Abstract
Background
Despite intense interest in the relationship between gut microbiota and brain development, longitudinal data from human studies are lacking. This study aimed to investigate the relationship between the composition of gut microbiota during infancy and subsequent behavioural outcomes.
Methods
A subcohort of 201 children with behavioural outcome measures was identified within a longitudinal, Australian birth-cohort study. The faecal microbiota were analysed at 1, 6, and 12 months of age. Behavioural outcomes were measured at 2 years of age.
Findings
In an unselected birth cohort, we found a clear association between decreased normalised abundance of Prevotella in faecal samples collected at 12 months of age and increased behavioural problems at 2 years, in particular Internalizing Problem scores. This association appeared independent of multiple potentially confounding variables, including maternal mental health. Recent exposure to antibiotics was the best predictor of decreased Prevotella.
Interpretation
Our findings demonstrate a strong association between the composition of the gut microbiota in infancy and subsequent behavioural outcomes; and support the importance of responsible use of antibiotics during early life.
Funding
This study was funded by the National Health and Medical Research Council of Australia (1082307, 1147980, 1129813), The Murdoch Children's Research Institute, Barwon Health, Deakin University, Perpetual Trustees, and The Shepherd Foundation. The funders had no involvement in the data collection, analysis or interpretation, trial design, recruitment or any other aspect pertinent to the study.
Keywords: Infant, Behaviour, Microbiota, Gut-brain axis
Research in context.
Evidence before this study
In rodents, gut microbiota composition has been shown to influence neurodevelopment and behaviour. In humans, cross-sectional associations have been observed between the composition of gut microbiota and neuropsychiatric outcomes including temperament, inattention and impulsivity, autism, schizophrenia, bipolar disorder, and depression; with recent evidence of an association between gut microbiota composition at 2.5 months and temperament at 6 months. However there is a lack of longitudinal data regarding gut microbiota and longer term behavioural outcomes.
Added value of this study
We present findings from an Australian birth cohort study demonstrating a prospective association between a decrease in a genus of bacteria in the faecal microbiota, Prevotella, at 1 year of age, and adverse behavioural outcomes at 2 years of age. Strengths of the study include the unselected sampling strategy, large sample size, and comprehensive assessment of potential confounding factors. The study adds novel evidence from a human birth cohort study regarding a longitudinal association between gut microbiota and behaviour.
Implications of all the available evidence
Accumulating evidence supports the role of the infant gut microbiota for neurodevelopment and mental health in later life. The findings of this study suggest a developmental window in late infancy in which increased Prevotella in the gut microbiota may predict a reduced risk of subsequent anxiety.
Alt-text: Unlabelled box
1. Introduction
The gut microbiome appears to play an important role in stimulating neurodevelopment via neuronal, hormonal, and immunological signalling [1]. Disruptions to gut microbiota cause aberrant hypothalamic-pituitary-adrenal axis stress-response, decreased expression of brain-derived neurotrophic factor, and impaired social behaviour in germ-free mice [2]. Both gut microbiota and the central nervous system mature rapidly during early life, and in mice, this appears to be a key period of gut-brain interaction [3].
Clinical studies show altered gut microbiota in people with established conditions such as autism and schizophrenia [4]; however, gut-brain associations in normal human neurodevelopment remain understudied. One study examining cross-sectional relationships between behaviour and gut microbiota in 77 human infants at age 18–27 months reported an association between phylogenetic diversity and temperamental problems, particularly in boys [5]. The only published prospective study of microbiota composition and a subsequent neurodevelopmental outcome in humans demonstrated an inverse association between phylogenetic alpha diversity at 12 months of age and overall cognitive and language measures at 2 years of age [6]. More recently, a Finish study found evidence of an association between gut microbiota composition at 2.5 months and maternal report of infant temperament at 6 months [7]. Further studies are required regarding the relationship between gut microbiota during early life and subsequent neuropsychiatric measures. Our aim was to investigate the association between faecal microbiota during infancy and subsequent child behavioural outcomes during early childhood.
2. Materials & methods
2.1. Sample and study design
The Barwon Infant Study (BIS) is a longitudinal, Australian birth cohort study (n = 1074 infants) [8]. Sociodemographic, family history, and maternal health data were collected during pregnancy, along with faecal samples from infants at 1, 6, and 12 months of age. 16S rRNA gene sequencing was performed in a random subsample (n = 324), of whom 201 had sufficient data available and were included in the present study. Parents completed the Child behaviour Checklist [9] (CBCL) when children were two years old.
3. Ethics
The study was approved by the ethics committee at Barwon Health (reference 10/24) and mothers of all infants gave written informed consent before participating.
3.1. Microbiota
Stool samples were stored at −80C. In a random subsample of n = 324, microbial DNA was extracted and 16S rRNA sequencing conducted. DNA was extracted using the Qiagen PowerSoil® DNA Isolation Kit, Cat# 12888–100. The lysis of the bacteria was optimised by performing two bead shearing steps (5 min each), in conjunction with the treatment with lysis buffer, and two heat incubation steps (85 °C × 2). The DNA was transported to the J. Craig Venter Institute, Rockville, MD, USA. The V4 hypervariable region of the 16S rRNA gene was sequenced on the Illumina MiSeq platform. USEARCH software was used to merge corresponding paired-end reads, filter, cluster into OTUs at 97% identity, identify OTU representative sequences, and remove chimeras. Mothur software was used to assign representative sequences to taxa described in the SILVA v123 Nr99 taxonomic database. The final descriptions of OTUs present in each sample were composed in USEARCH. Samples with fewer than 2500 read pairs were excluded. Additional aliquots of faecal samples were transported on dry ice to the Commonwealth Science and Industrial Research Organisation laboratories, Adelaide, Australia, where short-chain fatty acids (SCFAs) were quantified by capillary gas chromatography (GC; 5890 series II Hewlett Packard, Australia). For SCFA determination, samples (0.5–1 g) were diluted 3-fold with an internal standard (1.68 mmol/L heptanoic acid, Sigma, NSW), centrifuged (3000 × gfor 15 min at 5 °C) and the pH of the resultant supernatant measured by inserting an appropriate glass probe. An aliquot (150 μL) of supernatant were then acidified with 30 μL of 0.16 mol/L orthophosphoric acid and distilled under vacuum. Individual SCFA in the distillates were separated and quantified by capillary gas chromatography (GC; 5890 series II Hewlett Packard, NSW, Australia). The GC was equipped with a flame ionisation detector, split-less injector and a Zebron ZB-FFAP 30 m × 0.53 μm capillary column with 0.1 μm film thickness (Phenomenex, NSW, Australia). Injector and detector temperatures were both 210 °C, and the column temperature program was 120 °C held for 0.5 min and then raised at 30 °C/min to reach a final column temperature of 190 °C. Helium was used as the carrier gas (head pressure 50 kPa) and an injection volume of 0.2 μL was used. Faecal SCFA concentrations were calculated as (mmol/L) × wet faecal weight x faecal moisture content (g/100 g) × 10.
3.2. Behavioural measurement and case definition
The CBCL is a validated 99-item screening questionnaire consisting of problem items within Internalizing, Externalizing, and Total Problems subscales [9]. The Total Problems subscale is a separate subscale, not simply the combination of Externalizing and Internalizing problems scores. In the present study, the cut-off of T ≥ 60 on any subscale (one standard deviation above the mean of a reference population) to define a behaviour case group was based on prior validation in a longitudinal study of conversion to psychiatric disorders diagnosed later in childhood, which showed high specificity (88–96%) and moderate sensitivity (25–34%) with a cut-off of T ≥ 60 [10].
Infant temperament at ages 1, 6, and 12 months was measured by parent report using a 5-point Likert scale [11].
3.3. Statistical analysis
Statistical analysis was conducted in the statistical software environment R, using the phyloseq package for microbiome data management [12], and the vegan package for beta-diversity [13]. Alpha diversity within samples was computed as the Shannon, Simpson, Chao1, and Observed species indices. Beta diversity between samples was computed as both weighted and unweighted UniFrac distance [14]. The association between beta diversity and the binary behavioural outcome was examined via the PERMANOVA test; PERMDISP2 was used to examine whether the findings of PERMANOVA could have arisen from differing dispersion between groups. Both tests were based on 999 permutations.
The voom method from the limma package [15] was used for differential normalised abundance testing. Voom outperforms competing methods (e.g. DESeq2) for highly variable library sizes, which are typical in 16S sequencing studies [16]. The log-fold change reported by voom is based on abundances normalised using relative log expression with pseudo-counts added to avoid taking the log of 0 and including voom's precision weighting scheme (see Supplement 1). This metric is described as normalised abundance. We adjusted p-values for multiple testing using the Benjamini-Hochberg method and refer to corresponding false-discovery rates as q-values. As the SILVA taxonomy divides accepted genera into multiple clades (e.g. Tyzzerella_x, where x can be 3 or 4), we generated an intermediate taxonomic level between the SILVA family and genus levels by truncating the genus name before its first underscore. This has a secondary effect of consolidating genus level groupings of unnamed clades within families, e.g. Ruminococcaceae_UCG-xxx where xxx can be 013, 014, 010 etc. Logistic and linear regression and chi square tests were used to assess associations between categorical and linear outcomes as appropriate.
Analyses of differential normalised abundance were adjusted for the methodological variables:
(i) any storage of faecal samples in the home freezer (typically −18C) prior to delivery to the laboratory, and (ii) duration of storage at −80C.
To evaluate adjustment for potential covariates, twenty six candidate domains of relevance to infant microbiota and/or early childhood behaviour were identified and used to construct a directed acyclic graph (DAG; Supplement 2a) using DAGitty v3.0. For each of these domains, a number of candidate variables available in the BIS dataset were specified. Correlation matrices were constructed to inspect linear relationships between candidate variables, candidate microbial exposures, and behavioural outcomes of interest. For each construct, the one with the strongest associative relationships with exposures and/or outcomes was selected for inclusion in the directed acyclic graph (Supplement 2b). Minimal sufficient adjustment sets for estimating the total effect of the microbial exposure on the behavioural outcome, were then generated from DAGitty. We investigated potential confounding using the minimum adjustment set with the most complete data, which comprised: gestational age, mode of birth, antibiotic use during labour, breastfeeding at four weeks, number of siblings, and household pet ownership. Sex and child's age in months at time of questionnaire completion were additionally adjusted for in separate analyses given sex differences in neurodevelopment [17] and reduce potential outcome measurement variability respectively.
3.4. Data statement
Regarding access of data, the microbiome sequencing data is available from the Sequence Read Archive under accession number PRJNA576314. Anonymised data relating to the additional variables used in the analysis, results and conclusions reported in this paper will be made available upon reasonable requests for purposes of reproducing or extending the analysis.
4. Results
4.1. Descriptive statistics of study sample
The baseline characteristics of the inception birth cohort (n = 1074) and randomly selected subgroup with adequate 12 month faecal microbiota and behavioural outcome data (n = 201) were similar (Table 1). Of the 201 participants in the study sample, 22 were classified as cases on the basis of ‘elevated’ behavioural problems (T ≥ 60; n = 14 Externalizing subscale, n = 9 Internalizing subscale, n = 10 Total Problems subscale; see Supplement 3 for density plot of T score distributions and scores in case and non-case groups). 182 infants had one-month and 190 had six-month faecal microbiota samples available.
Table 1.
Descriptive characteristics of inception birth cohort and sample in the current study.
| Characteristic | Inception birth cohort (n = 1074) | Study sample: Random subcohort with 12-month 16S and neurocognitive data (n = 201) |
|---|---|---|
| Sex of child: | ||
| Male | 555 (51·7%) | 106 (52·7%) |
| Female | 519 (48·3%) | 95 (47·3%) |
| Twins (sets) | 10 (1%) | 2 (1%) |
| Maternal country of birth | ||
| Australia | 961 (89·5%) | 179 (89·1%) |
| Other | 110 (10·2%) | 21 (10·4%) |
| Unknown | 3 (0·3%) | 1 (0·5%) |
| Paternal country of birth | ||
| Australia | 915 (85·2%) | 169 (84·1%) |
| Other | 108 (10·1%) | 19 (9·4%) |
| Unknown | 51 (4·8%) | 13 (6·5%) |
| Maternal age, years (mean & SD) | 31.3 (4·8) | 32.4 (4·2) |
| Paternal age, years (mean & SD) | 33.5 (5·9) | 34.2 (5·1) |
| Maternal education | ||
| < year 10 of high school | 12 (1·1%) | 2 (1·0%) |
| Year 10 of high school | 80 (7·5%) | 14 (7·0%) |
| Year 12 of high school | 162 (15·1%) | 28 (13·9%) |
| Trade, certificate, diploma | 266 (24·8%) | 39 (19·4%) |
| Bachelor degree | 354 (33%) | 76 (37·8%) |
| Postgraduate degree | 194 (18·1%) | 42 (20·1%) |
| Unknown | 6 (6%) | 0 (0·0%) |
| SEIFA | ||
| Low | 357 (33·2%) | 59 (29·4%) |
| Middle | 353 (32·9%) | 73 (36·3%) |
| High | 351 (32·7%) | 69 (34·3%) |
| Unknown | 13 (1·9%) | 0 (0·0%) |
| Number of siblings including twins | ||
| 0 | 473 (44·0%) | 77 (36·3%) |
| 1 | 383 (35·7%) | 87 (44·8%) |
| 2 | 177 (16·5%) | 32 (16·4%) |
| 3 or more | 41 (3·8%) | 5 (2·5%) |
| Maternal cigarette smoking in pregnancy: | ||
| Yes | 169 (15·7%) | 19 (9·5%) |
| No | 892 (83·1%) | 182 (90·5%) |
| Unknown | 13 (1·2%) | 0 (0·0%) |
| Pet ownership | ||
| Yes | 790 (73·6%) | 143 (71·1%) |
| No | 278 (25·9%) | 58 (28·9%) |
| Unknown | 6 (0·6%) | 0 (0%) |
| Livestock ownership | ||
| Yes | 73 (6·8%) | 10 (5·0%) |
| No | 985 (91·7%) | 188 (93·5%) |
| Unknown | 16 (1·5%) | 3 (1·5%) |
| Delivered in a government hospital | ||
| Yes | 780 (72·6%) | 121 (60·2%) |
| No | 294 (27·4%) | 80 (39·8%) |
| Delivery via Caesarean section | 333 (31%) | 70 (34·9%) |
| Gestational age at birth: | ||
| 32 to 36 completed weeks | 47 (4·4%) | 4 (2%) |
| 37 to 42 completed weeks | 1027 (95·6%) | 197 (98%) |
| > 42 completed weeks | 0 (0·0%) | 0 (0·0%) |
| Birth weight in grams (mean & SD) | 3528 (519) | 3522 (522) |
| Internalising subscale t score (mean & IQR) | 41 (14) | 41 (14) |
| Externalising subscale t score (mean & IQR) | 44 (12) | 46 (13) |
| Total problems subscale t score (mean & IQR) | 43 (13) | 44 (13) |
4.2. Sequencing summary
Faecal samples from the three time-points were sequenced across two runs. Summary statistics of the sequencing are presented in Supplement 4a. A small number of biological and technical duplicates were sequenced. amongst the 12-month samples, all replicates of the same sample clustered together according to weighted UniFrac distance (Supplement 4b).
4.3. Child microbiota and behavioural outcomes
4.3.1. One- and six-month child microbiota
There was no evidence of associations between one- or six-month microbiota alpha diversity (as measured by Shannon Index), or beta diversity (weighted and unweighted UniFrac distances) and the behavioural outcome at age 2 (see Supplements 5, 6). Nor was there evidence of differential normalised abundance in the one-month faecal microbiota of behavioural case infants versus non-case infants (n = 182). In the six-month faecal samples (n = 190), the normalised abundance of the genus Sutterella appeared to be lower in the case group (logFC=−0·37, p = 0·0002, q = 0·02) but this association was attenuated following adjustment for any storage in a domestic freezer and duration of storage at −80 °C (p = 0·0016, q = 0·18).
4.4. 12-month child microbiota
4.4.1. Alpha and beta diversity
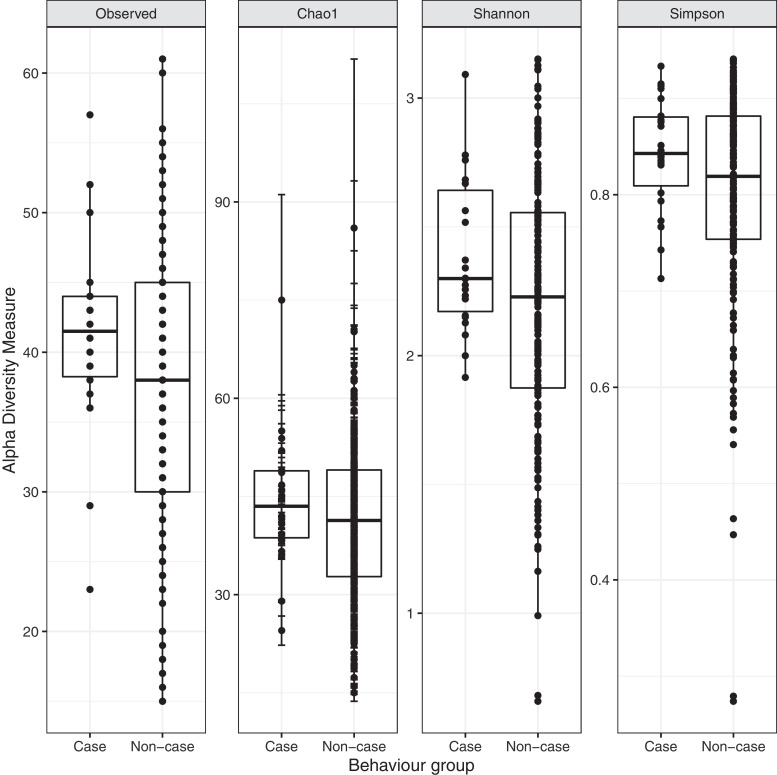
There was weak evidence that a higher Shannon index alpha diversity at 12 months was associated with increased risk of elevated behaviour problems at two years (OR: 2·42 [0·92– 6·97], p = 0·087). A similar pattern was observed using alternative measures of alpha diversity (see Fig. 1). None of the processing or potentially confounding variables caused a change of >10% to the estimate of the odds ratio.
Fig. 1.
Number of observed OTUs, Chao1, Shannon and Simpson indices of alpha diversity of 12-month faecal microbiota of infants with elevated behaviour problems (case) and normative behaviour (non-case) at two years of age.
PERMANOVA applied to unweighted UniFrac distances suggested differences in microbiota community structure between behavioural groups (R2=0.0092, p = 0·018). PERMDISP2 indicated that this may reflect differential multivariate dispersions (F = 9.33(1, 199), p = 0·003). Differences were not apparent in weighted UniFrac distance (R2=0.0047, p = 0.499; see figures in Supplements 7,8).
4.4.2. Differential normalised abundance
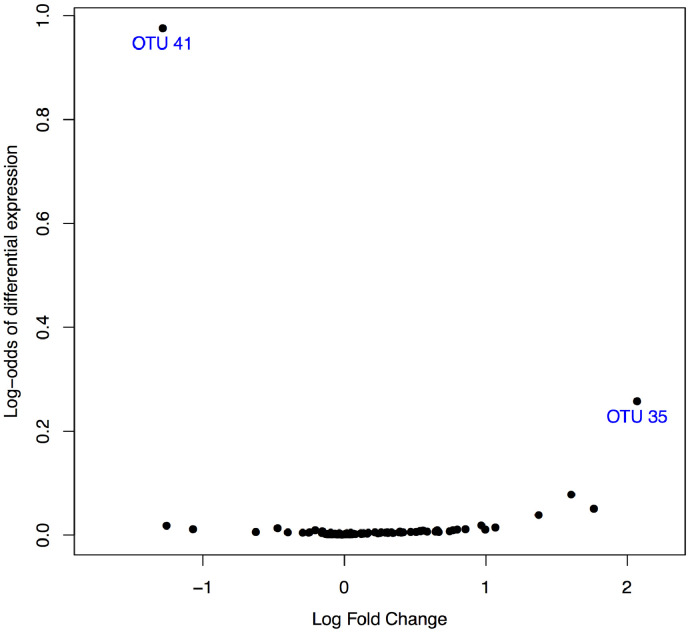
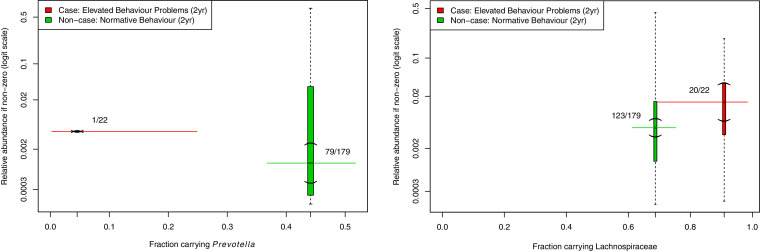
The normalised abundance of two bacterial groups was substantially different in the 12-month faecal microbiota of behavioural case infants versus non-case infants: Prevotella (logFC= −1·46, p < 0·0001, q < 0·0001) and the unspecified genera of the Lachnospiraceae family (logFC = 2·09, p = 0.0009, q = 0·054; see Fig. 2). Prevotella was detected in only 4% (1/22) of case infants compared with 44% (79/179) of non-case infants (Fig. 3, left-hand side). Unspecified Lachnospiraceae genera were detected in 91% (20/22) of case infants compared with 69% (123/179) of non-case infants (Fig. 3, right-hand side). Table 2 shows the differential normalised abundance of OTUs on the basis of behavioural case status for all q values under, and two above q = 0·2 (unadjusted analysis).
Fig. 2.
Volcano plot showing the magnitude (log-fold change) versus evidence (log-odds) of differential normalised abundance of all OTUs at 12 months between case and non-case behavioural groups. Evidence of difference was clearly strongest for OTU41 (of genus Prevotella) and OTU35 (of the Lachnospiraceae family).
Fig. 3.
Carriage and relative abundance of Prevotella and the genus-unspecified OTUs from the Lachnospiraceae family in faecal microbiota of 12-month old infants who have elevated behaviour problems at age two (n = 22) versus those with normative range behaviour problems (n = 179). Horizontal solid lines = 95% confidence intervals. Vertical dashed line = range of count values, with parentheses indicating the 95% CI of the median. Fractions represent number of individuals with any Prevotella and Lachnospiraceae carriage detected in each group.
Table 2.
Evidence of differential normalised abundance at the family/genus level on the basis of two year behaviour group*.
| Family/Genus | Log fold change | p | q |
|---|---|---|---|
| Prevotella | −1·46 | <0·001 | <0·001 |
| Lachnospiraceae | 2·09 | <0·001 | 0·05 |
| Bacteroides | 1·64 | 0·008 | 0·28 |
| Incertae** | 1·46 | 0·01 | 0·28 |
| Ruminococcus | 1·70 | 0·02 | 0·28 |
| uncultured** | 1·10 | 0·03 | 0·37 |
Note: Log fold change indicates change in case infants relative to non-case group; positive values indicate greater abundance in the case group, negative values indicate greater abundance in the non-case group.
Both OTUs 77 and 1106 are identified as Lachnospiraceae at the family level.
4.4.3. Investigation of potential confounding
Evidence of an association between low normalised abundance of Prevotella at one year and the behaviour case group persisted following adjustment for the processing variables (for any storage in a domestic freezer and duration of storage at −80 °C) and the minimum adjustment set identified (gestational age, mode of birth, antibiotic use during labour, breastfeeding at four weeks, number of siblings, household pet ownership) (all p < 0·0001, q < 0·0001). In addition, we confirmed that evidence of association persisted following adjustment for sex and child's age in months at time of questionnaire completion, maternal prenatal smoking, maternal perceived stress or maternal depressive symptoms at child age two, and household income (adjustment models run one at a time in addition to minimum adjustment set variables; q < 0·001 in all cases). By contrast, the association between increased normalised abundance of Lachnospiraceae at one year and the case group was substantially attenuated by adjustment for the processing and potential confounding variables (p = 0·002, q = 0·1).
4.4.4. Investigation of reverse causation
To assess reverse causation we considered associations between early temperament and both candidate bacteria at subsequent timepoints. There were however no associations evident between child temperament at one, six, and 12 months and presence or abundance of subsequent Prevotella or Lachnospiraceae at 12 months of age. Nor was the association between the normalised abundance of Prevotella and behaviour at two years attenuated by adjusting for infant temperament at 1-, 6-, or 12 months of age (q < 0·001 in all cases). For Lachnospiraceae, q values were 0·15, 0·01, and 0·01 respectively (for 1-, 6-, and 12-month infant temperament). Thus, the prospective association between Prevotella and subsequent behaviour persisted after accounting for early life infant temperament.
4.4.5. Child behaviour checklist subscales
The association between decreased Prevotella at 12 months and two-year behaviour outcomes was primarily related to the Internalizing subscale (Supplement 9). There was an analogous trend for Lachnospiraceae.
4.5. OTU members of the Prevotella genus and Lachnospiraceae family
Amongst 12-month samples from the random subcohort, OTU41 comprised 95% of all OTUs identified as belonging to the genus Prevotella. The sequence of 253 base pairs characterising OTU41 was 100% identical to base pairs 529–781 of the P. copri strain JCM 13,464 16S rRNA gene (Accession No: AB649279). The next most common Prevotella OTU was OTU697 at 1·7%. The sequence characterising OTU697 differed from that of OTU41 by only eight base pairs (96·8% identity). OTU697 was only evident in samples in which OTU41 was identified, suggesting that OTU697 may have arisen from sequencing errors in reads otherwise destined to be classified as OTU41.
The group of Lachnospiraceae OTUs was mostly composed of OTU35 (56%) and OTU70 (22%), both classified to the Lachnospiraceae NK4A136 group. A BLAST search for the representative sequences of these two OTUs was inconclusive. The NK4A136 group accounted for only 5% of all family Lachnospiraceae OTUs counted in 12-month samples.
4.6. Predictors of 12-month Prevotella and Lachnospiraceae carriage
There was no evidence of a relationship between infant feeding practices, sibling number, or pet ownership and carriage of Prevotella at 12 months (all p > 0·05). Having a pet in the household during the first postnatal year was positively associated with Lachnospiraceae carriage (OR: 2·03 [1.05–3.89], p = 0·033). Antibiotic use from 9 to 12 months (i.e. the three months prior to faecal sample collection) was associated with reduced presence of Prevotella (OR 0·37 [0·17–0·75], p = 0·007), but not Lachnospiraceae (OR 0·67 [0·34–1·33], p = 0·24; Supplement 11). However, antibiotic use did not relate to 2 year behaviour (χ2 = 0.047, df = 1, p = 0.83).
4.7. Faecal short-chain fatty acids
There was no evidence of associations between the concentration of faecal SCFAs and child behaviour, presence of Prevotella genus in 12-month faecal microbiota or antibiotic use from 9–12 months (butyrate, propionate, acetate, caproate, valerate, isobutyrate, isovalerate; and a sum of butyrate, acetate, propionate, and valerate; Supplement 10).
5. Discussion
In a study of 201 infants from an Australian birth cohort assembled using an unselected sampling frame, we report a clear association between reduced normalised abundance of the genus Prevotella in infancy and increased risk of being in a behaviour case group at 2 years of age. This association primarily related to the Internalizing subscale, and was independent of a range of potential confounding factors. Recent exposure to antibiotics was the best predictor of Prevotella absence. Although Prevotella abundance has been associated with both autism [18] and Parkinson's disease [19] in cross-sectional studies, this is the first study to show an association between a low abundance of Prevotella in early life and subsequent adverse behavioural outcomes.
The mechanisms by which Prevotella may influence brain development and behaviour are poorly characterised but may include stimulation of the vagus nerve, release of cytokines or enzymes, tryptophan metabolism, interaction with the peripheral immune system, effects on brain-derived neurotrophic factor and the production of SCFAs [20,21]. We found no evidence of associations between short-chain fatty acid abundance in stool and Prevotella, recent use of antibiotics (a predictor of Prevotella carriage in this sample) or behaviour problems. Of relevance, one study of women demonstrated positive associations between Prevotella abundance, complexity of frontal cortex and insula connections as measured by functional magnetic resonance imaging, and reduced emotional responses to distressing stimuli [22]. It is of course possible that Prevotella is simply a biomarker for broader aspects of the microbiome, host genotype, or metabolism. Further studies are required to assess causality and underlying mechanisms.
Prevotella is a gram-negative bacterial genus that is more abundant amongst populations living in non-westernised environments [23]. The low rate and abundance of Prevotella carriage amongst westernised populations is thought to relate to low dietary intake of plant polysaccharides, which provide important cellulose and xylans substrates [24]. High rates of antibiotic exposure, as reported in our cohort [25], may also be relevant; consistent with evidence from mouse studies demonstrating that antibiotic exposure in early life influences gut microbiota, and alters brain cytokines and behaviour [26]. Estimates regarding the duration of disruption to the gut microbiota following exposure to antibiotics vary widely, and are likely to depend on the nature of the antibiotic agent [27]. However here, antibiotic exposure between ages 9 and 12 months, but not earlier, predicted lower carriage of Prevotella at 12 months, suggesting that Prevotella carriage may recover over longer intervals. Host, diet, and environmental factors influencing the likelihood and rate of reconstitution are yet to be determined.
We found weak evidence of an association between higher alpha diversity and subsequent behavioural problems. Although decreased microbial diversity is typically associated with westernised populations and adverse health outcomes in adults, we note that associations between diversity and health status is mixed in children [7,28]. Our finding is consistent with recent evidence of an association between increased microbiota diversity at 12 months of age and subsequent adverse cognitive outcomes [6]. We acknowledge that microbial diversity provides a summary of a highly dimensional dataset, and it is widely recognised that more biologically meaningful descriptors are required.
The association we observed between increased normalised abundance of Lachnospiraceae and adverse behavioural outcomes is noteworthy. Lachnospiraceae is a family of anaerobic bacteria from the order Clostridiales. Some members of this family are ‘fibre fermenters’ and produce butyrate, a SCFA shown to have positive neurological effects on tight junction proteins of the blood brain barrier and to reduce neuroinflammation [29]. Decreased carriage of Lachnospiraceae has been associated with depression [30] and Parkinson's disease [31]. Our findings however highlight the likely complexity of gut-brain relationships and the importance of considering the adverse outcomes of strategies to promote carriage of specific taxa. The single finding to emerge from the six-month microbiota of reduced normalised abundance of Sutterella in the case group requires replication in a larger sample as the effect did not persist beyond adjustment for processing variables.
The only previous study to relate gut microbiota and behaviour beyond infancy in humans was limited by cross-sectional design [5]. We found no evidence of a cross-sectional association between gut microbiota and child temperament at 12 months of age, suggesting the longitudinal association between decreased Prevotella and adverse behaviour is unlikely to reflect reverse causation. Further, temperament measured during the first postnatal year did not predict Prevotella carriage.
In contrast to recent evidence of an association between microbiota composition at 2.5 months and temperament at 6 months [7], we found little evidence linking faecal microbiota composition at one or six months to behaviour at 2 years. The composition of gut microbiota develops rapidly over the course of infancy, prompted in particular by changes to feeding and the introduction of solid foods [32]. The relative lack of diversity, and low rate of carriage of Prevotella at one and six months, is likely to have limited our statistical power to identify associations at these earlier time points.
The strengths of this study include an unselected sampling frame, longitudinal design, collection and analysis of faecal samples at multiple timepoints, and detailed consideration of potential confounding factors. Limitations include insufficient information to estimate dietary intake of fermentable fibre, relatively small numbers of cases with elevated behaviour problems, and the absence of metagenomic or transcriptomic data from which to infer potential mechanisms underlying the observed associations. Studies are now required to replicate the current findings, and to further assess causality and mechanism. Studies using metagenomic techniques to improve taxonomic and functional characterisation are required to investigate the potential relevance of these earlier timepoints.
Here we have identified potential microbial targets for improving behaviour, and factors which may affect them, such as antibiotic use. This study adds support to evidence that the human infant gut microbiota may have long-term neurodevelopmental consequences, conferring protection or vulnerability to behavioural and mental health outcomes in later life.
Declaration of competing interest
The findings described in this paper are of potential relevance to Prevatex Pty Ltd, in which the following authors have a financial interest: PV, MOH, SR, FC and ALP.
In addition, the following interests have been declared:
- Michael Conlon – Patent PI2018702907 (Malaysia). Provisional patent AU2019902828 (Australia). Provisional patent AU2019901142 (Australia). Patent all related to microbes. With his CSIRO colleagues, Dr Conlon has had extensive discussions with the Australian company Microba, who provide gut microbiome testing services or the general population, around potential collaboration and allowed them to sell (along with their kits) for a short period one of the books he has co-authored entitled The CSIRO Healthy Gut Diet.
- Michael Berk – Dr. Berk reports grants from NHMRC Senior Principal Research Fellowship and personal fees from Allergan, RANZCP Hong Kong, Servier, Southern Star Research, Lundbeck, RANZCP Hobart, Livanova, Grunbiotics, Janssen, Catalyst NZ, Norwegian Psychiatry Assoc, Otsuka, Controversias Barcelona, Medisquire India, HealthEd, ANZJP, and Medplan Communications Canada. In addition, Dr. Berk has a patent Modulation of Physiological processes and agents useful for same. pending, and a patent Modulation of diseases of the central nervous system and related disorders (pending).
- Mimi Tang – Dr. Tang reports grants from National Health and Medical Research Council of Australia, from The Murdoch Childrens Research Institute, from Barwon Health, from Deakin University, from Perpetual Trustees, from The Shepherd Foundation, during the conduct of the study; personal fees from Abbot Nutrition, personal fees from Nestle Health Science, other from Nestle Nutritian Institute, other from Nutricia, personal fees from Bayer Pharmaceuticals, outside the submitted work; In addition, Dr. Tang has a patent Behavioural Treatment licensed to MCRI, and a patent Method of Inducing tolerance licensed to MCRI.
Acknowledgments
Acknowledgments
We would like to thank the study participants, as well as the entire BIS team which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists. We also thank the obstetric and midwifery teams at Barwon Health and Saint John of God Hospital Geelong for their assistance in recruitment and collection of biological specimens. We thank the following collaborators for their contributions: Karen Nelson, Manolito Torralba, Andres Gomez, and Sanjay Vashee from the J. Craig Venter Institute who conducted the 16S sequencing and preliminary bionformatic analyses.
Funding
This study was funded by the National Health and Medical Research Council of Australia (1082307, 1147980, 1129813), The Murdoch Children's Research Institute, Barwon Health, Deakin University, Perpetual Trustees, and The Shepherd Foundation. The funders had no involvement in the data collection, analysis or interpretation, trial design, recruitment or any other aspect pertinent to the study. Relevant grant funding applications were prepared by and awarded to: PV, ALP, JC, CS, FC, MT, SR, KA, RS, LH, PS, and the BIS Investigator Group.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102640.
Appendix. Supplementary materials
References
- 1.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudo N., Chida Y., Aiba Y., Sonoda J., Oyama N., Yu X.N. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tognini P. Gut microbiota: a potential regulator of neurodevelopment. Front Cell Neurosci. 2017;11:1–8. doi: 10.3389/fncel.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly J.R., Minuto C., Cryan J.F., Clarke G., Dinan T.G. Cross talk: the microbiota and neurodevelopmental disorders. Front Neurosci. 2017;11:1–31. doi: 10.3389/fnins.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian L.M., Galley J.D., Hade E.M., Schoppe-Sullivan S., Kamp Dush C., Bailey M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav Immun. 2015;45:118–127. doi: 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson A.L., Xia K., Azcarate-Peril M.A., Goldman B.D., Ahn M., Styner M.A. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83:148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aatsinki A.-.K., Lahti L., Uusitupa H.-.M., Munukka E., Keskitalo A., Nolvi S. Gut microbiota composition is associated with temperament traits in infants. Brain Behav Immun. 2019;80:849–858. doi: 10.1016/j.bbi.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Vuillermin P., Saffery R., Allen K.J., Carlin J.B., Tang M.L.K., Ranganathan S. Cohort profile: the barwon infant study. Int J Epidemiol. 2015;44:1148–1160. doi: 10.1093/ije/dyv026. [DOI] [PubMed] [Google Scholar]
- 9.Achenbach T.M. Child behavior checklist and related instruments. In: Maruish M.E., editor. The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers; 1994. pp. 429–466. [Google Scholar]
- 10.Petty C.R., Rosenbaum J.F., Hirshfeld-Becker D.R., Henin A., Hubley S., LaCasse S. The child behavior checklist broad-band scales predict subsequent psychopathology: a 5-year follow-up. J Anxiety Disord. 2008;22:532–539. doi: 10.1016/j.janxdis.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponsonby A.L., Dwyer T., Couper D. Factors related to infant apnoea and cyanosis: a population-based study. J Paediatr Child Health. 1997;33:317–323. doi: 10.1111/j.1440-1754.1997.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 12.McMurdie P.J., Holmes S. Phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oksanen J., Blanchet F.G., Kindt R., Legendre P., O'hara R., Simpson G.L. Vol. 23. 2010. p. 2010.http://cran r-project org>Acesso em (Vegan: community ecology package). R package version 1.17-4. [Google Scholar]
- 14.Lozupone C., Lladser M.E., Knights D., Stombaugh J., Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucl Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law C.W., Chen Y., Shi W., Smyth G.K. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung W.C., Auger A.P. Gender differences in neurodevelopment and epigenetics. Pflügers Archiv-Eur J Physiol. 2013;465(5):573–584. doi: 10.1007/s00424-013-1258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang D.-.W., Park J.G., Ilhan Z.E., Wallstrom G., LaBaer J., Adams J.B. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 20.Prykhodko O., Sandberg J., Burleigh S., Björck I., Nilsson A., Fåk Hållenius F. Impact of rye kernel-based evening meal on microbiota composition of young healthy lean volunteers with an emphasis on their hormonal and appetite regulations, and blood levels of brain-derived neurotrophic factor. Front Nutr. 2018;5:45. doi: 10.3389/fnut.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinan T.G., Cryan J.F. Microbes immunity and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology. 2017;42:178–192. doi: 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tillisch K., Labus J., Kilpatrick L., Jiang Z., Stains J., Ebrat B. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. doi: 10.1053/j.gastro.2013.02.043. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits S.A., Leach J., Sonnenburg E.D., Gonzalez C.G., Lichtman J.S., Reid G. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science. 2017;357:802–806. doi: 10.1126/science.aan4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson H., Vuillermin P., Jachno K., Allen K.J., Tang M.L.K., Collier F. Prevalence and determinants of antibiotic exposure in infants: a population-derived Australian birth cohort study. J Paediatr Child Health. 2017;53:942–949. doi: 10.1111/jpc.13616. [DOI] [PubMed] [Google Scholar]
- 26.Leclercq S., Mian F., Stanisz A., Bindels L.B., Cambier E., Ben-Amram H. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat Commun. 2017;8:15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahamsson T., Jakobsson H., Andersson A.F., Björkstén B., Engstrand L., Jenmalm M. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850. doi: 10.1111/cea.12253. [DOI] [PubMed] [Google Scholar]
- 29.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naseribafrouei A., Hestad K., Avershina E., Sekelja M., Linlokken A., Wilson R. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil: Offic J Eur Gastrointest Motil Soc. 2014;26(8):1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 31.Trasande L., Zoeller R.T., Hass U., Kortenkamp A., Grandjean P., Myers J.P. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1245–1255. doi: 10.1210/jc.2014-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig J.E., Spor A., Scalfone N., Fricker A.D., Stombaugh J., Knight R. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci. 2011;108:4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.