found insignificant. Current data in this study were not sufficient to reveal definitive clinical correlates of accelerated brain aging.
4.3. Cognitive correlates
Table 1 depicts the dynamic nature of the relationships between chronological and brain ages with crystallized and fluid cognitive abilities. In regard to crystalized abilities, only chronological age predicted improvement on one of the two measures (Picture Vocabulary, r = 0.293, corrected P = 0.006), predictably showing improving naming ability with age. In contrast, the interplay of chronological age with brain ages was more dynamic for fluid abilities. Mediation analyses indicated that structural brain age did not mediate any relationships between chronological age and cognition. In contrast, functional brain age partially mediated the relationship for three measures including memory (Picture Sequence Memory) and selected measures of executive function (Dimensional Change Card Sort, Flanker Inhibitory Control and Attention) (Fig. 6). The relationship between chronological age and processing speed was not mediated by brain age.
Importantly, these brain age relationships are detected in a predominantly young to middle age sample (mean age = 40.3) who have yet to enter the epoch where age exerts stronger and more diverse effects. To that point, the relationships reported (Table 1), while significant, are modest in explanatory power. It will be important to continue to monitor these relationships prospectively to confirm their change over time and linkages to changing cognition.
4.4. Limitations
One limitation of this investigation is the relatively small sample sizes. In order to control for the scanner variability, scan protocols and procedures, only data from the two Disease Connectome Studies (ECP and ADCP) were used. This resulted in a smaller training sample size compared to previous studies (Pardoe et al., 2017; Sone et al., 2019), while allowing us to expand the study to investigate the functional brain aging and other clinical and cognitive traits in TLE.
The age range of our TLE population (19 – 60 years) was towards the younger spectrum of that of our control population (18 – 89 years). The results using this dataset should remain valid, since 1) the age range of the training set covered that of the testing set, and 2) the testing results on the healthy controls confirmed the performance of the linear correction. Before the linear correction, the bias in the regression model over-estimated the ages of young test subjects, making the prediction of TLE brain ages unreliable. The correction mitigated, if not completely removed, this bias effect (more discussion can be found in Le et al., 2018). Use of larger training sample sizes in conjunction with accurate non-linear regression models will create more robust age-predicting models. Future work is also desired to confirm the findings from the current study in older TLE population.
5. Conclusion
Accelerated aging is evident not only in the structural brains of patients with TLE, but also in their functional brains. Functional brain age seems to be a better measure of the brain age because of the smaller variance and better predictive power of cognitive ability, compared to the structural age. Functional brain age of TLE partially mediated the relationship between the chronological age and threeof the fluid cognition scores. On the other hand, structural brain age was not a good predictor of their crystallized cognition. Future work is desired to reveal the causes of accelerated aging in TLE in order to potentially prevent or mitigate their cognitive deficits.
CRediT authorship contribution statement
Gyujoon Hwang: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing. Bruce Hermann: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing. Veena A. Nair: Data curation, Investigation, Project administration, Validation, Visualization, Writing - review & editing. Lisa L. Conant: Data curation, Investigation, Writing - review & editing. K. Dabbs: Data curation, Investigation, Writing - review & editing. Jed Mathis: Data curation, Investigation, Writing - review & editing. Cole J. Cook: Data curation, Investigation, Writing - review & editing. Charlene N. Rivera-Bonet: Data curation, Investigation, Writing - review & editing. Rosaleena Mohanty: Data curation, Investigation, Writing - review & editing. Gengyan Zhao: Data curation, Investigation, Writing - review & editing. Dace N. Almane: Data curation, Investigation, Writing - review & editing. Andrew Nencka: Data curation, Investigation, Writing - review & editing. Elizabeth Felton: Data curation, Investigation, Writing - review & editing. Aaron F. Struck: Data curation, Investigation, Writing - review & editing. Rasmus Birn: Data curation, Investigation, Writing - review & editing. Rama Maganti: Data curation, Investigation, Writing - review & editing. Colin J. Humphries: Data curation, Investigation, Writing - review & editing. Manoj Raghavan: Data curation, Investigation, Writing - review & editing. Edgar A. DeYoe: Data curation, Investigation, Writing - review & editing. Barbara B. Bendlin: Data curation, Investigation, Writing - review & editing. Vivek Prabhakaran: Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Writing - review & editing. Jeffrey R. Binder: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing - review & editing. Mary E. Meyerand: Funding acquisition, Investigation, Project administration, Resources, Software, Supervision, Validation, Writing - review & editing.
Declaration of Competing Interest
None of the authors have any conflict of interest to disclose.
Acknowledgments
This study was supported by grant numbers U01NS093650 (Epilepsy Connectome Project) and 1UF1AG051216-01A1 (Alzheimer's Disease Connectome Project) from the National Institutes of Health. Funding for healthy control subjects’ data acquisition was provided in part by the Department of Radiology at the University of Wisconsin – Madison. The authors would like to thank all the participants and their families. Additionally, the authors would like to thank Taylor McMillan, Courtney Forseth, Peter Kraegel, Anna Freiberg, Neelima Tellapragada for recruitment and data collection, MRI technologists for their assistance in scanning and other support staff.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102183.
Appendix. Supplementary materials
References
- Akshoomoff N., Beaumont J.L., Bauer P.J., Dikmen S.S., Gershon R.C., Mungas D., Heaton R.K. VIII. NIH toolbox cognition battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child Dev. 2013;78(4):119–132. doi: 10.1111/mono.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim M.K., Coan A.C., Campos B.M., Yasuda C.L., Oliveira M.C., Morita M.E., Cendes F. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia. 2016;57(4):621–629. doi: 10.1111/epi.13334. [DOI] [PubMed] [Google Scholar]
- Amoroso N., La Rocca M., Bellantuono L., Diacono D., Fanizzi A., Lella E., Bellotti R. Deep learning and multiplex networks for accurate modeling of brain age. Front. Aging Neurosci. 2019;11(115) doi: 10.3389/fnagi.2019.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.M., Kenny D.A. The moderator mediator variable distinction in social psychological-research - Conceptual, strategic, and statistical considerations. J Pers. Soc. Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baxendale S., Heaney D., Thompson P.J., Duncan J.S. Cognitive consequences of childhood-onset temporal lobe epilepsy across the adult lifespan. Neurology. 2010;75(8):705–711. doi: 10.1212/WNL.0b013e3181eee3f0. [DOI] [PubMed] [Google Scholar]
- Baxendale S., Thompson P. The new approach to epilepsy classification: cognition and behavior in adult epilepsy syndromes. Epilepsy Behav. 2016;64(Pt A):253–256. doi: 10.1016/j.yebeh.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate - a Practical and powerful approach to multiple testing. J. Roy. Stat. Soc. Ser. B. 1995;57(1):289–300. [Google Scholar]
- Breteler M.M., van Duijn C.M., Chandra V., Fratiglioni L., Graves A.B., Heyman A. Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. Eurodem risk factors research group. Int. J. Epidemiol. 1991;20 Suppl(2):S36–S42. doi: 10.1093/ije/20.supplement_2.s36. [DOI] [PubMed] [Google Scholar]
- Breuer L.E., Boon P., Bergmans J.W., Mess W.H., Besseling R.M., de Louw A., Aldenkamp A.P. Cognitive deterioration in adult epilepsy: does accelerated cognitive ageing exist. Neurosci. Biobehav. Rev. 2016;64:1–11. doi: 10.1016/j.neubiorev.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Caciagli L., Bernasconi A., Wiebe S., Koepp M.J., Bernasconi N., Bernhardt B.C. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain. Neurology. 2017;89(5):506–516. doi: 10.1212/WNL.0000000000004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constable R.T., Scheinost D., Finn E.S., Shen X., Hampson M., Winstanley F.S., Papademetris X. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Front. Neurol. 2013;4:39. doi: 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C.J., Hwang G., Mathis J., Nair V.A., Conant L., Allen L., Meyerand M.E. Effective connectivity within the default mode network in left temporal lobe epilepsy: findings from the epilepsy connectome project. Brain Connect. 2018 doi: 10.1089/brain.2018.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dabbs K., Becker T., Jones J., Rutecki P., Seidenberg M., Hermann B. Brain structure and aging in chronic temporal lobe epilepsy. Epilepsia. 2012;53(6):1033–1043. doi: 10.1111/j.1528-1167.2012.03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gershon R.C., Wagster M.V., Hendrie H.C., Fox N.A., Cook K.F., Nowinski C.J. NIH toolbox for assessment of neurological and behavioral function. Neurology. 2013;80(11 Suppl 3):S2–S6. doi: 10.1212/WNL.0b013e3182872e5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Consortium W.U.-M.H. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C., Elger C.E. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease. Brain. 2009;132(Pt 10):2822–2830. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C., Witt J.A. Clinical neuropsychology in epilepsy: theoretical and practical issues. Handb. Clin. Neurol. 2012;107:437–459. doi: 10.1016/B978-0-444-52898-8.00036-7. [DOI] [PubMed] [Google Scholar]
- Hermann B.P., Loring D.W., Wilson S. Paradigm shifts in the neuropsychology of epilepsy. J. Int. Neuropsychol. Soc. 2017;23(9–10):791–805. doi: 10.1017/S1355617717000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge M.R., Horton W., Brown T., Herrick R., Olsen T., Hileman M.E., Marcus D.S. ConnectomeDB–Sharing human brain connectivity data. Neuroimage. 2016;124(Pt B):1102–1107. doi: 10.1016/j.neuroimage.2015.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang G., Cook C.J., Nair V.A., Alexander A., Antuono P.G., Asthana S., Prabhakaran V. Characterizing structural brain alterations in alzheimer’s disease patients with machine learning. Alzheimer Dement. 2018;14(7):P135–P136. [Google Scholar]
- Hwang G., Nair V.A., Mathis J., Cook C.J., Mohanty R., Zhao G., Prabhakaran V. Using low-frequency oscillations to detect temporal lobe epilepsy with machine learning. Brain Connect. 2019;9(2):184–193. doi: 10.1089/brain.2018.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Le T.T., Kuplicki R.T., McKinney B.A., Yeh H.W., Thompson W.K., Paulus M.P., Tulsa I. A nonlinear simulation framework supports adjusting for age when analyzing brainage. Front. Aging Neurosci. 2018;10:317. doi: 10.3389/fnagi.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Zhang F., Niu X. Investigating systematic bias in brain age estimation with application to post-traumatic stress disorders. Hum. Brain Mapp. 2019;40(11):3143–3152. doi: 10.1002/hbm.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D.S., Harms M.P., Snyder A.Z., Jenkinson M., Wilson J.A., Glasser M.F., Consortium W.U.-M.H. Human connectome project informatics: quality control, database services, and data visualization. Neuroimage. 2013;80:202–219. doi: 10.1016/j.neuroimage.2013.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S., Yacoub E., Olman C.A., Auerbach E., Strupp J., Harel N., Ugurbil K. Multiband multislice GE-EPI at 7 T, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn. Reson. Med. 2010;63(5):1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H.R., Berg A.T., Jackson G.D. Sodium valproate use is associated with reduced parietal lobe thickness and brain volume. Neurology. 2013;80(20):1895–1900. doi: 10.1212/WNL.0b013e318292a2e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe H.R., Cole J.H., Blackmon K., Thesen T., Kuzniecky R., Human Epilepsy Project, I Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017;133:28–32. doi: 10.1016/j.eplepsyres.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Patriat R., Molloy E.K., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Birn R.M. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Duchesnay E. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- Preacher K.J., Rucker D.D., Hayes A.F. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav. Res. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Seabold S., Perktold J. Paper presented at the Proceedings of the 9th Python in Science Conference. 2010. Statsmodels: Econometric and statistical modeling with python. [Google Scholar]
- Sen A., Capelli V., Husain M. Cognition and dementia in older patients with epilepsy. Brain. 2018;141(6):1592–1608. doi: 10.1093/brain/awy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591. [Google Scholar]
- Smola A.J., Scholkopf B. A tutorial on support vector regression. Stat. Comput. 2004;14(3):199–222. [Google Scholar]
- Sone D., Beheshti I., Maikusa N., Ota M., Kimura Y., Sato N., Matsuda H. Neuroimaging-based brain-age prediction in diverse forms of epilepsy: a signature of psychosis and beyond. Mol. Psychiatry. 2019 doi: 10.1038/s41380-019-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakol S., Royer J., Lowe A.J., Bonilha L., Tracy J.I., Jackson G.D., Bernhardt B.C. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: from focal lesions to macroscale networks. Epilepsia. 2019 doi: 10.1111/epi.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Zenteno J.F., Hernandez-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res. Treat. 2012;2012 doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy J.I., Doucet G.E. Resting-state functional connectivity in epilepsy: growing relevance for clinical decision making. Curr. Opin. Neurol. 2015;28(2):158–165. doi: 10.1097/WCO.0000000000000178. [DOI] [PubMed] [Google Scholar]
- Weintraub S., Dikmen S.S., Heaton R.K., Tulsky D.S., Zelazo P.D., Bauer P.J., Gershon R.C. Cognition assessment using the NIH toolbox. Neurology. 2013;80(11 Suppl 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D., Anderson J.E., Richler J., Wallner-Allen K., Beaumont J.L., Weintraub S. II. NIH toolbox cognition battery (CB): measuring executive function and attention. Monogr. Soc. Res. Child Dev. 2013;78(4):16–33. doi: 10.1111/mono.12032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Efforts are ongoing to release raw DICOM data from the ECP through the CCF (Connectome Coordination Facility, www.humanconnectome.org/software/connectomedb) (Hodge et al., 2016) at Washington University in St. Louis, by the end of 2019.