Abstract
Stanniocalcin-1 (STC-1) is a pro-survival factor that protects tissues against stressors, such as hypoxia and inflammation. STC-1 is co-expressed with the endometrial receptivity markers, and recently endometrial STC-1 was reported to be dysregulated in endometriosis, a condition linked with endometrial progesterone resistance and inflammation. These features are also common in the endometrium in women with polycystic ovary syndrome (PCOS), the most common endocrine disorder in women. Given that women with PCOS present with subfertility, pregnancy complications, and increased risk for endometrial cancer, we investigated endometrial STC-1 expression in affected women. Endometrial biopsy samples were obtained from women with PCOS and controls, including samples from overweight/obese women with PCOS before and after a 3-month lifestyle intervention. A total of 98 PCOS and 85 control samples were used in immunohistochemistry, reverse-transcription polymerase chain reaction, or in vitro cell culture. STC-1 expression was analyzed at different cycle phases and in endometrial stromal cells (eSCs) after steroid hormone exposure. The eSCs were also challenged with 8-bromo-cAMP and hypoxia for STC-1 expression. The findings indicate that STC-1 expression is not steroid hormone mediated although secretory-phase STC-1 expression was blunted in PCOS. Lower expression seems to be related to attenuated STC-1 response to stressors in PCOS eSCs, shown as downregulation of protein kinase A activity. The 3-month lifestyle intervention did not restore STC-1 expression in PCOS endometrium. More studies are warranted to further elucidate the mechanisms behind the altered endometrial STC-1 expression and rescue mechanism in the PCOS endometrium.
Keywords: stanniocalcin-1, polycystic ovary syndrome, human endometrium, primary cell culture, endometrial stromal cells, hypoxia, stress, lifestyle intervention
Summary sentence Endometrial expression of STC-1 in the secretory phase is blunted in women with PCOS, suggesting impaired protection against stress.
Introduction
Stanniocalcin-1 (STC-1) is a 56-kD homodimeric cellular glycoprotein that carries out autocrine and paracrine regulatory functions with pleiotropic rather than classical endocrine hormonal effects [1, 2]. Mammalian STC-1 is considered to be a pro-survival factor, protecting against hypoxic, hypercalcemic, and ischemic damage, suppressing inflammatory responses, and reducing oxidative stress [3–6]. STC-1 has been detected in several human tissues, including human endometrium, and its tissue expression fluctuates during the menstrual cycle. Levels of STC-1 are high in mid-secretory-phase endometrium (MSE) [7–9], and the high-expression levels coincide with the receptivity markers related to the window of implantation (WOI) [10]. Even though STC-1 knockout mice have been shown to be fertile, STC-1 expression has been reported to be downregulated during the mid-secretory phase in women with unexplained infertility and in women with endometriosis, a condition underpinned by perturbed progesterone signal transduction and chronic inflammation [8]. Furthermore, the results of several studies indicate a role for STC-1 in cancer progression, including endometrial cancer [11–13].
Given that STC-1 modulates hypoxic and inflammatory responses by serving as a protective factor against these stressors, the role of STC-1 in human endometrium is worth exploring. Hypoxia in mammalian endometrium modifies the estrogen- and progesterone-driven decidualization process, thus contributing to the process of embryo implantation [14–16]. Furthermore, hypoxic and inflammatory responses in human endometrium are robust, especially during endometrial shedding [17], and their involvement in endometrial cancer progression has also been noted [18, 19]. Therefore, dysregulated expression of STC-1 in human endometrium could induce a more stressful environment for the implanting embryo and eventually lead to chronic endometrial stress with impaired protection against deoxyribonucleic acid (DNA) damage and cancer [4, 20–22].
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age, affecting approximately 8–12% of this population. The syndrome is characterized by oligo-anovulation, hyperandrogenism, and dysregulated metabolism [23]. All these characteristics may also affect endometrial receptivity and induce endometrial cancer in time. Indeed, women with PCOS have been shown to have an increased risk of pregnancy complications such as miscarriage, preeclampsia, and premature delivery but also a higher risk of endometrial cancer [24–28]. Whether dysregulated STC-1 expression is one of the mediating factors of these adverse outcomes in PCOS has yet to be established.
Given that accumulation of stress factors can be detected in the endometrium of women with PCOS, likely altering embryo implantation and placental formation and promoting endometrial cancer, investigation of the possible endometrial rescue mechanism is of importance. In the present study, our primary aim was to investigate endometrial STC-1 expression in women with PCOS in different cycle phases and after steroid hormone exposure. Second, as STC-1 has been previously shown to be triggered by cyclic adenosine monophosphate (cAMP) and hypoxia, we used them as in vitro stress tests for endometrial stromal cells (eSCs) to investigate STC-1 outcomes. As for the mechanism, cAMP-mediated protein kinase A (PKA) activity was also evaluated. Last, we investigated the effect of lifestyle intervention on STC-1 expression in women with PCOS.
Materials and methods
Study subjects and tissues
All biopsy specimens were collected using an endometrial suction curette (Pipet Curet; CooperSurgical, USA). The distribution of the samples used in each experiment is presented in Figure 1.
Figure 1.
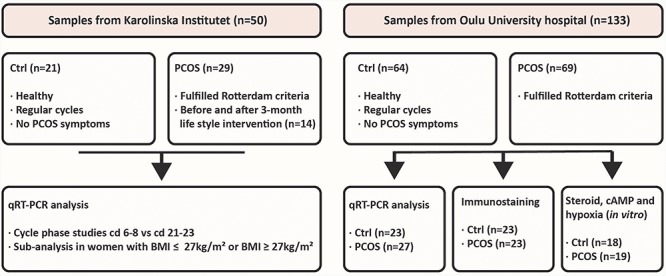
Endometrial tissue samples used in each experiment. The samples obtained from the Karolinska Institutet (ntotal = 50) comprised 21 samples from controls and 29 samples from women with PCOS. These samples were obtained during cd 6–8 and 21–23. Fourteen of the PCOS samples were obtained before and after 3-month lifestyle intervention. Samples obtained from Oulu University Hospital were taken at the various cd of the menstrual cycle based on LH testing (ntotal = 133). There were 64 control samples and 69 from women with PCOS.
Samples from Oulu University Hospital
Endometrial biopsy samples for cycle phase-dependent STC-1 expression and in vitro studies were obtained from the Department of Obstetrics and Gynecology, Oulu University Hospital, Oulu, Finland. All subjects were Caucasian and had not used any hormonal medication for at least 3 months prior to the sampling. The control women (n = 64) were healthy and reported regular menstrual cycles with no indication of having PCOS. For all experiments, body mass index (BMI) values were matched for both study groups. The mean BMI of the controls was 24.2 ± 2.6 kg/m2, and the mean age was 35.0 ± 5.9 (range 23–42) years. Women with PCOS (n = 69) were selected from the hospital records by tracing their former PCOS diagnoses established by a gynecologist or reproductive endocrinologist using Rotterdam criteria. Their mean BMI was 24.8 ± 3.3 kg/m2, and their mean age was 34.2 ± 3.8 (range 26–41) years.
The samples used in immuno-scoring and whole biopsy analyses were collected in 2013–2019. The biopsies were taken on cycle days (cd) 6–10 (proliferative phase, PE), luteinizing hormone (LH) peak + 2–4 days (early secretory phase, ESE), LH + 7–9 days (MSE), and LH + 10–12 days (late secretory phase, LSE). The day of the LH peak (LH + 0) was confirmed with a urinary ovulation prediction test (Clearblue Advanced Digital Ovulation Test kit). The corpus luteum and secretory phase were also confirmed by a gynecologist with an ultrasound on the day of the biopsy. The histology was dated by a pathologist. Women with PCOS with amenorrhea were only included in the PE endometrium group, and for them, no ovulation tracing was done. The samples used in in vitro testing were obtained in PE.
Samples from Karolinska Institutet
To investigate STC-1 expression in women with PCOS and in controls, we used samples (PCOS n = 29, controls n = 21) derived from a study conducted previously (2008–2012) at the Women’s Health Research Unit of Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden [29, 30]. All women with PCOS fulfilled all three Rotterdam criteria, with a mean BMI of 31.5 ± 8.8 kg/m2 and a mean age of 28.3 ± 5.1 (range 19–38) years. The controls were age and BMI matched: mean BMI 27.9 ± 7.7 kg/m2; mean age 30.2 ± 5.8 (range 18–38) years. The two study groups were further divided according to BMI: BMI ≤ 27 kg/m2 (normal weight/overweight PCOS and control; nN-PCOS = 11, nN-Ctrl = 11) or >27 kg/m2 (overweight/obese PCOS and control, nO-PCOS = 18, nO-Ctrl = 10) on the basis of the results of prior studies regarding the threshold for insulin resistance [31, 32]. All women with PCOS and BMI > 27 kg/m2 presented with ovulatory dysfunction, and all control women had regular menstrual cycles with no indication of having PCOS. All women were Caucasian, non-smokers, and free of any hormonal treatment for at least 3 months prior to the sampling. Endometrial biopsy samples were obtained during a single menstrual cycle between cd 6–8 (PE) and 21–23 (secretory, SE).
Samples from the lifestyle intervention study
The intervention part of the study concerning obese women with PCOS has already been published [29, 30]. Briefly, 15 women in this group underwent a 3-month lifestyle intervention program aimed at weight reduction [29, 33]. Before and after this period, the women were examined on cd 6–8 and 21–23 after spontaneous or medroxyprogesterone acetate-induced menstruation (10 mg/day for 7 days). For the present study, samples from 14 women were available both before and after intervention.
Ethics approval
All participants gave written informed consent. The study protocols and sample collection were approved by the ethics committees of Oulu University Hospital, Oulu, Finland, and Karolinska Institutet, Stockholm, Sweden. The experiments were conducted in accordance with the principles of the Declaration of Helsinki.
Immunohistochemistry
All samples were fixed with formalin and embedded in paraffin. Sections of endometrial biopsy samples (4 μm) were deparaffinized and washed. Antigen retrieval was performed with citrate buffer in a microwave oven at 800 W for 2 min and 150 W for 10 min. Neutralization of endogenous peroxidase was performed in peroxidase-blocking solution (Dako S2023) for 5 min. Sections were then incubated with anti-STC-1 (Atlas Antibodies; HPA023918) in antibody diluent (Dako S2023) for 5 min at room temperature, followed by incubation with Envision polymer (Dako K5007, Denmark) for 30 min. Peroxidase activity was induced with 3, 3’-diaminobenzidine (DAB) working solution (DAKO K5007) that was added to the slides. The slides were rinsed with distilled water, counterstained in hematoxylin for 15 s, and then mounted with mounting medium. Negative staining was performed on two samples using Phosphate-buffered saline (PBS) buffer instead of primary antibody. An Aperio ImageScope (Leica Biosystems, USA) microscope was used to evaluate and photograph the slides. Semi-quantitative evaluation of immunohistochemistry was performed by three independent observers (MKh, RKA, and AH) who were blinded to the identity of the slides; they used a grading system, and each sample was analyzed twice by each observer. Staining intensity and numbers of stained cells were graded on the following simple numeric scale: 0 = negative, 1 = faint, 2 = moderate, and 3 = intense. Average values obtained by the three observers are presented.
Isolation and culture of endometrial stromal cells
Endometrial stromal cells were prepared using a previously described optimized protocol [34, 35]. The technique produces a pure culture of eSCs, as confirmed previously by vimentin [36]. The eSCs were cultured in growth medium (phenol red-free medium at a 3:1 ratio with high-glucose Dulbecco Modified Eagle Medium (DMEM): a modified basal medium (MCDB)-105, 0.676 mM sodium pyruvate, 10% (v/v) charcoal-stripped fetal bovine serum (FBS), 1% (w/v) penicillin–streptomycin mix, gentamicin at 50 mg/mL, and insulin at 5 mg/mL). The media were replaced twice weekly until the desired confluency was reached prior to the experiment.
Steroid hormone exposure and decidualization
Estrogen and progesterone exposure to test stromal cell decidualization capacity
One-hundred thousand (105) eSCs were plated in 12 well plates in duplicate and cultured in growth medium (3:1 high-glucose DMEM/MCDB-105, 0.75 mM sodium pyruvate, 50 mg/mL gentamycin, and 10% FBS). When the cells showed 100% confluency, they were incubated for 24 h in low-serum medium (2% FBS) prior to the decidualization experiment. For these cultures, eSCs from the control women (n = 12) and from the women with PCOS (n = 13) were treated with 0.1% ethanol vehicle, and a combination of estradiol (E2; 10 nM, Sigma-Aldrich, Finland) and progesterone (P4; 1 μM, Sigma-Aldrich) in low-serum medium for 14 days. The culture medium and cells were collected at 0 h and at 14 days after E2 and P4 exposure to check the decidualization marker.
Steroid hormones, 8-bromo-cAMP, and hypoxia challenges as triggers for STC-1
Estrogen and progesterone exposure
One-hundred thousand (105) eSCs from the control women (n = 12) were treated with 0.1% ethanol vehicle, in a combination of E2 (10 nM, Sigma-Aldrich) and P4 (1 uM, Sigma-Aldrich) in low-serum medium for 48 h and 14 days. The cells were collected after 48 h and 14 days to check STC-1 expression.
5 Alpha-dihydrotestosterone exposure
One hundred thousand (105) eSCs from control women (n = 6) and women with PCOS (n = 6) were plated in six well plates in duplicate and cultured until confluent. After 24 h of starvation in low-serum medium, cells were treated with 0.1% ethanol vehicle, and E2 (10 nM, Sigma-Aldrich) alone or a combination of E2 and 5α-dihydrotestosterone (DHT; 100 nM, Sigma-Aldrich) in low-serum medium for 24 h [37]. The culture medium and cells were collected at 0 h and 24 h after steroid hormone (E2 or E2 + DHT) exposure.
8-Bromo-cAMP challenge
One hundred thousand (105) eSCs (nctrl = 12, nPCOS = 13) were plated in duplicate in 12 well plates. After the cells had reached confluency, they were maintained in low-serum medium for 24 h followed by an 8-bromo-cAMP (8-Br-cAMP; 0.5 mM, Sigma-Aldrich) challenge for 96 h [8, 38, 39]. The culture media and cells were collected after 96 h of incubation with 8-Br-cAMP and tested for STC-1 Ribonucleic acid (RNA) and protein expression.
Hypoxia challenge
One hundred thousand (105) eSCs (nCtrl = 12, nPCOS = 13) were plated in 12 well plates in duplicate and cultured in growth medium. At confluency, the cells were grown in low-serum medium for 24 h prior to treatment with 10% FBS-supplemented DMEM/F-12 medium under hypoxic conditions (1% O2, 5% CO2 and 94% N2; InVivo2 400 Hypoxia work station SCI-tive N; Ruskinn Technology Ltd., Bridgend, UK) or normoxic conditions (21% O2, 5% CO2, and 74% N2) for 24 h and 48 h as previously described [40]. The cells and supernatants were collected, and each experiment was repeated.
RNA isolation, complementary DNA synthesis, and quantitative reverse-transcription polymerase chain reaction
RNA from cultured eSCs and endometrial tissue samples was isolated using the QIAcube automated RNA isolation system according to the manufacturer’s instructions (Qiagen RNeasy Mini kit: (Hilden, Germany) including DNase treatment). Five hundred nanograms of RNA was converted to complementary DNA (cDNA) (Thermo Fisher cDNA synthesis kit: thermos Fisher Scientific Baltics UAB, Lithunia). Duplicate messenger RNAs (mRNAs) were pooled from each set of treatments. The comparative Ct method was used to obtain the relative expression according to the Livak 2−ΔΔCt method (Real-Time PCR, Bio-Rad, Finland) [41]. Expression of the target gene was normalized to that of glyceraldehyde 3-phosphate dehydrogenase, which is stably expressed in endometrial tissue, as previously shown [40, 42]. The primer sequences for quantitative reverse-transcription polymerase chain reaction (qRT-PCR) are presented in Supplementary Table S1.
Enzyme-linked immunosorbent assay for STC-1
STC-1 proteins from the culture media (E2/P4-, 8-Br-cAMP-, and hypoxia-treated) were measured according to the manufacturer’s instructions (R&D systems; Antibodies-online.com). All samples were assayed in duplicate, and a standard curve was run for each experiment.
Protein kinase A activity assay
Endometrial eSCs from both study groups (n = 4/group) were plated in six well plates and cultured until confluency and thereafter grown in a low-serum medium for 24 h. Subsequently, they were treated with 0.5 mM 8-Br-cAMP or 0.25% dimethyl sulfoxide (DMSO) for monitoring basal activity of PKA for 96 h. The cells were then lysed on ice as previously described [43], and the supernatants were collected.
The total protein concentration in obtained lysates was established using a Coomassie Plus (Bradford) Assay Kit (Thermo Scientific) according to the manufacturer’s instructions. Within each experiment, the total protein concentration of all collected lysates was equalized by suitable dilution with kinase assay buffer (50 mM 4-(2-Hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) pH 7.5, 150 mM NaCl, 0.005% Tween-20, 5 mM dithiothreitol, and 0.5 mg/mL Bovine serum albumin (BSA) fraction V). The assay was carried out as previously described [44]; the final total concentrations of ARC-1139 and H89 (Cayman Chemical) were 2 nM and 2–4 μM, respectively. The incubation times for binding and displacement were 30–60 min, and the time-delayed photoluminescence was measured using a PheraStar microplate reader (BMG Labtech) with the following parameters: excitation 337 (300–360) nm, emission 675 (50) nm, integration start 60 μs, and integration time 400 μs. For each lysate, the difference in the ARC-1139 signal prior to and after the addition of H89 was calculated. Within a single independent experiment, the values calculated for different lysates were then normalized to the value calculated for lysate from a sample of healthy eSCs treated with 0.25% DMSO (PKA activity set to 100%). The results of all measurements were then pooled.
Statistical evaluation
Statistical analyses were performed using non-parametric one-way analysis of variance (Kruskal–Wallis test) for differences among more than two groups, whereas differences between two groups were examined using the Mann–Whitney test. For the PKA data analysis, an unpaired t-test with the Welchs´ correction was carried out. The results are presented as mean ± SEM. Values of P value less than 0.05 were considered statistically significant. IBM SPSS statistics software version 22.0 and GraphPad Prism 6 were used for statistical analyses and for preparing images, respectively.
Results
Cyclic expression of STC-1 in endometrium from women with polycystic ovary syndrome and non-polycystic ovary syndrome controls
The LH-timed endometrial tissue analyses from controls and women with PCOS showed highest STC-1 expression at LSE compared with that at PE (P = 0.003 and 0.001, respectively). However, in Karolinska samples obtained from younger women with more severe PCOS phenotype and higher BMI, no such increase was observed (Figures 2A, 3A and B).
Figure 2.
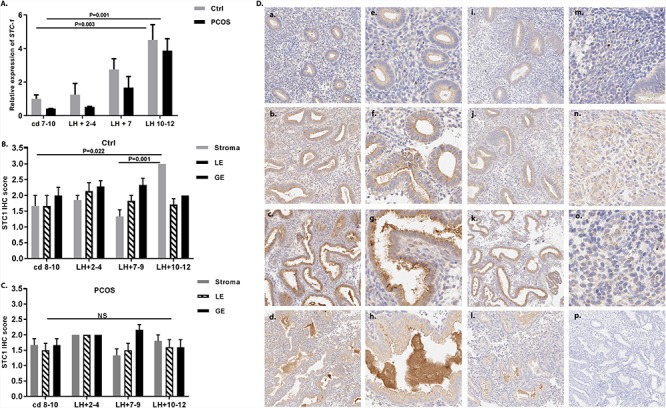
Expression of STC-1 in human endometrium. (A) Quantitative reverse-transcription polymerase chain reaction analysis of endometrial samples from healthy control women (gray bar) and women with PCOS (black bar) collected at cd 7–10 (nctrl = 7, nPCOS = 7), LH + 2–4 (nctrl = 5, nPCOS = 5), LH + 7 (nctrl = 7, nPCOS = 7), and LH + 10–12 (nctrl = 5, nPCOS = 7) revealed a significant increase in endometrial STC-1 gene expression from the PE (cd 7–10) toward the LSE (LH + 10–12) in both control samples (P = 0.003) and samples from women with PCOS (P = 0.001). Data are presented as mean ± standard error of the mean (SEM). (B) Semi-quantitative immunohistochemistry data on STC-1 protein expression in different endometrial cell compartments (stromal cells (stroma), luminal epithelium (LE), and GE) from control samples assessed at cd 8–10 (n = 6), LH + 2–4 (n = 7), LH + 7–9 (n = 6), and LH + 10–12 (n = 7) revealed the highest expression in the LSE (LH + 10–12) compared with the PE (cd 8–10; P = 0.022) and MSE (LH + 7–9; P = 0.001) in stromal cells, whereas (C) there was no significant difference between the cycle phases in cells from women with PCOS. (D) STC-1 expression in different cycle phases studied by immunohistochemistry in control women (a–h) and in women with PCOS (i–l). (a) PE (cd 8–10); (b) ESE (LH + 2–4); (c) MSE (LH + 7–9); (d) LSE (LH + 10–12). Higher magnification images from control women (40×) of GE shows that in PE, STC-1 is localized to the GE cells (e), and in ESE it is concentrated in the apical parts (f) from which it is gradually secreted into the lumen during the MSE (g) and LSE (h). The stromal STC-1 staining was weak in PE (m) and significantly stronger in LSE (n), but in PCOS, the staining intensity was similar across the cycle phases. (o) LSE stroma of a PCOS case; (p) negative control staining.
Figure 3.
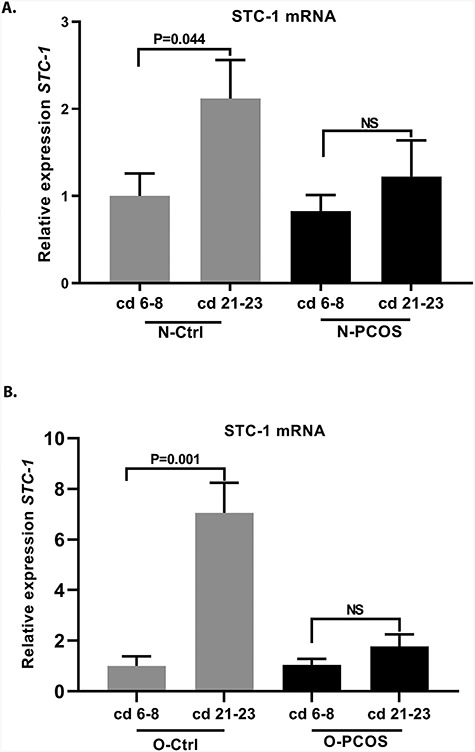
Cyclic expression of STC-1 in women with BMI ≤ 27 kg/m2 (N) and BMI > 27 kg/m2 (O) in controls and in women with PCOS. (A) STC-1 expression during cd 6–8 and 21–23 in controls with BMI ≤ 27 kg/m2 (N-Ctrl, n = 11 and n = 7, respectively) and in women with PCOS and BMI ≤ 27 kg/m2 (N-PCOS, n = 5 and n = 5, respectively). The control women presented with elevated STC-1 expression at cd 21–23 compared with cd 6–8 (P = 0.044), whereas there was no significant increase in STC-1 expression toward cd 21–23 in women with PCOS. (B) STC-1 expression at cd 6–8 and 21–23 in controls with BMI > 27 kg/m2 (O-Ctrl, n = 5 and n = 5, respectively) and in women with PCOS and BMI > 27 kg/m2 (O-PCOS, n = 8 and n = 15, respectively). Control women presented with elevated STC-1 expression at cd 21–23 compared with cd 6–8 (P = 0.001), but in women with PCOS the effect was again blunted.
In line with the mRNA results, STC-1 protein staining became stronger toward LSE in control women (Figure 2D, a–d). In more detail, intracellular STC-1 was detected in glandular epithelium (GE) during PE, and it gradually became concentrated in the apical part of the epithelial cells and secreted into the lumen in the LSE (Figure 2D, e–h 40×). During PE, only weak, punctuated STC-1 staining was detected in the stroma (Figure 2D, m), whereas stromal cells undergoing decidual transformation in LSE displayed increased cytoplasmic accumulation of STC-1 in the control samples. This accumulation was missing in PCOS samples (Figure 2D, n and o). As for the semi-quantitative protein data, STC-1 expression was higher in the stroma LSE vs PE in the controls (P = 0.022), with no difference in the epithelial cells in different cycle phases (Figure 2B). Differently from controls, PCOS endometrium showed blunted STC-1 protein expression with no differences in STC-1 between cycle phases in different cell compartments (Figure 2C).
Cyclic expression of STC-1 according to BMI in control vs polycystic ovary syndrome
In controls, in both women with BMI ≤ 27 kg/m2 (N-Ctrl) and those with BMI > 27 kg/m2 (O-Ctrl), the STC-1 expression was higher in secretory-phase samples at cd 21–23 compared with expression in the PE at cd 6–8 (P = 0.044 and 0.001, respectively; Figure 3A and B). Women with PCOS failed to present elevated STC-1 expression toward the secretory phase in both BMI groups (cd 6–8 vs cd 21–23; Figure 3A and B).
Confirmation of endometrial stromal cells decidualization and steroid hormone exposure for induction of STC-1
All control and PCOS eSCs used in in vitro experiments were able to decidualize successfully after E2/P4 exposure for 14 days according to insulin-like growth factor-1 and prolactin measurements (data not shown).
To find out whether STC-1 expression in eSCs is related to steroid hormone actions, cultures of eSCs from control women were subjected to a combination of E2 and P4 for 48 h and 14 days. No increased expression of STC-1 was observed after either time point (Supplementary Figure S1A). Therefore, no further testing was executed with the PCOS samples.
To assess the role of androgens in eSC STC-1 expression in controls and women with PCOS, the eSCs were treated for 24 h with E2 alone or with a combination of E2 and DHT. No significant change in STC-1 expression was detected in eSCs in either study group, confirming that steroid hormones do not directly induce STC-1 in eSCs (Supplementary Figure S1B).
STC-1 expression during 8-bromo-cAMP challenge in polycystic ovary syndrome vs control endometrial stromal cells
Cultures of eSCs from control women and women with PCOS were exposed to 8-Br-cAMP for 96 h to induce STC-1 expression. STC-1 mRNA was highly upregulated (~140 fold) in cultured eSCs from control subjects, while eSCs from women with PCOS showed significantly lower expression (P = 0.001; Figure 4A). Quantitative protein measurements from the growth media revealed similar stromal cell basal secretion of STC-1 in the two study groups. However, 8-Br-cAMP-induced STC-1 expression was lower in the eSCs from women with PCOS vs controls, supporting the qRT-PCR findings (P = 0.01; Figure 4B).
Figure 4.
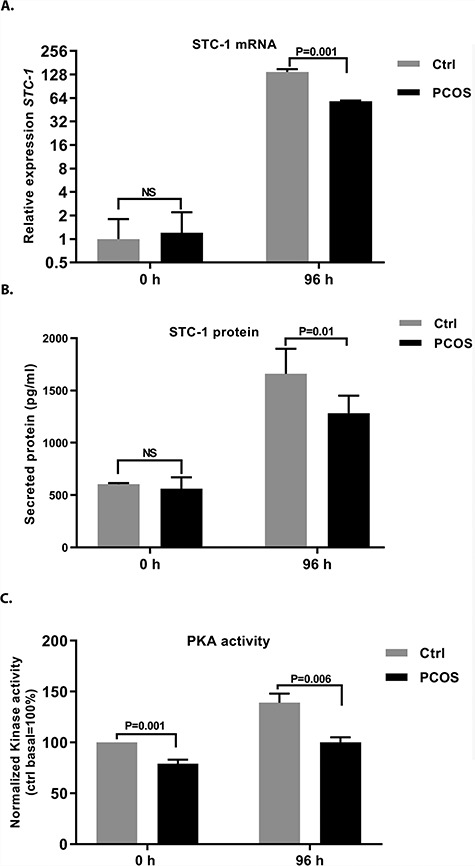
(A) STC-1 mRNA expression, (B) protein expression, and (C) PKA activity analysis in response to 8-Br-cAMP challenge in cultured eSCs obtained from control women and from women with PCOS. (A) Expression of STC-1 mRNA in stromal cells from control women (n = 10, gray bars) and women with PCOS (n = 12, black bars) after treatment with 0.5 mM 8-Br-cAMP for 96 h. STC-1 expression was blunted in the stromal cells obtained from women with PCOS (P = 0.001) compared with the controls. (B) Similarly, STC-1 protein secretion from stromal cells into culture media after 96 h of 0.5 mM 8-Br-cAMP administration was lower (P = 0.01) in women with PCOS (n = 12, black bars) compared with controls (n = 10, gray bars). Basal STC-1 secretion, however, did not differ between the study groups. (C) PKA activity was significantly higher in stromal cells from control women (n = 4, gray bars) compared to women with PCOS (n = 4, black bars; P = 0.006) after 96 h of 0.5 mM 8-Br-cAMP treatment. Interestingly, stromal cells from control women also presented with higher basal PKA activity than the stromal cells from women with PCOS (P = 0.001). Data are shown as mean values ± SEM.
Basal and cyclic adenosine monophosphate-dependent protein kinase A activity in polycystic ovary syndrome vs control endometrial stromal cells
To establish whether differences in STC-1 expression in control women and women with PCOS could be explained by differences in PKA activity (basal or 8-Br-cAMP induced), eSCs from control subjects and women with PCOS were treated with 8-Br-cAMP or DMSO for 96 h. The eSCs from women with PCOS showed lower basal and 8-Br-cAMP-induced PKA activity at 96 h compared with the controls (P = 0.001 and 0.006, respectively; Figure 4C).
STC-1 expression during hypoxia challenge in polycystic ovary syndrome vs control endometrial stromal cells
The eSCs from control women and from women with PCOS were subjected to hypoxic (1% O2) conditions for 24 h and 48 h. Hypoxic conditions induced STC-1 mRNA expression in control eSCs—8-fold and 2.5-fold higher expression at 24 h and 48 h, respectively (Figure 5A). Interestingly, we found that the expression was impaired in stromal cells from women with PCOS at both time points compared with the controls (24 h: P < 0.0001, 48 h: P = 0.001; Figure 5A). STC-1 expression was lower in the eSC culture media from women with PCOS compared with that from the controls (P = 0.001) after 48 h of exposure to hypoxia (Figure 5B), supporting the mRNA data.
Figure 5.
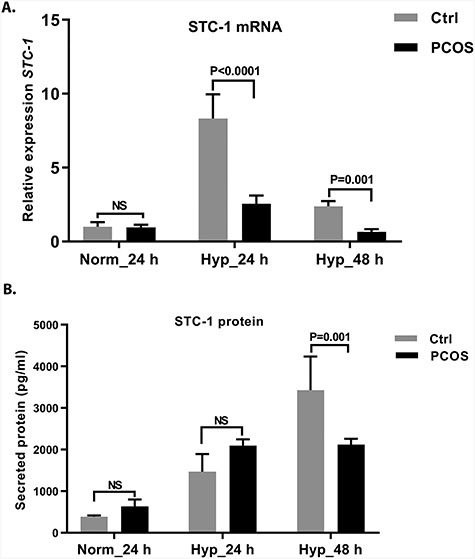
Expression of (A) STC-1 mRNA and (B) protein in cultured eSCs during hypoxia challenge (1% oxygen). (A) STC-1 mRNA expression of eSCs obtained from control women (n = 12, gray bars) and from women with PCOS (n = 13, black bars) after 24 hand 48 h of hypoxic challenge. The stromal cells from women with PCOS showed lower STC-1 mRNA expression at both 24 h (P < 0.0001) and 48 h (P = 0.001). (B) STC-1 protein secretion into eSC culture supernatant under normoxic (24 h) and hypoxic (24 and 48 h) conditions in cells from healthy controls (n = 12, gray bars) and from women with PCOS (n = 13, black bars). No significant changes were observed between the study groups under normoxic or hypoxic conditions after 24 h. However, at 48 h, the STC-1 response to hypoxia was blunted in stromal cells obtained from women with PCOS compared with the cells from controls (P = 0.001). Data are shown as mean ± SEM.
Effect of weight and lifestyle intervention on STC-1 expression
Control women with BMI > 27 kg/m2 (O-Ctrl) presented higher secretory phase STC-1 expression compared with the controls with BMI ≤ 27 kg/m2 (P = 0.047). However, no such difference was observed in women with PCOS (Figure 6A). Interestingly, 3-month lifestyle intervention did not restore endometrial STC-1 mRNA expression in the O-PCOS group (P = 0.223; Figure 6B).
Figure 6.
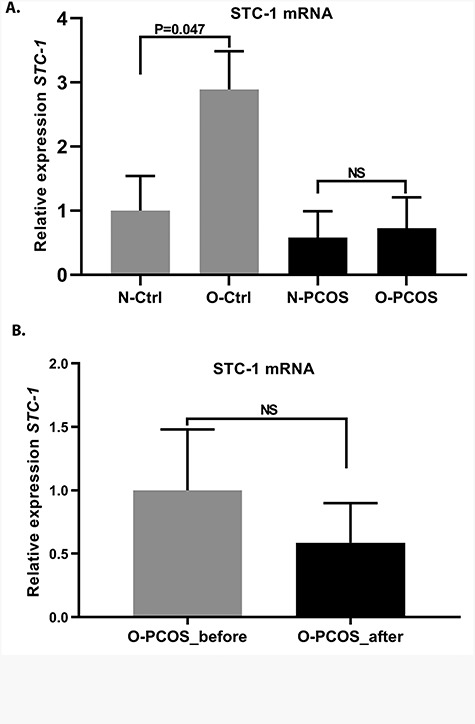
Effects of weight and lifestyle intervention on endometrial STC-1 expression. (A) Endometrial STC-1 mRNA expression at cd 21–23 in control women with BMI ≤ 27 kg/m2 (N-Ctrl, n = 11) and in controls with BMI > 27 kg/m2 (O-Ctrl, n = 5), showing increased endometrial STC-1 expression in O-Ctrl women (P = 0.047). No significant difference in STC-1 mRNA expression was found between the two weight groups of women with PCOS (N-PCOS, n = 5 vs O-PCOS, n = 15). (B) Relative expression of STC-1 on cd 21–23 in women with PCOS and BMI > 27 kg/m2 before (O-PCOS, before intervention, n = 14) and after (O-PCOS, after intervention, n = 14) 3-month lifestyle intervention. Lifestyle intervention failed to restore endometrial STC-1 expression. All values are means ± SEM.
Discussion
This is the first study to investigate STC-1 expression in the endometrium of women with PCOS. We used both in vivo and in vitro approaches and found that women with PCOS presented with reduced STC-1 expression during the secretory phase of the menstrual cycle. Furthermore, stromal cells from women with PCOS showed blunted STC-1 responses when challenged with 8-Br-cAMP and hypoxia. These results suggest that women with PCOS have altered STC-1 expression even during the secretory phase and in response to stress that may result in a defective rescue mechanism against endometrial stressors. Lifestyle intervention was not able to restore endometrial STC-1 responses in women with PCOS.
STC-1 has been shown to be expressed not only in human endometrium during the WOI but also in the placenta, suggesting a role in maternal–fetal interaction [45–47]. Since STC-1 knockout mice have been shown to be fertile, STC-1 seems to serve as a pro-survival factor modifying the endometrial environment rather than as an imperative factor for implantation [48–50]. In line with previous reports showing increased endometrial STC-1 secretion toward the secretory phase [8], we detected increased endometrial STC-1 expression toward MSE and LSE not only in the control group but also in the LH-timed PCOS samples. Interestingly, the endometrial samples from younger women with PCOS with a more severe phenotype and higher BMI failed to show an increase in STC-1 expression toward the secretory phase. Moreover, STC-1 protein expression in LH-timed in vivo samples was increased from PE to LSE stromal cells only in control women and not in PCOS, possibly indicating a defect in the stromal cells in women with PCOS. Given that STC-1 has been suggested to serve as a protective factor against oxidative stress and inflammation [51], and the fact that the endometrium in women with PCOS has been shown to display an abnormal inflammatory profile [35], the blunted STC-1-mediated rescue mechanism may be of clinical importance. The fact that blunted STC-1 was shown in the secretory phase endometrium suggests that progestin treatment or endogenous progesterone may not be sufficient to reduce the risk of endometrial dysfunction in PCOS, as commonly thought. On a related note, although the eSCs were able to decidualize in response to steroid hormones in both study groups, the steroid hormones per se did not stimulate STC-1 expression, in line with data published by Aghajanova et al. [8]. Moreover, even though many of the metabolic derangements in PCOS are driven by hyperandrogenism, the difference in STC-1 expression in stromal cells was not promoted by androgens, as shown by the experiment with DHT exposure.
As women with PCOS presented with an altered STC-1 expression in in vivo samples, next we challenged eSCs with cAMP, a trigger for STC-1 expression. We observed that stromal cells from controls presented a strong increase in STC-1 in response to 8-Br-cAMP, whereas the expression was blunted in PCOS samples. As cAMP action has been described to occur through activation of the canonical PKA signaling pathway, a blunted cAMP-induced STC-1 response may suggest a defect in this signaling cascade [8, 52]. Therefore, PKA signaling was tested in stromal cells from women with PCOS and from controls. The experiment confirmed, for the first time, blunted cAMP-mediated PKA signaling in stromal cells in women with PCOS.
In the present study, hypoxia was not able to trigger STC-1 in stromal cells from women with PCOS, in contrast to controls. In addition, secreted protein levels were lower in the stromal cell culture media from women with PCOS. Again, this might be crucial, given that hypoxia is an important factor in embryo implantation and cancer development; however, it has to be restricted for successful maternal–fetal interaction [15, 16]. Moreover, STC-1 has been identified as one of the hypoxia-responsive genes involved in hypoxia-driven angiogenesis in various cancer cell types; thus, blunted STC-1 could promote hypoxia-mediated cancer progression [53, 54].
The role of obesity was assessed in the present study using the samples obtained from Karolinska Institutet. The data showed increased STC-1 expression from PE to SE and higher STC-1 expression in overweight/obese control women compared to women with BMI < 27 kg/m2. Interestingly, no such increases from PE to SE or with overweight/obesity were detected in endometrial samples from women with PCOS. The elevated STC-1 expression in the secretory phase in controls with BMI ≥27 kg/m2 suggests that overweight/obesity might be one of the stress factors inducing endometrial STC-1, a rescue mechanism that seems to be missing in PCOS.
Women with PCOS are prone to systemic metabolic alterations with dysregulated glucose uptake and lipid metabolism and elevated low-grade inflammation. In PCOS, these alterations are commonly promoted by obesity; thus, lifestyle intervention is recommended as the first-line therapy for affected women (as also stated by the recently published International PCOS Guideline) [23]. We therefore assessed STC-1 expression before and after a 3-month lifestyle intervention. Previously, this intervention was reported to normalize menstrual cycles and to improve endometrial steroid receptor expression in women with PCOS [29, 30]. The intervention was not, however, able to restore STC-1. As lifestyle interventions are commonly known to be expensive and difficult due to high drop-out rates, other approaches should also be considered. Some studies have suggested that metformin could improve metabolic and hormonal profiles in PCOS endometrium [55, 56]. Whether this also applies to STC-1 remains to be investigated.
In the present study, we utilized rare endometrial tissue samples to assess STC-1 expression in women with PCOS. To increase the number of samples, we were able to pool samples from two study centers including well-characterized patients, LH-timed samples, and the possibility to separate women by BMI and assess the effects of an intervention. Power calculations were not feasible as no previous data on STC-1 in cases of PCOS were available. Limitations include the fact that patient recruitment for endometrial studies is known to be challenging, and for this reason, the number of endometrial tissue samples remains limited. In addition, the samples from Karolinska Institutet were collected in 2008–2012 and stored as RNA after extraction. However, we estimated the quality of the samples to be acceptable for the methods used for the present study. We are also aware of the difficulty of comparing in vitro results with in vivo findings. Moreover, even though we tested androgen effects in an in vitro environment, we could not consider the roles of systemic hyperandrogenism or hyperinsulinemia, both common in PCOS, in an in vitro environment. As for cellular STC-1 signaling, more profound pathway analyses and mechanistic approaches are warranted.
Conclusion
This is the first study reporting blunted STC-1 expression in the endometrium of women with PCOS. We also report decreased STC-1 expression in response to 8-Br-cAMP and hypoxic challenge in PCOS stromal cells in vitro. The basal and cAMP-driven PKA pathway activation was significantly lower in women with PCOS compared with controls, which may explain the STC-1 outcome in affected women. Defective endometrial STC-1 expression in PCOS may result in an impaired rescue mechanism, promoting endometrial dysfunction or even cancer in these women (Supplementary Figure S2).
Conflict of interest
The authors have declared that no conflict of interest exists.
Supplementary Material
Acknowledgements
The authors would like to thank Elina Huikari and Riitta Vuento for skilful technical support. This work was supported by the Sigrid Jusélius Foundation, the Academy of Finland, the Finnish Medical Foundation, the Estonian Ministry of Education and Research (grants IUT34-16 and PRG454), Enterprise Estonia (grant EU48695), the Horizon 2020 innovation programme (WIDENLIFE, 692065), Finska Läkaresällskapet, the Swedish Research Council (ALH 20324), and Karolinska Institutet.
Conference presentation: Presented in part at the Annual Meeting of European Society of Human Reproduction and Embryology, 1–4 July 2018, Barcelona, Spain.
References
- 1. Yoshiko Y, Aubin JE. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides 2004; 25:1663–1669. [DOI] [PubMed] [Google Scholar]
- 2. Pan JS, Huang L, Belousova T, Lu L, Yang Y, Reddel R, Chang A, Ju H, DiMattia G, Tong Q, Sheikh-Hamad D. Stanniocalcin-1 inhibits renal ischemia/reperfusion injury via an AMP-activated protein kinase-dependent pathway. J Am Soc Nephrol 2015; 26:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke 2007; 38:1025–1030. [DOI] [PubMed] [Google Scholar]
- 4. Mohammadipoor A, Lee RH, Prockop DJ, Bartosh TJ. Stanniocalcin-1 attenuates ischemic cardiac injury and response of differentiating monocytes/macrophages to inflammatory stimuli. Transl Res 2016; 177:127–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang L, Belousova T, Chen M, DiMattia G, Liu D, Sheikh-Hamad D. Overexpression of stanniocalcin-1 inhibits reactive oxygen species and renal ischemia/reperfusion injury in mice. Kidney Int 2012; 82:867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Westberg JA, Serlachius M, Lankila P, Andersson LC. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol 2007; 293:H1766–H1771. [DOI] [PubMed] [Google Scholar]
- 7. Boggavarapu NR, Lalitkumar S, Joshua V, Kasvandik S, Salumets A, Lalit kumar PG, Gemzell-Danielsson K. Compartmentalized gene expression profiling of receptive endometrium reveals progesterone regulated ENPP3 is differentially expressed and secreted in glycosylated form. Sci Rep 2016; 6:33811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aghajanova L, Altmae S, Kasvandik S, Salumets A, Stavreus-Evers A, Giudice LC. Stanniocalcin-1 expression in normal human endometrium and dysregulation in endometriosis. Fertil Steril 2016; 106:681, e1–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suhorutshenko M, Kukushkina V, Velthut-Meikas A, Altmae S, Peters M, Magi R, Krjutskov K, Koel M, Codoner FM, Martinez-Blanch JF, Vilella F, Simon C, et al. Endometrial receptivity revisited: endometrial transcriptome adjusted for tissue cellular heterogeneity. Hum Reprod 2018; 33:2074–2086. [DOI] [PubMed] [Google Scholar]
- 10. Allegra A, Marino A, Coffaro F, Lama A, Rizza G, Scaglione P, Sammartano F, Santoro A, Volpes A. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle? Hum Reprod 2009; 24:2549–2557. [DOI] [PubMed] [Google Scholar]
- 11. Guo F, Li Y, Wang J, Li Y, Li Y, Li G. Stanniocalcin1 (STC1) inhibits cell proliferation and invasion of cervical cancer cells. PLoS One 2013; 8:e53989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y, Yin S, Li S, Chen Y, Yang L. Stanniocalcin 1 in tumor microenvironment promotes metastasis of ovarian cancer. Onco Targets Ther 2019; 12:2789–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Md Fuzi AA, Omar SZ, Mohamed Z, Mat Adenan NA, Mokhtar NM. High throughput silencing identifies novel genes in endometrioid endometrial cancer. Taiwan J Obstet Gynecol 2018; 57:217–226. [DOI] [PubMed] [Google Scholar]
- 14. Gou J, Jia J, Feng J, Zhao X, Yi T, Cui T, Li Z. Stathmin 1 plays a role in endometrial decidualisation by regulating hypoxia inducible factor-1alpha and vascular endothelial growth factor during embryo implantation. Reprod Fertil Dev 2017; 29:1530–1537. [DOI] [PubMed] [Google Scholar]
- 15. Daikoku T, Matsumoto H, Gupta RA, Das SK, Gassmann M, DuBois RN, Dey SK. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem 2003; 278:7683–7691. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto L, Hirota Y, Saito-Fujita T, Takeda N, Tanaka T, Hiraoka T, Akaeda S, Fujita H, Shimizu-Hirota R, Igaue S, Matsuo M, Haraguchi H, et al. HIF2alpha in the uterine stroma permits embryo invasion and luminal epithelium detachment. J Clin Invest 2018; 128:3186–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol Biomarkers Prev 2005; 14:2840–2847. [DOI] [PubMed] [Google Scholar]
- 19. Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, Stegger J, Overvad K, Chabbert-Buffet N, Jimenez-Corona A, Clavel-Chapelon F, Rohrmann S, et al. Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study. Endocr Relat Cancer 2010; 17:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol 2005; 3: 56-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang AC, Jellinek DA, Reddel RR. Mammalian stanniocalcins and cancer. Endocr Relat Cancer 2003; 10:359–373. [DOI] [PubMed] [Google Scholar]
- 22. Chakraborty A, Brooks H, Zhang P, Smith W, McReynolds MR, Hoying JB, Bick R, Truong L, Poindexter B, Lan H, Elbjeirami W, Sheikh-Hamad D. Stanniocalcin-1 regulates endothelial gene expression and modulates transendothelial migration of leukocytes. Am J Physiol Renal Physiol 2007; 292:F895–F904. [DOI] [PubMed] [Google Scholar]
- 23. Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ. International PCOS network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018; 33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katulski K, Czyzyk A, Podfigurna-Stopa A, Genazzani AR, Meczekalski B. Pregnancy complications in polycystic ovary syndrome patients. Gynecol Endocrinol 2015; 31:87–91. [DOI] [PubMed] [Google Scholar]
- 25. Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015; 21:575–592. [DOI] [PubMed] [Google Scholar]
- 26. El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol 2016; 7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piltonen TT, Chen J, Erikson DW, Spitzer TL, Barragan F, Rabban JT, Huddleston H, Irwin JC, Giudice LC. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J Clin Endocrinol Metab 2013; 98:3765–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDonnell R, Hart RJ. Pregnancy-related outcomes for women with polycystic ovary syndrome. Womens Health (Lond) 2017; 13:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hulchiy M, Nybacka A, Sahlin L, Hirschberg AL. Endometrial expression of Estrogen receptors and the androgen receptor in women with polycystic ovary syndrome: a lifestyle intervention study. J Clin Endocrinol Metab 2016; 101:561–571. [DOI] [PubMed] [Google Scholar]
- 30. Paulson M, Sahlin L, Hirschberg AL. Progesterone receptors and proliferation of the endometrium in obese women with polycystic ovary syndrome-a lifestyle intervention study. J Clin Endocrinol Metab 2017; 102:1244–1253. [DOI] [PubMed] [Google Scholar]
- 31. Stepto NK, Cassar S, Joham AE, Hutchison SK, Harrison CL, Goldstein RF, Teede HJ. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum Reprod 2013; 28:777–784. [DOI] [PubMed] [Google Scholar]
- 32. Morin-Papunen L, Rantala AS, Unkila-Kallio L, Tiitinen A, Hippelainen M, Perheentupa A, Tinkanen H, Bloigu R, Puukka K, Ruokonen A, Tapanainen JS. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab 2012; 97:1492–1500. [DOI] [PubMed] [Google Scholar]
- 33. Ujvari D, Hulchiy M, Calaby A, Nybacka A, Bystrom B, Hirschberg AL. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod 2014; 29:1526–1535. [DOI] [PubMed] [Google Scholar]
- 34. Khatun M, Sorjamaa A, Kangasniemi M, Sutinen M, Salo T, Liakka A, Lehenkari P, Tapanainen JS, Vuolteenaho O, Chen JC, Lehtonen S, Piltonen TT. Niche matters: the comparison between bone marrow stem cells and endometrial stem cells and stromal fibroblasts reveal distinct migration and cytokine profiles in response to inflammatory stimulus. PLoS One 2017; 12:e0175986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piltonen TT, Chen JC, Khatun M, Kangasniemi M, Liakka A, Spitzer T, Tran N, Huddleston H, Irwin JC, Giudice LC. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum Reprod 2015; 30:1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen JC, Erikson DW, Piltonen TT, Meyer MR, Barragan F, McIntire RH, Tamaresis JS, Vo KC, Giudice LC, Irwin JC. Coculturing human endometrial epithelial cells and stromal fibroblasts alters cell-specific gene expression and cytokine production. Fertil Steril 2013; 100:1132–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babayev SN, Park CW, Keller PW, Carr BR, Word RA, Bukulmez O. Androgens upregulate endometrial epithelial progesterone receptor expression: potential implications for endometriosis. Reprod Sci 2017; 24:1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178:357–372. [DOI] [PubMed] [Google Scholar]
- 39. Okada H, Tsuzuki T, Murata H. Decidualization of the human endometrium. Reprod Med Biol 2018; 17:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiong Y, Liu Y, Xiong W, Zhang L, Liu H, Du Y, Li N. Hypoxia-inducible factor 1 alpha-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum Reprod 2016; 31:1327–1338. [DOI] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 42. Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC, Giudice LC. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 2012; 86:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kestav K, Viht K, Konovalov A, Enkvist E, Uri A, Lavogina D. Slowly on, slowly off: bisubstrate-analogue conjugates of 5-Iodotubercidin and histone H3 peptide targeting protein kinase Haspin. Chembiochem 2017; 18:790–798. [DOI] [PubMed] [Google Scholar]
- 44. Sinijarv H, Wu S, Ivan T, Laasfeld T, Viht K, Uri A. Binding assay for characterization of protein kinase inhibitors possessing sub-picomolar to sub-millimolar affinity. Anal Biochem 2017; 531:67–77. [DOI] [PubMed] [Google Scholar]
- 45. Uuskula L, Mannik J, Rull K, Minajeva A, Koks S, Vaas P, Teesalu P, Reimand J, Laan M. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLoS One 2012; 7:e49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, Sali A, Fisher SJ. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology 2007; 148:1059–1079. [DOI] [PubMed] [Google Scholar]
- 47. Juhanson P, Rull K, Kikas T, Laivuori H, Vaas P, Kajantie E, Heinonen S, Laan M. Stanniocalcin-1 hormone in nonpreeclamptic and preeclamptic pregnancy: clinical, life-style, and genetic modulators. J Clin Endocrinol Metab 2016; 101:4799–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chang AC, Cha J, Koentgen F, Reddel RR. The murine stanniocalcin 1 gene is not essential for growth and development. Mol Cell Biol 2005; 25:10604–10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147:1097–1121. [DOI] [PubMed] [Google Scholar]
- 50. Altmae S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod 2010; 16:178–187. [DOI] [PubMed] [Google Scholar]
- 51. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 2012; 20:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kikuchi M, Nakano Y, Nambo Y, Haneda S, Matsui M, Miyake Y, Macleod JN, Nagaoka K, Imakawa K. Production of calcium maintenance factor stanniocalcin-1 (STC1) by the equine endometrium during the early pregnant period. J Reprod Dev 2011; 57:203–211. [DOI] [PubMed] [Google Scholar]
- 53. Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CK. Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 2005; 146:4951–4960. [DOI] [PubMed] [Google Scholar]
- 54. Chi JT, Wang Z, Nuyten DS, Rodriguez EH, Schaner ME, Salim A, Wang Y, Kristensen GB, Helland A, Borresen-Dale AL, Giaccia A, Longaker MT, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med 2006; 3:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab 2010; 1:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol 2008; 199:596–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


