Abstract
The ubiquitin-proteasome pathway and autophagy-lysosome pathway are two major routes for clearance of aberrant cellular components to maintain protein homeostasis and normal cellular functions. Accumulating evidence shows that these two pathways are impaired during cerebral ischemia, which contributes to ischemic-induced neuronal necrosis and apoptosis. This review aims to critically discuss current knowledge and controversies on these two pathways in response to cerebral ischemic stress. We also discuss molecular mechanisms underlying the impairments of these protein degradation pathways and how such impairments lead to neuronal damage after cerebral ischemia. Further, we review the recent advance on the understanding of the involvement of these two pathways in the pathological process during many therapeutic approaches against cerebral ischemia. Despite recent advances, the exact role and molecular mechanisms of these two pathways following cerebral ischemia are complex and not completely understood, of which better understanding will provide avenues to develop novel therapeutic strategies for ischemic stroke.
1. Introduction
Protein homeostasis, the correct balance between production and degradation of proteins, is essential for cell function, development, and survival. Protein homeostasis also plays a critical role in cerebral ischemia. Protein homeostasis dysfunction has been implicated in the development and progression of neurological impairment in cerebral ischemia. Pharmacologic activation of the ATF6 arm of the unfolded protein response (UPR) reprograms cellular protein homeostasis and confers global neuroprotection in cerebral ischemia-reperfusion injury [1]. L-2-Oxothiazolidine-4-carboxylic acid (OTC), a cysteine precursor, improves proteostasis and protects against ischemic stroke injury [2].
The ubiquitin-proteasome pathway and autophagy-lysosome pathway are two major protein degradation systems in all eukaryotic cells to degrade a broad array of misfolded proteins. The ubiquitin-proteasome pathway is extremely efficient for the degradation of short-lived proteins and misfolded soluble proteins, while the autophagy-lysosome pathway is responsible for eliminating long-lived proteins, insoluble proteins, and certain whole organelles. The two highly regulated pathways do not only maintain protein homeostasis, but also mediate necrosis and apoptosis. In this review, we will focus on recent discoveries of the ubiquitin-proteasome pathway and autophagy-lysosome pathway and the crosstalk between them in the pathogenesis and treatment of cerebral ischemia and attempt to shed new light on the design of effective therapy strategies against cerebral ischemia injury-associated diseases.
2. The Ubiquitin-Proteasome Pathway in Cerebral Ischemia
2.1. Change of the Ubiquitin-Proteasome Pathway in Cerebral Ischemia
2.1.1. Change of Ubiquitin in Cerebral Ischemia
The expression level of ubiquitin conjugates is greatly increased in plaques, especially unstable plaques, indicating that the ubiquitin-proteasome system is involved in the development of atherosclerotic plaques in intracranial arteries [3]. After hypoxia-ischemia, ubiquitinated proteins accumulate and proteasome biology in white matter of neonatal piglets is compromised [4]. The expression of ubiquitin increases in the peri-ischemic area after transient middle cerebral artery occlusion, with a peak at 72 hours and returning to the baseline levels until 7 days [5].
After transient cerebral ischemia, ubiquitin-conjugated proteins accumulate in Triton-insoluble aggregates. 763 peptides to 272 proteins, including proteins involved in important neuronal functions and signaling pathways, were highly enriched in these aggregates [6]. After middle cerebral artery occlusion, the formation of ubiquitin aggregates is driven by reperfusion rather than ischemia. Ubiquitin aggregates formed in potentially viable brain tissue may be later recruited into infarction by factors independent of ubiquitination [7].
2.1.2. Change of Immunoproteasome in Cerebral Ischemia
Immunoproteasome, a subtype of proteasome, contains three major catalytic subunits: β1i, β2i, and β5i. The expression of the immunoproteasomal subunits, 20S β1i and β5i, is increased remarkably in the parietal cortex and hippocampus after transient cerebral ischemia, which is associated with a great increase in ubiquitinated proteins. The immunoproteasome induction after cerebral ischemia is supposed to play a critical role in coping with the damaged proteins and thus may be of great importance in neuronal survival and death [8]. The plasma level of the immunoproteasome is supposed to be a useful indicator in the early prediction of hemorrhagic transformation after acute ischemic stroke [9].
Immunoproteasome inhibition reduces infarction volume and attenuates inflammatory reaction after cerebral ischemia in rats, suggesting that selective immunoproteasome inhibitors may be promising candidates for the treatment of ischemic stroke [10]. In a rat model of focal cerebral ischemia, immunoproteasome inhibition also promotes angiogenesis, which is through enhancing hypoxia-inducible factor-1α (HIF-1α) abundance [11]. PR-957, a specific inhibitor of the immunoproteasome subunit low molecular weight polypeptide 7 (LMP7), confers neuroprotection in a mouse model of ischemic stroke, which is via modulating cytokine production and inhibiting Th17 differentiation [12].
2.2. The Role of the Ubiquitin-Proteasome Pathway in Cerebral Ischemia
After transient cerebral ischemia, the depletion of ubiquitin and the accumulation of its mutant form (ubiquitin(+1)) may inhibit the ubiquitin-proteasome pathway, which results in delayed neuronal death of CA1 pyramidal neurons directly or indirectly [13]. Meanwhile, proteasome activity may play a role in contralateral cortical plasticity occurring after focal cerebral ischemia [14]. Proteasome subunit type 6 (PSMA6) gene polymorphisms are associated with susceptibility to ischemic stroke, while PSMA6 (-C8G) gene polymorphism may play a protective role with the susceptibility of ischemic stroke [15]. The expression of key players of the ubiquitin-proteasome pathway (MuRF1 (muscle RING finger-1), MAFbx (muscle atrophy F-box), Musa1 (muscle ubiquitin ligase of SCF complex in atrophy-1)) is increased in both paretic and nonparetic skeletal muscles of patients with ischemic stroke and is critically associated with skeletal muscle atrophy after cerebral ischemia [16].
2.3. The Ubiquitin-Proteasome Pathway and the Treatment of Cerebral Ischemia
2.3.1. The Ubiquitin-Proteasome Pathway Activation and the Treatment of Cerebral Ischemia
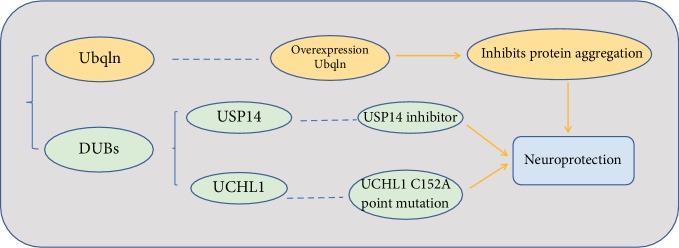
Protein aggregation has been proven to be a pathological basis responsible for ischemia-induced neuronal death. Therefore, enhanced removal of damaged proteins is supposed to be a promising therapeutic strategy for the treatment of ischemia-caused neuronal injury. Ubqln, a ubiquitin-like protein, mediates degradation of damaged proteins. Ubqln overexpression greatly inhibited the accumulation of protein aggregates and conferred neuroprotection against ischemia-induced cerebral injury [17]. Overexpression of Ubqln also protected mice from ischemic stroke and oxidative stress-caused neuronal injury, which was mediated by facilitating removal of misfolded proteins [18] (Figure 1).
Figure 1.
The ubiquitin-proteasome pathway activation strategy in the treatment of cerebral ischemia.
Deubiquitinating enzymes (DUBs) are a large group of proteases that negatively regulates proteasome activity. The ubiquitin-specific protease 14 (USP14), one of the DUBs, is associated with the proteasome and regulates protein degradation. The USP14 inhibitor ameliorates neuronal injury and confers neuroprotection after cerebral ischemia-reperfusion insult in mice [19]. Ubiquitin C-terminal hydrolase L1 (UCHL1) is a unique brain-specific deubiquitinating enzyme. UCHL1 activity is of great importance for function after ischemic stroke, and the C152 site of UCHL1 plays a critical role in functional recovery after cerebral ischemia [20]. Cyclopentenone prostaglandins (CyPGs) are induced in the brain after ischemic stroke and disrupt the ubiquitin-proteasome system. The point mutation UCHL1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity, indicating that it may also confer neuroprotection for postischemic neuronal injury. The neuroprotective effect was associated with less UCHL1 aggregation, as well as significantly less ubiquitinated protein accumulation and aggregation [21] (Figure 1).
Proteasome dysfunction leads protein aggregation after cerebral ischemia. Trehalose inhibits transient cerebral ischemia-induced protein aggregation via preservation of proteasome activity [22]. Propofol promotes PTEN degradation after cerebral ischemia injury via activating the ubiquitin-proteasome system and then attenuates hippocampal neuronal loss and memory impairment [23]. NSA administration inhibits the expression of mixed lineage kinase domain-like (MLKL) through the ubiquitination proteasome pathway and then greatly reduces infarct volume and improves neurological recovery after cerebral ischemia-reperfusion [24].
MicroRNA-124 confers neuroprotection in focal cerebral ischemia through inhibiting the deubiquitinating enzyme USP14 expression and reducing the expression level of REST [25]. Parkin promotes Drp1 degradation and exerts neuroprotective effect in oxygen-glucose deprivation/reperfusion injury [26]. However, the ischemia-induced ubiquitin E3 ligase tumor necrosis factor receptor-associated factor 6 (TRAF6) exacerbates cerebral ischemia injury through ubiquitinating and activating Rac1 [27] (Table 1).
Table 1.
Agents that can activate proteasome activity in ischemic stroke models.
| Agents | Effects on ischemic stroke | Possible mechanisms | Models | References |
|---|---|---|---|---|
| Trehalose | Protective | Preservation of proteasome activity | In vitro and in vivo | [22] |
| Propofol | Protective | Promoting PTEN degradation | In vivo | [23] |
| NSA | Protective | Promoting MLKL degradation | In vivo | [24] |
| MicroRNA-124 | Protective | Inhibiting the deubiquitinating enzyme USP14 expression and reducing the expression of REST | In vitro and in vivo | [25] |
| Parkin | Protective | Promotes Drp1 degradation | In vitro | [26] |
| TRAF6 | Detrimental | Ubiquitinating and activating Rac1 | In vivo | [27] |
Abbreviations: PTEN: phosphatase and tensin homology deleted on chromosome ten; USP14: ubiquitin-specific protease 14; MLKL: mixed lineage kinase domain-like; REST: RE1-silencing transcription factor; Drp1: dynamin-related protein 1; TRAF6: tumor necrosis factor receptor-associated factor 6.
Ischemic preconditioning protects against transient cerebral ischemic-induced damage through inhibiting ubiquitin aggregation [28]. Ischemic postconditioning also increases the activities of proteasomes and rescues focal ischemia-reperfusion-induced cerebral injury [29]. Meanwhile, elevated proteasome activity might contribute to the neuroprotective effect of glutamate preconditioning against cerebral ischemia-reperfusion-induced damage [30]. Excitotoxic stimulation with glutamate has multiple effects on the ubiquitin-proteasome system, which may account for the demise process in cerebral ischemia [31].
2.3.2. The Ubiquitin-Proteasome Pathway Inhibition and the Treatment of Cerebral Ischemia
Hypoxia-inducible factor-1 (HIF-1) is a key regulator of cellular responses to hypoxia and can determine the fate of neurons during cerebral ischemia. HIF-1α degradation in ischemic neurons is mediated by 20S proteasomes. Proteasomal inhibition confers neuroprotection against cerebral ischemia through HIF-1α stabilization [32]. The novel proteasome inhibitor BSc2118 enhances angioneurogenesis and protects against cerebral ischemia-induced damage, which is via HIF-1α accumulation [33]. Preservation of blood-brain barrier integrity and reversal of peripheral immunosuppression also contributes to the neuroprotective effect of proteasome inhibitor BSc2118 against cerebral ischemia [34].
Early 2-methoxyestradiol (2ME2) administration inhibits neuronal HIF-1α through ubiquitin-proteasome system-mediated degradation [35]. Dexmedetomidine administration upregulates HIF-1α expression, reduces neuronal autophagy, and thus protects neurons against ischemia-reperfusion-induced damage [36].
Cellular prion protein protects neurons against ischemic-induced damage, promotes angioneurogenesis, and enhances neural progenitor cell homing through inhibiting proteasome activity [37]. Ginsenoside Rd confers neuroprotection and alleviates neurological deficits in ischemic stroke, and this neuroprotective effect is attributed to its capability of inhibiting microglial proteasome activity and sequential inflammation [38]. Ginsenoside Rg1 also attenuates ubiquitinated protein aggregation, suppresses inflammatory responses, and thus protects against cerebral ischemia-reperfusion-induced injury [39].
rAAV8-733-mediated gene transfer of CHIP/Stub-1 reduces the expression of ubiquitinated proteins, which contributes to the prevention of hippocampal neuronal death in an animal model of brain ischemia [40]. The γ-secretase blocker DAPT reduces blood-brain barrier (BBB) permeability during permanent brain ischemia, which is the result from decreased ubiquitination and degradation of occludin [41]. The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway is a promising target for ischemic stroke therapy. The herb-derived compound, Britanin, selectively binds to a conserved cysteine residue, cysteine 151, of Keap1 and reduces Keap1-mediated ubiquitination of Nrf2, which results in activation of the Nrf2 pathway and attenuating cerebral ischemia-reperfusion-induced damage [42] (Table 2).
Table 2.
Agents that can inhibit proteasome activity in ischemic stroke models.
| Agents | Effects on ischemic stroke | Possible mechanisms | Models | References |
|---|---|---|---|---|
| Cellular prion protein | Protective | Inhibiting proteasome activity, promoting angioneurogenesis, and enhancing neural progenitor cell homing | In vivo | [37] |
| Ginsenoside Rd | Protective | Inhibiting microglial proteasome activity and sequential inflammation | In vivo and in vitro | [38] |
| Ginsenoside Rg1 | Protective | Attenuating ubiquitinated protein aggregation and inflammation | In vivo | [39] |
| rAAV8-733-mediated gene transfer of CHIP/Stub-1 | Protective | Reducing the expression of ubiquitinated proteins | In vivo and in vitro | [40] |
| γ-secretase blocker DAPT | Protective | Decreasing ubiquitination and degradation of occludin | In vivo | [41] |
| Britanin | Protective | Reducing Keap1-mediated ubiquitination of Nrf2 | In vivo and in vitro | [42] |
Abbreviations: rAAV: recombinant adenoassociated virus; Nrf2: nuclear factor erythroid 2-related factor 2.
Proteasome inhibition preconditioning promotes ER stress and autophagy but inhibits inflammation and apoptosis. Therefore, the combination of an autophagy promoter and a proteasome inhibitor is supposed to be a new potential strategy for the therapy of disorders related to hypoxia-reperfusion or ischemia-reperfusion injury [43]. Meanwhile, ischemic postconditioning confers neuroprotection against cerebral ischemic insult through Hsp70-mediated proteasome inhibition and promotes neural progenitor cell transplantation [44].
2.4. Small Ubiquitin-Like Modifier (SUMO) in Cerebral Ischemia
2.4.1. Change of SUMO in Cerebral Ischemia
The small ubiquitin-like modifier (SUMO), a ubiquitin-like protein, mediates posttranslational protein modifications and is involved in the regulation of a myriad of stress responses. Cerebral ischemia-reperfusion increases the aggregation of SUMO, which plays an important role in the altered protein dynamics after brain ischemia [45]. Focal cerebral ischemia also induces the expression of SUMO-conjugated proteins. After middle cerebral artery occlusion, SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex [46]. Maintenance of a globally decreased expression of SUMO2/3 conjugation is a component of ischemic tolerance [47].
2.4.2. The Role of SUMO in Cerebral Ischemia
Neuron-specific knockdown of SUMO inhibits global gene expression response and exacerbates the functional outcome in a mouse model of transient forebrain ischemia [48]. SUMOylation enhances neural stem cell graft survival and integration in ischemic stroke. Overexpression of the SUMO E2-conjugase Ubc9 in neural stem cells promotes neuronal differentiation and enhances resistance to cerebral ischemia-reperfusion-induced damage [49]. Ubc9 is also accounted for isoflurane preconditioning-induced neuroprotection in cerebral ischemic injury [50].
SUMO-specific protease 1 (SENP1) deconjugates SUMO from modified proteins and confers neuroprotection in cerebral ischemia. SENP1 inhibits SUMO1 conjugation and thus protects against ischemia-reperfusion-induced apoptosis [51]. URB597 inhibits SUMO-specific protease 3 (SENP3) and attenuates chronic cerebral hypoperfusion-induced neurovascular unit dysfunction in rats [52].
SUMOylation of NaV1.2 channels contributes to the early response to acute hypoxia in central neurons [53]. Ischemic preconditioning confers neuroprotection in ischemic stroke, which is mediated by SUMO1-induced SUMOylation of LYS590 of NCX3 f-Loop [54] (Table 3). E2-25K is an E2-conjugating enzyme and is SUMOylated during oxidative stress. E2-25K inhibits proteasome activity through its SUMOylation and thus plays a critical role in cerebral ischemia-reperfusion insult [55] (Table 3).
Table 3.
The role of SUMOylation pathway in cerebral ischemia.
| Molecular sites targeting SUMO | Possible mechanisms | Models | References |
|---|---|---|---|
| Neuron-specific knockdown of SUMO | Exacerbating functional outcome | In vivo | [48] |
| Overexpression the SUMO E2-conjugase Ubc9 | Promoting neuronal differentiation and enhancing resistance | In vitro | [49] |
| SUMO-specific protease 1 (SENP1) | Inhibiting SUMO1 conjugation and conferring neuroprotection | In vivo | [51] |
| URB597 | Inhibiting SENP3 and attenuating chronic cerebral hypoperfusion | In vivo | [52] |
| SUMOylation of LYS590 of NCX3 f-Loop | Conferring neuroprotection | In vivo | [54] |
| SUMOylation of E2-25K | Inhibiting proteasome activity | In vivo | [55] |
2.4.3. SUMO and Hypothermia in Cerebral Ischemia
Hypothermia confers early neuroprotective effects after middle cerebral artery occlusion in rats, which is involved in the protein conjugation of SUMO2/3 [56]. Hypothermia also increases cellular tolerance to cerebral ischemia, which substantially results from increases in global SUMOylation [57]. Moderate hypothermia promotes bone marrow stromal cell resistance to hypoxia through enhancing protein SUMOylation. Therefore, bone marrow stromal cell transplantation along with moderate hypothermia is supposed to be a promising candidate for the treatment of ischemic stroke [58].
3. The Autophagy-Lysosome Pathway in Cerebral Ischemia
3.1. Change of the Autophagy-Lysosome Pathway in Cerebral Ischemia
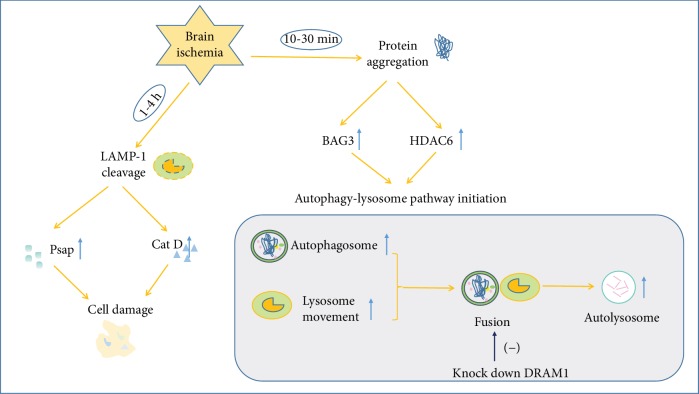
When the ubiquitin-proteasome pathway is overloaded, excessive misfolded proteins will be removed by the autophagy-lysosome pathway. Depending on the BAG1/BAG3 ratio and expression level of HDAC6, a switch from the ubiquitin-proteasome pathway to the autophagy-lysosome pathway occurs between 10 and 30 min during cerebral ischemia [59]. The lysosomal enzymes are spilled into the cytoplasm 1-4 h after ischemia-reperfusion, indicating the rapid loss of lysosomal membrane integrity after ischemic stroke [60] (Figure 2). The dynamics of lysosomal movements is revealed by the phagocytic state of brain myeloid cells in the ischemic brain. Lysosomal clustering is proximal to the cell membrane initially and will be perinuclear finally, consistent with the initial stage of active target internalization and the final stage of phagocytosis or autophagy [61].
Figure 2.
Change of the autophagy-lysosome pathway after brain ischemia.
Lysosomal Psap processing is found to be changed after cerebral ischemia from proteomic analysis of synaptosomal protein expression. Cerebral ischemia adversely affects the synapse, which may be involved in regulating the neuronal viability after ischemic stroke [62]. The distribution of lysosomal proteins is also changed after hypoxia-ischemia in the rat hippocampus and cortex. The lysosomal proteins Psap and Cat D are released abnormally to the cytosol after hypoxia-ischemia injury, which is caused by LAMP-1 cleavage, and will result in cell damage [63] (Figure 2). Chronic brain hypoperfusion leads to cognitive decline and abnormal cellular protein accumulation, accompanied by the abnormal autophagic-lysosomal system. Reduced expression of LAMP-2 protein by mir-27a leads to inefficient lysosomal clearance after chronic brain hypoperfusion in rats [64].
Cerebral ischemia significantly increases autophagosomes and autolysosomes in hippocampal CA1 and DG neurons. Transient cerebral ischemia-induced protein aggregation on subcellular organelle membranes could result in multiple organelle damage, accompanied by delayed neuronal death [65]. DNA damage-regulated autophagy modulator protein 1 (DRAM1) is a multipass membrane lysosomal protein and is involved in autophagy. Cerebral ischemia-reperfusion injury induces the expression of DRAM1 protein and leads to autophagy activation. Knockdown of DRAM1 blocks autophagosome-lysosome fusion, inhibits autophagy, and aggravates cerebral ischemia-induced cell damage [66] (Figure 2). The membrane-bound water channel aquaporin-4 (AQP4) is of great importance in maintaining water homeostasis in the brain. Ischemia induces AQP4 internalization in the brain, and the internalized AQP4 will be degraded by the lysosome [67].
3.2. The Role of the Autophagy-Lysosome Pathway in Cerebral Ischemia
3.2.1. The Neuroprotective Role of the Autophagy-Lysosome Pathway in Cerebral Ischemia
Absence of the circadian clock protein PER1 inhibits the hippocampal autophagic machinery and contributes to vulnerability to cerebral ischemia injury [68]. Neonatal hypoxic-ischemia initially activates autophagy but later impairs autophagosome clearance and leads to neuronal death in a piglet model [69].
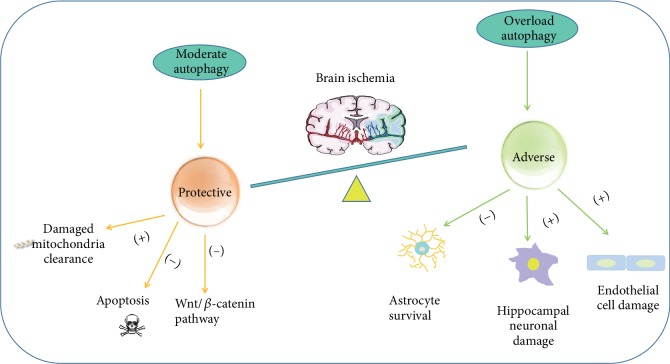
Autophagy plays diverse roles in cerebral ischemia-reperfusion injury (Figure 3). Mitophagy-related mitochondrial clearance and downstream apoptosis inhibition account for the neuroprotective effect of autophagy in cerebral ischemia-reperfusion injury. PARK2 may participate in the mitophagy process responsible for the protective role of autophagy [70]. Autophagy negatively regulates the Wnt/β-catenin pathway and thus exerts protective effect in neural ischemia and hypoxia [71].
Figure 3.
Dual roles of the autophagy-lysosome pathway in brain ischemia.
3.2.2. The Adverse Role of the Autophagy-Lysosome Pathway in Cerebral Ischemia
The autophagic-lysosomal pathway is activated in injured astrocytes following glucose and oxygen deprivation and focal cerebral ischemia, which at least partly account for decreased survival of astrocytes [72]. Asphyxia cardiac arrest-induced cerebral ischemia-reperfusion enhances the expression of lysosomal proteins and autophagosome numbers in hippocampal cells, as well as leads to hippocampal neuronal damage, which is attenuated by autophagy inhibition and lysosome inhibition [73].
RIP1K activates the autophagic-lysosomal pathway and induces necroptosis, which may account for the neuronal and the astrocytic cell death after cerebral ischemia [74]. The endothelial cell is damaged after sustained ischemic injury, and the autophagy-lysosome signaling is at least partially activated by peroxynitrite-mediated nitrosative stress [75] (Figure 3).
3.3. The Autophagy-Lysosome Pathway and the Treatment of Cerebral Ischemia
3.3.1. The Autophagy-Lysosome Pathway Activation and the Treatment of Cerebral Ischemia
Ischemia-reperfusion induces autophagosome formation and inhibits autolysosome degradation. Mitofusin 2, a mitochondrial fusion protein, enhances autophagosome formation and promotes the fusion of autophagosomes and lysosomes and, thus, attenuates ischemia-reperfusion-induced damage [76]. Rab7b, a lysosome-associated small Rab GTPase, regulates autophagy during cerebral ischemia and confers neuroprotection against ischemic brain damage [77]. The autophagic flux is found to be impaired in an animal model of ischemic stroke. Pseudoginsenoside-F11 ameliorates ischemic brain damage through attenuating autophagic-lysosomal defects [78].
Sphingosine kinase 2 exerts a neuroprotective effect in cerebral ischemia. It could interact with Bcl-2 via its BH3 domain, thereby dissociating it from Beclin-1, and activating autophagy subsequently [79]. Phosphorylated CAV1 binds to the BECN1/VPS34 complex under oxidative stress, which thereby induces the activation of autophagy, which contributes to its neuroprotection effect in ischemic injury [80]. Neuronal Rho GTPase Rac1 ablation protects against ischemic brain damage through maintenance of lysosomes [81].
Resveratrol induces Sirt1-dependent autophagy, which thereby suppresses NLRP3 inflammasome activation, and thus protects against cerebral ischemia-reperfusion injury [82]. Hyperhomocysteinemia (HHcy) is a common risk factor for cerebral ischemia. HHcy inhibits lysosomal membrane protein expression, which thereby leads to lysosomal dysfunction and autophagic defect, which can be alleviated by vitamin B supplementation [83]. CysC is a critical determinant accounting for endogenous neuroprotection and is a promising agent for the therapy of stroke, which is mediated by preserving lysosomal membrane integrity [84]. Acute ethanol exposure alleviates acidosis-induced neurotoxicity after cerebral ischemia, which is mediated by increased acid-sensing ion channel 1a (ASIC1a) protein degradation through the autophagy-lysosome pathway [85] (Table 4).
Table 4.
Agents that can activate the autophagy-lysosome pathway in ischemic stroke models.
| Agents | Effects on ischemic stroke | Possible mechanisms | Models | References |
|---|---|---|---|---|
| Mitofusin | Protective | Enhancing autophagosome formation and promoting the fusion of autophagosomes | In vitro | [76] |
| Rab7b | Protective | Regulating the lysosomal degradation of TLR4 | In vivo | [77] |
| PF-11 | Protective | Attenuating autophagic-lysosomal defects | In vivo | [78] |
| Sphingosine kinase 2 | Protective | Activating autophagy | In vitro | [79] |
| Phosphorylated CAV1 | Protective | Activating autophagy | In vivo and in vitro | [80] |
| Neuronal rho GTPase Rac1 ablation | Protective | Maintenance of lysosomes | In vivo | [81] |
| Resveratrol | Protective | Suppressing NLRP3 inflammasome activation | In vivo | [82] |
| HHcy | Detrimental | Promoting lysosomal dysfunction and autophagic defect | In vivo and in vitro | [83] |
| CysC | Protective | Preserving lysosomal membrane integrity | In vivo | [84] |
| Acute ethanol exposure | Protective | Increased ASIC1a protein degradation | In vitro | [85] |
Abbreviations: TLR4: toll-like receptor 4; PF-11: pseudoginsenoside-F11; CAV1: caveolin1; NLRP3: NOD-like receptor family pyrin domain-containing 3; HHcy: hyperhomocysteinemia; CysC: cystatin C; ASIC1a: acid-sensing ion channel 1a.
Autophagosome accumulation is increased after oxygen-glucose deprivation insult, which is mediated by increased autophagosome formation and decreased autophagosome clearance. Sevoflurane postconditioning protects against oxygen-glucose deprivation injury through inhibition of autophagosome accumulation [86]. Cerebral ischemia-induced CA1 neuronal death is exacerbated after inhibition of autophagosome-lysosome fusion. Hypoxic preconditioning protects neurons against transient global cerebral ischemia injury through Rab7-mediated autophagosome maturation [87].
Mild hypothermia confers protection against hippocampal neuronal injury after oxygen-glucose deprivation/reperfusion, which is mediated by promoting lysosomal function and autophagic flux [88]. Remote ischemic preconditioning protects against cerebral ischemia-reperfusion injury through activation of the autophagy-lysosome pathway [89].
3.3.2. The Autophagy-Lysosome Pathway Inhibition and the Treatment of Cerebral Ischemia
miR-207 and miR-352 play important roles in regulating cerebral ischemic damage and spontaneous recovery through the lysosomal pathway. miR-207 mimics could decrease the expression of cellular lysosome and autophagosome and, thus, induce the expression of autophagic vacuoles [90]. Dapsone (DDS), an anti-inflammation and antioxidation drug, suppresses abnormal degradation of tight junction ZO-1 through autophagy and inhibits lysosome accumulation and, thus, confers neuroprotection on cerebral microvessels [91].
2-(3′,5′-Dimethoxybenzylidene) cyclopentanone (DMBC), a novel synthetic small-molecule compound, inhibits the release of cathepsin B from the lysosomes into the cytoplasm after cerebral ischemia, thereby protecting neurons against ischemic injury [92]. CA-074Me or Clik148, a selective inhibitor of cathepsin B or cathepsin L, inhibits the release of cathepsin B or L from the lysosomes into the cytoplasm and activation of caspase-3 in astrocytes after the permanent middle cerebral artery occlusion (pMCAO). Suppression of cysteine cathepsin B and L activation could inhibit the tBid-mitochondrial apoptotic signaling pathway, thereby protecting astrocytes against ischemic injury [93]. 3-MA and Wort inhibit autophagy and protect astrocytes against ischemic injury. Inhibition of autophagy induces the expression of the lysosomal Hsp70.1B in ischemic astrocytes, which thereby stabilizes lysosomal membranes, and subsequently blocks the cathepsin-tBid-mitochondrial apoptotic signaling pathway [94]. Besides cathepsin B inhibition, CA074-me may exert the neuroprotective effect by preserving lysosomal membrane integrity and suppressing lysosomal rupture [95].
Fingolimod inhibits ischemia-induced neuronal autophagy via the mTOR/p70S6K signaling pathway and confers neuroprotection against cerebral ischemic insult [96]. Lycium barbarum polysaccharide protects against ischemia-reperfusion injury in primary cultured hippocampal neurons, which is mediated by inhibition of apoptosis and autophagic cell death through the PI3K/Akt/mTOR pathway [97]. Cornin also suppresses autophagy in SH-SY5Y cells after oxygen and glucose deprivation, which is via regulation of the PI3K/Akt/mTOR signaling pathway [98] (Table 5).
Table 5.
Agents that can inhibit the autophagy-lysosome pathway in ischemic stroke models.
| Agents | Effects on ischemic stroke | Possible mechanisms | Models | References |
|---|---|---|---|---|
| miR-207 mimics | Protective | Decreasing the expression of cellular lysosome and autophagosome | In vivo | [90] |
| DDS | Protective | Inhibiting lysosome accumulation | In vivo and in vitro | [91] |
| DMBC | Protective | Inhibiting the release of cathepsin B from the lysosomes into the cytoplasm | In vivo and in vitro | [92] |
| Clik148 | Protective | Inhibiting the release of cathepsin L from the lysosomes into the cytoplasm and activation of caspase-3 | In vivo and in vitro | [93] |
| 3-MA and Wort | Protective | Inhibiting autophagy and stabilizing lysosomal membranes | In vivo and in vitro | [94] |
| CA074-me | Protective | Inhibiting the release of cathepsin B, preserving lysosomal membrane integrity, and suppressing lysosomal rupture | In vivo | [93, 95] |
| Fingolimod | Protective | Inhibiting autophagy via the mTOR/p70S6K pathway | In vivo | [96] |
| Lycium barbarum polysaccharide | Protective | Inhibiting apoptosis and autophagy through the PI3K/Akt/mTOR pathway | In vitro | [97] |
| Cornin | Protective | Inhibiting autophagy through the PI3K/Akt/mTOR pathway | In vitro | [98] |
Abbreviations: DDS: dapsone; DMBC: 2-(3′,5′-dimethoxybenzylidene) cyclopentanone; 3-MA: 3-methyladenine; Wort: wortmannin.
Hyperbaric oxygen (HBO) suppresses autophagy activity and inhibits apoptosis and necrosis levels, thereby conferring neuroprotection against ischemic brain injury [99].
Sevoflurane postconditioning suppresses the activation and release of lysosomal cathepsin B and alleviates reactive astrogliosis and glial scar formation after cerebral ischemia-reperfusion [100].
4. The Crosstalk between the Ubiquitin-Proteasome Pathway and the Autophagy-Lysosome Pathway in Cerebral Ischemia
Ubiquitylation is a common component that guides misfolded protein to the proper degradation system for both the ubiquitin-proteasome pathway and the autophagy-lysosome pathway [101]. Besides, the two pathways recognize their substrates through corresponding ubiquitin tags. K48-linked ubiquitin chains are predominantly introduced to be a signal for the ubiquitin-proteasome pathway, while K63-linked ubiquitin chains are known as a signal for autophagic degradation [102, 103]. Despite their mode of function and that their ubiquitin tags for substrate recognition are different, there is crosstalk between the ubiquitin-proteasome pathway and the autophagy-lysosome pathway in the pathogenesis of cerebral ischemia.
On the one hand, initial evidences about functional links between the ubiquitin-proteasome pathway and the autophagy-lysosome pathway reveal that inhibition of one can result in a compensatory upregulation of the other pathway [104]. This can be observed in in vivo and in vitro models of cerebral ischemia. Liu et al. demonstrate that the high level of the HDAC6 and BAG1/BAG3 ratio decides the switch from the proteasome pathway to autophagy between 10 min and 30 min of cerebral ischemia [59]. Besides, inhibition of the ubiquitin-proteasome pathway by MG132 could activate autophagy and induce aggresome formation in primary neurons [105]. In addition, Quiroga et al. propose that Herp (an ER stress-induced stress protein) plays a key role in mediating the proteasomal degradation of autophagy regulator Beclin-1 through the regulation of the E3 ubiquitin ligase Hrd1. They further demonstrate that in a cell glucose starvation model, depletion or possibly inhibition of Herp leads to decreased Beclin-1 proteasomal degradation, which triggers a compensatory autophagy upregulation [106].
On the other hand, mitophagy is also a prominent biological phenomenon that involves both the ubiquitin-proteasome pathway and the autophagy-lysosome pathway since the ubiquitin-proteasome activity has been proven to be a prerequisite in the preparation of selective autophagy for mitochondria [107]. This scenario is also observed in cerebral ischemia. Lan et al. demonstrate that severe cellular stress following cerebral ischemia can cause significant aggregation of PINK1 in the mitochondrial outer membrane and subsequent Parkin phosphorylation, which in turn ubiquitylates mitochondrial targets as well as promotes the autophagic degradation of mitochondria [106].
5. Conclusions and Future Directions
Collectively, this review provides novel insights into the role of the ubiquitin-proteasome pathway and the autophagy-lysosome pathway after cerebral ischemia. Cerebral ischemia leads to dysfunction of these two pathways, which contributes to misfolded protein accumulation and neuronal necrosis and apoptosis ultimately. Regulation of these two pathways may be an important mechanism for the neuroprotective effects of many therapeutic agents and approaches against cerebral ischemia.
The challenge in the future research is to determine the key factors that mediate degradation of apoptosis and oxidative damage-related proteins, as well as the specific receptors that mediate the degradation of mitochondria, the endoplasmic reticulum, and Golgi apparatus through the autophagy-lysosome pathway in cerebral ischemic injury. To achieve this, mass spectrometry can be employed to quantitatively analyze proteins that are altered on organelles during cerebral ischemia, especially those whose expression is increased.
At the same time, future researches will also need to clarify the dynamic changes of the ubiquitin-proteasome pathway and the autophagy-lysosome pathway at different time points during cerebral ischemia, which will help us to determine their specific role in cerebral ischemic injury. As we know, there are transgenic mice expressing UbG76V-GFP, an ubiquitin-proteasome system reporter, for monitoring the role of ubiquitin-proteasome-dependent proteolysis [108], transgenic mice systemically expressing RFP-EGFP-LC3 for real-time observation of autophagy [109] and a mito-QC reporter mouse model for mammalian mitophagy detection [110], which will help us to solve these issues. These future studies will discover new measures targeting these two pathways to maintain protein homeostasis and provide new avenues to treat cerebral ischemic-related diseases.
Acknowledgments
This article has been supported by the National Natural Science Foundation of China (Grant no. 81771423 and 81974212).
Conflicts of Interest
The authors confirm that this article content has no conflicts of interest.
References
- 1.Blackwood E. A., Azizi K., Thuerauf D. J., et al. Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nature Communications. 2019;10(1):p. 187. doi: 10.1038/s41467-018-08129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Min J. W., Feng S., et al. Therapeutic role of a cysteine precursor, OTC, in ischemic stroke is mediated by improved proteostasis in mice. Translational Stroke Research. 2020;11(1):147–160. doi: 10.1007/s12975-019-00707-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun R., Xiao L., Duan S. High expression of ubiquitin conjugates and NF-κB in unstable human intracranial atherosclerotic plaques. Journal of Cellular Physiology. 2012;227(2):784–788. doi: 10.1002/jcp.22790. [DOI] [PubMed] [Google Scholar]
- 4.Santos P. T., O'Brien C. E., Chen M. W., et al. Proteasome biology is compromised in white matter after asphyxic cardiac arrest in neonatal piglets. Journal of the American Heart Association. 2018;7(20, article e009415) doi: 10.1161/JAHA.118.009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Nakano Y., Shang J., et al. Temporal profiles of stress protein inductions after focal transient ischemia in mice brain. Journal of Stroke and Cerebrovascular Diseases. 2016;25(10):2344–2351. doi: 10.1016/j.jstrokecerebrovasdis.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Iwabuchi M., Sheng H., Thompson J. W., et al. Characterization of the ubiquitin-modified proteome regulated by transient forebrain ischemia. Journal of Cerebral Blood Flow and Metabolism. 2014;34(3):425–432. doi: 10.1038/jcbfm.2013.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochrainer K., Jackman K., Anrather J., Iadecola C. Reperfusion rather than ischemia drives the formation of ubiquitin aggregates after middle cerebral artery occlusion. Stroke. 2012;43(8):2229–2235. doi: 10.1161/STROKEAHA.112.650416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu L., Wang H. Transient focal cerebral ischemia upregulates immunoproteasomal subunits. Cellular and Molecular Neurobiology. 2012;32(6):965–970. doi: 10.1007/s10571-012-9854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Wang Y., Fu M., Lei H., Cheng Q., Zhang X. Plasma immunoproteasome predicts early hemorrhagic transformation in acute ischemic stroke patients. Journal of Stroke and Cerebrovascular Diseases. 2017;26(1):49–56. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Chen X., Zhang X., Wang Y., et al. Inhibition of immunoproteasome reduces infarction volume and attenuates inflammatory reaction in a rat model of ischemic stroke. Cell Death & Disease. 2015;6(1, article e1626) doi: 10.1038/cddis.2014.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Zhang X., Chen T., et al. Inhibition of immunoproteasome promotes angiogenesis via enhancing hypoxia- inducible factor-1α abundance in rats following focal cerebral ischaemia. Brain, Behavior, and Immunity. 2018;73:167–179. doi: 10.1016/j.bbi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y., Chen X., Li D., et al. PR-957 mediates neuroprotection by inhibiting Th17 differentiation and modulating cytokine production in a mouse model of ischaemic stroke. Clinical and Experimental Immunology. 2018;193(2):194–206. doi: 10.1111/cei.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn H. C., Yoo K. Y., Hwang I. K., et al. Ischemia-related changes in naive and mutant forms of ubiquitin and neuroprotective effects of ubiquitin in the hippocampus following experimental transient ischemic damage. Experimental Neurology. 2009;220(1):120–132. doi: 10.1016/j.expneurol.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Savchuk O. I., Orlovsky M. O., Iarmoliuk I. S., Goncharov S. V., Dosenko V. E., Skibo G. G. Proteasomal activity in brain tissue following ischemic stroke in Wistar rats. Fiziologicheskiĭ Zhurnal. 2015;61(5):11–20. doi: 10.15407/fz61.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Misra S., Kumar P., Kumar A., Sagar R., Chakravarty K., Prasad K. Genetic association between inflammatory genes (IL-1α, CD14, LGALS2, PSMA6) and risk of ischemic stroke: a meta-analysis. Meta Gene. 2016;8:21–29. doi: 10.1016/j.mgene.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desgeorges M. M., Devillard X., Toutain J., et al. Molecular mechanisms of skeletal muscle atrophy in a mouse model of cerebral ischemia. Stroke. 2015;46(6):1673–1680. doi: 10.1161/STROKEAHA.114.008574. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y., Qiao F., Wang H. Enhanced proteostasis in post-ischemic stroke mouse brains by ubiquilin-1 promotes functional recovery. Cellular and Molecular Neurobiology. 2017;37(7):1325–1329. doi: 10.1007/s10571-016-0451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Lü L., Hettinger C. L., et al. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. The Journal of Neuroscience. 2014;34(8):2813–2821. doi: 10.1523/JNEUROSCI.3541-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min J. W., Lü L., Freeling J. L., Martin D. S., Wang H. USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. Journal of Neurochemistry. 2017;140(5):826–833. doi: 10.1111/jnc.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Povysheva N., Rose M. E., et al. Role of UCHL1 in axonal injury and functional recovery after cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(10):4643–4650. doi: 10.1073/pnas.1821282116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H., Li W., Rose M. E., et al. The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post- ischemic neuronal injury. Cell Death & Disease. 2015;6(11, article e1966) doi: 10.1038/cddis.2015.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Luo Y., Luo T., et al. Trehalose inhibits protein aggregation caused by transient ischemic insults through preservation of proteasome activity, not via induction of autophagy. Molecular Neurobiology. 2017;54(9):6857–6869. doi: 10.1007/s12035-016-0196-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen X., du Y. M., Xu F., Liu D., Wang Y. L. Propofol prevents hippocampal neuronal loss and memory impairment in cerebral ischemia injury through promoting PTEN degradation. Journal of Molecular Neuroscience. 2016;60(1):63–70. doi: 10.1007/s12031-016-0791-y. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y., Zhou B., Tu H., et al. The degradation of mixed lineage kinase domain-like protein promotes neuroprotection after ischemic brain injury. Oncotarget. 2017;8(40):68393–68401. doi: 10.18632/oncotarget.19416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doeppner T. R., Doehring M., Bretschneider E., et al. MicroRNA-124 protects against focal cerebral ischemia via mechanisms involving Usp14-dependent REST degradation. Acta Neuropathologica. 2013;126(2):251–265. doi: 10.1007/s00401-013-1142-5. [DOI] [PubMed] [Google Scholar]
- 26.Tang J., Hu Z., Tan J., Yang S., Zeng L. Parkin protects against oxygen-glucose deprivation/reperfusion insult by promoting Drp1 degradation. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/8474303.8474303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T., Qin J. J., Yang X., et al. The ubiquitin E3 ligase TRAF6 exacerbates ischemic stroke by ubiquitinating and activating Rac1. The Journal of Neuroscience. 2017;37(50):12123–12140. doi: 10.1523/JNEUROSCI.1751-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J. C., Kim I. H., Cho G. S., et al. Ischemic preconditioning-induced neuroprotection against transient cerebral ischemic damage via attenuating ubiquitin aggregation. Journal of the Neurological Sciences. 2014;336(1-2):74–82. doi: 10.1016/j.jns.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Li Z. Y., Liu B., Yu J., Yang F. W., Luo Y. N., Ge P. F. Ischaemic postconditioning rescues brain injury caused by focal ischaemia/reperfusion via attenuation of protein oxidization. The Journal of International Medical Research. 2012;40(3):954–966. doi: 10.1177/147323001204000314. [DOI] [PubMed] [Google Scholar]
- 30.Badawi Y., Pal R., Hui D., Michaelis E. K., Shi H. Ischemic tolerance in an in vivo model of glutamate preconditioning. Journal of Neuroscience Research. 2015;93(4):623–632. doi: 10.1002/jnr.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldeira M. V., Curcio M., Leal G., et al. Excitotoxic stimulation downregulates the ubiquitin-proteasome system through activation of NMDA receptors in cultured hippocampal neurons. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2013;1832(1):263–274. doi: 10.1016/j.bbadis.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Badawi Y., Shi H. Relative contribution of prolyl hydroxylase-dependent and -independent degradation of HIF-1alpha by proteasomal pathways in cerebral ischemia. Frontiers in Neuroscience. 2017;11:p. 239. doi: 10.3389/fnins.2017.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doeppner T. R., Mlynarczuk-Bialy I., Kuckelkorn U., et al. The novel proteasome inhibitor BSc2118 protects against cerebral ischaemia through HIF1A accumulation and enhanced angioneurogenesis. Brain. 2012;135(Part 11):3282–3297. doi: 10.1093/brain/aws269. [DOI] [PubMed] [Google Scholar]
- 34.Doeppner T. R., Kaltwasser B., Kuckelkorn U., et al. Systemic proteasome inhibition induces sustained post-stroke neurological recovery and Neuroprotection via mechanisms involving reversal of peripheral immunosuppression and preservation of blood-brain-barrier integrity. Molecular Neurobiology. 2016;53(9):6332–6341. doi: 10.1007/s12035-015-9533-3. [DOI] [PubMed] [Google Scholar]
- 35.Schaible E. V., Windschügl J., Bobkiewicz W., et al. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1α response after traumatic brain injury in mice. Journal of Neurochemistry. 2014;129(6):940–954. doi: 10.1111/jnc.12708. [DOI] [PubMed] [Google Scholar]
- 36.Luo C., Ouyang M. W., Fang Y. Y., et al. Dexmedetomidine protects mouse brain from ischemia-reperfusion injury via inhibiting neuronal autophagy through up-regulating HIF-1α. Frontiers in Cellular Neuroscience. 2017;11:p. 197. doi: 10.3389/fncel.2017.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doeppner T. R., Kaltwasser B., Schlechter J., et al. Cellular prion protein promotes post-ischemic neuronal survival, angioneurogenesis and enhances neural progenitor cell homing via proteasome inhibition. Cell Death & Disease. 2015;6(12, article e2024) doi: 10.1038/cddis.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G., Xia F., Zhang Y., et al. Ginsenoside Rd is efficacious against acute ischemic stroke by suppressing microglial proteasome-mediated inflammation. Molecular Neurobiology. 2016;53(4):2529–2540. doi: 10.1007/s12035-015-9261-8. [DOI] [PubMed] [Google Scholar]
- 39.Zheng T., Jiang H., Jin R., et al. Ginsenoside Rg1 attenuates protein aggregation and inflammatory response following cerebral ischemia and reperfusion injury. European Journal of Pharmacology. 2019;853:65–73. doi: 10.1016/j.ejphar.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Cabral-Miranda F., Nicoloso-Simões E., Adão-Novaes J., et al. rAAV8-733-mediated gene transfer of CHIP/Stub-1 prevents hippocampal neuronal death in experimental brain ischemia. Molecular Therapy. 2017;25(2):392–400. doi: 10.1016/j.ymthe.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang G. S., Tian Y., Huang J. Y., et al. The γ-secretase blocker DAPT reduces the permeability of the blood-brain barrier by decreasing the ubiquitination and degradation of occludin during permanent brain ischemia. CNS Neuroscience & Therapeutics. 2013;19(1):53–60. doi: 10.1111/cns.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu G., Zhu L., Yuan X., et al. Britanin ameliorates cerebral ischemia-reperfusion injury by inducing the Nrf2 protective pathway. Antioxidants & Redox Signaling. 2017;27(11):754–768. doi: 10.1089/ars.2016.6885. [DOI] [PubMed] [Google Scholar]
- 43.Fan T., Huang Z., Chen L., et al. Associations between autophagy, the ubiquitin-proteasome system and endoplasmic reticulum stress in hypoxia-deoxygenation or ischemia-reperfusion. European Journal of Pharmacology. 2016;791:157–167. doi: 10.1016/j.ejphar.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Doeppner T. R., Doehring M., Kaltwasser B., et al. Ischemic post-conditioning induces post-stroke neuroprotection via Hsp70-mediated proteasome inhibition and facilitates neural progenitor cell transplantation. Molecular Neurobiology. 2017;54(8):6061–6073. doi: 10.1007/s12035-016-0137-3. [DOI] [PubMed] [Google Scholar]
- 45.Kahl A., Blanco I., Jackman K., et al. Cerebral ischemia induces the aggregation of proteins linked to neurodegenerative diseases. Scientific Reports. 2018;8(1):p. 2701. doi: 10.1038/s41598-018-21063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hochrainer K., Jackman K., Benakis C., Anrather J., Iadecola C. SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex after middle cerebral artery occlusion. Journal of Cerebral Blood Flow and Metabolism. 2015;35(1):1–5. doi: 10.1038/jcbfm.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Huang D., Zhou J., Yue Y., Wang X. SUMOylation participates in induction of ischemic tolerance in mice. Brain Research Bulletin. 2019;147:159–164. doi: 10.1016/j.brainresbull.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L., Liu X., Sheng H., et al. Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience. 2017;343:190–212. doi: 10.1016/j.neuroscience.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstock J. D., Peruzzotti-Jametti L., Leonardi T., et al. SUMOylation promotes survival and integration of neural stem cell grafts in ischemic stroke. EBioMedicine. 2019;42:214–224. doi: 10.1016/j.ebiom.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong L., Wu Z., Ran M., et al. The role of SUMO-conjugating enzyme Ubc9 in the neuroprotection of isoflurane preconditioning against ischemic neuronal injury. Molecular Neurobiology. 2015;51(3):1221–1231. doi: 10.1007/s12035-014-8797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H., Wang Y., Zhu A., et al. SUMO-specific protease 1 protects neurons from apoptotic death during transient brain ischemia/reperfusion. Cell Death & Disease. 2016;7(11, article e2484) doi: 10.1038/cddis.2016.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D. P., Liu K. J., Kasper G., Lin Q., Hai J. Inhibition of SENP3 by URB597 ameliorates neurovascular unit dysfunction in rats with chronic cerebral hypoperfusion. Biomedicine & Pharmacotherapy. 2017;91:872–879. doi: 10.1016/j.biopha.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 53.Plant L. D., Marks J. D., Goldstein S. A. N. SUMOylation of NaV1.2 channels mediates the early response to acute hypoxia in central neurons. eLife. 2016;5, article e20054 doi: 10.7554/eLife.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuomo O., Pignataro G., Sirabella R., et al. Sumoylation of LYS590 of NCX3 f-loop by SUMO1 participates in brain neuroprotection induced by ischemic preconditioning. Stroke. 2016;47(4):1085–1093. doi: 10.1161/STROKEAHA.115.012514. [DOI] [PubMed] [Google Scholar]
- 55.Jeong E. I., Chung H. W., Lee W. J., et al. E2-25K SUMOylation inhibits proteasome for cell death during cerebral ischemia/reperfusion. Cell Death & Disease. 2016;7(12, article e2573) doi: 10.1038/cddis.2016.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G., Liu X., Su Z., Zhang D. Hypothermia exerts early neuroprotective effects involving protein conjugation of SUMO-2/3 in a rat model of middle cerebral artery occlusion. Molecular Medicine Reports. 2017;16(3):3217–3223. doi: 10.3892/mmr.2017.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y. J., Mou Y., Klimanis D., Bernstock J. D., Hallenbeck J. M. Global SUMOylation is a molecular mechanism underlying hypothermia-induced ischemic tolerance. Frontiers in Cellular Neuroscience. 2014;8:p. 416. doi: 10.3389/fncel.2014.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren W., Ma X., Liu X., et al. Moderate hypothermia induces protein SUMOylation in bone marrow stromal cells and enhances their tolerance to hypoxia. Molecular Medicine Reports. 2017;16(5):7006–7012. doi: 10.3892/mmr.2017.7425. [DOI] [PubMed] [Google Scholar]
- 59.Liu X., Yamashita T., Shang J., et al. Molecular switching from ubiquitin-proteasome to autophagy pathways in mice stroke model. Journal of Cerebral Blood Flow & Metabolism. 2020;40(1):214–224. doi: 10.1177/0271678x18810617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kilinc M., Gürsoy-Ozdemir Y., Gürer G., et al. Lysosomal rupture, necroapoptotic interactions and potential crosstalk between cysteine proteases in neurons shortly after focal ischemia. Neurobiology of Disease. 2010;40(1):293–302. doi: 10.1016/j.nbd.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Fumagalli S., Fiordaliso F., Perego C., et al. The phagocytic state of brain myeloid cells after ischemia revealed by superresolution structured illumination microscopy. Journal of Neuroinflammation. 2019;16(1):p. 9. doi: 10.1186/s12974-019-1401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costain W. J., Haqqani A. S., Rasquinha I., et al. Proteomic analysis of synaptosomal protein expression reveals that cerebral ischemia alters lysosomal Psap processing. Proteomics. 2010;10(18):3272–3291. doi: 10.1002/pmic.200900447. [DOI] [PubMed] [Google Scholar]
- 63.Troncoso M., Bannoud N., Carvelli L., Asensio J., Seltzer A., Sosa M. A. Hypoxia-ischemia alters distribution of lysosomal proteins in rat cortex and hippocampus. Biology Open. 2018;7(10, article bio036723) doi: 10.1242/bio.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Che H., Yan Y., Kang X. H., et al. MicroRNA-27a promotes inefficient lysosomal clearance in the hippocampi of rats following chronic brain hypoperfusion. Molecular Neurobiology. 2017;54(4):2595–2610. doi: 10.1007/s12035-016-9856-8. [DOI] [PubMed] [Google Scholar]
- 65.Liu C., Gao Y., Barrett J., Hu B. Autophagy and protein aggregation after brain ischemia. Journal of Neurochemistry. 2010;115(1):68–78. doi: 10.1111/j.1471-4159.2010.06905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu M., Jiang Y., Feng Q., Ouyang Y.'., Gan J. DRAM1 protects neuroblastoma cells from oxygen-glucose deprivation/reperfusion-induced injury via autophagy. International Journal of Molecular Sciences. 2014;15(10):19253–19264. doi: 10.3390/ijms151019253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang J., Sun S. Q., Lu W. T., et al. The internalization and lysosomal degradation of brain AQP4 after ischemic injury. Brain Research. 2013;1539:61–72. doi: 10.1016/j.brainres.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 68.Rami A., Fekadu J., Rawashdeh O. The hippocampal autophagic machinery is depressed in the absence of the circadian clock protein PER1 that may lead to vulnerability during cerebral ischemia. Current Neurovascular Research. 2017;14(3):207–214. doi: 10.2174/1567202614666170619083239. [DOI] [PubMed] [Google Scholar]
- 69.Cui D., Sun D., Wang X., et al. Impaired autophagosome clearance contributes to neuronal death in a piglet model of neonatal hypoxic-ischemic encephalopathy. Cell Death & Disease. 2017;8(7, article e2919) doi: 10.1038/cddis.2017.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X., Yan H., Yuan Y., et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy. 2014;9(9):1321–1333. doi: 10.4161/auto.25132. [DOI] [PubMed] [Google Scholar]
- 71.Shi Z. Y., Deng J. X., Fu S., et al. Protective effect of autophagy in neural ischemia and hypoxia: negative regulation of the Wnt/β-catenin pathway. International Journal of Molecular Medicine. 2017;40(6):1699–1708. doi: 10.3892/ijmm.2017.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qin A. P., Liu C. F., Qin Y. Y., et al. Autophagy was activated in injured astrocytes and mildly decreased cell survival following glucose and oxygen deprivation and focal cerebral ischemia. Autophagy. 2010;6(6):738–753. doi: 10.4161/auto.6.6.12573. [DOI] [PubMed] [Google Scholar]
- 73.Cui D., Shang H., Zhang X., Jiang W., Jia X. Cardiac arrest triggers hippocampal neuronal death through autophagic and apoptotic pathways. Scientific Reports. 2016;6(1, article 27642) doi: 10.1038/srep27642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni Y., Gu W. W., Liu Z. H., et al. RIP1K contributes to neuronal and Astrocytic cell death in ischemic stroke via activating autophagic-lysosomal pathway. Neuroscience. 2018;371:60–74. doi: 10.1016/j.neuroscience.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 75.Han F., Chen Y. X., Lu Y. M., et al. Regulation of the ischemia-induced autophagy-lysosome processes by nitrosative stress in endothelial cells. Journal of Pineal Research. 2011;51(1):124–135. doi: 10.1111/j.1600-079X.2011.00869.x. [DOI] [PubMed] [Google Scholar]
- 76.Peng C., Rao W., Zhang L., et al. Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cellular Physiology and Biochemistry. 2018;46(6):2311–2324. doi: 10.1159/000489621. [DOI] [PubMed] [Google Scholar]
- 77.Qi J., Rong Y., Wang L., Xu J., Zhao K. Rab7b overexpression-ameliorated ischemic brain damage following tMCAO involves suppression of TLR4 and NF-κB p65. Journal of Molecular Neuroscience. 2019;68(2):163–170. doi: 10.1007/s12031-019-01295-y. [DOI] [PubMed] [Google Scholar]
- 78.Liu Y. Y., Zhang T. Y., Xue X., et al. Pseudoginsenoside-F11 attenuates cerebral ischemic injury by alleviating autophagic/lysosomal defects. CNS Neuroscience & Therapeutics. 2017;23(7):567–579. doi: 10.1111/cns.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Song D. D., Zhang T. T., Chen J. L., et al. Sphingosine kinase 2 activates autophagy and protects neurons against ischemic injury through interaction with Bcl-2 via its putative BH3 domain. Cell Death & Disease. 2017;8(7, article e2912) doi: 10.1038/cddis.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nah J., Yoo S. M., Jung S., et al. Phosphorylated CAV1 activates autophagy through an interaction with BECN1 under oxidative stress. Cell Death & Disease. 2017;8(5, article e2822) doi: 10.1038/cddis.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karabiyik C., Fernandes R., Figueiredo F. R., et al. Neuronal rho GTPase Rac1 elimination confers neuroprotection in a mouse model of permanent ischemic stroke. Brain Pathology. 2018;28(4):569–580. doi: 10.1111/bpa.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He Q., Li Z., Wang Y., Hou Y., Li L., Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. International Immunopharmacology. 2017;50:208–215. doi: 10.1016/j.intimp.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 83.Tripathi M., Zhang C. W., Singh B. K., et al. Hyperhomocysteinemia causes ER stress and impaired autophagy that is reversed by vitamin B supplementation. Cell Death & Disease. 2016;7(12, article e2513) doi: 10.1038/cddis.2016.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang Z., Deng J., Wu Z., et al. Cystatin C is a crucial endogenous protective determinant against stroke. Stroke. 2017;48(2):436–444. doi: 10.1161/STROKEAHA.116.014975. [DOI] [PubMed] [Google Scholar]
- 85.Zhou R. P., Leng T. D., Yang T., Chen F. H., Xiong Z. G. Acute ethanol exposure promotes autophagy-lysosome pathway-dependent ASIC1a protein degradation and protects against acidosis-induced neurotoxicity. Molecular Neurobiology. 2019;56(5):3326–3340. doi: 10.1007/s12035-018-1289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng A., Lu Y., Huang Q., Zuo Z. Attenuating oxygen-glucose deprivation-caused autophagosome accumulation may be involved in sevoflurane postconditioning-induced protection in human neuron-like cells. European Journal of Pharmacology. 2019;849:84–95. doi: 10.1016/j.ejphar.2019.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan L., Chen S., Li K., et al. Autophagosome maturation mediated by Rab7 contributes to neuroprotection of hypoxic preconditioning against global cerebral ischemia in rats. Cell Death & Disease. 2017;8(7, article e2949) doi: 10.1038/cddis.2017.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou T., Liang L., Liang Y., Yu T., Zeng C., Jiang L. Mild hypothermia protects hippocampal neurons against oxygen-glucose deprivation/reperfusion-induced injury by improving lysosomal function and autophagic flux. Experimental Cell Research. 2017;358(2):147–160. doi: 10.1016/j.yexcr.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 89.Su J., Zhang T., Wang K., Zhu T., Li X. Autophagy activation contributes to the neuroprotection of remote ischemic perconditioning against focal cerebral ischemia in rats. Neurochemical Research. 2014;39(11):2068–2077. doi: 10.1007/s11064-014-1396-x. [DOI] [PubMed] [Google Scholar]
- 90.Tao J., Liu W., Shang G., et al. MiR-207/352 regulate lysosomal-associated membrane proteins and enzymes following ischemic stroke. Neuroscience. 2015;305:1–14. doi: 10.1016/j.neuroscience.2015.07.064. [DOI] [PubMed] [Google Scholar]
- 91.Zhan R., Zhao M., Zhou T., et al. Dapsone protects brain microvascular integrity from high-fat diet induced LDL oxidation. Cell Death & Disease. 2018;9(6):p. 683. doi: 10.1038/s41419-018-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gu W. W., Lu S. Q., Ni Y., et al. 2-(3′,5′-Dimethoxybenzylidene) cyclopentanone, a novel synthetic small-molecule compound, provides neuroprotective effects against ischemic stroke. Neuroscience. 2016;316:26–40. doi: 10.1016/j.neuroscience.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 93.Xu M., Yang L., Rong J. G., et al. Inhibition of cysteine cathepsin B and L activation in astrocytes contributes to neuroprotection against cerebral ischemia via blocking the tBid-mitochondrial apoptotic signaling pathway. Glia. 2014;62(6):855–880. doi: 10.1002/glia.22645. [DOI] [PubMed] [Google Scholar]
- 94.Zhou X. Y., Luo Y., Zhu Y. M., et al. Inhibition of autophagy blocks cathepsins-tBid-mitochondrial apoptotic signaling pathway via stabilization of lysosomal membrane in ischemic astrocytes. Cell Death & Disease. 2017;8(2, article e2618) doi: 10.1038/cddis.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Y., Wang J., Song X., et al. Protective mechanisms of CA074-me (other than cathepsin-B inhibition) against programmed necrosis induced by global cerebral ischemia/reperfusion injury in rats. Brain Research Bulletin. 2016;120:97–105. doi: 10.1016/j.brainresbull.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Li X., Wang M. H., Qin C., Fan W. H., Tian D. S., Liu J. L. Fingolimod suppresses neuronal autophagy through the mTOR/p70S6K pathway and alleviates ischemic brain damage in mice. PLoS One. 2017;12(11, article e0188748) doi: 10.1371/journal.pone.0188748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu Y., Wu X., Pu J., et al. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochemical and Biophysical Research Communications. 2018;495(1):1187–1194. doi: 10.1016/j.bbrc.2017.11.165. [DOI] [PubMed] [Google Scholar]
- 98.Ding C., Zhang J., Li B., et al. Cornin protects SH‑SY5Y cells against oxygen and glucose deprivation-induced autophagy through the PI3K/Akt/mTOR pathway. Molecular Medicine Reports. 2018;17(1):87–92. doi: 10.3892/mmr.2017.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu K., Wang H. R., Ge X. L., et al. Hyperbaric oxygen protects against cerebral damage in permanent middle cerebral artery occlusion rats and inhibits autophagy activity. Neurocritical Care. 2019;30(1):98–105. doi: 10.1007/s12028-018-0577-x. [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y. M., Gao X., Ni Y., et al. Sevoflurane postconditioning attenuates reactive astrogliosis and glial scar formation after ischemia-reperfusion brain injury. Neuroscience. 2017;356:125–141. doi: 10.1016/j.neuroscience.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Dikic I. Proteasomal and autophagic degradation systems. Annual Review of Biochemistry. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 102.Herhaus L., Dikic I. Expanding the ubiquitin code through post-translational modification. EMBO Reports. 2015;16(9):1071–1083. doi: 10.15252/embr.201540891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Welchman R. L., Gordon C., Mayer R. J. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nature Reviews Molecular Cell Biology. 2005;6(8):599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 104.Korolchuk V. I., Menzies F. M., Rubinsztein D. C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Letters. 2010;584(7):1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 105.Liu H., Li W., Ahmad M., et al. Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons. Neurotoxicity Research. 2013;24(2):191–204. doi: 10.1007/s12640-013-9377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quiroga C., Gatica D., Paredes F., et al. Herp depletion protects from protein aggregation by up-regulating autophagy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833(12):3295–3305. doi: 10.1016/j.bbamcr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Yoshii S. R., Kishi C., Ishihara N., Mizushima N. Parkin mediates proteasome-dependent protein degradation and rupture of the outer mitochondrial membrane. The Journal of Biological Chemistry. 2011;286(22):19630–19640. doi: 10.1074/jbc.M110.209338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindsten K., Menéndez-Benito V., Masucci M. G., Dantuma N. P. A transgenic mouse model of the ubiquitin/proteasome system. Nature Biotechnology. 2003;21(8):897–902. doi: 10.1038/nbt851. [DOI] [PubMed] [Google Scholar]
- 109.Li L., Wang Z. V., Hill J. A., Lin F. New autophagy reporter mice reveal dynamics of proximal tubular autophagy. Journal of the American Society of Nephrology. 2014;25(2):305–315. doi: 10.1681/ASN.2013040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McWilliams T. G., Prescott A. R., Allen G. F. G., et al. mito-QC illuminates mitophagy and mitochondrial architecture in vivo. The Journal of Cell Biology. 2016;214(3):333–345. doi: 10.1083/jcb.201603039. [DOI] [PMC free article] [PubMed] [Google Scholar]