Abstract
Background:
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions (SCARs). There is scant literature on the characteristics and causes of these conditions among the Nigerian population. Here, we describe the epidemiology, associated morbidity and mortality, and culpable drugs in SJS and TEN cases using the National Pharmacovigilance (NPC) database in Nigeria.
Methods:
A retrospective review of the NPC database was done to analyze SJS and TEN cases reported over a period of 14 years. Annual reports, age and sex of patients, type of reporter, suspects and concomitant drugs, time to onset (TTO) of the reactions, and outcome of SJS and TEN were evaluated.
Results:
The NPC received a total of 24,015 adverse drug reaction (ADR) reports. SJS and TEN accounted for 284 (0.1%) of the total reports, of which 254 (89.4%) were SJS and the remainder were TEN. Females (n = 184, 64.8%) and individuals aged 19–40 years (n = 181, 63.7%) were the most affected by SJS and TEN. Antiretrovirals, followed by antibiotics, were the most common drug classes reported to cause SJS and TEN, with nevirapine (n = 174, 40.7%) and co-trimoxazole (n = 143, 33.5%) being the most widely implicated drugs. Among patients with reported outcomes, 73 (28.7%) SJS and 3 (10.0%) TEN cases recovered without sequelae, at the time of reporting. Severity of the SCAR was reported for only 171 (69.0%) cases, of which 12 (4.7%) and 8 (26.7%) resulted in death (Grade 5) among SJS and TEN cases, respectively.
Conclusions:
Antiretroviral and antibiotics were the commonly reported offending group of drugs for SJS and TEN cases. Nevirapine and co-trimoxazole were the commonly reported suspect drugs. SJS and TEN were reported most frequently in females and in patients aged 19–40 years, indicating that drug surveillance and counseling in these groups of patients may be beneficial.
Keywords: Severe, adverse drug reactions, Stevens- Johnson syndrome, toxic epidermal necrolysis, pharmacovigilance, database, Nigeria
Introduction
Adverse drug reaction (ADR) is a global public health problem accounting for varying proportion of hospital admissions across different countries.1 The economic burden of ADRs is enormous, directly or indirectly impacting the patient’s healthcare, health professional, and hospital services.2 Drug-induced allergic reactions are immunologically mediated and typically account for a minority of all ADRs.3 Severe cutaneous adverse reactions (SCARs) are among the various forms of untoward drug effects with high fatality, which may necessitate prolonged hospital admission.4
The occurrence of SCARs in association with various medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, antiepileptic drugs, antimalarial drugs, and antiretroviral drugs have been reported.5–7 Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and drug rash with eosinophilia and systemic symptoms (DRESS) are the commonest SCARs.8 Mucocutaneous tenderness, erythema, hemorrhagic erosions, and necrotic epidermal detachment presenting as blisters and areas of denuded skin are major characteristics of SJS and TEN.9 Involvement of nonblistered skin in TEN can result in sloughing with direct lateral pressure (Nikolsky sign).10 Patient with SJS may subsequently evolve into TEN or SJS-TEN overlap. TEN is more severe than SJS, and is characterized by detached or detachable skin of more than 30% of the total body surface area. Less than 10% of the body surface area is affected in SJS, while SJS-TEN overlap involves 10–30% of the body surface area.11
Several risk factors for SCAR have been identified, including female gender, older age, genetic predisposition, viral infections [particularly human immune deficiency virus (HIV)], iatrogenic immunosuppression, underlying immune-mediated diseases, and malignancy.12–15 Previous studies describing the epidemiology of SJS and TEN in low- and middle-income countries (LMICs) were hospital based and involved a small number of patients.16–19 Consequently, only a narrow range of the potential culpable drugs and drug classes were captured in the studies.16,17,19 The rarity of SJS and TEN limits the practicability of large population studies to estimate the incidence; therefore, hospital-data-based incidence assumptions would have accuracy limitations. This may have contributed to the dearth of such studies in Nigeria. Difficulties in obtaining definitive diagnoses of SJS and TEN are also another major challenge in estimating an accurate incidence.
Being considered rare conditions in Nigeria, studies on SJS and TEN should be considered a priority. Such studies would be beneficial for basic and clinical pharmacologists, clinical toxicologists, and clinicians in various medical and surgical specialties such as dermatologists, pediatricians, and critical care physicians. One method to enrich literature about SJS and TEN in Nigeria is to provide detailed and critical database review and analysis of cases reported to the National Pharmacovigilance Center (NPC) in Nigeria. Therefore, we conducted this study to describe the epidemiology, associated morbidity and mortality, and culpable drugs in SJS and TEN cases using the NPC database in Nigeria.
Material and methods
Data source
Spontaneous reporting of ADRs is practiced in Nigeria using a standard structured yellow form as recommended by the World Health Organization-Uppsala Monitoring Center (WHO-UMC) in Sweden.20 The yellow form captures information about the details of patients, ADRs, suspected drug(s), concomitant drugs, and the reporter. A duly filled yellow or ADR form is called an individual case study report (ICSR). Healthcare providers, healthcare institutions, marketing authorization holders, and patients can send ADR reports to the NPC, zonal pharmacovigilance centers (ZPCs), or National Agency for Food and Drug Administration and Control (NAFDAC) state offices nationwide. Alternative means of spontaneous ADR reporting in Nigeria is through the Pharmacovigilance Rapid Alert System for Consumer Reporting (PRASCOR).21 This system allows a consumer who uses a medicine and experiences an untoward or unexpected effect to send a prepaid short text message (SMS) containing the name of the medicine and the reaction to a short code (20543) on any of the four major mobile networks in Nigeria.
Pharmacovigilance experts and staff of the NPC, using the WHO-UMC causality assessment system,22 perform causality assessment for each ADR and the suspected drug(s). The National Drug Safety Advisory Committee comprising of clinical pharmacologists and toxicologists, clinical pharmacists, and clinicians, with expertise in pharmacovigilance, assesses complex cases of ADRs for causality. The ADRs are coded on the basis of standard terms for Medical Dictionary for Regulatory Activities (MedDRA).23 All reports judged to be ADRs at the NPC are sent to UMC, which receives anonymized reports from over 124 member countries. These are then entered into the WHO Global Individual Case Safety Report database, VigiBase®.
Data abstraction
In our study, SJS-TEN was defined on the basis of an ADR report in the NPC and a description that satisfied one of the following criteria:
Spontaneous reporting including the SJS and TEN terminologies or SJS-TEN overlap, or ADR description including the following low-level term definition of SJS-TEN, based on WHO-ART search criteria: ‘Dermatitis necrotizing’, ‘Epidermal necrolysis’, ‘Lyell syndrome’, ‘Toxic epidermal necrolysis’, ‘Mucocutaneous ulceration’, and ‘Stevens-Johnson syndrome’.23,24
Spontaneous reporting of the ADR involving atypical targeted lesion, vesicular bulbous eruption, denuded or detached skin, positive Nikolsky’s sign, and at least a case of mucous area affectation such as the genital, eyes, nose, mouth, or throat.25
The ICSR of patients who experienced SJS, TEN, or SJS-TEN overlap after treatment with any drug, between January 2004 and December 2017, were sourced from the NPC in Nigeria and reviewed to obtain the following information: patient’s characteristics (age and sex), suspect drug (class and specific name) for the ADRs, SCARs (SJS or TEN, onset, causality, and outcome), and type of reporter. It is probable that the suspect drug for either SJS or TEN will vary from case to case. The period between intake of the suspect drug and the onset of clinical symptoms manifesting as SJS or TEN is the time to onset (TTO).26 Outcome of SJS or TEN refers to the extent of resolution of the signs and symptoms of the SCARs and its sequelae as at the time the report was submitted to NPC. The outcomes were categorized as full recovery with or without any sequelae, ongoing if patient was still experiencing the problems, or death.
Additional search for suspect drugs and SCAR rating
The suspect drug causing SJS or TEN and the concomitant drug with a potential to cause SJS or TEN are categorized by Wong and colleagues according to their risk as high, moderate, low, and no risk,27 and based on the frequency or rarity of the drug listing on the United States Food Drug Administration (US-FDA) database for SJS or TEN reports,28 or previous review of the drugs implicated in SJS and TEN.7,9,29–58 Given the idiosyncratic nature of SJS and TEN, and the wide range of drugs previously implicated in their causalities, we sought for evidence documenting the causality between the suspect drug or concomitant drug and SJS or TEN through an extensive literature search.29–58 The evidence is classified as excellent (existence of the causality is clearly established by randomized controlled trial studies), good (reports in the literature strongly suggests that the causality exists, but not supported by well-controlled studies), fair (the causality is scarcely documented in the literature; however, SJS or TEN is suspected based on some pharmacologic considerations of the suspect drugs), poor (only few studies and limited reported cases support the existence of the causality), or unlikely (insufficient documentation of the causality in the literature and no pharmacological basis). Concomitant drugs with high risk for SJS and TEN were included in this study as additional suspect drugs, thus minimizing causality bias. Therefore, more than one suspect drug was implicated in some of the SJS and TEN cases.
In the NPC database, the causalities were categorized as certain, probable, possible, unlikely, conditional/unclassified, or inaccessible/unclassifiable.21 Severity rating was based on the intensity of the specific ADR, and is generally categorized as mild, moderate, severe, life threatening or disabling, and fatal or death (Grades 1–5) according to the Common Terminology Criteria for Adverse Events (CTCAE) scale.59
Ethical considerations
The NAFDAC Director General approved the study.
Analysis
We analyzed the data with IBM SPSS statistics software, Version 21.0 (Armonk, NY, USA: IBM Corp, Released 2012). Descriptive statistics was used to summarize patients’ demographic and SJS or TEN characteristics. The annual number of SJS or TEN reports and the number of suspect drugs per SJS or TEN cases were presented pictorially.
Results
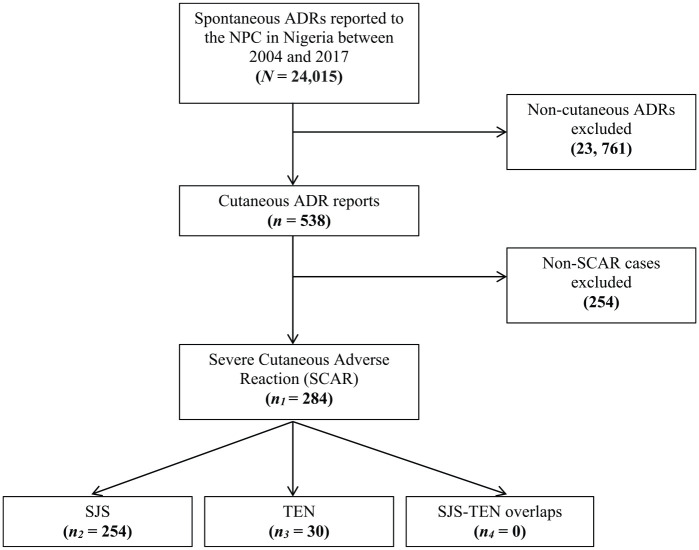
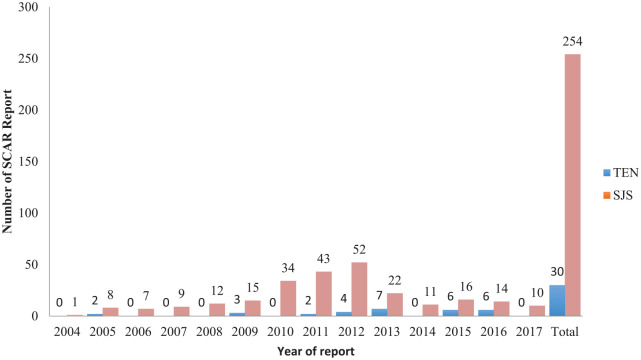
From January 2004 to December 2017, only 24,015 spontaneous ADR reports were recorded in the NPC local database, of which 284 (0.1%) cases were related to SCARs. Overall, 254 reports were registered as SJS and the remaining 30 reports were registered as TEN (Figure 1). None of the reports was registered as SJS-TEN overlaps. According to the data presented in Figure 2, the number of SJS reports increased annually, peaked in 2012 and, thereafter, gradually decreased. However, the trend for TEN reports fluctuated throughout the study period. The number of SJS reports per year was consistently higher than those of TEN.
Figure 1.
Flow chart for SJS and TEN reports in the NPC database in Nigeria.
ADR, Adverse drug reaction; NPC, National Pharmacovigilance Center; SCAR, severe cutaneous adverse reactions; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
Figure 2.
SCARs reported to the NPC in Nigeria between 2004 and 2017.
NPC, National Pharmacovigilance Center; SCAR, severe cutaneous adverse reactions; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
The demographic of the patients and SCAR characteristics reported for SJS and TEN are presented in Table 1. Female patients accounted for 64.8% of combined cases of SJS and TEN, and the highest number of cases of SJS and TEN were recorded for 19–40 year old patients. Pharmacists were the major healthcare practitioners (HCPs) to report SJS and TEN cases (n = 216, 76.1%), followed by medical practitioners (n = 48, 16.9%). In terms of SCAR outcome, it was reported for 59.4% and 69.7% cases of SJS and TEN, respectively. Among this group of patients, 73 (28.7%) SJS cases recovered without sequelae, while 8 (26.7%) TEN cases had not recovered fully at the time of reporting. At the time of reporting, full recovery without sequelae was more common among SJS than TEN patients (28.7% versus 10.0%, respectively). Severity of the SCAR was reported for only 171 (69.0%) cases, of which 12 (4.7%) and 8 (26.7%) resulted in deaths (Grade 5) among SJS and TEN cases, respectively. In terms of latency time estimates, the TTO of SJS and TEN was recorded for only 159 (56.0%) cases. About one-quarter (n = 55, 21.6%) of the SJS cases and nearly one-half (n = 13, 43.3%) of the TEN cases experienced early onset, occurring within 1–7 days.
Table 1.
Comparison of the demographic and SCAR characteristics reported for SJS and TEN.
| Characteristic | SJS (n = 254) n (%) |
TEN (n = 30) n (%) |
Total cases of SJS and TEN n (%) |
|---|---|---|---|
| Sex | |||
| Male | 82 (32.3) | 8 (26.7) | 90 (31.7) |
| Female | 162 (63.8) | 22 (73.3) | 184 (64.8) |
| Not specified | 10 (3.9) | 0 (0.0) | 10 (3.5) |
| Age category (years) | |||
| 0–18 | 23 (9.1) | 6 (20.0) | 29 (3.5) |
| 19–40 | 163 (64.2) | 18 (60.0) | 181 (63.7) |
| 41–60 | 47 (18.4) | 6 (20.0) | 53 (18.7) |
| >60 | 6 (2.4) | 0 (0.0) | 6 (2.1) |
| Not specified | 15 (5.9) | 0 (0.0) | 15 (5.3) |
| Reporter | |||
| Pharmacist | 195 (76.8) | 21 (70.0) | 216 (76.1) |
| Medical practitioner | 41 (16.1) | 7 (23.4) | 48 (16.9) |
| Nurse | 4 (1.6) | 0 (0.0) | 4 (1.4) |
| Individual | 4 (1.6) | 1 (3.3) | 5 (1.8) |
| Dentist | 1 (0.4) | 0 (0.0) | 1 (0.3) |
| Not specified | 9 (3.5) | 1 (3.3) | 10 (3.5) |
| Outcome | |||
| Not yet recovered/resolved | 13 (5.1) | 8 (26.7) | 21 (7.4) |
| Recovered/resolved with sequelae | 33 (13.0) | 7 (23.3) | 40 (14.1) |
| Recovered/resolved without sequelae | 73 (28.7) | 3 (10.0) | 76 (26.8) |
| Recovering/resolving | 32 (12.6) | 2 (6.7) | 34 (12.0) |
| Not specified | 103 (40.6) | 10 (30.3) | 113 (39.8) |
| Severity | |||
| Grade 3: severe | 107 (42.1) | 5 (16.7) | 112 (39.4) |
| Grade 4: life-threatening or disability | 32 (12.6) | 7 (23.3) | 39 (13.7) |
| Grade 5: death | 12 (4.7) | 8 (26.7) | 20 (7.0) |
| Not specified | 103 (40.6) | 10 (30.3) | 113 (39.8) |
| TTO | |||
| <24 h | 31 (12.2) | 7 (20.3) | 38 (13.4) |
| 1–7 days | 24 (9.4) | 6 (20.0) | 30 (10.6) |
| 8–28 days | 53 (20.9) | 21 (6.7) | 74 (26.1) |
| 29–56 days | 30 (11.8) | 5 (16.7) | 35 (12.3) |
| >56 days | 1 (0.4) | 0 (0.0) | 1 (0.3) |
| Not specified | 115 (45.3) | 10 (33.3) | 125 (44.0) |
| Class of suspected drug | SJS (n = 384)a | TEN (n = 41)a | |
| Antiretroviral | 174 (45.3) | 9 (21.9) | 183 (43.1) |
| Antibiotic | 149 (38.8) | 10 (24.4) | 159 (37.4) |
| Antimalarial | 36 (9.4) | 13 (31.7) | 49 (11.5) |
| Antiepileptic | 7 (1.8) | 4 (9.7) | 11 (2.6) |
| Analgesic/antipyretic | 5 (1.3) | 0 (0.0) | 5 (1.2) |
| Herbal medicine product or concoction | 5 (1.3) | 1 (2.4) | 6 (1.4) |
| Antituberculosis | 4 (1.0) | 0 (0.0) | 4 (0.9) |
| Antiparasitic | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Antipsychotic | 1 (0.3) | 2 (4.9) | 3 (0.7) |
| Antilipidemic | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Antiulcer | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Vaccine | 0 (0.0) | 2 (4.9) | 2 (0.5) |
SJS/TEN indicates one or more drugs are responsible for the severe cutaneous adverse reaction.
SCAR, severe cutaneous adverse reactions; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; TTO, time to onset.
Overall, 427 suspect drugs administered as single or multiple drugs were implicated in the 284 SJS/TEN reports. A total of 386 (90.4%) suspect drugs were responsible for the 254 SJS cases while 41 (9.6%) suspect drugs were responsible for the 30 TEN cases. In Table 1, antiretroviral (n = 174, 45.3%), followed by antibiotic (n = 19, 38.8%) class of drugs, were the most implicated in SJS. By contrast, antimalarial (n = 13, 31.7%), followed by antibiotic (n = 10, 24.4%) class of drugs, were the most implicated in TEN cases. For combined cases of SJS and TEN, antiretroviral (n = 183, 43.1%) and antibiotic (n = 159, 37.4%) were the most implicated classes of drugs.
Nearly one-half of the entire SJS cases resulted from use of a single or two different suspect drugs (Table 2). However, use of three different suspect drugs resulted in five (2.0%) SJS cases. Concomitant use of co-trimoxazole and nevirapine accounted for 97 (38.2%) SJS cases, while use of nevirapine only accounted for 61 (24.0%) SJS cases. Regarding TEN, use of a single suspect drug accounted for 21 (70.0%) cases, while use of two different suspect drugs accounted for seven (23.3%) cases (Table 3). Only two TEN cases resulted from use of three suspect drugs. Sulfadoxime-pyrimethamine only was implicated in most TEN cases. The specific suspect drugs reported for SJS and TEN are presented in Table 4. Nevirapine (174; 40.7%) and cotrimoxazole (143; 33.5%) were the two commonly reported suspect drugs.
Table 2.
The 386 suspected drugs involved in the 254 cases of SJS reported to the NPC between 2004 and 2017.
| Drug name | Number of suspected drug per SJS case | Frequency of occurrence | Number of suspected drug involved in SJS n (%) |
|---|---|---|---|
| Cotrimoxazole, sulfadoxime-pyrimethamine, and nevirapine | 3 | 2 | 6 (1.6) |
| Cotrimoxazole, arteether, and amodiaquine | 1 | 3 (0.8) | |
| Cotrimoxazole, nevirapine, and artemether-lumefantrine | 1 | 3 (0.8) | |
| Ciprofloxacin, cephalexin, and erythromycin | 1 | 3 (0.8) | |
| Subtotal | 5 | 15 (3.9) | |
| Cotrimoxazole and nevirapine | 2 | 97 | 194 (50.3) |
| Cotrimoxazole and efavirenz | 7 | 14 (3.6) | |
| Cotrimoxazole and sulfadoxime-pyrimethamine | 2 | 4 (1.0) | |
| Co-trimoxazole and herbal product | 2 | 4 (1.0) | |
| Sulfadoxime-pyrimethamine and nevirapine | 2 | 4 (1.0) | |
| Cotrimoxazole and dihydroartemisinin-piperaquin | 1 | 2 (0.5) | |
| Sulfadoxime-pyrimethamine and piroxicam | 1 | 2 (0.5) | |
| Sulfadoxime-pyrimethamine and carbamazepine | 1 | 2 (0.5) | |
| Ciprofloxacin and herbal product | 1 | 2 (0.5) | |
| Ciprofloxacin and piroxicam | 1 | 2 (0.5) | |
| Co-trimoxazole and amodiaquine | 1 | 2 (0.5) | |
| Nevirapine and artesunate | 1 | 2 (0.5) | |
| Nevirapine, crystalline- penicillin injection | 1 | 2 (0.5) | |
| Nevirapine, cimetidine | 1 | 2 (0.5) | |
| Cefixime and dihydroartemisinin-piperaquin | 1 | 2 (0.5) | |
| Phenobarbitone and crystalline-penicillin injection | 1 | 2 (0.5) | |
| Phenytoin and arteether | 1 | 2 (0.5) | |
| Subtotal | 122 | 244 (63.2) | |
| Nevirapine | 1 | 61 | 61 (15.8) |
| Cotrimoxazole | 22 | 22 (5.7) | |
| Sulfadoxime-pyrimethamine | 12 | 12 (3.1) | |
| Artemether-lumefantrine | 5 | 5 (1.3) | |
| Rifampicin | 4 | 4 (1.0) | |
| Carbamazepine | 3 | 3 (0.8) | |
| Acetaminophen | 2 | 2 (0.5) | |
| Erythromycin | 2 | 2 (0.5) | |
| Efavirenz | 2 | 2 (0.5) | |
| Herbal product | 2 | 2 (0.5) | |
| Ampicillin-cloxacillin | 1 | 1 (0.3) | |
| Amoxicillin-clavulanate | 1 | 1 (0.3) | |
| Artovastatin | 1 | 1 (0.3) | |
| Artesunate | 1 | 1 (0.3) | |
| Ceftriaxone injection | 1 | 1 (0.3) | |
| Chloroquine | 1 | 1 (0.3) | |
| Dipyrone injection | 1 | 1 (0.3) | |
| Doxycycline | 1 | 1 (0.3) | |
| Ivermectin | 1 | 1 (0.3) | |
| Olazapine | 1 | 1 (0.3) | |
| Phenytoin | 1 | 1 (0.3) | |
| Proguanil | 1 | 1 (0.3) | |
| Subtotal | 127 | 127 (32.9) | |
| TOTAL | 254 | 386 (100.0) |
NPC, National Pharmacovigilance Center; SJS, Stevens-Johnson syndrome.
Table 3.
The 41 suspected drugs involved in the 30 cases of TEN reported to the NPC between 2004 and 2017.
| Drug name | Number of suspected drug per TEN case | Frequency of occurrence | Number of suspected drug involved in TEN n (%) |
|---|---|---|---|
| Camoquin, carbamazepine, and chlorpromazine | 3 | 1 | 3 (7.3) |
| Cotrimoxazole, sulfadoxime-pyrimethamine, and nevirapine | 1 | 3 (7.3) | |
| Subtotal | 2 | 6 (14.6) | |
| Cotrimoxazole and nevirapine | 2 | 3 | 6 (14.6) |
| Cotrimoxazole and efavirenz | 1 | 2 (4.9) | |
| Carbamazepine and chlorpromazine | 1 | 2 (4.9) | |
| Carbamazepine and artemether-lumefantrine | 1 | 2 (4.9) | |
| Ceftazidime and levofloxacin | 1 | 2 (4.9) | |
| Subtotal | 7 | 14 (34.2) | |
| Sulfadoxime-pyrimethamine | 1 | 6 | 6 (14.6) |
| Nevirapine | 4 | 4 (9.8) | |
| Artemether-lumefantrine | 3 | 3 (7.3) | |
| Cotrimoxazole | 2 | 2 (4.9) | |
| Tetanus toxoid | 2 | 2 (4.9) | |
| Chloroquine | 1 | 1 (2.4) | |
| Ciprofloxacin | 1 | 1 (2.4) | |
| Phenytoin | 1 | 1 (2.4) | |
| Herbal product | 1 | 1 (2.4) | |
| Subtotal | 21 | 21 (51.2) | |
| TOTAL | 30 | 41 (100.0) | |
NPC, National Pharmacovigilance Center; TEN, toxic epidermal necrolysis.
Table 4.
Classes and specific drugs suspected to be responsible for SJS and TEN reported to the NPC between 2004 and 2017.
| Class and specific drug name | SJS | TEN | Total for SJS and TEN n (%) |
|---|---|---|---|
| Antiretroviral | |||
| Nevirapine | 166 | 8 | 174 (40.7) |
| Evafirenz | 9 | 1 | 10 (2.3) |
| Antibiotic | |||
| Cotrimoxazole | 136 | 7 | 143 (33.5) |
| Ciprofloxacin | 3 | 1 | 4 (0.9) |
| Erythromycin | 3 | 0 | 3 (0.7) |
| Crystalline- penicillin injection | 2 | 0 | 2 (0.5) |
| Cephalexin | 1 | 0 | 1 (0.2) |
| Ampicillin-cloxacillin | 1 | 0 | 1 (0.2) |
| Amoxicillin-clavulanate | 1 | 0 | 1 (0.2) |
| Doxycycline | 1 | 0 | 1 (0.2) |
| Cefixime | 1 | 0 | 1 (0.2) |
| Ceftriaxone injection | 1 | 0 | 1 (0.2) |
| Ceftazidime injection | 0 | 1 | 1 (0.2) |
| Levofloxacin | 0 | 1 | 1 (0.2) |
| Antimalarial | |||
| Sulfadoxime-pyrimethamine | 21 | 7 | 28 (6.6) |
| Artemether-lumefantrine | 6 | 4 | 10 (2.3) |
| Dihydroartemisinin-piperaquin | 2 | 0 | 2 (0.5) |
| Arteether | 2 | 0 | 2 (0.5) |
| Amodiaquine | 2 | 0 | 2 (0.5) |
| Artesunate | 2 | 0 | 2 (0.5) |
| Chloroquine | 1 | 1 | 2 (0.5) |
| Proguanil | 1 | 0 | 1 (0.5) |
| Camoquin | 0 | 1 | 1 (0.2) |
| Antiepileptic | |||
| Carbamazepine | 4 | 3 | 7 (1.6) |
| Phenytoin | 2 | 1 | 3 (0.7) |
| Analgesic/antipyretic | |||
| Piroxican | 2 | 0 | 2 (0.5) |
| Acetaminophen | 2 | 0 | 2 (0.5) |
| Dipyrone | 1 | 0 | 1 (0.2) |
| Antituberculosis | |||
| Rifampicin | 4 | 0 | 4 (0.9) |
| Antiulcer | |||
| Cimetidine | 1 | 0 | 1 (0.2) |
| Antipsychotic | |||
| Olanzapine | 1 | 0 | 1 (0.2) |
| Chlorpromazine | 0 | 2 | 2 (0.5) |
| Herbal medicine | |||
| Herbal product or concoction | 5 | 1 | 6 (1.4) |
| Antiparasitic | |||
| Ivermectin | 1 | 0 | 1 (0.2) |
| Antilipidemic | |||
| Artovastatin | 1 | 0 | 1 (0.2) |
| Vaccine | |||
| Tetanus toxoid | 0 | 2 | 2 (0.5) |
| TOTAL | 386 (90.4%) | 41 (9.6%) | 427 (100.0%) |
NPC, National Pharmacovigilance Center; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
The concomitant drugs used with the suspect drugs were categorized according to their potential risk for SJS and TEN as medium, low, and no risk (Table 5). Of the 456 concomitant drugs reportedly used by patients with SJS or TEN, 204 (44.7%) were of low risk, 197 (43.2%) had no risk, while 55 (12.1%) were of medium risk. Antiretroviral drugs were the most common concomitant drugs used by the patients; lamivudine (n = 167, 36.6%) being the most common low risk drug, and zidovudine (n = 141, 30.9%) the most common no risk drug.
Table 5.
The 456 concomitant drugs with potential risk for SJS and TEN.
| Drugs name and their potential risk category | Number of patients that used concomitant drugs n (%) |
|---|---|
| Medium potential risk | |
| Acetaminophen | 15 (3.3) |
| Ciprofloxacin | 7 (1.5) |
| Isoniazid | 6 (1.3) |
| Ethambutol | 5 (1.1) |
| Pyrazinamide | 4 (0.9) |
| Amoxicillin/cloxacillin | 4 (0.9) |
| Amoxicillin | 2 (0.4) |
| Amiloride-hydrochlorthiazide | 2 (0.4) |
| Cefuroxime | 2 (0.4) |
| Fluconazole | 2 (0.4) |
| Glibenclamide | 2 (0.4) |
| Azithromycin | 1 (0.2) |
| Doxycycline | 1 (0.2) |
| Codeine | 1 (0.2) |
| Hydrochlorthiazide | 1 (0.2) |
| Subtotal | 55 (12.1) |
| Low potential risk | |
| Lamivudine | 167 (36.6) |
| Multivitamin | 20 (43.9) |
| Nifedipine | 3 (0.7) |
| Ibuprofen | 2 (0.4) |
| Cetrizine | 2 (0.4) |
| Prednisolone | 2 (0.4) |
| Amlodipine | 1 (0.2) |
| Amprenavir | 1 (0.2) |
| Tramadol | 1 (0.2) |
| Losartan | 1 (0.2) |
| Ketoconazole | 1 (0.2) |
| Dexamethasone | 1 (0.2) |
| Quinine | 1 (0.2) |
| Tetanus toxoid | 1 (0.2) |
| Subtotal | 204 (44.7) |
| No potential risk | |
| Zidovudine | 141 (30.9) |
| Stavudine | 20 (4.4) |
| Tenofovir | 13 (2.8) |
| Ferrous sulphate | 5 (1.1) |
| Vitamin C | 4 (0.9) |
| Emtricitabine | 4 (0.9) |
| Chlorpheniramine | 2 (0.4) |
| Folic acid | 2 (0.4) |
| Ceftriaxone | 1 (0.2) |
| Chloramphenicol | 1 (0.2) |
| Metronidazole | 1 (0.2) |
| Gentamicin injection | 1 (0.2) |
| Loratidine | 1 (0.2) |
| Vitamin B complex | 1 (0.2) |
| Subtotal | 197 (43.2) |
| Total | 456 (100.0%) |
NPC, National Pharmacovigilance Center; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
Discussion
This study highlights the first application of a large database to evaluate drug-induced SJS and TEN among the general population in Nigeria. This is similar to previous studies where a reporting database from Vietnamese pharmacovigilance system, Japanese Adverse Drug Event Report database, and US-FDA Adverse Event Reporting System (AERS) database were used to generate drug safety signals at population level.25,28,60 Some unique features characterized our study: the high proportion of patients on antiretroviral, suggesting that HIV infection was common among patients who had SJS and TEN; the high number of HIV-infected patients with or without opportunistic infection that were treated with sulfonamide antibiotic, a high risk drug for SJS and TEN; and antimalarial drugs including sulfadoxime-primethamine and artemisinin-based combination therapy also contributed to a considerable number of SJS and TEN cases.
Our findings suggested antiretroviral, antibiotics, and antimalarial being the three commonest drug classes reported in SJS and TEN cases. This is in accordance with earlier reports from other developing countries. In a single hospital-based study in Kenya reporting 115 cases of SJS and TEN, antibiotics (47.8%), antiretroviral (16.5%), and antimalarial (10.0%) constituted the three commonest suspect drug classes.18 Sulfonamide (co-trimoxazole), nevirapine and sulfadoxime-pyrimethamine were the three commonest specific drugs that induced SJS and TEN among Kenyan patients, which agrees with our findings. Sulfonamide antibiotic, followed by nevirapine, accounted for 38.4% and 19.8%, respectively, of the 177 cases of SJS and TEN reported in a hospital-based study involving four sub-Saharan African countries (Benin, Burkina Faso, Central African Republic, and Togo).17 In another hospital-based study in India involving 45 patients with SJS and TEN, antibiotics and anticonvulsants were responsible for 35.5% and 28.9% of the cases, respectively.16 However, anti-gout (allopurinol and colchicine), anti-epileptic (carbamazepine and valproic acid), and NSAIDs (meloxicam) were the commonest suspect drug classes and specific drugs reported to have induced SJS and TEN among patients in Europe and the United States.28,61 Despite the fact that most of the suspect drugs are well known, their involvement in most SJS and TEN cases reflect the specific feature of drug utilization in low income countries, which is at variance with the pattern of use among middle- and high-income countries.25,28,61
Nearly one-half (44.4%) of our patients had SJS and TEN attributable to use of more than one drug. For each of these drugs, a high probability of causality was assigned due to their potential high risk documented in the literature.29–59 Furthermore, their median TTO of symptoms was 8 days with a range of 1–28 days, matching other studies.18,25 This is consistent with the latency period for the T-lymphocyte-mediated type IV hypersensitivity reaction notable for causing SJS and TEN.62
The prevalence of SJS and TEN in our database review was 284 cases within 14 years, which represents an average frequency of 20 cases per year. Similar low prevalence (11–16 cases per year) had been reported in a multicenter sub-Saharan African study and a Kenyan study.17,18 This result further confirms the extreme scarcity or under-reporting of this SCAR.63 The study also showed a high proportion of patients on antiretroviral drugs, suggesting a high prevalence of HIV infection among SJS and TEN cases. This is in accordance with previous studies documenting HIV infection as a major risk for SJS and TEN.12,17,18 By contrast, only 7% of patients in the European multicenter studies had SJS/TEN and HIV comorbidity.9,64 The very high prevalence of HIV infection in Nigeria could explain the high proportion of patients on antiretroviral drugs in our study. In fact, in late 2017, Nigeria had the second highest burden of HIV infection in the world, with about 3.6 million people infected.65 In line with the WHO 2006 guidelines on co-trimoxazole prophylaxis in resource-limited settings, the drug was used concomitantly with antiretroviral drugs to reduce HIV-related morbidity and mortality.66 However, sulfonamide is a high-risk drug for SJS and TEN, which has contributed to the high number of cases reported in our study. In spite of this untoward effect, the high benefit-risk ratio may have justified the use of co-trimoxazole among HIV-infected patients.
A female preponderance of SJS and TEN was observed in our study in a manner similar to a previous hospital-based study in Nigeria and a multicenter sub-Saharan African study.17,19 Many other studies from the United States, Japan, and Portugal also reported a similar trend.28,67,68 However, the reason SCARs (especially SJS and TEN) are more common among women than men has not been investigated to date.69 By contrast, several Indian studies have shown that male patients were more likely to suffer SJS and TEN than their female counterparts.70
Over one-half of our patients that experienced SJS (64.2%) or TEN (60.0%) were in the age group 19–40 years. This is comparable to the 20–40 year age group of Kenyan and sub-Saharan African patients that experienced the highest number of SJS and TEN cases.17,18 This affected age group constitutes a major work force in Nigeria, thus constituting both economic and financial loss to the nation as well as to families of SJS and TEN victims.71 Our findings on the most prevalent age group were at variance with other studies reporting most patients to be elderly and attributing this to reduced drug clearance, or reporting most patients to be children because of immature organs of drug clearance or poor immune status. For instance, a study reported a preponderance of SJS and TEN among children <5 years old, which was attributed to viral etiologies that are more common in this age group, while some findings in a German study reported more TEN in patients aged >63 years.72,73
One notable finding in our study was that a considerable number of the patients used one or more concomitant drugs that were potential risks (medium or low) for SJS and TEN. According to the European case control surveillance of SCAR (EuroSCAR) study, the odds ratios for SJS and TEN increased in patients on concomitant medications with potential risk for both severe cutaneous adverse reactions.61 Therefore, caution should be exercised when prescribing multiple drugs with risks for SJS and TEN for patients with a primary ailment and comorbidities.
Finally, we recorded 20 (7.0%) deaths, of which eight were due to sulfa-containing drugs (co-trimoxazole and sulfadoxime-pyrimethamine) and four were linked to nevirapine use in HIV-infected patients. This rate is slightly higher than that reported in Japan,67 but lower than rates reported in sub-Saharan African countries,17 Europe,61 and Senegal.72 HIV infection, with its many opportunistic infections, is a known risk factor for poor outcome of SJS and TEN.17,18 The majority of the deaths in our study were linked to sulfa-containing drugs and nevirapine, which mirrors the sub-Saharan African study,17 but at variance with the anticonvulsants, allopurinol, antibiotics, and anti-tuberculosis drugs reported in other studies.25,61,67,74
Several limitations characterized our study and are noteworthy. Important details for some patients and the SCAR, such as age, sex, indications for the suspect drugs, TTO, and outcomes were not reported. Similar incomplete filling of the ADR forms submitted to pharmacovigilance centers in Mexico and Saudi Arabia had been reported.75,76 Incomplete ADR information may limit the effectiveness and full potential of analysis of such reports.
There is a potential risk of not being able to adequately assess SCARs due to confounding factors such as environmental factors and comorbid illnesses. This bias may have resulted in over- or under-estimation of SJS and TEN cases in our study. Being a retrospective chart review study, the entire drugs used by the patients could not be evaluated based on the Algorithm of Drug Causality for Epidermal Necrolysis (ALDEN) protocol to exclude cases in which causality was weak.77 We relied on unverifiable reported data, which may have biased some patients who suffered other skin illnesses. We could not ascertain the past drug and medical history of the patients. Patients with a history of allergy to the suspect drug or had ever used a drug in the same suspected pharmacological group may also be at increased risk of allergy to any of the concomitant drugs.78
Conclusion
Antiretroviral and antibiotics were the commonly reported offending group of drugs for SJS and TEN in patients. Nevirapine and co-triomoxazole were the commonly reported culprit drugs by individual drug category. Most cases involved females, and individuals aged 19–40 years were the most affected groups within our study population. The findings suggest that clinicians should be aware of the adverse effects of sulfa-based drugs and nevirapine for effective pharmacovigilance among patients prescribed these drugs. Due to the severity of SJS and TEN, and the wide range of drugs implicated, all patients across age groups taking new drugs should undergo close monitoring for adverse events, particularly among females and individuals between 19 and 40 years. Further studies are suggested in a larger sample size across Nigeria, comparing patient characteristics with the general population, in order to identify any other predisposing factors to SJS and TEN.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Kazeem Adeola Oshikoya  https://orcid.org/0000-0003-3662-8476
https://orcid.org/0000-0003-3662-8476
Research ethics and patient consent: The NPC database contains de-identified information; thus, ethics approval is not required for the study.
Contributor Information
Kazeem Adeola Oshikoya, Department of Pharmacology, Therapeutics and Toxicology, Lagos State University College of Medicine, 1-5, Oba Akinjobi Street, Ikeja, Lagos 234, Nigeria.
Ibrahim Abayomi Ogunyinka, Department of Clinical Pharmacy and Pharmacy Practice, Usmanu Danfodiyo University, Sokoto, Nigeria.
Comfort Kunak Ogar, National Pharmacovigilance Center, National Agency for Food and Drug Administration and Control (NAFDAC), Abuja, FCT, Nigeria; Medicines, Technologies and Pharmaceutical Services (MTaPS) project, Management Sciences for Health, Abuja, Nigeria.
Abiodun Abiola, National Pharmacovigilance Center, National Agency for Food and Drug Administration and Control (NAFDAC), Abuja, FCT, Nigeria.
Ali Ibrahim, National Pharmacovigilance Center, National Agency for Food and Drug Administration and Control (NAFDAC), Abuja, FCT, Nigeria.
Ibrahim Adekunle Oreagba, Department of Pharmacology, Therapeutics and Toxicology, College of Medicine, University of Lagos, Idiaraba, Lagos, Nigeria.
References
- 1. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 2. Oshikoya KA, Chukwura H, Njokanma OF, et al. Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J 2011; 129: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Böhm R, Proksch E, Schwarz T, et al. Drug hypersensitivity-diagnosis, genetics and prevention. Dtsch Arztebl Int 2018; 115: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sasidharanpillai S, Riyaz N, Khader A, et al. Severe cutaneous adverse drug reactions: a clinicoepidemiological study. Indian J Dermatol 2015; 60: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marzano AV, Borghi A, Cugno M. Adverse drug reactions and organ damage: the skin. Eur J Intern Med 2016; 28: 17–24. [DOI] [PubMed] [Google Scholar]
- 6. Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: clinical pattern and causative agents: a 6 year series from Chandigarh, India. J Postgrad Med 2001; 47: 95–99. [PubMed] [Google Scholar]
- 7. Patel PP, Gandhi AM, Desai CK, et al. An analysis of drug induced Stevens-Johnson syndrome. Indian J Med Res 2012; 136: 1051–1053. [PMC free article] [PubMed] [Google Scholar]
- 8. Phillips EJ, Chung WH, Mockenhaupt M, et al. Drug hypersensitivity: pharmacogenetics and clinical syndromes. J Allergy Clin Immunol 2011; 127: S60–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995; 333: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 10. White KD, Abe R, Ardern-Jones M, et al. SJS/TEN 2017: building multidisciplinary networks to drive science and translation. J Allergy Clin Immunol Pract 2018; 6: 38–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993; 129: 92–96. [PubMed] [Google Scholar]
- 12. Martin T, Li H. Severe cutaneous adverse drug reactions: a review on epidemiology, etiology, clinical manifestation and pathogenesis. Chin Med J 2008; 121: 756–761. [PubMed] [Google Scholar]
- 13. Kim SH, Ye YM, Palikhe NS, et al. Genetic and ethnic risk factors associated with drug hypersensitivity. Curr Opin Allergy Clin Immunol 2010; 10: 280–290. [DOI] [PubMed] [Google Scholar]
- 14. Khan DA. Cutaneous drug reactions. J Allergy Clin Immunol 2012; 130: 1225e6. [DOI] [PubMed] [Google Scholar]
- 15. Wu R, Cheng YJ, Zhu LL, et al. Impact of HLA-B*58:01 allele and allopurinol-induced cutaneous adverse drug reactions: evidence from 21 pharmacogenetic studies. Oncotarget 2016; 7: 81870–81879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lihite RJ, Lahkar M, Borah A, et al. A study on drug induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and SJS-TEN overlap in a tertiary care hospital of Northeast India. J Young Pharm 2016; 8: 149–153. [Google Scholar]
- 17. Saka B, Barro-Traoré F, Atadokpédé FA, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in Sub-Saharan Africa: a multicentric study in four countries. Int J Dermatol 2013; 52: 575–579. [DOI] [PubMed] [Google Scholar]
- 18. Irungu K, Nyamu D, Opanga S. Characterization of Stevens-Johnson syndrome and toxic epidermal necrolysis among patients admitted to Kenyatta national hospital: a retrospective cross-sectional study. Drugs Real World Outcomes 2017; 4: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fadeyibi IO, Ademiluyi SA, Ajose FO, et al. Severe idiosyncratic drug reactions with epidermal necrolysis: a 5-year study. Indian J Plast Surg 2011; 44: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Uppsala Monitoring Centre. About UMC, https://www.who-umc.org/media/164372/ur69_final.pdf (2016, accessed 19 April 2019).
- 21. Ogar CK, Ibrahim A, Osakwe AI, et al. Pharmacovigilance rapid alert system for consumer reporting (PRASCOR): a look at its quantitative contribution to spontaneous reporting in Nigeria from August 2012 to February 2017. Pharm Med 2018; 32: 131–141. [Google Scholar]
- 22. The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment, https://www.who-umc.org/media/164372/ur69_final.pdf (2016, accessed 19 April 2019).
- 23. MedDRA - The Medical Dictionary for Regulatory Activities, https://www.meddra.org/faq/meddra-general (accessed 19 April 2019).
- 24. WHO Collaborating Centre for International Drug Monitoring. WHO adverse reaction terminology (WHO-ART), http://www.umc-products.com/. (2012, accessed 18 April 2019).
- 25. Nguyen KD, Tran TN, Nguyen MLT, et al. Drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Vietnamese spontaneous adverse drug reaction database: a subgroup approach to disproportionality analysis. J Clin Pharm Ther 2019; 44: 69–77. [DOI] [PubMed] [Google Scholar]
- 26. Riedl MA, Casillas AM. Adverse drug reactions: types and treatment options. Am Fam Physician 2003; 68: 1781–9170. [PubMed] [Google Scholar]
- 27. Wong A, Malvestiti AA, Hafner MFS. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review. Rev Assoc Med Bras 2016; 62: 468–473 [DOI] [PubMed] [Google Scholar]
- 28. Papay J, Yuen N, Powell G, et al. Spontaneous adverse event reports of Stevens-Johnson syndrome/toxic epidermal necrolysis: detecting associations with medications. Pharmacoepidemiol Drug Saf 2012; 21: 289–96. [DOI] [PubMed] [Google Scholar]
- 29. Modak D, Guha SK. Severe skin rash with lamivudine in HIV infected patients: some unusual case reports. Indian J Pharmacol 2013; 45: 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mawson AR, Eriator I, Karre S. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN): could retinoids play a causative role? Med Sci Monit 2015; 21: 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raucci U, Rossi R, Da Cas R, et al. Stevens-Johnson syndrome associated with drugs and vaccines in children: a case-control study. PLoS One 2013; 8: e68231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JY, Sohn KH, Song WJ, et al. A case of drug reaction with eosinophilia and systemic symptoms induced by ethambutol with early features resembling Stevens-Johnson syndrome. Acta Derm Venereol 2013; 93: 753–754. [DOI] [PubMed] [Google Scholar]
- 33. Eloranta K, Karakorpi H, Jeskanen L, et al. Photo-distributed Stevens-Johnson syndrome associated with oral itraconazole. Int J Dermatol 2016; 55: e508–e510. [DOI] [PubMed] [Google Scholar]
- 34. Yang SC, Hu S, Zhang SZ, et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in China. J Immunol Res 2018; 2018: 4320195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Petrou E, Karali V, Papadakis E. Simvastatin-induced toxic epidermal necrolysis. J Acute Dis 2014; 3: 335–336. [Google Scholar]
- 36. Sanmarkan AD, Sori T, Thappa DM, et al. Retrospective analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis over a period of 10 years. Indian J Dermatol 2011; 56: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strom BL, Carson JL, Halpern AC, et al. Using a claims database to investigate drug-induced Stevens-Johnson syndrome. Stat Med 1991; 10: 565–576. [DOI] [PubMed] [Google Scholar]
- 38. Sengupta S, Chetia DJ, Nath S, et al. Olanzapine induced Stevens-Johnson syndrome. Delhi Psychiatry J 2015; 18: 206–207. [Google Scholar]
- 39. Rotunda A, Hirsch RJ, Scheinfeld N, et al. Severe cutaneous reactions associated with the use of human immunodeficiency virus medications. Acta Derm Venereol 2003; 83: 1–9. [DOI] [PubMed] [Google Scholar]
- 40. Drug Lib.com. Zyrtec (Cetirizine) - Stevens-Johnson syndrome - suspected cause - side effect reports, http://www.druglib.com/reported-side-effects/zyrtec/reaction_stevens-johnson_syndrome/ (accessed 25 April 2019).
- 41. Mazumdar G, Shome K. Stevens-Johnson syndrome following use of metronidazole in a dental patient. Indian J Pharmacol 2014; 46: 121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Das SK, Jana PK, Bandyopadhyay AK, et al. Ethambutol and pyrazinamide-induced toxic epidermal necrolysis in an immunocompetent adult with tuberculosis. Lung India 2012; 29: 87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar N, Walia NS, Sandhu MS, et al. Toxic epidermal necrolysis: a case report. Med J Armed Forces India 2006; 62: 271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bygum A, Gregersen JW, Buus SK. Acetaminophen-induced toxic epidermal necrolysis in a child. Pediatr Dermatol 2004; 21: 236–238. [DOI] [PubMed] [Google Scholar]
- 45. Patel JB, Agrawal P, Soitawala S, et al. Amoxicillin-induced toxic epidermal necrolysis (TEN): a case report. Int J Res Med Sci 2015; 3: 1011–1014. [Google Scholar]
- 46. Saiag P, Caumes E, Chosidow O, et al. Drug-induced toxic epidermal necrolysis (Lyell syndrome) in patients infected with the human immunodeficiency virus. J Am Acad Dermatol 1992; 26: 567–574. [DOI] [PubMed] [Google Scholar]
- 47. Tagami H, Tatsuta K, Iwatski K, et al. Delayed hypersensitivity in ampicillin-induced toxic epidermal necrolysis. Arch Dermatol 1983; 119: 910–913. [PubMed] [Google Scholar]
- 48. Khaldi N, Miras A, Gromb S. Toxic epidermal necrolysis and clarithromycin. Can J Clin Pharmacol 2005; 12: e264–e268. [PubMed] [Google Scholar]
- 49. Seegobin K, Bueno E, Maharaj S, et al. Toxic epidermal necrolysis after ivermectin. Am J Emerg Med 2018; 36: 887–889. [DOI] [PubMed] [Google Scholar]
- 50. Chen KT, Twu SJ, Chang HJ, et al. Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan. Am J Public Health 2003; 93: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Purcell P, Valmana A. Toxic epidermal necrolysis following chlorpromazine ingestion complicated by SIADH. Postgrad Med J 1996; 72: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rajagopalan S, Kaur S, Dogra S, et al. Toxic epidermal necrolysis induced by rarely implicated drugs. Indian J Pharmacol 2012; 44: 272–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar A, Sarkar S, Praharaj SK, et al. Stevens–Johnson syndrome progressing to toxic epidermal necrolysis with haloperidol and carbamazepine combination. Ind Psychiatry J 2011; 20: 131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chahal D, Aleshin M, Turegano M, et al. Vaccine-induced toxic epidermal necrolysis: a case and systematic review. Dermatol Online J 2018; 24: 1–10. [PubMed] [Google Scholar]
- 55. Nagge JJ, Knowles SR, Juurlink DN, et al. Pseudoephedrine-induced toxic epidermal necrolysis. Arch Dermatol 2005; 141: 907–908. [DOI] [PubMed] [Google Scholar]
- 56. Boffa MJ, Chalmers RJG. Toxic epidermal necrolysis due to chloroquine phosphate. Br J Dermatol 1994; 131: 444–445. [DOI] [PubMed] [Google Scholar]
- 57. Weedon D. The lichenoid reaction pattern (‘interface dermatitis’). In: Weedon’s skin pathology. 3rd ed. London: Churchill Livingstone Elsevier, 2010. [Google Scholar]
- 58. Kanwar AJ, Dogra S, Kumar B. Changing pattern of drug-induced toxic epidermal necrolysis in developing countries. Clin Exp Dermatol 2004; 29: 425–426. [DOI] [PubMed] [Google Scholar]
- 59. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) - version 4.03, https://evs.nci.nih.gov/ftp1/CTCAE/About.html (2010, accessed 20 April 2019).
- 60. Abe J, Umetsu R, Mataki K, et al. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis using the Japanese adverse drug event report database. J Pharm Health Care Sci 2016; 2: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens–Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Investig Dermatol 2008; 128: 35–44. [DOI] [PubMed] [Google Scholar]
- 62. Harr T, French LE. Adverse cutaneous drug eruptions. Chem Immunol Allergy 2012; 97: 149–166. [DOI] [PubMed] [Google Scholar]
- 63. Wolkenstein P, Revuz J. Drug-induced severe skin reactions. Incidence, management and prevention. Drug Saf 1995; 13: 56–68. [DOI] [PubMed] [Google Scholar]
- 64. Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, et al. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS 2001; 15: 1843–1848. [DOI] [PubMed] [Google Scholar]
- 65. Federal Republic of Nigeria. National HIV and AIDS strategic plan 2017–2021, https://naca.gov.ng/wp-content/uploads/2018/05/National-HIV-and-AIDS-Strategic-Plan-FINAL1.pdf (accessed 4 June 2019).
- 66. World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV related infections among children, adolescents and adults: recommendations for a public health approach. Geneva: World Health Organization, 2006. [Google Scholar]
- 67. Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens–Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to 2006. Allergol Int 2007; 56: 419–425. [DOI] [PubMed] [Google Scholar]
- 68. Sousa-Pinto B, Araújo L, Freitas A, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis and erythema multiforme drug-related hospitalisations in a national administrative database. Clin Transl Allergy 2018; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li LF, Ma C. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Clin Exp Dermatol 2006; 31: 646–647. [DOI] [PubMed] [Google Scholar]
- 70. De Rojas MV, Dart JK, Saw VP. The natural history of Stevens - Johnson syndrome: patterns of chronic ocular disease and the role of immunosuppressive therapy. J Opthamol 2007; 91: 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Owoyemi OA, Elegbede T, Gbajumo-Sheriff M. Age diversity and the future of workforce in Nigeria. Eur J Econ Fin Admin Scs 2011; 30: 65–75. [Google Scholar]
- 72. Chan HL. The incidence of erythema multiforme, Stevens– Johnson syndrome, and toxic epidermal necrolysis. Arch Dermatol Am Med Assoc 1990; 126: 43. [PubMed] [Google Scholar]
- 73. Schopf E, Stuhmer A, Rzany B, et al. Toxic epidermal necrolysis and Stevens–Johnson syndrome: an epidemiologic study from West Germany. Arch Dermatol 1991; 127: 839–842. [DOI] [PubMed] [Google Scholar]
- 74. Mame Thierno D, On S, Thierno Ndiaye S, et al. Lyell syndrome in Senegal: responsibility of thiacetazone. Ann Dermatol Venereol 2001; 128: 1305–1307. [PubMed] [Google Scholar]
- 75. Sánchez-Sánchez B, Altagracia-Martínez M, Kravzov-Jinich J, et al. Evaluation of completeness of suspected adverse drug reaction reports submitted to the Mexican national pharmacovigilance centre: a cross-sectional period-prevalence study. Drug Saf 2012; 35: 837–844. [DOI] [PubMed] [Google Scholar]
- 76. Alshammari TM, Al-Kathiri WH, Louet HL, et al. Completeness of adverse drug reactions reports of the Saudi adverse event reporting system. Saudi Med J 2015; 36: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case- control analysis. Clin Pharmacol Ther 2010; 88: 60–68. [DOI] [PubMed] [Google Scholar]
- 78. Frew A. General principles of investigating and managing drug allergy. Br J Clin Pharmacol 2011; 71: 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]