Abstract
Background/objective:
Reduction of brain temperature remains the most common method of neuroprotection against ischemic injury employed during cardiac surgery. However, cooling delivered via the cardiopulmonary bypass circuit is brief and cooling the body core along with the brain has been associated with a variety of unwanted effects. This study investigated the feasibility and safety of a novel selective brain cooling approach to induce rapid, brain-targeted hypothermia independent of the cardiopulmonary bypass circuit.
Methods:
This first-in-human feasibility study enrolled five adults undergoing aortic valve replacement with cardiopulmonary bypass support. During surgery, the NeuroSave system circulated chilled saline within the pharynx and upper esophagus. Brain and body core temperature were continuously monitored. Adverse effects, cardiopulmonary function, and device function were noted.
Results:
Patient 1 received cooling fluid for an insignificant period, and Patients 2-5 successfully underwent the cooling procedure using the NeuroSave system for 56-89 minutes. Cooling fluid was 12°C for Patients 1-3, 6°C for Patient 4, and 2°C for Patient 5. There were no NeuroSave-related adverse events and no alterations in cardiopulmonary function during NeuroSave use. Brain temperature decreased by 3°C within 15 minutes and remained at least 3.5°C colder than the body core. During a brief episode of hypotension in one patient, the brain cooled an additional 4°C in 2 minutes, briefly reaching 27.4°C.
Conclusion:
The NeuroSave system can induce rapid brain-targeted hypothermia and simultaneously maintain a favorable body–brain temperature gradient, even during hypotension. Further studies are required to evaluate the function of the system during longer periods of use.
Keywords: therapeutic hypothermia, cardiac surgical procedures, stroke, circulatory arrest, heart arrest, neuroprotection
Introduction
Brain injury is a major source of patient morbidity after cardiac surgery and is associated with prolonged hospitalization, excessive operative mortality, high hospital costs, decreased quality of life, early withdrawal from the workforce, and increased dependence on society.1–10 Brain injury can be found in approximately half of all patients after cardiac surgery with reported rates depending on the sensitivity of the measurements used.2–4 Diffuse cerebral injury can be detected on magnetic resonance imaging (MRI) within an hour of cardiac surgery, with diffuse blood-brain barrier (BBB) breakdown evident in 67% of patients within 24 hours.11,12 Hundreds of emboli are delivered to the brain during cardiac surgery with the number of emboli strongly correlated with hospital length of stay after surgery.13 Hospital length of stay is doubled, and discharge not to home is sixfold more likely, for patients with clinically evident cerebral injury.14 The annual global cost of neurological injury from coronary artery bypass graft (CABG) surgery alone is $2–4 billion (USD).14–16
The only neuroprotectant in clinical use today is cooling. Cooling is guideline recommended for cardiac arrest17 and brain injury during birth,18 and is frequently used to prevent brain injury during cardiac surgery.4,19,20 In a contemporary Canadian survey, cooling is standard care in 94% of cardiac surgery centers for CABG and in 97% of centers for “high risk” surgery.19 Still, the effectiveness of hypothermic neuroprotection in cardiac surgery is debated as evidence from clinical trials is mixed.21
The depth and duration of cooling for neuroprotection are limited because body core hypothermia carries risk. In patients undergoing cardiac surgery, core hypothermia is associated with an increased risk of death, vasoplegia, bleeding and transfusion, extended periods on cardiopulmonary bypass (CPB), prolonged mechanical ventilation, increased oxygen consumption from shivering, and increased length of stay in the intensive care unit and hospital.22–30 The American College of Chest Physicians guidelines have recommended minimizing core hypothermia during cardiac surgery as a means of reducing the risk of postoperative atrial fibrillation.26 The ability to cool the brain while preventing cooling of the body would allow for effective neuroprotection without risking serious systemic injury from cooling of the core.
In this feasibility trial, a novel system (NeuroSave™, San Francisco, CA, USA) of brain-targeted cooling was evaluated for safety and performance in five patients undergoing cardiac surgery.
Methods
Study design and patients
This prospective first-in-man single-center trial was conducted from February to August 2014 at the Alfred Hospital in Melbourne, Australia. The study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all patients. The protocol of this study was approved by the Alfred Hospital Human Research Ethics Committee and registered at ClinicalTrials.gov (identifier number: NCT01894724). Patients scheduled to undergo aortic valve replacement surgery were screened for eligibility. Inclusion criteria were as follows: ⩾18 years of age, undergoing coronary revascularization or valvular cardiac surgery, the study patient or the study patient’s legal representative has been informed of the nature of the study, agrees to its provisions and has provided written informed consent as approved by the Human Research Ethics Committee (HREC) of the respective clinical site, and the study patient agrees to comply with all study-related procedures. Exclusion criteria were as follows: women known or suspected to be pregnant (as confirmed by a pregnancy test for all women of childbearing age); past history of cerebrovascular accident (stroke or transient ischemic attack [TIA]); history of clinically diagnosed active psychiatric conditions; emergency or salvage cardiac valve operations; body weight <50 kg; leukopenia (white blood cell [WBC] <3,000 cell/mL); anemia (Hg <11 g/dL); thrombocytopenia (Plt <50,000 cell/mL); active upper gastrointestinal bleeding within 3 months (90 days) prior to procedure; renal insufficiency (creatinine >265 µmol/L) and/or renal replacement therapy at the time of screening; estimated life expectancy <12 months; structural abnormality or any known pathological disease of nose, mouth, pharynx, and esophagus (excluding gastric reflux esophagus disease); and currently participating in an investigational drug or another device study that would potentially impact the results of this study as determined by the Principal Investigator. Five patients consented to participate and undergo intraoperative selective brain cooling with the NeuroSave system.
NeuroSave system configuration
The NeuroSave system was developed in accordance with ISO 13485 compliant quality system and consists of a base unit and disposable components that contact the patient and connect the patient and the base unit. The electrically isolated base unit is mounted on a wheeled aluminum frame cart 112 cm long by 61 cm wide and 102 cm high, and provides the necessary pumping and cooling of the fluid delivered to the patient. The disposable components consist of tubing, a fluid reservoir, two 0.2 µm filters, a heat exchanger, and patient-contacting components. The disposable components create a single-use fluid pathway that transports cooling fluid to the esophagus and nose of the patient and returns cooling fluid from the mouth. The reservoir is kept under moderate vacuum thereby facilitating the return of the cooling fluid from the patient to the reservoir via the oral return tubing. Cooling fluid is drawn from the reservoir, passed through a heat exchanger and a 0.2 µm filter, and delivered to the patient’s nose and esophagus by two independent roller pumps. The nasal line delivers fluid to a nasal interface that creates a seal at each nare. The nasal interface is held in place by a strap around the back of the head. The esophageal line delivers fluid to a Linton tube—a balloon-tipped, multi-lumen rubber catheter that is placed through the mouth with the distal end and balloon residing in the stomach. The Linton tube is designed to create a seal at the gastroesophageal junction when the balloon is inflated, and the proximal end of the tube is placed under tension. A cuffed endotracheal tube prevents cooling fluid from entering the lungs. Cooling fluid flow within the patient is illustrated in Figure 1.
Figure 1.
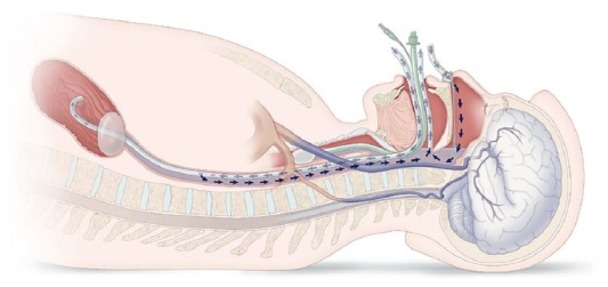
Flow path of the cooling fluid. Cold fluid is delivered into both the nose and esophagus and exits via the mouth. Balloons in trachea and stomach prevent loss of cooling fluid into the stomach and lungs.
NeuroSave system use
All patients received standard intraoperative care, including general anesthesia, mechanical ventilator support delivered by endotracheal tube, vasoactive drugs, and CPB support. After initiation of anesthesia, a 5-French pediatric Swan–Ganz catheter was placed in the right jugular vein with the tip in the jugular bulb (JB) by standard placement technique.31 Connector tubing was connected to the base unit at the roller pumps and heat exchanger, wall vacuum was connected to the reservoir, and the chiller was activated and set to a predetermined temperature (Table 1). The nasal interface was sealed at the nares, and the esophageal interface (Linton tube) was placed into the esophagus and stomach through the mouth. The Linton tube balloon was inflated with 500 mL of air and placed under tension with a 1 kg weight to make a liquid-tight seal at the lower esophageal junction. The esophageal and nasal interfaces were connected to the disposable connector tubing, and the roller pumps were activated, starting at 0.5 L/minute each and gradually increased. Total cooling fluid flow was maintained at 1-3 L/minute.
Table 1.
Demographic and clinical characteristics and response to cooling procedure.
| Patient Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Decade of life | Seventh | Seventh | Fifth | Seventh | Ninth |
| Clinical presentation | Coronary artery disease | Aortic stenosis, coronary artery disease | Coronary artery disease | Coronary artery disease | Coronary artery disease |
| Body weight (kg) | 68 | 136 | 105 | 77 | 102 |
| Length of surgery (minutes) | 62 | 97 | 67 | 68 | 110 |
| Length of X-clamp (minutes) | 47 | 70 | 45 | 49 | 89 |
| Body core temperature before cooling (°C) | 35.0 | 36.4 | 35.9 | 35.9 | 36 |
| Brain temperature before cooling (°C) | 36.7 | 35.7 | 35.4 | 35 | 34.9 |
| Setting of heater/chiller T (°C) | 12 | 12 | 12 | 6 | 2 |
| Duration of cooling by NeuroSave system (minutes) | NA | 65 | 89 | 79 | 56 |
| Adverse events and serious adverse eventsa | Blurred vision Pulmonary edema Peripheral edema Paroxysmal atrial fibrillation |
Anemia Rapid atrial fibrillation Left pneumothoraxa |
Hypotension Patient fall Rapid atrial fibrillation Wheezing Right pneumothoraxa |
Low hemoglobin Sinus tachycardia Confusion and hallucination |
Confusion Peripheral edema Fluid overload |
All adverse events and serious adverse events occurred post-procedure, and none of the events were deemed device-related based upon an independent medical review.
Saline temperature set point of 12°C was used in Patients 1, 2, and 3; 6°C in Patient 4; and 2°C in Patient 5. A temperature of 12°C was chosen as our mathematical models suggested it would likely provide a clearly measurable selective cooling effect; 2°C was chosen as lower fluid temperatures are expected to result in improved brain cooling; and 2°C is the intranasal temperature safely achieved during evaporative nasal cooling using the RhinoChill device.32
Duration of brain cooling via the NeuroSave system was determined by the investigator for each patient. Circulation of saline was halted if the JB temperature dropped below 30°C.
At the end of the procedure, saline circulation was discontinued and the remaining liquid was suctioned from the pharynx via the oral cavity. After discontinuation of the NeuroSave system, temperature management continued via CPB per usual care. The NeuroSave device was not active during patient rewarming. Nasal and esophageal delivery catheters and JB catheter were removed at the end of surgery.
Monitoring and safety evaluation
The brain and core temperatures were recorded at 1-minute intervals for the first 15 minutes and then at 5-minute intervals thereafter. Brain temperature was measured in the right JB, and core temperature was measured in the bladder. Cardiopulmonary parameters were continuously monitored during surgery. Site staff collected data related to adverse events/complications during the procedure and through to patient discharge. All adverse events were reviewed by an Independent Safety Committee and assessed for device relatedness.
Results
A total of five patients completed the required procedures and were discharged from the hospital on schedule. Baseline characteristics of patients and their response to the cooling procedure are shown in Table 1. Patients were cooled via the NeuroSave system between 56 and 89 minutes. For Patient 1, device-related noise required early discontinuation of NeuroSave system use. Adjustment of vacuum delivered to the system reservoir mitigated noise in the remaining patients.
Sixteen adverse events and an additional two serious adverse events were observed after surgery (Table 1). An Independent Safety Committee reviewed all the adverse events and determined that none were device-related and all were reasonable events post-cardiac surgery. Specifically, none of the following events occurred: alterations in cardiopulmonary function during NeuroSave system use; injuries to the nose, mouth, esophagus, or stomach; aspiration of cooling fluid into the lungs; delay to extubation; difficulty swallowing; or any JB catheter-related complications.
Brain temperature decreased by 3°C within 15 minutes in Patients 4 and 5 (Figure 2). Use of colder saline resulted in deeper and faster cooling of the brain and a larger temperature difference between the body core and the brain. Maximum brain–body temperature differences for Patients 2, 3, 4, and 5 were 2.6°C, 1.8°C, 3.2°C, and 4.5°C (except during hypotension as noted below).
Figure 2.
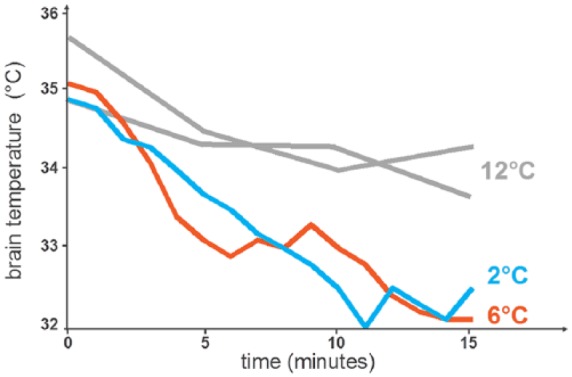
Reduction in brain temperature in the first 15 minutes in four patients. Patient 1 not displayed due to limited cooling system use.
Patient 5 experienced a 3-minute episode of hypotension (mean arterial pressure = 45 mm Hg) while transitioning to CPB support, 55 minutes after initiation of NeuroSave cooling. During this transient hypotensive episode, the brain temperature decreased by an additional 4°C in 2 minutes, while the body core temperature did not change, resulting in a transient brain-core temperature difference of 7.5°C (Figure 3). Per protocol, circulation of cooling fluid to the patient was discontinued once brain temperature decreased below 30°C.
Figure 3.
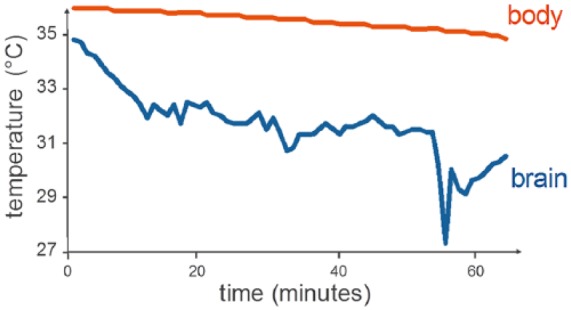
Brain and body temperature for Patient 5. Brain temperature decreased 4°C in 2 minutes during a 3-minute episode of hypotension (mean arterial pressure [MAP] = 45 mm Hg). Circulation of cooling fluid (2°C) was discontinued when brain temperature decreased below 30°C.
Discussion
This feasibility trial of five patients demonstrated that use of the NeuroSave system to irrigate the pharynx and upper esophagus with chilled saline was well tolerated and cooled the brain faster than any currently marketed device other than a CPB circuit.32–35 Several adverse events, common in the studied population, were observed and none were deemed to be related to the NeuroSave system (Table 1).
The observed rate of brain cooling, 3°C in 15 minutes, compares favorably to cooling rates of 1.0-1.5°C/hour for invasive and non-invasive whole body cooling technologies.34 The degree of brain cooling was dependent on cooling fluid temperature. The brain was cooled while maintaining a warmer body core; active body counter-warming was not used.
Demonstration of significantly enhanced (deeper and faster) brain-targeted cooling during episodic hypotension appears to be the first observation of this effect in humans, confirming the phenomenon observed in primates during use of a pharyngeal balloon cooling device and in pigs treated with nasal evaporative cooling.36,37 This beneficial effect is due to the location of the zone of cooling along the length of the carotid and vertebral arteries.
Performance of the NeuroSave system is maximized by the following : 1. cooling the mucosa of the pharynx and esophagus, the closest accessible surfaces to the carotid and vertebral arteries and the circle of Willis; 2. establishing a cooling zone encompassing the entire length of the carotid and vertebral arteries, and the circle of Willis; 3. maximizing the surface area cooled utilizing a free-flowing fluid; 4. avoiding insulating materials such as balloons; and 5. applying a biocompatible chilling liquid, that is, saline with high thermal capacity. Systemic body warming may also be employed to maintain a desired body core temperature, though this was not performed during this study and is not required for NeuroSave function.
Recognition of the trade-off between neuroprotection and the complications of body core cooling has led to several attempts to provide neuroprotection by brain-targeted cooling in cardiac surgery. The most notable of them is a two-circuit CPB system employing a specialized aortic cannula (Cobra, Cardeon Corp., Cupertino, CA, USA) to direct cold blood to the cerebral circulation and warmer blood to the systemic circulation during CPB.38 Unfortunately, in spite of rapid brain-targeted cooling, clinical results were disappointing with Cobra.39,40 Cardeon’s technology required deployment of a larger than normal bore catheter into the aorta, followed by inflation of baffles within the aortic arch to direct blood flow which may have increased embolic burden. Also, Cardeon’s technology is CPB-based and does not offer brain protection prior to CPB initiation, during active rewarming via CPB, nor after CPB conclusion.
Unlike the Cardeon solution to brain-targeted cooling, NeuroSave use is independent of the CPB circuit, and one of its potential benefits is lowering brain temperature before and after CPB use when hypothermic neuroprotection is not currently offered. In the future, it may be possible to use NeuroSave to protect the brain during the entire surgical procedure and early recovery period. Also, CPB-independent brain-targeted cooling could allow for slower rates of brain rewarming, consistent with guidelines for temperature management from The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology.41 And CPB-independent brain-targeted cooling would prevent brain hyperthermia and associated injury during rewarming and could reduce time on CPB circuit because active rewarming via CPB would be brief if required at all.20
Limitations
This first-in-human feasibility study had several limitations. Only five patients were studied, and only two patients were treated with deeply chilled saline. More patients will be needed to confirm the brain cooling rate and depth observed in this study, as well as the performance and safety of the NeuroSave system during prolonged use and rewarming. Future clinical trials could also evaluate NeuroSave safety and efficacy with brain temperatures of less than 30°C.
Brain temperature was not measured by direct probe but rather inferred from a JB temperature measurement. The JB, which receives 99% of cerebral venous blood flow, has been validated as the site that most accurately reflects intracerebral temperature and is the reference when evaluating the accuracy and reliability of brain temperature estimation from temperature readings at other bodily sites.20,42–44 Since the NeuroSave system cools the pharynx by circulating chilled saline in close anatomical proximity to the jugular veins, the use of JB as a measure of cerebral temperature might introduce the possibility of overestimating the magnitude of brain cooling. However, according to a Peclet Number Analysis (Supplement 1), it is unlikely that this potentially confounding conductive heat transfer would outweigh the convective contribution of the blood flow to the temperature field at the JB.
This study was not powered for clinical outcomes measurement. Endoscopy was not performed to evaluate the mucosa of the pharynx or esophagus. Patients were not followed after hospital discharge. Larger clinical trials with longer duration of follow-up will be required to assess complications and outcomes following NeuroSave device use.
Another limitation to the device’s applicability in cardiac surgery is related to its concurrent use with transesophageal echocardiography (TEE), which might modify NeuroSave function during periods of TEE use. Further investigation is warranted to assess the effect of the esophageal catheter tension and low esophageal temperatures on TEE imaging.
New onset atrial fibrillation developed in three of the five enrolled patients. All episodes of atrial fibrillation occurred postoperatively, none during NeuroSave use. There was no association between duration of NeuroSave use or cooling fluid temperature and development of atrial fibrillation. This rate is consistent with retrospective studies reporting new-onset atrial fibrillation in 40-60% of patients having surgical aortic valve replacement.45,46 The proximity of the esophagus to the left atrium may increase the risk of cooling-related heart complications such as arrhythmia; however, during cardiac surgery, cold-cardioplegia does not increase the rate of atrial fibrillation compared to warm-cardioplegia.47
Conclusion
The NeuroSave selective brain cooling system offers a potentially feasible and efficient method for inducing brain-targeted hypothermia. Findings from this first-in-human feasibility study suggest that rapid brain cooling in humans may be applied in time frames similar to those employed in animal studies, raising the possibility that reduction in brain injury in humans may someday be on par with that consistently demonstrated in animal studies. Further studies are required to evaluate the function of the NeuroSave system during longer periods of use and during rewarming. The faster and deeper brain cooling observed during hypotension deserves further study.
Supplemental Material
Supplemental material, Supplement_1 for Selective brain hypothermia: feasibility and safety study of a novel method in five patients by Seyed Mohammad Seyedsaadat, Silvana F Marasco, David J Daly, Robin McEgan, James Anderson, Seth Rodgers, Thomas Kreck, Ramanathan Kadirvel and David F Kallmes in Perfusion
Acknowledgments
We are grateful to all the patients for their participation in the study and Margaret Quayle for study management support.
Footnotes
Authors’ Note: This study was performed in the Alfred Hospital, Melbourne, Australia.
Authors’ Contribution: All authors have made substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; drafting of the work; or revising it critically for important intellectual content. All authors have provided final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. S.F.M. was the principal investigator. D.J.D. induced selective brain cooling using NeuroSave system. R.M. and J.A. collected and analyzed data. S.M.S., R.K., and D.F.K. analyzed data and wrote the manuscript. S.R. and T.K. operated the NeuroSave System.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.K. and S.R. founded and invested in NeuroSave, Inc. All other authors have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the Mayo Clinic Radiology Departmental internal funding through grant and by NeuroSave, Inc.
ORCID iD: Seyed Mohammad Seyedsaadat  https://orcid.org/0000-0002-8219-6144
https://orcid.org/0000-0002-8219-6144
Supplemental material: Supplemental material for this article is available online.
References
- 1. Grogan K, Stearns J, Hogue CW. Brain protection in cardiac surgery. Anesthesiol Clin 2008; 26: 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Messe SR, Acker MA, Kasner SE, et al. Stroke after aortic valve surgery: results from a prospective cohort. Circulation 2014; 129: 2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. New Engl J Med 2001; 344: 395–402. [DOI] [PubMed] [Google Scholar]
- 4. Swain JA. Cardiac surgery and the brain. New Engl J Med 1993; 329: 1119–1120. [DOI] [PubMed] [Google Scholar]
- 5. Seco M, Edelman JJ, Van Boxtel B, et al. Neurologic injury and protection in adult cardiac and aortic surgery. J Cardiothorac Vasc Anesth 2015; 29: 185–195. [DOI] [PubMed] [Google Scholar]
- 6. Royston D, Cox F. Anaesthesia: the patient’s point of view. Lancet 2003; 362: 1648–1658. [DOI] [PubMed] [Google Scholar]
- 7. van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia 2012; 67: 280–293. [DOI] [PubMed] [Google Scholar]
- 8. Newman MF, Grocott HP, Mathew JP, et al. Report of the substudy assessing the impact of neurocognitive function on quality of life 5 years after cardiac surgery. Stroke 2001; 32: 2874–2881. [DOI] [PubMed] [Google Scholar]
- 9. Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009; 110: 548–555. [DOI] [PubMed] [Google Scholar]
- 10. Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology 2008; 108: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson RE, Li TQ, Hindmarsh T, et al. Increased extracellular brain water after coronary artery bypass grafting is avoided by off-pump surgery. J Cardiothorac Vasc Anesth 1999; 13: 698–702. [DOI] [PubMed] [Google Scholar]
- 12. Merino JG, Latour LL, Tso A, et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol 2013; 34: 518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbut D, Lo YW, Gold JP, et al. Impact of embolization during coronary artery bypass grafting on outcome and length of stay. Ann Thorac Surg 1997; 63: 998–1002. [DOI] [PubMed] [Google Scholar]
- 14. Roach GW, Kanchuger M, Mangano CM, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter study of perioperative ischemia research group and the ischemia research and education foundation investigators. N Engl J Med 1996; 335: 1857–1863. [DOI] [PubMed] [Google Scholar]
- 15. Cumbler E. In-hospital ischemic stroke. The Neurohospitalist 2015; 5: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogue CW, Jr, Murphy SF, Schechtman KB, et al. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100: 642–647. [DOI] [PubMed] [Google Scholar]
- 17. Geocadin RG, Wijdicks E, Armstrong MJ, et al. Practice guideline summary: reducing brain injury following cardiopulmonary resuscitation: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology 2017; 88: 2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiang MC, Jong YJ, Lin CH. Therapeutic hypothermia for neonates with hypoxic ischemic encephalopathy. Pediatr Neonatol 2017; 58: 475–483. [DOI] [PubMed] [Google Scholar]
- 19. Belway D, Tee R, Nathan HJ, et al. Temperature management and monitoring practices during adult cardiac surgery under cardiopulmonary bypass: results of a Canadian national survey. Perfusion 2011; 26: 395–400. [DOI] [PubMed] [Google Scholar]
- 20. Nussmeier NA. Management of temperature during and after cardiac surgery. Tex Heart Inst J 2005; 32: 472–476. [PMC free article] [PubMed] [Google Scholar]
- 21. Rees K, Beranek-Stanley M, Burke M, et al. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database Syst Rev 2001; 2001: CD002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Insler SR, O’Connor MS, Leventhal MJ, et al. Association between postoperative hypothermia and adverse outcome after coronary artery bypass surgery. Ann Thorac Surg 2000; 70: 175–181. [DOI] [PubMed] [Google Scholar]
- 23. Levin MA, Lin HM, Castillo JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 2009; 120: 1664–1671. [DOI] [PubMed] [Google Scholar]
- 24. DeFoe GR, Krumholz CF, DioDato CP, et al. Lowest core body temperature and adverse outcomes associated with coronary artery bypass surgery. Perfusion 2003; 18:127–133. [DOI] [PubMed] [Google Scholar]
- 25. Vallabhajosyula P, Jassar AS, Menon RS, et al. Moderate versus deep hypothermic circulatory arrest for elective aortic transverse hemiarch reconstruction. Ann Thorac Surg 2015; 99: 1511–1517. [DOI] [PubMed] [Google Scholar]
- 26. Creswell LL, Alexander JC, Jr, Ferguson TB, Jr, et al. Intraoperative interventions: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005; 128: 28S–35S. [DOI] [PubMed] [Google Scholar]
- 27. Chelazzi C, Villa G, De Gaudio AR. Postoperative atrial fibrillation. ISRN Cardiol 2011; 2011: 203179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurz A. Thermal care in the perioperative period. Best Pract Res Clin Anaesthesiol 2008; 22: 39–62. [DOI] [PubMed] [Google Scholar]
- 29. Baker WL, Colby JA, Tongbram V, et al. Neurothrombectomy devices for treatment of acute ischemic stroke. Rockville, MD: Agency for Healthcare Research and Quality, 2011. [PubMed] [Google Scholar]
- 30. Grocott HP, Nussmeier NA. Neuroprotection in cardiac surgery. Anesthesiol Clin North America 2003; 21: 487–509, viii. [DOI] [PubMed] [Google Scholar]
- 31. Schell RM, Cole DJ. Cerebral monitoring: jugular venous oximetry. Anesth Anal 2000; 90: 559–566. [DOI] [PubMed] [Google Scholar]
- 32. Abou-Chebl A, Sung G, Barbut D, et al. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke 2011; 42: 2164–2169. [DOI] [PubMed] [Google Scholar]
- 33. Gladen A, Iaizzo PA, Bischof JC, et al. A head and neck support device for inducing local hypothermia. J Med Device 2014; 8: 0110021–0110029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoedemaekers CW, Ezzahti M, Gerritsen A, et al. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care 2007; 11: R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nybo L, Wanscher M, Secher NH. Influence of intranasal and carotid cooling on cerebral temperature balance and oxygenation. Front Physiol 2014; 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takeda Y, Hashimoto H, Fumoto K, et al. Effects of pharyngeal cooling on brain temperature in primates and humans: a study for proof of principle. Anesthesiology 2012; 117: 117–125. [DOI] [PubMed] [Google Scholar]
- 37. Tsai MS, Barbut D, Tang W, et al. Rapid head cooling initiated coincident with cardiopulmonary resuscitation improves success of defibrillation and post-resuscitation myocardial function in a porcine model of prolonged cardiac arrest. J Am Coll Cardiol 2008; 51: 1988–1990. [DOI] [PubMed] [Google Scholar]
- 38. Cook DJ, Slater JM, Orszulak TA, et al. First clinical report of differential perfusion during cardiac surgery using the Cardeon Cobra aortic cannula. Ann Thorac Surg 2002; 73: S375. [Google Scholar]
- 39. Cook DJ, Orszulak TA, Zehr KJ, et al. Effectiveness of the Cobra aortic catheter for dual-temperature management during adult cardiac surgery. J Thorac Cardiovasc Surg 2003; 125: 378–384. [DOI] [PubMed] [Google Scholar]
- 40. Kaukuntla H, Walker A, Harrington D, et al. Differential brain and body temperature during cardiopulmonary bypass—a randomised clinical study. Eur J Cardiothorac Surg 2004; 26: 571–579. [DOI] [PubMed] [Google Scholar]
- 41. Engelman R, Baker RA, Likosky DS, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: clinical practice guidelines for cardiopulmonary bypass—temperature management during cardiopulmonary bypass. Ann Thorac Surg 2015; 100: 748–757. [DOI] [PubMed] [Google Scholar]
- 42. Akata T, Setoguchi H, Shirozu K, et al. Reliability of temperatures measured at standard monitoring sites as an index of brain temperature during deep hypothermic cardiopulmonary bypass conducted for thoracic aortic reconstruction. J Thorac Cardiovasc Surg 2007; 133: 1559–1565. [DOI] [PubMed] [Google Scholar]
- 43. Crowder CM, Tempelhoff R, Theard MA, et al. Jugular bulb temperature: comparison with brain surface and core temperatures in neurosurgical patients during mild hypothermia. J Neurosurg 1996; 85: 98–103. [DOI] [PubMed] [Google Scholar]
- 44. Grigore AM, Murray CF, Ramakrishna H, et al. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: does rewarming rate matter? Anesth Analg 2009; 109: 1741–1751. [DOI] [PubMed] [Google Scholar]
- 45. Swinkels BM, de Mol BA, Kelder JC, et al. New-onset postoperative atrial fibrillation after aortic valve replacement: Effect on long-term survival. J Thorac Cardiovasc Surg 2017; 154: 492–498. [DOI] [PubMed] [Google Scholar]
- 46. Tanawuttiwat T, O’Neill BP, Cohen MG, et al. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J Am Coll Cardiol 2014; 63: 1510–1519. [DOI] [PubMed] [Google Scholar]
- 47. Fan Y, Zhang AM, Xiao YB, et al. Warm versus cold cardioplegia for heart surgery: a meta-analysis. Eur J Cardiothorac Surg 2010; 37: 912–919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement_1 for Selective brain hypothermia: feasibility and safety study of a novel method in five patients by Seyed Mohammad Seyedsaadat, Silvana F Marasco, David J Daly, Robin McEgan, James Anderson, Seth Rodgers, Thomas Kreck, Ramanathan Kadirvel and David F Kallmes in Perfusion


