Abstract
Purpose:
The aim of this study was to compare operative duration and total hospital costs incurred for patients undergoing elective cranioplasty with a variety of materials, including manually shaped autogenous bone graft and titanium mesh, custom patient-specific titanium mesh, polymethyl methacrylate (PMMA) acrylic, and polyetheretherketone (PEEK) implants.
Methods:
A single-centre retrospective chart review was used. Patient demographics, defect characteristics, total operative time, and length of hospital stay were obtained. Total costs were sourced from Sunnybrook and standardized to the 2014 to 2015 year. Bivariate and age-controlled multivariate analyses were performed with (n = 119) and without (n = 101) outliers.
Results:
When outliers were removed, an age-controlled analysis revealed that autogenous implants resulted in an operative time of 178 ± 37 minutes longer than manually shaped titanium implants (P < .01). The average cost of cranioplasty was CAD$18 335 ± CAD$10 265 for manually shaped titanium implants, CAD$31 956 ± CAD$31 206 for custom patient-specific titanium implants, CAD$20 786 ± CAD$13 075 for PMMA, CAD$14 291 ± CAD$5562 for autogenous implants, and CAD$27 379 ± CAD$4945 for PEEK implants (P = .013). When outliers were removed, cranioplasty with PMMA and PEEK incurred greater costs, CAD$4442 ± CAD$2100 and CAD$13 372 ± CAD$2728, respectively, more than manually shaped titanium implants (P < .01).
Conclusions:
Manually shaped titanium mesh is the most cost-effective implant choice for small cranial defects. Large unknown defects and frontal paranasal sinus defects are most effectively treated with autogenous bone or titanium mesh. Despite prolonged operative duration and inpatient admission, total costs were not significantly increased. Both PMMA and PEEK implants were significantly more costly, which may be a result of higher complications necessitating reoperation.
Keywords: cranioplasty, autogenous bone, PMMA, PEEK, titanium implants, cost, operative time
Abstract
Objectif :
La présente étude visait à comparer la durée de l’opération et les coûts hospitaliers totaux engagés pour les patients qui subissaient une cranioplastie non urgente faisant appel à divers matériaux : greffon osseux autologue et treillis de titane façonnés à la main, implant PMMA et implant PEEK.
Méthodologie :
Les chercheurs ont réalisé une analyse rétrospective monocentrique des dossiers. Ils ont colligé les renseignements démographiques sur les patients, les caractéristiques de l’anomalie, la durée totale de l’opération et la durée du séjour hospitalier. Ils ont extrait les coûts totaux de Sunnybrook et les ont standardisés pour l’année 2014-2015. Ils ont effectué des analyses bivariées et multivariées contrôlées selon l’âge en incluant (n=119) et en excluant (n=101) les valeurs aberrantes.
Résultats :
Après l’élimination des valeurs aberrantes, une analyse contrôlée selon l’âge a révélé que les implants autologues s’associaient à une opération plus longue de 178 ± 37 min que les implants de titane façonnés à la main (p<0,01). Le coût moyen de la cranioplastie s’élevait à 18 335 CAD$ ± 10 265 CAD$ pour les implants de titane façonnés à la main, à 31 956 CAD$ ± 31 206 CAD$ pour les implants de titane adaptés aux patients, à 20 786 CAD$ ± 13 075 CAD$ pour les implants en PMMA, à 14 291 CAD$ ± 5 562 CAD$ pour les implants autologues et à 27 379 CAD$ ± 4 945 CAD$ pour les implants en PEEK (p=0,013). Une fois les valeurs aberrantes éliminées, la cranioplastie par PMMA ou PEEK étaient les plus coûteuses, à 4 442 CAD$ ± 2 100 CAD$ et 13 372 CAD$ ± 2 728 CAD$ de plus que les implants de titane façonnés à la main (p<0,01).
Conclusions :
Les treillis de titane façonnés à la main présentent le meilleur rapport coût-efficacité en cas d’anomalies crâniennes bénignes. Le traitement des graves anomalies d’origine inconnue et des anomalies des sinus paranasaux frontaux les plus efficaces sont l’os autologue ou le treillis de titane. Malgré une opération et un séjour hospitalier prolongés, les coûts totaux n’augmentaient pas de manière significative. Les implants de PMMA et de PEEK étaient considérablement plus cher, peut-être à cause du plus fort taux de complications donnant lieu à une réopération.
Introduction
Cranioplasty is a reconstructive procedure that repairs functional and aesthetic defects of the skull. It aims to restore skull integrity,1 improve cerebral hemodynamics,2,3 and relieve psychological and social disabilities.4-6
The material used for cranioplasty must obliterate the skull defect while restoring cranial surface morphology. It should be cost-effective, radiolucent and non-ferromagnetic, infection resistant, lightweight, biologically inert,7 osteoinductive, and osteoconductive.5,7,8 At present, there are various materials available for cranioplasty, including autogenous bone, polymethyl methacrylate (PMMA) acrylic, polyetheretherketone (PEEK), and titanium implants.
The potential for revascularization and consolidation of autogenous split skull bone grafts results in the preference of this material for cranioplasty, particularly in reconstruction of frontal skull defects adjacent to paranasal sinuses.1,9-12 Nonetheless, pitfalls of autogenous implants include long operative durations, donor site morbidity, and a high risk of reabsorption resulting in visible surface contour irregularities.9,10,13,14
Advances in biocompatible materials and custom computer-generated implants (CCGIs)1,15 have broadened the reconstructive options available to surgeons. Polymethyl methacrylate is a moldable acrylic resin that is lightweight, radiolucent, and permanently maintains both shape and volume.13,16 Polymethyl methacrylate however lacks osteoconductive properties and incurs an exothermic reaction that poses a threat to adjacent tissues. More importantly, it is a polymer that does not tolerate contamination and is therefore contraindicated in frontal reconstruction with potential exposure to paranasal sinuses.1,9,17 Polyetheretherketone is easily shaped, radiolucent, and lightweight.5,18,19 However, PEEK implants are expensive7 and, similar to all polymer implants, are contraindicated in frontal reconstruction. Finally, titanium implants are an excellent choice for large cranioplasties due to biocompatibility, handling characteristics, strength, and low infection rates.8,20 Titanium mesh is more tolerant of contamination and is the preferred material in the reconstruction of frontal defects adjacent to sinuses. Titanium mesh is versatile, allowing intraoperative modification in the size and shape of the implant and incorporation of bone graft or bone segments within the reconstructive construct. Reported aesthetic outcomes and long-term, patient-reported satisfaction are excellent.21 However, titanium implants can result in erosion of the overlying soft tissues with implant exposure in 14% of patients22; possibly due to fluctuations in the pressure gradient between the atmosphere and the intracranial space acting through a mesh structure.23 The risk of mesh exposure may be particularly high in patients undergoing postoperative radiotherapy.20,24
Presently, there is no consensus on which material is superior, and the choice is typically based on surgeon preference.5 One key consideration in the choice of implant material is cost. Cranioplasty with CCGI or patient-specific implants (PSIs) is significantly costlier than cranioplasty with autogenous bone grafts.12,25,26 Yet, when costs associated with operative duration or intensive care unit (ICU) admission are considered, Gilardino et al15 observed no significant difference in costs between autogenous and CCGI. When the costs of reoperation for complications are included in the cost–benefit analysis,15 cranioplasty with autogenous implants was found to be more expensive.
Overall, the cost-effectiveness of different cranioplasty implant materials is unclear. Previous studies are limited by small sample size, the comparison of few implant materials, or incomplete analysis of costs.20 For this reason, we aimed to elucidate the costs incurred in a large sample of cranioplasty patients treated with several implant materials including manually shaped titanium implants, autogenous split skull autografts, custom patient-specific (CPS) titanium implants, PMMA acrylic implants, and PEEK implants. The present investigation is a single-centre retrospective chart review of patients who underwent elective cranioplasty by a single surgeon (O.A.). The primary objective of this study is to summarize the total hospital costs incurred for a series of cranioplasty patients, with the interest of comparing operative time, inpatient stay, complications, and the material of reconstruction.
Materials and Methods
Participants
Following Research Ethics Board approval, a single-centre retrospective chart review was used. Consecutive patients between January 2000 and July 2016 of 1 surgeon (O.A.) were assessed for inclusion. Patients were eligible if they underwent elective cranial vault reconstruction with autologous split skull bone graft, manually shaped or CPS titanium implants, PMMA, or PEEK. Patients were excluded if (1) a postoperative computed tomography scan was unavailable, (2) they failed to attend follow-ups (>1 month), (3) they incurred a primary cranial defect, or (4) calcium phosphate bone cement exclusively or in association with any of the above-named implant materials was used.
Cranioplasty Techniques
Five different cranioplasty techniques were used and are compared in this study:
Manually shaped titanium implants require direct intraoperative manipulation of a sheet of titanium mesh to restore a 3-dimensional anatomical shape. Although simple and versatile, this technique is only applicable in small defects (Figure 1).
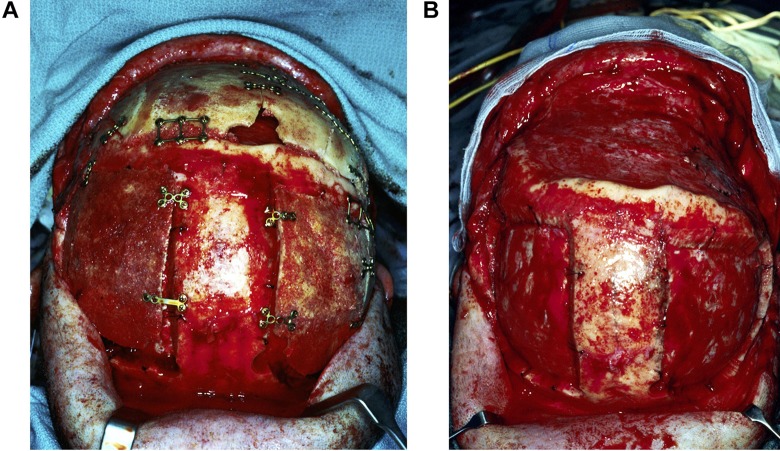
Split skull autogenous cranioplasty relies on the harvesting of autograft, manual carving, and assembly of bone segments into a 3-dimensional construct. It is a laborious and technically challenging procedure (Figure 2).
Custom PEEK implants are prefabricated commercially available (Depuy Synthes, Raynham, Massachusetts) polymer implants that are suitable for stable, unalterable skull defects (Figure 3).
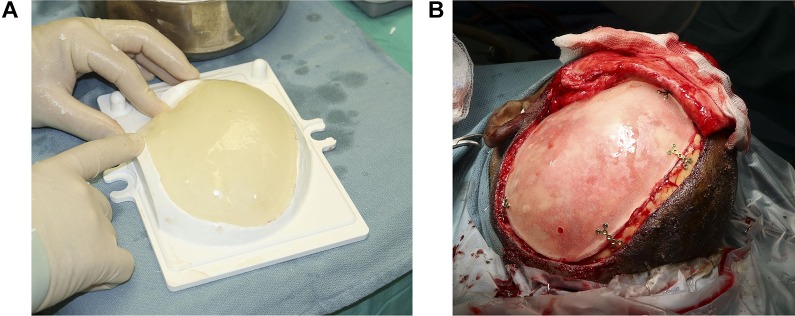
Custom PMMA implants are cast intraoperatively using prefabricated patient-specific molds and forming tools (Calavera Surgical Design, Toronto, Canada; Figure 4).
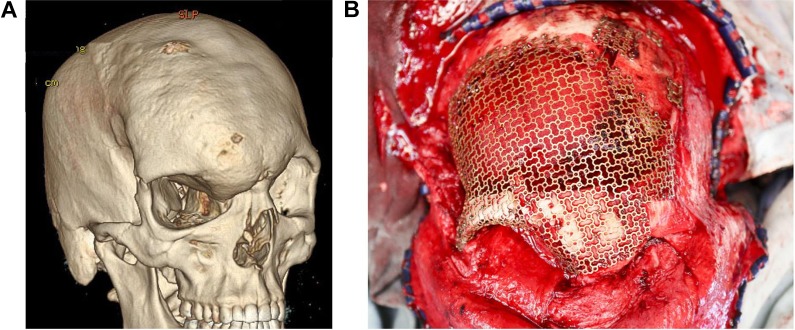
Custom patient-specific titanium implants are shaped intraoperatively using patient-specific molds, forming tools, and a press to shape a sheet of titanium mesh into a 3-dimensional anatomical implant (Calavera Surgical Design). These implants are indicated in the reconstruction of “unknown defects,” that is, ablative defects where the extent of ablation cannot be predicted and in the reconstruction of craniofrontal defects with potential paranasal sinus exposure (Figure 5).
Figure 1.
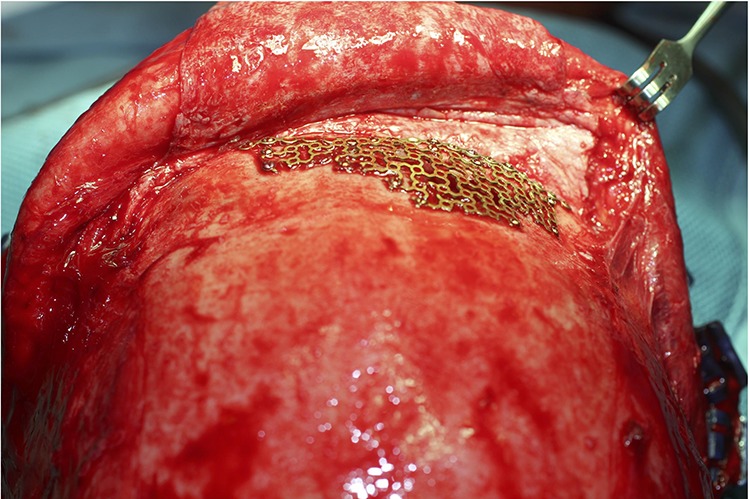
Manually shaped titanium mesh relies on intraoperative manipulation of a 2-dimensional sheet of titanium mesh into a 3-dimensional shape. It is a simple and effective technique for small defects.
Figure 2.
Autogenous cranioplasty necessitates harvesting of split skull bone graft. For a large bifrontal defect, bilateral parietal craniotomies are performed to harvest bone graft (A), which is split into inner and outer tables. The inner table bone reconstructs the craniotomy donor sites, while outer table grafts are carved and assembled to reconstruct the skull defect (B).
Figure 3.
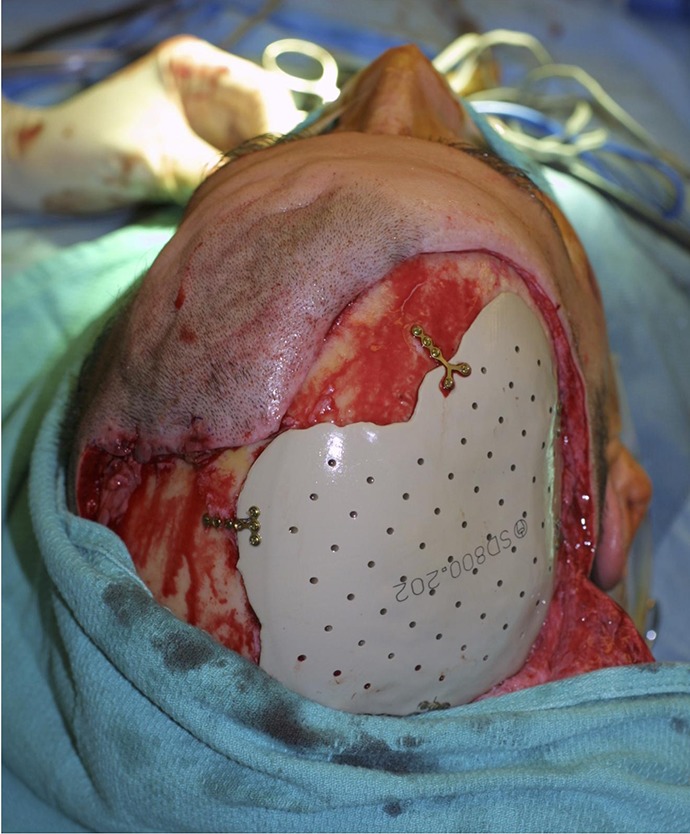
Polyetheretherketone (PEEK) cranioplasty employs a prefabricated polymer implant to resurface a stable skull defect.
Figure 4.
Intraoperative fabrication of a patient-specific polymethyl methacrylate (PMMA) implant using a custom mold (A; Calavera Surgical Design, Toronto, Canada) provides a polymer implant which fits the defect and restores normal skull shape (B).
Figure 5.
Three-dimensional computed tomography (CT) demonstrating a right craniofrontal dysplasia (A). The extent of tumour ablation and therefore the size and shape of the skull defect are “unknown” prior to surgery. A patient-specific titanium mesh can be shaped intraoperatively (B) using a press and custom mold and forming tool (Calavera Surgical Design, Toronto, Canada) and trimmed appropriately to fit any defect. The titanium mesh is versatile in that it can be adapted to any shape and size of defect, can incorporate bone grafts, and can be used in reconstructing paranasal sinus regions.
Data Collection
Patient demographics (age, sex), defect characteristics (etiology, size), soft tissue coverage (free flap or transposition flap), perioperative radiotherapy, complication rates, operative time, ICU admission, and hospital inpatient stay (recovery on ward) were obtained.
Total costs were sourced from Sunnybrook Health Sciences Centre (Alliance Decision Support Case Costing System) and standardized. The 2014 to 2015 cost data were obtained for the implant, the ICU (CAD$4596) and ward stay (CAD$639) per day, and the operating room cost per minute (CAD$20.5). Indirect costs (wages lost) and intangible costs (pain) were not assessed.15 Complication costs were calculated as procedural costs and did not include outpatient medication or the costs associated with a new implant. When a reoperation was required, the cases were treated separately. All data were managed with a database software (FilemakerPro 12; Filemaker Inc, Santa Clara, California).
Of the 132 consecutive patients reviewed, 119 met the inclusion criteria (Table 1). Outliers were identified (A.B. and O.A.), removed, and a separate analysis was performed (Table 2). These included surgical cases where operative duration was prolonged for extraneous reasons (frozen section for cancer ablation and free tissue transfer for resurfacing).
Table 1.
Participant Characteristics (Outliers Included).a
| n (%) | Implant Type | P Value | ||||
|---|---|---|---|---|---|---|
| Manually Shaped Titanium | Custom Patient- Specific Titanium | PMMA | Autogenous Bone | PEEK | ||
| N = 32 | N = 32 | N = 31 | N = 13 | N = 11 | ||
| Gender | .019b | |||||
| Male | 14 (43) | 24 (75) | 23 (74) | 5 (38) | 6 (55) | |
| Female | 18 (57) | 8 (25) | 8 (26) | 8 (62) | 5 (45) | |
| Age, years | 58 ± 21 | 49 ± 20 | 43 ± 16 | 48 ± 10 | 40 ± 12 | .008b |
| Total costs, CAD$ | ||||||
| n = 118 | 18 355 ± 10 265 | 31 956 ± 31 206 | 20 786 ± 13 075 | 14 291 ± 5562 | 27 379 ± 4945 | .013b |
| Operation time (minutes) | ||||||
| n = 119 | 214 ± 131 | 308 ± 190 | 177 ± 87 | 348 ± 138 | 197 ± 88 | <.001b |
| Recovery, days | ||||||
| ICU, n = 115 | 1.2 ± 1.5 | 2.2 ± 3.4 | 1.1 ± 1.6 | 1.2 ± 1.0 | 1.4 ± 0.8 | .302 |
| Ward, n = 114 | 2.7 ± 4.0 | 13.4 ± 41.3 | 7.9 ± 18.2 | 2.9 ± 1.6 | 2.7 ± 1.7 | .397 |
| Complications | .435 | |||||
| None | 23 (72) | 23 (72) | 20 (65) | 11 (85) | 9 (82) | |
| Minor | 5 (16) | 3 (9) | 2 (6) | 2 (15) | 1 (9) | |
| Major | 4 (12) | 6 (19) | 9 (29) | 0 (0) | 1 (9) | |
| Size of defectc | <.001b | |||||
| Small | 20 | 0 | 1 | 0 | 0 | |
| Medium | 6 | 9 | 8 | 8 | 0 | |
| Large | 2 | 12 | 14 | 3 | 4 | |
| Massive | 0 | 8 | 8 | 1 | 6 | |
Abbreviations: ICU, intensive care unit; PMMA, polymethyl methacrylate; PEEK, polyetheretherketone; SD, standard deviation.
Note. Bold values p<.05.
a Values are presented as mean ± SD.
b Statistically significant difference (P < .05).
c Not all patient data were available.
Table 2.
Participant Characteristics (Outliers Removed).a
| n (%) | Implant Type | P Value | ||||
|---|---|---|---|---|---|---|
| Manually Shaped Titanium | Custom Patient- Specific Titanium | PMMA | Autogenous Bone | PEEK | ||
| N = 25 | N = 23 | N = 26 | N = 13 | N = 11 | ||
| Gender | .014b | |||||
| Male | 11 (44) | 19 (90) | 20 (77) | 5 (38) | 6 (55) | |
| Female | 15 (56) | 4 (10) | 8 (33) | 8 (62) | 5 (45) | |
| Age, years | 55 ± 21 | 43 ± 17 | 42 ± 16 | 48 ± 10 | 40 ± 12 | .027b |
| Total costs | ||||||
| n = 100 | 14 626 ± 4676 | 17 536 ± 7719 | 18 540 ± 10 080 | 14 291 ± 5562 | 27 379 ± 4945 | <.001b |
| Operation duration (minutes) | ||||||
| N = 101 | 170 ± 82 | 227 ± 137 | 179 ± 90 | 348 ± 138 | 197 ± 88 | <.001b |
| Recovery (days) | ||||||
| ICU, n = 97 | 0.8 ± 0.6 | 0.9 ± 1.3 | 1.2 ± 1.6 | 1.2 ± 1.0 | 1.4 ± 0.8 | .604 |
| Ward, n = 96 | 1.8 ± 1.3 | 3.8 ± 6.5 | 3.3 ± 3.2 | 2.9 ± 1.6 | 2.7 ± 1.7 | .429 |
| Complications | .099 | |||||
| None | 21 (84) | 20 (87) | 16 (62) | 11 (85) | 9 (82) | |
| Minor | 3 (12) | 2 (9) | 2 (8) | 2 (15) | 1 (9) | |
| Major | 1 (4) | 1 (4) | 8 (31) | 0 (0) | 1 (9) | |
Abbreviations: ICU, intensive care unit; PMMA, polymethyl methacrylate; PEEK, polyetheretherketone; SD, standard deviation.
Note. Bold values p<.05.
a Values are presented as mean ± SD.
b Statistically significant difference (P < .05).
Data Analysis
Summary statistics were calculated for all variables. Continuous variables were assessed for normality using the Kolmogorov-Smirnov test, skewness, and kurtosis. Data are displayed as mean ± standard deviation. One-way analysis of variance and χ2 tests (categorical variables) were performed to compare demographics, defect characteristics, complication rates, operative duration, total costs, and recovery (ICU or Ward) between implant types. A bivariate analysis was performed with (n = 119) and without outliers (n = 101). Multiple regression analysis adjusting for age was conducted for total costs (n = 118), operative duration (n = 119), recovery in ICU (n = 115), and recovery on ward (n = 114). An age-controlled multivariate analysis was also performed in the absence of outliers: total costs (n = 100), operative duration (n = 101), recovery in ICU (n = 97), and recovery on ward (n = 96). Multiple regression analysis utilized manually shaped titanium implants as the reference group. Data are reported as mean ± standard error with the 95% confidence intervals (95% CIs). A P value of .05 was used to denote statistical significance. All analysis were performed using SPSS statistical package version 20 (version 23.0; IBM SPSS Statistics for Windows, Armonk, New York) or SAS version 9.4.
Results
Defect Etiology
There was no statistically significant difference in defect location between the groups (P = .111; Table 1). There was, however, a significant difference in the size of the defect between groups (P < .001). Manually shaped titanium implants were primarily used to treat small defects, whereas CPS titanium implants and PMMA were used for large cranial defects. In most ablative, postinfection cases or in close proximity to paranasal sinus, autogenous bone or manually shaped titanium implants were used.
Operative Time
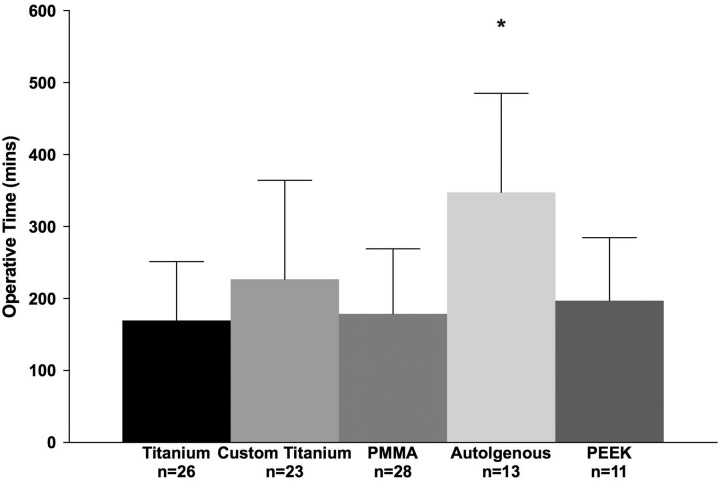
Operative time was significantly different between the groups (n = 119, P < .001). The average operative time was 214 ± 131 minutes for manually shaped titanium implants, 308 ± 190 minutes for CPS titanium implants, 177 ± 87 minutes for PMMA, 348 ± 138 minutes for autogenous implants, and 197 ± 88 minutes for PEEK (Figure 6). After adjusting for age, CPS titanium implants, autogenous implants, PMMA, and PEEK groups were compared to manually shaped titanium implants (reference group, 214 ± 131 minutes). Cranioplasty with CPS titanium implants and autogenous implants resulted in a significantly longer operative duration, 113 ± 34 (95% CI: 46-181) minutes and 154 ± 45 (95% CI: 66-242) minutes, compared to manually shaped titanium implants (P < .01; Table 3). When outliers were removed, only autogenous implants were associated with an operative duration 178 ± 37 (95% CI: 105-252) minutes longer than manually shaped titanium implants (P < .01; Table 4).
Figure 6.
Operative time (minutes) for cranial vault reconstruction. Outliers removed; after adjusting for age, autogenous implants were associated with a greater operative time compared to manually shaped titanium implants (P < .01). *Significant difference (P < .05).
Table 3.
Multiple Regression Analysis Adjusted for Age (Outliers Included).a
| Outcome and Implant Type | Unstandardized Coefficient | P Value | |
|---|---|---|---|
| Estimate ± SE | 95% CI | ||
| Total estimate costs (CAD$, n = 118) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 15 147 ± 4693 | 5849 to 24 445 | .002 b |
| PMMA | 4879 ± 4893 | −4815 to 14 574 | .321 |
| Autogenous bone | −2391 ± 6137 | −14 550 to 9768 | .698 |
| PEEK | 12 107 ± 6683 | −1133 to 25 351 | .073 |
| Operation duration (minutes, n = 119) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific Titanium | 113 ± 34 | 46 to 181 | .001 b |
| PMMA | −6 ± 35 | −76 to 63 | .855 |
| Autogenous bone | 154 ± 45 | 66 to 242 | <.0001 b |
| PEEK | 21 ± 49 | −75 to 118 | .659 |
| Recovery in ICU (days, n = 115) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 1.24 ± 0.54 | 0.16 to 2.32 | .025 b |
| PMMA | 0.35 ± 0.56 | −0.76 to 1.47 | .533 |
| Autogenous bone | 0.23 ± 0.70 | −1.16 to 1.62 | .744 |
| PEEK | 0.70 ± 0.76 | −0.82 to 2.21 | .36 |
| Recovery in ward (days, n = 114) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 10.4 ± 6.1 | −1.6 to 22.4 | .089 |
| PMMA | 4.6 ± 6.3 | −7.9 to 17.1 | .464 |
| Autogenous bone | −0.1 ± 7.8 | −15.6 to 15.3 | .985 |
| PEEK | −0.7 ± 8.5 | −17.6 to 16.1 | .931 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; PMMA, polymethyl methacrylate; PEEK, polyetheretherketone; SE, standard error; SD, standard deviation.
Note. Bold values p<.05.
a Values are presented as estimate ± SE.
b Statistically significant difference (P < .05).
Table 4.
Multiple Regression Analysis Adjusted for Age (Outliers Removed).a
| Outcome and Implant Type | Unstandardized Coefficient | P Value | |
|---|---|---|---|
| Estimate ± SE | 95% CI | ||
| Total estimate costs (CAD$, n = 100) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 3378 ± 2165 | −920 to 7677 | .122 |
| PMMA | 4442 ± 2100 | 272 to 8,613 | .037 b |
| Autogenous bone | −601 ± 2513 | −5051 to 4929 | .981 |
| PEEK | 13 372 ± 2728 | 7955 to 18 788 | <.0001 b |
| Operation duration (minutes, n = 101) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 58 ± 32 | −5 to 121 | .071 |
| PMMA | 10 ± 31 | −51 to 71 | .739 |
| Autogenous bone | 178 ± 37 | 105 to 252 | <.0001 b |
| PEEK | 29 ± 40 | −51 to 108 | .478 |
| Recovery in ICU (days, n = 97) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 0.2 ± 0.4 | −0.50 to 0.93 | .548 |
| PMMA | 0.5 ± 0.3 | −0.19 to 1.18 | .158 |
| Autogenous bone | 0.4 ± 0.4 | −0.42 to 1.21 | .339 |
| PEEK | 0.6 ± 0.4 | −0.24 to 1.53 | .151 |
| Recovery in ward (days, n = 95) | |||
| Manually shaped titanium | Reference | Reference | Reference |
| Custom patient-specific titanium | 2.4 ± 1.1 | 0.30 to 4.58 | .026 b |
| PMMA | 2.1 ± 1.0 | −0.01 to 4.11 | .051 b |
| Autogenous bone | 1.4 ± 1.2 | −1.06 to 3.82 | .265 |
| PEEK | 1.6 ± 1.3 | −1.05 to 4.24 | .233 |
Abbreviations: CI, confidence interval; ICU, intensive care unit; PMMA, polymethyl methacrylate; PEEK, polyetheretherketone; SE, standard error; SD, standard deviation.
Note. Bold values p<.05.
a Values are presented as estimate ± SE.
b Statistically significant difference (P < .05).
Recovery in ICU and on Ward
There was no significant difference between implant type and recovery in ICU (P = .302) with (n = 115; Table 1) or without outliers (n = 97; Table 2). However, an age-controlled multivariate analysis revealed that participants treated with CPS titanium implants remained in ICU 1.24 ± 0.54 (95% CI: 0.16-2.32) days longer than those treated with manually shaped titanium implants (P = .025; Table 3). When outliers were removed, there was no statistically significant association between implant type and recovery in the ICU (P > .05; Table 4).
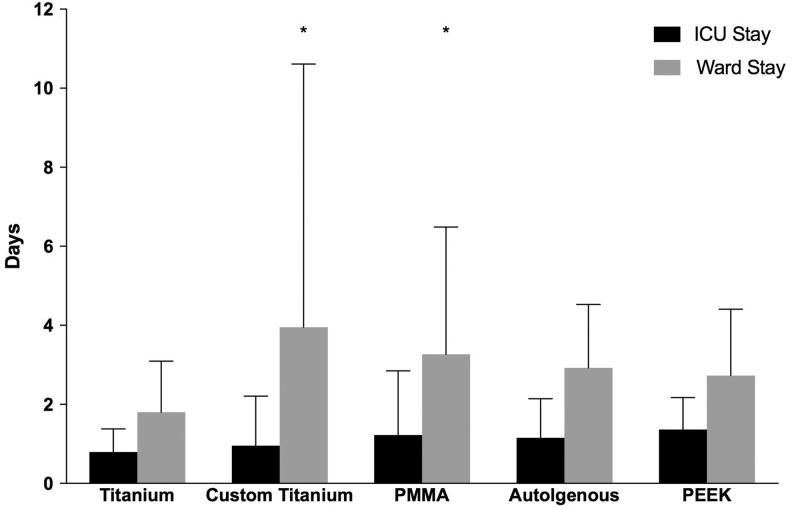
There was no significant difference between implant type and recovery on ward with (n = 114; Table 1) or without outliers (n = 96, P = .397). The relationship remained non-significant after adjusting for age (P > .05; Table 3). However, when outliers were removed and the data adjusted for age, CPS titanium implants resulted in a longer recovery on ward 2.4 ± 1.1 (95% CI: 0.3-4.58) days compared to manually shaped implants (P = .026; Table 4). In addition, those treated with PMMA remained on ward 2.1 ± 1.0 (95% CI: −0.01 to 4.11) days longer than those treated with manually shaped titanium implants (P = .051; Figure 7).
Figure 7.
Intensive care unit (ICU) and ward stay for cranial vault reconstructive patients treated with various implant types. Data are presented as mean ± standard deviation (SD) days. When outliers were removed and the data adjusted for age, patients treated with custom patient-specific titanium implants and polymethyl methacrylate (PMMA) implants remained in the ward longer (2.4 ± 1.1 and 2.1 ± 1.0 days, respectively) than those treated with manually shaped titanium implants (P < .05). After adjusting for age, there was no statistically significant difference between implant types and ICU stay. *Significant difference (P < .05).
Total Costs
Total costs were significantly different between the groups (Table 1; P = .013). The average cost was CAD$18 335 ± CAD$10 265 for manually shaped titanium implants, CAD$31 956 ± CAD$31 206 for CPS titanium implants, CAD$20 786 ± CAD$13 075 for PMMA, CAD$14 291 ± CAD$5562 for autogenous implants, and CAD$27 379 ± CAD$4945 for PEEK (Figure 8). After adjusting for age, cranioplasty with CPS titanium implants cost CAD$15 147 ± CAD$4693 (95% CI: 5849-24 445) more than cranioplasty with manually shaped titanium implants (P = .002; Table 3).
Figure 8.
Total cost (CAD$CAD) for cranial vault reconstruction. Outliers removed; after adjusting for age, polymethyl methacrylate (PMMA), and polyetheretherketone (PEEK) implants are associated with greater costs compared to manually shaped titanium implants (P < .05). *Significant difference (P < .05).
Upon the removal of outliers (n = 100), total costs were significantly different between the groups (P < .001). The average cost was CAD$14 626 ± CAD$4676 for manually shaped titanium implants, CAD$17 536 ± CAD$7719 for CPS titanium implants, CAD$18 540 ± CAD$10 080 for PMMA, CAD$14 291 ± CAD$5562 for autogenous, and CAD$27 379 ± CAD$4945 for PEEK (Figure 8). An age-adjusted multivariate analysis performed without outliers (n = 100) revealed that cranioplasty with PMMA incurred greater costs, CAD$4442 ± CAD$2100 (95% CI: 272-8613) more than cranioplasty with manually shaped titanium implants (P = .037; Table 4). Similarly, cranioplasty with PEEK was CAD$13 372 ± 2728 (95% CI: 7955-18 788) more than cranioplasty with manually shaped titanium implants (P < .01; Table 4).
Complication Rates
Complications associated with each implant type are listed in Table 5. There was no significant difference between implant type and complication rates with or without outliers (P > .05). The proportion of participants who experienced a complication requiring surgical intervention was 19% titanium implants, 29% for PMMA, 15% for autogenous bone, and 27% for PEEK (Figure 9).
Table 5.
Incidence of Complications by Implant Material.
| Material | Total Complications | Complication Type | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Complications | Complications Requiring Surgical Intervention | Poor Contour | Implant Exposure | Infection | Fluid Collection | Scar | CSF Leak | |
| Titaniuma | 64 | 21 | 12 | 5 | 1 | 2 | 3 | 1 | |
| PMMA | 31 | 13 | 9 | 4 | 5 | ||||
| Autogenous | 13 | 5 | 2 | 2 | |||||
| PEEK | 11 | 5 | 3 | 1 | 1 | 1 | |||
Abbreviations: CSF, cerebral spinal fluid; PMMA, polymethyl methacrylate; PEEK, polyetheretherketone.
a Data reported included manually shaped and custom patient-specific titanium implants.
Figure 9.
Complication rates for patients treated with various cranioplasty implant types (n = 119). Data are presented as the total incidence of complications including headache, seizures, and pain, some of which may not be related to cranioplasty. Complications requiring surgical intervention are all related to the cranioplasty and range from scar revision, fluid aspiration, lumbar drain to implant removal. Polymethyl methacrylate (PMMA; acrylic) and polyetheretherketone (PEEK) were associated with higher complication rates (29% and 27%, respectively).
Discussion
This investigation is a single-centre retrospective chart review that compares operative duration, inpatient stay, and total hospital costs incurred for patients undergoing elective cranioplasty with manually shaped or CPS titanium implants, PMMA, autogenous bone implants, or PEEK. It is of vital importance to recognize that these patient populations are not strictly comparable because the clinical indications for the use of a specific implant type varies with etiology, size, and location of skull defect. Specifically, manually shaped titanium implants were used in small cranial defects only. Prefabricated PMMA or PEEK implants can only be used in the elective reconstruction of defined skull defects, where the size and/or shape of the defect will not be modified at the time of surgery. Autogenous split skull bone grafts and CPS titanium mesh implants are specifically designed for reconstruction of the “unknown defect,” that is, ablative surgery where the size and shape of the skull defect cannot be predicted. Autogenous split skull bone grafts and CPS titanium mesh implants are also preferentially used in frontal reconstruction, where there is a potential for encroachment on or exposure to paranasal sinuses. Certain reconstructive modalities, therefore, are used in more challenging recipient sites.
In terms of total hospital costs, our results indicate that cranioplasty using CPS titanium implants costs CAD$15 147 ± CAD$4693 more than cranioplasty using manually shaped titanium implants. However, if cases requiring cancer excision under frozen section control or free tissue transfer for resurfacing of extensive composite defects are removed, cranioplasty using PMMA and PEEK results in total costs CAD$4442 ± CAD$2100 and CAD$13 372 ± CAD$2728 more than cranioplasty with manually shaped titanium implants.
One factor contributing to total hospital costs is operative duration. The present investigation observed that cranioplasty with autogenous bone graft and CPS titanium implants resulted in a longer operative duration compared to manually shaped titanium implants. However, upon the removal of outliers, the average operative duration of CPS titanium implants decreased from 308 ± 190 minutes to 227 ± 137 minutes, leaving only cranioplasty with autogenous implants with a significantly longer operative duration. These data are similar to previous investigations that observed a longer operative duration for cranioplasty with autogenous compared to PSI or CCGI.15,25,26 The longer operative duration for autogenous implants is driven, primarily, by the technical requirements of the procedure, including harvesting of bone, manual carving, shaping, and assembly of bone segments.20
In addition to operative duration, total hospital costs are greatly influenced by the length of hospital stay. Some investigations report hospital stays up to 6 days for custom titanium implants,27 2 days for autogenous implants,25 and 4.5 days for PEEK.19 In the present investigation, we observed an average ICU stay of 1.4 days, with no significant difference between the groups. However, a multivariate analysis revealed that CPS titanium implants resulted in an ICU stay 1.24 days longer than cranioplasty with manually shaped titanium implants. Further, upon the removal of outliers, cranioplasty with CPS titanium implants and PMMA resulted in a longer recovery on ward (2.4 and 2.1 days, respectively) compared to manually shaped titanium implants.
In contrast, recent investigations report that cranioplasty with autogenous implants results in a longer inpatient stay compared to CCGI, but this does not translate into statistically significant differences in total hospital costs.15,25 Of note, despite a longer operative duration, hospital stay, implant issues, or infection, previous studies have documented that cranioplasty with an autogenous bone graft is cheaper than custom PEEK implants24 or PSI in any other material.25 In the present investigation, an age-controlled analysis revealed that cranioplasty with CPS titanium implants costs more than cranioplasty with manually shaped titanium implants. However, when outliers were removed, cranioplasty with PMMA and PEEK is more expensive than manually shaped titanium implants. Although not accounted for in our analysis, it is worth noting that CPS titanium implants and PMMA were used to treat large cranial defects, which may influence total costs and complication rates.20,28 Of note, Li et al28 analyzed 8275 cranioplasty patients and observed that defect size, time of cranioplasty, and previous infection, rather than implant material, increased complication rates and cost. Specifically, large cranioplasties (>5 cm) and delayed cranioplasties (>90 days) resulted in a higher complication rate, whereas the use of autographs did not.28 Similar to Li et al28 and despite the high complication rate associated with cranioplasty (10%-40%),4 we report no difference in major complication rates between the groups.
Although our sample size was substantially smaller than Li et al,28 a strength of our investigation was the decision to perform the analysis twice. The decision to do so was 2-fold; first, the medium sample size encouraged the inclusion of outliers to increase the number of data points for the analysis. Second, we sought to examine the effect of complex cases (cancer resection with frozen section and free tissue transfer for associated scalp defect reconstruction) on our outcomes. Our results indicate that the inclusion of outliers differentially influenced operative duration, recovery in ICU or on ward, and total hospital costs. As such, our data suggest that investigations with small sample sizes (n < 50) should consider if the inclusion of outliers is appropriate and recognize the consequences of their removal.
To the best of our knowledge, this is the largest cost analysis to date examining various materials used for cranioplasty. Our aim was to provide objective evidence to inform reconstructive surgeons on the operative time, inpatient stay, and total costs associated with various implant materials. Although we performed a thorough analysis of various outcomes with and without outliers, this investigation is not without limitations. We used a single-centre retrospective chart review, and so the cost associated with cranioplasty may vary across Canadian centres. Further, we did not assess aesthetic or patient-reported satisfaction, and wages lost due to inpatient stay were not included in analyses.
In summary, small cranial defects are amenable to reconstruction with manually shaped titanium or autogenous split skull bone graft, and both of these techniques are the most cost-effective. Large paranasal sinus defects and large ablative “unknown” defects can only be reconstructed with autogenous bone or CPS titanium mesh. Autogenous bone reconstruction results in significantly prolonged operative duration, while CPS titanium mesh is associated with prolonged hospital ward admission. Despite this, the cost of autogenous bone or CPS titanium mesh reconstruction is not significantly greater than manually shaped titanium implants. Elective cranioplasty of large stable skull defects far removed from potential sinus contamination can be repaired by any type of implant material (titanium, PMMA, PEEK). Of these, custom PMMA and PEEK implants are significantly more costly, and the incidence of complications necessitating reoperations may contribute to this.
Supplementary Material
Footnotes
Level of Evidence: Level 3, Therapeutic
Authors’ Note: Ethics approval was obtained from Sunnybrook Health Sciences Centre.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Josie Jakubowski, MSc  https://orcid.org/0000-0002-0743-7913
https://orcid.org/0000-0002-0743-7913
References
- 1. De Bonis P, Frassanito P, Mangiola A, Nucci CG, Anile C, Pompucci A. Cranial repair: how complicated is filling a “hole”? J Neurotrauma. 2012;29(6):1071–1076. doi:10.1089/neu.2011.2116 PubMed PMID: 22059899. [DOI] [PubMed] [Google Scholar]
- 2. Kuo JR, Wang CC, Chio CC, Cheng TJ. Neurological improvement after cranioplasty—analysis by transcranial doppler ultrasonography. J Clin Neurosci. 2004;11(5):486–489. doi:10.1016/j.jocn.2003.06.005 PubMed PMID: 15177389. [DOI] [PubMed] [Google Scholar]
- 3. Dujovny M, Aviles A, Agner C, Fernandez P, Charbel FT. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47(3):238–241. PubMed PMID: 9068693. [DOI] [PubMed] [Google Scholar]
- 4. Piazza M, Grady MS. Cranioplasty. Neurosurg Clin N Am. 2017;28(2):257–265. doi:10.1016/j.nec.2016.11.008 PubMed PMID: 28325460. [DOI] [PubMed] [Google Scholar]
- 5. Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC. Cranioplasty: review of Materials. J Craniofac Surg. 2016;27(8):2061–2072. doi:10.1097/SCS.0000000000003025 PubMed PMID: 28005754. [DOI] [PubMed] [Google Scholar]
- 6. Wiggins A, Austerberry R, Morrison D, Ho KM, Honeybul S. Cranioplasty with custom-made titanium plates—14 years experience. Neurosurgery. 2013;72(2):248–256; discussion 56. doi:10.1227/NEU.0b013e31827b98f3 PubMed PMID: 23149967. [DOI] [PubMed] [Google Scholar]
- 7. Badhey A, Kadakia S, Mourad M, Inman J, Ducic Y. Calvarial Reconstruction. Semin Plast Surg. 2017;31(4):222–226. doi:10.1055/s-0037-1606557 PubMed PMID: 29075161; PubMed Central PMCID: PMCPMC5656441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg Focus. 2014;36(4):E19 doi:10.3171/2014.2.FOCUS13561 PubMed PMID: 24684331. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein JA, Paliga JT, Bartlett SP. Cranioplasty: indications and advances. Curr Opin Otolaryngol Head Neck Surg. 2013;21(4):400–409. doi:10.1097/MOO.0b013e328363003e PubMed PMID: 23770828. [DOI] [PubMed] [Google Scholar]
- 10. Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann Maxillofac Surg. 2014;4(1):9–18. doi:10.4103/2231-0746.133065 PubMed PMID: 24987592; PubMed Central PMCID: PMCPMC4073471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnaoutakis D, Bahrami A, Cohn JE, Smith JE. Cranioplasty using a mixture of biologic and nonbiologic agents. JAMA Facial Plast Surg. 2018;20(1):9–13. doi:10.1001/jamafacial.2017.0437 PubMed PMID: 29098278; PubMed Central PMCID: PMCPMC5833661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogers GF, Greene AK. Autogenous bone graft: basic science and clinical implications. J Craniofac Surg. 2012;23(1):323–327. doi:10.1097/SCS.0b013e318241dcba PubMed PMID: 22337435. [DOI] [PubMed] [Google Scholar]
- 13. Cho YR, Gosain AK. Biomaterials in craniofacial reconstruction. Clin Plast Surg. 2004;31(3):377–385. doi:10.1016/j.cps.2004.03.001. PubMed PMID: 15219744. [DOI] [PubMed] [Google Scholar]
- 14. Bobinski L, Koskinen LO, Lindvall P. Complications following cranioplasty using autologous bone or polymethylmethacrylate—retrospective experience from a single center. Clin Neurol Neurosurg. 2013;115(9):1788–1791. doi:10.1016/j.clineuro.2013.04.013 PubMed PMID: 23725651. [DOI] [PubMed] [Google Scholar]
- 15. Gilardino MS, Karunanayake M, Al-Humsi T, et al. A comparison and cost analysis of cranioplasty techniques: autologous bone versus custom computer-generated implants. J Craniofac Surg. 2015;26(1):113–117. doi:10.1097/SCS.0000000000001305 PubMed PMID: 25534061. [DOI] [PubMed] [Google Scholar]
- 16. Huang GJ, Zhong S, Susarla SM, Swanson EW, Huang J, Gordon CR. Craniofacial reconstruction with poly(methyl methacrylate) customized cranial implants. J Craniofac Surg. 2015;26(1):64–70. doi:10.1097/SCS.0000000000001315 PubMed PMID: 25376145. [DOI] [PubMed] [Google Scholar]
- 17. Marchac D, Greensmith A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J Plast Reconstr Aesthet Surg. 2008;61(7):744–752; discussion 53. doi:10.1016/j.bjps.2007.10.055 PubMed PMID: 18474454. [DOI] [PubMed] [Google Scholar]
- 18. Wolff A, Santiago GF, Belzberg M, et al. Adult cranioplasty reconstruction with customized cranial implants: preferred technique, timing, and biomaterials. J Craniofac Surg. 2018;29(4):887–894. doi:10.1097/SCS.0000000000004385 PubMed PMID: 29489570. [DOI] [PubMed] [Google Scholar]
- 19. Punchak M, Chung LK, Lagman C, et al. Outcomes following polyetheretherketone (PEEK) cranioplasty: systematic review and meta-analysis. J Clin Neurosci. 2017;41:30–35. doi:10.1016/j.jocn.2017.03.028 PubMed PMID: 28377284. [DOI] [PubMed] [Google Scholar]
- 20. Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: review over the last 11 years. J Plast Reconstr Aesthet Surg. 2010;63(10):1615–1623. doi:10.1016/j.bjps.2009.06.003. PubMed PMID: 19577527. [DOI] [PubMed] [Google Scholar]
- 21. Cabraja M, Klein M, Lehmann TN. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26(6):E10 doi:10.3171/2009.3.FOCUS091 PubMed PMID: 19485714. [DOI] [PubMed] [Google Scholar]
- 22. Maqbool T, Binhammer A, Binhammer P, Antonyshyn OM. Risk Factors for titanium mesh implant exposure following cranioplasty. J Craniofac Surg. 2018;29(5):1181–1186. doi:10.1097/SCS.0000000000004479 PubMed PMID: 29533254. [DOI] [PubMed] [Google Scholar]
- 23. Yoshioka N, Tominaga S. Titanium mesh implant exposure due to pressure gradient fluctuation. World Neurosurg. 2018;119:e734–e739. doi:10.1016/j.wneu.2018.07.255 PubMed PMID: 30092473. [DOI] [PubMed] [Google Scholar]
- 24. Thien A, King NK, Ang BT, Wang E, Ng I. Comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2015;83(2):176–180. doi:10.1016/j.wneu.2014.06.003 PubMed PMID: 24909393. [DOI] [PubMed] [Google Scholar]
- 25. Mrad MA, Murrad K, Antonyshyn O. Analyzing the cost of autogenous cranioplasty versus custom-made patient-specific alloplastic cranioplasty. J Craniofac Surg. 2017;28(5):1260–1263. doi:10.1097/SCS.0000000000003708 PubMed PMID: 28582300. [DOI] [PubMed] [Google Scholar]
- 26. Lethaus B, Bloebaum M, Koper D, Poort-Ter Laak M, Kessler P. Interval cranioplasty with patient-specific implants and autogenous bone grafts—success and cost analysis. J Craniomaxillofac Surg. 2014;42(8):1948–1951. doi:10.1016/j.jcms.2014.08.006 PubMed PMID: 25443869. [DOI] [PubMed] [Google Scholar]
- 27. Williams LR, Fan KF, Bentley RP. Custom-made titanium cranioplasty: early and late complications of 151 cranioplasties and review of the literature. Int J Oral Maxillofac Surg. 2015;44(5):599–608. doi:10.1016/j.ijom.2014.09.006 PubMed PMID: 25482456. [DOI] [PubMed] [Google Scholar]
- 28. Li A, Azad TD, Veeravagu A, et al. Cranioplasty complications and costs: a national population-level analysis using the marketscan longitudinal database. World Neurosurg. 2017;102:209–220. doi:10.1016/j.wneu.2017.03.022 PubMed PMID: 28315803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.