Summary
Background
Closure of an abdominal stoma, a common elective operation, is associated with frequent complications; one of the commonest and impactful is incisional hernia formation. We aimed to investigate whether biological mesh (collagen tissue matrix) can safely reduce the incidence of incisional hernias at the stoma closure site.
Methods
In this randomised controlled trial (ROCSS) done in 37 hospitals across three European countries (35 UK, one Denmark, one Netherlands), patients aged 18 years or older undergoing elective ileostomy or colostomy closure were randomly assigned using a computer-based algorithm in a 1:1 ratio to either biological mesh reinforcement or closure with sutures alone (control). Training in the novel technique was standardised across hospitals. Patients and outcome assessors were masked to treatment allocation. The primary outcome measure was occurrence of clinically detectable hernia 2 years after randomisation (intention to treat). A sample size of 790 patients was required to identify a 40% reduction (25% to 15%), with 90% power (15% drop-out rate). This study is registered with ClinicalTrials.gov, NCT02238964.
Findings
Between Nov 28, 2012, and Nov 11, 2015, of 1286 screened patients, 790 were randomly assigned. 394 (50%) patients were randomly assigned to mesh closure and 396 (50%) to standard closure. In the mesh group, 373 (95%) of 394 patients successfully received mesh and in the control group, three patients received mesh. The clinically detectable hernia rate, the primary outcome, at 2 years was 12% (39 of 323) in the mesh group and 20% (64 of 327) in the control group (adjusted relative risk [RR] 0·62, 95% CI 0·43–0·90; p=0·012). In 455 patients for whom 1 year postoperative CT scans were available, there was a lower radiologically defined hernia rate in mesh versus control groups (20 [9%] of 229 vs 47 [21%] of 226, adjusted RR 0·42, 95% CI 0·26–0·69; p<0·001). There was also a reduction in symptomatic hernia (16%, 52 of 329 vs 19%, 64 of 331; adjusted relative risk 0·83, 0·60–1·16; p=0·29) and surgical reintervention (12%, 42 of 344 vs 16%, 54 of 346: adjusted relative risk 0·78, 0·54–1·13; p=0·19) at 2 years, but this result did not reach statistical significance. No significant differences were seen in wound infection rate, seroma rate, quality of life, pain scores, or serious adverse events.
Interpretation
Reinforcement of the abdominal wall with a biological mesh at the time of stoma closure reduced clinically detectable incisional hernia within 24 months of surgery and with an acceptable safety profile. The results of this study support the use of biological mesh in stoma closure site reinforcement to reduce the early formation of incisional hernias.
Funding
National Institute for Health Research Research for Patient Benefit and Allergan.
Introduction
Stoma closure is a frequent and widely performed operation, with 6295 stoma closures done in England during 2017–18.1 The procedure has a bimodal age incidence, being needed in both young patients with inflammatory bowel disease and in older patients, typically with colorectal cancer. Closure of these wounds is associated with a high risk of complications for patients. Bowel content contamination at the stoma closure site is inevitable, increasing wound infections, which are key risk factors for wound breakdown. This breakdown can create both short-term and long-term morbidity, which can be severe.2 Incisional hernias are a direct consequence of this wound failure, resulting in an accumulating incidence of pain, reoperation, and emergency surgery over many years.
Cohort studies show that at least 30% of patients have a clinically detectable hernia in the first 2 years following stoma closure surgery.3 These hernias are associated with substantial long-term morbidity. Over the years, the need for complex reoperation accumulates, which patients might choose not to undergo until in extremis or until their daily symptoms become too severe; nearly half of patients who develop an incisional hernia at a closed stoma site ultimately require subsequent surgical repair.4 Studying contaminated wound closure (failure) is challenging due to the heterogeneity of wound size and underlying pathology.5 Stoma closure sites can be considered a controlled model for the study of contaminated wound healing.
Research in context.
Evidence before this study
A systematic review of studies reporting on the incidence of incisional hernias at the site of previously closed ileostomy or colostomy identified 34 relevant studies. These were small and heterogeneous studies with short follow-up. The overall reported hernia rate was 7%, but with a wide range among the studies (0–48%). Three studies specifically assessed incisional hernia as a primary endpoint and reported a clinical hernia rate of 30% at 3 years. In 11 studies reporting reoperation rates, 51% (34 of 66) of patients who developed a hernia ultimately required a surgical repair. No randomised trials were identified evaluating prophylactic mesh reinforcement of the stoma closure site to reduce hernia rates.
Added value of this study
In this patient and assessor masked, international, multicentre, randomised controlled trial, we allocated 790 patients in a 1:1 ratio to either biological mesh reinforcement of the stoma closure site or standard closure without mesh. After 2 years of follow-up, the clinically detectable hernia rate was 12% in the mesh repair group versus 20% in the standard repair group, with a relative risk reduction of 38%. The mesh reinforcement technique was delivered with an acceptable safety profile. There were reductions in symptomatic hernia and surgical re-intervention at 2 years, but these did not reach statistical significance.
Implications of all the available evidence
Biological mesh is not currently used on a routine basis for prophylaxis in abdominal wound closure. The results of this study support the use of biological mesh in stoma closure site reinforcement to reduce the early formation of incisional hernias. As time progresses, the prevalence of symptomatic hernias and consequent need for reoperation will rise, increasing the clinical effect of hernia prevention. Data from ROCSS, particularly incorporating longer follow-up and future meta-analyses with ongoing randomised studies will inform international guidelines for prevention of incisional hernia in contaminated wounds. Stoma site closure is an elective procedure, which is a controlled model for contaminated wounds, as it involves uniform wounds that have consistent contamination levels. Evidence of benefit in stoma closure supports the case for evaluation of prophylactic biological mesh in broader groups of patients undergoing high-risk abdominal wound closure, exemplified by patients undergoing emergency laparotomy.
The evidence base for the use of mesh for prophylaxis of incisional hernias is weak when wound contamination with faeces or small bowel contents is substantial, such as that which occurs at stoma closure sites.6, 7 Insertion of prosthetic material in a contaminated field can result in major long-term morbidity by providing a focus for persistent infection.8 Biological mesh, commonly made from collagen, might carry a lower infection risk as it becomes incorporated into host tissue, while still providing reinforcement to wounds at high risk of hernia.9 Some retrospective case-series have suggested that biological meshes can be safely used in complex and contaminated settings;5, 9 high quality or randomised evidence is however absent and so they are not used routinely in this setting.10
Prophylactic biological mesh implantation at time of stoma closure (to prevent incisional hernia) has not been tested in multicentre randomised studies.10 The IDEAL framework describes a pathway for testing surgical devices and innovation.11 We described proof of concept in a single case (stage 1),12 development in a short case series (stage 2A), exploration within a feasibility randomised trial (stage 2B),13 and here describe assessment within a phase 3 randomised controlled trial (stage 3).14 The primary objective of the Reinforcement of Closure of Stoma Site (ROCSS) study was to assess whether the addition of a biological mesh (collagen tissue matrix) reduced the incidence of clinically detectable stoma closure site hernias at 2 years compared with standard closure techniques.
Methods
Study design and participants
The ROCSS trial, a prospective, multicentre, two arm parallel group, randomised controlled trial, with an internal pilot study was done in 37 hospitals (35 in the UK, one in Denmark, and one in the Netherlands). The trial compared biological mesh (non-crosslinked porcine collagen tissue matrix) reinforcement of closure against standard closure techniques (no mesh) in patients undergoing elective stoma closure and was designed by the West Midlands Research Collaborative (Birmingham, UK), a trainee-led research group who have published previously in the design and delivery of randomised controlled trials in surgery,15, 16 with senior support and mentorship from the Birmingham Clinical Trials Unit.
At participating centres, patients aged 18 years or older undergoing elective ileostomy or colostomy closure (loop or end) were eligible for participation. Patients were eligible irrespective of the operative approach that had originally been used to construct the stoma (open or laparoscopic), or the planned operative technique for stoma closure (trephine, midline, or laparoscopic approach). Exclusion criteria were: (1) the surgeon pre-operatively anticipated that a mesh repair would be required, for example if there was a known large parastomal hernia; (2) participation in another clinical trial of surgical technique during the same operation; (3) allergy to porcine or collagen products; (4) history of familial adenomatous polyposis (due to increased risk of cutaneous desmoid tumours, mimicking clinical diagnosis of hernia); and (5) being unable or unwilling to provide written informed consent.
Potentially eligible patients were approached for entry into ROCSS, provided with a patient information sheet and given the opportunity to ask questions. Once eligibility was confirmed, the patient was consented for participation in the trial in the outpatient clinic or following admission for their operation. Consent was obtained by suitably trained and delegated consultant surgeons, surgical registrars, or research nurses. Patients were able to withdraw consent to remain in the trial at any time.
This study is reported in accordance with the guidance set out in the CONSORT statement.15 In the UK, the study was approved by the West Midlands Research Ethics Committee (MREC number: 12/WM/0187), with appropriate ethical approvals in Denmark and the Netherlands.
The study protocol has been previously published.14 A report on the recruitment and safety data for the first 90 patients in the internal pilot has also been published (IDEAL stage 2B).13 This Article reports the results from the phase 3 multicentre randomised controlled trial (IDEAL stage 3), which was undertaken in European centres doing colorectal surgery.
Randomisation and masking
Patients were randomly assigned in a 1:1 ratio to either biological mesh reinforcement of the stoma closure site (experimental group) or standard closure without mesh (control group). Randomisation was done by a ROCSS team member who would not participate in the patient's follow-up for ROCSS. Patients were randomly assigned using a computer-based algorithm with minimisation variables judged likely to influence the rate of clinical herniation: planned surgical incision (reopening of midline wound or trephine only), stoma type (ileostomy or colostomy), and planned skin closure type (primary or secondary). Randomisation was made available to site investigators. Randomisation was recommended to be done as close to the time of skin incision as possible, to optimise allocation concealment and maximise adherence, ensuring availablity of mesh in theatre (for example, randomising a month in advance of the surgery date introduces risks of theatre cancellation and variation in mesh stock). Patients’ randomised allocation was not recorded in their clinical notes to prevent unmasking of outcome assessment teams. The operating surgeon, surgical assistant, and theatre team were aware of the treatment allocation, but the patient and outcome assessors remained masked to treatment allocation.
Procedures
In all patients, prophylactic preoperative antibiotics were given according to local protocol. The ileostomy or colostomy (including bowel, fascia, and skin) was closed in accordance with the surgeon's preferred technique (ie, stapled or hand sewn), choice of suture material, and suture technique.
To be eligible to participate in the trial, the local principal investigator and each participating surgeon was required to have previously done at least 20 stoma closures. Dissemination of the surgical technique was standardised for quality assurance. A mandatory site initiation teleconference with pictorial explanation of the surgical technique was supplemented by a freely available online video demonstration and pictorial aide-memoir (also available on a DVD). To ensure homogeneity of technique, every site was visited by a senior surgeon from the trial management group. During these visits, a demonstration case was undertaken with the local principal investigator and team; a non-trial consented patient underwent standardised mesh placement via a circumstomal approach. The trained site surgeon or surgeons then trained other surgeons participating at their site in the technique. This novel closure technique with biological mesh was not otherwise offered to patients outside of the trial.
For the intervention group, Strattice Reconstructive Tissue Matrix (Allergan, Oxford, UK) was provided for use as the biological mesh for ROCSS, although the specific mesh used was at the operating surgeon's preference. Mesh was stored and handled in accordance with the manufacturer's standard operating procedure. The closure technique for ROCSS was refined and protocolised during IDEAL stage 1.12 This required an intra-abdominal placement, with the mesh fixed within the abdominal cavity, deep to the peritoneum. Anchoring transfascial bites (using 2-0 polydioxanone [PDS], a slowly absorbable suture) were taken circumferentially and the mesh parachuted (see video) into place. A minimum circumferential fascial overlap of 3 cm was required. Once correctly sited, the fascia above was closed using a synthetic, non-absorbable, or slowly absorbable suture according to the surgeon's usual technique. Infiltration of up to 40 mL of local anaesthetic into the fascial layer was permitted. The remainder of the closure was at the surgeon's discretion, including primary (skin edges together) or secondary (skin edges apart) skin closure.
In the control group, surgeons were permitted to undertake their usual closure technique for patients randomly assigned to the control group. The only stipulations were that mesh was not placed and rapidly absorbable sutures (eg, Vicryl [polyglactin 910]) were not used for fascial closure.
For the assessment of clinical hernia, assessors were asked to follow a standardised clinical examination technique for the closed stoma site. The patient was examined in both standing and lying positions. Either the examiner did a Valsalva manoeuvre or the patient made a forceful cough, while the assessor placed a hand over the closed stoma site. The examiner recorded if the patient had either a palpable fascial defect with or without protrusion of bowel or fat or a global weakness around the stoma scar, without palpable fascial defect.
If the assessor was uncertain, a second masked clinician examined the patient and a consensus decision was made.
Outcomes
Outcome data were collected at 30 days postoperatively, and at 1 and 2 years post-randomisation. Clinical follow-up assessments and abdominal wall examinations were done by trained doctors holding Membership of the Royal College of Surgeons or equivalent qualification or an appropriately trained research nurse (assessed and delegated by the local principal investigator) who was masked to treatment allocation.
The primary outcome measure was the rate of clinically detected hernia at 2 years post-randomisation. A clinical hernia was defined as a palpable or visible discrete protrusion at the site of the stoma closure, associated with a palpable fascial defect. A 3-month time window was applied to the primary analysis. To be included in the primary analysis, the clinical examination to assess for hernia presence needed to be done within 2 years (with 3 months’ flexibility either side) from randomisation. Any clinical hernias reported after this time window (ie, after 27 months) were not included in the primary analysis.
The secondary outcome measures were: (1) radiological hernia rate at 1 year post-randomisation. A radiological hernia is defined as any breach in the abdominal wall muscles or fascia visible on CT scan, with or without the passage of bowel, omentum, or fat through it. Outcome assessment was independently done by two gastrointestinal consultant radiologists who were masked to randomised allocation. Any discrepancies in radiological assessment were resolved through discussion. This outcome measure tested the performance of a CT scan at 12 months as a surrogate for 24-month clinical hernia rate; (2) symptomatic hernia rate at 1 and 2 years post-randomisation. This outcome measure was based on patient-reported hernia symptoms including a local lump or pain at the site of the stoma closure; (3) surgical re-intervention rates at 2 years post-randomisation; (4) surgical complications, including surgical site infections (30 days post-operation and 1 year post-randomisation) and seroma formation (1 year post-randomisation); (5) quality of life assessed using EuroQol EQ-5D (3 level) at 30 days post-operation, and 1 and 2 years post-randomisation; (6) pain assessed using a 100 point visual analogue scale at 30 days post-operation, and 1 and 2 years post-randomisation; and (7) health economic analysis, which was pre-planned to be reported in a subsequent paper.
Time windows were also applied to the clinical secondary outcome measures as per the primary outcome. A 10 day time window either side was applied to secondary outcome measures at 30 days (wound infection) and a 3 month time window either side was applied to secondary outcome measures at 1 and 2 years (radiological and symptomatic hernia, surgical re-intervention, wound infection, and seroma formation).
The study included a within trial economic evaluation (including costs per hernia clinically detected at 2 years post-randomisation and 2-year and long-term costs per additional quality adjusted life year gained). These findings will be reported separately.
Statistical analysis
The original sample size was based on detecting a 40% relative reduction in the 2-year clinical hernia rate (or 10% absolute reduction from 25%17, 18, 19 to 15% with the biological mesh) with 80% power (α=0·05) and a 10% attrition rate, which required 560 patients (280 per group). Following recommendation of the Data Monitoring and Ethics Committee, after a routine review of progress, the sample size was increased to 790 patients to give 90% power and allow for a 15% attrition rate.
All primary analyses of the primary and secondary outcomes were based on the intention-to-treat principle. The analyses were adjusted for the minimisation variables used in the randomisation algorithm (ileostomy vs colostomy, midline vs trephine incision, primary vs secondary skin closure) and baseline score (where appropriate). All treatment effects are presented with 95% CIs and two-sided p values with p<0·05 considered statistically significant. Analyses were done using SAS version 9.4 or Stata 15.
The number of clinically detectable hernias at 2 years in the two treatment groups (biological mesh and control) was compared and a log-binomial regression model fitted to obtain an adjusted relative risk (RR) and 95% CI. An RR less than 1 favoured the mesh group. Categorical secondary outcome measures (eg, radiological hernia rate, symptomatic hernia rate, reoperation rate, wound infection, seroma formation) were analysed in the same manner as the primary outcome. Continuous data (eg, EuroQoL EQ-5D, pain scores) were analysed using linear regression models at each timepoint to obtain an adjusted mean difference and 95% CI. In line with the primary outcome, the 2-year data were considered the main analysis timepoint. Because data were collected over multiple timepoints, a mixed effects repeated measures analysis was done across all timepoints. Time was included as a continuous variable in the model. A treatment by time cross-term was also included and if this was not significant, then it was considered that the treatment effect was constant over time, and models without the treatment by time cross-term were fitted. An unstructured covariance structure was used.
Various sensitivity analyses were undertaken including a per-protocol analysis (ie, those actually receiving mesh vs those not) and analyses ignoring any time windows applied to the data analysis. Subgroup analyses were planned for the primary outcome with respect to the three variables that were used for minimisation (stoma site [ileostomy or colostomy]; surgical incision [reopening of midline wound or stoma site only]; and skin closure type [primary or secondary]) and an additional pre-specified subgroup relating to size of fascial defect (≤7 cm vs >7 cm). Subgroup analyses used a test of interaction to explore whether there was evidence that the treatment effects differed across subgroups.
An independent Data Monitoring and Ethics Committee and Trial Steering Committee were convened. The trial was prospectively registered at ISCTRN (ISRCTN46330337) and ClinicalTrials.gov (NCT02238964).
Role of the funding source
The academic investigators retained full independence and autonomy for study conduct, including design, data collection, interpretation, and reporting. The statistical analysis group (SM, KH, NI) from Birmingham Clinical Trials Unit held and had access to the full dataset. The writing committee (see contributions) were responsible for the decision to submit. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
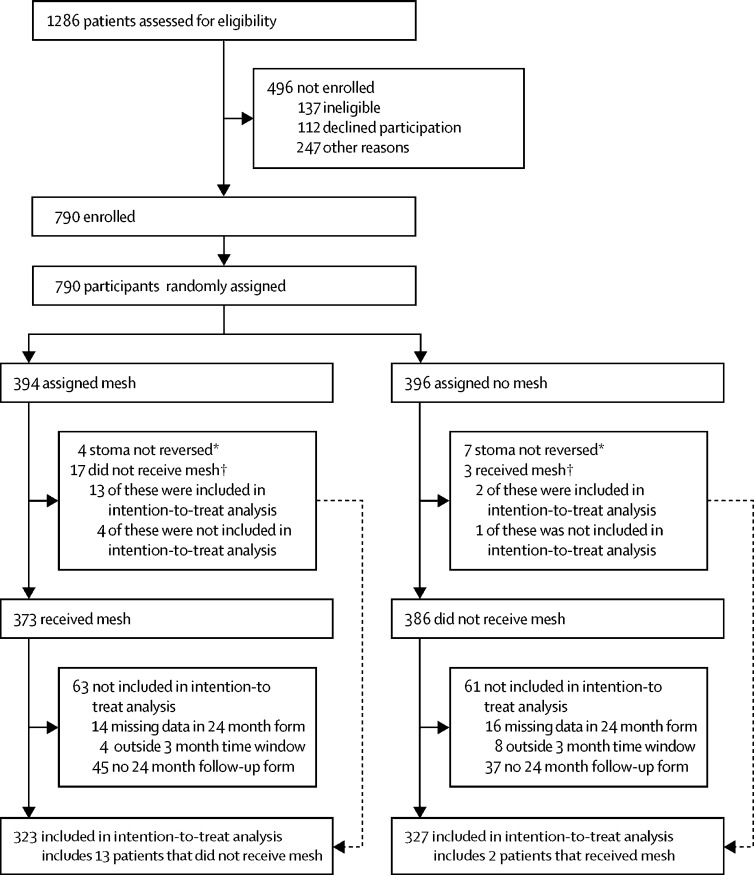
Between Nov 28, 2012, and Nov 11, 2015, we screened 1286 patients for the trial, of whom 790 patients were randomly assigned. Of the screened patients who were not randomly assigned, 137 were ineligible, 112 eligible patients declined participation, and 247 eligible patients were not randomly assigned for other reasons. The acceptance rate among patients who were potentially eligible was 68·8% (790 of 1149, appendix p 2).
From the 790 patients, 394 patients were randomly assigned to mesh and 396 to control (figure). Within the mesh group, four patients did not have their stoma reversed and 17 did not receive mesh, resulting in 373 mesh placements (95% compliance, appendix pp 3–4). In the control group, seven patients did not have their stoma reversed and three received mesh (97% compliance). At 2-year follow-up, 95% of expected patients completed follow-up in the mesh group (344 of 364) and 94% in the control group (349 of 370; figure). The intention-to-treat primary outcome analysis was then restricted on the basis of stomas not reversed (n=11), missing data in the 24 month form (n=32), follow-up outside the 3-month time window either side (n=12), and no 24-month follow-up form (n=85). 82% of randomly assigned patients in the mesh group (323 of 394) and 83% of randomly assigned patients in the control group (327 of 396, appendix p 6) contributed to the analysis of the primary outcome.
Figure.
Trial profile
*For detailed reasons, see appendix p 3. †For detailed reasons, see appendix p 4.
The mean age of trial participants was 58·7 years (range 18–89) and 514 (65%) of 790 were male (table 1). Most stomas had two lumens (605 [77%] of 790) and just more than half were initially formed for cancer treatment (444 [56%] of 790). The parastomal hernia rate, assessed clinically before surgery, was 26% (205 of 790).
Table 1.
Baseline characteristics
| Mesh (n=394) | Control (n=396) | Total (n=790) | |
|---|---|---|---|
| Age, years | |||
| Mean | 58·4 (16·0) | 59·0 (16·0) | 58·7 (16·0) |
| Range | 18·0–89·0 | 19·0–89·0 | 18·0–89·0 |
| Sex | |||
| Male | 263 (67%) | 251 (63%) | 514 (65%) |
| Female | 131 (33%) | 145 (37%) | 276 (35%) |
| Body-mass index | |||
| Mean | 26·8 (4·8) | 26·6 (5·2) | 26·7 (5·0) |
| Diabetes | |||
| No | 351 (89%) | 357 (90%) | 708 (90%) |
| Yes | 42 (11%) | 37 (9%) | 79 (10%) |
| Missing | 1 (<1%) | 2 (<1%) | 3 (<1%) |
| Steroid medications | |||
| No | 377 (96%) | 382 (97%) | 759 (96%) |
| Yes | 15 (4%) | 12 (3·0%) | 27 (3%) |
| Missing | 2 (<1%) | 2 (<1%) | 4 (<1%) |
| Original indication for stoma? | |||
| Cancer | 227 (58%) | 217 (55%) | 444 (56%) |
| Non-cancer | 167 (42%) | 179 (45%) | 346 (44%) |
| Type of stoma opening | |||
| Loop | 295 (75%) | 310 (78%) | 605 (77%) |
| End | 99 (25%) | 86 (22%) | 185 (23%) |
| Type of stoma being closed* | |||
| Ileostomy | 315 (80%) | 316 (80%) | 631 (80%) |
| Colostomy | 79 (20%) | 80 (20%) | 159 (20%) |
| Side of stoma | |||
| Right side | 307 (78%) | 306 (77%) | 613 (78%) |
| Left side | 87 (22%) | 90 (23%) | 177 (22%) |
| Parastomal hernia evident | |||
| No | 284 (72%) | 301 (76%) | 585 (74%) |
| Yes | 110 (28%) | 95 (24%) | 205 (26%) |
| Midline incisional hernia evident | |||
| No | 372 (94%) | 380 (96%) | 752 (95%) |
| Yes | 22 (6%) | 16 (4%) | 38 (5%) |
| Midline laparotomy planned* | |||
| No | 339 (86%) | 341 (86%) | 680 (86%) |
| Yes | 55 (14%) | 55 (14%) | 110 (14%) |
| Planned skin closure* | |||
| Primary | 274 (70%) | 274 (69%) | 548 (70%) |
| Secondary | 120 (30%) | 120 (30%) | 240 (30%) |
| Missing | 0 | 2 (1%) | 2 (<1%) |
Data are mean (SD) or n (%).
Minimisation variables.
The median duration of surgery was 90 min (IQR 70–130) in the mesh group and 70 min (50–100) in the control group (table 2). A midline incision was done in 124 (16%) of 790 individuals and a parastomal hernia was confirmed at surgery in 293 (37%) of 790 individuals. The mesh was placed in the recommended (intraperitoneal) surgical plane in 363 (92%) of 394 individuals, with five meshes placed above posterior rectus sheath and three meshes between peritoneum and posterior rectus sheath (data missing for two patients, mesh not placed for 21 patients; appendix pp 3–4).
Table 2.
Intraoperative findings and procedures
| Mesh (n=394) | Control (n=396) | Total (n=790) | ||
|---|---|---|---|---|
| Number of intraoperative forms available | 393 | 396 | 789 | |
| Duration of surgery (to nearest 10 min) | 90 (70–130) | 70 (50–100) | 80 (60–120) | |
| Surgical access | ||||
| Non-midline | 326 (83%) | 337 (85%) | 663 (84%) | |
| Midline | 66 (17%) | 58 (15%) | 124 (16%) | |
| Missing | 1 (<1%) | 1 (<1%) | 2 (<1%) | |
| Evidence of midline hernia | ||||
| No | 342 (87%) | 351 (89%) | 693 (88%) | |
| Yes | 32 (8%) | 25 (6%) | 57 (7%)* | |
| Suture repair | 13 | 13 | 26 | |
| Mesh repair† | 2 | 1 | 3 | |
| Missing | 19 (5%) | 20 (5%) | 39 (5%) | |
| Evidence of parastomal hernia | ||||
| No | 233 (59%) | 231 (58%) | 464 (59%) | |
| Yes | 142 (36%) | 151 (38%) | 293 (37%) | |
| Missing | 18 (5%) | 14 (4%) | 32 (4%) | |
| Size of fascial defect | ||||
| ≤7 cm | 274 (70%) | 265 (67%) | 539 (68%) | |
| >7 cm | 11 (3%) | 20 (5%) | 31 (4%) | |
| Missing | 108 (27%) | 111 (28%) | 219 (28%)‡ | |
| Skin closure | ||||
| Fully closed | 200 (51%) | 192 (49%) | 392 (50%) | |
| Left partially or completely open | 192 (49%) | 199 (50%) | 391 (49%) | |
| Missing | 1 (<1%) | 5 (1%) | 6 (1%) | |
Data are n, median (IQR), or n (%).
Data were available on method of midline hernia repair for 29 of 57 patients, and were missing for 28 patients.
Repaired using a separate mesh to that used for trial.
The missing data are presented because size of fascial defect was added to the case report form a third of the way through the trial.
A significant difference was observed in the clinically detectable hernia rate at 2 years between the two groups: 12% (39 of 323) in the mesh group versus 20% (64 of 327) in the control group (adjusted RR 0·62, 95% CI 0·43–0·90; p=0·012; table 3). A per-protocol analysis gave similar results (0·60, 0·41–0·88; p=0·009), as did the other sensitivity analyses undertaken (appendix p 7).
Table 3.
Primary and secondary outcomes
| Mesh | Control | Adjusted relative risk*(95% CI) | p value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Clinical hernia at 2 years | 39/323 (12%) | 64/327 (20%) | 0·62 (0·43–0·90) | 0·012 |
| Secondary outcome at 30 days | ||||
| Wound infection | 60/371 (16%) | 49/369 (13%) | 1·19 (0·84–1·68) | 0·32 |
| Secondary outcomes at 12 months | ||||
| Radiological hernia | 20/229 (9%) | 47/226 (21%) | 0·42 (0·26–0·69) | <0·001 |
| Symptomatic hernia | 27/316 (9%) | 36/315 (11%) | 0·75 (0·47–1·21) | 0·24 |
| Wound infection | 63/364 (17%) | 53/362 (15%) | 1·16 (0·83–1·60) | 0·39 |
| Seroma formation | 10/353 (3%) | 8/355 (2%) | 1·26 (0·51–3·14) | 0·61 |
| Secondary outcomes at 24 months | ||||
| Symptomatic hernia | 52/329 (16%) | 64/331 (19%) | 0·83 (0·60–1·16) | 0·29 |
| Surgical re-intervention at stoma site | 42/344 (12%) | 54/346 (16%) | 0·78 (0·54–1·13) | 0·19 |
Data are n/N (%) unless otherwise specified.
Adjusted for minimisation variables (midline laparotomy planned; planned skin closure; type of stoma being closed). An adjusted relative risk value of less than 1 favours mesh.
The primary analysis for radiological hernia rate was based on the 455 patients (58%) who had a CT scan within 3 months of the 1-year timepoint. A significant difference was seen in the radiological hernia rate at 1 year, with rates of 9% (20 of 229) in patients in the mesh group versus 21% (47 of 226) in patients in the control group (adjusted RR 0·42, 95% CI 0·26–0·69; p<0·001; table 3). Demographic details for patients included in the analysis for the radiological hernia outcome at 1 year (n=455) are shown in the appendix (p 8), showing no clinically important differences.
There was a reduction in symptomatic hernia (52 [16%] of 329 vs 64 [19%] of 331, adjusted RR 0·83, 0·60–1·16; p=0·29) and surgical re-intervention (42 [12%] of 344 vs 54 [16%] of 346, adjusted RR 0·78, 0·54–1·13; p=0·19), but these did not reach statistical significance within the 2-year follow-up period (table 3). Insertion of mesh was not associated with any measurable increase in pain in the first 30 days after surgery (appendix p 9) and no associated increase in wound infection or seroma (table 3). No significant differences were seen between groups for patient reported quality of life in the first 2 years of follow-up (appendix p 10). Of the 96 surgical re-interventions, 32 (33%) occurred within 30 days of randomisation; 14 in the mesh group and 18 in the control group.
418 serious adverse events were reported in 274 patients (appendix p 11). At least one serious adverse event was reported in 141 [36%] of 394 patients randomly assigned to the mesh group versus 133 [34%] of 396 in the control group (adjusted RR 1·06, 95% CI 0·88–1·28; p=0·53). Four patients died within 30 days (two in each group), and during the trial 29 deaths were reported (13 [3%] of 394 in the mesh group vs 16 [4%] of 396 in the control group).
No evidence was found that the treatment effect differed according to surgical incision, skin closure type, or size of fascial defect. For stoma type (ileostomy vs colostomy), there was possible evidence of a difference (test for interaction p=0·060). For those randomised to mesh who had an ileostomy, the risk of a clinical hernia was reduced (adjusted RR 0·51, 95% CI 0·33–0·79) compared with those who had a colostomy (adjusted RR 1·11, 95% CI 0·55–2·22; appendix p 5).
Discussion
This study found that reinforcement of the abdominal wall with a biological mesh at the time of stoma closure prevented the development of clinical and radiological incisional hernia within 2 years of surgery. Biological mesh insertion was not associated with additional early complications, either during surgery or in the follow-up period.
Abdominal wall reinforcement with mesh was associated with an absolute reduction in symptoms and reoperation in the first 2 years after surgery, but this was not significant. These findings were unidirectional (reduction with mesh) with wide CIs, and small reductions in these areas might still be important to patients. The natural history of a hernia is to gradually increase in both size and symptoms, and to accumulate complications over a patient's lifetime.17 Although the majority of incisional hernias will start to form within 2 years of surgery,17 this timepoint is early to assess the subsequent development of symptoms and need for reoperation. Thus, clinically detectable hernia is a more suitable primary endpoint than patient reported symptomatic hernia at 2 years, as at that point it is too early for the full spectrum of symptoms to have been reported. The mesh insertion added 20 min to the median duration of surgery. This additional resource use should be balanced against an accumulating number of reoperations for symptomatic hernias. A full health economic analysis of ROCSS will be published separately.
There remains controversy over the precise placement of the mesh with respect to the layers of the abdominal wall. In the ROCSS trial, intra-abdominal placement (deep to the abdominal wall) was agreed following a series of consensus meetings with clinicians. It was selected because of reduced technical complexity and was therefore believed to be more widely acceptable across the surgical community. In practice, no centres withdrew from the study and adherence to study protocol was higher than we anticipated. The intraperitoneal position was associated with a lower seroma rate than has been previously reported in cohorts in which mesh placement was within the layers of the abdominal wall.5, 9 This mesh positioning was not associated with increased pain. These data will be informative in the design of future hernia prevention studies.
Subgroup analyses suggested a possible greater benefit for mesh in patients undergoing ileostomy closure than those undergoing colostomy, although the result should be interpreted with caution. The potential reason for this finding is not obvious, although it seemed unrelated to the size of wound. It could be related to underlying disease (ileostomy is most common in inflammatory bowel disease), patient age (ileostomy patients tend to be younger), or to a chance finding. Further analysis in a subsequent report will help detect these differences, but was beyond the scope of this primary analysis for which we followed a pre-planned, published statistical analysis plan.
Unfortunately, there are not yet any published randomised controlled trials that are comparable with the current trial. Two ongoing randomised trials will add to the knowledge base of reinforcement of stoma site closure. A three-group French trial of 381 patients is assessing a synthetic mesh, a biological mesh, and a control group, with mesh placement in a retrorectus position (NCT02576184). A Dutch trial is assessing a synthetic mesh in a retrorectus position in 130 patients (NCT03750942). Although both trials are smaller than ROCSS, they will enhance the evidence base and allow for correlation of findings between different mesh types and precise placement within the abdominal wall.
A strength of ROCSS is that it shows a structured evaluation of surgical innovation, following the IDEAL framework from stage 1 to stage 3.11 The research team developed the surgical technique, and evaluated safety in a published series of patients. Acceptability, reproducibility, and generalisability were tested in an internal pilot study. The pragmatic design of the randomised trial allowed inclusion of patients with small parastomal hernias and showed delivery across a multicentre, international setting, indicating that the results are generalisable for routine clinical practice. The quality assurance of delivery of technique through both in-person training and active monitoring during the trial contributed to standardisation of intervention and reproducibility of the outcome across a multicentre setting. Randomisation before incision, no longer than 1 week before surgery meant that supply of mesh could be ensured (sites had different pathways for storing mesh), but also meant that intraoperative preferences were avoided (eg, surgeon preference during challenging procedures) and eligibility was maximised. Mandating follow-up in a well patient population was a particular challenge in ROCSS. Its completion required input from many clinical staff and was greatly facilitated by trainee collaborative support.
There might have been a learning curve effect of mesh placement that was not detected within this analysis. However, eliminating such an effect should serve to increase the clinical effect rather than reduce it. Any effect on safety was so low as to be undetectable, so any learning curve effect is likely to be minor. Most colorectal surgeons might need to become competent in mesh placement, as it will benefit patients with inflammatory bowel disease, colorectal cancer, and those needing emergency surgery; these patients are part of the clinical workload of most colorectal surgeons. The implementation of ROCSS across 32 centres shows a model for broader dissemination, with the training video openly accessible online and local champions disseminating the technique. Wide scale monitoring will help establish the safety of both the learning curve and use in routine practice, in keeping with IDEAL stage IV assessment (long-term monitoring). This study included three European countries although the majority were from the UK (35 of 37). Dissemination across western Europe is likely to be similar, although beyond this region dissemination might vary, especially regarding ability of local champions to train other surgeons, depending on local practices.
Limitations of the study include that ROCSS encompassed only 2 years of follow-up. Further follow-up of this randomised population is possible and should provide valuable data as to the medium term (5 year) effect on patients and health-care providers of increasing hernia complications, notable patient symptoms, and further surgery. Follow-up in the community would also enable measurement of resource use outside the hospital.
Secondly, the observed hernia rate in ROCSS was lower than that anticipated by an earlier systematic review.3 The predicted control event rate was 25% but the observed rate was 20%. The most probable overall factor affecting this result is the additional training in both procedures that standardised practice. However, other causes need to be considered. It is possible that patients entering the trial could have been of lower risk than the average population undergoing stoma closure; for example patients with parastomal hernia, who have a high risk of stoma site hernia, might have been less likely to be recruited. This selection bias might have reduced the subsequent hernia rate. Additionally, there might have been an overall decline in hernia rates since when the original studies included in the systematic reviews were done, although this speculation is beyond the scope of the collected data.
Thirdly, ROCSS used a general quality of life questionnaire (EQ-5D) that might have missed differences in stoma related symptoms. No validated hernia questionnaires were available at the time the study was designed. However, newly validated questionnaires specific to abdominal wall quality of life are now available for future studies.18 It is the future burden of symptoms and reoperations that will drive the long-term health-care resource use and quality of life for incisional hernias at stoma sites. 2 years was too early to detect the maximum differences that will occur once hernias have enlarged and started to cause greater symptoms. However, it was a suitable timeframe for the primary endpoint and long-term follow-up is planned.
Fourthly, we did not capture the exact type of fascial closure that was done, although ROCSS did stipulate synthetic, non-absorbable, or slowly absorbable suture had to be used. It is feasible that fascial closure technique has an effect on incisional hernia rate, as shown through the STITCH trial.19 However, any bias here (eg, variability in individual surgeon preference) should have been distributed across trial groups. This equal distribution is indicated by skin closure techniques, which were similar between groups, with a 51% fully closed skin closure technique in the mesh group and a 49% rate in the control group.
Fifthly, obtaining CT scans during correct time windows in all patients and centralising scans nationally and internationally was highly challenging because of timing constraints (eg, cancer follow-up requirements). We have presented an analysis of patients for whom we did not receive scans that showed no clinically important differences. However, the analysis of 1 year CT should be considered as exploratory.
Finally, we did not test interobserver reliability of the assessment of the primary endpoint, nor did we stipulate a second examination if the first revealed no hernia. It is possible that there was variation in application of the definition and examination technique, although the training package around this assessment sought to minimise variation. However, since the outcome assessors were masked, any biases in examination techniques should have been evenly distributed across both groups.
ROCSS has established that biological mesh insertion can be safely implemented across different health systems and prevent a substantial proportion of hernias following closure of a complex contaminated wound. Longer follow-up will establish the size of effect on hernia complications and reoperation. Although there are long-term concerns with prosthetic mesh placement in other indications (eg, female genitourinary prolapse surgery), the same findings are far less probable with biological mesh. Long-term follow-up will ultimately provide the data patients and commissioners need to make decisions. The findings raise the question as to whether biological mesh should now be evaluated in closure of other high-risk wounds.
Data sharing
Acknowledgments
Acknowledgments
Birmingham Experimental Cancer Centre provided support for developing the pilot study. Funding was provided by Royal College of Surgeons of England Clinical Research Initiative, Birmingham Surgical Trials Consortium, and NIHR/Wellcome Trust Clinical Research Facility. An unrestricted medical education grant for the feasibility study was provided by Allergen, who also offered biological mesh for the study, without additional charge. The phase 3 study was funded by the National Institute of Health Research for Patient Benefit (project number PB-PG-1014-35079).
Contributors
Writing Committee: Aneel Bhangu, Dmitri Nepogodiev, Natalie Ives, Laura Magill, James Glasbey, Colm Forde, Thue Bisgaard, Kelly Handley, Samir Mehta, Dion Morton, Thomas Pinkney; Statistical analysis: Samir Mehta, Kelly Handley, Natalie Ives; Trial Management Group: Aneel Bhangu, James Brown, Colm Forde, Kaori Futaba, James Glasbey, Kelly Handley, Natalie Ives, Sarah Khan, Laura Magill, Samir Mehta, Dion Morton, Dmitri Nepogodiev, Arvind Pallan, Abhilasha Patel, Steve Ashdown-Phillips, Tracy Roberts, Susan Jowett, Lorraine Munetsi, Thomas Pinkney, Andrew Torrance; Birmingham Clinical Trial Unit staff: James Brown, Kelly Handley, Nicholas Hilken, Matt Hill, Mark Hunter, Natalie Ives, Sarah Khan, Susan Leek, Helen Lilly, Laura Magill, Samir Mehta, Amruta Sawant, Alexandra Vince, Michael Walters; Trial Steering Committee: Rachel Hargest (Chair), Richard Jackson, Arumugam Rajesh; Data Monitoring Committee: Olagunju Ogunbiyi, Andrew Slater, Ly-Mee Yu (Chair).
The following centres and investigators (listed in alphabetical order) were involved in the trial.
Academic Medical Center, University of Amsterdam, Netherlands (Willem Bemelman*, Marjolein Blussé, Wernard Borstlap, Olivier RC Busch, Christianne Buskens, Charlotte Klaver, Hendrik Marsman, Oddeke van Ruler, Pieter Tanis, Emma Westerduin, Dennis Wicherts); Ashford and St Peter's Hospitals NHS Foundation Trust, Ashford, UK (Parthasarathi Das, Sharadah Essapen, Victoria Frost, Ana Glennon, Catherine Gray, Anwar Hussain, Lisa McNichol, Pasha Nisar*, Humphrey Scott, Jonathan Trickett, Prateesh Trivedi, Daniel White); Chesterfield Royal Hospital NHS Foundation Trust, Chesterfield, UK (Talakalukoppa Amarnath*, Rohan Ardley, Robin Gupta, Emily Hall, Kathryn Hodgkins, Harjeet Narula, Terri-Ann Sewell, John M Simms, Julie Toms, Tim White); Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust, Doncaster, UK (Angela Atkinson, Dan Beral, Nicola Lancaster, Felicity Mackenzie, Tim Wilson*); Dorset County Hospital NHS Foundation Trust, Dorset, UK (David Cruttenden-Wood, Jackie Gibbins, Mark Halls, Dennise Hill, Karen Hogben, Stephanie Jones, Michael J Lamparelli, Mark Lewis*, Sarah Moreton, Paul Ng, Arabis Oglesby, James Orbell, Benjamin Stubbs*, Krishnan Subramanian, Anjay Talwar, Simon Wilsher); East Cheshire NHS Trust, Cheshire, UK (Mohammed Al-Rashedy, Catherine Fensom, Muhammed Gok, Lisa Hardstaff, Kamran Malik, Mohammed Sadat*, Barbara Townley, Lesley Wilkinson); East Kent Hospitals University NHS Foundation Trust, Kent, UK (Tracey Cosier, Sudhakar Mangam*, Mohamed Rabie); Heart of England NHS Foundation Trust, Birmingham, UK (Graham Broadley, John Canny, Simon Fallis, Nikki Green, Ahmed Hawash, Sharad Karandikar, Mehboob Mirza, Edward Rawstorne, Julie Reddan, Jonathan Richardson, Chris Thompson, Kathryn Waite, Haney Youssef*); Hvidovre Hospital, Copenhagen, Denmark (Thue Bisgaard*, Lindsey De Nes, Steffen Rosenstock, Pernille Strandfelt, Mikkel Westen; James Paget University Hospitals NHS Foundation Trust, Great Yarmouth, UK (Kamal Aryal, Kirosh Shankar Kshatriya, Roshan Lal*, Vamsi Velchuru*, Elva Wilhelmsen); London North West Healthcare NHS Trust, London, UK (Ayesha Akbar, Anthony Antoniou*, Sue Clark, Pooja Datt, Jason Goh, Ian Jenkins, Robin Kennedy, Yasuko Maeda, Piero Nastro, Harriet Owen, Robin K S Phillips, Janindra Warusavitarne); Manchester University NHS Foundation Trust, Manchester, UK (Joanne Bradley-Potts, Peter Charleston, Hamish Clouston, Sarah Duff*, Taher Fatayer, Anna Gipson, Nick Heywood, Muneer Junejo, James Kennedy, Helen Lalor, Chris Manning, Richard McCormick, Kathryn Parmar, Stephen Preston, Aswatha Ramesh, Abhiram Sharma, Karen Telford); Mid Essex Hospital Services NHS Trust, Essex, UK (Abidemi Adeosun, Toby Hammond*, Susan Smolen, Joanne Topliffe); NHS Highland, Inverness, UK (James G. Docherty, Michael Lim, Michael Lim, Kathleen Macleod, Eimar Monaghan, Lesley Patience, Ian Thomas, Kenneth G Walker, Michael Walker, Angus JM Watson*); Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich, UK (Amanda Burgess, Yasser Ghanem, Georgina Glister, Sandeep Kapur, Abhilash Paily, Atanu Pal, Rathinam Ravikumar, Melissa Rosbergen, Kevin Sargen, Christopher Speakman*); North Tees and Hartlepool Hospitals NHS Foundation Trust, Hartlepool, UK (Anil K Agarwal, Amlan Banerjee, David Borowski, Dharmendra Garg*, Talvinder Gill, Tracey Johnston, Susan Kelsey, Phanibhushana Chakravarthy Munipalle, Mohamed Tabaqchali, Deborah Wilson); Nottingham University Hospitals NHS Trust, Nottingham, UK (Austin Acheson*, Heather Cripps, Ahmed El-Sharkawy, Oliver Ng, Parveen Sharma, Kym Ward); Royal United Hospital Bath NHS Trust, Bath, UK (Dawne Chandler, Edward Courtney, John Bunni, Katrina Butcher, Stephen Dalton*, Ian Flindall, Joyce Katebe, Pratik Roy, Jeremy Tate, Tracy Vincent, Michael E R Williamson, James Wood); Salisbury NHS Foundation Trust, Salisbury, UK (Mark Bignell, Graham Branagan*, Jack Broardhurst, Helen Chave, Hilary Dean, Nigel D’Souza, Gemma Foster, Simon Sleight, Rupesh Sutaria); Sandwell and West Birmingham NHS Foundation Trust, Birmingham, UK (Ishmail Ahmed, Misra Rai Budhoo, Julie Colley, Neil Cruickshank*, Kathryn Gill, Anne Hayes, Howard Joy*, Carrington Kamabjha, James Plowright, Simon Radley, Mr Rea, Vijay Thumbe, Andrew Torrance*, Philip Varghese, Richard Wilkin, Elzbieta Zulueta); Sherwood Forest Hospitals NHS Foundation Trust, Nottingham, UK (Lynne Allsop, Bence Atkari, Krishnamurthy Badrinath, Prita Daliya, Mukul Dube, Cheryl Heeley, Richard Hind, Dominic Nash, Andrea Palfreman, Oliver Peacock, Nicholas Watson*); Tameside and Glossop Integrated Care NHS Foundation Trust, Ashton-Under-Lyne, UK (Michelle Blodwell, Ali Javaid, Ashiq Mohamad, Karim Muhammad*, Nafees Qureshi, Stephanie Ridgway, Kamran Siddiqui, Mamoon Solkar, Joanne Vere, Alexander Wordie); The Royal Wolverhampton NHS Trust, Wolverhampton, UK (Jessica Chang, Sanaa Elgaddal, Marie Green, Marianne Hollyman, Nazzia Mirza*, Jayne Rankin, Graham Williams); United Lincolnshire Hospitals NHS Trust, Lincoln, UK (Wadah Ali, Anne Hardwick, Zakir Mohamed*, Ahmad Navid, Kimberley Netherton, Mugurel Obreja, Milind Rao*, Joanna Stringer, Athula Tennakoon); University Hospital of North Midlands NHS Trust, Stoke, UK (Timothy Bullen, Mansoor Butt, Robin Dawson, Sarah Dawson, Martin Farmer, Veerabhadram Garimella, Zoe Gates, Lisa Wilkings, Neil Yeomans*); University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK (Olufunso Adedeji, Rashid Alalawi, Aamed Al Araimi, Shazad Ashraf, Simon Bach, Andrew Beggs, Carmen Cagigas, Mit Dattani, Nikolletta Dimitriou, Kaori Futaba, Manijeh Ghods- Ghorbani, James Glasbey, David Gourevitch, Geoffrey Haydon, Tariq Ismail, Christopher Keh, Dion G Morton, Mandip Narewal, Dmitri Nepogodiev, Trifonas Papettas, Thomas Pinkney*, Aaron Poh, Edward Ranstorne, Timothy James Royle, Tahir Shah, Jagdeep Singh, Chris Smart, Nigel Suggett, Muhammad Tayyab, Deepak Vijayan, Ravinder Vohra, Naseem Wairaich, Denise Yeung); University Hospitals Bristol NHS Foundation Trust, Bristol, UK (Richard Bamford, Joanne Chambers, David Cotton, Rebecca Houlihan, James Kynaston, Rob Longman, Amelia Lowe, David Messenger, Anwar Owais, Catherine Phillpott, Jamshed Shabbir*); University Hospitals Coventry and Warwickshire NHS Trust, Coventry, UK (Phil Baragwanath, Charlotte El-Sayed, Anne Gaunt, Chetan Khatri, Peter McCullough, Abhilasha Patel, Stephen Ward, Richard Wilkin, Ross Obukofe, Robert Stroud, David Mason, Nigel Williams*, Ling S Wong); University Hospitals of Leicester NHS Trust, Leicester, UK (Sanjay Chaudhri*, Jill Cooke, Melissa Cunha, Howard Fairey, Michael Norwood, Baljit Singh, Sarah Thomasset); Walsall Healthcare NHS Trust, Walsall, UK (Sian Abbott, Sarah Addison*, James Archer, Aneel Bhangu, Robert Church*, Erika Holford, Fionnaula Lenehan, Steve Odogwu, Lisa Richardson, Jane Sidebotham, Elaine Swan, Alison Tilley, Lynda Wagstaff); Western Sussex Hospitals NHS Foundation Trust, Sussex, UK (Isobel Amey, Yolanda Baird, Neil Cripps, Susanna Greenslade, Guy Harris, Bruce Levy*, Paul Mckenzie, Alison Misselbrook, Sally Moore, Angela Skull); Worcestershire Acute Hospitals NHS Trust, Worcester, UK (Deborah Nicol*, Bala Reddy, Jessica Thrush, Miriam Iglesias Vecchio); Wrightington, Wigan and Leigh NHS Foundation Trust, Wigan, UK (Yvonne Dunn, Claire Williams (nee Fairhurst), Sanjay Furtado, Michael Gill, Lydia Gilmore, Paul Goldsmith, Cezary Kocialkowski, Sadasivam Loganathan, Rabindra Nath, Marius Paraoan*, Tracey Taylor); Yeovil Hospital NHS Foundation Trust, Yeovil, UK (Andrew Allison, Joanna Allison, Nathan Curtis, Richard Dalton, Conrad D’Costa, Godwin Dennison, Jake Foster, Nader Francis*, James Gibbons, Mohammed Hamdan, Alison Lewis, Jonathan Ockrim, Rishabha Sharma, Katie Spurdle, Saseendran Varadharajan); York Teaching Hospital NHS Foundation Trust, York, UK (Assad Aghahoseini, David John Alexander, Dibyendu Bandyopadhyay*, Ian Bradford, Praminthra Chitsabesan, Zoe Coleman, Andrew Gibson, Kostas Lasithiotakis, Dimitrios Panagiotou, Konstantinos Polyzois, Stevan Stojkovic, Nicholas Woodcock, Monica Wright).
*Principal Investigator at each centre.
Declaration of interests
This was an independent investigator led study with no input into the study design, delivery or data analysis by the funders. Mesh was provided free of charge by Allergan who also supported the pilot phase of the study through an unconditional medical education grant. The main study was funded by the National Institute of Health Research through a Research for Patient Benefit grant.
Data sharing requests will be considered by the trial management group on written request to rocss@trials.bham.ac.uk. De-identified participant data or other pre-specified data will be available, subject to a written proposal and an agreed data sharing agreement.
Contributor Information
Reinforcement of Closure of Stoma Site (ROCSS) Collaborative and West Midlands Research Collaborative:
Aneel Bhangu, Dmitri Nepogodiev, Natalie Ives, Laura Magill, James Glasbey, Colm Forde, Thue Bisgaard, Kelly Handley, Samir Mehta, Dion Morton, Thomas Pinkney, Samir Mehta, Kelly Handley, Natalie Ives, Aneel Bhangu, James Brown, Colm Forde, Kaori Futaba, James Glasbey, Kelly Handley, Natalie Ives, Sarah Khan, Laura Magill, Samir Mehta, Dion Morton, Dmitri Nepogodiev, Arvind Pallan, Abhilasha Patel, Steve Ashdown-Phillips, Tracy Roberts, Susan Jowett, Lorraine Munetsi, Thomas Pinkney, Andrew Torrance, James Brown, Kelly Handley, Nicholas Hilken, Matt Hill, Mark Hunter, Natalie Ives, Sarah Khan, Susan Leek, Helen Lilly, Laura Magill, Samir Mehta, Amruta Sawant, Alexandra Vince, Michael Walters, Willem Bemelman, Marjolein Blussé, Wernard Borstlap, Olivier RC Busch, Christianne Buskens, Charlotte Klaver, Hendrik Marsman, Oddeke van Ruler, Pieter Tanis, Emma Westerduin, Dennis Wicherts, Parthasarathi Das, Sharadah Essapen, Victoria Frost, Ana Glennon, Catherine Gray, Anwar Hussain, Lisa McNichol, Pasha Nisar, Humphrey Scott, Jonathan Trickett, Prateesh Trivedi, Daniel White, Talakalukoppa Amarnath, Rohan Ardley, Robin Gupta, Emily Hall, Kathryn Hodgkins, Harjeet Narula, Terri-Ann Sewell, John M Simms, Julie Toms, Tim White, Angela Atkinson, Dan Beral, Nicola Lancaster, Felicity Mackenzie, Tim Wilson, David Cruttenden-Wood, Jackie Gibbins, Mark Halls, Dennise Hill, Karen Hogben, Stephanie Jones, Michael J Lamparelli, Mark Lewis, Sarah Moreton, Paul Ng, Arabis Oglesby, James Orbell, Benjamin Stubbs, Krishnan Subramanian, Anjay Talwar, Simon Wilsher, Mohammed Al-Rashedy, Catherine Fensom, Muhammed Gok, Lisa Hardstaff, Kamran Malik, Mohammed Sadat, Barbara Townley, Lesley Wilkinson, Tracey Cosier, Sudhakar Mangam, Mohamed Rabie, Graham Broadley, John Canny, Simon Fallis, Nikki Green, Ahmed Hawash, Sharad Karandikar, Mehboob Mirza, Edward Rawstorne, Julie Reddan, Jonathan Richardson, Chris Thompson, Kathryn Waite, Haney Youssef, Thue Bisgaard, Lindsey De Nes, Steffen Rosenstock, Pernille Strandfelt, Mikkel Westen, Kamal Aryal, Kirosh Shankar Kshatriya, Roshan Lal, Vamsi Velchuru, Elva Wilhelmsen, Ayesha Akbar, Anthony Antoniou, Sue Clark, Pooja Datt, Jason Goh, Ian Jenkins, Robin Kennedy, Yasuko Maeda, Piero Nastro, Harriet Owen, Robin KS Phillips, Janindra Warusavitarne, Joanne Bradley-Potts, Peter Charleston, Hamish Clouston, Sarah Duff, Taher Fatayer, Anna Gipson, Nick Heywood, Muneer Junejo, James Kennedy, Helen Lalor, Chris Manning, Richard McCormick, Kathryn Parmar, Stephen Preston, Aswatha Ramesh, Abhiram Sharma, Karen Telford, Abidemi Adeosun, Toby Hammond, Susan Smolen, Joanne Topliffe, James G Docherty, Michael Lim, Michael Lim, Kathleen Macleod, Eimar Monaghan, Lesley Patience, Ian Thomas, Kenneth G Walker, Michael Walker, Angus JM Watson, Amanda Burgess, Yasser Ghanem, Georgina Glister, Sandeep Kapur, Abhilash Paily, Atanu Pal, Rathinam Ravikumar, Melissa Rosbergen, Kevin Sargen, Christopher Speakman, Anil K Agarwal, Amlan Banerjee, David Borowski, Dharmendra Garg, Talvinder Gill, Tracey Johnston, Susan Kelsey, Phanibhushana Chakravarthy Munipalle, Mohamed Tabaqchali, Deborah Wilson, Austin Acheson, Heather Cripps, Ahmed El-Sharkawy, Oliver Ng, Parveen Sharma, Kym Ward, Dawne Chandler, Edward Courtney, John Bunni, Katrina Butcher, Stephen Dalton, Ian Flindall, Joyce Katebe, Pratik Roy, Jeremy Tate, Tracy Vincent, Michael ER Williamson, James Wood, Mark Bignell, Graham Branagan, Jack Broardhurst, Helen Chave, Hilary Dean, Nigel D'Souza, Gemma Foster, Simon Sleight, Rupesh Sutaria, Ishmail Ahmed, Misra Rai Budhoo, Julie Colley, Neil Cruickshank, Kathryn Gill, Anne Hayes, Howard Joy, Carrington Kamabjha, James Plowright, Simon Radley, Mr Rea, Vijay Thumbe, Andrew Torrance, Philip Varghese, Richard Wilkin, Elzbieta Zulueta, Lynne Allsop, Bence Atkari, Krishnamurthy Badrinath, Prita Daliya, Mukul Dube, Cheryl Heeley, Richard Hind, Dominic Nash, Andrea Palfreman, Oliver Peacock, Nicholas Watson, Michelle Blodwell, Ali Javaid, Ashiq Mohamad, Karim Muhammad, Nafees Qureshi, Stephanie Ridgway, Kamran Siddiqui, Mamoon Solkar, Joanne Vere, Alexander Wordie, Jessica Chang, Sanaa Elgaddal, Marie Green, Marianne Hollyman, Nazzia Mirza, Jayne Rankin, Graham Williams, Wadah Ali, Anne Hardwick, Zakir Mohamed, Ahmad Navid, Kimberley Netherton, Mugurel Obreja, Milind Rao, Joanna Stringer, Athula Tennakoon, Timothy Bullen, Mansoor Butt, Robin Dawson, Sarah Dawson, Martin Farmer, Veerabhadram Garimella, Zoe Gates, Lisa Wilkings, Neil Yeomans, Olufunso Adedeji, Rashid Alalawi, Aamed Al Araimi, Shazad Ashraf, Simon Bach, Andrew Beggs, Carmen Cagigas, Mit Dattani, Nikolletta Dimitriou, Kaori Futaba, Manijeh Ghods-Ghorbani, James Glasbey, David Gourevitch, Geoffrey Haydon, Tariq Ismail, Christopher Keh, Dion G Morton, Mandip Narewal, Dmitri Nepogodiev, Trifonas Papettas, Tom Pinkney, Aaron Poh, Edward Ranstorne, Timothy J Royle, Tahir Shah, Jagdeep Singh, Chris Smart, Nigel Suggett, Muhammad Tayyab, Deepak Vijayan, Ravinder Vohra, Naseem Wairaich, Denise Yeung, Richard Bamford, Joanne Chambers, David Cotton, Rebecca Houlihan, James Kynaston, Rob Longman, Amelia Lowe, David Messenger, Anwar Owais, Catherine Phillpott, Jamshed Shabbir, Phil Baragwanath, Charlotte El-Sayed, Anne Gaunt, Chetan Khatri, Peter McCullough, Abhilasha Patel, Stephen Ward, Richard Wilkin, Ross Obukofe, Robert Stroud, David Mason, Nigel Williams, Ling S Wong, Sanjay Chaudhri, Jill Cooke, Melissa Cunha, Howard Fairey, Michael Norwood, Baljit Singh, Sarah Thomasset, Sian Abbott, Sarah Addison, James Archer, Aneel Bhangu, Robert Church, Erika Holford, Fionnaula Lenehan, Steve Odogwu, Lisa Richardson, Jane Sidebotham, Elaine Swan, Alison Tilley, Lynda Wagstaff, Isobel Amey, Yolanda Baird, Neil Cripps, Susanna Greenslade, Guy Harris, Bruce Levy, Paul Mckenzie, Alison Misselbrook, Sally Moore, Angela Skull, Deborah Nicol, Bala Reddy, Jessica Thrush, Miriam Iglesias Vecchio, Yvonne Dunn, Claire Williams, Sanjay Furtado, Michael Gill, Lydia Gilmore, Paul Goldsmith, Cezary Kocialkowski, Sadasivam Loganathan, Rabindra Nath, Marius Paraoan, Tracey Taylor, Andrew Allison, Joanna Allison, Nathan Curtis, Richard Dalton, Conrad D'Costa, Godwin Dennison, Jake Foster, Nader Francis, James Gibbons, Mohammed Hamdan, Alison Lewis, Jonathan Ockrim, Rishabha Sharma, Katie Spurdle, Saseendran Varadharajan, Assad Aghahoseini, David J Alexander, Dibyendu Bandyopadhyay, Ian Bradford, Praminthra Chitsabesan, Zoe Coleman, Andrew Gibson, Kostas Lasithiotakis, Dimitrios Panagiotou, Konstantinos Polyzois, Stevan Stojkovic, Nicholas Woodcock, Monica Wright, Rachel Hargest, Richard Jackson, Arumugam Rajesh, Olagunju Ogunbiyi, Andrew Slater, and Ly-Mee Yu
Supplementary Materials
The video shows the surgical technique used in the ROCSS trial. The demonstration was provided to ensure homogeneity of technique between surgeons.
Youtube url: https://youtu.be/GR5rMQFZvqM
References
- 1.NHS Digital Hospital Episode Statistics. 2019. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics
- 2.Doussot A, Abo-Alhassan F, Derbal S. Indications and outcomes of a cross-linked porcine dermal collagen mesh (permacol) for complex abdominal wall reconstruction: a multicenter audit. World J Surg. 2019;43:791–797. doi: 10.1007/s00268-018-4853-x. [DOI] [PubMed] [Google Scholar]
- 3.Bhangu A, Nepogodiev D, Futaba K. Systematic review and meta-analysis of the incidence of incisional hernia at the site of stoma closure. World J Surg. 2012;36:973–983. doi: 10.1007/s00268-012-1474-7. [DOI] [PubMed] [Google Scholar]
- 4.Bhangu A, Fletcher L, Kingdon S, Smith E, Nepogodiev D, Janjua U. A clinical and radiological assessment of incisional hernias following closure of temporary stomas. Surgeon. 2012;10:321–325. doi: 10.1016/j.surge.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Rosen MJ, Bauer JJ, Harmaty M. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg. 2017;265:205–211. doi: 10.1097/SLA.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhangu A, Fitzgerald JE, Singh P, Battersby N, Marriott P, Pinkney T. Systematic review and meta-analysis of prophylactic mesh placement for prevention of incisional hernia following midline laparotomy. Hernia. 2013;17:445–455. doi: 10.1007/s10029-013-1119-2. [DOI] [PubMed] [Google Scholar]
- 7.Borab ZM, Shakir S, Lanni MA. Does prophylactic mesh placement in elective, midline laparotomy reduce the incidence of incisional hernia? A systematic review and meta-analysis. Surgery. 2017;161:1149–1163. doi: 10.1016/j.surg.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Bondre IL, Holihan JL, Askenasy EP. Suture, synthetic, or biologic in contaminated ventral hernia repair. J Surg Res. 2016;200:488–494. doi: 10.1016/j.jss.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Itani KM, Rosen M, Vargo D, Awad SS, Denoto G, 3rd, Butler CE. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery. 2012;152:498–505. doi: 10.1016/j.surg.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kamarajah SK, Chapman SJ, Glasbey J. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open. 2018;2:371–380. doi: 10.1002/bjs5.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCulloch P, Altman DG, Campbell WB. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 12.Bhangu A, Futaba K, Patel A, Pinkney T, Morton D. Reinforcement of closure of stoma site using a biological mesh. Tech Coloproctol. 2014;18:305–308. doi: 10.1007/s10151-013-1001-3. [DOI] [PubMed] [Google Scholar]
- 13.Reinforcement of Closure of Stoma Site (ROCSS) Collaborative and the West Midlands Research Collaborative Feasibility study from a randomized controlled trial of standard closure of a stoma site vs biological mesh reinforcement. Colorectal Dis. 2016;18:889–896. doi: 10.1111/codi.13310. [DOI] [PubMed] [Google Scholar]
- 14.Reinforcement of Closure of Stoma Site (ROCSS) Collaborative and the West Midlands Research Collaborative Randomized controlled trial of standard closure of a stoma site vs biological mesh reinforcement: study protocol of the ROCSS trial. Colorectal Dis. 2018;20:O46–O54. doi: 10.1111/codi.13997. [DOI] [PubMed] [Google Scholar]
- 15.DREAMS Trial Collaborators and West Midlands Research Collaborative Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial) BMJ. 2017;357 doi: 10.1136/bmj.j1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinkney TD, Calvert M, Bartlett DC. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI trial) BMJ. 2013;347 doi: 10.1136/bmj.f4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–583. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krpata DM, Schmotzer BJ, Flocke S. Design and initial implementation of HerQLes: a hernia-related quality-of-life survey to assess abdominal wall function. J Am Coll Surg. 2012;215:635–642. doi: 10.1016/j.jamcollsurg.2012.06.412. [DOI] [PubMed] [Google Scholar]
- 19.Deerenberg EB, Harlaar JJ, Steyerberg EW. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015;386:1254–1260. doi: 10.1016/S0140-6736(15)60459-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The video shows the surgical technique used in the ROCSS trial. The demonstration was provided to ensure homogeneity of technique between surgeons.
Youtube url: https://youtu.be/GR5rMQFZvqM