ABSTRACT
Background
Diets higher in protein have been reported to improve age-related changes in body composition via increased energy expenditure, shifts in substrate oxidation (SO), and decreased appetite. However, how protein source (e.g., animal compared with plant protein) affects energy expenditure, appetite, and food intake as we age is unknown.
Objectives
The objective of this study was to evaluate the effect of protein source as part of a high-protein breakfast on appetite, food intake, energy expenditure, and fat oxidation in young men (YM) compared with older men (OM).
Methods
This study used a randomized, single-blinded crossover design, with a 1-wk washout period between testing days. Fifteen YM (mean ± SD age: 25.2 ± 2.8 y) and 15 OM (67.7 ± 4.5 y), healthy adults, participated in the study. Participants arrived fasted and consumed an isocaloric, volume-matched, high-protein (40-g) test beverage made with either an animal [whey protein isolate (WPI)] or plant [pea protein isolate (PPI)] protein isolate source. Markers of appetite and energy expenditure were determined at baseline and over 4 h postprandial.
Results
There was a significant effect of time, age, and protein source on appetite (P < 0.05). There was no effect of protein source on plasma markers of appetite, food intake, energy expenditure, and SO. After controlling for body weight, OM had decreased energy expenditure (P < 0.05) and lower fat oxidation (P < 0.001) compared with YM.
Conclusions
This study indicates that a high-protein breakfast containing WPI or PPI exerts comparable effects on appetite, energy expenditure, and 24-h energy intake in both young and older healthy adult men.
This trial was registered at clinicaltrials.gov as NCT03399812.
Keywords: aging, whey protein, pea protein, protein source, energy expenditure, appetite, food intake, body composition
Introduction
Life expectancy continues to increase in the United States and adults 65 y of age and older are projected to more than double from 600 million to 1.6 billion worldwide between 2015 and 2050 (1). Successful aging is commonly defined by high levels of physiological function (2), which is strongly associated with body composition, strength, and appetite (3, 4). Skeletal muscle mass and strength begin to decrease in the third decade of life and these losses are accelerated in the sixth decade of life (5). In the midst of skeletal muscle loss, older adults commonly experience concurrent fat mass gain (6). These shifts in body composition are often accompanied by changes in energy homeostasis via decreased energy expenditure (7), shifts in substrate oxidation (SO) (8), and decreases in appetite (9). Age-related shifts in appetite contribute to energy imbalance and altered body composition often observed with age (10). Age-related decreases in appetite are largely attributed to alterations in appetite hormones (11), changes in gastrointestinal motility (12), and losses in lean body mass (13–15). Research suggests nutritional strategies focused on higher-protein diets containing high-quality proteins are a potential way to mitigate the decrease in energy expenditure and change in body composition observed with age (6).
Dietary patterns promoting plant-based protein have gained significant attention in recent years (16). However, studies examining the effect of plant-based protein sources compared with animal-based protein sources on markers of appetite, energy expenditure, and markers of metabolism offer conflicting results (17–20). For example, high-protein meals containing varying protein sources have been shown to influence appetite differently (18, 21, 22), albeit previous work from our laboratory did not see a difference in postprandial appetite responses in participants consuming an animal protein– compared with a plant protein–based breakfast (17).
Although research exists comparing the effects of protein source on appetite and energy expenditure in healthy young adults, there are scarce data looking at the effect of animal and plant protein sources on energy expenditure, appetite, and food intake in young men (YM) compared with older men (OM). Therefore, the primary objective of this study was to compare the acute effects of a high-protein breakfast containing either animal protein or plant protein on energy expenditure, appetite, and food intake in YM compared with OM. Whey protein isolate (WPI) was used as the animal protein source because of the high concentration of branched-chain amino acids (leucine, isoleucine, and valine) and its ability to increase satiety in response to a mixed meal (23). Pea protein isolate (PPI) was used as the plant protein source because of its complete amino acid profile and its potential to suppress appetite compared with animal proteins (24).
Methods
Participants and ethical approval
From December 2017 to May 2018, YM between 18 and 29 y of age and OM 60–85 y of age were recruited to participate in this study (NCT03399812). Participants were recruited from the Northwest Arkansas area via the daily University of Arkansas digital newsletter, flyers throughout the community, word-of-mouth, and social media to participate in this study. The initial screening was carried out via phone interview. Participants who consumed protein-related supplements, did not regularly consume breakfast (<5 times/wk), smoked, had dietary restrictions, disliked chocolate, were actively trying to lose weight, participated in vigorous activity for ≥4 h/wk, were competitive athletes, had any pre-existing metabolic conditions (e.g., type 1 or 2 diabetes, cancer, cardiovascular disease), were taking medications that would influence protein or energy metabolism, were claustrophobic, and/or were uncomfortable with needles were excluded from participating in the study.
Sixty-one men underwent an initial screening, 17 YM and 20 OM met the screening criteria, and 15 YM and 15 OM completed all study procedures (May 2018). Of those who did not complete the study, participants dropped out because of claustrophobia under the metabolic canopy hood, time constraints, and personal reasons. Each individual agreed to participate by signing the study consent form, then completed 2 test days and an additional final body composition assessment. Written consent was obtained from participants before starting the study. Ethical approval for the study protocol was given by the Office of Research Compliance Institutional Review Board of the University of Arkansas (Fayetteville, AR).
Study design
The study was conducted as a single-blinded randomized crossover design study in which each participant was allocated to the YM (18–29 y of age; n = 15) or OM (60–85 y of age; n = 15) intervention group. Refer to Table 1 for participant characteristics. On the 2 test days, the participants arrived fasted (10–12 h) at the Center for Human Nutrition at the University of Arkansas before 08:00 for data collection. Each participant followed a randomized crossover comparison design as they received both breakfast beverages, WPI and PPIon subsequent test days with each participant serving as their own control. A 1-wk washout period separated the test days. Refer to Figure 1 for study design.
TABLE 1.
Baseline characteristics of the study population by age group1
| Characteristic | Young (n = 15) | Older (n = 15) | P value |
|---|---|---|---|
| Age, y | 25.2 ± 2.8 | 67.65 ± 4.5 | <0.0001**** |
| Anthropometrics | |||
| Height, m | 1.80 ± 0.1 | 1.81 ± 0.1 | 0.59 |
| Weight, kg | 78.4 ± 11.3 | 88.9 ± 10.4 | 0.01* |
| BMI, kg/m2 | 25.1 ± 3.3 | 27.9 ± 3.0 | 0.02* |
| DXA | |||
| Total body fat mass, kg | 17.5 ± 6.4 | 26.3 ± 9.8 | 0.01** |
| Body fat, % | 23.5 ± 7.8 | 30.5 ± 9.7 | 0.04* |
| Total lean mass, kg | 57.6 ± 11.1 | 58.3 ± 7.0 | 0.84 |
| Total fat-free mass, kg | 60.9 ± 11.6 | 58.0 ± 16.6 | 0.59 |
| Fat-to-lean ratio (total fat mass/total lean mass) | 0.32 ± 0.1 | 0.46 ± 0.2 | 0.03* |
| Ethnicity2 | |||
| American Asian/Asian | 4 of 15 | — | |
| Indian | 1 of 15 | — | |
| Caucasian | 10 of 15 | 15 of 15 | |
Values are means ± SDs unless otherwise indicated. *,**,****Significant differences: *P < 0.05; **P < 0.01; ****P < 0.0001.
Ethnicity is expressed as number of participants within age group.
FIGURE 1.
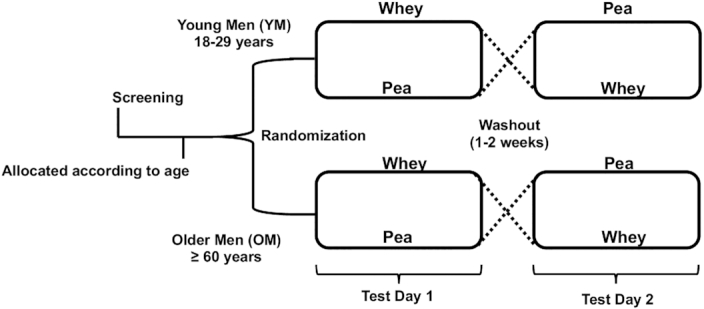
Randomized, controlled, single-blinded study design.
Upon arrival, anthropometrics were recorded and an intravenous catheter was inserted into an antecubital arm vein. Fasting measurements of subjective appetite via visual analog scales (VASs) (25), resting energy expenditure (REE) and SO via indirect calorimetry (26), and venous blood via an intravenous catheter were collected before the consumption of the protein-based breakfast test beverage. Participants were then served 1 of 2 breakfast test beverages. Each protein-based breakfast test beverage was served with a straw inserted into an opaque disposable cup and lid to prevent visual and olfactory influence. Participants consumed the protein-based breakfast test beverage during the next 10 min. The cups were evaluated by research staff to confirm the contents were fully consumed. Subsequently, the participants completed VASs on subjective appetite and the palatability of the protein-based breakfast test beverage. Assessment of subjective appetite using a VAS was repeated at 30, 60, 90, 120, 180, and 240 min after the ingestion of the protein-based breakfast test beverage. REE, thermic effect of feeding (TEF), and SO via indirect calorimetry were measured at 30, 60, 120, 180, and 240 min after the ingestion of the protein-based breakfast test beverage. In addition, 10 mL blood were collected via a syringe from an intravenous catheter at 30, 60, 90, 120, and 240 min after the ingestion of the protein-based breakfast test beverage. At the conclusion of the 4-h test day, a 24-h food log was administered and detailed instructions were given to participants to record their food intake until 11:59 p.m.
Dietary intervention
The protein-based breakfast test beverage contained 40 g dietary supplementary chocolate WPI or chocolate PPI. The WPI (BiPRO; Davisco Foods International) and PPI (NOW Foods—sourced from yellow peas, Lathyrus aphaca species) were commercially purchased. The test beverages were isocaloric, volume matched, and macronutrient matched (refer to Table 2 for nutrient composition of the test beverages). Supplemental Table 1 lists the amino acid profile of the test beverages.
TABLE 2.
Ingredient composition, macronutrient profile, palatability, and viscosity of breakfast test beverages1
| WPI | PPI | |
|---|---|---|
| Ingredient composition | ||
| Protein isolate, g | 50.00 | 73.33 |
| Cane sugar, g | 13.00 | — |
| Canola oil, g | 0.75 | — |
| Inulin, g | 3.60 | — |
| Water, mL | 350.00 | 350.00 |
| Nutrient profile | ||
| Calories, kcal | 265.8 | 263.8 |
| Protein, g | 40.0 | 40.0 |
| Carbohydrate, g | 15.0 | 15.0 |
| Fiber, g | 3.6 | 3.3 |
| Fat, g | 4.4 | 4.2 |
| Palatability,2 mm | 56.2 ± 16.6 | 37.9 ± 17.9* |
| Viscosity, cP | 62.5 | 10,500.0* |
*Significant difference: *P < 0.05. cP, centipoise; PPI, pea protein isolate; WPI, whey protein isolate.
Palatability is expressed as means ± SDs. Palatability measurements were collected from participants at time point 15 min.
Palatability of the test beverages was measured using VASs. Viscosity was measured using a Brookfield Synchro-Lectric Viscometer (Brookfield Engineering Laboratories, Inc.). Viscosity of the pea and whey protein drinks was measured at ambient conditions in separate 16-oz (473.2 mL). opaque serving containers. Samples were thoroughly mixed immediately before measurement. The viscosity samples were measured after the immersion of the spindle and a minimum of 5 revolutions. When the motor was activated, the spindle rotated at a constant speed of 4 rpm. Table 2 presents the palatability and viscosity of the protein-based breakfast test beverages.
Anthropometric measurements
Height was measured to the nearest 0.01 cm using a standard stadiometer (Detecto) without shoes, in the free-standing position. Body weight was measured to the nearest 0.05 kg using a calibrated scale (Detecto) in the fasted state. Body composition was determined using DXA analysis (Lunar Prodigy, GE Healthcare) at the Exercise Science Research Center at the University of Arkansas.
Appetite response
Subjective appetite and palatability were assessed using a traditional 100-mm VAS (25) with opposing anchors at 0, 15, 30, 60, 90, 120, 180, and 240 min postprandial. Participants were asked to place an “X” on the 100-mm VAS that most accurately reflected their perceived feeling of appetite according to a series of 7 questions (e.g., “How HUNGRY do you feel at this moment” and “How FULL do you feel at this moment”).
Dietary records and assessment
Participants completed a total of two 24-h food logs, 1 after each test day. The energy and macronutrient composition of the breakfast test beverages and the remaining 24 h of the test day were analyzed using the Genesis R&D nutrient analysis software package (version 9.10.2; ESHA Research).
Energy expenditure and SO
REE (kcal/min), TEF (kcal/min), and SO (kcal/min) were measured by indirect calorimetry using the validated (26) ventilated hood technique with the TrueOne 2400 metabolic cart (Parvo Medics) (27).
Plasma biomarkers
Six blood samples (10 mL/sample, 60 mL/testing day) were collected after a 10- to 12-h fast and during the 4-h postprandial meal time response period. The samples were collected in EDTA-coated vacutainer tubes. Samples were immediately centrifuged at 4°C for 15 min at 1800 × g. The plasma was separated and stored at −80°C until analysis. Plasma glucose (mg/dL), cholecystokinin (CCK) (pg/mL), and peptide YY (PYY) (pg/mL) concentrations were determined via colorimetry (Cayman Chemical Company) and enzyme immunoassay (RayBiotech, Inc.) using commercially available kits as per the manufacturer's instructions.
Statistical analysis
Summary statistics were calculated for all data and data are expressed as means ± SDs. Two-sample independent t tests were used to analyze baseline measurements of participant characteristics and body composition. The 2-factor repeated-measures design was analyzed as a generalized linear mixed model with protein source and age as fixed effects and subjects as a random effect nested within age categories. Appetite ratings, REE, SO, food intake, and metabolic biomarker concentrations (glucose, PYY, and CCK) that could only take on positive values were assumed to follow a γ distribution. TEF was analyzed as a proportion and was assumed to follow a β distribution. For appetite ratings, energy expenditure, SO, and plasma markers of glucose, PYY, and CCK there was a third main effect of time. In our model we analyzed main effects of time, age, and protein source. Where appropriate, 2-way and 3-way interactions of age × protein source, age × time, and protein source × time and age × protein source × time, respectively, were tested for significance. Where appropriate, follow-up least-squares mean comparisons for protein source, age, and time main effects were declared significantly different if the corresponding ANOVA F statistic was significant. For any significant interactions, mean comparisons were carried out using the protected least significant difference (LSD). Subjective rating of palatability was analyzed as a generalized linear mixed model with protein source and age as fixed effects and subjects as a random effect nested within age categories without repeated measures. Viscosity of the test beverages was analyzed using independent t tests. Net incremental AUC (niAUC) was calculated for appetite ratings, REE, TEF, SO, and metabolic biomarker concentrations. Where significance was found, follow-up least-squares mean comparisons for protein source and age categories was conducted. For any significant interactions, mean comparison was carried out using the protected LSD. Statistical analyses involving generalized linear mixed models were performed using PROC GLIMMIX in SAS version 9.4 (SAS Institute, Cary, NC). All graphs were made using GraphPad Prism Software version 7.0 (GraphPad Software, San Diego, CA). P < 0.05 was considered significant. To verify the appropriateness of the sample sizes we carried out a post hoc power analysis using the SAS procedure PROC POWER with the paired t test option. The observed sample means and SDs were used to determine that 15 participants per group had a statistical power of 0.987 (based on an overall level of significance of 0.05) to detect an accurate postprandial difference in TEF after supplementation of WPI and PPI protein-based breakfast test beverages.
Results
Participant characteristics
Table 1 presents the demographics and physical characteristics of the participants who completed the study. The YM and OM had a mean ± SD age of 25.2 ± 2.8 y and 67.7 ± 4.5 y, respectively (P < 0.0001). There were significant differences in fat mass (P < 0.01), body fat percentage (P < 0.05), and fat-to-lean ratio (P < 0.05) between groups with no significant differences in lean body mass and fat-free mass.
Energy expenditure and SO
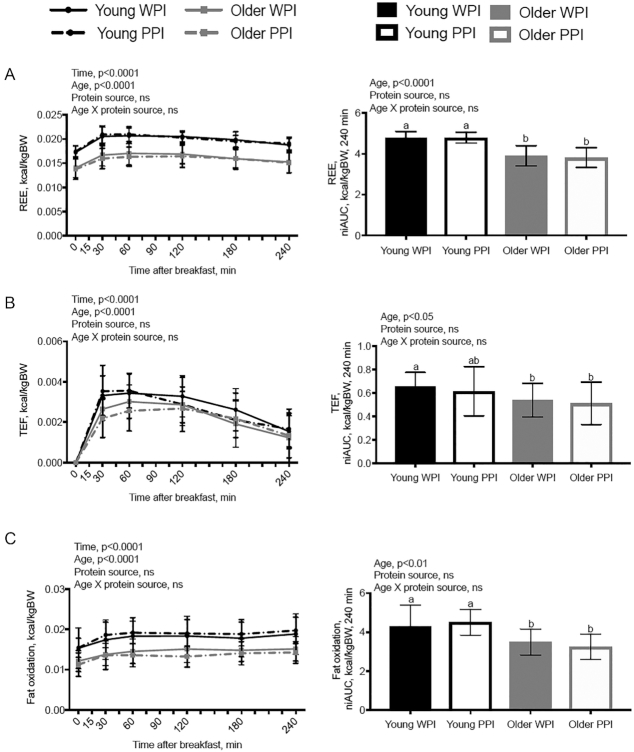
Results for energy expenditure and SO are presented in the line (individual time points) and bar graphs (niAUC) in Figure 2. After controlling for body weight (kg), there was a significant effect of age (P < 0.0001) and time (P < 0.0001) on REE (kcal/min), TEF (kcal/min), and fat oxidation (kcal/min) with no effect of protein source. There was an effect of age on REE, TEF, and fat oxidation with YM having significantly higher REE (P < 0.0001), TEF (P < 0.05), and fat oxidation (P < 0.01) than OM. There was no effect of age or protein source on carbohydrate oxidation (Supplemental Figure 1). There was a significant age × time interaction on TEF (kcal/min) (P < 0.01). All other 2- and 3-way interactions of REE, TEF, and SO were nonsignificant.
FIGURE 2.
Energy expenditure and substrate oxidation after ingestion of either a WPI-based or PPI-based breakfast test beverage in young men (n = 15) or older men (n = 15) using indirect calorimetry. (A) REE over time and niAUC. (B) Postprandial energy expenditure (TEF) over time and niAUC. (C) Fat oxidation over time and niAUC. Data are shown as means ± SDs and controlled for body weight in kilograms. Means without a common letter are significantly different (P < 0.05). niAUC, net incremental AUC; PPI, pea protein isolate; REE, resting energy expenditure; TEF, thermic effect of feeding; WPI, whey protein isolate.
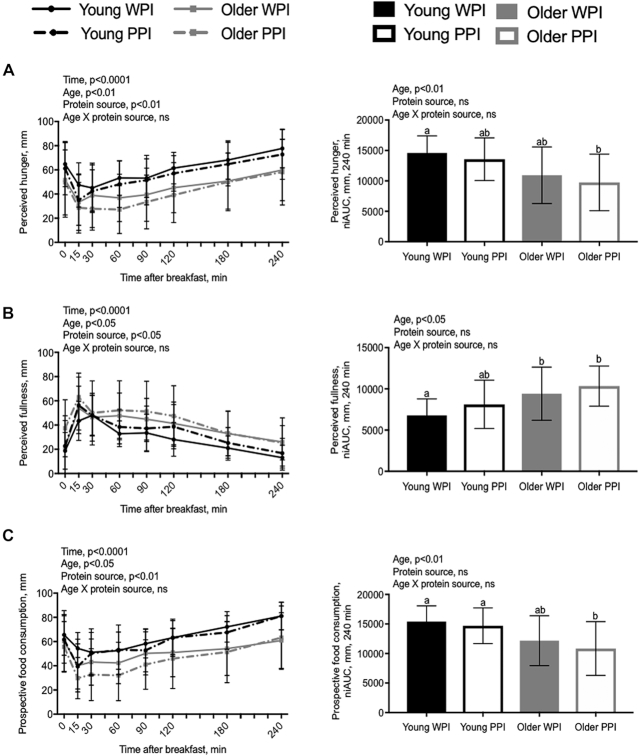
Subjective appetite and palatability
Figure 3 presents results for perceived hunger, perceived fullness, prospective food consumption (PFC), and perceived desire to eat. Fasting values of perceived hunger, fullness, PFC, and desire to eat were not significantly different between the YM and OM when consuming either of the protein-based breakfast test beverages. There was a significant effect of time, age, and protein source on subjective hunger (P < 0.01), fullness (P < 0.01), PFC (P < 0.01), desire to eat (P < 0.01), and desire for a snack (P < 0.05). There was a significant interaction effect of age × time (P < 0.01) and protein source × time (P < 0.05) on desire for a snack. All other interactions of age × protein source, age × time, protein source × time, and age × protein source × time were nonsignificant. There were no significant differences in the effect of desire for something sweet on time, age, or protein source (Supplemental Figure 2). However, there was a significant effect of age on the desire for something salty (P < 0.001) (Supplemental Figure 2). Palatability was higher for the WPI than for the PPI protein-based breakfast test beverage (P < 0.01), with no significant difference between age groups (Table 2).
FIGURE 3.
Ratings of perceived appetite assessment after ingestion of either a WPI-based or PPI-based breakfast test beverage in young men (n = 15) or older men (n = 15) using visual analog scales. (A) Perceived hunger over time and niAUC. (B) Perceived fullness over time and niAUC. (C) Perceived prospective food consumption over time and niAUC. Data are shown as means ± SDs. Means without a common letter are significantly different (P < 0.05). niAUC, net incremental AUC; PPI, pea protein isolate; WPI, whey protein isolate.
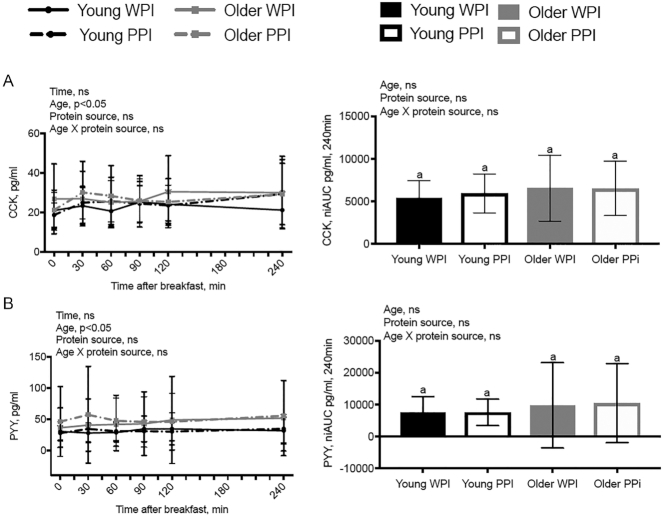
Plasma biomarkers
Figure 4 depicts the plasma CCK and PYY responses to the breakfast test beverage. There was an effect of age (P < 0.05), but not protein source, with OM having higher concentrations of all tested biomarkers. There was a significant time × age interaction on glucose (P < 0.05) (Supplemental Figure 3) with no significant effect of age × time × protein source. All other interactions of age × time, protein source × time, and age × protein source × time for plasma glucose, CCK, and PYY were nonsignificant.
FIGURE 4.
PYY and CCK responses after ingestion of either a WPI-based or PPI-based breakfast test beverage in young men (n = 15) or older men (n = 15). (A) CCK response over time and niAUC. (B) PYY response over time and niAUC. Data are shown as means ± SDs. Means without a common letter are significantly different (P < 0.05). CCK, cholecystokinin; niAUC, net incremental AUC; PPI, pea protein isolate; PYY, postprandial peptide YY; WPI, whey protein isolate.
Twenty-four-hour dietary assessment
Table 3 shows the 24-h energy and macronutrient intakes. No significant differences were observed in 24-h total food intake between either protein source or age groups.
TABLE 3.
Twenty-four-hour energy and macronutrient intakes after consumption of breakfast test beverages1
| Young | Older | |||
|---|---|---|---|---|
| WPI (n = 15) | PPI (n = 15) | WPI (n = 15) | PPI (n = 15) | |
| Calories, kcal | 2248.6 ± 703.0 | 2328.6 ± 903.7 | 2078.0 ± 542.3 | 2120.7 ± 850.1 |
| Protein, g | 129.4 ± 44.9 | 141.4 ± 51.4 | 117.9 ± 26.3 | 115.7 ± 29.5 |
| Fat, g | 88.7 ± 40.1 | 87.1 ± 53.8 | 80.1 ± 31.2 | 80.0 ± 43.7 |
| Carbohydrate, g | 236.5 ± 73.1 | 244.9 ± 82.7 | 217.7 ± 87.1 | 211.0 ± 91.9 |
| Sugar, g | 73.5 ± 26.7 | 64.0 ± 26.7 | 94.5 ± 52.2 | 64.5 ± 40.4 |
| Fiber, g | 21.4 ± 7.6 | 22.3 ± 9.2 | 19.5 ± 5.3 | 21.1 ± 11.1 |
| Sodium, mg | 3934.3 ± 1937.8 | 3895.0 ± 1482.4 | 2577.3 ± 1329.8 | 3611.3 ± 1976.9 |
| Protein, % | 23.2 ± 1 | 24.97 ± 1 | 23.4 ± 1 | 24.1 ± 1 |
| Carbohydrate, % | 43.3 ± 1 | 43.9 ± 1 | 41.3 ± 1 | 40.0 ± 1 |
| Fat, % | 34.1 ± 1 | 32.3 ± 1 | 34.5 ± 1 | 32.9 ± 0 |
Values are means ± SDs. PPI, pea protein isolate; WPI, whey protein isolate.
Discussion
To our knowledge, this is the first study to examine the short-term effect of a high-protein breakfast from plant- or animal-derived protein sources on energy expenditure and appetite response in healthy YM and OM. The present study tested the hypothesis that WPI, when compared with PPI, would have a greater effect on energy expenditure and appetite in OM than in YM when supplemented as a 40-g protein-based breakfast beverage. Collectively, the results of this study suggest that age, not protein source, affects postprandial energy expenditure and appetite responses.
A breakfast containing high-protein foods has been shown to increase energy expenditure and fat oxidation in healthy, young adults (18, 27). However, the impact of protein source as part of a high-protein breakfast on energy expenditure and fat oxidation in aging adults still needs to be established. For example, consumption of whey, casein, and soy protein-based beverages compared with a carbohydrate-based control beverage increased TEF and fat oxidation in YM over a 5-h period (18). One likely mechanism for the increase in TEF could be due to protein turnover and the favoring of protein synthesis or deamination and urea synthesis associated with protein breakdown (28). However, in this clinical trial, we did not observe any differences between protein source with respect to energy expenditure and SO. This may have been due to the 40 g protein used in the breakfast test beverages, which was a larger dose than the doses used in other studies demonstrating differences in energy metabolism between protein sources (18, 29).
The majority of clinical trials investigating the short-term effect of animal- and plant-based proteins on appetite and food intake have used soy as the plant-based protein source (20, 30), whey as the animal-based protein source (18, 31, 32), or a complete mixed meal (30, 33–35). In agreement with our study, Diepvens et al. (21), investigating the effects of 15 g protein sourced from either WPI, PPI, or a combination of WPI and PPI on appetite, postprandial changes in satiety hormones, and energy intake, found that the pea protein resulted in a modest increase in satiety, with no differences in energy intake. In addition, a randomized single-blind crossover study investigating the role of a meal preload of 20 g casein, whey, pea protein, egg albumin, or maltodextrin compared with water found that casein and pea protein increased satiety significantly more than did the other sources of protein (24). In contrast, casein and pea protein also lowered energy intake, albeit food intake was recorded 30 min after the meal preload.
There are a limited number of studies investigating the differences in energy expenditure and SO between protein sources. In 1 study, 3 isoenergetic 30% protein test meals using meat, dairy, and soy protein sources found no significant differences in energy expenditure, carbohydrate oxidation, or fat oxidation between test meals (30), similar to the results found in this study. In contrast, a second study tested 3 meals with 50% protein coming from either whey, casein, or soy protein and found that TEF and fat oxidation were greater after the consumption of the whey protein meal (18).
To our knowledge, this is the first short-term meal response study to demonstrate the effect of WPI and PPI on energy expenditure and appetite in YM compared with OM at breakfast. However, there are several limitations to this study. This study had strict inclusion and exclusion criteria and we only recruited healthy YM and OM, which could be the reason that there was no difference in lean or fat-free mass between the YM and OM. Women were excluded from this study, which means the results may not apply to the overall population. The sample size, although powered correctly, was small. The breakfast test beverages varied in viscosity, which may have contributed to differences seen in participant appetite response (36). The test beverages also varied in palatability despite controlling for nutrient content and sensory properties of smell and sight, which may have influenced appetite (37). We also relied on self-reported 24-h food intake for the 24-h dietary assessment, which may provide inaccurate measurements of food intake (38). In addition, we did not provide the pea protein and whey protein in a mixed-meal context. Therefore, the results cannot be directly translated into a plant-based or animal-based protein complete diet. Finally, there was a racial imbalance in the young compared with the older participants. The 15 OM were Caucasian, whereas the YM were Caucasian, Indian, and American Asian/Asian. However, because this was a crossover design the racial imbalance was unlikely to affect our primary outcomes.
In conclusion, isocaloric, isovolumetric, macronutrient- and fiber-matched protein-based breakfast beverages from an animal-based WHI and a plant-based PPI exert comparable effects on appetite, energy expenditure, and 24-h energy intake in both young and older healthy adult men.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JIB and ALH: conceptualized and designed the study and drafted and critically reviewed the manuscript; ALH, AMT, SW, AM, and RB: conducted the intervention; EG: was responsible for the statistical analysis; and all authors: read and approved the final manuscript.
Notes
Supported by the Arkansas Biosciences Institute (to JIB); a grant from the Honors College at the University of Arkansas, Fayetteville; and a USDA National Institute of Food and Agriculture Hatch project.
Author disclosures: the authors report no conflicts of interest.
The funding agencies had no role in the design, analysis, or writing of this article.
Supplemental Figures 1–3 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: CCK, cholecystokinin; LSD, least significant difference; niAUC, net incremental AUC; OM, older men; PFC, prospective food consumption; PPI, pea protein isolate; PYY, peptide YY; REE, resting energy expenditure; SO, substrate oxidation; TEF, thermic effect of feeding; VAS, visual analog scale; WPI, whey protein isolate; YM, young men.
References
- 1. Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA. The population 65 years and older in the United States: 2016. US Department of Commerce, editor. Washington (DC): United States Census Bureau; 2018. [Google Scholar]
- 2. Cosco TD, Prina AM, Perales J, Stephan BC, Brayne C. Operational definitions of successful aging: a systematic review. Int Psychogeriatr. 2014;26:373–81. [DOI] [PubMed] [Google Scholar]
- 3. Donini LM, Savina C, Cannella C. Eating habits and appetite control in the elderly: the anorexia of aging. Int Psychogeriatr. 2003;15:73–87. [DOI] [PubMed] [Google Scholar]
- 4. Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Y et al.. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev. 2015;24:304–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D et al.. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018;13:913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baum JI, Wolfe RR. The link between dietary protein intake, skeletal muscle function and health in older adults. Healthcare (Basel). 2015;3:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–5. [DOI] [PubMed] [Google Scholar]
- 8. Gheller BJ, Riddle ES, Lem MR, Thalacker-Mercer AE. Understanding age-related changes in skeletal muscle metabolism: differences between females and males. Annu Rev Nutr. 2016;36:129–56. [DOI] [PubMed] [Google Scholar]
- 9. Pilgrim AL, Robinson SM, Sayer AA, Roberts HC. An overview of appetite decline in older people. Nurs Older People. 2015;27:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli S et al.. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. [DOI] [PubMed] [Google Scholar]
- 11. Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86:651–67. [DOI] [PubMed] [Google Scholar]
- 12. Serra-Prat M, Mans E, Palomera E, Clave P. Gastrointestinal peptides, gastrointestinal motility, and anorexia of aging in frail elderly persons. Neurogastroenterol Motil. 2013;25:291–e245. [DOI] [PubMed] [Google Scholar]
- 13. Blundell JE, Finlayson G, Gibbons C, Caudwell P, Hopkins M. The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake?. Physiol Behav. 2015;152:473–8. [DOI] [PubMed] [Google Scholar]
- 14. Blundell JE, Caudwell P, Gibbons C, Hopkins M, Naslund E, King NA, Finlayson G. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–9. [DOI] [PubMed] [Google Scholar]
- 15. Hopkins M, Finlayson G, Duarte C, Whybrow S, Ritz P, Horgan GW, Blundell JE, Stubbs RJ. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int J Obes (Lond). 2016;40:312–8. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services (US HHS) and USDA. 2015–2020 Dietary Guidelines for Americans. 8th ed Washington (DC): US HHS and USDA; 2015. [Google Scholar]
- 17. Crowder CM, Neumann BL, Baum JI. Breakfast protein source does not influence postprandial appetite response and food intake in normal weight and overweight young women. J Nutr Metab. 2016:6265789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Acheson KJ, Blondel-Lubrano A, Oguey-Araymon S, Beaumont M, Emady-Azar S, Ammon-Zufferey C, Monnard I, Pinaud S, Nielsen-Moennoz C, Bovetto L. Protein choices targeting thermogenesis and metabolism. Am J Clin Nutr. 2011;93:525–34. [DOI] [PubMed] [Google Scholar]
- 19. Hochstenbach-Waelen A, Veldhorst MA, Nieuwenhuizen AG, Westerterp-Plantenga MS, Westerterp KR. Comparison of 2 diets with either 25% or 10% of energy as casein on energy expenditure, substrate balance, and appetite profile. Am J Clin Nutr. 2009;89:831–8. [DOI] [PubMed] [Google Scholar]
- 20. Veldhorst MA, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ, Deutz NE, Westerterp-Plantenga MS. Effects of high and normal soyprotein breakfasts on satiety and subsequent energy intake, including amino acid and ‘satiety’ hormone responses. Eur J Nutr. 2009;48:92–100. [DOI] [PubMed] [Google Scholar]
- 21. Diepvens K, Haberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond). 2008;32:510–18. [DOI] [PubMed] [Google Scholar]
- 22. Veldhorst M, Smeets A, Soenen S, Hochstenbach-Waelen A, Hursel R, Diepvens K, Lejeune M, Luscombe-Marsh N, Westerterp-Plantenga M. Protein-induced satiety: effects and mechanisms of different proteins. Physiol Behav. 2008;94:300–7. [DOI] [PubMed] [Google Scholar]
- 23. Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. [DOI] [PubMed] [Google Scholar]
- 24. Abou-Samra R, Keersmaekers L, Brienza D, Mukherjee R, Macé K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr J. 2011;10:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 26. Cooper JA, Watras AC, O'Brien MJ, Luke A, Dobratz JR, Earthman CP, Schoeller DA. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J Am Diet Assoc. 2009;109:128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neumann BL, Dunn A, Johnson D, Adams JD, Baum JI. Breakfast macronutrient composition influences thermic effect of feeding and fat oxidation in young women who habitually skip breakfast. Nutrients. 2016;8(8):490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morales FEM, Tinsley GM, Gordon PM. Acute and long-term impact of high-protein diets on endocrine and metabolic function, body composition, and exercise-induced adaptations. J Am Coll Nutr. 2017;36:295–305. [DOI] [PubMed] [Google Scholar]
- 29. Bendtsen LQ, Lorenzen JK, Gomes S, Liaset B, Holst JJ, Ritz C, Reitelseder S, Sjödin A, Astrup A. Effects of hydrolysed casein, intact casein and intact whey protein on energy expenditure and appetite regulation: a randomised, controlled, cross-over study. Br J Nutr. 2014;112:1412–22. [DOI] [PubMed] [Google Scholar]
- 30. Tan SY, Batterham M, Tapsell L. Energy expenditure does not differ, but protein oxidation rates appear lower in meals containing predominantly meat versus soy sources of protein. Obes Facts. 2010;3:101–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tahavorgar A, Vafa M, Shidfar F, Gohari M, Heydari I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr Res. 2014;34:856–61. [DOI] [PubMed] [Google Scholar]
- 32. Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A, Phillips SM. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One. 2017;12:e0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Douglas SM, Lasley TR, Leidy HJ. Consuming beef vs. soy protein has little effect on appetite, satiety, and food intake in healthy adults. J Nutr. 2015;145:1010–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nielsen LV, Kristensen MD, Klingenberg L, Ritz C, Belza A, Astrup A, Raben A. Protein from meat or vegetable sources in meals matched for fiber content has similar effects on subjective appetite sensations and energy intake—a randomized acute cross-over meal test study. Nutrients. 2018;10(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kristensen MD, Bendsen NT, Christensen SM, Astrup A, Raben A. Meals based on vegetable protein sources (beans and peas) are more satiating than meals based on animal protein sources (veal and pork) - a randomized cross-over meal test study. Food Nutr Res. 2016;60:32634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camps G, Mars M, de Graaf C, Smeets PA. Empty calories and phantom fullness: a randomized trial studying the relative effects of energy density and viscosity on gastric emptying determined by MRI and satiety. Am J Clin Nutr. 2016;104:73–80. [DOI] [PubMed] [Google Scholar]
- 37. Johnson F, Wardle J. Variety, palatability, and obesity. Adv Nutr. 2014;5:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sørensen TIA, Speakman JR, Jeansonne M, Allison DB; Energy Balance Measurement Working Group. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond). 2015;39:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.