Abstract
Background:
Endoscopic transaxillary augmentation mammaplasty breast augmentation offers several advantages over other augmentation methods. Nonetheless, this procedure is fraught with some problems, including greater surgical trauma due to the longer separation area. We hypothesized that cold separation of the interpectoral space could reduce surgical injury in comparison to the electrosurgical method. This study aimed to compare the outcomes of endoscopic-assisted transaxillary augmentation mammaplasty using cold separation versus electrosurgical separation of the interpectoral space.
Methods:
In this prospective clinical trial, cold and electrosurgical separation of the interpectoral space were achieved using a separation shovel and monopolar electrotome, respectively. A total of 20 patients who visited our department in Beijing, China, for primary breast augmentation surgeries from October 1, 2017, and May 31, 2018, were included. The primary outcome was total postoperative drainage volume. The secondary outcomes were operative time, daily drainage volume, daily pain as assessed using the visual analogue scale (VAS), and reoperation rate. Quantitative data were compared using independent-samples t test. Chi-square test was used to compare 2 classified indexes.
Results:
The total drainage volume was significantly lower in the cold separation group than in the electrosurgical separation group (170.45 ± 75.40 mL vs 281.05 ± 148.43 mL; P = .005). The VAS score on the first postoperative day was significantly lower in the cold separation group than in the electrosurgical separation group (6.45 ± 1.93 vs 7.55 ± 1.43; P = .048). Two (20%) reoperations owing to postoperative pain or implant stiffness were performed in the electrosurgical separation group.
Conclusions:
Cold separation is more conducive to reducing drainage, relieving postoperative pain, and causing less damage than the electrosurgical method in endoscopic-assisted transaxillary dual-plane augmentation mammaplasty.
Keywords: mammoplasty, transaxillary augmentation, interpectoral space
Abstract
Historique:
L’augmentation mammaire transaxillaire par voie endoscopique comporte plusieurs avantages par rapport aux autres méthodes d’augmentation. Cette intervention se heurte toutefois à certains problèmes, y compris des traumatismes chirurgicaux plus importants à cause de la zone de séparation plus longue. Les auteurs ont postulé que la séparation de l’espace interpectoral par le froid réduirait davantage la lésion que la méthode électrochirurgicale. La présente étude visait à comparer les résultats cliniques de l’augmentation mammaire transaxillaire assistée par endoscopie au moyen de la séparation par le froid plutôt que par la séparation électrochirurgicale de l’espace interpectoral.
Méthodologie:
Dans la présente étude clinique prospective, la séparation par le froid et la séparation électrochirurgicale de l’espace interpectoral ont été assurées par une pince de séparation et une électrode monopolaire, respectivement. Au total, 20 participants qui ont consulté le département des auteurs à Beijing, en Chine, afin de subir une augmentation mammaire primaire entre le 1er octobre 2017 et le 31 mai 2018 ont participé à l’étude. Le résultat primaire était le volume total de drainage postopératoire. Les résultats secondaires étaient la durée de l’opération, le volume de drainage quotidien, la douleur quotidienne évaluée à l’aide de l’échelle analogique visuelle (ÉAV) et le taux de réopérations. Les chercheurs ont utilisé le test du chi carré pour comparer deux indices répertoriés.
Résultats:
Le volume de drainage total était considérablement plus faible dans le groupe de séparation par le froid que dans celui de séparation électrochirurgicale (170,45 ± 75,40 mL par rapport à 281,05 ± 148,43 mL; P = 0,005). Le score d’ÉAV le premier jour postopératoire était considérablement plus faible dans le groupe de séparation par le froid que dans celui de séparation électrochirurgicale (6,45 ± 1,93 par rapport à 7,55 ± 1,43; P = 0,048). Deux réopérations (20 %) causées par la douleur postopératoire ou la rigidité de l’implant ont été exécutées dans le groupe de séparation électrochirurgicale.
Conclusions:
La séparation par le froid favorise la diminution du drainage, le soulagement de la douleur postopératoire et la réduction des dommages davantage que la méthode életrochirurgicale en cas d’augmentation mammaire transaxillaire biplan assistée par endoscopie.
Introduction
Transaxillary augmentation mammaplasty was first described in 1970s.1 Its most important advantage is prevention of any visible scar on the breasts.2-4 Moreover, the addition of endoscopy enables accurate pocket positioning and precise hemostasis.5 Dissimilar to Western women for whom lower fold incisions tend to be used, some conservative Chinese women are more likely to opt for an axillary incision owing to the concern about their sexual partners’ awareness of their breast augmentation history.6-10
Endoscopic-assisted transaxillary augmentation mammaplasty offers several advantages over other methods. Nonetheless, this procedure is fraught with some problems, including more pain, prolonged recovery time, and the requirement for a supportive bra or pectoral band to be worn for months after surgery.11,12 Unfortunately, little recent progress in the strategies for further surgical procedure optimization to reduce patients’ discomfort has been achieved.
Reducing surgical trauma is an important aspect of surgical procedure optimization. The underlying space between the pectoralis major and the minor muscles, commonly referred to as the interpectoral space or foam layer, contains numerous small blood vessels, nerves, and lymphatic vessels.11 The larger separation of this space poses an inherent challenge to endoscopic-assisted transaxillary augmentation mammaplasty compared to other approaches.10,13 Thus, we speculate that updating the technique for the separation of the interpectoral space, which has long been neglected, may be key to reducing surgical trauma, thereby promoting patients’ recovery.
Monopolar electrotomes are traditional instruments typically used in surgical separation during endoscopic-assisted transaxillary augmentation mammaplasty. Their use can reduce blood loss due to the coagulative effect of diathermy on the microcirculation in the area immediately adjacent to the incision.14,15 However, due to extreme heat, monopolar electrotomes can cause substantial thermal injury to the surrounding tissues, which may result in significant postoperative pain and poor wound healing.16 Therefore, an update on the application of surgical techniques is not only essential to achieve effective separation during procedures and but also imperative to obtain optimal intra- and postoperative results.17 Blunt separation during transaxillary breast augmentation has been rejected by some scholars because it is usually used under blinded condition, which may result in imprecise tissue dissection.18 However, the use of an endoscope to separate the foam layer can avoid this risk.
We hypothesized that cold separation of the interpectoral space could reduce the accumulation of acute inflammatory exudates and prevent capillary and lymphatic leakage, hence decreasing the duration and quantity of serosanguineous drainage and alleviating patients’ pain. Drainage volume is a common index used for the assessment of surgical trauma and for the prediction of seroma risk after breast surgery.19 Thus, total postoperative drainage volume was selected as the primary outcome in this study. Furthermore, pain level, postoperative complication, and reoperation rate were analyzed and compared to those in the literature. This prospective clinical trial aimed to compare the outcomes of endoscopic-assisted transaxillary augmentation mammaplasty using cold separation versus electrosurgical separation of the interpectoral space.
Patients and Methods
Cold separation and electrosurgical separation of the interpectoral space were achieved using a separation shovel and monopolar electrotome (ERBE Elektromedizin GmbH, Tuebingen, Germany), respectively. Inpatients aged 18 to 70 years who underwent surgery at our hospital and signed informed consent were included in this study. Patients who (1) had already participated in other clinical trials conducted within 4 weeks prior to the start of the study; (2) were treated with anticoagulants; (3) had respiratory depression, (pulmonary) airway obstruction or tissue hypoxia, biliary tract disease, cardiac disease (ie, ≥grade II cardiac function), systemic diseases, liver and kidney dysfunction (ie, index more than twice the normal value), and neurological disorders; (4) had above normal blood pressure and serum levels within 2 weeks prior to study initiation; (5) had abnormal judgment ability; (6) had a history of drug and/or alcohol abuse; and (7) were pregnant or lactating were excluded from the analysis. The elimination criteria included noncompliance with the enrollment criteria, nonstandard case report forms, and withdrawal from the trial without adverse reactions or poor efficacy. Furthermore, patients were withdrawn from the study if the researchers considered the discontinuation of the test as necessary for the patients from a medical point of view or if patients requested for the discontinuation of the test themselves (Table 1).
Table 1.
Inclusion, Exclusion, Elimination, and Withdrawal Criteria.
| Inclusion criteria |
| Participants: a patient undergoing surgery in our hospital |
| Age: 18-70 years old |
| Female |
| Hospitalized patients |
| Signature of informed consent |
| Exclusion criteria: |
| 4 weeks before the start of this study, I participated in other clinical trials |
| Taking anticoagulant drugs within 2 weeks before taking the drug or starting the trial |
| Respiratory depression, airway obstruction, or hypoxia |
| Biliary tract diseases |
| Heart disease (grade 2 and grade 2 cardiac function) |
| Blood pressure is above normal |
| Hematological diseases |
| The liver and kidney function were obviously abnormal (ie, the index was more than twice the normal value) |
| Brain disorders, abnormal ability to determine |
| Drugs and/or alcohol abuse |
| Pregnant women or lactating women |
| Elimination criteria |
| Cases that do not conform to the inclusion criteria and case reports are not standardized |
| Cases not withdrawn from trial due to adverse reactions or poor efficacy |
| Midway withdrawal criteria for patients |
| From the perspective of medicine, researchers consider that it is necessary for the patients to stop the experiment |
| The patient himself asked to stop the experiment |
Patients who met the inclusion criteria were randomly divided into 2 groups: the cold separation group and the electrosurgical separation group. Randomization was conducted using a random number table. The sample size was 20 breasts, with the cold separation and the electrosurgical separation groups each comprising 10 patients. To ensure the accuracy of separation of the interpectoral space in the 2 groups, an electrotome was used to sever the muscle attachment points, and endoscopy was performed to confirm the separation boundary; additionally, the pectoralis major muscle was cut to ensure the formation of accurate biplane. In other words, the surgical separations performed in the 2 groups differed only with respect to the interpectoral space. For standardization, all surgeries were completed by senior surgeons with similar experience (250-300 endoscopic-assisted transaxillary dual-plane augmentation mammaplasty case per year) from the same surgical team. Drainage was recorded by nurses blinded to the intervention, whereas other outcomes were recorded by a senior resident. The primary outcome was total drainage volume. The secondary outcomes were operative time, daily drainage volume in postoperative drainage days, postoperative daily pain as assessed using the visual analogue scale (VAS), postoperative complications, and reoperation rate.
Ethics
This study received approval from the ethics committee of Plastic Surgery Hospital. Following provision of information about the trial, appropriate informed consent was obtained from all patients.
Statistical Analysis
Double entry of data from case report forms was performed, and the database was verified after confirmation. Measurement data were expressed as mean and standard deviation, and the number and percentage of counts were determined. Baseline demographic characteristics of the 2 groups were analyzed, and balance and comparability were investigated. Subsequently, outcome indicators of the 2 groups were compared.
Quantitative data on age, drainage volume, drainage days, and degree of pain were compared between the 2 groups using independent samples t test. Chi-square test was used to compare 2 classified indexes such as implant brands. The incidence of complications was compared using 2-sample rank-sum test. Statistical analysis was performed using Prism 8 (GraphPad Software, San Diego, California) and SPSS (IBM Corp, Armonk, New York). All statistical tests were conducted as 2-sided, and P values <.05 were considered statistically significant.
Data Collection
Preoperative data
All patients underwent standard preoperative examination. The following items were recorded to determine the existence of any known or unknown hemorrhagic factors: history of hematologic diseases, hematocrit count, hemoglobin count, international normalized ratio, fibrinogen level, activated partial thromboplastin time, and prothrombin time. Preoperative images of patients were acquired.
Intraoperative data
The amount of blood loss in the operating room was not estimated, as it was too small to be accurately determined. Data on the following were collected: operative time, brand, shape, surface, and size of plants, dual-plane type, position of muscle amputated.
Postoperative data
The drainage tube was removed when the drainage volume reached <50 mL within 24 hours. In the event of bright red drainage, the drainage tube was extended based on clinical conditions. Daily drainage volume (mL) in each drainage tube and duration of drainage tube placement (days) were recorded by a blind reviewer (breast care nurse).
Using VAS, patients in both groups rated their pain in each breast from 0 (no pain) to 10 (severe pain) on each day after surgery. Pain scores on all postoperative days were obtained. A senior resident assessed postoperatively the patients for wound hematoma, effusion, or seroma in the outpatient department. If hematoma was present, and its diameter between the 2 farthest points was measured and recorded. Any wound infection requiring antibiotic treatment and other complications were recorded. Postoperative images of patients were acquired.
Telephone or outpatient follow-up was performed to confirm revision surgery. The reasons for reoperation as well as revision time and procedures were recorded. Postoperative images of patients were acquired.
Results
A total of 20 patients who visited our department for primary breast augmentation surgeries from October 2017 to May 2018 and met the inclusion criteria were randomly divided into either cold separation or electrosurgical separation group, with 20 breasts of 10 patients each. The 2 study groups were well balanced and exhibited similar baseline demographic characteristics. The average age of the cold and electrosurgical separation groups was 33.50 ± 6.47and 32.00 ± 9.58 years, respectively. Furthermore, both groups showed similar body mass index (18.90 ± 1.43 and 18.71 ± 1.22 in the cold and electrosurgical separation groups, respectively); diagnosis, marriage status, and medical history (Table 2); and hematological parameters (Table 3).
Table 2.
Demographic Data.
| Cold Separation Group | Electrosurgical Separation Group | P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 33.50 | 6.47 | 32.00 | 9.58 | .686 |
| BMI | 18.90 | 1.43 | 18.71 | 1.22 | .762 |
| Number of Patients | Breast dysplasia | Breast atrophy | Breast dysplasia | Breast atrophy | |
| 4 (40.0%) | 6 (60.0%) | 6 (60.0%) | 4 (40.0%) | .371 | |
| Medical diseases | Yes | No | Yes | No | |
| 0 (0.0%) | 10 (100.0%) | 1 (10.0%) | 9 (90.0%) | .304 | |
| 0.00 | 10.00 | 0.00 | 10.00 | ||
| Marital status | Married | Unmarried | Married | Unmarried | |
| 7 (70.0%) | 3 (30.0%) | 6 (60.0%) | 4 (40.0%) | .639 | |
Abbreviations: BMI, body mass index; SD, standard deviation.
Table 3.
Hematological Indexes.
| Cold Separation Group | Electrosurgical Separation Group | P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HCT, % | 37.990 | 3.376 | 44.740 | 17.480 | .246 |
| Hb, g/L | 126.300 | 7.718 | 126.600 | 14.968 | .956 |
| APTT, s | 23.030 | 3.750 | 23.460 | 3.737 | .800 |
| PT, s | 12.380 | 0.798 | 12.100 | 1.150 | .535 |
| INR | 1.032 | 0.057 | 1.003 | 0.085 | .383 |
| FBG, g/L | 2.380 | 0.388 | 2.530 | 0.406 | .409 |
Abbreviations: APTT, activated partial thromboplastin time; FBG, fibrinogen; HCT, hematocrit; Hb, hemoglobin; INR, international normalized ratio; PT, prothrombin time; SD, standard deviation.
With respect to baseline intraoperative data, no significant differences in operative time, implant brands and surface, dual-plane type, and position of muscle amputated were observed between the 2 groups (Table 4). Implant volume was significantly larger in the cold separation group (278.50 ± 28.66 cc) than in the electrosurgical separation group (253.25 ± 34.00 cc). Moreover, 70% and 100% of implants had an anatomic shape in the cold and electrosurgical separation groups, respectively (Table 4).With respect to baseline postoperative data, no significant difference in the type of hemostasis drugs and their time of administration were noted between the 2 groups.
Table 4.
Intraoperative Indexes.
| Cold Separation Group | Electrosurgical Separation Group | P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Operative time | 117.50 | 36.00 | 126.00 | 24.01 | .542 |
| Implant brand | Mentor | Others | Mentor | Others | |
| 10 (100.0%) | 0 (0.0%) | 9 (90.0%) | 1 (10.0%) | .305 | |
| Implant surface | Textured | Smooth | Textured | Smooth | |
| 10 (100.0%) | 0 (0.0%) | 10 (100.0%) | 0 (0.0%) | ||
| Implant shape | Round | Anatomically shaped | Round | Anatomically shaped | |
| 6 (30%) | 14 (70%) | 0 (0%) | 20 (100.0%) | .008 | |
| Implant volume | 278.50 | 28.66 | 253.25 | 34.00 | .015 |
| Dual-plane type | I type | II type | I type | II type | |
| 7 (70%) | 3 (30%) | 8 (80%) | 2 (20%) | .606 | |
| Position of muscle amputation | 1.35 | 0.24 | 1.40 | 0.32 | .696 |
Abbreviation: SD, standard deviation.
Primary Outcome
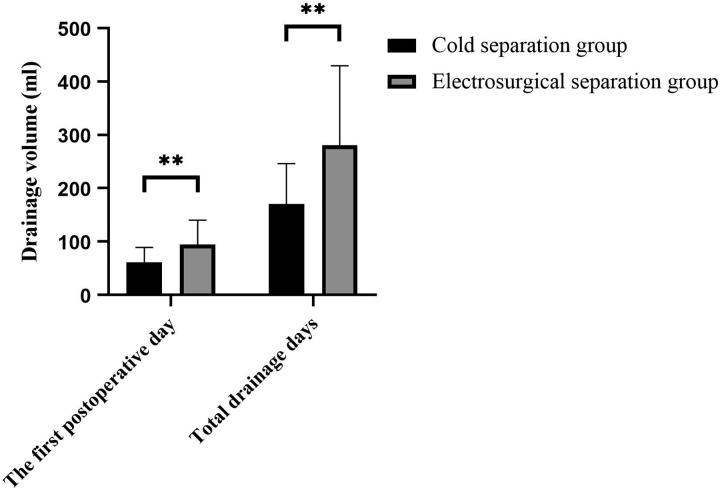
The primary outcome measure was the total drainage volume. The total drainage volume was significantly lower in the cold separation group than in the electrosurgical separation group (170.45 ± 75.40 mL vs 281.05 ± 148.43 mL; P = .005; Table 5, Figure 1).
Table 5.
Postoperative Indexes of Drainage.
| Cold Separation Group | Electrosurgical Separation Group | P Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
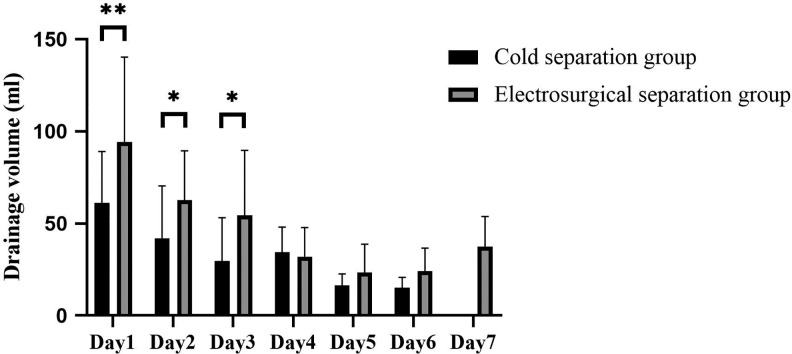
| Drainage volume of day 1 | 61.25 | 27.76 | 94.25 | 46.09 | .009 |
| Drainage volume of day 2 | 42.00 | 28.49 | 62.65 | 26.84 | .024 |
| Drainage volume of day 3 | 29.67 | 23.55 | 54.41 | 35.26 | .019 |
| Drainage volume of day 4 | 34.44 | 13.60 | 32.00 | 15.79 | .636 |
| Drainage volume of day 5 | 16.25 | 6.41 | 23.43 | 15.37 | .226 |
| Drainage volume of day 6 | 15.00 | 5.77 | 24.17 | 12.58 | .188 |
| Drainage volume of day 7 | 37.50 | 16.36 | |||
| Postoperative total drainage volume, mL | 170.45 | 75.40 | 281.05 | 148.43 | .005 |
| Postoperative drainage days, mL | 4.40 | 1.14 | 5.60 | 2.72 | .077 |
Abbreviation: SD, standard deviation.
Figure 1.
Daily drainage volume on the first day after surgery was significantly less in the cold separation group than in the electrosurgical separation group (61.25 ± 27.76 mL vs 94.25 ± 46.09 mL), and the total drainage volume was significantly lower in the cold separation group than in the electrosurgical separation group (170.45 ± 75.40 mL vs 281.05 ± 148.43mL; P = .005). P value <.05, *; ≤.01, **.
Secondary Outcomes
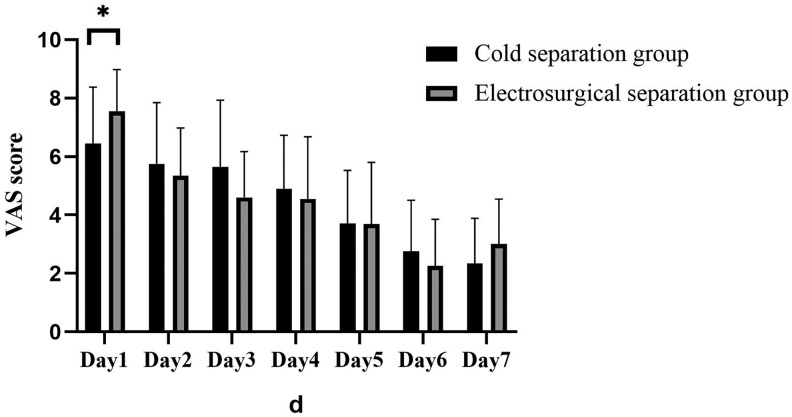
With respect to secondary outcomes, no difference in operative time was observed between the cold and the electrosurgical separation groups (117.50 ± 36.00 minutes and 126.00 ± 24.01 minutes, respectively; Table 4). Daily drainage volume on the first 3 days after surgery was significantly less in the cold separation group than in the electrosurgical separation group, especially on the first day postoperatively (61.25 ± 27.76 mL vs 94.25 ± 46.09 mL; Table 5, Figure 2). There was no significant difference in drainage days and postoperative aesthetic outcome (Figure 3) between the cold and the electrosurgical separation groups. VAS score of the electrosurgical separation group was significantly higher than that of the cold separation group on the first postoperative day (7.55 ± 1.43 vs 6.45 ± 1.93, p = 0.048; Figure 4). Hematoma formation and wound infection were not detected in both groups during the observation period. Two (20%) reoperations were performed in the electrosurgical separation group because of postoperative pain (3 months after the first operation) or feeling of tightness with the implant (8 months after the first operation). In contrast, no reoperation (0%) was performed in the cold separation group.
Figure 2.
In either group, drainage production peaked at 24 hours after surgery and subsequently decreased rapidly, which is congruent with the theoretical process of acute inflammatory response. P value <.05, *; .05, e, **.
Figure 3.
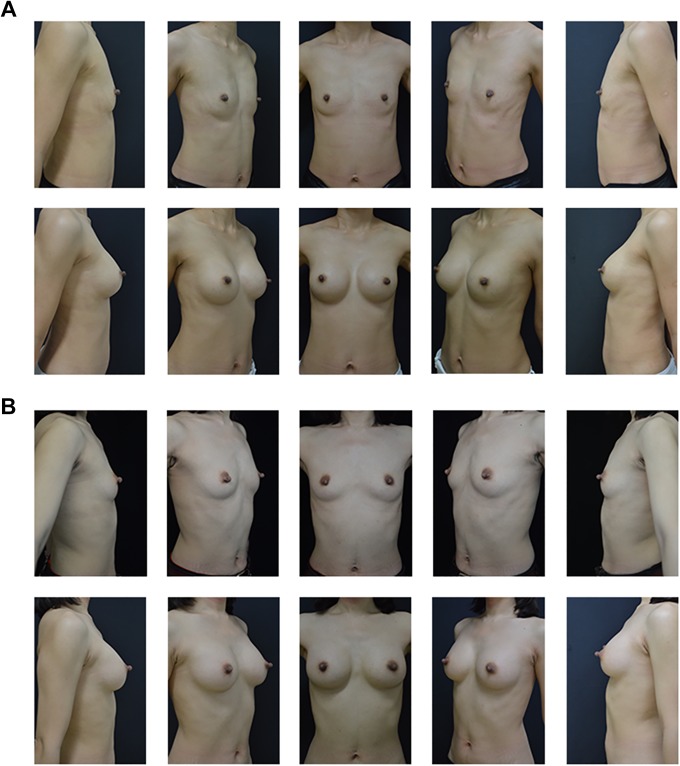
There was no significant difference in postoperative aesthetic outcomes between the 2 groups. A, preoperative and postoperative morphology of cold separation group after 6 months. B, Preoperative and postoperative morphology of electrosurgical separation group after 6 months.
Figure 4.
There was significant difference between 2 groups in terms of VAS score on the first day (cold method group 6.45 + 1.93 vs electrosurgical separation group 7.55 + 1.43, p = 0.048. And the trend of VAS score was similar to that of drainage. P value <.05, *; .01, **. VAS indicates visual analogue scale.
Briefly, there existed strong evidence that drainage was lesser when the foam layer was bluntly separated using cold method. The total drainage volume on the first 3 days after surgery was significantly different between the 2 groups. As indicated by the VAS score, patients in the cold separation group experienced less pain on the first postoperative day. The rate of revision surgery was higher in the electrosurgical separation group than in the cold separation group. Nonetheless, there was no evidence that any other secondary outcomes were different in the two groups.
Discussion
In this study, we used the cold and the electrosurgical methods to separate the interpectoral space and mainly compared the postoperative drainage pattern and pain level. We observed that the cold separation group had lower total drainage volume and experienced less pain within 24 hours after surgery. No significant complications were identified in either group. Good aesthetic outcomes were achieved using both methods, which was consistent with the results of previous studies on endoscopic-assisted transaxillary dual-plane augmentation mammaplasty in the literature.20-22
Several studies have shown the association between inflammatory factors and exudate formation, which may be the body’s natural response to tissue damage.19,23-26 In our study, drainage production peaked at 24 hours after surgery and subsequently decreased rapidly, which is congruent with the theoretical process of acute inflammatory response.27,28 Thus, our results supported the proinflammatory theory that seroma formation has an inflammatory component that seems to be acute-phase inflammatory reaction.25 Both cold and electrosurgical separation method seemed to follow the development of inflammatory response; however, the drainage volume was higher with the electrosurgical method, particularly on the first postoperative day, which most closely resembles that with surgical methods. This might have resulted from the increased thermal injury caused by electrosurgical separation, which promoted the inflammatory response process. Wu et al reported that thermal injury is associated with greater histologic disturbance,29 which is always less reversible than that caused by traction injury.30 In the study of Szecsi et al, significantly higher levels of cytokines, interleukin 6, and tumor necrosis factor in drain fluids were observed in the electrocautery group than in the scalpel dissection group.25
According to prior studies in the literature, 10 to 50 mL of serosanguinous or frankly sanguineous fluid is drained overnight for the first 24 hours after breast augmentation with blunt separation,31 and 30 to 150 mL of serosanguinous fluid is usually drained for 24 to 48 hours.32 The results of previous studies are in accordance with our findings.33-36 Furthermore, the drainage time was mostly longer than 72 hours after surgery in our study, which was greater than the time reported in the literature (ie, 24-72 hours).31,34-36 Unfortunately, the use of postoperative drainage in breast augmentation is seldom specified in reports describing the electrosurgical method.33-36 This may be because most augmentation mammaplasties are performed in the clinic, and no drainage tube is placed. However, our study showed that the total drainage volume could reach 392 mL and 710 mL after surgery using the cold and the electrosurgical methods, respectively. Whether exudates will be absorbed, how long can exudates be absorbed, and whether the slow absorption process will lead to seroma, capsular contracture, or other complications if drainage tubes are not placed are worth discussing.
Caputo et al investigated the trend of postoperative daily serum collection after acellular dermal matrix-assisted breast reconstruction to further explore the pathogenesis of seroma formation; they showed that drainage volume could aid in predicting the risk of seroma after breast surgery.28 Jiang et al reported that placement of drainage tubes after breast augmentation could reduce complications such as seroma.37 Therefore, identifying the exact cause of seroma formation by drainage analysis and controlling it are very important to reduce seroma after augmentation mammaplasty. In addition, studies have shown the relation between seroma formation and capsular contracture, which is another problem encountered in endoscopic-assisted transaxillary dual-plane augmentation mammaplasty.38-41 Interestingly, Hipps et al suggested that placement of drainage tubes after augmentation mammaplasty could reduce the incidence of capsular contracture.42 However, whether this results from the placement of drainage tubes, which reduces serum production, remains unknown. We suggest that pulling out the drainage tube based on the change in drainage volume and color is more reliable, as it can help us estimate the drainage rate, determine the presence of a bleeding wound, prevent the occurrence of seroma and hematoma, and investigate the pathogenesis of some complications.
Visual analogue scale is the most frequently used tool for pain assessment during the perioperative period and is utilized to evaluate pain after augmentation mammoplasty.34-43 The precision of measurement and its ratio scale properties are the 2 most advantages of this method.44-47 Interestingly, the tendency for pain intensity was similar to that for drainage (Figure 2); hence, we speculate that pain also reflected inflammation.48 Moreover, in our study, the 2 groups statistically differed in perioperative assessment using VAS on the first postoperative day only. We speculate that the inflammation caused by tissue damage was more apparent within 24 hours. Since then, although pain could reflect inflammation, it was not as sensitive as drainage. Similar to our research, Rzymski et al reported that the VAS scores on the first days after augmentation mammaplasty were relatively high and that patients often required opioid treatment for up to 7 to 8 days to reduce pain. Furthermore, they suggested that early pain after breast augmentation is associated with inflammation, which could result in the high activation of nociceptors in traumatized nerves from the pectoralis major muscle and subglandular fascia.34 Nonetheless, Pacik et al showed that postoperative pain after augmentation mammaplasty might be unrelated to blunt or sharp dissection, although the inframammary fold approach was used in their study, and urethral sounds were used for blunt separation.48 The differences in our results may be attributable to the various surgical approaches and blunt separation methods used. During the follow-up, we identified 1 case of chronic pain in the electrosurgical separation group. Causes of chronic pain vary and include direct brachial plexus compression and damage to the long thoracic nerve; the pain may also be referred from implants or due to focal nerve injuries resulting from short patches of demyelination, microneuroma, or neuroma. However, capsule formation, which leads to nerve compression and ischemia, is a more typical reason.34 Heat injury leads to more severe inflammation, resulting in more severe capsule formation; we speculate that this could be the reason for the chronic pain caused by electrosurgical method and that this can be avoided using the cold method for cases with mild inflammation.
Aside from chronic pain, we identified a case of implant stiffness in the electrosurgical separation group during follow-up, which led to a revision. This might have been caused by the thermal effects, which resulted in inflammation leading to more pronounced capsule formation and greater thickness.34,49,50 Although no capsular contracture was detected during the follow-up period, it might have been due to the insufficient number of cases and short follow-up time.
The present study has some limitations. Inflammatory indicators and hemoglobin in the drainage fluid, as well as other qualitative and quantitative indicators, have not been measured. Further studies should be performed in the future to analyze the composition of drainage fluid, which will help us more accurately understand the effect of different methods on the surgical area. Some postoperative evaluations, such as VAS, were not blinded to the intervention, and it could introduce observer bias.
In conclusion, based on our findings and previous studies in the literature, seroma formation or drainage seems to be a natural inflammatory response after tissue injury.51 The cold method for foam layer separation could more effectively reduce seroma formation and pain than the electrosurgical method. This might have been due to the decreased stimulation to the foam layer by the cold method, thus reducing the inflammatory reaction. Therefore, we recommend the use of the cold method for foam layer separation in endoscopic-assisted transaxillary dual-plane augmentation mammaplasty and the placement of drainage tubes so as to reduce possible complications due to seroma formation. However, these results should be validated by future multicenter randomized controlled trials.
Footnotes
Level of Evidence: Level 2, Prognostic
Authors’ Note: All human tissue collections were approved by the ethical committee of Plastic Surgery Hospital.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zifei Li, MD, PhD  https://orcid.org/0000-0001-6097-3494
https://orcid.org/0000-0001-6097-3494
Boyang Xu, MD  https://orcid.org/0000-0002-7351-8412
https://orcid.org/0000-0002-7351-8412
References
- 1. Pereira LH, Sterodimas A. Transaxillary breast augmentation: a prospective comparison of subglandular, subfascial, and submuscular implant insertion. Aesthetic Plast Surg. 2009;33(5):752–759. [DOI] [PubMed] [Google Scholar]
- 2. Hoehler H. Breast augmentation: the axillary approach. Br J Plast Surg. 1973;26(4):373–376. [DOI] [PubMed] [Google Scholar]
- 3. Tebbetts JB. Transaxillary subpectoral augmentation mammaplasty: a 9-year experience. Clin Plast Surg. 1988;15(4):557–568. [PubMed] [Google Scholar]
- 4. Tebbetts JB. Transaxillary subpectoral augmentation mammaplasty: long-term follow-up and refinements. Plast Reconstr Surg. 1984;74(5):636–649. [DOI] [PubMed] [Google Scholar]
- 5. Eaves FF, III, Bostwick J, III, Nahai F, Murray DR, Styblo TM, Carlson GW. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg. 1995;22(4):683–695. [PubMed] [Google Scholar]
- 6. Sun J, Luan J. Response to the comments of Dr. Chen on Chinese women’s preferences and concerns regarding incision location for breast augmentation surgery: a survey of 216 patients. Aesthetic Plast Surg. 2016;40(1):184–185. [DOI] [PubMed] [Google Scholar]
- 7. Reece EM, Ghavami A, Hoxworth RE, et al. Primary breast augmentation today: a survey of current breast augmentation practice patterns. Aesthetic Surg J. 2009;29(2):116–121. [DOI] [PubMed] [Google Scholar]
- 8. Sevin A, Sevin K, Senen D, Deren O, Adanali G, Erdogan B. Augmentation mammaplasty: retrospective analysis of 210 cases. Aesthetic Plast Surg. 2006;30(6):651–654. [DOI] [PubMed] [Google Scholar]
- 9. Spear SL, Bulan EJ, Venturi ML. Breast augmentation. Plast Reconstr Surg. 2006;118(suppl 7):188S–196S. [DOI] [PubMed] [Google Scholar]
- 10. Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133(4):567e–583e. [DOI] [PubMed] [Google Scholar]
- 11. Strock LL. Transaxillary endoscopic silicone gel breast augmentation. Aesthet Surg J. 2010;30(5):745–755. [DOI] [PubMed] [Google Scholar]
- 12. Strock LL. Surgical approaches to breast augmentation: the transaxillary approach. Clin Plast Surg. 2015;42(4):585–593. [DOI] [PubMed] [Google Scholar]
- 13. Niechajev I. Improvements in transaxillary breast augmentation. Aesthetic Plast Surg. 2010;34(3):322–329. [DOI] [PubMed] [Google Scholar]
- 14. Siraj A, Dar MF, Gilani AAS, Raziq S. Elective midline laparotomy: comparison of diathermy and scalpel incisions. Professional Med J. 2011;18(1):106–111. [Google Scholar]
- 15. Ayandipo OO, Afuwape OO, Irabor D, Oluwatosin OM, Odigie V. Diathermy versus Scalpel incision in a heterogeneous cohort of general surgery patients in a Nigerian teaching hospital. Niger J Surg. 2015;21(1):43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aird LN, Bristol SG, Phang PT, Raval MJ, Brown CJ. Randomized double-blind trial comparing the cosmetic outcome of cutting diathermy versus scalpel for skin incisions. Br J Surg. 2015;102(5):489–494. [DOI] [PubMed] [Google Scholar]
- 17. Yang X, Cao J, Yan Y, et al. Comparison of the safety of electrotome, Harmonic scalpel, and LigaSure for management of thyroid surgery. Head Neck. 2017;39(6):1078–1085. [DOI] [PubMed] [Google Scholar]
- 18. Tebbetts JB. Axillary endoscopic breast augmentation: processes derived from a 28-year experience to optimize outcomes. Plast Reconstr Surg. 2006;118(suppl 7):53S–80S. [DOI] [PubMed] [Google Scholar]
- 19. Kuroi K, Shimozuma K, Taguchi T, et al. Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol. 2006;36(4):197–206. [DOI] [PubMed] [Google Scholar]
- 20. Jie L, Mu D, Mu L. Transaxillary dual-plane augmentation mammaplasty: experience with 98 breasts. J Plast Reconstru Aesthet Surg. 2008;62(11):1459–1463. [DOI] [PubMed] [Google Scholar]
- 21. Momeni A, Padron NT, Föhn M, et al. Safety, complications, and satisfaction of patients undergoing submuscular breast augmentation via the inframammary and endoscopic transaxillary approach. Aesthetic Plast Surg. 2005;29(6):558–564. [DOI] [PubMed] [Google Scholar]
- 22. Villafane O, Garcia-Tutor E, Taggart I. Endoscopic transaxillary subglandular breast augmentation using silicone gel textured implants. Aesthetic Plast Surg. 2000;24(3):212–215. [DOI] [PubMed] [Google Scholar]
- 23. Srivastava V, Basu S, Shukla VK. Seroma formation after breast cancer surgery: what we have learned in the last two decades. J Breast Cancer. 2012;15(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zieliński J, Jaworski R, Irga N, Kruszewski JW, Jaskiewicz J. Analysis of selected factors influencing seroma formation in breast cancer patients undergoing mastectomy. Arch Med Sci. 2013;9(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szecsi PB, Larsen J, Horby J, Axelsson CK. Seroma production after breast cancer surgery has a pro-inflammatory component. Open Breast Cancer J. 2012;4(1):11–17. [Google Scholar]
- 26. Sampathraju S, Rodrigues G. Seroma formation after mastectomy: pathogenesis and prevention. Indian J Surg Oncol. 2010;1(4):328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toby L, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2(10):787–795. [DOI] [PubMed] [Google Scholar]
- 28. Caputo GG, Franchini Z, Maritan M, Dalla Pozza E, et al. Daily serum collection after acellular dermal matrix-assisted breast reconstruction. Arch Plast Surg. 2015;42(3):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu CW, Dionigi G, Sun H, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: a porcine model. Surgery. 2014;155(2):329–339. [DOI] [PubMed] [Google Scholar]
- 30. Dionigi G, Alesina PF, Barczynski M, et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc. 2012;26(9):2601–2608. [DOI] [PubMed] [Google Scholar]
- 31. Fanous N, Salem I, Tawilé C, Bassas A. Absence of capsular contracture in 319 consecutive augmentation mammaplasties: dependent drains as a possible factor. Can J Plast Surg. 2004;12(4):193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldberg HM. Drains and breast implants. Plast Reconstr Surg. 1988;81(6):990. [DOI] [PubMed] [Google Scholar]
- 33. Khan SM, Smeulders MJ, Van der Horst CM. Wound drainage after plastic and reconstructive surgery of the breast. Cochrane Database Syst Rev. 2015;10(3):CD007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rzymski P, Kubasik M, Gaca M, Opala T. Is the shear wave sonographic elastography correlated with pain after breast augmentation with silicone implants an indication of inflammatory activity? A preliminary report. Wideochir Inne Tech Maloinwazyjne. 2011;6(4):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. El-Haddad R, Lafarge-Claoue B, Garabedian C, Staub S. A 10-year prospective study of implant-based breast augmentation and reconstruction. Eplasty. 2018;18:e7. [PMC free article] [PubMed] [Google Scholar]
- 36. Shi H, Cao C, Li X, Chen L, Li S. A retrospective study of primary breast augmentation: recovery period, complications and patient satisfaction. Int J Clin Exp Med. 2015;8(10):18737–18743. [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang T, Li J, Ren J. Continuous negative pressure drain is associated with better outcome: a randomized prospective trial in plastic surgery patients. Aesthetic Plast Surg. 2019;43(1):91–97. [DOI] [PubMed] [Google Scholar]
- 38. Jacobson JM, Gatti ME, Schaffner AD, Hill LM, Spear SL. Effect of incision choice on outcomes in primary breast augmentation. Aesthet Surg J. 2012;32(4):456–62. [DOI] [PubMed] [Google Scholar]
- 39. Sim H.B, Sun SH. Transaxillary endoscopic breast augmentation with shaped gel implants. Aesthet Surg J. 2015;35(8):952–961. [DOI] [PubMed] [Google Scholar]
- 40. Calobrace MB, Stevens WG, Capizzi PJ, Cohen R, Godinez T, Beckstrand M. Risk factor analysis for capsular contracture: a 10-year Sientra study using round, smooth, and textured implants for breast augmentation. Plast Reconstr Surg. 2018;141(4S Sientra Shaped and Round Cohesive Gel Implants):20S–28S. [DOI] [PubMed] [Google Scholar]
- 41. Listed N. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation: correction. Plast Reconstr Surg. 2013;132(6):1764. [DOI] [PubMed] [Google Scholar]
- 42. Hipps CJ, Raju R, Straith RE. Influence of some operative and postoperative factors on capsular contracture around breast prostheses. Plast Reconstr Surg. 1978;61(3):384–389. [DOI] [PubMed] [Google Scholar]
- 43. Aubrun F, Langeron O, Quesnel C, Coriat P, Riou B. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003;98(6):1415–1421. [DOI] [PubMed] [Google Scholar]
- 44. Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485–489. [DOI] [PubMed] [Google Scholar]
- 45. Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–638. [DOI] [PubMed] [Google Scholar]
- 46. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. [DOI] [PubMed] [Google Scholar]
- 47. Donald D Harkins, Stephen W. Combined use of experimental pain and visual analogue scales in providing standardized measurement of clinical pain. Clin J Pain. 1987;3(1):1–8. [Google Scholar]
- 48. Pacik PT, Nelson CE, Werner C. Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: data analysis. Aesthet Surg J. 2008;28(6):631–641. [DOI] [PubMed] [Google Scholar]
- 49. Mazaheri MK, Schultz GS, Blalock TD, Caffee HH, Chin GA. Role of connective tissue growth factor in breast implant elastomer capsular formation. Ann Plast Surg. 2003;50(3):263–268. [DOI] [PubMed] [Google Scholar]
- 50. Wyatt LE, Sinow JD, Wollman JS, Sami DA, Miller TA. The influence of time on human breast capsule histology: smooth and textured silicone-surfaced implants. Plast Reconstr Surg. 1998;102(6):1922–1931. [DOI] [PubMed] [Google Scholar]
- 51. Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma formation following breast cancer surgery. Breast J. 2003;9(5):385–388. [DOI] [PubMed] [Google Scholar]