Abstract
The term episomal induced pluripotent stem cells (EiPSCs) refers to somatic cells that are reprogrammed into induced pluripotent stem cells (iPSCs) using non-integrative episomal vector methods. This reprogramming process has a better safety profile compared with integrative methods using viruses. There is a current trend toward using episomal plasmid reprogramming to generate iPSCs because of the improved safety profile. Clinical reports of potential human cell sources that have been successfully reprogrammed into EiPSCs are increasing, but no review or summary has been published. The functional applications of EiPSCs and their potential uses in various conditions have been described, and these may be applicable to clinical scenarios. This review summarizes the current direction of EiPSC research and the properties of these cells with the aim of explaining their potential role in clinical applications and functional restoration.
Keywords: Episomal induced pluripotent stem cells, EiPSCs, iPS, therapeutic application
Introduction
Application of the reprogramming techniques first developed by Yamanaka et al.1 has made possible the conversion of somatic cells to pluripotent stem cells that resemble embryonic stem cells (ESCs). ESCs have been touted as the “Holy Grail” for unrestricted regeneration because of their potential to differentiate into any cell lineage in the body and to replace damaged tissue. Opponents of this technique commonly cite the potential for unethical use or donation of ESCs, which are potential sources of life and embryo formation. With the development of a method to create induced pluripotent stem cells (iPSCs), it is possible to harness the regenerative properties of iPSCs, which resemble ESCs, yet without the ethical controversies associated with the sources of ESCs.
For research purposes, iPSCs can be readily cultured in the laboratory from various somatic cells. Somatic cells of various lineages have been shown to be capable of changing both their morphology and pluripotent potential through the overexpression of four main pluripotent factors: Oct3/4, Sox2, Klf4, and c-Myc (OSKM). The replacement of c-Myc and Klf4 by Nanog and Lin28 has also been shown to be possible when used in conjunction with Oct3/4 and Sox2 during the reprogramming of cells into iPSCs2. Overexpression of Oct4, Sox2, and Nanog can also reprogram human fetal gut mesentery-derived cells into iPSCs3. Although Nanog is a dispensable reprogramming factor4, it has been reported to be essential for the ability for self-renewal5 and generation of stable iPSCs6. In some cases, such as adult mouse neural stem cells, expression of only one factor (Oct4) is sufficient for the generation of iPSCs7, even using episomal reprogramming8. A possible reason is the endogenous expression of Sox2, c-Myc, and Klf4 in neural stem cells. In addition, downregulation of p53 using knockdown9 and knockout10 methods can markedly improve the efficiency of iPSC generation11, and only Oct4 and Sox2 are sufficient for iPSC generation under conditions of p53 loss12. One possible mechanism to explain the suppression of iPSC generation by p53 is through the inhibition of the expression of Nanog and Oct413. Certain somatic and adult stem cells, such as keratinocytes14 and dental pulp stem cells15, have a greater propensity to be reprogrammed into iPSCs compared with fibroblasts.
The introduction of these pluripotent reprogramming techniques can be divided into integrative and non-integrative methods. When first discovered, viral transduction was used for the insertion of the OSKM genes into the somatic cell genome. However, this may cause disruption to the host’s genome and, with the use of the proto-oncogene c-Myc, gene reactivation could increase the risk of transgene-derived tumor formation16,17. Other methods have been used to reprogram somatic cells into iPSCs. In particular, non-integrative methods such as episomal plasmid delivery of pluripotent OSK genes without c-Myc have been touted as a safer alternative for iPSC generation18,19. Episomal plasmid vectors are also combined with p53 knockdown to generate iPSCs10,20,21. This review focuses on studies of the use of iPSCs generated using episomal plasmids, which are termed “episomal iPSCs” (EiPSCs) to reflect their origin.
Integrative Methods of iPSC Production
Potential issues with integrative methods of iPSC production include the inadvertent introduction of potentially harmful viral components that express certain oncogenes that may lead to tumor formation. Insertion of genes also carries the risk of disrupting the expression of host cell tumor suppressor genes, especially if the insertions occur in an open reading frame or alter the expression of oncogenes in close proximity17. Several viral systems have been introduced to circumvent this issue such as Cre-deletable22 or inducible lentiviruses, which reduce the risk of integration, although concerns have been raised about the use of viral vectors for therapeutic applications. Cre-deletable lentiviruses refer to a Cre recombinase-mediated deletion of a viral genome once integration is complete. This allows for the time-controlled integration, which is self-limiting and hence minimizes the chance of permanent viral genomic integration. Inducible lentivirus commonly refers to a tetracycline- or doxycycline-inducible expression system in lentiviruses, which allows the activation or deactivation of viral genomic DNA in the presence of tetracycline. This also provides some control over the duration of the viral genomic presence and, therefore, the risk of integration with the host genome.
Non-Integrative Methods of iPSC Production
The use of plasmids for the introduction of OSK pluripotent transcription factors has been described, and this method has been successful for reprogramming somatic and adult stem cells. The use of integration-defective viral delivery systems (adenoviruses23, Sendai viruses24), piggyBac systems25, minicircle vectors26, episomal delivery19, mRNA delivery27, protein delivery28, and chemical induction29 in the production of iPSCs has been described. The piggyBac transposition system allows the direct cutting and pasting of sections of DNA to allow insertion and removal of certain sections. Specific deletion and insertion of reprogramming genes can be performed. Minicircle vectors are small circular plasmid derivatives that are freed from all prokaryotic vector parts or bacterial plasmid DNA. However, these are not in widespread use because of the intensive production process. The delivery of mRNA and protein can be much more costly for experimental purposes, and the mRNA or protein is not always inducible downstream. Chemical induction uses specific sets of chemicals and mutagens to allow the reprogramming of cells. By subjecting cells to a specific set of chemicals, researchers have shown that cells can be reprogrammed to their pluripotent state. However, this method is extremely laborious and has unknown safety parameters at present. Episomal plasmid delivery is cost effective, and the plasmid does not integrate when used with common transfection methods. This method provides an effective method for the delivery of plasmids into cells for reprogramming30. The plasmids are readily available on multiple gene platforms and are removed from the host cell through cell division and serial dilution. The use of non-integrative methods of iPSC production has the advantage of producing iPSCs that are free of transgene integration. This has been confirmed by polymerase chain reaction (PCR) analysis, which shows no residual transgenes of viral origin31. For example, the Yamanaka group reported that no residual episomal plasmid DNA could be detected in clones after 11–20 passages20.
Two components of the oriP and Epstein–Barr nuclear antigen-1 (EBNA-1) are also used widely in episomal plasmids32–38. Yu et al. reported that human iPSCs that are completely free of plasmids and transgene sequences could be derived from fibroblasts by a single transfection with oriP/EBNA-1-based episomal plasmids39–42. The footprint-free iPSCs make them safer for clinical application because of the loss of plasmids and transgenes. OriP/EBNA-1 plasmids have a wide host cell range for episomal reprogramming, and a single transfection of episomal plasmids is sufficient for iPSC generation. Compared with the original episomal plasmids without EBNA-1, oriP/EBNA-1 can improve the efficiency of iPSC generation43 through the oriP/EBNA-1-mediated nuclear import and retention of vector DNA44. It can replicate only once per cell cycle. Episomal DNA is lost from cells at a rate of 5% per cell generation because of defects in plasmid synthesis and partitioning45. Subsequently, episome-free iPSCs can be easily harvested. Although plasmids appear in the first few cell passages immediately after transfection, many studies have used PCR analysis to show complete loss of plasmids and transgenes during extended cell culture20,39–42,46–58. The time point of successful loss of episomes varies between different somatic cell types. The oriP/EBNA-1-based episomal plasmids have been proven to generate iPSCs very efficiently without the risk of transgenic sequences inserted into the somatic cell genome. Certain comments exist saying iPSCs generated using episomal plasmids with EBNA-1 expressed have residual episomal DNA. This does not appear to be true after reviewing the data presented by Yu et al.39, where the group demonstrated that in fact that there were no residual plasmids and transgenes in iPSCs generated by EBNA-based plasmids using PCR and Southern blot analysis.
In an interesting experiment, the efficiency of reprogramming was compared between various methods for generating iPSCs. Reprogramming success rates were similarly high, at around 80%, with Sendai-viral, episomal, and lentivirus methods. mRNA methods alone were reported to have a lower success rate because of massive cell death and detachment. Episomal methods seem to be a good method for producing iPSCs, and the materials can be manufactured using current good manufacturing practice compatible processes (cGMP). As such, episomal methods remain very useful in the clinical setting59.
Human EiPSC Sources
Multiple cell lineages from humans have been reprogrammed into iPSCs using episomal plasmids. Episomal plasmid reprogramming differs markedly from other forms of reprogramming techniques, such as retroviral transduction, and varied success rates have been reported. Recent trends include the increasing use of episomal plasmid methods for reprogramming various types of human cells for research use and potential clinical applications. The human cells reported to have been successfully reprogrammed into EiPSCs include fibroblasts, epithelial cells, keratinocytes, mononuclear cells from adult peripheral blood, cord blood cells, amniotic fluid stem cells, mesenchymal stromal cells, lymphoblasts, lamina propria progenitor cells from oral mucosa, and urothelial cells obtained from urine. A summary of these reported human EiPSC sources is shown in Table 1 8,20,21,32–43,47–52,55,56,58,60–207. The variety of cell lineages that can be reprogrammed by episomal techniques demonstrates the versatility of this technique, which has been used in many laboratories around the world.
Table 1.
Summary of Reported Human EiPSC sources available in the Literature.
| Type of cell | Source | Patient conditions | Method of transfection | Further differentiation | Author |
|---|---|---|---|---|---|
| Fibroblasts | Fetal foreskin | Healthy | Electroporation | N/A | Yu et al.39,40, Matz and Adjave62, Tandon et al.63, Tidball et al.64, Kamath et al.65, Kim et al.66, Schmitt et al.67, Mah et al.68 |
| Fibroblasts | Fetal foreskin | Healthy | Electroporation | CD235a+CD45− leukocyte-free red blood cells | Dias et al.69 |
| Fibroblasts | Fetal foreskin | Healthy | Electroporation | Cardiomyocytes | Mehta et al.70 |
| Fibroblasts | Fetal foreskin | Healthy | Electroporation | Hepatocyte-like cells | Wruck and Adjave71 |
| Fibroblasts; Fibroblasts | Fetal lung | Healthy | Poly(beta-amino ester) nanoparticles; Electroporation | Neuronal cells | Bhise et al.72 |
| Fibroblasts | Fetal foreskin | Healthy | Lipofectamine 3000 reagent | N/A | Skrzypczyk et al.73 |
| Fibroblasts | Fetal right musculus quadriceps femoris | Healthy | Lipofectamine 3000 reagent | N/A | Csobonyeiova et al.74 |
| Fibroblasts | Fetal foreskin | Homozygous α-thalassemia (−SEA/−SEA) | Electroporation | N/A | Tangprasittipap et al.75 |
| Fibroblasts | Fetal epidermal tissue from rim of open neural placode and spinal cord | Spina bifda aperta (SBA) | Electroporation | Neurospheres | Bamba et al.76 |
| Fibroblasts | Adult skin | Healthy | Electroporation | N/A | Bharathan et al.36, Weltner et al.37, Yu et al.40, Wong et al.77, Bang et al.78, Hey et al.79, Jaffer et al.80, Trevisan et al.81, Fidan et al.82, Wang et al.83, Chen et al.84, Polanco et al.85, Willmann et al.86, Høffding et al.87 |
| Fibroblasts | Adult skin | Healthy | Electroporation; Lipofectamine 3000 reagent; Nucleofector system | N/A | Manzini et al.88 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Neural stem cells | Capetian et al.89 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Motor neurons | Hu et al.51 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Neural cells | Wang et al.90 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Neural stem cells, motor neurons, cardiomyocytes, and fibroblasts | Requena et al.91 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Dopaminergic neurons and retinal pigment epithelial cells | Okita et al.20 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Smooth muscle progenitor cells | Zhou et al.92 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Retinal pigment epithelial cells | Li et al.93 |
| Fibroblasts | Adult skin | Healthy | Electroporation | Cardiomyocytes | Sequiera et al.94 |
| Fibroblasts | Adult skin | Healthy | FuGENE HD reagent | Hepatocyte-like and cardiac myocyte-like cells | Si-Tayeb et al.95 |
| Fibroblasts | Adult gingival tissues | Healthy | Electroporation | Periodontal cells | Yin et al.96 |
| Fibroblasts | Adult skin | Huntington’s disease | Electroporation | N/A | Tidball et al.21 |
| Fibroblasts | Adult skin | Fibrodysplasia ossificans progressiva caused by a missense mutation in ACVR1 gene | Electroporation | N/A | Hayashi et al.35 |
| Fibroblasts | Adult skin | Spinocerebellar ataxia type 3 | Electroporation | N/A | Hansen et al.97,98 |
| Fibroblasts | Adult skin | Frontotemporal dementia caused by mutations in microtubule-associated protein tau (MAPT) gene | Electroporation | N/A | Rasmussen et al.99–101 |
| Fibroblasts | Adult skin | Maturity-onset diabetes of the young 4 and type 2 diabetes mellitus caused by mutations in PDX1 gene | Electroporation | N/A | Wang et al.52,102 |
| Fibroblasts | Adult skin | Autosomal recessive Stargardt disease caused by compound heterozygous mutations in ABCA4 gene | Electroporation | N/A | Claassen et al.103 |
| Fibroblasts | Adult skin | X-Chromosomal disease | Electroporation | N/A | Hinz et al.121 |
| Fibroblasts | Adult skin | Becker muscular dystrophy (BMD) caused by mutations in dystrophin gene on chromosome Xp21 | Electroporation | N/A | Gowran et al.105 |
| Fibroblasts | Adult skin | Spinocerebellar ataxia type 3 (SCA3, also known as Machado-Joseph disease) caused by a CAG trinucleotide repeat expansion in ATXN3 gene | Electroporation | N/A | Hayer et al.106 |
| Fibroblasts | Adult skin | Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) caused by a heterozygous mutation in CSF1R gene | Electroporation | N/A | Hayer et al.107 |
| Fibroblasts | Adult skin | Healthy and kidney disease caused by an autosomal dominant mutation in HNF4A gene | Electroporation | N/A | Howden et al.108 |
| Fibroblasts | Adult skin | Prostate adenocarcinoma (PCa) | Electroporation | N/A | Kahounová et al.109 |
| Fibroblasts | Adult skin | Spinocerebellar ataxia type 3 (SCA3) | Electroporation | N/A | Ritthaphai et al.110 |
| Fibroblasts | Adult skin | Dravet syndrome caused by a heterozygous R1525X mutation in SCN1A gene | Electroporation | N/A | Tanaka et al.111 |
| Fibroblasts | Adult skin | Mucoplysaccharydosis IIIA (MPSIIIA) | Electroporation | N/A | Vallejo et al.112 |
| Fibroblasts | Adult skin | Mucopolysaccharydosis IIIB (MPSIIIB) | Electroporation | N/A | Vallejo-Diez et al.113 |
| Fibroblasts | Adult skin | Late-onset non-syndromic retinitis pigmentosa caused by compound heterozygous mutations in CLN3 gene | Electroporation | N/A | Zhang et al.114 |
| Fibroblasts | Adult skin | Autosomal recessive Alport syndrome (ARAS) caused by a homozygous COL4A3 mutation | Electroporation | N/A | Kuebler et al.115 |
| Fibroblasts | Adult skin | X-linked Alport syndrome (XLAS) caused by hemizygous COL4A5 mutations in exon 41 or exon 46 | Electroporation | N/A | Kuebler et al.116 |
| Fibroblasts | Adult skin | Duchenne muscular dystrophy (DMD) lacking DMD exons 49 and 50 | Electroporation | N/A | Spaltro et al.117 |
| Fibroblasts | Adult skin | Leber’s hereditary optic neuropathy (LHON) | Electroporation | N/A | Hung et al.118 |
| Fibroblasts | Adult skin | Low-grade steatosis | Electroporation | N/A | Kawala et al.119 |
| Fibroblasts | Adult skin | Fibrodysplasia ossificans progressiva syndrome caused by a mutation in ACVR1 gene | Electroporation | N/A | Kim et al.120 |
| Fibroblasts | Adult skin | Alzheimer’s disease caused by mutations in PSEN1 gene | Electroporation | N/A | Li et al.121,122, Poon et al.123, Tubsuwan et al.124 |
| Fibroblasts | Adult skin | Familial Mediterranean Fever (FMF) | Electroporation | N/A | Fidan et al.125 |
| Fibroblasts | Adult skin | Turner syndrome (TS) caused by monosomy X | Electroporation | N/A | Luo et al.126 |
| Fibroblasts | Adult skin; Fetal skin | Retinitis pigmentosa; Severe combined immunodeficiency | Electroporation | N/A | Howden et al.127 |
| Fibroblasts | Adult skin | Ankylosing spondylitis; Sjögren’s syndrome; Systemic lupus erythematosus | Electroporation | Hematopoietic and mesenchymal lineages | Son et al.47 |
| Fibroblasts | Adult skin | Retinitis pigmentosa-11 caused by a dominant nonsense mutation in PRPF31 gene | Electroporation | Retinal organoids | McLenachan et al.128 |
| Fibroblasts | Adult skin | Rare neurodevelopmental disorders (NDDs) | Electroporation | Forebrain neurons | Bell et al.129 |
| Fibroblasts | Adult skin | Down syndrome | Electroporation | Neuronal cells | Briggs et al.130 |
| Fibroblasts | Adult skin | Alzheimer’s disease caused by mutations in PSEN1 gene | Electroporation | Mature neurons with amyloidogenic properties | Mahairaki et al.131 |
| Fibroblasts | Adult skin | Low-density lipoprotein receptor (LDLR) deficiency familial hypercholesterolemia (FH) | Electroporation | Hepatocyte-like cells | Ramakrishnan et al.132 |
| Keratinocytes | Adult skin | Healthy | FuGENE HD reagent | N/A | Piao et al.133 |
| Mononuclear cells | Fetal peripheral blood | Healthy | Electroporation | N/A | Dowey et al.134 |
| Mononuclear cells | Neonatal peripheral blood and cord blood | Lung disease | Electroporation | N/A | Kamath et al.135 |
| Mononuclear cells | Adult peripheral blood | Healthy | Electroporation | N/A | Okita et al.43, Wen et al.136,137, Wang et al.138,139, Su et al.140, Tangprasittipap et al.141, Mack et al.142, Chou et al.143 |
| Mononuclear cells | Adult peripheral blood | Healthy | Electroporation | Cardiovascular progenitor cells | Hu et al.61 |
| Mononuclear cells | Adult peripheral blood | Healthy | Electroporation | Ventricular cardiomyocyte | Weng et al.144 |
| Mononuclear cells | Adult peripheral blood | Healthy | Electroporation | Hepatocytes | Liu et al.145 |
| Mononuclear cells | Adult bone marrow | Healthy | Electroporation | Mesenchymal stem cells | TheinHan et al.146 |
| Mononuclear cells | Adult peripheral blood | Alzheimer’s disease | Electroporation | N/A | Wang et al.147–150 |
| Mononuclear cells | Adult peripheral blood | Bipolar disorder (BD) | Electroporation | N/A | Wang et al.151 |
| Mononuclear cells | Adult peripheral blood | Obsessive compulsive disorder (OCD) | Electroporation | N/A | Wang et al.152 |
| Mononuclear cells | Adult peripheral blood | Parkinson disease | Electroporation | N/A | Zhao et al.153 |
| Mononuclear cells | Adult peripheral blood | Complete dopa-responsive dystonia (DYT5) caused by a GCH1 mutation | Electroporation | N/A | Murakami et al.154 |
| Mononuclear cells | Adult peripheral blood | Hypertrophic cardiomyopathy caused by mutations in beta-myosin heavy chain (MYH7) gene | Electroporation | N/A | Ross et al.155 |
| Mononuclear cells | Adult peripheral blood | Sickle cell anemia (SCA) | Electroporation | CD34+CD45+ hematopoietic stem and progenitor cells | Junqueira Reis et al.156 |
| Mononuclear cells | Adult peripheral blood | Myocardial infarction | Electroporation | Cardiomyocytes | Malecki et al.157 |
| Mononuclear cells | Adult bone marrow | Healthy | Electroporation | Mesenchymal stem cells, adipocytes, chondrocytes, and osteoblasts | Tang et al.158 |
| Mononuclear cells | Adult peripheral blood and bone marrow | Myelodysplastic syndromes (MDS) | Electroporation | CD34+CD45+ hematopoietic stem and progenitor cells (HPC), and CD71+CD235a+ erythroid cells | Hsu et al.159 |
| Mononuclear cells | Fetal cord blood and neoplastic bone marrow; Adult patient | Healthy; Chronicmyeloid leukemia | Electroporation | CD34+CD43+ hematopoietic progenitors, CD34+CD31+CD43− endothelial cells, and CD34+CD31−CD43− mesenchymal cells; N/A | Hu et al.41,160 |
| Erythroblast | Adult peripheral blood | Healthy | Electroporation | N/A | Varga et al.161 |
| Erythroblast | Adult peripheral blood | Ataxia-Telangiectasia (A-T) caused by compound heterozygous null mutations in ATM kinase gene at chromosome 11q22 | Electroporation | Neural Stem Cells | Bhatt et al.162,163 |
| Cord blood CD34+ cells | Fetal cord blood | Healthy | Electroporation | N/A | Chou et al.48, Meng et al.56, Su et al.164, Fernandes et al.165–167 |
| Amniotic fluid cells | Fetal amniotic fluid | Healthy | Electroporation | N/A | Slamecka et al.168, He et al.169 |
| Amniotic fluid cells | Fetal amniotic fluid | Healthy | Fugene HD reagent | Neural cells | Wilson et al.170 |
| Amniotic fluid cells | Fetal amniotic fluid | Trisomy 18 (18T) | Electroporation | N/A | Xing et al.171 |
| Mesenchymal stromal cells | Fetal amnion | Healthy | Electroporation | N/A | Slamecka et al.172 |
| Mesenchymal stromal cells | Fetal femur | Healthy | Electroporation | N/A | Megges et al.60 |
| Mesenchymal stromal cells | Adult femur | Healthy | Electroporation | N/A | Göbel et al.173, Foja et al.174 |
| Mesenchymal stromal cells | Adult subcutaneous fat | Healthy | Electroporation | N/A | Qu et al.175 |
| Mesenchymal stromal cells | Adult dental pulp | Healthy | Electroporation | Neural progenitor cells | Thekkeparambil Chandrabose et al.176, Saitoh et al.177 |
| Mesenchymal stromal cells | Adult parotid gland | Squamous cell carcinoma of oral cavity | Electroporation | N/A | Yan et al.178 |
| Neural stem cells | Neonate | Healthy | Electroporation | N/A | Marchetto et al.58 |
| Neural stem cells | Fetal cortical tissue | Healthy | Electroporation | Neural cells | Zhou et al.8 |
| Lymphoblast | Adult peripheral blood | Healthy | Electroporation | N/A | Schroter et al.179 |
| Lymphoblast | Adult peripheral blood | Healthy | Electroporation | Neurons, spinal motor neurons, and intestinal organoids | Barrett et al.180 |
| Lymphoblast | Adult peripheral blood | Parkinson’s disease | Electroporation | N/A | Kumar et al.49 |
| Lymphoblast | Adult peripheral blood | Alzheimer’s disease caused by a TREM2 missense mutation | Electroporation | N/A | Schroter et al.181 |
| Lymphoblast | Adult peripheral blood | Alzheimer’s disease caused by a homozygous APOE4 allele mutation | Electroporation | N/A | Zulfiqar et al.182,183 |
| Lymphoblast | Adult peripheral blood | Alzheimer’s disease with different genotypes of a functional copy number variation in the AD risk gene CR1; AD with TREM2 p.R47H variant | Electroporation | N/A | Schröter et al.184,185 |
| Lymphoblast | Adult peripheral blood | APOE ∊3/∊3 genotype and expressing CR1 isoform F/F (low risk of Alzheimer’s disease) | Electroporation | N/A | Martins et al.186 |
| T cells | Adult peripheral blood | Healthy | Electroporation | Neuronal cells | Tsai et al.33 |
| T cells | Adult peripheral blood | Age-related macular degeneration | Electroporation | Retinal pigment epithelial cells | Chang et al.34 |
| B cells | Adult peripheral blood | Healthy | Electroporation | N/A | Choi et al.55 |
| B cells | Adult peripheral blood | Healthy | Electroporation | Hematopoietic, cardiac, neural, and hepatocyte-like lineages | Rajesh et al.42 |
| B cells | Adult peripheral blood | Parkinson’s disease | Electroporation | Neurospheres, and neural cells | Fujimori et al.187 |
| Lamina propria progenitor cells | Adult oral mucosal | Healthy | Electroporation | N/A | Howard-Jones et al.188 |
| Oral mucosa epithelial stem cells | Adult oral mucosal | Healthy | Electroporation | N/A | Alvisi et al.189 |
| Oral mucosa epithelial stem cells | Adult oral mucosal | Ectrodactyly-ectodermal dysplasia-clefting (EEC) syndrome caused by a R279H mutation in TP63 gene | Electroporation | N/A | Trevisan et al.190 |
| Urine cells | Adult urine | Healthy | Electroporation | N/A | Wang et al.191 |
| Urine cells | Adult urine | Healthy | Electroporation | Hepatocyte-like cells | Si-Tayeb et al.192 |
| Urine cells | Adult urine | Multiple endocrine neoplasia type 1 (MEN1) (also termed Wermer syndrome) caused by mutations in tumor suppressor gene MEN1 | Electroporation | N/A | Guo et al.193 |
| Urine cells | Adult urine | Type 2 long QT syndrome caused by a mutation in HERG A561P gene | Electroporation | Cardiomyocytes | Jouni et al.194 |
| Urine progenitor cells | Adult urine | Healthy | Lipofectamine 3000 reagent | N/A | Steichen et al.195 |
| Urine epithelial cells | Adult urine | Healthy | Electroporation | N/A | Ju et al.196 |
| Urine epithelial cells | Adult urine | Healthy | Electroporation | Hepatocytes | Sauer et al.50 |
| Urine epithelial cells | Adult urine | Phenylketonuria (PKU) | Electroporation | N/A | Qi et al.197 |
| Urine epithelial cells | Adult urine | Spinal muscular atrophy (SMA) caused by mutations in survival motor neuron 1 (SMN1) gene | Electroporation | Motor neurons | Zhou et al.198 |
| SIX2-positive renal cells | Adult urine | An African male expressing the CYP2D6 *4/*17 variant which confers intermediate drug metabolizing activity | Electroporation | N/A | Bohndorf et al.199 |
| Epicardium-derived cells | Adult atrial biopsy | Healthy | Electroporation | N/A | Paulitschek et al.200 |
| Neonatal fibroblasts; Adult skin fibroblasts; Urine epithelial cells; Amniotic fluid cells | Neonatal and adult skin; adult urine | Healthy | PEI reagent | N/A | Drozd et al.32 |
| Fibroblasts; Urine epithelial cells | Adult skin; adult urine | Healthy | PEI reagent | Insulin producing cells | Walczak et al.38 |
| Fibroblasts; Mononuclear cells | Adult skin; Adult peripheral blood | Healthy | Electroporation | Cardiomyocytes, endothelial cells, and neuronal cell | Diecke et al.201 |
| Fibroblasts; Mononuclear cells | Adult skin; Adult peripheral blood | Kawasaki disease (KD) | Electroporation | Vascular endothelial cells | Ikeda et al.202 |
| Mononuclear cells; Mesenchymal stromal cells | Adult peripheral blood and bone marrow | Healthy | Electroporation | N/A | Cheng et al.203 |
| Fetal fibroblasts; Adult fibroblasts; Keratinocytes; Cord blood CD34+ cells | Fetal skin; Adult skin; Adult skin; Fetal cord blood | Healthy | Electroporation | N/A | Park et al.204 |
| Fibroblasts; Cord blood CD34+ cells | Adult skin; Fetal cord blood | Healthy | Electroporation | Vascular progenitor cells | Park et al.205 |
| Fibroblasts; Keratinocytes | Adult skin; Hair follicle | Timothy syndrome with cardiac arrhythmias | Lipofectamine 2000 reagent | Cardiomyocytes | Song et al.206 |
| Cancer cells | Adult lung | Adenocarcinoma | X-tremeGENE transfection reagent | N/A | Zhao et al.207 |
The ease of obtaining several of the human cell types, such as those in peripheral blood or urine, for reprogramming make this method less invasive for donors, and there is an equivalent success rate for producing EiPSCs of similar nature. Obtaining EiPSCs from donors with known specific genetic manipulations also allows for the establishment of cell lines for further research, especially with regards to future developments of gene therapy for these specific conditions. Episomal plasmids have been used to generate EiPSCs from various somatic cells of differing origins. Human mesenchymal stromal cells have been developed into EiPSCs through the episomal plasmid-based expression of Oct4, Sox2, Nanog, Lin28, SV40LT, Klf4, and c-Myc60. Other sources reported include human fetal foreskin fibroblasts, and CD34+ cells from cord and peripheral human blood, which shows that peripheral blood mononuclear cells may be a good source of cells for iPSC reprogramming especially because of the low invasiveness when obtaining samples.
Gene Delivery Methods
Delivery of plasmids into cells for reprogramming has been described. These methods include electroporation techniques (using Nucleofector kits), liposomal magnetofection, and Lipofectamine transfection reagents. Liposomal transfection using magnetofection208 or Lipofectamine refers to the delivery of plasmid DNA via liposomes and allows the merging of cationic liposomes carrying the DNA into the cells. Electroporation is the use of an electric current across cell membranes, which forcibly opens their channels to allow entry of reagents. This method has been described widely and has a higher rate of delivery efficiency but is known to cause cell damage209. The use of non-liposomal transfection reagents for episomal plasmid delivery, such as FuGENE HD, has also been reported to have good transfection efficiencies210. The company produces a proprietary formula that is touted as non-liposomal but can still deliver DNA and plasmids into cells and therefore works as a transfection kit. Recent work has also shown promise in producing EiPSCs using small molecules instead of feeder cells. A cocktail of molecules has been described for the reprogramming of human somatic cells to iPSCs. These include the MEK inhibitor PD0325901, GSK3β inhibitor CHIR99021, TGF-β/activin/nodal receptor inhibitor A-83-01, ROCK inhibitor HA-100, and human leukemia inhibitory factor40.
In Vivo Animal Model Applications
Cardiogenic Regeneration
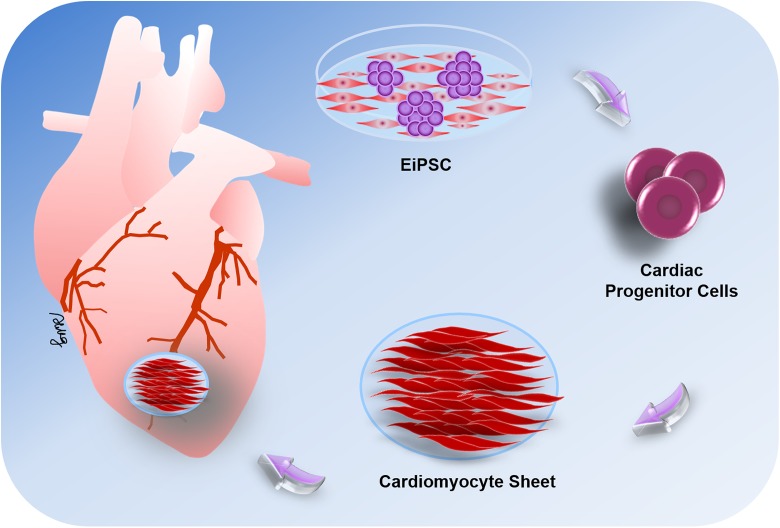
Much attention has focused on the use of iPSCs in cardiac regeneration especially after cardiac infarction, which causes loss of cardiomyocytes that cannot regenerate and are replaced with scar tissue. Both EiPSCs and iPSCs have been differentiated into cardiomyocytes, which shows their potential use in both autologous and allogeneic therapies. A recent study demonstrated that allogeneic EiPSCs cultured from cynomolgus monkeys, when differentiated into cardiomyocytes and injected intramuscularly infarcted cardiac muscle, induced remuscularization of infarcted muscle tissue. Fibroblasts obtained from the monkeys were reprogrammed using episomal plasmids into EiPSCs, and the EiPSCs-derived cardiomyocytes were then injected into the infarcted cardiac muscle. After a clinical regimen of immunosuppression using methylprednisolone and tacrolimus, the hearts showed improvement in cardiac contractile function without any signs of rejection on postoperative week 12211. The results are promising in showing that direct application of EiPSCs-derived cardiomyocytes is possible. The local environment and conditions under which the EiPSCs were directly injected allowed for their direct use and differentiation according to clinical need. A diagram of the potential application for an EiPSCs-engineered cardiac cell sheet is shown in Fig. 1.
Figure 1.
The potential application for cardiac cell sheet strategies using EiPSC-derived cardiomyocytes. EiPSCs can be differentiated into cardiac progenitor cells, which are then induced to form cardiomyocytes in vitro. These cardiomyocytes can then be organized into a cell sheet and applied to damaged areas of cardiac muscle in vivo via intracoronary or intracardiac injections or epicardially by tissue-engineered cardiac patches. The cell sheets exhibit regenerative capabilities and induce the restoration of cardiac function after muscle damage.
One problem with bioengineered tissue is that it cannot be used to create a large structure, which requires thorough oxygenation, because of the lack of vascularization in the bioengineered construct. EiPSCs were reported to regenerate vascular tissue if some were first converted to patient-specific cardiovascular progenitor cells, which then differentiated into vascular smooth muscle cells to make up the vascular scaffold present in blood vessels. This new development heralds the potential for integration and creation of larger bioengineered constructs that can become vascularized. This suggests the potential ability to design whole organs with vascularized networks made from the patient’s cells, which are then attached using conventional surgical methods. This may allow the organ to be manufactured in the laboratory and vascularized61.
Peripheral Nerve Regeneration
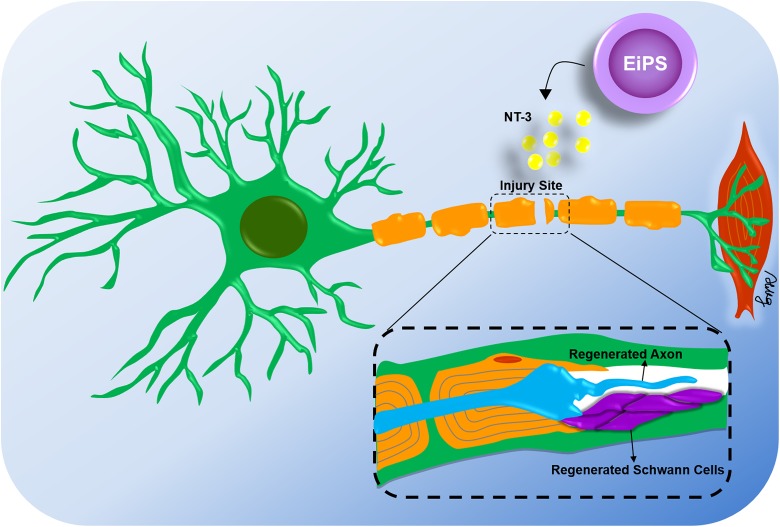
EiPSCs have shown promise in promoting the regeneration of peripheral nerves in a mouse sciatic transection model212. Transection or neurotmesis of peripheral nerves is notoriously difficult to recover and usually leads to wasting of motor end plates, muscle atrophy, and functional loss, which markedly impairs the patient’s quality of life. In this mouse model, undifferentiated EiPSCs were applied to the transected ends of the sciatic nerves after coaptation of both ends by suturing. Compared with the negative control without cell administration, sciatic nerves treated with EiPSCs displayed significantly faster axonal regeneration and a ration of the degree of myelination to axonal diameter. These positive changes were similar to those observed in the ESC group, which acted as a positive control. The results of this study demonstrate the neuroregenerative potential of EiPSCs. One possible mechanism includes the increased expression of neutrotrophin-3, a neuronal growth factor, which can accelerate axonal regeneration and myelination. Direct application of EiPSCs to the site of injury and nerve transection presumably allowed the EiPSCs to act through a paracrine mechanism due to its direct effect and fast nature; they probably differentiate but rather, when applied to the environment, promoted sciatic nerve recovery through the upregulation of neutrotrophin-3 and subsequent secretion of neuronal growth factor by the EiPSCs themselves. The diagram in Fig. 2 shows a depiction of the actions of EiPSCs on mouse transected peripheral nerve regeneration.
Figure 2.
Topical application of EiPSCs to transected peripheral nerves. After surgical repair of transected peripheral nerves in a mouse sciatic nerve model, axonal regeneration was accelerated by topical application of EiPSCs to the site of injury. The increased production of neurotrophic factor-3 as a growth factor was one of the causes of acceleration of axonal growth and maintenance of muscle function and gait. Compared with negative controls without cell administrations, the regenerated axons exhibited a higher quality of myelination and more cells were obtained.
Ischemic Stroke Therapy
Mouse embryonic fibroblasts reprogrammed into EiPSCs using episomal plasmid transfection were delivered and used to treat mice in an ischemic stroke model213. To avoid oncogenic and virus integration, while generating EiPSCs, two expression plasmids, Oct4 and Sox2, were repeatedly transfected into fibroblasts under hypoxic condition. The EiPSCs were first differentiated into neural precursor cells before being injected into the brain of mice after the induced ischemic stroke. The authors observed evidence of the differentiation of precursor cells into neurons and astrocytes. They concluded that these observed changes resulted in better behavioral recovery including locomotor activity, beam walking, and rotarod movement compared with control mice.
Smooth Muscle Regeneration
The use of EiPSCs in the regeneration of smooth muscle cells for the treatment of stress-induced urinary incontinence has also been described214. Inadequate muscle sphincter function is a possible cause for urinary incontinence, and regeneration of smooth muscles at this sphincter may increase sphincter tone and hence urinary continence and control. The group here conducted experiments in rat urethral sphincters which were surgically weakened, resulting in urinary incontinence. Smooth muscle precursor cells were then differentiated from human EiPSCs and injected periurethrally to enhance muscle tone. Leak pressure and sphincter muscle electromyography were measured as markers of recovery. The group with the EiPSCs-derived smooth muscle injection showed recovery of the sphincter compared with the control without cell administration, which suggested that this method of treatment may be possible for restoration of urethral sphincter function.
EiPSCs currently have the potential for cardiac regeneration to replace damaged myocardium. The topical application of cells to regenerate a limited area appears promising, but reconstructing an entire cardiac structure requires further bioengineering advances to deliver blood supply to the entire organ. The use of EiPSCs in peripheral nerve regeneration can improve transected nerve recovery. Direct applications after surgical repair can enhance recovery through nerve growth factor secretion. In ischemic stroke therapy, EiPSCs may have potential for improving the recovery of damaged neural cells in the brain by differentiating into neurons and astrocytes, which should result in better motor recovery. EiPSCs can also play a role in regeneration of smooth muscle cells, leading to restoration of muscle sphincter function in urinary incontinence. A summary of the EiPSCs used in various functional studies is shown in Table 2 211–214.
Table 2.
Summary of EiPSCs used in Various Functional Studies.
| Type of cell | Animal model | Method of transfection | Differentiated cell type | Functional application | Author |
|---|---|---|---|---|---|
| Mouse fibroblasts | Mouse | FuGENE HD reagent | Neural precursor cells | Ischemic stroke therapy | Liu et al.213 |
| Mouse fibroblasts | Mouse | Lipofectamine 3000 reagent | N/A | Transected peripheral nerve recovery | Loh et al.212 |
| Primate fibroblasts | Cynomolgus monkey | Electroporation | Cardiomyocytes | Myocardial infarction recovery | Shiba et al.211 |
| Human fibroblasts | Rat | Electroporation | Smooth muscle cells | Urethral spincter recovery | Wang et al.214 |
Clinical Trials
Current clinical trials known at the time of writing this review all involve iPSCs derived from retroviral transduction. A trial of the replacement of retinal pigment epithelium cells (RPEs) in age-related macular degeneration (ARMD) was recently continued and is in progress. The human iPSCs used in this case were derived from retroviral reprogramming215. Human iPSC-derived RPE cell sheets were generated without any artificial scaffolds, express typical RPE cell markers, form tight junctions that exhibit polarized secretion of growth factors, and show phagocytotic ability and gene-expression patterns similar to those of native RPE cells. The monolayer cell sheets have potential use as a graft for tissue replacement therapy for ARMD.
The trial was temporarily halted because spontaneous genetic mutations were found in the generation of iPSCs216. The spontaneous mutations comprised six mutations, in which three genes had been deleted and another three nucleotides changed. One of the mutations was an “oncogene,” which has a low-risk link to cancer. None of these mutations were present in the patient’s original DNA makeup. The appearance of mutations was deemed to be either the result of the iPSC induction procedure or the presence at undetectable levels in the patient’s somatic skin cells initially. As the risk of carcinogenesis was low, the trial was continued as planned217.
Currently registered trials focus on platelet generation for treating various anemias. However, the research group faced the problem of the mass production of platelets required for effective clinical use. Other trials involve the differentiation of dopaminergic neurons piloted for use in Parkinson’s disease. Retinal ganglion cells are also being used for treatment of glaucoma and optic neuropathies. Except for the current trial in Japan of the use of RPE cells for wet ARMD mentioned above, all of these trials are in the preclinical or animal model stages218. Guidelines for further clinical trials for stem cell research involving patients and iPSCs were issued by the International Society for Stem Cell Research in 2016. These guidelines suggest that the donor cell procurement should be checked for iPSCs intended for use in human, and that these cells should be excluded from specialized reviews because they are now acknowledged to have different implications in the treatment of disease compared with human ESCs219.
Safety
The evidence for the safety of the use of EiPSCs in animal models is only now emerging, and there is a paucity of evidence in this area. Mice given EiPSCs at the site of the transected sciatic nerve displayed no formation of tumors locally at the site of application212. Distant sites and major organs were also examined histologically for the presence of tumors after 1 year of follow-up. Normal behavior and health of the mice were recorded, and no ill effects of EiPSCs were reported in this group. Another study found no aberrant growths from differentiated EiPSCs that were added to primate hearts regeneration after a myocardial infarction211. Neither macroscopic nor microscopic analysis revealed any evidence of tumor formation at 12 weeks after transplantation of the EiPSCs-derived cardiomyocytes. One possible reason is that the EiPSCs used were completely differentiated, with minimal residual EiPSCs present remaining after grafting, which resulted in no tumor formation. Because viral or integrative reprogramming techniques may alter gene expression, the use of episomal reprogramming techniques in the production of EiPSCs may reduce the risk of tumor transformation. Further evidence is required to understand more about the safety of the use of EiPSCs and to substantiate the hypothesized mechanisms of action. One possible future direction is to compare the formation of tumors in two groups—undifferentiated EiPSCs and iPSCs generated from other methods such as retroviruses grafted onto recipients. The possible lack of tumor formation seen in EiPSC-transplanted groups from such a study design would provide evidence of its safety.
Yamanaka Cell Bank: A Future in Autologous and Allo-iPSC Therapy
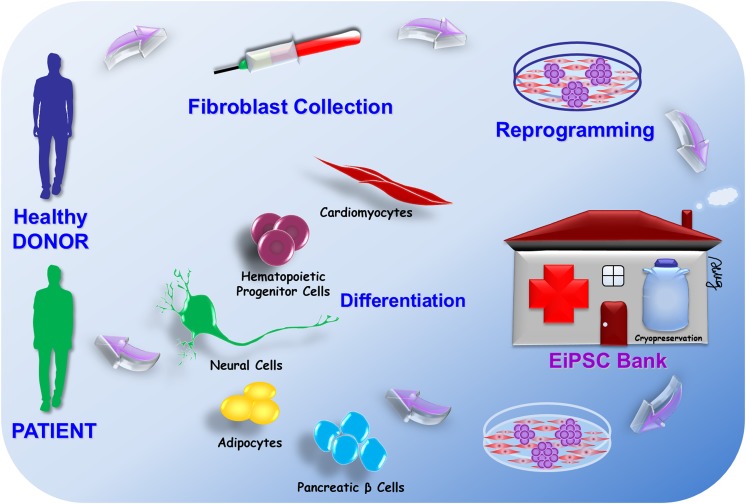
With the recent establishment of the Yamanaka stem cell bank at the RIKEN BioResource Center in Japan, several human iPSC cell lines have been produced in preparation for future research or clinical application. A switch to episomal methods of iPSC production is evident from the most recent human iPSC cell lines formed at the Yamanaka stem cell bank220. As mentioned previously, to enable use of the cells clinically, particular attention should be paid to the methods of iPSC production that favor episomal methods. The Yamanaka stem cell bank has successfully produced multiple human cell lines of EiPSCs using transformation of human cells from various types. EiPSCs have been produced from human skin fibroblasts (cell numbers HPS0076, HPS0077)221,222, human cord blood (cell numbers HPS0328, HPS0331), and human peripheral blood (cell number HPS0360)43. Other than the two initial cell lines produced from human, which were obtained using retroviral transduction without c-Myc, the five more recently developed human EiPSC cell lines were produced purely via episomal vectors. A diagram showing the potential for an EiPSC bank is depicted in Fig. 3.
Figure 3.
The potential for an EiPSC bank. The pluripotent potential of EiPSCs allows them to differentiate into various cell lineages for repair and regeneration. Fibroblasts from healthy donors can be harvested and reprogrammed into EiPSCs and stored in a cell bank. These EiPSCs can then be differentiated into various cell lineages for repair and regeneration according to the needs of individual patients. So far, these cells include cardiomyocytes, hematopoietic progenitor cells, neural cells, adipocytes, and pancreatic islet cells. Each of these cells can be used to replace damaged cells in patients and provide a novel therapeutic potential for each clinical scenario. For any allogeneic transfer of EiPSCs, MHC mismatch typing can first be performed to minimize any chance of MHC mismatch incompatibility before selecting the least antigenic EiPSC bank sample to be transferred to the patient.
The immunogenicity of iPSCs generated remains an unknown area of research. A recent study showed a possible immune response toward smooth muscle cells derived from iPSCs but not the RPE from iPSCs223. Possible strategies for bypassing a possible immune response include the development of humanized iPSCs-derived RPE cells for transplantation. In a humanized mouse model, Zhao et al. found that the smooth muscle cells induced a strong antigenic response by the host’s immune system which was not evident when RPE cells were used. The potential use of immunosuppression must be considered for the clinical use and clinical trials of iPSCs. If there is evidence of rejection of tissue as for a foreign host, immunosuppressants should be administered when the cells are delivered to the recipient. The use of iPSCs for treating Parkinson’s disease has been reported in Japan. However, certain clinical considerations, such as the safety and therapeutic use of iPSCs in patients with Parkinson’s disease, are needed. Clinical trials are underway for examining this aspect of iPSC use and treatment. Current rapid integration into its clinical use should continue to surface within the next few years224.
Conclusion
The future of EiPSCs lies in the increasing trend for the use of cell therapy in the treatment of various diseases because of their regenerative properties. Differentiating these cells before use or their direct application requires further investigation. The immunogenicity of allogeneic EiPSCs will also need to be determined for the tissue both derived and differentiated from these cells.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Chang Gung Medical Foundation, Chang Gung Memorial Hospital, Taiwan (CMRPG1F0082, CMRPG3F1431, CMRPG1H0081 and CMRPG1H0082) and Ministry of Science and Technology, Taiwan (MOST 108-2314-B-182A-009-).
ORCID iD: Aline Yen Ling Wang  https://orcid.org/0000-0001-6272-6948
https://orcid.org/0000-0001-6272-6948
References
- 1. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 2. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. [DOI] [PubMed] [Google Scholar]
- 3. Li Y, Zhao H, Lan F, Lee A, Chen L, Lin C, Yao Y, Li L. Generation of human-induced pluripotent stem cells from gut mesentery-derived cells by ectopic expression of OCT4/SOX2/NANOG. Cell Reprogram. 2010;12(3):237–247. [DOI] [PubMed] [Google Scholar]
- 4. Schwarz BA, Bar-Nur O, Silva JC, Hochedlinger K. Nanog is dispensable for the generation of induced pluripotent stem cells. Curr Biol. 2014;24(3):347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olariu V, Lövkvist C, Sneppen K. Nanog, Oct4 and Tet1 interplay in establishing pluripotency. Sci Rep. 2016;6:25438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sumer H, Liu J, Malaver-Ortega LF, Lim ML, Khodadadi K, Verma PJ. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J Anim Sci. 2011;89(9):2708–2716. [DOI] [PubMed] [Google Scholar]
- 7. Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. [DOI] [PubMed] [Google Scholar]
- 8. Zhou S, Liu Y, Feng R, Wang C, Jiang S, Zhang X, Lan F, Li Y. Survivin improves reprogramming efficiency of human neural progenitors by single molecule OCT4. Stem Cells Int. 2016. doi:10.1155/2016/4729535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, Zhang Q, Xiang C, Hou P, Song Z, Liu Y, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3(5):475–479. [DOI] [PubMed] [Google Scholar]
- 10. Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y, Feng H, Gu H, Lewis DW, Yuan Y, Zhang L, Yu H, Zhang P, Cheng H, Miao W, Yuan W, et al. The p53-PUMA axis suppresses iPSC generation. Nat Commun. 2013;4:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin T, Lin Y. p53 switches off pluripotency on differentiation. Stem Cell Res Ther. 2017;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solozobova V, Blattner C. p53 in stem cells. World J Biol Chem. 2011;2(9):202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G, Edel M, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26(11):1276–1284. [DOI] [PubMed] [Google Scholar]
- 15. Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2010;19(4):469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. [DOI] [PubMed] [Google Scholar]
- 17. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. [DOI] [PubMed] [Google Scholar]
- 18. Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–953. [DOI] [PubMed] [Google Scholar]
- 19. Okita K, Hong H, Takahashi K, Yamanaka S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat Protoc. 2010;5(3):418–428. [DOI] [PubMed] [Google Scholar]
- 20. Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8(5):409–412. [DOI] [PubMed] [Google Scholar]
- 21. Tidball AM, Neely MD, Chamberlin R, Aboud AA, Kumar KK, Han B, Bryan MR, Aschner M, Ess KC, Bowman AB. Genomic instability associated with p53 knockdown in the generation of Huntington’s disease human induced pluripotent stem cells. PLoS One. 2016;11(3):e0150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318(5858):1920–1923. [DOI] [PubMed] [Google Scholar]
- 23. Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27(11):2667–2674. [DOI] [PubMed] [Google Scholar]
- 24. Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, Cowling R, Wang W, Liu P, Gertsenstein M, Kaji K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458(7239):766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, et al. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7(3):197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4(1):16–19. [DOI] [PubMed] [Google Scholar]
- 30. Vdovin AS, Lupatov AY, Kholodenko IV, Yarygin KN. Comparison of the efficiency of viral transduction and episomal transfection in human fibroblast reprogramming. Bull Exp Biol Med. 2015;160(1):123–128. [DOI] [PubMed] [Google Scholar]
- 31. O’Doherty R, Greiser U, Wang W. Nonviral methods for inducing pluripotency to cells. Biomed Res Int. 2013;2013:705902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drozd AM, Walczak MP, Piaskowski S, Stoczynska-Fidelus E, Rieske P, Grzela DP. Generation of human iPSCs from cells of fibroblastic and epithelial origin by means of the oriP/EBNA-1 episomal reprogramming system. Stem Cell Res Ther. 2015;6(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai PH, Chang YC, Lee YY, Ko YL, Yang YH, Lin CF, Chang YL, Yu WC, Shih YH, Chen MT. Differentiation of blood T cells: reprogramming human induced pluripotent stem cells into neuronal cells. J Chin Med Assoc. 2015;78(6):353–359. [DOI] [PubMed] [Google Scholar]
- 34. Chang YC, Chang WC, Hung KH, Yang DM, Cheng YH, Liao YW, Woung LC, Tsai CY, Hsu CC, Lin TC, Liu JH, et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front Aging Neurosci. 2014;6:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayashi Y, Hsiao EC, Sami S, Lancero M, Schlieve CR, Nguyen T, Yano K, Nagahashi A, Ikeya M, Matsumoto Y, Nishimura K, et al. BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence. Proc Natl Acad Sci U S A. 2016;113(46):13057–13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bharathan SP, Manian KV, Aalam SM, Palani D, Deshpande PA, Pratheesh MD, Srivastava A, Velayudhan SR. Systematic evaluation of markers used for the identification of human induced pluripotent stem cells. Biol Open. 2017;6(1):100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weltner J, Balboa D, Katayama S, Bespalov M, Krjutškov K, Jouhilahti EM, Trokovic R, Kere J, Otonkoski T. Human pluripotent reprogramming with CRISPR activators. Nat Commun. 2018;9(1):2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walczak MP, Drozd AM, Stoczynska-Fidelus E, Rieske P, Grzela DP. Directed differentiation of human iPSC into insulin producing cells is improved by induced expression of PDX1 and NKX6.1 factors in IPC progenitors. J Transl Med. 2016;14(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 2011;6(3):e17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117(14):e109–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rajesh D, Dickerson SJ, Yu J, Brown ME, Thomson JA, Seay NJ. Human lymphoblastoid B-cell lines reprogrammed to EBV-free induced pluripotent stem cells. Blood. 2011;118(7):1797–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31(3):458–466. [DOI] [PubMed] [Google Scholar]
- 44. Middleton T, Sugden B. Retention of plasmid DNA in mammalian cells is enhanced by binding of the Epstein-Barr virus replication protein EBNA1. J Virol. 1994;68(6):4067–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26(19):4252–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ross SB, Fraser ST, Bagnall RD, Semsarian C. Peripheral blood derived induced pluripotent stem cells (iPSCs) from a female with familial hypertrophic cardiomyopathy. Stem Cell Res. 2017;20:76–79. [DOI] [PubMed] [Google Scholar]
- 47. Son MY, Lee MO, Jeon H, Seol B, Kim JH, Chang JS, Cho YS. Generation and characterization of integration-free induced pluripotent stem cells from patients with autoimmune disease. Exp Mol Med. 2016;48:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21(3):518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kumar S, Curran JE, Glahn DC, Blangero J. Utility of lymphoblastoid cell lines for induced pluripotent stem cell generation. Stem Cells Int. 2016;2016(9):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sauer V, Tchaikovskaya T, Wang X, Li Y, Zhang W, Tar K, Polgar Z, Ding J, Guha C, Fox IJ, Roy-Chowdhury N, et al. Human urinary epithelial cells as a source of engraftable hepatocyte-like cells using stem cell technology. Cell Transplant. 2016;25(12):2221–2243. [DOI] [PubMed] [Google Scholar]
- 51. Hu W, He Y, Xiong Y, Lu H, Chen H, Hou L, Qiu Z, Fang Y, Zhang S. Derivation, expansion, and motor neuron differentiation of human-induced pluripotent stem cells with non-integrating episomal vectors and a defined xenogeneic-free culture system. Mol Neurobiol. 2016;53(3):1589–1600. [DOI] [PubMed] [Google Scholar]
- 52. Wang X, Chen S, Burtscher I, Sterr M, Hieronimus A, Machicao F, Staiger H, Häring HU, Lederer G, Meitinger T, Lickert H. Generation of a human induced pluripotent stem cell (iPSC) line from a patient with family history of diabetes carrying a C18 R mutation in the PDX1 gene. Stem Cell Res. 2016;17(2):292–295. [DOI] [PubMed] [Google Scholar]
- 53. Gallego Romero I, Pavlovic BJ, Hernando-Herraez I, Zhou X, Ward MC, Banovich NE, Kagan CL, Burnett JE, Huang CH, Mitrano A, Chavarria CI, et al. A panel of induced pluripotent stem cells from chimpanzees: a resource for comparative functional genomics. Elife. 2015;4:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jung-Klawitter S, Blau N, Sebe A, Ebersold J, Göhring G, Opladen T. Generation of an iPSC line from a patient with tyrosine hydroxylase (TH) deficiency: TH-1 iPSC. Stem Cell Res. 2016;17(3):580–583. [DOI] [PubMed] [Google Scholar]
- 55. Choi SM, Liu H, Chaudhari P, Kim Y, Cheng L, Feng J, Sharkis S, Ye Z, Jang YY. Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood. 2011;118(7):1801–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meng X, Neises A, Su RJ, Payne KJ, Ritter L, Gridley DS, Wang J, Sheng M, Lau KH, Baylink DJ, Zhang XB. Efficient reprogramming of human cord blood CD34+ cells into induced pluripotent stem cells with OCT4 and SOX2 alone. Mol Ther. 2012;20(2):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pollini D, Loffredo R, Cardano M, Conti L, Lattante S, Notarangelo A, Sabatelli M, Provenzani A. Generation and characterization of a human iPSC line from an ALS patient carrying the Q66K-MATR3 mutation. Stem Cell Res. 2018;33:146–150. [DOI] [PubMed] [Google Scholar]
- 58. Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4(9):e7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, et al. A comparison of non-integrating reprogramming methods. Nat Biotechnol. 2015;33(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Megges M, Oreffo RO, Adjaye J. Episomal plasmid-based generation of induced pluripotent stem cells from fetal femur-derived human mesenchymal stromal cells. Stem Cell Res. 2016;16(1):128–132. [DOI] [PubMed] [Google Scholar]
- 61. Hu J, Wang Y, Jiao J, Liu Z, Zhao C, Zhou Z, Zhang Z, Forde K, Wang L, Wang J, Baylink DJ, et al. Patient-specific cardiovascular progenitor cells derived from integration-free induced pluripotent stem cells for vascular tissue regeneration. Biomaterials. 2015;73:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matz P, Adjaye J. Episomal-based generation of an iPS cell line from human fetal foreskin fibroblasts. Stem Cell Res. 2016;16(1):67–69. [DOI] [PubMed] [Google Scholar]
- 63. Tandon R, Brändl B, Baryshnikova N, Landshammer A, Steenpaß L, Keminer O, Pless O, Müller FJ. Generation of two human isogenic iPSC lines from fetal dermal fibroblasts. Stem Cell Res. 2018;33:120–124. [DOI] [PubMed] [Google Scholar]
- 64. Tidball AM, Swaminathan P, Dang LT, Parent JM. Generating loss-of-function iPSC lines with combined CRISPR indel formation and reprogramming from human fibroblasts. Bio Protoc. 2018;8(7):e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kamath A, Ternes S, McGowan S, English A, Mallampalli R, Moy AB. Efficient method to create integration-free, virus-free, Myc and Lin28-free human induced pluripotent stem cells from adherent cells. Future Sci OA. 2017;3(3):FSO211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim KM, Heo DR, Lee JY, Seo CS, Chung SK. High-efficiency generation of induced pluripotent stem cells from human foreskin fibroblast cells using the Sagunja-tang herbal formula. BMC Complement Altern Med. 2017;17(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schmitt CE, Morales BM, Schmitz EMH, Hawkins JS, Lizama CO, Zape JP, Hsiao EC, Zovein AC. Fluorescent tagged episomals for stoichiometric induced pluripotent stem cell reprogramming. Stem Cell Res Ther. 2017;8(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mah N, Wang Y, Liao MC, Prigione A, Jozefczuk J, Lichtner B, Wolfrum K, Haltmeier M, Flöttmann M, Schaefer M, Hahn A, et al. Molecular insights into reprogramming-initiation events mediated by the OSKM gene regulatory network. PLoS One. 2011;6(8):e24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dias J, Gumenyuk M, Kang H, Vodyanik M, Yu J, Thomson JA, Slukvin II. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20(9):1639–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mehta A, Chung YY, Ng A, Iskandar F, Atan S, Wei H, Dusting G, Sun W, Wong P, Shim W. Pharmacological response of human cardiomyocytes derived from virus-free induced pluripotent stem cells. Cardiovasc Res. 2011;91(4):577–586. [DOI] [PubMed] [Google Scholar]
- 71. Wruck W, Adjaye J. Human pluripotent stem cell derived HLC transcriptome data enables molecular dissection of hepatogenesis. Sci Data. 2018;5:180035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhise NS, Wahlin KJ, Zack DJ, Green JJ. Evaluating the potential of poly(beta-amino ester) nanoparticles for reprogramming human fibroblasts to become induced pluripotent stem cells. Int J Nanomedicine. 2013;8:4641–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Skrzypczyk A, Giri S, Bader A. Generation of induced pluripotent stem cell line from foreskin fibroblasts. Stem Cell Res. 2016;17(3):572–575. [DOI] [PubMed] [Google Scholar]
- 74. Csobonyeiova M, Krajciova L, Nicodemou A, Polak S, Danisovic L. Induction of pluripotency in long-term cryopreserved human neonatal fibroblasts in feeder-free condition. Cell Tissue Bank. 2017;18(1):45–52. [DOI] [PubMed] [Google Scholar]
- 75. Tangprasittipap A, Satirapod C, Jittorntrum B, Lertritanan S, Anurathaphan U, Phanthong P, Borwornpinyo S, Kitiyanant N, Hongeng S. Generation of iPSC line MU011.A-hiPS from homozygous alpha-thalassemia fetal skin fibroblasts. Stem Cell Res. 2015;15(3):506–509. [DOI] [PubMed] [Google Scholar]
- 76. Bamba Y, Nonaka M, Sasaki N, Shofuda T, Kanematsu D, Suemizu H, Higuchi Y, Pooh RK, Kanemura Y, Okano H, Yamasaki M. Generation of induced pluripotent stem cells and neural stem/progenitor cells from newborns with spina bifida aperta. Asian Spine J. 2017;11(6):870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wong RCB, Hung SS, Jackson S, Singh V, Khan S, Liang HH, Kearns LS, Nguyen T, Conquest A, Daniszewski M, Hewitt AW, et al. Generation of a human induced pluripotent stem cell line CERAi001-A-6 using episomal vectors. Stem Cell Res. 2017;22:13–15. [DOI] [PubMed] [Google Scholar]
- 78. Bang JS, Choi NY, Lee M, Ko K, Lee HJ, Park YS, Jeong D, Chung HM, Ko K. Optimization of episomal reprogramming for generation of human induced pluripotent stem cells from fibroblasts. Anim Cells Syst (Seoul). 2018;22(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hey CAB, Saltõkova KB, Bisgaard HC, Møller LB. Comparison of two different culture conditions for derivation of early hiPSC. Cell Biol Int. 2018;42(11):1467–1473. [DOI] [PubMed] [Google Scholar]
- 80. Jaffer S, Goh P, Abbasian M, Nathwani AC. Mbd3 promotes reprogramming of primary human fibroblasts. Int J Stem Cells. 2018;11(2):235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Trevisan M, Desole G, Costanzi G, Lavezzo E, Palù G, Barzon L. Reprogramming methods do not affect gene expression profile of human induced pluripotent stem cells. Int J Mol Sci. 2017;18(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fidan K, Ebrahimi A, Çağlayan ÖH, Özçimen B, Önder TT. Transgene-free disease-specific iPSC generation from fibroblasts and peripheral blood mononuclear cells. Methods Mol Biol. 2016;1353:215–231. [DOI] [PubMed] [Google Scholar]
- 83. Wang PY, Hung SS, Thissen H, Kingshott P, Wong RC. Binary colloidal crystals (BCCs) as a feeder-free system to generate human induced pluripotent stem cells (hiPSCs). Sci Rep. 2016;6 doi:10.1038/srep36845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8(5):424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Polanco JC, Ho MS, Wang B, Zhou Q, Wolvetang E, Mason E, Wells CA, Kolle G, Grimmond SM, Bertoncello I, O’Brien C, et al. Identification of unsafe human induced pluripotent stem cell lines using a robust surrogate assay for pluripotency. Stem Cells. 2013;31(8):1498–1510. [DOI] [PubMed] [Google Scholar]
- 86. Willmann CA, Hemeda H, Pieper LA, Lenz M, Qin J, Joussen S, Sontag S, Wanek P, Denecke B, Schüler HM, Zenke M, et al. To clone or not to clone? Induced pluripotent stem cells can be generated in bulk culture. PLoS One. 2013;8(5):e65324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hoffding MK, Hyttel P. Ultrastructural visualization of the Mesenchymal-to-Epithelial Transition during reprogramming of human fibroblasts to induced pluripotent stem cells. Stem Cell Res. 2015;14(1):39–53. [DOI] [PubMed] [Google Scholar]
- 88. Manzini S, Viiri LE, Marttila S, Aalto-Setälä K. A comparative view on easy to deploy non-integrating methods for patient-specific iPSC production. Stem Cell Rev. 2015;11(6):900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Capetian P, Azmitia L, Pauly MG, Krajka V, Stengel F, Bernhardi EM, Klett M, Meier B, Seibler P, Stanslowsky N, Moser A, et al. Plasmid-based generation of induced neural stem cells from adult human fibroblasts. Front Cell Neurosci. 2016;10:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang J, Gu Q, Hao J, Bai D, Wang L, Liu Z, Zhou Q. Efficient derivation of human induced pluripotent stem cells with a c-myc-free non-integrating episomal vector. J Genet Genomics. 2016;43(3):161–164. [DOI] [PubMed] [Google Scholar]
- 91. Requena J, Alvarez-Palomo AB, Codina-Pascual M, Delgado-Morales R, Moran S, Esteller M, Sal M, Juan M, Boronat Barado A, Consiglio A, Bogle OA, et al. Global proteomic and methylome analysis in human induced pluripotent stem cells reveals overexpression of a human tlr3 affecting proper innate immune response signaling. Stem Cells. 2019;37(4):476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zhou Y, Kang G, Wen Y, Briggs M, Sebastiano V, Pederson R, Chen B. Do induced pluripotent stem cell characteristics correlate with efficient in vitro smooth muscle cell differentiation? A comparison of three patient-derived induced pluripotent stem cell lines. Stem Cells Dev. 2018;27(20):1438–1448. [DOI] [PubMed] [Google Scholar]
- 93. Li P, Sun X, Ma Z, Liu Y, Jin Y, Ge R, Hao L, Ma Y, Han S, Sun H, Zhang M, et al. Transcriptional reactivation of OTX2, RX1 and SIX3 during reprogramming contributes to the generation of RPE cells from human iPSCs. Int J Biol Sci. 2016;12(5):505–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sequiera GL, Mehta A, Ooi TH, Shim W. Ontogenic development of cardiomyocytes derived from transgene-free human induced pluripotent stem cells and its homology with human heart. Life Sci. 2013;92(1):63–71. [DOI] [PubMed] [Google Scholar]
- 95. Si-Tayeb K, Noto FK, Sepac A, Sedlic F, Bosnjak ZJ, Lough JW, Duncan SA. Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Dev Biol. 2010;10(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yin X, Li Y, Li J, Li P, Liu Y, Wen J, Luan Q. Generation and periodontal differentiation of human gingival fibroblasts-derived integration-free induced pluripotent stem cells. Biochem Biophys Res Commun. 2016;473(3):726–732. [DOI] [PubMed] [Google Scholar]
- 97. Hansen SK, Borland H, Hasholt LF, Tümer Z, Nielsen JE, Rasmussen MA, Nielsen TT, Stummann TC, Fog K, Hyttel P. Generation of spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cell line SCA3.B11. Stem Cell Res. 2016;16(3):589–592. [DOI] [PubMed] [Google Scholar]
- 98. Hansen SK, Borland H, Hasholt LF, Tümer Z, Nielsen JE, Rasmussen MA, Nielsen TT, Stummann TC, Fog K, Hyttel P. Generation of spinocerebellar ataxia type 3 patient-derived induced pluripotent stem cell line SCA3.A11. Stem Cell Res. 2016;16(3):553–556. [DOI] [PubMed] [Google Scholar]
- 99. Rasmussen MA, Hjermind LE, Hasholt LF, Waldemar G, Nielsen JE, Clausen C, Hyttel P, Holst B. Induced pluripotent stem cells (iPSCs) derived from a patient with frontotemporal dementia caused by a R406 W mutation in microtubule-associated protein tau (MAPT). Stem Cell Res. 2016;16(1):75–78. [DOI] [PubMed] [Google Scholar]
- 100. Rasmussen MA, Hjermind LE, Hasholt LF, Waldemar G, Nielsen JE, Clausen C, Hyttel P, Holst B. Induced pluripotent stem cells (iPSCs) derived from a patient with frontotemporal dementia caused by a P301 L mutation in microtubule-associated protein tau (MAPT). Stem Cell Res. 2016;16(1):70–74. [DOI] [PubMed] [Google Scholar]
- 101. Rasmussen MA, Hjermind LE, Hasholt LF, Waldemar G, Nielsen JE, Clausen C, Hyttel P, Holst B. Induced pluripotent stem cells (iPSCs) derived from a pre-symptomatic carrier of a R406 W mutation in microtubule-associated protein tau (MAPT) causing frontotemporal dementia. Stem Cell Res. 2016;16(1):105–109. [DOI] [PubMed] [Google Scholar]
- 102. Wang X, Chen S, Burtscher I, Sterr M, Hieronimus A, Machicao F, Staiger H, Häring HU, Lederer G, Meitinger T, Lickert H. Generation of a human induced pluripotent stem cell (iPSC) line from a patient carrying a P33 T mutation in the PDX1 gene. Stem Cell Res. 2016;17(2):273–276. [DOI] [PubMed] [Google Scholar]
- 103. Claassen JN, Zhang D, Chen SC, Moon SY, Lamey T, Thompson JA, McLaren T, De Roach JN, McLenachan S, Chen FK. Generation of the induced pluripotent stem cell line from a patient with autosomal recessive ABCA4-mediated Stargardt Macular Dystrophy. Stem Cell Res. 2019;34:101352. [DOI] [PubMed] [Google Scholar]
- 104. Hinz L, Hoekstra SD, Watanabe K, Posthuma D, Heine VM. Generation of isogenic controls for in vitro disease modelling of X-chromosomal disorders. Stem Cell Rev. 2019;15(2):276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gowran A, Spaltro G, Casalnuovo F, Vigorelli V, Spinelli P, Castiglioni E, Rovina D, Paganini S, Di Segni M, Gervasini C, Nigro P, et al. Generation of induced pluripotent stem cells from a Becker muscular dystrophy patient carrying a deletion of exons 45-55 of the dystrophin gene (CCMi002BMD-A-9 ▵45-55). Stem Cell Res. 2018;28:21–24. [DOI] [PubMed] [Google Scholar]
- 106. Hayer SN, Schelling Y, Huebener-Schmid J, Weber JJ, Hauser S, Schöls L. Generation of an induced pluripotent stem cell line from a patient with spinocerebellar ataxia type 3 (SCA3): HIHCNi002-A. Stem Cell Res. 2018;30:171–174. [DOI] [PubMed] [Google Scholar]
- 107. Hayer SN, Schelling Y, Hoeflinger P, Hauser S, Schöls L. Generation of an induced pluripotent stem cell line from a patient with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP): HIHCNi003-A. Stem Cell Res. 2018;30:206–209. [DOI] [PubMed] [Google Scholar]
- 108. Howden SE, Thomson JA, Little MH. Simultaneous reprogramming and gene editing of human fibroblasts. Nat Protoc. 2018;13(5):875–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kahounová Z, Slabáková E, Binó L, Remšík J, Fedr R, Bouchal J, Kurfűrstová D, Vrtěl R, Študent V, Jurečková L, Porokh V, et al. Generation of human iPSCs from human prostate cancer-associated fibroblasts IBPi002-A. Stem Cell Res. 2018;33:255–259. [DOI] [PubMed] [Google Scholar]
- 110. Ritthaphai A, Wattanapanitch M, Pithukpakorn M, Heepchantree W, Soi-Ampornkul R, Mahaisavariya P, Triwongwaranat D, Pattanapanyasat K, Vatanashevanopakorn C. Derivation of an induced pluripotent stem cell line (MUSIi004-A) from dermal fibroblasts of a 48-year-old spinocerebellar ataxia type 3 patient. Stem Cell Res. 2018;30:113–116. [DOI] [PubMed] [Google Scholar]
- 111. Tanaka Y, Higurashi N, Shirasu N, Yasunaga S, Moreira KM, Okano H, Hirose S. Establishment of a human induced stem cell line (FUi002-A) from Dravet syndrome patient carrying heterozygous R1525X mutation in SCN1A gene. Stem Cell Res. 2018;31:11–15. [DOI] [PubMed] [Google Scholar]
- 112. Vallejo S, Fleischer A, Martín JM, Sánchez A, Palomino E, Bachiller D. Generation of two induced pluripotent stem cells lines from Mucopolysaccharydosis IIIA patient: IMEDEAi004-A and IMEDEAi004-B. Stem Cell Res. 2018;32:110–114. [DOI] [PubMed] [Google Scholar]
- 113. Vallejo-Diez S, Fleischer A, Martín-Fernández JM, Sánchez-Gilabert A, Bachiller D. Generation of two induced pluripotent stem cells lines from a Mucopolysaccharydosis IIIB (MPSIIIB) patient. Stem Cell Res. 2018;33:180–184. [DOI] [PubMed] [Google Scholar]
- 114. Zhang X, Zhang D, Chen SC, Lamey T, Thompson JA, McLaren T, De Roach JN, Chen FK, McLenachan S. Generation of an induced pluripotent stem cell line from a patient with non-syndromic CLN3-associated retinal degeneration and a coisogenic control line. Stem Cell Res. 2018;29:245–249. [DOI] [PubMed] [Google Scholar]
- 115. Kuebler B, Aran B, Miquel-Serra L, Muñoz Y, Ars E, Bullich G, Furlano M, Torra R, Marti M, Veiga A, Raya A. Integration-free induced pluripotent stem cells derived from a patient with autosomal recessive Alport syndrome (ARAS). Stem Cell Res. 2017;25:1–5. [DOI] [PubMed] [Google Scholar]
- 116. Kuebler B, Aran B, Miquel-Serra L, Muñoz Y, Ars E, Bullich G, Furlano M, Torra R, Marti M, Veiga A, Raya A. Generation of integration-free induced pluripotent stem cell lines derived from two patients with X-linked Alport syndrome (XLAS). Stem Cell Res. 2017;25:291–295. [DOI] [PubMed] [Google Scholar]
- 117. Spaltro G, Vigorelli V, Casalnuovo F, Spinelli P, Castiglioni E, Rovina D, Paganini S, Di Segni M, Nigro P, Gervasini C, Pompilio G, et al. Derivation of the Duchenne muscular dystrophy patient-derived induced pluripotent stem cell line lacking DMD exons 49 and 50 (CCMi001DMD-A-3, 49, 50). Stem Cell Res. 2017;25:128–131. [DOI] [PubMed] [Google Scholar]
- 118. Hung SS, Van Bergen NJ, Jackson S, Liang H, Mackey DA, Hernández D, Lim SY, Hewitt AW, Trounce I, Pébay A, Wong RC. Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells. Aging (Albany NY). 2016;8(5):945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kawala MA, Bohndorf M, Graffmann N, Wruck W, Zatloukal K, Adjaye J. Characterization of dermal fibroblast-derived iPSCs from a patient with low grade steatosis. Stem Cell Res. 2016;17(3):547–549. [DOI] [PubMed] [Google Scholar]
- 120. Kim BY, Jeong S, Lee SY, Lee SM, Gweon EJ, Ahn H, Kim J, Chung SK. Concurrent progress of reprogramming and gene correction to overcome therapeutic limitation of mutant ALK2-iPSC. Exp Mol Med. 2016;48(6):e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Holst B, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying a M146I mutation in PSEN1. Stem Cell Res. 2016;16(2):334–337. [DOI] [PubMed] [Google Scholar]
- 122. Li T, Pires C, Nielsen TT, Waldemar G, Hjermind LE, Nielsen JE, Dinnyes A, Hyttel P, Freude KK. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying an A79 V mutation in PSEN1. Stem Cell Res. 2016;16(2):229–232. [DOI] [PubMed] [Google Scholar]
- 123. Poon A, Li T, Pires C, Nielsen TT, Nielsen JE, Holst B, Dinnyes A, Hyttel P, Freude KK. Derivation of induced pluripotent stem cells from a familial Alzheimer’s disease patient carrying the L282F mutation in presenilin 1. Stem Cell Res.2016;17(3):470–473. [DOI] [PubMed] [Google Scholar]
- 124. Tubsuwan A, Pires C, Rasmussen MA, Schmid B, Nielsen JE, Hjermind LE, Hall V, Nielsen TT, Waldemar G, Hyttel P, Clausen C, et al. Generation of induced pluripotent stem cells (iPSCs) from an Alzheimer’s disease patient carrying a L150P mutation in PSEN-1. Stem Cell Res. 2016;16(1):110–112. [DOI] [PubMed] [Google Scholar]
- 125. Fidan K, Kavaklıoğlu G, Ebrahimi A, Özlü C, Ay NZ, Ruacan A, Gül A, Önder TT. Generation of integration-free induced pluripotent stem cells from a patient with Familial Mediterranean Fever (FMF). Stem Cell Res. 2015;15(3):694–696. [DOI] [PubMed] [Google Scholar]
- 126. Luo Y, Zhu D, Du R, Gong Y, Xie C, Xu X, Fan Y, Yu B, Sun X, Chen Y. Uniparental disomy of the entire X chromosome in Turner syndrome patient-specific induced pluripotent stem cells. Cell Discov. 2015;1:15022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Howden SE, Maufort JP, Duffin BM, Elefanty AG, Stanley EG, Thomson JA. Simultaneous reprogramming and gene correction of patient fibroblasts. Stem Cell Reports. 2015;5(6):1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. McLenachan S, Zhang D, Zhang X, Chen SC, Lamey T, Thompson JA, McLaren T, De Roach JN, Fletcher S, Chen FK. Generation of two induced pluripotent stem cell lines from a patient with dominant PRPF31 mutation and a related non-penetrant carrier. Stem Cell Res. 2019;101357. [DOI] [PubMed] [Google Scholar]
- 129. Bell S, Peng H, Crapper L, Kolobova I, Maussion G, Vasuta C, Yerko V, Wong TP, Ernst C. A rapid pipeline to model rare neurodevelopmental disorders with simultaneous CRISPR/Cas9 gene editing. Stem Cells Transl Med. 2017;6(3):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Briggs JA, Sun J, Shepherd J, Ovchinnikov DA, Chung TL, Nayler SP, Kao LP, Morrow CA, Thakar NY, Soo SY, Peura T, et al. Integration-free induced pluripotent stem cells model genetic and neural developmental features of down syndrome etiology. Stem Cells. 2013;31(3):467–478. [DOI] [PubMed] [Google Scholar]
- 131. Mahairaki V, Ryu J, Peters A, Chang Q, Li T, Park TS, Burridge PW, Talbot CC, Jr, Asnaghi L, Martin LJ, Zambidis ET, et al. Induced pluripotent stem cells from familial Alzheimer’s disease patients differentiate into mature neurons with amyloidogenic properties. Stem Cells Dev. 2014;23(24):2996–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ramakrishnan VM, Yang JY, Tien KT, McKinley TR, Bocard BR, Maijub JG, Burchell PO, Williams SK, Morris ME, Hoying JB, Wade-Martins R, et al. Restoration of physiologically responsive low-density lipoprotein receptor-mediated endocytosis in genetically deficient induced pluripotent stem cells. Sci Rep. 2015;5:13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Piao Y, Hung SS, Lim SY, Wong RC, Ko MS. Efficient generation of integration-free human induced pluripotent stem cells from keratinocytes by simple transfection of episomal vectors. Stem Cells Transl Med. 2014;3(7):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dowey SN, Huang X, Chou BK, Ye Z, Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat Protoc. 2012;7(11):2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kamath A, Ternes S, McGowan S, Moy AB. Virus-free and oncogene-free induced pluripotent stem cell reprogramming in cord blood and peripheral blood in patients with lung disease. Regen Med. 2018;13(8):889–915. [DOI] [PubMed] [Google Scholar]
- 136. Wen W, Cheng X, Fu Y, Meng F, Zhang JP, Zhang L, Li XL, Yang Z, Xu J, Zhang F, Botimer GD, et al. High-level precise knockin of iPSCs by simultaneous reprogramming and genome editing of human peripheral blood mononuclear cells. Stem Cell Reports. 2018;10(6):1821–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wen W, Zhang JP, Xu J, Su RJ, Neises A, Ji GZ, Yuan W, Cheng T, Zhang XB. Enhanced generation of integration-free iPSCs from human adult peripheral blood mononuclear cells with an optimal combination of episomal vectors. Stem Cell Reports. 2016;6(6):873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wang Y, Zhang Y, Zhang J, Lu J, Yang C, Zhao J, Li G, Liu Z, Lei Y. Generation of a human control PBMC derived iPS cell line TUSMi001-A from a healthy male donor of Han Chinese genetic background. Stem Cell Res. 2017;25:22–25. [DOI] [PubMed] [Google Scholar]
- 139. Wang Y, Zhang J, Zhang Y, Huang D, Zhao J, Li G, Lei Y. Generation of a human induced pluripotent stem cell line from a 65-year old healthy female donor with Chinese Han genetic background. Stem Cell Res. 2017;24:33–35. [DOI] [PubMed] [Google Scholar]
- 140. Su RJ, Neises A, Zhang XB. Generation of iPS cells from human peripheral blood mononuclear cells using episomal vectors. Methods Mol Biol. 2016;1357:57–69. [DOI] [PubMed] [Google Scholar]
- 141. Tangprasittipap A, Jittorntrum B, Wongkummool W, Kitiyanant N, Tubsuwan A. Generation of induced pluripotent stem cells from peripheral blood CD34+ hematopoietic progenitors of a 31 year old healthy woman. Stem Cell Res. 2017;20:91–93. [DOI] [PubMed] [Google Scholar]
- 142. Mack AA, Kroboth S, Rajesh D, Wang WB. Generation of induced pluripotent stem cells from CD34+ cells across blood drawn from multiple donors with non-integrating episomal vectors. PLoS One. 2011;6(11):e27956 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chou BK, Gu H, Gao Y, Dowey SN, Wang Y, Shi J, Li Y, Ye Z, Cheng T, Cheng L. A facile method to establish human induced pluripotent stem cells from adult blood cells under feeder-free and xeno-free culture conditions: a clinically compliant approach. Stem Cells Transl Med. 2015;4(4):320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Weng Z, Kong CW, Ren L, Karakikes I, Geng L, He J, Chow MZ, Mok CF, Chan W, Keung W, et al. A simple, cost-effective but highly efficient system for deriving ventricular cardiomyocytes from human pluripotent stem cells. Stem Cells Dev. 2014;23(14):1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu J, Brzeszczynska J, Samuel K, Black J, Palakkan A, Anderson RA, Gallagher R, Ross JA. Efficient episomal reprogramming of blood mononuclear cells and differentiation to hepatocytes with functional drug metabolism. Exp Cell Res. 2015;338(2):203–213. [DOI] [PubMed] [Google Scholar]
- 146. TheinHan W, Liu J, Tang M, Chen W, Cheng L, Xu HH. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res. 2013;4:371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang Y, Zhang J, Hu J, Li G, Lei Y, Zhao J. Establishment of TUSMi008-A, an induced pluripotent stem cell (iPSC) line from a 76-year old Alzheimer’s disease (AD) patient with PAXIP1 gene mutation. Stem Cell Res. 2019;36:101391. [DOI] [PubMed] [Google Scholar]
- 148. Wang Y, Zhang J, Lei Y, Zhao J. Establishment of TUSMi007-A, an induced pluripotent stem cell (iPSC) line from an 83-year old Chinese Han patient with Alzheimer’s disease (AD). Stem Cell Res. 2018;33:265–268. [DOI] [PubMed] [Google Scholar]
- 149. Wang Y, Yu H, Zhang Y, Zhang J, Chen K, Sun H, Meng S, Li G, Lei Y, Zhao J. Establishment of TUSMi003-A, an induced pluripotent stem cell (iPSC) line from a 62-year old Chinese Han patient with Alzheimer’s disease with ApoE3/4 genetic background. Stem Cell Res. 2018;27:57–60. [DOI] [PubMed] [Google Scholar]
- 150. Wang Y, Yu H, Chen Y, Li G, Lei Y, Zhao J. Derivation of induced pluripotent stem cells TUSMi006 from an 87-year old Chinese Han Alzheimer’s disease patient carrying GRINB and SORL1 mutations. Stem Cell Res. 2018;31:127–130. [DOI] [PubMed] [Google Scholar]
- 151. Wang Y, Fen Q, Yu H, Qiu H, Ma X, Lei Y, Zhao J. Establishment of TUSMi005-A, an induced pluripotent stem cell (iPSC) line from a 32-year old Chinese Han patient with Bipolar Disorder (BD). Stem Cell Res. 2018;33:65–68. [DOI] [PubMed] [Google Scholar]
- 152. Wang Y, Kang C, Yu H, Fen J, Ma X, Lei Y, Zhao J. Establishment of TUSMi004-A, an induced pluripotent stem cell (iPSC) line from a 32-year old Chinese Han patient with Obsessive-Compulsive Disorder (OCD). Stem Cell Res. 2018;32:83–86. [DOI] [PubMed] [Google Scholar]
- 153. Zhao Z, Ji S, Shi Z, Liu H. Generation of CSi001-A, a transgene-free, induced pluripotent stem cell line derived from a Parkinson Disease (PD) patient. Stem Cell Res. 2018;33:1–5. [DOI] [PubMed] [Google Scholar]
- 154. Murakami N, Ishikawa T, Kondo T, Imamura K, Tsukita K, Enami T, Funayama M, Shibukawa R, Matsumoto S, Izumi Y, Ohta E, et al. Establishment of DYT5 patient-specific induced pluripotent stem cells with a GCH1 mutation. Stem Cell Res. 2017;24:36–39. [DOI] [PubMed] [Google Scholar]
- 155. Ross SB, Fraser ST, Nowak N, Semsarian C. Generation of induced pluripotent stem cells (iPSCs) from a hypertrophic cardiomyopathy patient with the pathogenic variant p.Val698Ala in beta-myosin heavy chain (MYH7) gene. Stem Cell Res. 2017;20:88–90. [DOI] [PubMed] [Google Scholar]
- 156. Junqueira Reis LC, Picanço-Castro V, Paes BCMF, Pereira OA, Gerdes Gyuricza I, de Araújo FT, Morato-Marques M, Moreira LF, Costa EBO, Dos Santos TPM, Covas DT, et al. Induced pluripotent stem cell for the study and treatment of sickle cell anemia. Stem Cells Int. 2017;2017:7492914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Malecki M, Putzer E, Sabo C, Foorohar A, Quach C, Stampe C, Beauchaine M, Malecki R, Tombokan X, Anderson M. Directed cardiomyogenesis of autologous human induced pluripotent stem cells recruited to infarcted myocardium with bioengineered antibodies. Mol Cell Ther. 2014;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A. 2014;20(7-8):1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hsu J, Reilly A, Hayes BJ, Clough CA, Konnick EQ, Torok-Storb B, Gulsuner S, Wu D, Becker PS, Keel SB, Abkowitz JL, et al. Reprogramming identifies functionally distinct stages of clonal evolution in myelodysplastic syndromes. Blood. 2019;134(2):186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hu K, Slukvin I. Generation of transgene-free iPSC lines from human normal and neoplastic blood cells using episomal vectors. Methods Mol Biol. 2013;997:163–176. [DOI] [PubMed] [Google Scholar]
- 161. Varga E, Hansen M, Wüst T, von Lindern M, van den Akker E. Generation of human erythroblast-derived iPSC line using episomal reprogramming system. Stem Cell Res. 2017;25:30–33. [DOI] [PubMed] [Google Scholar]
- 162. Bhatt N, Ghosh R, Roy S, Gao Y, Armanios M, Cheng L, Franco S. Robust reprogramming of Ataxia-Telangiectasia patient and carrier erythroid cells to induced pluripotent stem cells. Stem Cell Res. 2016;17(2):296–305. [DOI] [PubMed] [Google Scholar]
- 163. Bhatt N, Ghosh R, Roy S, Gao Y, Armanios M, Cheng L, Franco S. Integration-free erythroblast-derived human induced pluripotent stem cells (iPSCs) from an individual with Ataxia-Telangiectasia (A-T). Stem Cell Res. 2016;17(2):205–207. [DOI] [PubMed] [Google Scholar]
- 164. Su RJ, Baylink DJ, Neises A, Kiroyan JB, Meng X, Payne KJ, Tschudy-Seney B, Duan Y, Appleby N, Kearns-Jonker M, Gridley DS, et al. Efficient generation of integration-free ips cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS One. 2013;8(5):e64496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Fernandes S, Tembe S, Singh S, Vardhan S, Nair V, Kale V, Limaye L. Development and characterization of human iPSC line NCCSi004-A from umbilical cord blood (UCB) derived CD34(+)cells obtained from donor belonging to Indian ethnic population. Stem Cell Res. 2019;35:101392. [DOI] [PubMed] [Google Scholar]
- 166. Fernandes S, Shinde P, Khan N, Singh S, Vardhan S, Nair V, Kale V, Limaye L. Derivation of human iPSC line NCCSi002-A from umbilical cord blood (UCB) CD34+cells of donor from Indian ethnicity. Stem Cell Res. 2018;26:80–83. [DOI] [PubMed] [Google Scholar]
- 167. Fernandes S, Talwadekar M, Agarwal R, Nair V, Kale V, Limaye L. Generation and characterization of human iPSC line from CD34(+) cells isolated from umbilical cord blood belonging to Indian origin. Stem Cell Res. 2017;18:60–63. [DOI] [PubMed] [Google Scholar]
- 168. Slamecka J, Salimova L, McClellan S, van Kelle M, Kehl D, Laurini J, Cinelli P, Owen L, Hoerstrup SP, Weber B. Non-integrating episomal plasmid-based reprogramming of human amniotic fluid stem cells into induced pluripotent stem cells in chemically defined conditions. Cell Cycle. 2016;15(2):234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. He W, Kang X, Du H, Song B, Lu Z, Huang Y, Wang D, Sun X, Yu Y, Fan Y. Defining differentially methylated regions specific for the acquisition of pluripotency and maintenance in human pluripotent stem cells via microarray. PLoS One. 2014;9(9):e108350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wilson PG, Payne T. Genetic reprogramming of human amniotic cells with episomal vectors: neural rosettes as sentinels in candidate selection for validation assays. PeerJ. 2014;2:e668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Xing K, Cui Y, Luan J, Zhou X, Shi L, Han J. Establishment of a human trisomy 18 induced pluripotent stem cell line from amniotic fluid cells. Intractable Rare Dis Res. 2018;7(2):94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Slamecka J, McClellan S, Wilk A, Laurini J, Manci E, Hoerstrup SP, Weber B, Owen L. Induced pluripotent stem cells derived from human amnion in chemically defined conditions. Cell Cycle. 2018;17(3):330–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Göbel C, Goetzke R, Eggermann T, Wagner W. Interrupted reprogramming into induced pluripotent stem cells does not rejuvenate human mesenchymal stromal cells. Sci Rep. 2018;8(1):11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Foja S, Jung M, Harwardt B, Riemann D, Pelz-Ackermann O, Schroeder IS. Hypoxia supports reprogramming of mesenchymal stromal cells via induction of embryonic stem cell-specific microRNA-302 cluster and pluripotency-associated genes. Cell Reprogram. 2013;15(1):68–79. [DOI] [PubMed] [Google Scholar]
- 175. Qu X, Liu T, Song K, Li X, Ge D. Induced pluripotent stem cells generated from human adipose-derived stem cells using a non-viral polycistronic plasmid in feeder-free conditions. PLoS One. 2012;7(10):e48161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Thekkeparambil Chandrabose S, Sriram S, Subramanian S, Cheng S, Ong WK, Rozen S, Kasim NHA, Sugii S. Amenable epigenetic traits of dental pulp stem cells underlie high capability of xeno-free episomal reprogramming. Stem Cell Res Ther. 2018;9(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Saitoh I, Inada E, Iwase Y, Noguchi H, Murakami T, Soda M, Kubota N, Hasegawa H, Akasaka E, Matsumoto Y, Oka K, et al. Choice of feeders is important when first establishing iPSCs derived from primarily cultured human deciduous tooth dental pulp cells. Cell Med. 2015;8(1-2):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yan X, Xu N, Meng C, Wang B, Yuan J, Wang C, Li Y. Generation of induced pluripotent stem cells from human mesenchymal stem cells of parotid gland origin. Am J Transl Res. 2016;8(2):419–432. [PMC free article] [PubMed] [Google Scholar]
- 179. Schröter F, Sleegers K, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPS cell line from a 69-year-old male. Stem Cell Res. 2016;16(1):29–31. [DOI] [PubMed] [Google Scholar]
- 180. Barrett R, Ornelas L, Yeager N, Mandefro B, Sahabian A, Lenaeus L, Targan SR, Svendsen CN, Sareen D. Reliable generation of induced pluripotent stem cells from human lymphoblastoid cell lines. Stem Cells Transl Med. 2014;3(12):1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Schröter F, Sleegers K, Cuyvers E, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPS cell line from a female 67-year-old Alzheimer’s disease patient with TREM2 (R47 H) missense mutation. Stem Cell Res. 2016;17(3):553–555. [DOI] [PubMed] [Google Scholar]
- 182. Zulfiqar S, Fritz B, Nieweg K. Episomal plasmid-based generation of an iPSC line from a 79-year-old individual carrying the APOE4/4 genotype: i11001. Stem Cell Res. 2016;17(3):544–546. [DOI] [PubMed] [Google Scholar]
- 183. Zulfiqar S, Fritz B, Nieweg K. Episomal plasmid-based generation of an iPSC line from an 83-year-old individual carrying the APOE4/4 genotype: i10984. Stem Cell Res. 2016;17(3):523–525. [DOI] [PubMed] [Google Scholar]
- 184. Schröter F, Sleegers K, Van Cauwenberghe C, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPSC lines from a female and male Alzheimer’s disease patient expressing different copy numbers of a coding CNV in the Alzheimer risk gene CR1. Stem Cell Res. 2016;17(3):560–563. [DOI] [PubMed] [Google Scholar]
- 185. Schröter F, Sleegers K, Cuyvers E, Bohndorf M, Wruck W, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free iPS cell line from a 65-year-old Alzheimer’s disease patient expressing the TREM2 p.R47 H variant. Stem Cell Res. 2016;16(1):113–115. [DOI] [PubMed] [Google Scholar]
- 186. Martins S, Bohndorf M, Schröter F, Assar F, Wruck W, Sleegers K, Van Broeckhoven C, Adjaye J. Lymphoblast-derived integration-free ISRM-CON9 iPS cell line from a 75year old female. Stem Cell Res. 2018;26:76–79. [DOI] [PubMed] [Google Scholar]
- 187. Fujimori K, Tezuka T, Ishiura H, Mitsui J, Doi K, Yoshimura J, Tada H, Matsumoto T, Isoda M, Hashimoto R, Hattori N, et al. Modeling neurological diseases with induced pluripotent cells reprogrammed from immortalized lymphoblastoid cell lines. Mol Brain. 2016;9(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Howard-Jones RA, Cheung OK, Glen A, Allen ND, Stephens P. Integration-free reprogramming of lamina propria progenitor cells. J Dent Res. 2016;95(8):882–888. [DOI] [PubMed] [Google Scholar]
- 189. Alvisi G, Trevisan M, Masi G, Canel V, Caenazzo L, Nespeca P, Barzon L, Di Iorio E, Barbaro V, Palù G. Generation of a transgene-free human induced pluripotent stem cell line (UNIPDi001-A) from oral mucosa epithelial stem cells. Stem Cell Res. 2018;28:177–180. [DOI] [PubMed] [Google Scholar]
- 190. Trevisan M, Di Iorio E, Masi G, Riccetti S, Barzon L, Alvisi G, Caenazzo L, Barbaro V, Palù G. Induced pluripotent stem cells line (UNIPDi003-A) from a patient affected by EEC syndrome carrying the R279 H mutation in TP63 gene. Stem Cell Res. 2018;28:141–144. [DOI] [PubMed] [Google Scholar]
- 191. Wang L, Chen Y, Guan C, Zhao Z, Li Q, Yang J, Mo J, Wang B, Wu W, Yang X, Song L, et al. Using low-risk factors to generate non-integrated human induced pluripotent stem cells from urine-derived cells. Stem Cell Res Ther. 2017;8(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Si-Tayeb K, Idriss S, Champon B, Caillaud A, Pichelin M, Arnaud L, Lemarchand P, Le May C, Zibara K, Cariou B. Urine-sample-derived human induced pluripotent stem cells as a model to study PCSK9-mediated autosomal dominant hypercholesterolemia. Dis Model Mech. 2016;9(1):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Guo D, Wu F, Liu H, Gao G, Kou S, Yang F, Abbas N, Zhou T, Cai X, Zhang H, Qin D, et al. Generation of non-integrated induced pluripotent stem cells from a 59-year-old female with multiple endocrine neoplasia type 1 syndrome. Stem Cell Res. 2017;18:64–66. [DOI] [PubMed] [Google Scholar]
- 194. Jouni M, Si-Tayeb K, Es-Salah-Lamoureux Z, Latypova X, Champon B, Caillaud A, Rungoat A, Charpentier F, Loussouarn G, Baró I, Zibara K, et al. Toward personalized medicine: using cardiomyocytes differentiated from urine-derived pluripotent stem cells to recapitulate electrophysiological characteristics of type 2 long QT syndrome. J Am Heart Assoc. 2015;4(9):e002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Steichen C, Si-Tayeb K, Wulkan F, Crestani T, Rosas G, Dariolli R, Pereira AC, Krieger JE. Human Induced Pluripotent Stem (hiPS) cells from urine samples: a non-integrative and feeder-free reprogramming strategy. Curr Protoc Hum Genet. 2017;92:21.7.1–21.7.22. [DOI] [PubMed] [Google Scholar]
- 196. Ju Z, Ma J, Wang C, Yu J, Qiao Y, Hei F. Exosomes from iPSCs delivering siRNA attenuate intracellular adhesion molecule-1 expression and neutrophils adhesion in pulmonary microvascular endothelial cells. Inflammation. 2017;40(2):486–496. [DOI] [PubMed] [Google Scholar]
- 197. Qi Z, Cui Y, Shi L, Luan J, Zhou X, Han J. Generation of urine-derived induced pluripotent stem cells from a patient with phenylketonuria. Intractable Rare Dis Res. 2018;7(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhou M, Hu Z, Qiu L, Zhou T, Feng M, Hu Q, Zeng B, Li Z, Sun Q, Wu Y, Liu X, et al. Seamless genetic conversion of SMN2 to SMN1 via CRISPR/Cpf1 and single-stranded oligodeoxynucleotides in spinal muscular atrophy patient-specific induced pluripotent stem cells. Hum Gene Ther. 2018;29(11):1252–1263. [DOI] [PubMed] [Google Scholar]
- 199. Bohndorf M, Ncube A, Spitzhorn LS, Enczmann J, Wruck W, Adjaye J. Derivation and characterization of integration-free iPSC line ISRM-UM51 derived from SIX2-positive renal cells isolated from urine of an African male expressing the CYP2D6 *4/*17 variant which confers intermediate drug metabolizing activity. Stem Cell Res. 2017;25:18–21. [DOI] [PubMed] [Google Scholar]
- 200. Paulitschek C, Schulze-Matz P, Hesse J, Schmidt T, Wruck W, Adjaye J, Schrader J. Generation and characterization of two iPSC lines from human epicardium-derived cells. Stem Cell Res. 2017;20:50–53. [DOI] [PubMed] [Google Scholar]
- 201. Diecke S, Lu J, Lee J, Termglinchan V, Kooreman NG, Burridge PW, Ebert AD, Churko JM, Sharma A, Kay MA, Wu JC. Novel codon-optimized mini-intronic plasmid for efficient, inexpensive, and xeno-free induction of pluripotency. Sci Rep. 2015;5:8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ikeda K, Mizoro Y, Ameku T, Nomiya Y, Mae SI, Matsui S, Kuchitsu Y, Suzuki C, Hamaoka-Okamoto A, Yahata T, Sone M, et al. Transcriptional analysis of intravenous immunoglobulin resistance in Kawasaki disease using an induced pluripotent stem cell disease model. Circ J. 2016;81(1):110–118. [DOI] [PubMed] [Google Scholar]
- 203. Cheng L, Hansen NF, Zhao L, Du Y, Zou C, Donovan FX, Chou BK, Zhou G, Li S, Dowey SN, Ye Z, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell. 2012;10(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Park TS, Huo JS, Peters A, Talbot CC, Jr, Verma K, Zimmerlin L, Kaplan IM, Zambidis ET. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS One. 2012;7(8):e42838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Park TS, Bhutto I, Zimmerlin L, Huo JS, Nagaria P, Miller D, Rufaihah AJ, Talbot C, Aguilar J, Grebe R, Merges C, et al. Vascular progenitors from cord blood-derived induced pluripotent stem cells possess augmented capacity for regenerating ischemic retinal vasculature. Circulation. 2014;129(3):359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Song L, Awari DW, Han EY, Uche-Anya E, Park SH, Yabe YA, Chung WK, Yazawa M. Dual optical recordings for action potentials and calcium handling in induced pluripotent stem cell models of cardiac arrhythmias using genetically encoded fluorescent indicators. Stem Cells Transl Med. 2015;4(5):468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhao H, Davies TJ, Ning J, Chang Y, Sachamitr P, Sattler S, Fairchild PJ, Huang FP. A highly optimized protocol for reprogramming cancer cells to pluripotency using nonviral plasmid vectors. Cell Reprogram. 2015;17(1):7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Park HY, Noh EH, Chung HM, Kang MJ, Kim EY, Park SP. Efficient generation of virus-free iPS cells using liposomal magnetofection. PLoS One. 2012;7(9):e45812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Cao F, Xie X, Gollan T, Zhao L, Narsinh K, Lee RJ, Wu JC. Comparison of gene-transfer efficiency in human embryonic stem cells. Mol Imaging Biol. 2010;12(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Sueishi M. Dual gene expression in embryoid bodies derived from human induced pluripotent stem cells using episomal vectors. Tissue Eng Part A. 2014;20(23-24):3154–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016;538(7625):388–391. [DOI] [PubMed] [Google Scholar]
- 212. Loh CY, Wang AY, Kao HK, Cardona E, Chuang SH, Wei FC. Episomal induced pluripotent stem cells promote functional recovery of transected murine peripheral nerve. PLoS One. 2016;11(10):e0164696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu SP, Fu RH, Wu DC, Hsu CY, Chang CH, Lee W, Lee YD, Liu CH, Chien YJ, Lin SZ, Shyu WC. Mouse-induced pluripotent stem cells generated under hypoxic conditions in the absence of viral infection and oncogenic factors and used for ischemic stroke therapy. Stem Cells Dev. 2014;23(4):421–433. [DOI] [PubMed] [Google Scholar]
- 214. Wang Z, Wen Y, Li YH, Wei Y, Green M, Wani P, Zhang P, Pera RR, Chen B. Smooth muscle precursor cells derived from human pluripotent stem cells for treatment of stress urinary incontinence. Stem Cells Dev. 2016;25(6):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014;2(2):205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Cell Engineering Division / RIKEN BioResource Research Center. 2016. [accessed May 23]. https://www.newscientist.com/article/dn27986/.
- 217. Trounson A, DeWitt ND. Pluripotent stem cells progressing to the clinic. Nat Rev Mol Cell Biol. 2016;17(3):194–200. [DOI] [PubMed] [Google Scholar]
- 218. Garber K. Inducing translation. Nat Biotechnol. 2013;31(6):483–486. [DOI] [PubMed] [Google Scholar]
- 219. Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Reports. 2016;6(6):787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Health / New Scientist. 2015. [July 31]. http://cell.brc.riken.jp/en/hps/search_hps_en.
- 221. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 222. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. [DOI] [PubMed] [Google Scholar]
- 223. Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, Rong Z, Kim J, He J, Wang M, Clegg DO, et al. Humanized mice reveal differential immunogenicity of cells derived from autologous induced pluripotent stem cells. Cell Stem Cell. 2015;17(3):353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Xiao B, Ng HH, Takahashi R, Tan EK. Induced pluripotent stem cells in Parkinson’s disease: scientific and clinical challenges. J Neurol Neurosurg Psychiatry. 2016;87(7):697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]