Abstract
Ovarian cancer (OC) is the most lethal gynecologic cancer, and the incidence of OC has risen steadily worldwide. Numerous microRNAs (miRNAs) have been found to be involved in the progression of OC. miR-204-5p is down-regulated and functions as a tumor suppressor in various types of human malignant tumors. However, the biological roles and molecular mechanisms of miR-204-5p in OC still remain unclear. In this study, the aberrant down-regulation of miR-204-5p was detected in OC tissues. We also observed that miR-204-5p overexpression represses OC cell proliferation. Ubiquitin-specific peptidase 47 (USP47) is verified as the functional target of miR-204-5p, through which it plays an important biological role in OC. Our results uncover new functions and mechanisms for miR-204-5p in the progression of OC, and provide a potential therapeutic target for the treatment of OC.
Keywords: miR-204-5p, USP47, ovarian cancer, cell proliferation
Introduction
Ovarian cancer (OC) is the sixth most common cancer in women globally, and has the highest mortality rate and the poorest prognosis among gynecological malignancies1. OC is also the most common gynecological malignancy in China2. The number of cases is steadily increasing worldwide3. Nowadays, even with the advances in its treatment, including surgery, chemotherapy, and radiotherapy, survival rates remain poor for the majority of patients, with a 5-year survival rate of ∼30%4. Therefore, it is essential to develop novel therapeutic strategies.
An increasing number of interesting genes and pathways that may play essential roles in the pathogenesis of OC have already been identified at the molecular level. Among these, the recently discovered miRNAs constitute a novel layer of gene expression regulation and have been implicated in the etiology of OC. MiRNAs are approximately 22-nucleotide non-coding RNAs, which are generally involved in posttranscriptional gene regulation5. Currently, it is accepted that miRNAs are involved in processes including proliferation, cell cycle, differentiation, and development in various human cancers6–8. Of them, miR-204-5p functions as a tumor suppressor in various types of human tumors including glioblastoma, prostate cancer, and non-small-cell lung carcinoma9–11. However, the function and molecular mechanism of miR-204-5p in OC remains unclear.
In this study, we showed that miR-204-5p is down-regulated in OC tissues compared with paired adjacent non-cancerous tissues. We also demonstrated that the up-regulation of miR-204-5p suppressed the OC cell proliferation. Moreover, USP47 is identified as the direct functional target of miR-204-5p in OC. Based on these results, we report a novel molecular mechanism of the miR-204-5p/USP4 pathway in OC. miR-204-5p could serve as a potential therapeutic target for patients with OC.
Materials and Methods
Cell Culture and Tissue Collection
The human OC cell lines SKOV-3 and TOV-112D were purchased from ATCC (Manassas, VA, USA). Cells were maintained in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in 95% air and 5% CO2.
Human OC tissues were obtained from 20 patients diagnosed at the oncology division in the department of Gynecology and Obstetrics of the affiliated hospital of Hubei Polytechnic Institute (Xiaogan, China), between November 2017 and May 2018. All pathology specimens were reviewed in the pathology department and the relevant clinical data were collected by retrospective review of the patients’ files. Patients with non-epithelial-type neoplasia, patients treated before operation, and patients who were not treated surgically were excluded. Borderline tumors of the ovary were also excluded from this study. Biopsies were frozen in liquid nitrogen and stored at –80°C until subsequent analysis. This study was approved by the Ethics Committee of the medical faculty of Hubei Polytechnic Institute (Xiaogan, China), and informed consent was obtained from each patient.
Quantitative Real-Time PCR (qRT-PCR) for Genes and miRNAs
Total RNA from tissue samples and cells was extracted by TRIzol (Invitrogen) according to the protocols supplied by the manufacturer. After removal the residual DNA by DNase I (Invitrogen), RNAs were reverse-transcribed into cDNA using the high-capacity RNA-to-cDNA kit (Thermo Fisher Scientific, Waltham, MA, USA). Taqman qRT-PCR was performed to determine the USP47 gene expression on a QuantStudio 6 Flex system (Life Technologies, Gaithersburg, MD, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
To quantify the levels of the mature miRNAs, total RNA was reverse-transcribed using the Taqman advanced miRNA cDNA synthesis kit, according to manufacturer-recommended protocols (Applied Biosystems Materials, Richmond, VA, USA). The relative levels of miR-204-5p were normalized to the levels of U6, a ubiquitously expressed small nuclear RNA. U6 was reverse-transcribed by TaqmanTM microRNA reverse transcription kit according to the manufacturer’s protocol (Applied Biosystems Materials).
The relative gene and miRNA expression data using the 2(-Delta Delta C(T)) Method12. qRT-PCR primers for gene and miRNA expression were available from Applied Biosystems. All independent PCR-based reactions were performed in triplicate.
Western Blot Analysis
Total protein lysates were fractionated on 4–15% polyacrylamide gels and transferred onto nitrocellulose (Bio-Rad, Richmond, CA, USA). The levels of USP47 were analyzed by western blots using a rabbit anti-USP47 (Thermo Fisher Scientific) with dilution of 1:500. Normalization was performed by blotting the same samples with an antibody against GAPDH (Abcam, Cambridge, MA, USA). The secondary antibodies were goat anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000; Bio-Rad).
Overexpression and Knockdown
The lentiviral system of has-miR-204-5p (Applied Biological Materials, Forster City, CA, USA) was used to stably overexpress miR-204-5p in the SKOV-3 or TOV-112D cells. A non-relevant sequence insert lentivirus acted as a negative control (miR-Ctrl) (Applied Biological Materials). Similarly, USP47 lentivirus was used to stably overexpress the USP47 (USP47-OE) (OriGene Technologies, Rockville, MD, USA). The relative control lentivirus (USP47-Ctrl) was also available at OriGene Technologies.
The silencing lentiviral plasmid vector of USP47 (sh-USP47) and correlated control vector (sh-Ctrl) were commercially available at Applied Biological Materials. The lentiviruses were generated as previously described13.
The lentivirus was infected to cells with 5 μg/mL polybrene (Sigma-Aldrich). Cells were incubated in complete growth medium with 10 μg/mL puromycin (Sigma-Aldrich) for stable clone selection.
Target Prediction
The prediction of potential target genes of miR-204-5p were searched using the available databases TargetScan (http://www.targetscan.org/) and miRanda (microrna.org and miRbase).
Dual Luciferase Reporter Assay
SKOV-3 or TOV-112D cells were cultured in 96-well plates and co-transfected with 50 nM of miR-204-5p mimic or mirVanaTM miRNA mimic Negative Control # 1 and 50 ng of USP47 3’-UTR luciferase reporter construct (Switchgear genomics, Menlo Park, CA, USA) using Lipofectamine 2000 with Opti-MEM (Thermo Fisher Scientific). The luciferase activities were assayed using a luciferase assay kit (Promega, Madison, WI, USA) after 48 h transfection.
Cell Proliferation Assay
For the cell proliferation assay, SKOV-3 and TOV-112D cells transfected with different vectors were plated in 96-well plates (5×103 cells/well) for 48 h and detected with Cell Counting Kit-8 (CCK8) assay (Promega) according to the manufacturer’s instruction. Cellular proliferation was detected at 0 h, 24 h, 48 h, 72 h, 96 h and 120 h. The solution was then measured spectrophotometrically at 450 nm. Results came from three independent cell preparations.
Soft Agar Assay
The colony formations of SKOV-3 and TOV-112D cells transfected with different vectors were determined in soft agarose. The 6-well plates were coated with 0.5% (v/v) agarose in the DMEM media and allowed to set. Cells (5×103 cells/well) were resuspended in a heated soft agarose medium consisting of 0.4% (v/v) low-melt agarose. The incubation time for colony formation varies from 1 to 4 weeks. Colonies were fixed and stained with 0.5% crystal violet (Sigma-Aldrich).
Statistics
The results are expressed as means ± standard errors (SE). The data were subjected to the two-tailed, unpaired student’s t-test between two conditions. The Spearman’s rank correlation analysis was performed to analyzed the correlation. The P (probability) <0.05 was considered statistically significant. The SPSS software package (version 20.0, SPSS Inc, Chicago, IL, USA) was used for statistical analyses.
Results
miR-204-5p was Down-Regulated in OC Tissues
Based on our preliminary miRNA microarray study, miR-204-5p was one of the down-regulated miRNAs in OC tissues compared with the adjacent non-tumor tissues. qRT-PCR analysis revealed that miR-204-5p was down-regulated in 12 OC tissues than the corresponding adjacent non-tumor tissues by 2.1-fold (P < 0.05), which is consistent with the microarray data (Fig. 1). This result suggested that miR-204-5p may be involved in the progression of OC.
Figure 1.
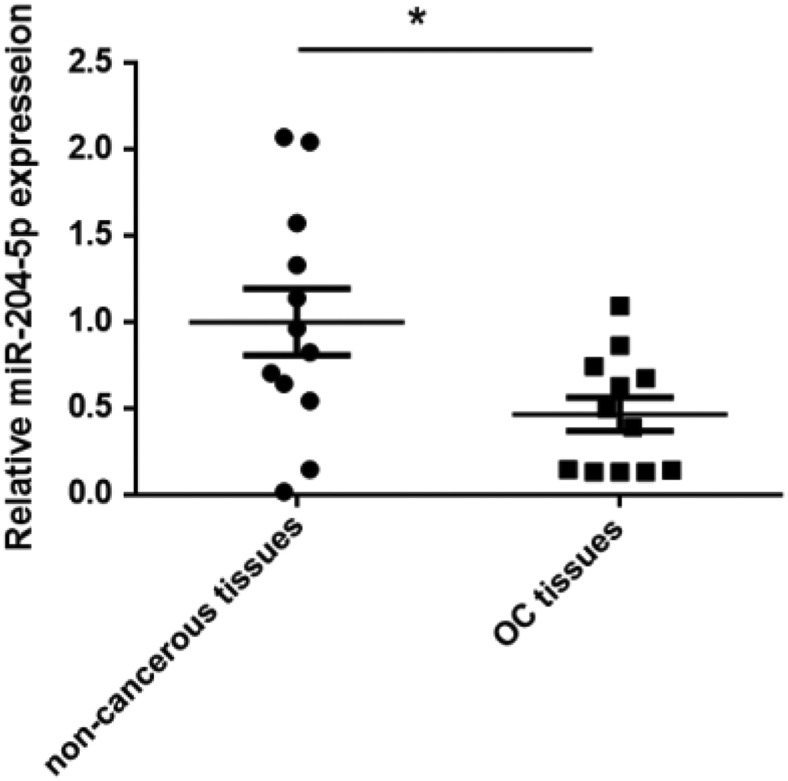
miR-204-5p expression was down-regulated in OC tissues. miR-204-5p expression was determined by qRT-PCR in 12 paired CRC and adjacent non-cancerous tissues. miR-204-5p expression was significantly down-regulated in tumor tissues compared with the paired non-cancerous tissues. *P < 0.05 vs. non-cancerous tissues.
miR-204-5p Inhibits OC Cell Proliferation in Vitro
To decipher the biological function of miR-204-5p, the miR-204-5p was stably overexpressed in SKOV-3 and TOV-112D cells. We first determined miR-204-5p expression levels after infecting SKOV-3 and TOV-112D cells by qRT-PCR (Fig. 2A). Cell proliferation assays revealed that miR-204-5p overexpression significantly reduced the growth rates of SKOV-3 and TOV-112D cells (P < 0.05, Fig. 2B). Soft agar colony formation assays confirmed the proliferation-repressing function of miR-204-5p in SKOV-3 and TOV-112D cells (P < 0.05, Fig. 2C). Collectively, these data indicate that miR-204-5p has a growth-suppressive function in OC.
Figure 2.
miR-204-5p inhibits OC cells proliferation in vitro. (A) miR-204-5p expression levels after infection in SKOV-3 and TOV-112D cells were examined by qRT-PCR; (B) miR-204-5p overexpression repressed the proliferation of SKOV-3 and TOV-112D cells. The CCK-8 assay was used to determine the cell growth rate (C) miR-204-overexpressed SKOV-3 and TOV-112D cells exhibited decreased colony formation rates compared with the control cells, which varies from 1-4 weeks. *P < 0.05 vs. cell without transduction (BC), **P < 0.05 vs. miR-control (n=3).
USP47 Expression is Increased in OC Tissues and Inversely Correlated with miR-204-5p Expression
To decipher the underlying molecular mechanism of how miR-204-5p regulates OC cell proliferation, we subsequently identified the direct target of miR-204-5p. The complementary sequence of miR-204-5p has already been identified in the 3’ UTR of USP47 mRNA14. To further study the relationship between miR-204-5p and USP47 in OC, we assessed the USP47 mRNA and protein expression in 12 OC tissues and the paired adjacent non-tumor tissues. The expression levels of USP47 mRNA and protein levels were significantly higher in the OC tissues compared with the matched adjacent non-cancerous tissues (P < 0.05, Fig. 3A, B). Furthermore, we executed a correlation analysis between the expression of USP47 and miR-204-5p to elucidate whether USP47 interacts with miR-204-5p in OC. The protein expression of USP47 levels inversely correlated with miR-204-5p expression (Fig. 3C). Our results suggested that miR-204-5p acts as a regulator of USP47 expression in OC tumors.
Figure 3.
USP47 mRNA and protein levels were overexpressed in OC tissues and inversely correlated with miR-204-5p levels. (A) USP47 mRNA expression was up-regulated in the OC tissues compared with the paired non-cancerous tissues by qRT-PCR examination. *P < 0.05 vs. non-cancerous tissues (n=12). (B) USP47 protein expression was up-regulated in the OC tissues compared with the paired non-cancerous tissues by western blot analysis; (C) the correlation between miR-204-5p expression and USP47 protein from 12 pairs of OC tissues and the corresponding non-cancerous tissues was determined by non-parametric correlation Spearman’s correlation test.
Identification of USP47 as a Target of miR-204-5p
Subsequently, the 3’-UTR luciferase assay confirmed that miR-204-5p directly binds USP47 mRNA in SKOV-3 and TOV-112D cells. MiR-204-5p significantly decreased the luciferase activities of USP47 (P < 0.05, Fig. 4A). However, we did not observe down-regulation of luciferase activities of USP47 by miR-Ctrl (Fig. 4A). In addition, the expression levels of USP47 mRNA and protein were also significantly suppressed in the miR-204-5p-overexpressed SKOV-3 and TOV-112D cells (P < 0.05, Fig. 4B, C). Taken together, these results imply that USP47 is directly suppressed by miR-92a-3p in OC cells.
Figure 4.
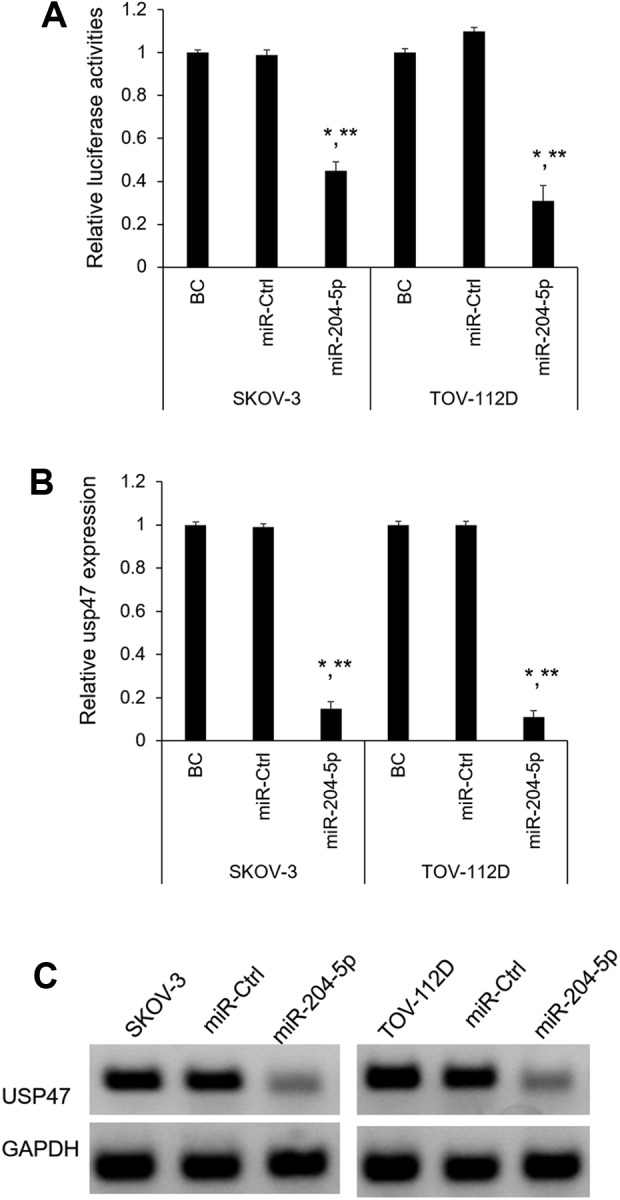
USP47 is the direct target of miR-204-5p. (A) Analysis of relative luciferase activity of SKOV-3 and TOV-112D cells after co-transfection with USP47 3′ UTR luciferase reporter and miR-204-5p or miR-Ctrl. The relative luciferase activity was normalized to Renilla activities. *P < 0.05 vs. BC, **P < 0.05 vs. miR-Ctrl (n=3); (B) USP47 mRNA expression levels after miR-204-5p overexpression in SKOV-3 and TOV-112D cells were determined by qRT-PCR (n=3); (C) The USP47 protein levels were examined by western blot in miR-Ctrl and miR-204-5p overexpressed SKOV-3 and TOV-112D cells.
Role of miR-204-5p in Cell Proliferation via Directly Targeting USP47
To further determine the functional significance of USP47 in the miR-204-5p-induced phenotype, USP47 was silenced by USP47 shRNA lentivirus in the SKOV-3 and TOV-112D cells (Fig. 5A, B). As for the cell proliferation assay and soft agar colony formation assay, shRNA-mediated USP47 silencing could phenocopy the proliferation-repressing effect of miR-204-5p (Fig. 5C, D). Subsequently, we rescued the USP47 in the miR-204-5p overexpressed SKOV-3 and TOV-112D cells (Fig. 5E, F). We revealed that USP47 overexpression could significantly abrogate the inhibitory effect of miR-204-5p on cell proliferation (Fig. 5G, H). Collectively, these results show that miR-204-5p exerts tumor-suppressive function in OC through directly regulating USP47.
Figure 5.
miR-204-5p inhibited SKOV-3 and TOV-112D cell growth by regulating USP47. (A) USP47 mRNA expression levels after USP47 silencing by shRNA in SKOV-3 and TOV-112D cells were determined by qRT-PCR (n=3); (B) The USP47 protein levels were examined by western blot in USP47 silenced SKOV-3 and TOV-112D cells. (C) Knockdown of USP47 by shRNA remarkedly inhibited SKOV-3 and TOV-112D cell proliferation. *P < 0.05 vs. mock cells, **P < 0.05 vs. sh-Ctrl (n=3); (D) USP47 silenced SKOV-3 and TOV-112D cells exhibited decreased colony formation rates compared with the control cells, which varies from 1 to 4 weeks. (E) USP47 mRNA expression levels after USP47 overexpression in miR-204-5p overexpressed SKOV-3 and TOV-112D cells were determined by qRT-PCR (n=3). *P < 0.05 vs. mock cells, **P < 0.05 vs. USP47-Ctrl (n=3); (F) USP47 mRNA expression levels after USP47 overexpression in miR-204-5p overexpressed SKOV-3 and TOV-112D cells were determined by qRT-PCR (n=3); (G) up-regulation of USP47 promoted cell growth and abrogated miR-204-5p-induced growth-inhibition in SKOV-3 and TOV-112D cells. *P < 0.05 vs. mock cells, **P < 0.05 vs sh-Ctrl (n=3); (H) USP47 overexpressed SKOV-3 and TOV-112D cells exhibited increased colony formation rates compared with the control cells, which varies from 1 to 4 weeks.
Discussion
Nowadays, miRNAs have been identified as key regulation factors for the tumor cell proliferation, migration, invasion, and apoptosis. Extensive reports have shown that many miRNAs act as onco-miRNAs or tumor suppressors in diverse human tumors. The role of miR-204-5p in tumor development has recently received much attention. Several studies have reported that miR-204-5p is down-regulated and functions as a tumor suppressor in different types of human tumors. miR-204-5p has been reported to contribute to BRAF inhibitor resistance in melanoma15. miR-204-5p has also been identified to suppress hepatocellular cancer proliferation by regulating homeoprotein SIX1 expression16. In addition, previous research has reported that miR-204-5p suppressed cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma17. However, little is known about the biological function and molecular mechanism of miR-204-5p in OC.
In this study, we observed that miR-204-5p expression is significantly lower in OC tissues compared with the paired non-cancerous tissues. These findings indicate that low expression of miR-204-5p may play an important role in the progression of OC. Furthermore, the biological function of miR-204-5p has been investigated in OC cells. It inhibited cell proliferation. Subsequently, we further investigated the molecular mechanism of miR-204-5p. We discovered that USP47 may be the functional target of miR-204-5p in OC. It reflects the number of living cells. However, the reduction of living cells might be due to increased cell death or extended cell cycle arrest; both possibilities are worth further research in the future.
USP47 has been verified as the direct target of miR-204-5p in human gastric cancer18. Reversible regulation of SATB1 ubiquitination by USP47 and SMURF2-mediated colon cancer cell proliferation and tumor progression has been reported19. The deubiquitinylase USP47 has been shown to promote RelA phosphorylation and survival in gastric cancer cells20. USP47 has been suggested as a possible therapeutic target, as USP47 depletion up-regulated levels of Cdc25A and decreased cell survival21. In this study, USP47 was found to be significantly up-regulated in OC tissues compared with their paired adjacent non-cancerous tissues. Our data also revealed a negative correlation between miR-204-5p and USP47 protein expression in OC tissues. Then, we demonstrated that USP47 is the functional targets of miR-204-5p in OC by luciferase reporter assays and cell proliferation assays. In vivo experiments are needed to verify this in vitro cell model in the future. The investigation of downstream effectors of USP47, invasion and/or progression results of miR-204-5p/USP47 regulation in human OC cell are also worth future investigation.
In conclusion, we demonstrated that the level of miR-204-5p is significantly lower in OC tissues than in the paired adjacent non-cancerous tissues. We also determined that up-regulated miR-204-5p inhibited OC cell proliferation. Low levels of miR-204-5p participate in OC progression. Furthermore, we revealed that USP47 is the direct functional target of miR-204-5p. Thus, miR-204-5p/ USP47 may be a potential therapeutic target for OC.
Acknowledgements
The authors would like to thank the Pathology section of the affiliated hospital of Hubei Polytechnic Institute for the pathologic diagnosis.
Footnotes
Author Contributions: QY and YX conceived the study. LH and QY designed the experiments and wrote the manuscript. LH collected the samples and completed the experimental part of the study. HN, HM and YH contributed to cell culture and sample collection. HK and YX analyzed the data and revised the manuscript. SZ and MC critically discussed the results.
Ethics Approval: Ethical approval was obtained for all experimental procedures by the Ethics Committee of the medical faculty of Hubei Polytechnic Institute, Xiaogan, Hubei, China.
Statement of Human and Animal Rights: All procedures with human subjects in this study were conducted in accordance with the human ethics committee of the medical faculty of Hubei Polytechnic Institute, Xiaogan, Hubei, China. This article does not contain any studies with animals.
Statement of Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Qinan Yin  https://orcid.org/0000-0001-8560-5751
https://orcid.org/0000-0001-8560-5751
References
- 1. Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol. 2016;13(4):255–261. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–332. [DOI] [PubMed] [Google Scholar]
- 3. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. [DOI] [PubMed] [Google Scholar]
- 4. Cliby WA, Powell MA, Al-Hammadi N, Chen L, Philip Miller J, Roland PY, Mutch DG, Bristow RE. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Contreras J, Rao DS. MicroRNAs in inflammation and immune responses. Leukemia. 2012;26(3):404–413. [DOI] [PubMed] [Google Scholar]
- 7. Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sokol NS. Small temporal RNAs in animal development. Curr Opin Genet Dev. 2012;22(4):368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin YC, Lin JF, Tsai TF, Chou KY, Chen HE, Hwang TI. Tumor suppressor miRNA-204-5p promotes apoptosis by targeting BCL2 in prostate cancer cells. Asian J Surg. 2017;40(5):396–406. [DOI] [PubMed] [Google Scholar]
- 10. Song S, Fajol A, Tu X, Ren B, Shi S. miR-204 suppresses the development and progression of human glioblastoma by targeting ATF2. Oncotarget. 2016;7(43):70058–70065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang P, Lv HY, Zhou DM, Zhang EN. miR-204 suppresses non-small-cell lung carcinoma (NSCLC) invasion and migration by targeting JAK2. Genet Mol Res. 2016;15(2). [DOI] [PubMed] [Google Scholar]
- 12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Huang C, Guo Y, Gou X, Hinsdale M, Lloyd P, Liu L. MicroRNA-26b modulates the NF-kappaB pathway in alveolar macrophages by regulating PTEN. J Immunol. 2015;195(11):5404–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang W, Dou C, Wang Y, Jia Y, Li C, Zheng X, Tu K. MicroRNA-92a contributes to tumor growth of human hepatocellular carcinoma by targeting FBXW7. Oncol Rep. 2015;34(5):2576–2584. [DOI] [PubMed] [Google Scholar]
- 15. Diaz-Martinez M, Benito-Jardon L, Alonso L, Koetz-Ploch L, Hernando E, Teixido J. miR-204-5p and miR-211-5p contribute to BRAF inhibitor resistance in melanoma. Cancer Res. 2018;78(4):1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu Y, Jiang M, Du F, Chen D, Ye T, Xu B, Li X, Wang W, Qiu Z, Liu H, Ne Y, et al. miR-204-5p suppresses hepatocellular cancer proliferation by regulating homeoprotein SIX1 expression. FEBS Open Bio. 2018;8(2):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu L, Wang J, Li X, Ma J, Shi C, Zhu H, Xi Q, Zhang J, Zhao X, Gu M. MiR-204-5p suppresses cell proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2015;457(4):621–626. [DOI] [PubMed] [Google Scholar]
- 18. Zhang B, Yin Y, Hu Y, Zhang J, Bian Z, Song M, Hua D, Huang Z. MicroRNA-204-5p inhibits gastric cancer cell proliferation by downregulating USP47 and RAB22A. Med Oncol. 2015;32(1):331. [DOI] [PubMed] [Google Scholar]
- 19. Yu L, Dong L, Wang Y, Liu L, Long H, Li H, Li J, Yang X, Liu Z, Duan G, Dai X, et al. Reversible regulation of SATB1 ubiquitination by USP47 and SMURF2 mediates colon cancer cell proliferation and tumor progression. Cancer Lett. 2019;448:40–51. [DOI] [PubMed] [Google Scholar]
- 20. Naghavi L, Schwalbe M, Ghanem A, Naumann M. Deubiquitinylase USP47 promotes RelA phosphorylation and survival in gastric cancer cells. Biomedicines. 2018;6(2):E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peschiaroli A, Skaar JR, Pagano M, Melino G. The ubiquitin-specific protease USP47 is a novel beta-TRCP interactor regulating cell survival. Oncogene. 2010;29(9):1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]





