Abstract
Background
Progressive peritoneal fibrosis is a common complication in patients on long-term peritoneal dialysis (PD). PD-associated peritonitis is a major exacerbating factor. We investigated the anti-fibrotic properties of decorin secreted by peritoneal mesothelial cells.
Methods
Dialysate decorin level in stable PD patients and those with peritonitis was measured. In vitro experiments were conducted to investigate the effect of decorin in fibrotic response in human peritoneal mesothelial cells (HPMC).
Findings
Increasing PD duration was associated with a progressive decrease of dialysate decorin and CA125 levels. Dialysate decorin level correlated with CA125 level. Peritonitis episodes were associated with a massive drop of dialysate decorin, which persisted for over three months despite clinical recovery. Dialysate decorin level correlated with that of TGF-β1, but was inversely related to IL-1β and IL-8. TGF-β1, IL-1β, IL-6, IL-8, or TNF-α reduced decorin secretion in HPMC, but induced fibronectin expression. The effects were mediated in part through increased p38 MAPK and AKT/PI3K phosphorylation. Decorin abrogated the induction of fibronectin expression in mesothelial cells by PD fluids or pro-fibrotic cytokines, through decreased TGF-βRI, p38 MAPK and AKT/PI3K phosphorylation and increased glycogen synthase kinase-3β phosphorylation. Decorin gene-silencing resulted in increased fibronectin expression under these conditions.
Interpretation
Our data demonstrate anti-fibrotic actions of decorin in HPMC, when these cells are subjected to the pro-fibrotic effect of peritoneal dialysate and pro-fibrotic cytokines in PD, especially during peritonitis.
Keywords: Peritoneal dialysis, Peritonitis, Decorin, Inflammation, Fibrosis, Mesothelial cells
Abbreviations: ELISA, Enzyme-linked immunosorbent assay; ERK, Extracellular signal-regulated kinase; FCS, Fetal calf serum; FN, Fibronectin; GSK-3β, Glycogen synthase kinase-3beta; IL, Interleukin; HPMC, Human peritoneal mesothelial cells; JNK, C-jun n-terminal kinase; MAPK, Mitogen-activated protein kinase; mTOR, Mammalian or mechanistic target of rapamycin; PD, peritoneal dialysis; PDF, peritoneal dialysis fluid; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase, PKC, protein kinase C; RIPA buffer, radioimmunoprecipitation assay buffer; SFM, serum free medium; TGF-β1, transforming growth factor beta1; TGFβRI, TGF-β receptor 1; TGFβRII, TGF-β receptor 2; TNF-α, tumor necrosis factor-alpha
Research in context.
Evidence before this study
Progressive peritoneal fibrosis and loss of the dialytic function affect all patients on long-term PD, and result in increased morbidity and mortality. A history of PD-associated peritonitis is a major risk factor for peritoneal fibrosis and failure to continue PD.
Added value of this study
Decorin is a soluble dermatan sulfate proteoglycan synthesized by peritoneal mesothelial cells. Our results from in vitro experiments show that decorin has anti-fibrotic properties and counters the pro-fibrotic effects of PD fluid, TGF-β1 and IL-1β that patients on long-term PD are exposed to perennially, through downregulation of distinct signaling pathways. Data from PD patients show that dialysate decorin level decreases with increasing duration of PD, especially during episodes of peritonitis. These findings indicate that the anti-fibrotic actions of decorin are of clinical relevance.
Implications of all the available evidence
Presently there is no effective means to prevent or treat peritoneal fibrosis in PD, which is essential in the preservation of structural and functional integrity of the peritoneal membrane. Our data provide evidence on the anti-fibrotic actions of decorin, and suggest a novel approach to tackle this unmet clinical need.
Alt-text: Unlabelled box
1. Introduction
More than one-tenth of the global dialysis population (i.e. over 200,000 patients) are treated with peritoneal dialysis (PD) [1]. Effective PD depends on the structural and functional integrity of the peritoneal membrane, yet progressive peritoneal fibrosis is a common complication in long-term PD, and this leads to inadequate dialysis and encapsulating peritoneal sclerosis which could be life-threatening. PD-associated peritonitis is an important exacerbating factor for peritoneal fibrosis [1], [2], [3].
Mesothelial cells lining the peritoneal cavity constitute the first line of defense against injury from unphysiological PD fluids and micro-organisms [4,5]. In contrast to low-grade inflammation induced by PD fluids, infective peritonitis triggers severe inflammatory responses in the peritoneum associated with inflammatory cell infiltration and local production of pro-inflammatory and pro-fibrotic cytokines and growth factors, and the secretion of proteases from infiltrating and resident peritoneal cells [6], [7], [8], [9], [10], [11]. These processes result in severe mesothelial cell injury and denudation, and peritoneal fibrosis occurs instead of normal wound healing.
Decorin is a 110 kDa, soluble dermatan sulfate proteoglycan that can bind and sequester TGF-β1, resulting in reduced biological activity of the latter [12,13]. Decorin is the predominant proteoglycan secreted by human peritoneal mesothelial cells (HPMC) in culture [14]. It has been reported that patients on long-term PD showed a reduction of peritoneal membrane decorin expression [15]. Data from a rodent PD model showed that transient overexpression of decorin was associated with reduced peritoneal collagen deposition [16]. While these observations suggest that decorin produced by mesothelial cells could have anti-fibrotic actions, there is little data on dialysate decorin level in patients on long-term PD. Also, the mechanisms through which decorin might modulate pro-fibrotic responses in peritoneal mesothelial cells upon injury or exposure to pro-fibrotic cytokines are obscure.
The aim of this study was to investigate the level of decorin in the effluent peritoneal dialysate of stable patients on long-term PD and to examine the impact of bacterial peritonitis. In vitro experiments were conducted to investigate the mechanisms of pro-fibrotic cellular responses in HPMC and the effect of decorin. Our results showed that decorin suppressed fibronectin deposition when HPMC were exposed to PD fluid, TGF-β1, or IL-1β, and this suppressive effect was mediated through down-regulation of TGF-β receptor I (TGF-βRI), p38 MAPK and AKT/PI3K phosphorylation, and increased glycogen synthase kinase-3β (GSK-3β) phosphorylation. Dialysate decorin level decreased with increasing duration of PD, and exposure of HPMC to high levels of pro-inflammatory cytokines, such as occurring during PD-associated peritonitis, resulted in marked reduction of decorin secretion.
2. Materials and methods
2.1. Chemicals and reagents
All chemicals and antibodies were of the highest purity and were purchased from Sigma Aldrich (Tin Hang Technology Ltd, Hong Kong) unless otherwise stated. Tissue culture flasks were purchased from Falcon (Becton-Dickinson, Gene Company Limited, Hong Kong) and Medium 199, supplements and Stealth RNAi™ Pre-designed decorin specific siRNA (assay ID: HSS175999) were purchased from Life Technologies (ThermoFisher Scientific (HK) Limited, Hong Kong). Taqman probes for human fibronectin (Hs01549976_m1) and decorin (Hs00754870_s1) were purchased from ThermoFisher Scientific (HK) Limited, Hong Kong. RNeasy mini kits and AllStars Negative Control siRNA complexed with HiPerfect transfection reagent were purchased from Qiagen Hong Kong Pte Limited, Hong Kong. Human recombinant decorin, TGF-β1, IL-1β, IL-6, IL-8, and TNF-α, and Duoset ELISAs for decorin and TGF-β1 were purchased from R&D Systems (Bio-Techne Hong Kong Limited). BD OptEIA ELISA kits for IL-1β, IL-6, IL-8 and TNF-α were purchased from BD Biosciences Pharmingen (Bio-Gene Technology Limited, Hong Kong). PD98059, SP600125 and LY294002 and antibodies to phosphorylated and total ERK, p38 MAPK, JNK, AKT/PI3K, mTOR, GSK-3β (Ser9), SMAD2 and SMAD3 were purchased from Cell Signaling Technology (Genetimes Technology International Holding Limited, Hong Kong). Antibodies to phosphorylated and total PKC-α were purchased from Santa Cruz, (Genetimes Technology International Holding Limited, Hong Kong). SB203580 and Gö6976 were purchased from Calbiochem, Merck (Onwon Trading Limited, Hong Kong), and rapamycin was purchased from Sigma-Aldrich. Anti-human TGF-βRI antibody was purchased from Abcam (Hong Kong) Limited. Antibodies to human decorin and TGF-βRII were purchased from Invitrogen (ThermoFisher Scientific (HK) Limited, Hong Kong). DC Protein Assay kits were purchased from Bio-Rad Pacific Limited, Hong Kong.
2.2. PD fluid samples
Overnight PD fluid (PDF) was prospectively collected from Chinese adult patients on continuous ambulatory PD (CAPD) attending the PD Units at Queen Mary Hospital and Tung Wah Hospital, Hong Kong during the period of November 2012 to December 2013. Single overnight PDF were obtained from stable PD patients who were free from peritonitis over the past 6 months or longer (Control PDF). Serial PDF were also obtained from PD patients who developed bacterial peritonitis at the onset of infection before antibiotic treatment (Peritonitis D0 PDF) and after 3 months (Peritonitis D100 PDF). All patients were on glucose-based PD regimen (Dianeal®). Aliquots of PDF (2 × 50 ml) were centrifuged at 3000 rpm at 4°C for 10 min to remove cell debris, filter-sterilized and stored at −80°C until analysis. This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. All subjects gave written informed consent before participation in this study.
2.3. Measurement of decorin, TGF-β1, IL-1β, IL-6, IL-8 and TNF-α levels in PDF
The levels of decorin, TGF-β1, IL-1β, IL-6, IL-8 and TNF-α were measured in spent dialysate, culture media and cell lysates using commercially available ELISAs according to the manufacturers’ instructions. The detection range was 31.2–2000 pg/ml for decorin and total TGF-β1, 4–250 pg/ml for IL-1β, 4–300 pg/ml for IL-6, 3–200 pg/ml for IL-8 and 8–500 pg/ml for TNF-α. Decorin level in culture media and cell lysates was normalized to cellular protein and expressed as pg/μg cellular protein for direct comparison between the different treatment groups.
2.4. Mesothelial cell culture
In vitro studies were conducted in primary HPMC obtained by enzymatic digestion of omental specimens from nonuremic patients who underwent abdominal surgery, and cultured in Medium 199 supplemented with 10% FCS as previously described [17,18]. All experiments were performed on cells of the second passage that had been growth arrested for 72 h. To determine the effect of PDF and pro-inflammatory and pro-fibrotic cytokines on mesothelial cell decorin and fibronectin expression and the beneficial effect or otherwise of decorin, HPMC were incubated with serum free medium (SFM), Control PDF, Peritonitis D0 PDF, TGF-β1, IL-1β, IL-6, IL-8, TNF-α, or TGF-β1 together with IL-1β (0.5 - 10 ng/ml for TGF-β1, 10 ng/ml for all other cytokines) in the presence or absence of decorin (1 µg/ml) for 24 h. In separate studies, HPMC were incubated with TGF-β1 neutralizing antibody or IL-1 receptor antagonist (1 µg/ml, for both) for 1 h prior to incubation with Peritonitis D0 PDF for 24 h to investigate the contribution of TGF-β1 and IL-1β in PDF-induced fibronectin expression. To determine the signaling pathways involved in decorin secretion and fibronectin synthesis, HPMC were incubated with inhibitors to ERK (PD98059, 75 μM), p38 MAPK (SB203580, 50 μM), JNK (SP600125, 50 μM), PKC (Gö6976, 20 μM), PI3K (LY294002, 50 μM) and mTOR (rapamycin, 20 ng/ml) for 1 h before incubation with SFM, Control PDF, Peritonitis PDF, TGF-β1 or IL-1β (10 ng/ml, for both cytokines) for 24 h. To determine whether spent dialysate, TGF-β1 or IL-1β could activate ERK, p38 MAPK, JNK, AKT/PI3K, GSK-3β, SMAD2 or SMAD3 signaling and the effect of decorin, HPMC were incubated with SFM, Control PDF, Peritonitis D0 PDF, TGF-β1 and IL-1β in the presence or absence of decorin (1 μg/ml) for selective time points up to 6 h. To determine the effect of decorin on TGF-βRI and TGF-βRII expression, HPMC were stimulated with TGF-β1 or IL-1β (10 ng/ml for both cytokines) in the presence or absence of decorin (0.1–1 µg/ml) for 24 h.
Met-5A cells (ATCC® CRL-9444™) are normal human mesothelial cells immortalized by transduction with the pRSV-T plasmid, and are often used in studies related to PD. Data obtained from Met-5A cells showed concordance with that from HPMC [10,19]. Decorin gene silencing was performed in Met-5A cells using Stealth RNAi followed by incubation with SFM, Control PDF, Peritonitis D0 PDF, TGF-β1 or IL-1β (10 ng/ml, for both cytokines) for 24 h to assess the effect of decorin deficiency on fibronectin expression.
Following stimulation, the culture media were decanted, cells washed with PBS and total mRNA extracted for real-time PCR, or cells lysed with 25 mM Tris–HCl, pH 7.0 containing 4 M urea and 1% Triton X-100 (200 μl) to determine decorin, fibronectin, TGF-βRI or TGFβRII protein expression by Western blot. To investigate phosphorylation of signaling pathways, cells were lysed with RIPA buffer. A cocktail of proteinase inhibitors was added to all culture media and cell lysates [14,20].
2.5. Decorin knockdown using stealth RNAi
Met-5A cells were seeded into 60 mm2 dishes in the presence or absence of Stealth RNAi™ Pre-designed decorin specific siRNA (20 nM) or AllStars Negative Control siRNA complexed with HiPerfect transfection reagent. After overnight incubation, the RNAi transfection reagents were removed and cells cultured in Medium 199 supplemented with 10% FCS for 72 h. Cells were growth arrested for 72 h and incubated with SFM, Control PDF, Peritonitis D0 PDF, TGF-β1 or IL-1β for 24 h. Decorin transfection efficiency was determined by real-time PCR and decorin ELISA.
2.6. Gene expression
To determine the effect of decorin gene silencing, PDF, TGF-β1 and IL-1β in the presence or absence of decorin on decorin and/or fibronectin gene expression, total mRNA was extracted from HPMC using RNeasy mini kits according to the manufacturer's instructions. Two micrograms of total RNA was reverse-transcribed into cDNA with M-MLV reverse transcriptase using the random hexamer method [20] and decorin and fibronectin transcripts assessed by quantitative real-time PCR using Taqman gene expression assay on a Lightcycler 480 II real time PCR system (Roche Diagnostics, DKSH Hong Kong Limited, Hong Kong). All samples were analyzed in triplicate, and decorin and fibronectin mRNA expression calculated using the delta delta Ct (2−ΔΔCt) method, normalized to GAPDH.
2.7. Western blot analysis
Cell lysates or culture media (20 μg total protein content) from control and stimulated cells were made up to 20 μl with water, mixed with 5X Laemmli buffer (5 μ1), and samples boiled for 5 min at 100°C. Samples were electrophoresed under denaturing conditions on 8% polyacrylamide gels to determine fibronectin expression, and on 10% polyacrylamide gels to determine β-actin, decorin, TGF-βRI, TGF-βRII and phosphorylated and total SMAD2, SMAD3, ERK, p38 MAPK, JNK, PKC-α, GSK-3β, AKT and mTOR expression. Samples were transferred onto nitrocellulose membranes by electroblotting, and membranes probed with primary antibodies to the aforementioned proteins followed by the relevant secondary antibodies [20,21]. Bands were visualized with ECL, semi-quantitated by densitometry using ImageJ (US National Institute of Health, Bethesda, Maryland, USA) and expressed as arbitrary densitometric units (DU). Expression of fibronectin, decorin, TGF-βRI and TGF-βRII was normalized to β-actin, and expression of phosphorylated SMAD2, SMAD3, ERK, p38 MAPK, JNK, PKC-α, GSK-3β, AKT/PI3K and mTOR normalized to total SMAD2, SMAD3, ERK, p38 MAPK, JNK, PKC-α, GSK-3β, AKT/PI3K and mTOR respectively [20,22].
2.8. Immunohistochemistry
Confluent, growth arrested HPMC cultured on 13 mm diameter glass coverslips were incubated with Control PDF, Peritonitis D0 PDF, TGF-β1 or IL-1β in the presence or absence of decorin for 24 h, after which time, cells were fixed in cold acetone:methanol (1:1 ratio) for 5 min. HPMC were washed thrice with PBS between each step and all incubations were for 1 h at 37°C. Cells were blocked with 3% BSA in PBS and incubated with mouse anti-human fibronectin antibody in a darkened, humidified chamber. Cells were then incubated with anti-mouse secondary antibody conjugated to FITC, mounted in fluorescent mountant, and epifluorescence viewed using a Nikon 80i upright fluorescent microscope and Spot RT3 slider digital camera system (Chintek Scientific (China) Ltd, Hong Kong).
2.9. Statistical analysis
All in vitro experiments were repeated at least three times and results expressed as mean ± SD unless otherwise stated. Statistical analysis was performed using Prism 6.07 for Windows (GraphPad Software, Inc., California, USA). Dialysate decorin and CA125 levels were expressed as mean ± SEM. Normal distribution was assessed using the D'Agostino-Pearson normality test. Mann-Whitney U test was used in the comparison of nonparametric unpaired data, and Wilcoxon signed-ranked test for nonparametric paired data. For intra-group and inter-group comparisons with three groups or more, one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison post-test was used for normally distributed data or Kruskal–Wallis test followed by Dunn's multiple comparison post-test for nonparametric data. Correlation between dialysate levels of decorin and CA125 and mediators of inflammation and fibrosis was examined using the Pearson's correlation coefficient for normally distributed variables and Spearman's correlation coefficient for data that were not normally distributed. Categorical variables between patients with or without peritonitis were compared using Fisher's Exact Test. Two-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. Dialysate decorin and CA125 levels
Overnight PDF was collected from 72 stable PD patients free from peritonitis for 6 months or longer (Control PDF), and from 31 PD patients with peritonitis (Gram-positive bacteria in 13 episodes, Gram-negative bacteria in 13 episodes, culture-negative in 5 episodes) at onset (Peritonitis D0 PDF) and 100 days later (Peritonitis D100 PDF) (Table 1). All patients were on Dianeal® PDF. Dialysate decorin and CA125 levels were 12.88 ± 0.48 ng/ml and 19.28 ± 1.41 U/ml respectively in stable PD patients. Both dialysate decorin and CA125 levels showed an inverse relationship with the time on PD, and both were significantly lower compared with baseline after two years of PD (P < 0.05 for decorin, P < 0.01 for CA125) (Fig. 1a & b). Dialysate decorin level showed a positive correlation with dialysate CA125 level (Fig. 1c).
Table 1.
Characteristics of PD patients.
| Stable PD patients (n = 72) | PD patients with peritonitis (n = 31) | P value | |
|---|---|---|---|
| Gender (male: female): | 36: 36 | 13: 18 | 0.522c |
| aAge (years): | 61.82 ± 11.59 | 67.68 ± 10.96 | 0.052d |
| bTime on PD (months): | 40.13 (22.08, 168.6) | 33.60 (11.50, 293.4) | 0.911d |
| Underlying kidney disease: | |||
| Diabetic nephropathy | 15 (20.8%) | 9 (29.0%) | 0.447c |
| Hypertension | 5 (6.9%) | 2 (6.5%) | 1.000c |
| Glomerulonephritis | 27 (37.5%) | 12 (38.7%) | 1.000c |
| Polycystic kidney disease | 3 (4.2%) | 1 (3.2%) | 1.000c |
| Unknown | 22 (30.6%) | 7 (22.6%) | 0.480c |
| Frequency of peritonitis: | 1 episode per 42.2 patient-months | 1 episode per 23.9 patient-months | 0.005d |
Results presented as mean ± SD.
Time on PD expressed as median (25% and 75% percentile).
Inter-group comparison using Fisher's Exact Test.
Inter-group comparison using Mann–Whitney U test.
Fig. 1.
Decorin and CA125 levels in overnight dialysate effluent from PD patients. Scatterplots showing dialysate (a) decorin and (b) CA125 levels in stable PD patients free from peritonitis grouped according to their duration on PD. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test for dialysate decorin level and Kruskal–Wallis test followed by Dunn's multiple comparison post-test for dialysate CA125 level. (c) Dialysate decorin level correlated with dialysate CA125 level. Data analyzed using the Spearman's correlation coefficient method. (d) Dialysate decorin level in stable PD patients (Control PDF) and PD patients at the onset of peritonitis (Peritonitis D0 PDF). Data analyzed using Mann–Whitney U test. (e) Dialysate decorin level in peritonitis PD patients at the onset of peritonitis (Peritonitis D0 PDF) and 100 days later (Peritonitis D100 PDF). Data analyzed using Wilcoxon signed-ranked test. Each data point represents an individual patient. Horizontal line represents mean value for each group.
The level of dialysate decorin at the onset of peritonitis was 37.8% of that in stable PD patients matched for the duration of PD (4.87 ± 0.90 for Peritonitis D0 vs 12.88 ± 0.48 ng/ml, P < 0.0001) (Fig. 1d). Following clinical resolution of peritonitis after antibiotic treatment, dialysate decorin level increased (Fig. 1e), but still appeared low compared with stable patients without peritonitis (7.73 ± 1.06 for Peritonitis D100 PDF vs 12.88 ± 0.48 ng/ml, P < 0.0002). There was no significant difference in dialysate decorin level between Gram-positive, Gram-negative, and culture-negative peritonitis (6.46 ± 2.47, 5.18 ± 1.35, and 5.31 ± 2.57 ng/ml respectively; P = 0.81, Gram-positive vs Gram-negative; P = 0.92, Gram-positive vs culture-negative; P = 0.96, Gram-negative vs culture-negative).
3.2. Dialysate TGF-β1, IL-1β, IL-6, IL-8 and TNF-α levels
Dialysate TGF-β1, IL-1β, IL-6, IL-8 and TNF-α levels were significantly increased at the onset of peritonitis compared to Control PDF obtained from stable patients (Supplementary Fig. 1). At the onset of peritonitis, dialysate decorin level correlated with that of TGF-β1 (r = 0.49, P = 0.006), and inversely correlated with the levels of IL-1β (r = −0.35, P < 0.05) and IL-8 (r = −0.40, P = 0.027). There was no relationship between the levels of decorin, IL-6 or TNF-α (data not shown).
3.3. Secreted and cell-associated decorin expression in mesothelial cells
Exogenous TGF-β1, IL-1β and TNF-α, but not IL-6 or IL-8 decreased decorin gene expression in cultured HPMC (Fig. 2a). TGF-β1 decreased decorin transcript in a dose-dependent manner and the reduction was more pronounced when HPMC were incubated with TGF-β1 (10 ng/ml) together with IL-1β (10 ng/ml) than with TGF-β1 or IL-1β alone (Fig. 2a). Incubation of cultured HPMC with exogenous TGF-β1, IL-1β, IL-6, IL-8 or TNF-α for 24 h decreased decorin secretion by 36.4%, 45.9%, 27.9%, 32.3%, and 46.2% respectively compared to cells incubated with SFM (P < 0.05, for IL-6 and IL-8 vs SFM; P < 0.01, for TGF-β1, IL-1β and TNF-α vs SFM) (Fig. 2b). Co-incubation of HPMC with TGF-β1 and IL-1β further reduced decorin secretion when compared to control cells or HPMC incubated with TGF-β1 or IL-1β alone (62.3% reduction compared to SFM, P < 0.001, for TGF-β1 + IL-1β vs SFM, P < 0.05, for TGF-β1 vs TGF-β1 + IL-1β, P=NS, for IL-1β vs TGF-β1 + IL-1β) (Fig. 2b). Secreted decorin accounted for ~98% of the total decorin synthesized by HPMC, whether under basal conditions or in the presence of TGF-β1 or IL-1β, and comprised solely the intact 110 kDa proteoglycan (Fig. 2c). Pre-incubation of mesothelial cells with SB203580 and LY294002, but not PD98059, SP600125, Gö6976 or rapamycin significantly decreased decorin secretion by 43.0% and 54.6% respectively (P < 0.05, for both) (Fig. 2d), suggesting that under basal conditions decorin secretion in mesothelial cells was regulated by p38 MAPK and PI3K pathways. Decorin production in HPMC stimulated with exogenous TGF-β1 or IL-1β was also regulated through p38 MAPK and PI3K pathways (Fig. 2d).
Fig. 2.
Effect of exogenous TGF-β1, IL-1β, IL-6, IL-8 and TNF-α on decorin gene expression and secretion in mesothelial cells. (a) Real-time PCR showing decorin gene expression in cultured mesothelial cells incubated with SFM, or exogenous TGF-β1 (0.5, 1 and 10 ng/ml), IL-1β, IL-6, IL-8, TNF-α (10 ng/ml, final concentration for all pro-inflammatory cytokines) or TGF-β1 (10 ng/ml) together with IL-1β (10 ng/ml) for 24 h. (b) Confluent, growth arrested mesothelial cells were incubated as described in panel (a), the culture medium was decanted, centrifuged to remove any cellular debris and decorin secretion determined by ELISA, normalized to cellular protein. (c) Representative Western blots showing the MW of decorin secreted by mesothelial cells following incubation with SFM or exogenous TGF-β1, IL-1β, or TGF-β1 + IL-1β (10 ng/ml, final concentration for all) for 24 h. (d) In a separate study, confluent, growth arrested mesothelial cells were pre-incubated with or without inhibitors to ERK (PD98059, 75 μM), p38 MAPK (SB203580, 50 μM), JNK (SP600125, 50 μM), PKC (Gö6976, 20 μM), PI3K (LY294002, 50 μM) and mTOR (rapamycin, 20 ng/ml) for 1 h at 37 °C and then incubated with SFM (upper panel), TGF-β1 (middle panel) or IL-1β (lower panel) (10 ng/ml, final concentration for TGF-β1 and IL-1β) for a further 24 h. The supernatant was decanted, centrifuged and decorin secretion measured by ELISA. Data are expressed as mean ± SD of 3 separate experiments. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. *P<0.05, **P<0.01, ***P<0.001, compared to SFM.
Cell-associated decorin accounted for ~2% of the total decorin synthesized by HPMC (Fig. 3a) and comprised both the intact proteoglycan and also the decorin core protein (Fig. 3b-e). TGF-β1 and IL-1β both decreased cell-associated decorin (P < 0.001, for all compared to SFM), with the latter showing a more pronounced effect (Fig. 3b & d).
Fig. 3.
Effect of exogenous TGF-β1 and IL-1β, either alone or in combination, on cell-associated decorin expression in mesothelial cells. (a) Confluent, growth arrested mesothelial cells were incubated with SFM or exogenous TGF-β1, IL-1β, or TGF-β1 + IL-1β (10 ng/ml, final concentration for all) for 24 h. Cells were lysed with 4 M urea buffer and decorin level determined by ELISA, normalized to cellular protein. (b) Representative Western blots showing the effect of TGF-β1, IL-1β, or TGF-β1 + IL-1β on cell-associated decorin expression. (c) The intensity of detected bands for intact decorin proteoglycan (PG) ( ), decorin intermediate (
), decorin intermediate ( ) and decorin core protein (
) and decorin core protein ( ) was analyzed by densitometric scanning, normalized to β-actin, and expressed as a percentage of total cell-associated decorin expression in control cells. The intensity of detected bands for (d) intact decorin proteoglycan and (e) decorin core protein, normalized to β-actin and expressed as arbitrary densitometric units (DU) are also shown. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. **P<0.01, ***P<0.001, SFM vs cytokine.
) was analyzed by densitometric scanning, normalized to β-actin, and expressed as a percentage of total cell-associated decorin expression in control cells. The intensity of detected bands for (d) intact decorin proteoglycan and (e) decorin core protein, normalized to β-actin and expressed as arbitrary densitometric units (DU) are also shown. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. **P<0.01, ***P<0.001, SFM vs cytokine.
3.4. Mesothelial cell expression of fibronectin
Fibronectin accumulation is an early event in tissue fibrosis, and fibronectin serves as a scaffold for the deposition of other matrix proteins [23]. Fibronectin gene and protein expression in HPMC significantly increased after incubation with Control PDF and Peritonitis D0 PDF, especially the latter (P < 0.001 for both) (Fig. 4a & b). Almost all of the fibronectin synthesized by HPMC, with or without exposure to dialysate, was deposited in the extracellular matrix (Fig. 4c). Constitutive fibronectin expression by HPMC was mediated through ERK, p38 MAPK, JNK, and PI3K signaling pathways, since PD98059, SB203580, SP600125 and LY294002, but not Gö6976 or rapamycin, abrogated fibronectin expression. Increased fibronectin expression in HPMC by Control PDF and Peritonitis D0 PDF was mediated through ERK, p38 MAPK, JNK, PKC, PI3K and mTOR signaling pathways (Fig. 4d). Fibronectin expression by HPMC that was stimulated by Control PDF and Peritonitis D0 PDF was reduced by decorin, at transcription and also translation (Fig. 4a–c). Decorin gene-silencing reduced basal decorin gene expression in mesothelial cells by 79.7% and 80.1% compared with cells incubated with SFM or negative RNAi control respectively (P < 0.01 for both) (Fig. 4e), and this was associated with a decrease in decorin secretion by 86.2% and 84.7% respectively (P < 0.01 for both). Decorin gene-silencing significantly increased fibronectin expression in HPMC exposed to SFM and also in cells exposed to Peritonitis D0 PDF (Fig. 4f).
Fig. 4.
Effect of dialysate in the presence or absence of decorin, on fibronectin expression in mesothelial cells. (a) Real-time PCR showing fibronectin (FN) gene expression in cultured mesothelial cells incubated with SFM, Control PDF or Peritonitis D0 PDF with or without decorin (1 μg/ml) for 24 h. ***P<0.001, SFM vs dialysate. (b) Representative Western blots showing FN expression in cultured mesothelial cells incubated with SFM, Control PDF or Peritonitis D0 PDF with or without decorin (1 μg/ml) for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). ***P<0.001, SFM vs dialysate. (c) Representative images showing FN deposition in mesothelial cells incubated with SFM, Control PDF and Peritonitis D0 PDF with or without decorin (1 μg/ml). (d) Representative Western blots showing the effect of ERK, p38 MAPK, JNK, PKC, PI3K and mTOR inhibition on FN expression in cultured mesothelial cells incubated with SFM, Control PDF or Peritonitis D0 PDF for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). *P<0.05, with vs without inhibitor for the same stimulation. (e) Real-time PCR showing decorin gene expression in cultured Met-5A cells, with or without decorin gene silencing, incubated with SFM, Control PDF or Peritonitis D0 PDF for 24 h. **P<0.01, SFM vs dialysate. (f) Representative Western blots showing the effect of SFM, Control PDF or Peritonitis D0 PDF on FN expression in cultured Met-5A cells with or without decorin gene silencing (upper panel). The intensity of detected bands for FN was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). ***P<0.001, SFM vs dialysate. Data presented as mean ± SD of three individual experiments. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test.
Induction of HPMC fibronectin expression by Peritonitis D0 PDF was mediated in part through TGF-β1 and IL-1β, since this was partially prevented by prior incubation of cells with TGF-β1 neutralizing antibody or IL-1 receptor antagonist, by 36.3% and 62.9% respectively (Fig. 5a). TGF-β1 at 10 ng/ml increased fibronectin expression to a level similar to that of Peritonitis D0 PDF (5.77-fold and 5.60-fold increase respectively compared to SFM) (Figs. 5b and 4d respectively). Exposure of cells to exogenous TGF-β1 or IL-1β significantly increased fibronectin gene and protein expression (Fig. 5c–e), and this effect was mediated through ERK, p38 MAPK, JNK, PKC, PI3K and mTOR signaling pathways, and was attenuated by PD98059, SB203580, SP600125, Gö6976, LY294002 and rapamycin respectively (Fig. 5f). Co-incubation of HPMC with both TGF-β1 and IL-1β resulted in a synergistic increase in fibronectin expression (Supplementary Figure 2a & b). Decorin significantly reduced the increased fibronectin expression in cells exposed to TGF-β1 or IL-1β (Fig. 5c–e).
Fig. 5.
Effect of exogenous TGF-β1 and IL-1β in the presence or absence of decorin, on fibronectin expression in mesothelial cells. (a) Representative Western blots showing fibronectin (FN) expression in cultured mesothelial cells incubated with SFM or Peritonitis D0 PDF in the presence or absence of TGF-β1 neutralizing antibody (TGF-β1 NA) or IL-1 receptor antagonist (IL-1 RA) for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). ***P<0.001, SFM vs dialysate. (b) Representative Western blots showing the effect of TGF-β1 (0.5, 1 and 10 ng/ml) on FN expression in mesothelial cells after 24 h incubation (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). *P<0.05, ***P<0.001, SFM vs TGF-β1. (c) Quantitative real-time PCR showing FN gene expression in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β (10 ng/ml, for both) with or without decorin (1 μg/ml) for 24 h. ***P<0.001, SFM vs TGF-β1 or IL-1β. (d) Representative Western blots showing FN expression in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β with or without decorin (1 μg/ml) for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). *P<0.05, ***P<0.001, SFM vs TGF-β1 or IL-1β. (e) Representative images showing FN deposition in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β with or without decorin (1 μg/ml). (f) Representative Western blots showing the effect of ERK, p38 MAPK, JNK, PKC, PI3K and mTOR inhibition on FN expression in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). **P<0.01, with vs without inhibitor for the same stimulation. Data presented as mean ± SD of three individual experiments. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test.
Gene-silencing with decorin-specific siRNA was associated with 79.0% and 88.5% reduction of decorin transcript and secretion respectively in mesothelial cells incubated with SFM (Fig. 6a & b). Decorin gene-silencing resulted in 85.3% and 90.3% reduction in decorin mRNA expression and secretion respectively in cells incubated with TGF-β1, and 80.6% and 87.9% reduction respectively in cells incubated with IL-1β (P < 0.05, for decorin mRNA expression, P < 0.01, for decorin secretion, following gene-silencing) (Fig. 6a & b). A decrease in decorin secretion was accompanied by a significant increase in fibronectin expression in cells incubated with SFM or TGF-β1 (Fig. 6c).
Fig. 6.
Effect of decorin gene silencing on decorin mRNA, secretion and fibronectin expression in mesothelial cells stimulated with TGF-β1 and IL-1β. (a) Real-time PCR showing decorin gene expression in cultured Met-5A cells, with or without decorin gene-silencing, incubated with SFM, TGF-β1 or IL-1β for 24 h. (b) Decorin secretion in Met-5A cells, with or without decorin gene-silencing, incubated with SFM, TGF-β1 or IL-1β for 24 h. (c) Representative Western blots showing FN expression in cultured Met-5A cells with or without decorin gene silencing, incubated with SFM, TGF-β1 or IL-1β for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). All data presented as mean ± SD of three individual experiments. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. *P<0.05, **P<0.01, SFM vs TGF-β1 or IL-1β.
3.5. TGF-βRI and TGF-βRII expression and signaling pathways in peritoneal mesothelial cells
Incubation of cultured HPMC with exogenous TGF-β1 or IL-1β for 24 h reduced TGF-βRI gene expression by 38.0% and 42.3% respectively compared with cells incubated with SFM (P < 0.01, for both) (Fig. 7a). TGF-β1 and IL-1β also reduced TGF-βRII gene expression by 36.0% and 57.0% respectively (P < 0.01, for both when compared to SFM) (Fig. 7b). TGF-βRI protein expression was predominantly in the form of a heterotetrameric complex (MW ~250 kDa), and to a lesser extent as a dimer (MW ~100 kDa), and HPMC did not constitutively express TGF-βRI in the monomeric form (MW 56 kDa) (Fig. 7c). Exogenous TGF-β1 had no effect on TGF-βRI expression while IL-1β significantly decreased TGF-βRI heterotetramer expression (P < 0.05). Decorin decreased TGF-βRI heterotetramer expression in HPMC incubated with SFM or TGF-β1, but had no effect on TGF-βRI expression in cells stimulated with IL-1β (Fig. 7c). In these experiments the expression of TGF-βRI in the dimeric form remained stable, irrespective of whether decorin was added (Fig. 7c).
Fig. 7.
Effect of exogenous TGF-β1 and IL-1β on TGF-β receptor I and II expression in mesothelial cells. Quantitative real-time PCR showing (a) TGF-βRI and (b) TGF-βRII gene expression in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β (10 ng/ml, for both) for 24 h. **P<0.01, SFM vs TGF-β1 or IL-1β. (c) Representative Western blots showing TGF-βRI expression in cultured mesothelial cells incubated with SFM, TGF-β1 or IL-1β in the presence or absence of decorin (0.1, 0.5 and 1 μg/ml) for 24 h (upper panel). The intensity of detected bands was analyzed by densitometric scanning, normalized to β-actin and expressed as arbitrary densitometric units (DU) (lower panel). The MW of TGF-βRI monomer, dimer and heterotetramer are 56, 112 and ~250 kDa respectively. *P<0.05, with or without decorin for the same stimulation. Data analyzed using ANOVA with Bonferroni's multiple comparison post-test.
Exposure of HPMC to effluent dialysate, especially Peritonitis D0 PDF, resulted in prolonged induction of p38 MAPK phosphorylation for over 1 h, compared with peak induction at 10 min in cells incubated with SFM (Fig. 8a). Exposure of cells to IL-1β was also associated with sustained induction of p38 MAPK phosphorylation (Fig. 8b). Adding decorin did not affect p38 MAPK phosphorylation in cells exposed to SFM, but significantly reduced p38 MAPK phosphorylation in cells stimulated with Control PDF, Peritonitis PDF, or exogenous IL-1β (Fig. 8a & b).
Fig. 8.
Effect of PDF and exogenous TGF-β1 or IL-1β, in the presence or absence of decorin, on p38 MAPK phosphorylation in mesothelial cells. Representative Western blots showing the effect of (a) SFM, Control PDF and Peritonitis D0 PDF and (b) SFM, TGF-β1 and IL-1β (10 ng/ml, final concentration for both) in the presence or absence of decorin (1 μg/ml) on p38 MAPK phosphorylation in cultured mesothelial cells (upper panels). The intensity of phospho-p38 MAPK bands was analyzed by densitometric scanning, normalized to total p38 MAPK and expressed as arbitrary densitometric units (DU). Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. *P<0.05, SFM vs Control PDF, Peritonitis D0 PDF, TGF-β1 or IL-1β for the same time-point.
In cells incubated with SFM, AKT phosphorylation peaked at 10–20 min then declined to negligible levels. Exposure of cells to Control PDF induced transient AKT phosphorylation for around 30 min, while Peritonitis PDF induced prolonged AKT activation for over 60 min (Fig. 9a). Decorin significantly reduced AKT activation in cells stimulated with Control PDF or Peritonitis PDF (Fig. 9a). TGF-β1 and IL-1β had no effect on PI3K phosphorylation (data not shown).
Fig. 9.
Effect of PDF in the presence or absence of decorin, on AKT and GSK-3β phosphorylation in mesothelial cells. Representative Western blots showing the effect of SFM, Control PDF and Peritonitis D0 PDF in the presence or absence of decorin (1 μg/ml) on (a) AKT and (b) GSK-3β phosphorylation in cultured mesothelial cells (upper panels). The intensity of bands for AKT and GSK-3β phosphorylation was analyzed by densitometric scanning, normalized to total AKT and GSK-3β respectively and expressed as arbitrary densitometric units (DU). Data analyzed using ANOVA with Bonferroni's multiple comparison post-test. *P<0.05, SFM vs Control PDF or Peritonitis D0 PDF for the same time-point.
In cells incubated with SFM, GSK-3β phosphorylation at serine 9 residue peaked at 10–30 min then declined to low levels. Exposure of cells to Control PDF or Peritonitis PDF significantly decreased GSK-3β phosphorylation (Fig. 9b). Decorin increased both constitutive and dialysate-induced GSK-3β phosphorylation, with the increase especially marked in cells exposed to dialysates (Fig. 9b).
Dialysate samples, TGF-β1, and IL-1β, had no effect on SMAD2, SMAD3, ERK, JNK, PKC or mTOR signaling, and the addition of decorin in these experiments also had no effect (data not shown).
4. Discussion
Peritoneal fibrosis remains an unavoidable consequence in long-term PD. The severity and progression of peritoneal fibrosis, as well as its clinical sequelae, vary considerably between patients, with the majority of patients showing a progressive deterioration of peritoneal dialytic function with increasing time on PD and a small number of patients may develop the potentially fatal complication of encapsulating peritoneal sclerosis [24,25]. Prevention and intervention of peritoneal fibrosis in PD is a major unmet clinical need. Decorin is a 110 kDa dermatan sulfate proteoglycan that has putative anti-fibrotic properties and regulates TGF-β1 bioactivity and bioavailability [12,13]. We previously reported that peritoneal mesothelial cells synthesize and secrete decorin into the peritoneal cavity [14,26,27]. Although it remains possible that transcapillary diffusion of decorin from the systemic circulation to the peritoneal cavity may contribute to dialysate decorin level, the main source of decorin is likely to be from local production [27], such as from peritoneal mesothelial cells. In this study, we demonstrated that dialysate decorin level correlated with dialysate CA125 level, a marker of mesothelial cell mass, and also showed an inverse relationship with time on PD, and the level was further decreased when patients developed peritonitis.
The peritoneum in patients on long-term PD is in a persistent state of low grade local inflammation and the inflammatory processes during stable PD and during peritonitis are distinctly different. In the absence of peritonitis, peritoneal inflammation in clinically stable PD patients is induced by long-term exposure to bioincompatible PDF, and is characterized by the activation of resident macrophages and release of pro-inflammatory mediators such as IL-6 that not only promulgate the inflammatory processes but also augment peritoneal fibrosis during unresolved inflammation [28]. This initiation phase is followed by the amplification phase where resident peritoneal cells such as mesothelial cells and fibroblasts amplify the inflammatory response through cell activation and local secretion of inflammatory and fibrotic mediators [29,30]. Despite the reduced incidence with improved connectology, peritonitis remains an important complication in PD, and it is an important risk factor predisposing patients to increased severity of peritoneal fibrosis. It is associated with increased infiltration of neutrophils and T cells and elevated levels of pro-inflammatory mediators such as LPS, IL-1β, IL-8, IFN-γ and TNF-α [31]. The mesothelium is exposed to a wide array of injurious substances, including inflammatory cells and their products, which are produced in an attempt to eliminate invading microorganisms. Despite apparent clinical resolution of peritonitis, dialysate levels of pro-inflammatory cytokines and growth factors may remain elevated for months [8], adding to the background peritoneal inflammation and fibrosis in long-term PD. In this study, we observed a significant decrease in dialysate decorin level at the onset of peritonitis, and although its level gradually increased after clinical resolution of peritonitis, it remained significantly lower than that detected in stable PD patients. At the onset of peritonitis, dialysate decorin level correlated with dialysate TGF-β1 level and inversely correlated with IL-1β and IL-8 levels suggesting that these cytokines play important roles in regulating decorin synthesis in the peritoneum.
We next performed in vitro studies using mesothelial cells to investigate the effects of pro-inflammatory cytokines on mesothelial cell decorin production. The results showed that TGF-β1, IL-1β, IL-6, IL-8 and TNF-α all reduced mesothelial cell decorin secretion. This reduction was more pronounced when TGF-β1 and IL-1β were added together to mesothelial cells. Almost all of the decorin synthesized by mesothelial cells, whether under basal conditions or when stimulated with TGF-β1 or IL-1β, was secreted as the intact proteoglycan, and was mediated through p38 MAPK and PI3K signaling pathways. Cell-associated decorin accounted for only 2% of the total decorin synthesized by mesothelial cells. Approximately 20% of cell-associated decorin was attributed to the intact proteoglycan, with the remainder detected as the free core protein. Whether this pool represents de novo synthesized core protein prior to dermatan sulfate glycosaminoglycan chain attachment, or decorin undergoing degradation warrants further investigation. Previous results from independent researchers showed that the regulation of decorin synthesis by cytokines and growth factors is dependent on the cell type and species [32], [33], [34], [35]. TGF-β1 has been shown to increase decorin synthesis in rat mesangial cells and to decrease decorin expression in mouse mesangial and proximal renal tubular epithelial cells [32,33]. IL-6 was shown to induce decorin synthesis in endothelial cells, whereas TNF-α inhibited decorin transcription in fibroblasts [34,35]. Whether peritoneal fibroblasts and endothelial cells contribute to decorin level in the peritoneum warrants further investigation.
Our in vitro experiments showed that TGF-β1 and IL-1β contributed to increased fibronectin expression induced by Peritonitis D0 PDF in mesothelial cells. Fibronectin is a large glycoprotein that is ubiquitous in all extracellular matrices. Deposition of fibronectin is an early event in renal tubulo-interstitial fibrosis, where fibronectin serves as a scaffold for the assembly of other matrix proteins [23]. It is possible that fibronectin may also play a similar role in peritoneal fibrosis in the setting of PD. Fibronectin exists either as a soluble dimer or as insoluble fibrils deposited in the extracellular matrix. Soluble fibronectin interacts with α5β1 integrin then polymerizes and forms fibronectin fibrils. Our results showed that fibronectin deposition in mesothelial cells was induced by PD fluid, TGF-β1 or IL-1β. We further demonstrated that TGF-β1 and IL-1β synergistically increased fibronectin expression, suggesting that pro-fibrotic mediators secreted into the peritoneal cavity may act in concert to promote peritoneal fibrosis. p38 MAPK and PI3K signaling pathways also played an important role in fibronectin expression in mesothelial cells under basal conditions and in the presence of TGF-β1 or IL-1β. Exogenous decorin decreased fibronectin expression at the transcriptional and translational levels, and these findings were corroborated by data from experiments of decorin gene silencing. The results showed that the suppression of fibronectin synthesis in PDF- or cytokine-stimulated mesothelial cells by decorin was mediated through down-regulation of p38 MAPK and AKT/PI3K signaling. How p38 MAPK and AKT/PI3K signaling regulates both fibrotic and anti-fibrotic pathways is intriguing. It is possible that downstream mediators that induce fibronectin expression are distinct from those that regulate decorin synthesis and further studies are required to confirm this. Overall, these data suggest that decorin might have a role in the prevention of peritoneal fibrosis.
Incubation of mesothelial cells with dialysate resulted in a significant reduction in GSK-3β phosphorylation at serine 9 residue, whereas decorin attenuated this decrease. GSK-3β is a serine/threonine kinase that plays an important role in EMT and fibrogenesis. It is constitutively phosphorylated in resting cells leading to the functional inhibition of GSK-3β [36] and conversely, decreased GSK-3β phosphorylation promotes stabilization and nuclear translocation of SNAIL, a transcription repressor of E-cadherin and promotor of EMT and fibrosis [37]. Given that both Control PDF and Peritonitis D0 PDF decreased GSK-3β phosphorylation in mesothelial cells, this would suggest that GSK-3β may contribute to mesothelial-to-mesenchymal transition and increase fibronectin expression. GSK-3β phosphorylation has been shown to exert anti-fibrotic effects through its direct interaction with SMAD3, inhibiting SMAD3 protein stability and enzymatic activity [38]. Genetic deletion of GSK-3β in cardiac fibroblasts promotes fibrogenesis and excessive scarring in the ischemic heart [39]. Independent researchers have also reported on the activation of GSK-3β in mesothelial cells exposed to glucose-based dialysate, whereas inhibition of GSK-3β activation using specific GSK-3β inhibitors was associated with mesothelial cell cytoprotection [40]. It has been shown that activation of AKT/PI3K and p38 MAPK signaling inactivates GSK-3β and promotes myocardial protection [41]. It is unknown whether a similar mechanism exists in mesothelial cells during PD-associated peritonitis or when exposed to pro-fibrotic cytokines in the presence of decorin.
TGF-β1 signals through SMAD-dependent (canonical) or SMAD-independent (non-canonical) pathways through binding to TGF-βRI and TGF-βRII present on the plasma membrane [42,43]. These receptors normally exist as monomers (MW 56 and ~65 kDa respectively), homodimers or heterodimers. TGF-β1 signaling is triggered by ligand binding to TGF-βRII, which binds and phosphorylates TGF-βRI, resulting in the assembly of a heterotetrameric complex comprising two molecules each of TGF-βRI and TGF-βRII [42]. The formation of the heterotetramer is indicative of receptor activation, and this complex mediates downstream TGF-β1 signaling. In our experiments, we observe that mesothelial cells constitutively express TGF-βRI and TGF-βRII transcripts and their expression is reduced by TGF-β1 and IL-1β. At the protein level, TGF-βRI expression is predominantly in the heterotetrameric form. TGF-β1 has no effect on TGF-βRI translation, whereas IL-1β reduces its translation. Decorin reduces TGF-βRI expression under basal conditions or in the presence of TGF-β1, but has no effect on IL-1β associated reduction of TGF-βRI expression. Our data suggest that decorin exerts its anti-fibrotic effects in mesothelial cells, at least in part, through inhibiting TGF-βRI heterotetramer expression, resulting in inhibition of TGF-β1 signaling. We did not detect TGF-βRII protein expression in mesothelial cells, although this could be related to the property of the antibody used in the experiments. Also, our results show that TGF-β1 signaling in mesothelial cells activates non-canonical pathways such as p38 MAPK and PI3K rather than the canonical pathways. Intriguingly, IL-1β reduces TGF-βRI expression as the heterotetrameric complex, and the mechanism remains to be defined. Whether this could be a means to counteract the anti-inflammatory properties of TGF-β1 or serve other biological purposes remains to be investigated.
Our data showed that decorin reduced fibronectin expression that was induced by IL-1β and mediated through activation of p38 MAPK and PI3K signaling. This suggests that decorin may have an anti-fibrotic effect under the setting of inflammation-induced fibrosis, characterized by increased level of IL-1β. The detailed mechanisms require further investigation, including whether decorin could regulate IL-1 receptor expression or function, or whether decorin could sequester IL-1β thereby preventing its binding to the receptor.
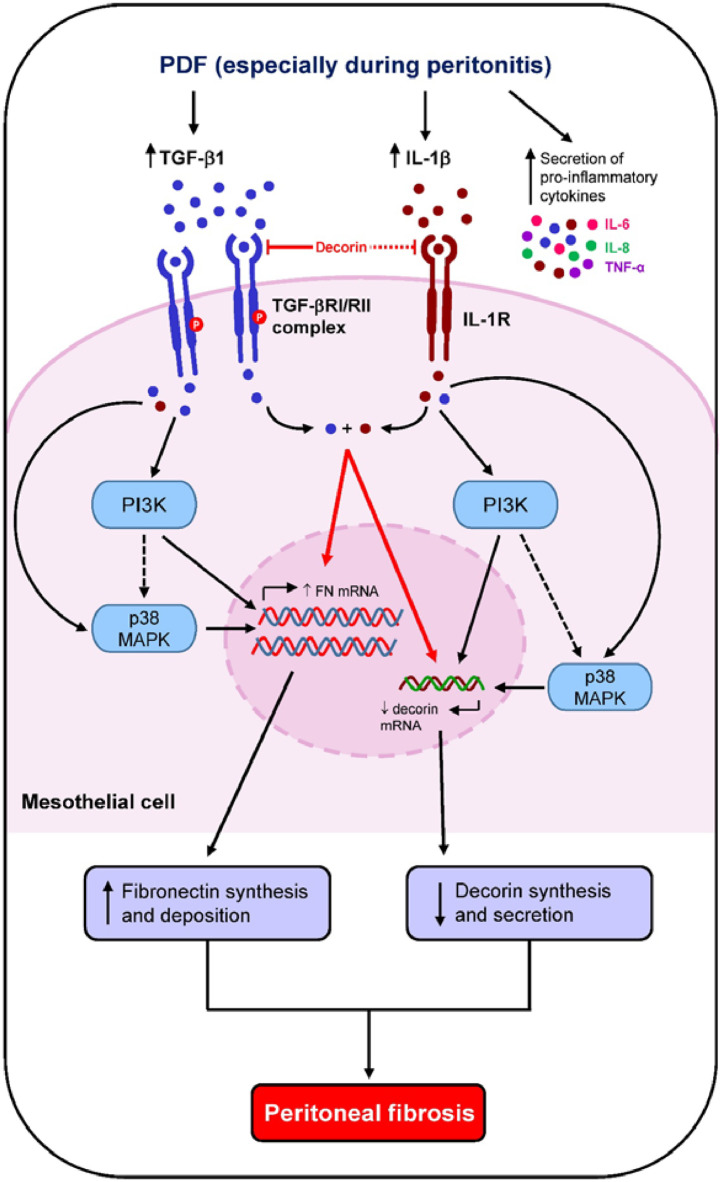
In conclusion, our original data demonstrate an important role of decorin in the regulation of peritoneal fibrosis in the setting of long-term PD especially during episodes of peritonitis, through down-regulation of p38 MAPK, AKT/PI3K and GSK-3β activation (Fig. 10). Whether adding decorin to PD fluid could be a therapeutic intervention to reduce peritoneal fibrosis deserves further investigation.
Fig. 10.
Schematic diagram showing how long-term PD and PD-associated peritonitis impact on peritoneal decorin level and downstream peritoneal fibrosis. Chronic exposure of the peritoneal membrane to glucose-based PD fluid induces low grade peritoneal inflammation with secretion of growth factors and pro-inflammatory cytokines by peritoneal mesothelial cells and infiltrating leukocytes. Inflammatory and fibrotic mediators that include TGF-β1 and IL-1β, decrease decorin mRNA and decorin secretion in mesothelial cells through increased p38 MAPK and AKT/PI3K activation resulting in increased peritoneal fibronectin deposition and subsequent peritoneal fibrosis. These fibrotic processes are exacerbated in patients with PD-associated peritonitis. Secreted TGF-β1 and IL-1β may also act in synergy to further augment fibrotic processes. Supplementation of PD fluids with decorin may delay peritoneal fibrosis by inhibiting TGF-βRI expression and downstream TGF-β1 signaling, decrease p38 MAPK and PI3K phosphorylation and attenuate fibronectin synthesis.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We would like to thank all PD patients for participating in this study. We are most grateful to the nurses at the Renal Units at Queen Mary Hospital and Tung Wah Hospital, Miss Vicky Ho and Miss Cindy Tang for coordinating the collection of PDF for this study and retrieval of clinical data, and to the nurses in the Operation Theatres at Queen Mary Hospital for the collection of omentum specimens. We thank Dr Kin Yi Au, Mr Andrew Yim, Dr Cheuk Chun Wan, Mr David Tam and Mr Martin Yip for technical assistance, and Mr Colin Tang for statistical analyses of clinical data.
Funding
This study was supported by the RGC General Research Fund (HKU 7830/09M and HKU 7848/12M), the Stanley Ho Alumni Challenge, the Department of Medicine Academic Activities Fund and kind donations from Mr C. S. Yung, Mr S. Ho, and Hui Hoy & Chow Sin Lan Charity Fund and the Family of Mr Hui Ming. S. Yung is supported by the Endowment Fund established for the 'Yu Chiu Kwong Professorship in Medicine' awarded to T. M. Chan, and the Wai Hung Charitable Foundation Limited. Na Jiang is a recipient of the Hong Kong PhD Fellowship Scheme established by the Research Grant Council (HKPFS ref no. PF10-15517). None of the funding bodies had any involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript and in the decision to submit the paper for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102661.
Contributor Information
Tak Mao Chan, Email: dtmchan@hku.hk.
Susan Yung, Email: ssyyung@hku.hk.
Appendix. Supplementary materials
References
- 1.Cho Y., Johnson D.W. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis. 2014;64(2):278–289. doi: 10.1053/j.ajkd.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Choi P., Nemati E., Banerjee A. Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis. 2004;43(1):103–111. doi: 10.1053/j.ajkd.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 3.Troidle L., Finkelstein F. Treatment and outcome of CPD-associated peritonitis. Ann Clin Microbiol Antimicrob. 2006;5:6. doi: 10.1186/1476-0711-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yung S., Chan T.M. Mesothelial cells. Perit Dial Int. 2007;27:S110–S115. [PubMed] [Google Scholar]
- 5.Mutsaers S.E., Bishop J.E., McGrouther G., Laurent G.J. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29(1):5–17. doi: 10.1016/s1357-2725(96)00115-x. [DOI] [PubMed] [Google Scholar]
- 6.Betjes M.G., Tuk C.W., Struijk D.G. Interleukin-8 production by human peritoneal mesothelial cells in response to tumor necrosis factor-alpha, interleukin-1, and medium conditioned by macrophages cocultured with staphylococcus epidermidis. J Infect Dis. 1993;168(5):1202–1210. doi: 10.1093/infdis/168.5.1202. [DOI] [PubMed] [Google Scholar]
- 7.Fukudome K., Fujimoto S., Sato Y., Hisanaga S., Eto T. Peritonitis increases MMP-9 activity in peritoneal effluent from CAPD patients. Nephron. 2001;87(1):35–41. doi: 10.1159/000045882. [DOI] [PubMed] [Google Scholar]
- 8.Lai K.N., Lai K.B., Lam C.W. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;35(4):644–652. doi: 10.1016/s0272-6386(00)70011-4. [DOI] [PubMed] [Google Scholar]
- 9.Yung S., Coles G.A., Davies M. IL-1 beta, a major stimulator of hyaluronan synthesis in vitro of human peritoneal mesothelial cells: relevance to peritonitis in CAPD. Kidney Int. 1996;50(4):1337–1343. doi: 10.1038/ki.1996.446. [DOI] [PubMed] [Google Scholar]
- 10.Rampino T., Cancarini G., Gregorini M. Hepatocyte growth factor/scatter factor released during peritonitis is active on mesothelial cells. Am J Pathol. 2001;159(4):1275–1285. doi: 10.1016/S0002-9440(10)62514-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Paolo N., Sacchi G., De Mia M. Morphology of the peritoneal membrane during continuous ambulatory peritoneal dialysis. Nephron. 1986;44(3):204–211. doi: 10.1159/000183987. [DOI] [PubMed] [Google Scholar]
- 12.Border W.A., Noble N.A., Yamamoto T. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y., Mann D.M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 14.Yung S., Thomas G.J., Stylianou E. Source of peritoneal proteoglycans. Human peritoneal mesothelial cells synthesize and secrete mainly small dermatan sulfate proteoglycans. Am J Pathol. 1995;146(2):520–529. [PMC free article] [PubMed] [Google Scholar]
- 15.Osada S., Hamada C., Shimaoka T. Alterations in proteoglycan components and histopathology of the peritoneum in uraemic and peritoneal dialysis (PD) patients. Nephrol Dial Transplant. 2009;24(11):3504–3512. doi: 10.1093/ndt/gfp268. [DOI] [PubMed] [Google Scholar]
- 16.Margetts P.J., Gyorffy S., Kolb M. Antiangiogenic and antifibrotic gene therapy in a chronic infusion model of peritoneal dialysis in rats. J Am Soc Nephrol. 2002;13(3):721–728. doi: 10.1681/ASN.V133721. [DOI] [PubMed] [Google Scholar]
- 17.Stylianou E., Jenner L.A., Davies M., Coles G.A., Williams J.D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990;37(6):1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- 18.Chan T.M., Leung J.K., Tsang R.C. Emodin ameliorates glucose-induced matrix synthesis in human peritoneal mesothelial cells. Kidney Int. 2003;64(2):519–533. doi: 10.1046/j.1523-1755.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 19.Bidmon B., Endemann M., Arbeiter K. Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis. Kidney Int. 2004;66(6):2300–2307. doi: 10.1111/j.1523-1755.2004.66040.x. [DOI] [PubMed] [Google Scholar]
- 20.Yung S., Cheung K.F., Zhang Q., Chan T.M. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol. 2010;21(11):1912–1927. doi: 10.1681/ASN.2009080805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yung S., Zhang Q., Zhang C.Z. Anti-DNA antibody induction of protein kinase C phosphorylation and fibronectin synthesis in human and murine lupus and the effect of mycophenolic acid. Arthritis Rheum. 2009;60(7):2071–2082. doi: 10.1002/art.24573. [DOI] [PubMed] [Google Scholar]
- 22.Yung S., Ng C.Y., Ho S.K. Anti-dsDNA antibody induces soluble fibronectin secretion by proximal renal tubular epithelial cells and downstream increase of TGF-beta1 and collagen synthesis. J Autoimmun. 2015;58:111–122. doi: 10.1016/j.jaut.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Eddy A.A. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7(12):2495–2508. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 24.Wong Y.Y., Wong P.N., Mak S.K. Persistent sterile peritoneal inflammation after catheter removal for refractory bacterial peritonitis predicts full-blown encapsulating peritoneal sclerosis. Perit Dial Int. 2013;33(5):507–514. doi: 10.3747/pdi.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambie M.R., Chess J., Summers A.M. Peritoneal inflammation precedes encapsulating peritoneal sclerosis: results from the global fluid study. Nephrol Dial Transplant. 2016;31(3):480–486. doi: 10.1093/ndt/gfv440. [DOI] [PubMed] [Google Scholar]
- 26.Yung S., Hausser H., Thomas G. Catabolism of newly synthesized decorin in vitro by human peritoneal mesothelial cells. Perit Dial Int. 2004;24(2):147–155. [PubMed] [Google Scholar]
- 27.Yung S., Lui S.L., Ng C.K. Impact of a low-glucose peritoneal dialysis regimen on fibrosis and inflammation biomarkers. Perit Dial Int. 2015;35(2):147–158. doi: 10.3747/pdi.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fielding C.A., Jones G.W., McLoughlin R.M. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40(1):40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yung S., Chan T.M. Intrinsic cells: mesothelial cells - central players in regulating inflammation and resolution. Perit Dial Int. 2009;29(Suppl 2):S21–S27. [PubMed] [Google Scholar]
- 30.Yung S., Chan T.M. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: the role of mesothelial cells. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/484167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin C.Y., Roberts G.W., Kift-Morgan A. Pathogen-specific local immune fingerprints diagnose bacterial infection in peritoneal dialysis patients. J Am Soc Nephrol. 2013;24(12):2002–2009. doi: 10.1681/ASN.2013040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Border W.A., Okuda S., Languino L.R., Ruoslahti E. Transforming growth factor-beta regulates production of proteoglycans by mesangial cells. Kidney Int. 1990;37(2):689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- 33.Mogyorosi A., Ziyadeh F.N. Increased decorin mRNA in diabetic mouse kidney and in mesangial and tubular cells cultured in high glucose. Am J Physiol. 1998;275(5 Pt 2):F827–F832. doi: 10.1152/ajprenal.1998.275.5.F827. [DOI] [PubMed] [Google Scholar]
- 34.Strazynski M., Eble J.A., Kresse H., Schonherr E. Interleukin (IL)-6 and IL-10 induce decorin mRNA in endothelial cells, but interaction with fibrillar collagen is essential for its translation. J Biol Chem. 2004;279(20):21266–21270. doi: 10.1074/jbc.M309782200. [DOI] [PubMed] [Google Scholar]
- 35.Mauviel A., Santra M., Chen Y.Q., Uitto J., Iozzo R.V. Transcriptional regulation of decorin gene expression. Induction by quiescence and repression by tumor necrosis factor-alpha. J Biol Chem. 1995;270(19):11692–11700. doi: 10.1074/jbc.270.19.11692. [DOI] [PubMed] [Google Scholar]
- 36.Lal H., Ahmad F., Woodgett J., Force T. The GSK-3 family as therapeutic target for myocardial diseases. Circ Res. 2015;116(1):138–149. doi: 10.1161/CIRCRESAHA.116.303613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aroeira L.S., Aguilera A., Sanchez-Tomero J.A. Epithelial to mesenchymal transition and peritoneal membrane failure in peritoneal dialysis patients: pathologic significance and potential therapeutic interventions. J Am Soc Nephrol. 2007;18(7):2004–2013. doi: 10.1681/ASN.2006111292. [DOI] [PubMed] [Google Scholar]
- 38.Guo X., Ramirez A., Waddell D.S. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 2008;22(1):106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lal H., Ahmad F., Zhou J. Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart. Circulation. 2014;130(5):419–430. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rusai K., Herzog R., Kuster L., Kratochwill K., Aufricht C. GSK-3beta inhibition protects mesothelial cells during experimental peritoneal dialysis through upregulation of the heat shock response. Cell Stress Chaperones. 2013;18(5):569–579. doi: 10.1007/s12192-013-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Xuan W., Yan R. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of beta-catenin. Clin Sci. 2011;120(10):451–462. doi: 10.1042/CS20100466. [DOI] [PubMed] [Google Scholar]
- 42.Heldin C.H., Moustakas A. Signaling receptors for TGF-beta family members. Cold Spring Harb Perspect Biol. 2016;8(8) doi: 10.1101/cshperspect.a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng X.M., Nikolic-Paterson D.J., Lan H.Y. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.