Short abstract
Background
Neural damage at early stages of multiple sclerosis (MS) can subtly affect gait muscle activation patterns. Detecting these changes using current clinical tools, however, is not possible. We propose using muscle coactivation measures to detect these subtle gait changes. This may also help in identifying people with MS (PwMS) that may benefit from strategies aimed at preventing further mobility impairments.
Objective
We aimed to determine if coactivation of ankle muscles during gait is greater in PwMS with Expanded Disability Status Scale (EDSS) score <3.5. A secondary aim is to determine whether coactivation increases are speed dependent.
Methods
For this study 30 PwMS and 15 healthy controls (HC) walked on a treadmill at 1.0 m/s, 1.2 m/s and 1.4 m/s. Electromyography was recorded from the tibialis anterior (TA), soleus (SO) and lateral gastrocnemius (LG). The coactivation index was calculated between SO/TA and LG/TA. Ankle kinematics data were also collected.
Results
Compared with HC, PwMS exhibited significantly greater SO/TA and LG/TA coactivation, which was greater during early stance and swing phases (p < .01). Speed did not affect coactivation except during early stance. Ankle kinematic changes were also observed.
Conclusion
PwMS exhibited greater ankle muscles coactivation than controls regardless of the speed of walking. These changes in muscle activation may serve as a biomarker of neurodegeneration occurring at early stages of the disease.
Keywords: Electromyography, coactivation, gait, movement, biomarkers
Introduction
Mobility and independence can be severely affected by gait deterioration in people with multiple sclerosis (PwMS) even in the early stages of the disease.1 Therefore, identifying early markers of disease progression from gait measures is important, not only to better target rehabilitation interventions but also to determine the effects of medications and rehabilitation on walking. Instrumented clinical tests of gait, for example, have found that PwMS exhibit slower speed, shorter steps and decreased cadence.2,3 Furthermore, laboratory measures of gait using 3D gait analysis and electromyography have found several kinematic (joint angles) and kinetic (joint forces) changes associated with speed reductions as well as changes in the timing of muscle activity, especially in the ankle plantarflexors and dorsiflexors,4 but also in knee flexors/extensors.5
Increased coactivation of agonist/antagonist muscles reflects impaired motor control due to nervous system injury, such as occurs in stroke and traumatic brain injury.6 Altered coactivation patterns of the ankle and knee flexors/extensors during gait have also been reported in PwMS mainly during stance.5,7 However, in these studies PwMS exhibited a wide range of disability as measured using the Expanded Disability Status Scale (EDSS) score7 or walked at a significantly slower speed than controls.5 These two factors can significantly affect comparisons between PwMS and controls, as EDSS>3.5 could imply emerging gait deterioration and speed is one of the main confounding variables in gait.8,9
It has been proposed that muscle coactivation during gait may be a compensatory mechanism to maintain stability during stance, as PwMS may also present with balance deterioration.5 However, coactivation during swing may predispose PwMS to falls by tripping, as inadequate timing of plantarflexor activation can reduce toe-clearance.7 Although the exact mechanisms underlying muscle coactivation are not clear, studies in the elderly have demonstrated decreases in spinal reciprocal inhibition and spread of motor commands within the motor cortex for the control of agonist-antagonist muscles that may partly explain this phenomenon.10 An additional mechanism that is often present in PwMS is that of damage of neural circuits in the spinal cord that may therefore also be responsible for increased muscle coactivation in the early stages of MS.11
Although the causes of increased coactivation are yet to be elucidated, it is known that altered agonist/antagonist activation patterns may serve to increase walking stability in the presence of weakness.12,13 The latter may also be a reflection of poor balance control, which has been widely reported in PwMS with a wide range of disability.3,14,15 Excessive coactivation has been associated with an increased energy cost of walking seen in older adults,16 hence it may partly explain the increased oxygen consumption and fatigability during mobility tasks observed in PwMS.17
The aims of this study were to compare coactivation of ankle agonist/antagonist musculature in PwMS in the early stages of the disease with that in healthy controls (HC) and the association with the speed of walking. We also investigated whether coactivation of plantar- and dorsiflexors was associated with kinematic changes at the ankle joint. We hypothesize that PwMS exhibit greater agonist/antagonist coactivation of ankle muscles than controls, and that this difference increases at faster speeds.
Methods
Patients
Thirty people with relapsing–remitting MS were recruited from the outpatient clinic at the Royal Melbourne Hospital. Inclusion criteria were: (a) <10–15 years since onset of first symptoms; (b) >18 years; (c) clinically definite MS diagnosis using the criteria of McDonald et al. (2001)18; (d) EDSS <4.5. Exclusion criteria included: (a) neurological condition other than MS; (b) coexisting cardiovascular disease; (c) orthopaedic conditions causing disability of the lower limbs; (d) recent laryngeal infection or laryngeal surgery. Fifteen healthy participants (controls) were recruited from amongst university and hospital staff. The inclusion criteria for the control participants were: (a) no history of neurological condition; (b) no history of orthopaedic conditions; and (c) >18 years. Table 1 presents demographics for both groups and relevant clinical data for PwMS, including overall EDSS and functional systems scores. This study was approved by the Melbourne Health Human Research Ethics Committee (2015.144). All participants provided signed informed consent prior to assessment.
Table 1.
Participants’ demographics and EDSS clinical scores for PwMS.
| MS (n = 30) |
HC (n = 15) |
|||
|---|---|---|---|---|
| Mean | sd | Mean | sd | |
| Age (years) | 42.5 | 9.2 | 36.8 | 7.0 |
| Height (cm) | 167.7 | 6.5 | 170.8 | 12.6 |
| Weight (kg) | 70.6 | 12.0 | 69.8 | 14.7 |
| Sex (f/m) | 25/5 | – | 9/6 | – |
|
|
Median |
Range |
||
| EDSS | 1.25 | [0–2.5] | ||
| Visual | 0 | [0–3] | ||
| Brainstem | 0 | [0–0] | ||
| Pyramidal | 0 | [0–1] | ||
| Cerebellar | 0 | [0–2] | ||
| Sensory | 0 | [0–2] | ||
| Bowel/Bladder | 0 | [0–2] | ||
| Cerebral | 0 | [0–2] | ||
| Ambulation | 0 | [0–0] | ||
Setup
Participants wore shorts or short tights and a singlet so that reflective spherical markers could be placed on specific body landmarks using a Plug-in-Gait model. The marker displacement during treadmill walking was collected at 200 Hz using an 8-camera infrared Vicon system (Vicon, Oxford, UK). Foot markers displacements were used to determine heel contact and toe-off events and cycle phases (stance and swing). A wireless surface electromyography (EMG) system (Cometa, Milano, Italy) was used to collect bilateral electromyographic signals at 1000 Hz from tibialis anterior (TA), lateral gastrocnemius (LG) and soleus (SO) muscles. Bipolar EMG electrodes were placed following SENIAM recommendations. Nexus 2 software (Vicon, Oxford, UK) was used to record all marker and EMG data and to obtain ankle kinematics.
Assessments
Participants were asked to walk barefoot on a Biodex RTM600 (Shirley, NY, USA) treadmill in three conditions representative of slow (1.0 ms−1), normal (1.2 ms−1) and fast (1.4 ms−1) walking speeds without holding the rails. Each condition was recorded for 20 s to ensure that the EMG data for at least 12 full gait cycles per side was obtained. Participants were instructed to stay in the middle of the belt and stop the treadmill by pressing the emergency button in case of balance loss or exhaustion. No participant (PwMS and controls) reported fatigue or exhaustion after the treadmill walking.
Data processing
All data were processed using a Matlab R2017a script (Natwick, MA, USA). Coactivation between LG/TA and SO/TA was assessed using the coactivation index (COI) as described by Chow et al. (2012).6 The raw EMG data were first filtered using second order bidirectional bandpass Butterworth filter (10–400 Hz). Data were then rectified and EMG average at rest (rectified) was subtracted before low-pass filtering (10 Hz, 2nd order Butterworth) for linear envelope. EMG data were then normalized to the average EMG for each gait cycle. The LG/TA and SO/TA curves overlap area was then divided by the overlap duration during the whole gait cycle, and also for the stance and swing phases separately, to obtain the COI for the LG/TA and SO/TA muscle pairs. For each subject the average between 10 left and 10 right gait cycles LG/TA and SO/TA COIs per speed condition was used for statistical analysis.
Ankle kinematic measures were calculated in Matlab R2017 using data exported from VICON-Nexus. Four angular peaks were identified: first ankle plantarflexion (PF1) occurring at early stance, first ankle dorsiflexion (DF1) occurring during late stance, second ankle plantarflexion (PF2, maximum plantarflexion) occurring around toe-off and second ankle dorsiflexion (DF2) occurring during swing. Kinematic peaks from the same 10 left and 10 right gait cycles per speed described above were used for statistical analysis.
Statistical analysis
A multivariate ANOVA using speed and disease as main factors was applied to determine their effect on LG/TA and SO/TA COIs during the whole gait cycle (GC), stance (ST) and swing (SW) phases and initial double-support (DS1), single-support (SS) and second double-support (DS2) subphases, separately. A second multivariate ANOVA was performed to determine the effects of disease and speed on kinematic measures (PF1, DF1, PF2 and DF2) across all left and right GCs analysed. For all statistical tests the alpha level was set at 0.05.
Results
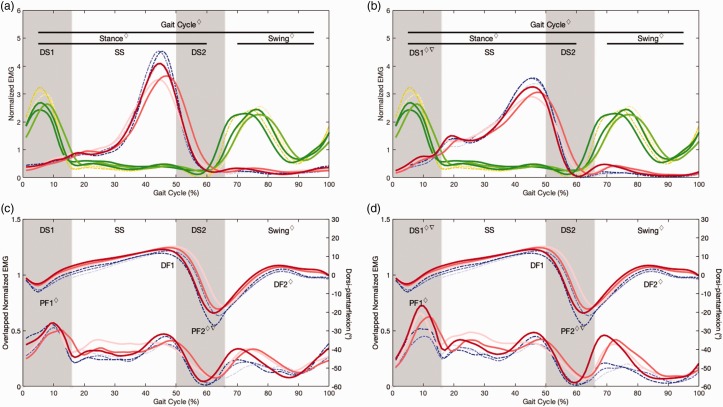
Although EDSS inclusion criteria was <4.5, all PwMS had EDSS ≤2.5 and with an ambulation sub score equals to 0. All PwMS were able to complete uninterrupted 20 s treadmill walking for the three speed conditions and without reporting fatigue. Overall, PwMS exhibited significantly greater LG/TA and SO/TA COI during the whole GC, stance and swing phases and significantly greater SO/TA COI during early stance (DS1) when compared with the control group. A speed effect was only found for SO/TA at DS1; however, no group*speed interaction for this or the remaining variables was found. Table 2 presents descriptive and comparative statistics for all measures and Figure 1 depicts the averaged EMG plots for all PwMS and HCs normalized to GC. For the ankle kinematics, PwMS exhibited significantly lower PF1 and PF2 than controls (p < .01). These differences, however, were not affected by the speed of walking.
Table 2.
Coactivation indices and kinematics (mean ± sd) for both muscle pairs (LG/TA and SO/TA) analysed for each gait cycle (GC) phase and subphase as well as for each speed condition.
| 1.0 ms−1 |
1.2 ms−1 |
1.4 ms−1 |
p-values |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS |
Control |
MS |
Control |
MS |
Control |
Group | Speed | Group* speed | ||||||||
| Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | Mean | sd | |||||
| LG/TA COI | GC | 0.34 | 0.10 | 0.27 | 0.08 | 0.33 | 0.11 | 0.27 | 0.09 | 0.34 | 0.11 | 0.30 | 0.09 | 0.00 | 0.91 | 0.99 |
| Stance | 0.38 | 0.14 | 0.32 | 0.08 | 0.37 | 0.14 | 0.31 | 0.10 | 0.38 | 0.15 | 0.35 | 0.09 | 0.02 | 0.83 | 0.99 | |
| Swing | 0.26 | 0.14 | 0.19 | 0.14 | 0.25 | 0.13 | 0.19 | 0.13 | 0.25 | 0.12 | 0.20 | 0.15 | 0.01 | 0.98 | 0.95 | |
| DS1 | 0.43 | 0.14 | 0.38 | 0.21 | 0.42 | 0.15 | 0.41 | 0.26 | 0.47 | 0.18 | 0.48 | 0.27 | 0.20 | 0.61 | 0.84 | |
| SS | 0.40 | 0.22 | 0.32 | 0.10 | 0.36 | 0.19 | 0.30 | 0.08 | 0.37 | 0.22 | 0.32 | 0.09 | 0.07 | 0.79 | 0.95 | |
| DS2 | 0.22 | 0.15 | 0.19 | 0.07 | 0.28 | 0.22 | 0.18 | 0.05 | 0.23 | 0.20 | 0.19 | 0.06 | 0.08 | 0.82 | 0.56 | |
| SO/TA COI | GC | 0.35 | 0.14 | 0.26 | 0.06 | 0.35 | 0.14 | 0.25 | 0.06 | 0.37 | 0.15 | 0.28 | 0.05 | 0.00 | 0.69 | 1.00 |
| Stance | 0.40 | 0.17 | 0.31 | 0.07 | 0.39 | 0.17 | 0.31 | 0.07 | 0.42 | 0.18 | 0.35 | 0.06 | 0.00 | 0.59 | 0.95 | |
| Swing | 0.23 | 0.12 | 0.14 | 0.12 | 0.25 | 0.14 | 0.13 | 0.08 | 0.26 | 0.13 | 0.14 | 0.07 | 0.00 | 0.89 | 0.82 | |
| DS1 | 0.46 | 0.14 | 0.33 | 0.10 | 0.45 | 0.17 | 0.35 | 0.14 | 0.53 | 0.17 | 0.43 | 0.14 | 0.00 | 0.03 | 0.89 | |
| SS | 0.44 | 0.24 | 0.34 | 0.10 | 0.40 | 0.23 | 0.33 | 0.08 | 0.41 | 0.25 | 0.35 | 0.10 | 0.05 | 0.84 | 0.93 | |
| DS2 | 0.22 | 0.18 | 0.20 | 0.08 | 0.28 | 0.23 | 0.18 | 0.05 | 0.23 | 0.19 | 0.19 | 0.06 | 0.08 | 0.86 | 0.58 | |
| Kinematics | PF1 (°) | −5.48 | 4.11 | −8.14 | 3.78 | −6.03 | 3.27 | −8.17 | 3.20 | −4.25 | 7.85 | −8.40 | 3.67 | 0.00 | 0.78 | 0.64 |
| DF1 (°) | 14.43 | 4.00 | 13.65 | 3.47 | 14.04 | 3.59 | 13.39 | 3.33 | 16.17 | 16.70 | 12.31 | 3.15 | 0.27 | 0.96 | 0.65 | |
| PF2 (°) | −16.13 | 9.04 | −19.58 | 9.42 | −18.52 | 8.03 | −22.31 | 8.61 | −20.30 | 9.13 | −25.61 | 9.92 | 0.01 | 0.05 | 0.89 | |
| DF2 (°) | 4.73 | 3.25 | 3.38 | 2.86 | 4.22 | 3.23 | 2.74 | 2.89 | 5.22 | 3.49 | 4.05 | 3.02 | 0.03 | 0.28 | 0.97 | |
The right side of the table presents p-values for the main effects of group (PwMS and HC) and speed as well as their interaction. Significant effects are highlighted in bold.
Figure 1.
(a) Normalized averaged EMG curves for TA in PwMS (green continuous) and HC (yellow dashed) and for LG in PwMS (red continuous) and HC (blue dashed). (b) Normalized averaged EMG curves for SO in PwMS (red continuous) and HC (blue dashed); TA also presented. (c) Right axis: normalized averaged overlapped EMG curves for LG/TA in PwMS (red continuous) and HC (blue dashed); Left axis: normalized ankle kinematic curves in PwMS (red continuous) and HC (blue dashed). (d) Right axis: normalized averaged overlapped EMG curves for SO/TA in PwMS (red continuous) and HC (blue dashed), ankle kinematics is also presented. For all plots slow (1.0 ms−1), normal (1.2 ms−1) and fast (1.4 ms−1) are presented from lighter to darker colour intensity. ⋄ significant group effect, ▽ significant speed effect, DS1: initial double-support, SS: single stance, DS2: second double-support, PF1: plantarflexion at DS1, DF1: peak dorsiflexion at stance, PF2: peak ankle plantarflexion, DS2: peak dorsiflexion at swing.
Discussion
The main aim of this study was to determine whether ankle muscle coactivation, as a marker of motor control deterioration during gait, was increased in PwMS at early stages of the disease (EDSS < 3.0) and whether this increase was affected by the speed of walking. A secondary aim was to determine whether SO/TA and LG/TA COIs were associated with kinematic changes. Although previous studies had already reported subtle changes in gait mechanics in the early stages of MS,2,3 this is the first using EMG coactivation measures. Overall, we found a significantly greater coactivation for both muscle pairs (SO/TA and LG/TA) in PwMS compared with HC. Reduced plantarflexion during early stance and around toe-off and greater dorsiflexion during swing were also found in PwMS compared with HC.
To our knowledge this is the first study reporting increases in ankle muscle coactivation during speed-controlled walking (treadmill) and in a relatively homogeneous group of PwMS with low levels of disability. This is important because the findings of previous studies may have been biased due to either the confounding effect of speed when comparing gait measures or due to the wide range of physical disability levels in the cohort of PwMS assessed.5,7 Furthermore, this study found that increased coactivation occurred even when PwMS walked at a range of speeds, which were representative of slow (1.0 ms−1), normal (1.2 ms−1) and fast (1.4 ms−1) walking. However, since no group-by-speed interaction was found, results indicate that coactivation did not increase with speed more in the PwMS than in HC as was expected. This hypothesis was based on previous studies reporting that the cost of walking and coactivation increases with speed in older adults.16 It is possible, therefore, that coactivation of ankle muscles is a constant underlying mechanism in PwMS that helps maintain motor function19 yet at the expense of a greater cost of walking.20,21
We found that coactivation between SO/TA and LG/TA was significantly greater throughout the whole GC, and for both stance and swing phases and for SO/TA during the initial double-support subphase (DS1). A previous study found significant differences between PwMS and HC for the SO/TA and MG/TA (medial gastrocnemius) pairs during stance, DS1, SS and DS2 but did not report COI during swing.5 Interestingly, our values for COI are comparatively lower than those in that study for both HC and PwMS groups. This may be due to differences between overground and treadmill walking,22 the number of strides analysed and the fact that our patients walked barefoot instead of shod.5 For the PwMS, lower COI values may be also due to our patients’ disability scores being lower than in previous studies (EDSS <5 vs. EDSS <3.0) and therefore possibly with less compromised gait performance.
During initial double-support (DS1) the leading limb (limb at the front) is accepting the weight being transferred from the trailing limb (limb at the back) during push-off (around DS2). Significantly greater COI between SO/TA in PwMS at this stage may be a mechanical strategy used to stiffen the ankle joint and increase stability.5,16,23 Increased coactivation may also be used as a compensatory mechanism to minimize feedback coming from expected inputs during foot landing (DS1) and increase the feedback due to potential perturbations that may threaten stability.11 The latter may serve as a sensory re-weighting mechanism that also increases proprioceptive inputs for the balance control system when other sources (i.e. somatosensory, vestibular, visual) are not completely reliable in PwMS.24 This is of great relevance for PwMS who exhibit poorer balance control during walking.25 Furthermore, the significant effect of speed on SO/TA COI during DS1 may indicate that this ‘joint stiffening’ mechanism is more relevant when speed increases.
Although the causes of increased coactivation of agonist/antagonist muscles are not fully understood, it has been proposed that they can be mediated by cortical and subcortical mechanisms.10,11,19 For example, patients with cerebellar disorders,26 stroke,6,23 Parkinson’s disease,27 acquired brain injury6 and diabetic neuropathy13 have all shown greater muscle coactivation during walking; nonetheless, it is most likely that different structures and circuitries are affected in these conditions. On the other hand, if coactivation is a mechanism to preserve motor function,19 coactivation increases may not indicate specific damage of the nervous system but instead may be the output of a ‘default’ compensatory mechanism that is triggered when nervous system damage alters normal motor control.
We also found significant kinematic changes such as lower plantarflexion during early stance and around toe-off (push-off) and greater dorsiflexion in swing in PwMS; however, these changes were not associated with COI increases around the same stage of the GC. Although plantarflexion changes found in this study are in agreement with previous findings,1,3,28 dorsiflexion during swing has been reported to be lower in PwMS.3,28 This may be due to the adoption of a more cautious pattern by adults when walking on a treadmill;29 in PwMS this may also involve increasing dorsiflexion to avoid tripping. Finally, our finding of significant increases in plantarflexion with speed is in line with previous studies.4
PwMS in this study had low disability as measured by EDSS (range: 0–2.5) and did not exhibit evident gait abnormalities (EDSS-ambulation = 0). Therefore, increases in ankle muscle coactivation may be an early indicator of the disease affecting the motor control system, which may potentially be used as an indicator of disease progression. Future studies should explore the use of inertial or EMG sensors to identify measures that can reflect coactivation of ankle musculature and that can be more easily implemented in clinical settings.
Limitations
It is noteworthy that direct comparisons with previous literature assessing coactivation in neurological populations is not possible, because calculations and methodologies varied amongst studies.30 However, we can partly compare our results with those reported in PwMS who walked overground and where the same method was used as in the present study.16,23 We used the average COI between left and right legs, which may be argued as a confounder in PwMS that report one leg more affected than the other; nevertheless, no significant differences between legs (more and less affected) have been previously found.5
Treadmill walking is the most used method to control the confounding effect of speed yet differs from overground walking at the same speed; for example, TA and MG EMG activity is lower.22 However, the results of this study showed that TA, SO and LG EMG activity gradually increased with speed until 1.4ms−1 (fast speed) was reached, hence a range of EMG activation levels was assessed. This study only focused on ankle dorsi-plantarflexor muscles (TA, SO and LG); however, the activation patterns of other muscles and joints may be also compromised in PwMS. We chose these distal muscles because it has been shown that the motor control of the distal musculature is more affected than the proximal in patients with upper motor neuron damage.31
Conclusions
PwMS exhibited greater ankle muscle coactivation than controls regardless of the speed of walking. These changes in muscle activation may serve as a biomarker of neurodegeneration occurring at early stages of the disease.
Acknowledgements
The authors would like to thank Myrte Stryk, Stacey Telianidis and Scott Kolbe for their help with recruitment and scheduling of patients.
Contributor Information
L Eduardo Cofré Lizama, Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Melbourne, Australia; Australian Rehabilitation Research Centre, Royal Melbourne Hospital, Melbourne, Australia.
Andisheh Bastani, Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Melbourne, Australia.
Anneke van der Walt, Department of Neurosciences, Alfred Health, Central Clinical School, Monash University, Melbourne, Australia.
Trevor Kilpatrick, Department of Medicine, Royal Melbourne Hospital, The University of Melbourne, Melbourne, Australia; Florey Institute of Neuroscience and Mental Health, Melbourne, Australia.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publicationof this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
L Eduardo Cofré Lizama https://orcid.org/0000-0002-3490-4521
Anneke van der Walt https://orcid.org/0000-0002-4278-7003
References
- 1.Benedetti MG, Piperno R, Simoncini Let al. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 1999; 5: 363–368. [DOI] [PubMed] [Google Scholar]
- 2.Galea MP, Cofre Lizama LE, Butzkueven Het al. Gait and balance deterioration over a 12-month period in multiple sclerosis patients with EDSS scores </= 3.0. NeuroRehabilitation 2017; 40: 277–284. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Phillips BA, Kilpatrick TJet al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 2006; 12: 620–628. [DOI] [PubMed] [Google Scholar]
- 4.Cofre Lizama LE, Khan F, Lee PVet al. The use of laboratory gait analysis for understanding gait deterioration in people with multiple sclerosis. Mult Scler 2016; 22: 1768–1776. [DOI] [PubMed] [Google Scholar]
- 5.Boudarham J, Hameau S, Zory Ret al. Coactivation of lower limb muscles during gait in patients with multiple sclerosis. PloS One 2016; 11: e0158267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow JW, Yablon SA, Stokic DS. Coactivation of ankle muscles during stance phase of gait in patients with lower limb hypertonia after acquired brain injury. Clin Neurophysiol 2012; 123: 1599–1605. [DOI] [PubMed] [Google Scholar]
- 7.Lencioni T, Jonsdottir J, Cattaneo Det al. Are modular activations altered in lower limb muscles of persons with multiple sclerosis during walking? Evidence from muscle synergies and biomechanical analysis. Front Human Neurosci 2016; 10: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remelius JG, Jones SL, House JDet al. Gait impairments in persons with multiple sclerosis across preferred and fixed walking speeds. Arch Phys Med Rehabil 2012; 93: 1637–1642. [DOI] [PubMed] [Google Scholar]
- 9.Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008; 28: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hortobagyi T, DeVita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev 2006; 34: 29–35. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen JB. Human spinal motor control. Annu Rev Neurosci 2016; 39: 81–101. [DOI] [PubMed] [Google Scholar]
- 12.Den Otter AR, Geurts AC, Mulder Tet al. Abnormalities in the temporal patterning of lower extremity muscle activity in hemiparetic gait. Gait Posture 2007; 25: 342–352. [DOI] [PubMed] [Google Scholar]
- 13.Kwon OY, Minor SD, Maluf KSet al. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture 2003; 18: 105–113. [DOI] [PubMed] [Google Scholar]
- 14.Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. Mult Scler 2009; 15: 59–67. [DOI] [PubMed] [Google Scholar]
- 15.Kalron A, Dvir Z, Achiron A. Effect of a cognitive task on postural control in patients with a clinically isolated syndrome suggestive of multiple sclerosis. Eur J Phys Rehabil Med 2011; 47: 579–586. [PubMed] [Google Scholar]
- 16.Peterson DS, Martin PE. Effects of age and walking speed on coactivation and cost of walking in healthy adults. Gait Posture 2010; 31: 355–359. [DOI] [PubMed] [Google Scholar]
- 17.Devasahayam AJ, Kelly LP, Wallack EMet al. Oxygen cost during mobility tasks and its relationship to fatigue in progressive multiple sclerosis. Arch Phys Med Rehabil 2019; 100: 2079–2088. [DOI] [PubMed] [Google Scholar]
- 18.McDonald W I, Compston A, Edan G, et al. . Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of neurology 2001; 50: 121–7. DOI: 10.1002/ana.1032. 11456302. [DOI] [PubMed] [Google Scholar]
- 19.Yamagata M, Falaki A, Latash ML. Effects of voluntary agonist-antagonist coactivation on stability of vertical posture. Motor Control 2019; 23: 304–326. [DOI] [PubMed] [Google Scholar]
- 20.Jeng B, Sandroff BM, Motl RW. Energetic cost of walking and spasticity in persons with multiple sclerosis with moderate disability. NeuroRehabilitation 2018; 43: 483–489. [DOI] [PubMed] [Google Scholar]
- 21.Sebastiao E, Bollaert RE, Hubbard EAet al. Gait variability and energy cost of overground walking in persons with multiple sclerosis: A cross-sectional study. Am J Phys Med Rehabil 2018; 97: 646–650. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol 2008; 104: 747–755. [DOI] [PubMed] [Google Scholar]
- 23.Lamontagne A, Richards CL, Malouin F. Coactivation during gait as an adaptive behavior after stroke. J Electromyogr Kinesiol 2000; 10: 407–415. [DOI] [PubMed] [Google Scholar]
- 24.Doty RL, MacGillivray MR, Talab Het al. Balance in multiple sclerosis: Relationship to central brain regions. Exp Brain Res 2018; 236: 2739–2750. [DOI] [PubMed] [Google Scholar]
- 25.Peebles AT, Bruetsch AP, Lynch SGet al. Dynamic balance is related to physiological impairments in persons with multiple sclerosis. Arch Phys Med Rehabil 2018; 99: 2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mari S, Serrao M, Casali Cet al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum 2014; 13: 226–236. [DOI] [PubMed] [Google Scholar]
- 27.Dietz V, Zijlstra W, Prokop Tet al. Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol 1995; 97: 408–415. [DOI] [PubMed] [Google Scholar]
- 28.van der Linden ML, Scott SM, Hooper JEet al. Gait kinematics of people with multiple sclerosis and the acute application of functional electrical stimulation. Gait Posture 2014; 39: 1092–1096. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, King GA. Dynamic gait stability of treadmill versus overground walking in young adults. J Electromyogr Kinesiol 2016; 31: 81–87. [DOI] [PubMed] [Google Scholar]
- 30.Souissi H, Zory R, Bredin Jet al. Comparison of methodologies to assess muscle co-contraction during gait. J Biomech 2017; 57: 141–145. [DOI] [PubMed] [Google Scholar]
- 31.Adams RW, Gandevia SC, Skuse NF. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain 1990; 113(Pt 5): 1459–1476. [DOI] [PubMed] [Google Scholar]