Abstract
Retroperitoneal fibrosis (RPF) causing large vessel stenosis and thrombosis is rare but well-described. We describe a 50-year-old man with rapid progression of central venous thrombosis in the presence of RPF and exogenous testosterone use. Therapeutic anticoagulation was initiated and catheter directed thrombolysis was performed after placement of an inferior vena cava (IVC) filter. Repeat venogram revealed severe focal retrohepatic IVC stenosis, which was treated with serial venoplasty and stenting. Clinical improvement was significant 48 hours after intervention. This case represents a rare presentation of IVC occlusion in the setting of RPF and exogenous testosterone administration successfully treated with endovascular interventions.
Keywords: Renal vein thrombosis, Inferior vena cava occlusion, Retroperitoneal fibrosis, Testosterone
Case report
A 50-year-old man was admitted for a 2-day history of progressive severe lower abdominal and suprapubic pain that improved slightly with urination. His past medical history was significant for arthritis, obstructive sleep apnea, hyperlipidemia, glaucoma, and fatty liver disease. He was taking minimal medications, but had recently started using anabolic steroids (intramuscular testosterone) obtained from Mexico. He had no personal or family history of venous thrombosis or hypercoagulability. His body mass index was 35 and he did not smoke. He was active and had no other known risk factors for venous thrombosis.
The initial workup was notable for an elevation in serum creatinine from 1.2 mg/dL to 2.0 mg/dL and nonspecific retroperitoneal inflammatory changes were seen on the computed tomography (CT) scan. During his hospital stay, the patient rapidly developed significant bilateral lower extremity edema and painful scrotal edema over a 2-day period. A lower extremity venous ultrasound examination was negative for deep venous thrombosis. Bilateral iliac veins seemed to be patent, but the study was significantly limited due to patient body habitus. A CT cystogram was performed which showed evidence of left renal vein thrombosis extending into the distal inferior vena cava (IVC) (Fig 1). A workup for nephrotic syndrome and renal/testicular malignancy was performed and found to be negative. Testicular ultrasound examination revealed bilateral partial thrombosis of the pampiniform plexus. Therapeutic anticoagulation was initiated using unfractionated heparin and the patient underwent a diagnostic ascending venogram. Fresh thrombus was seen in the distal IVC and bilateral common iliac veins. A Cook Celect Platinum IVC filter (Cook Medical Incorporated, Bloomington, Ind) was placed at the level of the hepatic veins and catheter-directed thrombolytic therapy was initiated via an Ekos catheter (Ekos Corporation, Bothell, Wash) from the distal superior vena cava to the left common iliac vein. The following day, the catheter was removed and a repeat venogram showed severe focal stenosis of the retrohepatic IVC (Fig 2). The stenosis was serially venoplastied to 16 mm (8-mm cutting balloon followed by 10-, 12-, 14-, and 16-mm balloons). This segment of IVC was then stented using a 20 × 55-mm WALLSTENT (Boston Scientific, Marlborough, Mass; Fig 3, A and B). Completion venogram showed a patent IVC with restored flow. Over the next 48 hours, the patient had significant improvement in his bilateral lower extremity and scrotal edema. Of note, IVC filter removal was attempted, but was aborted secondary to the tilted position and difficulty in snaring the hook.
Fig 1.
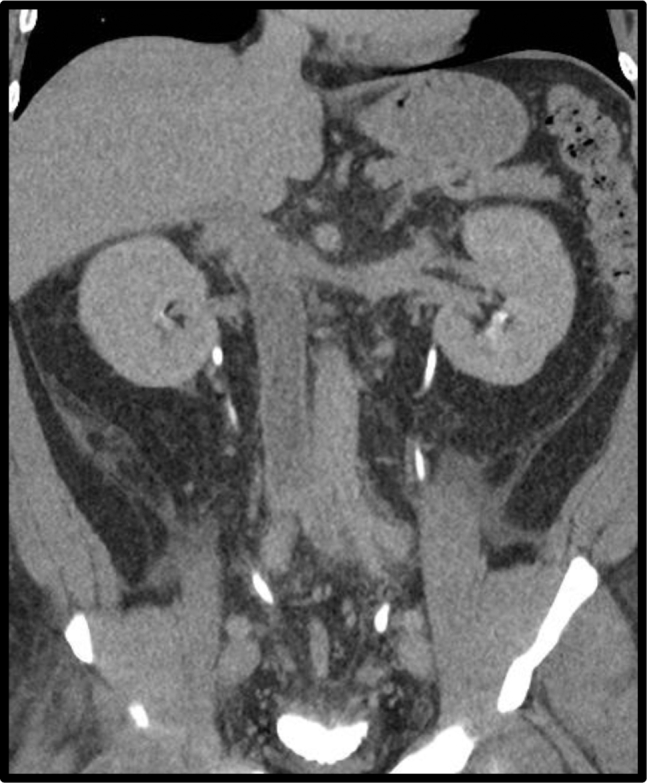
Initial computed tomography (CT) urogram demonstrating left renal vein thrombosis extending into the distal inferior vena cava (IVC).
Fig 2.
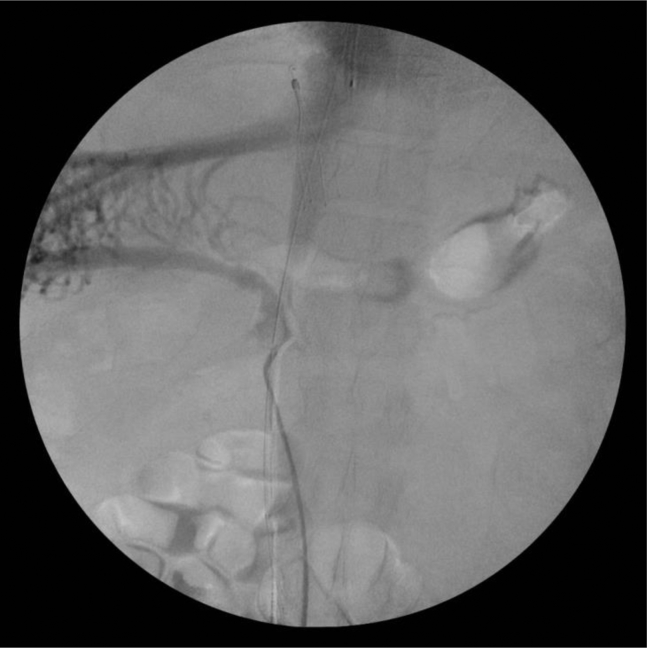
Repeat venogram following removal of Ekos catheter demonstrating severe focal stenosis of the retrohepatic inferior vena cava (IVC).
Fig 3.
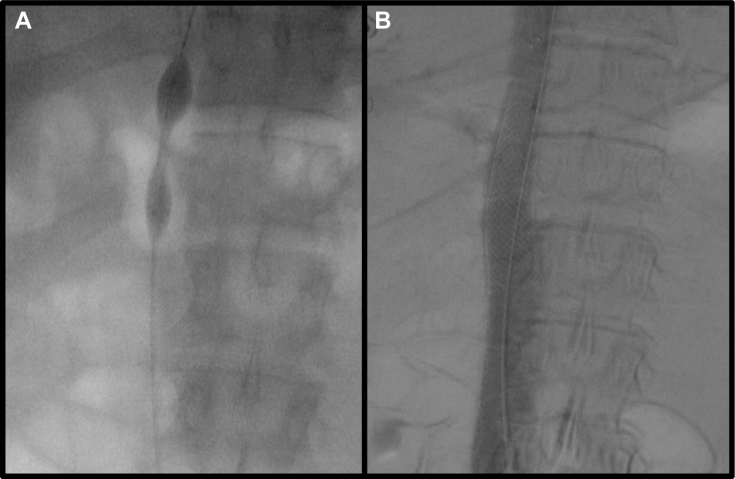
A, Initial balloon wasting during serial venoplasty of stenosis to 16 mm (8 mm cutting balloon followed by 10-, 12-, 14-, and 16-mm balloons). B, Stenotic area stented using a 20- × 55-mm WALLSTENT and further venoplastied to 18 mm.
An evaluation for inherited hypercoagulopathy was negative for disorders of factor V Leiden, antithrombin III, lupus anticoagulant, prothrombin 2021A mutation, protein C, protein S, homocysteine, JAK-2, and cardiolipin antibody. He was found to have positive antinuclear antibody levels and positive U1-ribonucleoprotein levels. Ultimately, the patient was discharged on therapeutic warfarin. His renal function has recovered, with his most recent serum creatinine being 1.1 mg/dL. Surveillance CT at the 6-month follow-up revealed a patent stent with maintained patency of his IVC and iliac veins with resolution of retroperitoneal fibrosis (RPF). He will be followed for continued CT surveillance yearly. Patient consent to share medical information and images was obtained before submission of this material.
Discussion
RPF is a rare disease process that typically presents in the fourth to sixth decades of life and is more prevalent in men.1 Typical symptoms include back, abdomen, and/or flank pain, similar to this patient's presentation.2 Like in our patient, up to 60% of patients will have elevated antinuclear antibody and the presence of this is often seen in conjunction with an autoimmune disease.3 However, there was no other evidence of autoimmune disease in our case. Although venous thrombosis is rare, RPF is known to cause compression of the retroperitoneal structures. Iliocaval thrombosis/occlusion occurs in only 2% of cases of RPF.4 Although most cases reported are at the level of the renal veins or inferior to this, one case reported intrahepatic and extrahepatic biliary obstruction secondary to RPF, suggesting that RPF can have significant inflammatory effects extending to the retrohepatic area.5 Although our patient self-reported a history of fatty liver disease, imaging showed only mild hepatic steatosis and hepatic laboratory values were within normal limits. Therefore, it is unlikely that nonalcoholic fatty liver disease contributed to the RPF in this case. Additionally, widespread retroperitoneal inflammatory changes would be highly unlikely from a focal retrohepatic IVC stenosis. One report in 2012 described an elderly man with lower extremity edema owing to IVC stenosis caused by IgG4-related RPF; his IVC patency improved after a course of corticosteroids and he did not have IVC thrombosis.6 An IgG workup was not pursued in our case because it was deemed unnecessary during the initial evaluation and was not pursued when the patient had symptom resolution after intervention and no evidence of RPF on follow-up imaging. Ureteral and renal compression are possible with RPF as well and can cause acute kidney injury or uropathy presenting as urinary urgency, urinary frequency, or dysuria.7 Renal vein thrombosis can also present with symptoms similar to pyelonephritis.8 Our patient also manifested with evidence of urologic involvement, which was evident by marked acute kidney injury, CT findings consistent with pericystic inflammation/fibrosis, and suprapubic pain with slight improvement after urination.
The uniqueness of this case is the degree of acuity of the clinical manifestations. Venous compression secondary to RPF is typically a chronic process with development of extensive collateral circulation.9 This did not seem to be the case for our patient. However, his clinical history is unique because he had recently started using anabolic steroids in the form of unregulated injectable testosterone obtained outside of the United States. It is well-known that oral contraceptives increase the risk of a venous thrombotic event, even being attributed to renal vein thrombosis in rare cases.10 A report published in 2016 on a population of 2.92 million men, showed a significantly increased risk of venous thrombotic event within the first 6 months of initiation of exogenous testosterone therapy.11 Our patient's use of unregulated intramuscular testosterone likely contributed to a hypercoagulable state that, in the presence of a chronic stenosis of the retrohepatic IVC secondary to RPF, led to IVC thrombosis.
There is minimal previous literature describing the surgical treatment of venous stenosis/thrombosis owing to RPF. One retrospective study of 42 patients with benign venous occlusive disease included four patients with RPF; however, all of these patients were treated with open venous reconstruction.12 Surgical treatment of IVC stenosis/thrombosis in general is well-described, as are the technical aspects of the endovascular treatments used in this case. Unlike the arterial system, balloon angioplasty in the venous system alone is insufficient owing to significant vein recoil causing an increased risk of early restenosis and thrombosis.13 Therefore, placement of a stent is strongly recommended.11 The recommended stent diameter for the IVC is 18 to 24 mm.13 After stent placement, it is recommended to further balloon dilate to improve apposition and minimize the risk of migration.13
Conclusions
Our case is a unique description of endovascular treatment of an IVC stenosis/thrombosis secondary to a hypercoagulable state caused by the administration of exogenous testosterone in the setting of RPF. We demonstrate successful use of a combination of endovascular therapies including catheter-directed thrombolysis, venoplasty, and venous stenting to treat a severe IVC stenosis/thrombosis. Clinical resolution of the patient's symptoms of scrotal and bilateral lower extremity edema were evident within 48 hours of treatment. By using endovascular treatment, we were able to avoid the morbidity of a large open venous reconstructive surgery as well as the morbidity of long-term chronic venous stasis disease.
Footnotes
Disclaimer: The views expressed in this paper are those of the author(s) and do not reflect the official policy or position of William Beaumont Army Medical Center, Department of the Army, Defense Health Agency, or the US Government.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.van Bommel E.F.H., Jansen I., Hendriksz T.R., Aarnoudse A.L. Idiopathic retroperitoneal fibrosis: prospective evaluation of incidence and clinicoradiologic presentation. Medicine. 2009;88:193–201. doi: 10.1097/MD.0b013e3181afc420. [DOI] [PubMed] [Google Scholar]
- 2.van Bommel E.F. Retroperitoneal fibrosis. Neth J Med. 2002;60:231–242. [PubMed] [Google Scholar]
- 3.Ceresini G., Urban M.L., Corradi D., Lauretani F., Marina M., Usberti E. Association between idiopathic retroperitoneal fibrosis and autoimmune thyroiditis: a case–control study. Autoimmun Rev. 2015;14:16–22. doi: 10.1016/j.autrev.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Rhee R.Y., Gloviczki P., Luthra H.S., Stanson A.W., Bower T.C., Cherry K.J., Jr. Iliocaval complications of retroperitoneal fibrosis. Am J Surg. 1994;168:179–183. doi: 10.1016/s0002-9610(94)80063-4. [DOI] [PubMed] [Google Scholar]
- 5.Khalil F., Ouslim H., Mhanna T., Barki A. Extensive primary retroperitoneal fibrosis (Ormond’s disease) with common bile duct and ureteral obstruction: a rare case report. Int J Surg Case Reports. 2015;13:5–7. doi: 10.1016/j.ijscr.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishimura M., Kamisawa T., Kitahara Y., Nishizawa A., Tabata T., Hara S. Improvement of a compressed inferior vena cava due to IgG4-related retroperitoneal fibrosis with steroid therapy. Intern Med. 2012;51:1705–1707. doi: 10.2169/internalmedicine.51.7378. [DOI] [PubMed] [Google Scholar]
- 7.Vaglio A., Palmisano A., Alberici F., Maggiore U., Ferretti S., Cobelli R. Prednisone versus tamoxifen in patients with idiopathic retroperitoneal fibrosis: an open-label randomised controlled trial. Lancet. 2011;378:338–346. doi: 10.1016/S0140-6736(11)60934-3. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary A., Majee P., Gupta R., Basu S., Das R.K. Adult idiopathic renal vein thrombosis mimicking acute pyelonephritis. J Clin Diagn Res. 2016;10:PD18. doi: 10.7860/JCDR/2016/20139.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaglio A., Salvarani C., Buzio C. Retroperitoneal fibrosis. Lancet. 2006;367:241–251. doi: 10.1016/S0140-6736(06)68035-5. [DOI] [PubMed] [Google Scholar]
- 10.Ajmera A., Joshi A., Weisberg L., Kamat B., Germaine P. Idiopathic acute renal vein thrombosis in a young healthy woman with no hypercoagulable state taking oral contraceptives. Am J Med Sci. 2010;339:380–382. doi: 10.1097/MAJ.0b013e3181ce4f34. [DOI] [PubMed] [Google Scholar]
- 11.Martinez C., Suissa S., Rietbrock S., Katholing A., Freedman B., Cohen A.T. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968. doi: 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jost C.J., Gloviczki P., Cherry K.J., Jr., McKusick M.A., Harmsen W.S., Jenkins G.D. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320–328. doi: 10.1067/mva.2001.112805. [DOI] [PubMed] [Google Scholar]
- 13.Cronenwett J.L., Johnston K.W. Elsevier Health Sciences; New York: 2014. Rutherford's vascular surgery e-book. [Google Scholar]