Abstract
A standardised, single-centre, longitudinal imaging protocol was used to evaluate longitudinal brainstem alterations in 100 patients with amyotrophic lateral sclerosis (ALS) with reference to 33 patients with primary lateral sclerosis (PLS), 30 patients with frontotemporal dementia (FTD) and 100 healthy controls. “Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: A longitudinal neuroimaging study” [1] ALS patients were scanned twice; 4 months apart. T1-weighted imaging data were acquired on a 3 T Philips Achieva MRI system, using a 3D Inversion Recovery prepared Spoiled Gradient Recalled echo (IR-SPGR) sequence. Raw MRI data underwent meticulous quality control before pre-processing. A Bayesian segmentation algorithm was utilised to parcellate the brainstem into the medulla oblongata, pons and mesencephalon before estimating the volume of each segment. Vertex-based shape analyses were carried out to characterise anatomical patterns of atrophy. Brainstem volume loss in ALS was dominated by medulla oblongata atrophy, but significant pontine pathology was also detected. Brainstem volume reductions were more significant in PLS than in ALS after correcting for demographic variables and total intracranial volume. Shape analyses revealed bilateral ‘flattening’ of the medullary pyramids in ALS compared to healthy controls. Our data demonstrate that computational neuroimaging readily detects brainstem pathology in vivo in both amyotrophic lateral sclerosis and primary lateral sclerosis.
Keywords: Amyotrophic lateral sclerosis, Primary lateral sclerosis, Frontotemporal dementia, Magnetic resonance imaging, Brainstem, Medulla oblongata, Pons, Mesencephalon
Specifications Table
| Subject | Neuroscience (Neurology) |
| Specific subject area | Neurology |
| Type of data | Raw volumetric data, Box plots, Radar graph, 3D anatomical figures |
| How data were acquired | Imaging data were acquired on a Philips Achieva 3T MRI scanner (Philips Medical Systems, Best, The Netherlands) with an 8-channel head coil. |
| Data format | Raw volumetric brainstem characteristics; medulla oblongata, pons and mesencephalon volume profiles |
| Parameters for data collection | 3D–T1-weighted sequence: spatial resolution: 1 × 1x1mm, Field of view: 256 × 256 × 160 mm, TR/TE = 8.5/3.9 ms, TI = 1060 ms, flip angle = 8°, SENSE factor = 1.5. |
| Description of data collection | The protocol, consent forms, recruitment procedures, and data management were approved by the institutional ethics committee. All participants provided informed consent prior to inclusion. Participating ALS patients were diagnosed according to the El Escorial research criteria, PLS patients were diagnosed according to the Gordon criteria, FTD patients were diagnosed according to the Rascovsky criteria. MRI data were acquired on a 3 T Philips Achieva system with uniform pulse sequence settings and anonymised. |
| Data source location | Institution: Computational neuroimaging group, Trinity Biomedical Sciences Institute, Trinity College Dublin City/Town/Region: Dublin Country: Ireland |
| Data accessibility | Raw brainstem volumes and segmental volumetric profiles have been uploaded to ‘Mendeley Data’ https://doi.org/10.17632/4t8c4bmw5p.3 |
| Related research article | Authors: Peter Bede, Rangariroyashe H. Chipika, Eoin Finegan, Stacey Li Hi Shing, Mark A. Doherty, Jennifer C. Hengeveld, Alice Vajda, Siobhan Hutchinson, Colette Donaghy, Russell L. McLaughlin, Orla Hardiman Title: Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: a longitudinal neuroimaging study Journal: Neuroimage Clinical https://doi.org/10.1016/j.nicl.2019.102054 |
Value of the Data
|
1. Data
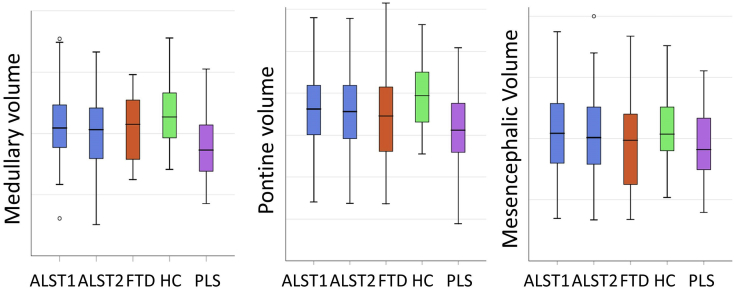
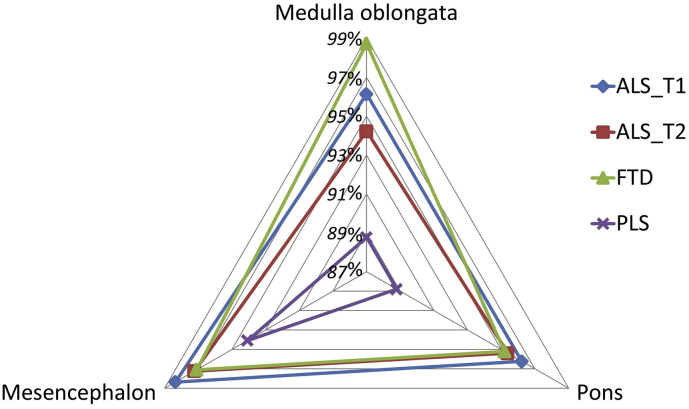
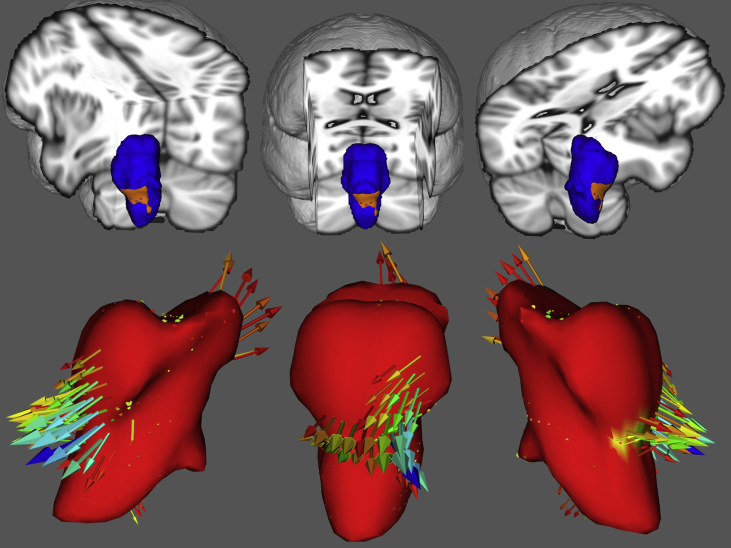
Despite pathognomonic bulbar involvement in motor neuron diseases [[2], [3], [4]], the majority of imaging studies in ALS and PLS focus on cortical and spinal cord signatures [[5], [6], [7], [8], [9]]. In this dataset we present the volumetric brainstem profile of 100 patients with amyotrophic lateral sclerosis (ALS), 33 patients with primary lateral sclerosis (PLS), 30 patients with frontotemporal dementia (FTD), and 100 age-matched healthy controls (HC) [1] (Table 1). Relevant demographic (age, gender, education and handedness) and clinical (ALSFRS-r) characteristics [10,11] are presented in the companion article [1]. Raw volumetric data for each brainstem segment are available online at Mendeley Data; https://doi.org/10.17632/4t8c4bmw5p.3 Longitudinal brainstem changes in ALS [12] are presented in boxplots (Fig. 1). Reference volumetric data are shown for PLS patients [13], disease controls [14,15] and healthy controls [16] to aid the interpretation of ALS-associated brainstem changes. Based on estimated marginal means corrected for age, gender, total intracranial volumes and education, the comparative profile of the study groups are further illustrated in radar plots with reference to healthy controls (Fig. 2). Focal atrophy patterns are presented as three-dimensional vertex projections (Fig. 3).
Table 1.
Data categories and measures.
| Data categories | Specific measures |
|---|---|
| Segmental brainstem volumes | Medulla oblongata volume (mm3) |
| Pons volume (mm3) | |
| Mesencephalon volume (mm3) | |
| Vertex contrast between ALS patients and healthy controls | Vertex locations of individual participants are projected on the surface of an average brainstem template as scalar values. Permutation based non-parametric statistics were used for group comparisons including age, gender and education. Resulting statistical maps are displayed on 3D mesh templates to showcase focal shape deformations. |
ALS = amyotrophic lateral sclerosis; ALSFRS-R = amyotrophic lateral sclerosis functional rating scale-revised; PLS = Primary lateral sclerosis.
Fig. 1.
The comparative volumetric brainstem profile of patients with amyotrophic lateral sclerosis at time-point 1 (ALST1), patients with amyotrophic lateral sclerosis at time-point 2 (ALST2), frontotemporal dementia (FTD), healthy controls (HC) and patients with primary lateral sclerosis (PLS).
Fig. 2.
The segmental brainstem profile of ALS, PLS and FTD with reference to healthy controls. 100% represents the estimated marginal mean of healthy controls for each structure. Estimated marginal means of volumes were calculated with the following values Age = 59.59, Gender = 1.43, Education = 13.63, TIV = 1435355.28.
Fig. 3.
Anatomical patterns of atrophy in ALS compared to healthy controls based on vertex-analyses after corrections for demographic variables. Top: Vertex analyses; brainstem mesh is shown in blue and shape deformations are highlighted in orange at p < 0.05 FWE Bottom: Surface-based vertex analyses; the brainstem mesh template is shown in red.
2. Experimental design, materials, and methods
This protocol was approved by the institutional ethics committee and each participant provided informed consent. Recruitment, anonymisation and data management procedures followed institutional and EU data handling guidelines (GDPR). Patients were recruited from a national motor neuron disease clinic to participate in a standardised imaging protocol [8]. Participating ALS patients were diagnosed according to the El Escorial research criteria, PLS patients were diagnosed according to the Gordon criteria and FTD patients according to the Rascovsky criteria [17]. The study was designed to characterise brainstem degeneration in ALS and PLS with a view to identify distinguishing imaging characteristics for diagnostic classification applications [18,19]. T1-weighted images were acquired with a spatial resolution of 1 × 1 × 1mm and field of view of 256 × 256 × 160 mm using a 3D Inversion Recovery prepared Spoiled Gradient Recalled echo (IR-SPGR) sequence; repetition time (TR) = 8.5 ms, echo time (TE) = 3.9 ms, Inversion time (TI) = 1060 ms, flip angle = 8°, SENSE factor = 1.5. Whole brainstem volumes and total intracranial volumes (TIV) were estimated using FSL-FIRST [20] of the FMRIB's Software Library (FSL) [7,14].
The brainstem of each participant was further segmented into the medulla oblongata, pons, and mesencephalon using a Bayesian parcellation algorithm implemented in version 6.0 of the FreeSurfer image analysis suite. Analyses of covariance (ANCOVA) were used to explore intergroup volumetric differences using age, education, gender and TIV as covariates [11]. To illustrate disease-specific volumetric traits in ALS and PLS, the estimated marginal mean of each segment was plotted on a radar chart with reference to healthy controls.
As volumetric profiling only detects global volume reductions, additional vertex analyses were performed to characterise focal shape deformations in ALS compared to healthy controls. FMRIB's subcortical segmentation and registration tool FIRST was used for shape analyses to map surface-projected atrophy patterns in the brainstem. ALS patients at their second time point exhibited considerable bilateral flattening of the medullary pyramids above the pyramidal decussation (Fig. 3).
Acknowledgments
We thank all the patients, their families and caregivers for participating in this research study. We are also grateful for the participation of the healthy controls. Peter Bede and the computational neuroimaging group is supported by the Health Research Board (HRB – Ireland; HRB EIA-2017-019), the Andrew Lydon scholarship, the Irish Institute of Clinical Neuroscience, the Iris O'Brien Foundation, the Research Motor Neuron (RMN-Ireland) Foundation and the Irish Motor Neurone Disease Association. Russell L McLaughlin is supported by the Motor Neurone Disease Association (957-799) and Science Foundation Ireland (17/CDA/4737). Mark A Doherty is supported by Science Foundation Ireland (15/SPP/3244). The sponsors had no role in the design of the study, data analyses, or the decision to submit these findings for publication.
Conflict of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Peter Bede is the associate editor of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, member of the UK Motor Neuron Disease Association (MNDA) Research Advisory Panel, the steering committee of the Neuroimaging Society in ALS (NiSALS) and the patron of the Irish Motor Neuron Disease Association (IMNDA).
References
- 1.Bede P., Chipika R.H., Finegan E., Li Hi Shing S., Doherty M.A., Hengeveld J.C., Vajda A., Hutchinson S., Donaghy C., McLaughlin R.L., Hardiman O. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: a longitudinal neuroimaging study. NeuroImage Clinical. 2019;24:102054. doi: 10.1016/j.nicl.2019.102054. Epub 2019/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yunusova Y., Plowman E.K., Green J.R., Barnett C., Bede P. Clinical measures of bulbar dysfunction in ALS. Front. Neurol. 2019;10:106. doi: 10.3389/fneur.2019.00106. Epub 2019/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finegan E., Chipika R.H., Li Hi Shing S., Hardiman O., Bede P. Pathological crying and laughing in motor neuron disease: pathobiology, screening, intervention. Front. Neurol. 2019;10:260. doi: 10.3389/fneur.2019.00260. Epub 2019/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bede P., Finegan E. Revisiting the pathoanatomy of pseudobulbar affect: mechanisms beyond corticobulbar dysfunction. Amyotroph Lateral Scler. frontotemporal degeneration. 2018;19:4–6. doi: 10.1080/21678421.2017.1392578. Epub 2017/11/03. [DOI] [PubMed] [Google Scholar]
- 5.Hardiman O., Doherty C.P., Elamin M., Bede P. Springer Cham Heidelberg New York Dordrecht London© Springer International Publishing Switzerland 2016. Springer International Publishing; 2016. Neurodegenerative disorders: a clinical guide. 2016; pp. 1–336. [Google Scholar]
- 6.Lebouteux M.V., Franques J., Guillevin R., Delmont E., Lenglet T., Bede P., Desnuelle C., Pouget J., Pascal-Mousselard H., Pradat P.F. Revisiting the spectrum of lower motor neuron diseases with snake eyes appearance on magnetic resonance imaging. Eur. J. Neurol. 2014;21:1233–1241. doi: 10.1111/ene.12465. Epub 2014/05/23. [DOI] [PubMed] [Google Scholar]
- 7.Bede P., Iyer P.M., Schuster C., Elamin M., McLaughlin R.L., Kenna K., Hardiman O. The selective anatomical vulnerability of ALS: 'disease-defining' and 'disease-defying' brain regions. Amyotroph Lateral Scler. frontotemporal degeneration. 2016;17:561–570. doi: 10.3109/21678421.2016.1173702. Epub 2016/04/19. [DOI] [PubMed] [Google Scholar]
- 8.Schuster C., Elamin M., Hardiman O., Bede P. The segmental diffusivity profile of amyotrophic lateral sclerosis associated white matter degeneration. Eur. J. Neurol. 2016;23:1361–1371. doi: 10.1111/ene.13038. Epub 2016/05/22. [DOI] [PubMed] [Google Scholar]
- 9.Bede P., Querin G., Pradat P.F. The changing landscape of motor neuron disease imaging: the transition from descriptive studies to precision clinical tools. Curr. Opin. Neurol. 2018;31:431–438. doi: 10.1097/WCO.0000000000000569. Epub 2018/05/12. [DOI] [PubMed] [Google Scholar]
- 10.Bede P., Elamin M., Byrne S., Hardiman O. Sexual dimorphism in ALS: exploring gender-specific neuroimaging signatures. Amyotroph Lateral Scler. frontotemporal degeneration. 2014;15:235–243. doi: 10.3109/21678421.2013.865749. Epub 2013/12/19. [DOI] [PubMed] [Google Scholar]
- 11.Schuster C., Hardiman O., Bede P. Survival prediction in Amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurol. 2017;17:73. doi: 10.1186/s12883-017-0854-x. Epub 2017/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bede P., Hardiman O. Longitudinal structural changes in ALS: a three time-point imaging study of white and gray matter degeneration. Amyotroph Lateral Scler. frontotemporal degeneration. 2018;19:232–241. doi: 10.1080/21678421.2017.1407795. Epub 2017/12/08. [DOI] [PubMed] [Google Scholar]
- 13.Finegan E., Chipika R.H., Li Hi Shing S., Doherty M.A., Hengeveld J.C., Vajda A., Donaghy C., McLaughlin R.L., Pender N., Hardiman O., Bede P. The clinical and radiological profile of primary lateral sclerosis: a population-based study. J. Neurol. 2019;266:2718–2733. doi: 10.1007/s00415-019-09473-z. Epub 2019/07/22. [DOI] [PubMed] [Google Scholar]
- 14.Bede P., Omer T., Finegan E., Chipika R.H., Iyer P.M., Doherty M.A., Vajda A., Pender N., McLaughlin R.L., Hutchinson S., Hardiman O. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imag. Behav. 2018;12:1696–1707. doi: 10.1007/s11682-018-9837-9. Epub 2018/02/10. [DOI] [PubMed] [Google Scholar]
- 15.Omer T., Finegan E., Hutchinson S., Doherty M., Vajda A., McLaughlin R.L., Pender N., Hardiman O., Bede P. Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2017:1–13. doi: 10.1080/21678421.2017.1332077. Epub 2017/06/01. [DOI] [PubMed] [Google Scholar]
- 16.Schuster C., Hardiman O., Bede P. Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PloS One. 2016;11 doi: 10.1371/journal.pone.0167331. Epub 2016/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finegan E., Chipika R.H., Shing S.L.H., Hardiman O., Bede P. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler. frontotemporal degeneration. 2019:1–13. doi: 10.1080/21678421.2018.1550518. Epub 2019/01/19. [DOI] [PubMed] [Google Scholar]
- 18.Bede P., Iyer P.M., Finegan E., Omer T., Hardiman O. Virtual brain biopsies in amyotrophic lateral sclerosis: diagnostic classification based on in vivo pathological patterns. NeuroImage Clinical. 2017;15:653–658. doi: 10.1016/j.nicl.2017.06.010. Epub 2017/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querin G., El Mendili M.M., Bede P., Delphine S., Lenglet T., Marchand-Pauvert V., Pradat P.F. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J. Neurol. Neurosurg. Psychiatr. 2018 doi: 10.1136/jnnp-2017-317214. Epub 2018/01/22. [DOI] [PubMed] [Google Scholar]
- 20.Christidi F., Karavasilis E., Rentzos M., Velonakis G., Zouvelou V., Xirou S., Argyropoulos G., Papatriantafyllou I., Pantolewn V., Ferentinos P., Kelekis N., Seimenis I., Evdokimidis I., Bede P. Hippocampal pathology in Amyotrophic Lateral Sclerosis: selective vulnerability of subfields and their associated projections. Neurobiol. Aging. 2019 doi: 10.1016/j.neurobiolaging.2019.07.019. [DOI] [PubMed] [Google Scholar]