Abstract
Background
The colony stimulating factors (CSFs), granulocyte‐macrophage colony stimulating factor (GM‐CSF) and granulocyte colony stimulating factor (G‐CSF), are naturally occurring cytokines that stimulate the production and antibacterial function of neutrophils and monocytes. Two strategies have been adopted for exploring whether CSFs can provide clinical benefit for preterm infants. The first has investigated their use as a treatment to improve outcome in established systemic infection, especially when complicated by a low neutrophil count. The alternative strategy has been to use CSFs prophylactically, to prevent sepsis prospectively through stimulation of neutrophil production and bactericidal function.
Objectives
To determine the efficacy and safety of the haemopoietic colony stimulating factors (G‐CSF or GM‐CSF) in newborn infants, when used for: a) treatment of suspected or proven systemic infection to reduce mortality, or b) prophylaxis, to prevent systemic infection in infants at high risk of nosocomial infection. To determine, in subgroup analysis, the influence of pre‐existing or high risk of neutropenia on the outcome of therapy.
Search methods
PubMed, EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 2, 2003) were searched in April 2003 using the keywords: G‐CSF, GM‐CSF, infant newborn, with and without the limit Clinical Trial. In addition, reference lists of identified RCTs, meta‐analyses and personal files were searched.
Selection criteria
The criteria used to select studies for inclusion were: Design: RCT. Subjects: Newborn infants in intensive care. Interventions: G‐CSF or GM‐CSF given as treatment in conjunction with antibiotics for suspected or microbiologically proven systemic infection. G‐CSF or GM‐CSF given as prophylaxis with the aim of reducing the incidence of systemic infection. Outcomes: Treatment studies reporting all cause mortality. Prophylaxis studies reporting subsequent incidence of sepsis and / or mortality.
Data collection and analysis
Relative risks (RR) and risk differences (RD) with 95% confidence intervals (CI) using the fixed effect model are reported. Number needed to treat (NNT) was calculated for the outcomes that showed a statistically significant reduction in RR.
Main results
Seven treatment studies of 257 infants with suspected systemic bacterial infection and three prophylaxis studies comprising 359 neonates are analysed. Treatment studies: There is no evidence that the addition of G‐CSF or GM‐CSF to antibiotic therapy in preterm infants with suspected systemic infection reduces immediate all cause mortality. No significant survival advantage was seen at 14 days from the start of therapy [typical RR 0.71 (95% CI 0.38,1.33); typical RD ‐0.05 (95% CI ‐0.14, 0.04)]. However all seven of the treatment studies were small, the largest recruiting only 60 infants. The subgroup analysis of 97 infants from three treatment studies who, in addition to systemic infection, had clinically significant neutropenia (< 1.7 x 109/l) at trial entry, does show a significant reduction in mortality by day 14 [RR 0.34 (95% CI 0.12, 0.92); RD ‐0.18 (95% CI ‐0.33, ‐0.03); NNT 6 (95% CI 3‐33)].
Prophylaxis studies have not demonstrated a significant reduction in mortality in neonates receiving GM‐CSF [RR 0.59 (95% CI 0.24,1.44); RD ‐0.03 (95% CI ‐0.08,0.02)]. The identification of sepsis as the primary outcome of prophylaxis studies has been hampered by inadequately stringent definitions of systemic infection. However, data from one study suggest that prophylactic GM‐CSF may provide protection against infection when given to preterm infants who are neutropenic or at high risk of developing postnatal neutropenia.
Authors' conclusions
There is currently insufficient evidence to support the introduction of either G‐CSF or GM‐CSF into neonatal practice, either as treatment of established systemic infection to reduce resulting mortality, or as prophylaxis to prevent systemic infection in high risk neonates. No toxicity of CSF use was reported in any study included in this review. The limited data suggesting that CSF treatment may reduce mortality when systemic infection is accompanied by severe neutropenia should be investigated further in adequately powered trials which recruit sufficient infants infected with organisms associated with a significant mortality risk.
Plain language summary
G‐CSF and GM‐CSF for treating or preventing neonatal infections
Infants born before 32 weeks and infants that are small for their gestational age are at high risk of developing infections while in hospital. These infections can cause death, disability (including cerebral palsy) as a result of damage to nervous tissue as well as contributing to chronic lung disease. Infection‐related deaths have remained constant for two decades and antibiotic resistance is increasing, emphasising the need for new ways to prevent infection. The haemopoietic colony stimulating factors (CSFs), granulocyte‐macrophage colony stimulating factor (GM‐CSF) and granulocyte colony stimulating factor (G‐CSF) are naturally occurring substances (cytokines) that can increase circulating white blood cells (neutrophils) and their ability to destroy bacteria. Common minor side effects are low grade fever and skeletal pain. The review authors identified seven treatment studies of 257 premature infants with suspected systemic bacterial infection. Adding G‐CSF or GM‐CSF to antibiotic therapy did not improve survival, overall. It may be, however, that infants who had clinically low neutrophils at the start of treatment did show some reduction in number of deaths by day 14 (taken from three studies). In three studies in which 359 low birthweight or premature neonates were treated preventatively (prophylaxis) no reduction in deaths was evident in those neonates receiving GM‐CSF. GM‐CSF was well tolerated with no adverse reactions in these small studies.
Background
Neonatal sepsis is a major cause of death. Published rates of sepsis in preterm newborns range from 25‐50% (Stoll 1996; Cooke 1997) and neonates in whom late onset sepsis develops are significantly more likely to die than those who avoid infection (17% vs 7%; p < 0.0001) (Stoll 1996). Sepsis also leads to disability, including cerebral palsy through damage to white matter and other brain injury (Damman 1998), and contributes to the aetiology of chronic lung disease. These conditions are powerful determinants of outcome after preterm birth. Sepsis related mortality has remained constant for two decades (Stoll 1996) and antibiotic resistance is increasing, emphasising the need for novel strategies to prevent infection and its consequences.
Neutropenia, which occurs frequently in infants born before 32 weeks and particularly in those with intrauterine growth restriction, adds substantially to the risk of sepsis (Gessler 1995; Engle 1984; Koening 1989). When sepsis is associated with severe neutropenia, mortality exceeds 50% (Rodwell 1993). Neutropenia is due to immaturity of neutrophil production (Christensen 1989; Carr 2000a), but preterm infant neutrophils are also functionally immature (Hill 1987; Carr 2000b); both factors contribute to infection risk and morbidity.
The colony stimulating factors (CSFs), granulocyte‐macrophage colony stimulating factor (GM‐CSF) and granulocyte colony stimulating factor (G‐CSF), are naturally occurring cytokines that are in routine clinical use in adults and children to accelerate neutrophil recovery following chemotherapy. In this setting both CSFs have been shown to be free from serious adverse effects. Common minor side effects are low grade fever and skeletal pain. Rarely, capillary leak and pulmonary infiltrates have been reported when high doses are administered rapidly. In neonates there is a theoretical risk that activation of neutrophils and monocytes in the circulation might exacerbate lung disease or necrotising enterocolitis.
The earliest published case of CSF use in a human neonate was in 1991 and reported the successful correction of persistent neutropenia and associated recurrent infections in a preterm infant following administration of G‐CSF (Roberts 1991). During the early 1990's several studies demonstrated the ability of both G‐ and GM‐CSF to protect newborn rat pups from sepsis‐related death (reviewed by Carr 1997). Encouraged by these data and following the two earliest phase I/II pilot studies in human neonates of G‐CSF (Gillan 1994) and GM‐CSF (Cairo 1995), a small number of randomised studies have been undertaken investigating the potential of CSFs to protect against infection and infection‐related death in preterm infants at high risk of sepsis.
Two strategies have been adopted for exploring whether CSFs can provide clinical benefit (Modi 2000). The first has investigated their use as a treatment to increase circulating neutrophils and improve outcome in established sepsis complicated by a low neutrophil count. G‐CSF has been used in these studies because of its powerful ability to mobilise preformed neutrophils from the marrow into the circulation and its effect on neutrophil precursor proliferation.
The alternative strategy has been to use CSFs prophylactically, to prevent sepsis by prospectively stimulating neutrophil production and enhancing phagocyte bactericidal function. Some prophylactic studies have taken established neutropenia as an indication for commencing CSFs. These studies have tended to use G‐CSF. Other investigators have adopted the approach that the prevention of neutropenia may be a more effective sepsis prophylaxis strategy and provide benefit to a more inclusive population of infants at risk. These studies have tended to use GM‐CSF, because in addition to its stimulating effect on neutrophil production, it upregulates bactericidal function of both neutrophils and monocytes to a greater extent than that achieved by G‐CSF (Lejeune 1999), and thus may correct the phagocytic functional defects believed to contribute to the high incidence of neonatal sepsis (Carr 2000b).
This is an appropriate time to undertake a systematic review of the subject as the first cohort of clinical studies has now been published. The review includes randomised controlled studies in which a CSF was used as prophylaxis to prevent infection, or used as a treatment adjunct to antibiotic therapy in established infection to reduce mortality. Studies using either G‐ or GM‐CSF are included.
Objectives
To determine the efficacy and safety of the haemopoietic colony stimulating factors (G‐CSF or GM‐CSF) in newborn infants, when used for: a) treating suspected or proven sepsis, to reduce mortality, or b) prophylaxis, to prevent sepsis in infants at high risk of nosocomial infection. To determine, in subgroup analysis, the influence of pre‐existing or high risk of neutropenia on the outcome of therapy.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials: randomised or quasi‐randomised with or without blinding placebo control not necessary
Types of participants
Newborn infants: a) with suspected or microbiologically proven sepsis (treatment studies) b) at high risk of developing nosocomial sepsis (prophylaxis studies)
Key subgroup: a) Treatment studies: i) infants with neutropenia at trial entry. (Neutropenia defined as less than or equal to 1.7 x 109/l) (Manroe 1979) ii) agent used: G‐CSF or GM‐CSF
b) Prophylaxis studies: i) infants with neutropenia at trial entry. (Neutropenia defined as less than or equal to 1.7 x 109/l) ii) infants at high risk of neutropenia. (Defined as infants born to mothers with pregnancy‐induced hypertension, or small for gestational age infants) iii) infants born < 32 weeks gestation, or < 1500g. iv) agent used: G‐CSF or GM‐CSF.
Types of interventions
a) Treatment studies: G‐CSF or GM‐CSF treatment in conjunction with antibiotics, compared with antibiotic treatment alone.
b) Prophylaxis studies: G‐CSF or GM‐CSF prophylactic administration, compared with control infants not receiving a CSF.
Types of outcome measures
a) Treatment studies:
Primary outcome: i) All cause mortality, directly related (in time) to septic episode. (This is likely to be defined differently in different studies. However, to achieve some comparability between studies, data have been sought to permit analysis of the mortality at 14 days and at 28 days from start of CSF therapy)
Secondary outcomes: i) Failure to correct pre‐existing neutropenia during treatment. (Correction of neutropenia defined as neutrophils increased to > 1.7 x 109/l) ii) Mortality at discharge from hospital. iii) Long term outcomes: Death and disability at or greater than one year from birth. iv) Toxicity: Adverse effects attributable to CSF therapy; eg. Increased incidence of chronic lung disease or necrotising enterocolitis.
b) Prophylaxis studies:
Primary outcome: i) Sepsis. Definition of sepsis: The diagnostic criteria used for identifying sepsis may differ and this has been taken into account when analysing trials. Trials have been grouped, and analysed separately, according to whether they used: a) Clinical plus microbiological criteria; b) Either clinical criteria, or microbiological criteria alone.
Definitions of short‐term and long‐term sepsis prevention: The study period during which sepsis is prevented by CSF therapy is likely to be defined differently in different studies and will depend on the treatment protocol and duration of CSF administration. However, to achieve some comparability between studies, data have been sought to permit analysis of a) short term sepsis prevention, defined as during CSF therapy and up to 10 days following discontinuation of CSF, and b) long term sepsis prevention, defined as during the subsequent reported follow up period, up to the time of discharge from hospital.
Secondary outcomes: i) Failure to prevent or correct pre‐exisiting neutropenia. (As defined above) ii) All cause mortality: Short term and long term. (Time periods as defined for sepsis prevention) iii) Long term outcomes: Death and disability at or greater than one year from birth iv) Toxicity: Adverse effects attributable to CSF therapy; eg. Increased incidence of chronic lung disease or necrotising enterocolitis
Search methods for identification of studies
Relevant trials in any language were identified through:
i) The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2003).
ii) Electronic journal reference databases, PubMed, MEDLINE, EMBASE, were searched in April 2003:
Pub Med search strategy: #1 Search G‐CSF [mesh heading] AND therapeutic use [sub‐heading] #2 Search Granulocyte colony stimulating factor [mesh heading] AND therapeutic use [sub‐heading] #3 Search #1 OR #2 #4 Search GM‐CSF [mesh heading] AND therapeutic use [sub‐heading] #5 Search Granulocyte macrophage colony stimulating factor [mesh heading] AND therapeutic use [sub‐heading] #6 Search #4 OR #5 #7 Search #3 OR #6 #8 Search Infant, newborn #9 Search Infant, premature #10 Search Infant, small for gestation #11 Search Infant, low birth weight #12 Search Infant, very low birth weight #13 Search #8 OR #9 OR #10 OR #11 OR #12 #14 Search #7 AND #13 #15 Search #7 AND #13 with Limits: Clinical Trial, Publication date from 1994 to April 2003.
MEDLINE and EMBASE were searched using a similar strategy and headings.
iii) Science Citation Index was searched, in April 2003, for citations of: a) Gillan 1994 b) Cairo 1995
iv) In addition, searches were made in major paediatric and haematology conference proceedings, up to December 2002, (including American Pediatric Society, Society for Pediatric Research, European Society for Paediatric Research, American Society of Haematology) to identify potentially relevant trials ongoing or awaiting full publication. However, studies will not be included in the analysis until reported in full peer reviewed publication.
v) Additional searches were made in reference lists of identified clinical trials, and in the reviewers' personal files.
Data collection and analysis
Full reports of studies identified from the above sources were screened independently by individual reviewers for the eligibility criteria stated above. Full text versions of all eligible studies were obtained for quality assessment and data extraction.
The methodological quality of the eligible trials was independently assessed by individual unblinded reviewers using the full text article and based on the following criteria: (See Additional table: Table 3 Methodological quality of included studies) i) Blinding of treatment allocation ii) Blinding of intervention iii) Blinding of outcome measure assessment iv) Completeness of follow‐up of all randomised subjects for short term outcomes v) Planned recruitment achieved
1. Methodological quality of included studies.
| Study | Allocation blind | Intervention blind | Outcome blind | Compl. follow‐up | Recruitment complete | Quality score |
| Ahmad 2002 | unclear | yes | yes | yes | yes | 4 |
| Bedford‐Russell 2001 | yes | yes | yes | yes | yes | 5 |
| Bilgin 2001 | no | no | no | unclear | yes | 1 |
| Cairo 1995 | unclear | no | no | yes | yes | 2 |
| Cairo 1999 | unclear | yes | yes | yes | yes | 4 |
| Carr 1999 | yes | no | yes | yes | yes | 4 |
| Drossou‐Agakidou '98 | unclear | no | no | unclear | yes | 1 |
| Gillan 1994 | unclear | no | unclear | yes | yes | 2 |
| Miura 2001 | unclear | yes | yes | yes | no | 3 |
| Schibler 1998 | unclear | yes | yes | yes | yes | 4 |
Data extraction was independently performed by all three reviewers using a paper based data extraction proforma. The results were compared and disagreements resolved by discussion.
ADDITIONAL DATA In order to increase comparability of data for analysis from the Treatment studies, the corresponding author of each published trial was contacted and sent the completed data extraction proforma for their trial with a request to check its accuracy and to request additional data not available from the trial publication. In particular, mortality data for all recruited infants at 14 days and 28 days from the start of the intervention and to discharge were requested. In addition a specific request was made for the same outcome data on the subgroup of infants, if any, who were neutropenic (less than or equal to 1.7 x 109/l) at trial entry. Additional data have been received only from Bedford‐Russell, who provided outcome data for the subgroup of infants who were neutropenic at trial entry and those who were not neutropenic.
ANALYSIS Trials were grouped for outcome according to the aim of the therapy: a) Treatment or b) Prophylaxis.
Within each of these study groups, trials using G‐CSF or GM‐CSF were analysed together and separately. However, data on adverse effects were analysed separately for G‐CSF and GM‐CSF, pooling the treatment and prophylaxis trials.
Additional analyses were undertaken of the subgroups as defined under Types of Participants.
Heterogeneity of treatment effects between trials was assessed graphically and tested using the standard chi‐squared test. A weighted estimate of the relative risk (RR) across trials was calculated using a fixed effects model. Risk difference (RD) and number needed to treat (NNT) were also calculated. 95% CIs were calculated for all outcomes. Sensitivity analysis was performed using a random effects model to allow for possible between trial heterogeneity. Sensitivity analysis was undertaken to assess the robustness of the results of the review to trial quality.
Results
Description of studies
TREATMENT STUDIES See: Table of included studies
Details of the included studies are provided in the Table "Characteristics of included studies". Seven studies met the inclusion criteria (Ahmad 2002; Bedford‐Russell 2001; Bilgin 2001; Drossou‐Agakidou '98; Miura 2001; Schibler 1998; Gillan 1994).
Ahmad 2002: Single centre, randomised, double‐blind, placebo controlled trial. Participants were 28 infants with early or late onset sepsis. Sepsis was defined as clinical signs indicative of sepsis (shock, or respiratory failure, or necrotising enterocolitis), plus either proven bacteraemia, or neutropenia less than 1 x 109/l. GA < 32w, BW 445 ‐ 1960g, no postnatal age limit.
Interventions: Infants were randomised into three groups, to receive G‐CSF, GM‐CSF or placebo. 10 infants received G‐CSF 10 mg/kg/day in two divided doses by iv infusion, 10 infants received GM‐CSF 8 mg/kg/day in two divided doses by iv infusion, eight infants received placebo. Treatment continued for six days, or until ANC > 10 x 109/l.
Outcomes: Mortality to discharge. Neutrophil counts on day two, five, seven from study entry. The number of infants with neutropenia at trial entry is not reported, nor is their outcome separately identified.
Bedford‐Russell 2001: A multi‐centre randomised double‐blind placebo controlled trial, stratified by centre (4) and age at infection onset (younger or older than 72h postnatal age).
Participants were 28 infants with a clinical diagnosis of sepsis and neutrophils less than or equal to 5 x 109/l. Sepsis was defined as two or more objective clinical signs of sepsis requiring treatment with antibiotics. Microbiological confirmation was not an entry criterion. GA > 25w, BW 500 ‐ 1500g, age at recruitment < 29d.
Interventions: 13 infants received 10 mcg/kg G‐CSF IV daily for a maximum of 14d or until discontinuation of antibiotics; 15 infants received placebo.
Outcomes: Mortality during the 42d from study entry and survival to 12m post‐menstrual age.
Bilgin 2001: A single centre, controlled, quasi‐randomised (alternate allocation) trial; randomisation stratified by growth status (small, appropriate, large for gestation).
Participant were 60 infants with a clinical and microbiological diagnosis of sepsis and neutrophils less than 1.5 x109/l. Sepsis was defined as the onset of new symptoms or objective clinical signs of sepsis, plus at least 1 positive blood culture. GA < 42w, BW 1100 ‐ 3500g, age < 29d. 25 treated and 24 controls had early onset sepsis.
Intervention: 30 infants received 5 mcg/kg GM‐CSF SC daily for 7d. The 30 controls received no intervention or placebo.
Outcomes: Mortality to discharge (all survivors were discharged on or before day 21 from admission).
Drossou‐Agakidou '98: A single centre randomised controlled trial, not stratified. Participants were 35 infants with a clinical and microbiological diagnosis of sepsis and neutrophils <5 x109/l. Sepsis was defined as the presence of objective clinical signs plus at least two laboratory findings indicative of sepsis, plus a positive blood culture. GA 24 ‐ 37w, BW 720‐2940g, age < 28d. Intervention: 19 infants received 10 mcg/kg G‐CSF SC daily for 3d. The 16 controls received no intervention or placebo.
Outcomes: Mortality within 10d of sepsis onset.
Miura 2001: A single centre, randomised, double‐masked, placebo controlled trial, not stratified.
Participants were 44 infants with a clinical diagnosis of sepsis. Sepsis was defined as three or more objective clinical signs of sepsis, plus at least one laboratory criterion for sepsis, plus blood culture taken and antibiotics commenced. Neutropenia was not an entry criterion. GA < 37w, BW 500 ‐ 2000g, age < 5d.
Intervention: 22 infants received 10 mcg/kg G‐CSF IV daily for 3d. 22 infants received placebo.
Outcomes: Mortality within 30d of sepsis onset; mortality to discharge. In addition, nosocomial infections during the 14d after the final dose were recorded.
Schibler 1998: A two centre randomised double‐blind placebo controlled trial, not stratified.
Participants were 20 infants with a clinical diagnosis of early onset sepsis and neutrophils <1.7 x 109/l. Sepsis was defined as neutrophils < 1.7 x 109/l, plus an elevated neutrophil I/T ratio, plus receiving mechanical ventilation. GA 24 ‐ 40w, BW 530 ‐ 3667g, age < 3d.
Intervention: 10 infants received 10 mcg/kg G‐CSF IV daily for 3d. 10 infants received placebo.
Outcomes: Mortality within 7d from recruitment; mortality to discharge.
Gillan 1994: A two centre, randomised, placebo controlled trial, stratified by GA (26 ‐ 30, 31 ‐ 35, 36 ‐ 40w).
Participants were 42 infants with suspected sepsis, defined as "blood cultures obtained and antibiotic therapy initiated". GA 26‐41w, BW > 800g, age < 3d.
Interventions: This was a phase I/II study in which nine infants received 1 mcg/kg/d G‐CSF, nine infants received 5 mcg/kg/d, nine infants received 10 mcg/kg/d, three infants received 5 mcg/kg every 12h, three infants received 10 mcg/kg every 12h, all for 3d. An additional nine infants received placebo daily for 3d.
Outcomes: Mortality to 15 months from study entry.
PROPHYLAXIS STUDIES See: Table of included studies
Details of the included studies are provided in the Table "Characteristics of included studies". Three studies met the inclusion criteria (Carr 1999, Cairo 1999, Cairo 1995).
Carr 1999: A multi‐centre, randomised controlled trial, stratified by growth status (small (< 10th centile) or appropriate for gestation). Participants were 75 infants without evidence of infection, who were recruited into the study at < 72h postnatal age. Neutropenia was not an entry criterion. BW 480 ‐ 1760g, GA < 32w.
Intervention: 36 infants received 10 mcg/kg GM‐CSF SC daily for 5d; the 39 control infants received no intervention or placebo.
Outcomes: The primary outcome was sepsis to 14 days from trial entry, based on clinical and microbiological criteria (three or more clinical signs of sepsis, plus a positive blood culture). Secondary outcome was mortality to 28d and to 6m.
Cairo 1999: A multi‐centre, randomised, double‐blind, placebo controlled trial, stratified by centre and BW (501 ‐ 750, 751 ‐ 1000g).
Participants were 264 infants without evidence of infection, who were recruited into the study at < 72h postnatal age. Neutropenia not an entry criterion. BW 501 ‐ 1000g. All were appropriately grown; small for gestation infants were excluded. Intervention: 134 infants received 8 mcg/kg GM‐CSF IV daily for 7d, then every other day for an additional 21d. Treatment discontinued if WBC > 20 x109/l or if venous access no longer available. The 130 control infants received placebo.
Outcomes: The primary outcome was the incidence of nosocomial infection during treatment (ie to day 28 from trial entry), based on either microbiological or clinical criteria, and mortality to 28d. The criterion for diagnosing "nosocomial infection" was one or more of: 1) positive blood culture: single positive for pathogenic bacteria; positive on two occasions four days apart for commensal organisms 2) positive urine culture: suprapubic or urethral catheter 3) positive CSF culture 4) positive culture from bone abscess or pleura; 5) radiological evidence of NEC
Cairo 1995: A multi‐centre, randomised, placebo controlled trial, not stratified.
Participants were 20 infants infants without evidence of infection, who were recruited into the study at < 72h postnatal age. Neutropenia not an entry criterion.
BW 500 ‐ 1500g, GA < 34w.
Intervention: This was a phase I/II study in which five infants received 5 mcg/kg GM‐CSF IV daily for 7d, five infants received 5 mcg/kg GM‐CSF IV twice daily for 7d, five infants received 10 mcg/kg GM‐CSF IV daily for 7d. Five infants received placebo daily for 7d.
Outcomes: The primary outcomes were: a positive blood culture during the 10d from the start of in intervention; mortality to 30d.
STUDIES POSSIBLY ELIGIBLE FOR INCLUSION The following studies were excluded from the current systematic review, but may be eligible for inclusion in future updates: See: Table of studies awaiting assessment.
Kucukoduk 2002: A randomised controlled trial of 40 infants with suspected sepsis. 20 infants received G‐CSF 5ug/kg/day for 3 days, 20 infants received placebo. Primary outcome: mortality. one treated and two control infants died. The study cannot be included at present, as the timing of the infant deaths in relation to study entry is not reported. The authors are being contacted.
La Gamma 2000: A multicentre randomised placebo controlled trial, stratified by birthweight and centre. Participants were 137 infants > 7d postnatal age, with "clinical and laboratory signs of sepsis requiring antibiotics". Neutropenia was not a recruitment criterion. BW 500 ‐ 1500g.
Intervention: 66 infants received G‐CSF 10 mcg/kg/d for up to five days, 71 infants received placebo.
Outcomes: Treatment failure was defined as all‐cause mortality and/or persistent organ dysfunction and/or new infection up to 28 days from study entry.
Results: Treatment failure occurred in 59% of treated and 52% of control infants. Mortality by 28 days occurred in 14% of each group. No information is provided on the numbers of infants with neutropenia at trial entry or the outcome of any neutropenic subgroup. Toxicity: G‐CSF was "well tolerated". There was no difference between groups in the incidence of post treatment chronic lung disease, NEC, or other organ dysfunction. The trial was terminated early for futility following a planned blinded safety analysis, which showed a much greater than expected treatment failure. This study is eligible for inclusion on the basis of methodology, but the results have, to date, only been reported in conference proceedings abstract form.
USE OF G‐CSF OR GM‐CSF
TREATMENT STUDIES: Six studies used G‐CSF (Filgrastim, "Neupogen" ‐ Amgen Corp. USA). One study (Bilgin 2001) used GM‐CSF (Molgramostim, "Leucomax" ‐ Novartis, UK). Administration was either by subcutaneous injection or by slow intravenous infusion. Apart from the phase I/II dose‐finding study (Gillan 1994), all the G‐CSF studies used a dose of 10 mcg/kg/day for 3 days, or in one study for the duration of antibiotic therapy (Bedford‐Russell 2001). The GM‐CSF study (Bilgin 2001) used 5 mcg/kg/day for seven days.
PROPHYLAXIS STUDIES: All published randomised studies have used GM‐CSF, but in two different preparations. Carr 1999 used Molgramostim ("Leucomax" Novartis) which is a non‐glycosylated recombinant protein. Cairo 1995 and Cairo 1999 used sargramostim ("Leukine", Immunex Corp. USA) which is a glycosylated recombinant protein. They are believed to have equivalent in vivo activity. The former study used 10 mcg/kg/day by SC injection; the latter 8 mcg/kg/day by IV infusion.
Risk of bias in included studies
See Additional table: Table 3 Methodological quality of included studies
Size: All seven of the treatment studies were small; the largest recruiting 60 infants. Only one study (Miura 2001) reported power or justified sample sizes.
Bias: Adequate allocation concealment (grade A) was described in only two of the 10 studies; in one study (Bilgin 2001) allocation was quasi‐random (alternate) and in the remainder the method of randomisation was unclear. In five studies a placebo was used to blind the intervention. However, because the administration of G‐CSF or GM‐CSF rapidly increases peripheral blood neutrophil counts, true blinding of treatment allocation can never be fully achieved. When outcomes other than mortality are used, blinding of outcome assessment is more important as in neonates the identification of systemic infection involves an element of clinical judgement. Eight of the ten studies reported results using an intention to treat approach, including all randomised infants. The two remaining treatment trials (Bilgin 2001; Drossou‐Agakidou '98) analyse infants with clinical signs of sepsis plus a positive blood culture. Our interpretation is that recruitment of infants into these two studies was delayed until culture results were available. However it is not entirely clear whether some infants were recruited on clinical suspicion of infection and those subsequently found to have negative blood cultures excluded from the analysis (Table 3: completeness of follow‐up unclear). Clarification could not be obtained though both authors were contacted. Recruitment to one study (Miura 2001) was stopped early following an interim analysis, because of lack of evidence of efficacy. This may produce a biased estimate of the treatment effect.
A quality score was produced by assigning one point for adequate handling of each of the following potential sources of bias: adequate allocation concealment, blinding of the intervention, blinding of outcome assessment, completeness of follow‐up of all randomised subjects for short term outcomes, and completing the planned recruitment. The quality score therefore ranges from 0 (worst) to 5 (best). There were five high quality trials (one score of 5, four scores of 4).
Treatment studies: The identification of systemic infection as a recruitment criterion was inadequate in one study, being based only on "the initiation of antibiotics" (Gillan 1994). In this study less than 5% of recruits had sepsis confirmed by positive blood culture. The remaining studies used appropriate clinical and/or laboratory criteria. Two studies (Bilgin 2001; Drossou‐Agakidou '98) required a positive blood culture for recruitment (however see above). The outcome of infants with culture positive "confirmed" systemic infection was not reported for any study.
Prophylactic studies: All studies reported the incidence of systemic infection as the primary outcome. However, all three used different criteria for identifying episodes of sepsis. The incidence of infection as an outcome will be very different depending on whether episodes are identified by both clinical and microbiological criteria together, or by either clinical criteria or microbiological criteria alone. Because of the difficulty of diagnosing systemic infection in neonates, high quality studies should pre‐define this outcome on the basis of both objective clinical and microbiological criteria, and use assessors that are blind to the randomised intervention. This standard was not met by any study.
Toxicity: Only two studies (Bedford‐Russell 2001; Carr 1999) discussed individual clinical features that might indicate toxicity. The general statements concerning lack of toxicity in other studies gives no indication of how closely information on toxicity was sought. No studies included long term follow up of all recruits.
Countries undertaking studies: The location of studies involving infection may affect the outcome, because of differences in the incidence of nosocomial infection, the spectra of causative bacteria and the nature of the newborn population studied. Treatment studies: three studies recruited infants in the US, and one each in Greece, Mexico, Turkey and UK. Prophylaxis studies: two studies in US, one in UK.
Effects of interventions
See: List of comparisons
616 infants have been enrolled into fully published RCTs to evaluate the potential benefit of G‐CSF or GM‐CSF when used as treatment or prophylaxis of sepsis in preterm neonates. The analyses have been grouped under 3 headings: 1) treatment studies 2) prophylaxis studies 3) toxicity of G‐CSF and GM‐CSF
TREATMENT STUDIES [Comparisons and Data Tables 01.] These are studies that have examined the ability of G‐CSF or GM‐CSF to reduce mortality in suspected or microbiologically proven systemic bacterial infection. Seven studies (n = 257) met the inclusion criteria and reported at least one outcome of interest. Five used G‐CSF, one used GM‐CSF and one (Ahmad 2002) had both a G‐CSF and a GM‐CSF treatment arm. In two studies (n = 95) (Bilgin 2001, Drossou‐Agakidou '98) a positive blood culture plus clinical signs of sepsis were criteria for recruitment. In the remaining studies 30% (48/162) of recruited infants with clinically suspected sepsis were subsequently found to be blood culture positive. The outcomes of the culture positive subgroup were not specifically identified in any study and therefore separate analysis was not possible.
Primary outcomes ‐ mortality to day 14 and day 28 from study entry: Mortality to day 14 [comparison 01.01]:
All cause mortality to day 14 was available for all seven studies (n = 257). Overall there was no significant reduction in mortality in treated infants [RR 0.71 (95% CI 0.38,1.33); RD ‐0.05 (95% CI ‐0.14, 0.04)]. Only one study showed a significant benefit from CSF therapy (Bilgin 2001) [RR 0.22 (95% CI 0.05, 0.94); RD ‐0.23 (95% CI ‐0.42, ‐0.05)]. This study had features which may be of relevance to its efficacy: all infants had microbiologically proven infection, which in over 75% of cases was due to gram negative organisms, and all subjects were neutropenic. It was also the largest study to use GM‐CSF. If this study is excluded, the 6 remaining treatment studies showed almost no effect of G‐CSF treatment on mortality [RR 1.09 (95% CI 0.52, 2.29); RD 0.02 (95% CI ‐0.09, 0.12)].
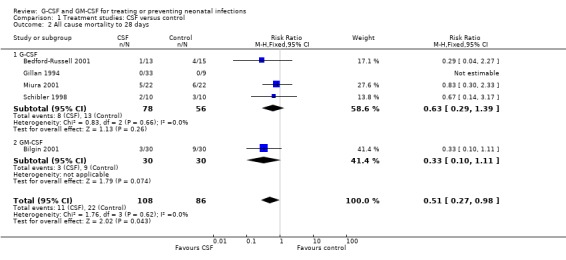
Mortality to day 28 [comparison 01.02]: Data on mortality to day 28 could be extracted from five studies (n = 194). Between D14 and D28 there were five additional control and two additional treatment group deaths in studies with mortality outcome reported for both timepoints. This increased the difference between the groups to show an overall reduction in mortality in treated infants which just reaches significance [RR 0.51 (95% CI 0.27, 0.98); RD ‐0.12 (‐0.23, ‐0.01); NNT 9 (95% CI 5‐100)]. However this result was affected to a large extent by the Bilgin GM‐CSF study described above. If this is excluded, the four G‐CSF studies did not show significant reduction in mortality [RR 0.63 (95% CI 0.29, 1.39); RD ‐0.08 (95% CI ‐0.21, 0.06)].
Primary outcomes ‐ mortality to day 14 and day 28 from trial entry ‐ neutropenic subgroup: Mortality to day 14, in infants neutropenic at trial entry [comparison 01.05]: Neutropenia, for the purpose of this subgroup analysis was defined as < 1.7 x 109/l. In two studies neutropenia of this degree was a recruitment criterion (Bilgin 2001; Schibler 1998). While all but one study noted that some recruited infants were neutropenic, their outcomes were not separately reported. The study authors were contacted to obtain outcome data on those infants who had neutropenia, as defined, at study entry. To date, additional data have been received only from Bedford‐Russell.
Thus three studies (n = 97) (Bedford‐Russell 2001; Schibler 1998; Bilgin 2001) contribute to this subgroup analysis. Together they show a significant reduction in mortality to day 14 when a CSF is given to neonates with systemic infection and neutropenia [RR 0.34 (95% CI 0.12, 0.92); RD ‐0.18 (95% CI ‐0.33, ‐0.03); NNT 6 (95% CI 3 ‐ 33)]. However this result is again influenced to a major extent by the Bilgin study, which contributed 67% of the infants in this subgroup. In sensitivity analyses using either a random effects model or only the high quality studies, there is no longer a significant reduction.
Mortality to day 28: in infants neutropenic at trial entry [comparison 01.06]: The same three studies were analysed for mortality at day 28 from recruitment. There was one additional death in each treatment group between day 14 and 28, the overall outcome is therefore similar [RR 0.38 (95% CI 0.16, 0.95); RD ‐0.18 (95% CI ‐0.34, ‐0.03); NNT 6 (95% CI 3 ‐ 34)].
Secondary outcomes Mortality to discharge: Five studies reported the number of infants who survived to discharge. In one study [Gillan 1994] there were no deaths and thus four studies contribute to this analysis (Ahmad 2002; Bedford‐Russell 2001; Schibler 1998; Bilgin 2001) (n = 136) [RR 0.53 (95% CI 0.25, 1.16); RD ‐0.09 (95% CI ‐0.20, 0.02)]; [comparison 01.03]. When only those infants with neutropenia at the start of the trial intervention are included (n = 97) there is a significantly lower all cause mortality following CSF treatment [RR 0.38 (95% CI 0.16, 0.95); RD ‐0.18 (95% CI ‐0.34, ‐0.03); NNT 6 (95% CI 3 ‐ 33)]; [comparison 01.07].
Failure to correct neutropenia within 48h from trial entry [comparison 01.04]: This was reported in three studies (Bilgin 2001; Miura 2001; Schibler 1998) (n = 88).There was a significant difference in the risk of failure to correct neutropenia between infants treated with CSF and controls by 48h from the start of the intervention [RR 0.13 (95% CI 0.04, 0.46); RD ‐0.39 (95% CI ‐0.55, ‐0.24)]. The greatest effect was seen in the GM‐CSF treatment study, however the number of infants in whom this outcome was reported following G‐CSF treatment (n = 13) was small. An additional study (Ahmad 2002) specifically compared the neutrophil counts at day two, five, seven between those infants treated with G‐CSF and GM‐CSF. G‐CSF resulted in a significantly more rapid correction of neutropenia by day 7 (p = 0.03).
Long term follow‐up; mortality and disability reported beyond discharge: This has only been reported in one study (Gillan 1994), and then only for part of the study population. Twenty one of the original 33 treated and nine control infants were examined at median two years (range 15 to 36m) (Rosenthal 1996). There had been no deaths since the primary report. The cognition, language and social developmental performance scores were within the normal range for age and motor deficits were "typical of high‐risk, low birth weight neonates". However there was no comparison made between G‐CSF and control infants.
PROPHYLAXIS STUDIES (Comparisons and Data Tables 02.) These are studies in which G‐CSF or GM‐CSF have been given prophylactically, starting as soon as possible after birth, with the aim of enhancing neonatal immunity in order to reduce the incidence of systemic infection and infection related mortality. Only three studies have been published (n = 359). All three used GM‐CSF and all restricted recruitment to infants < 1500g or < 32w gestation. The primary outcome of prophylactic studies for this review is a reduction in systemic infection. One study (Carr 1999) (n = 75) identified episodes of sepsis by the onset of indicative clinical signs supported by a positive blood culture. The other two studies (Cairo 1995, Cairo 1999) (n = 284) used either clinical or microbiological criteria alone for identifying sepsis as an outcome. Only the secondary outcome short term mortality is comparable and can be combined for all three studies.
Primary outcomes: Sepsis incidence to 10 days after intervention complete [comparison 02.03]: Short term sepsis prevention, where sepsis is defined by clinical plus microbiological criteria, is reported only by (Carr 1999) (n = 75). Although there was a trend to reduce sepsis in the GM‐CSF prophylaxis group, the reduction in sepsis incidence observed did not reach significance [RR 0.66 (95% CI 0.36, 1.20); RD ‐0.16 (95% CI ‐0.37, 0.06)]; [comparison 02.03.01]. In studies where sepsis was defined by either clinical or microbiological criteria alone (Cairo 1995;Cairo 1999) (n = 284) sepsis incidence did not differ between treated and control infants [RR 1.02 (95% CI 0.76, 1.37); RD 0.01 (95% CI ‐0.11, 0.12)]; [comparison 02.03.02].
Sepsis from start of intervention to discharge: Longer term sepsis prevention was only reported by (Carr 1999) [RR 0.81 (95% CI 0.75, 1.24); RD 0.10 (95% CI ‐0.32, 0.13)].
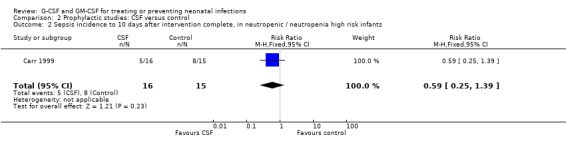
Sepsis incidence to 10 days after intervention complete, in infants with pre‐existing neutropenia or at high risk of developing neutropenia at trial entry [comparison 02.02]: Neutropenia, for the purpose of this subgroup analysis was defined as < 1.7 x 109/l; high risk of postnatal neutropenia was defined as infants with a birth weight below the tenth centile, or born to mothers with pregnancy‐induced hypertension. Only one study separately identified neutropenic or high neutropenia‐risk infants (Carr 1999). The number of these infants developing sepsis following GM‐CSF (5/16), was less than controls (8/15) but the difference, based on such small numbers, was not significant [RR 0.59 (95% CI 0.25, 1.39); RD ‐0.22 (95% CI ‐0.56, 0.12)].
Secondary outcome: Mortality to 10 days after intervention complete [comparison 02.01]: Short term all cause mortality is reported for all studies. In the phase I/II study (Cairo 1995) there were no deaths, thus two studies (n = 339) contribute to the analysis. Overall mortality was 5.3%. There was no significant mortality reduction in treated infants [RR 0.59 (95% CI 0.24, 1.44); RD ‐0.03 (95% CI ‐0.08, 0.02)].
Correction of existing, or prevention of subsequent neutropenia: Only one study addressed this issue (Carr 1999) and observed that prophylactic GM‐CSF completely prevented the occurrence of neutropenia for up to 28 days following GM‐CSF prophylaxis.
Long term outcomes: These have not been reported for any prophylaxis study.
Toxicity: G‐CSF: Six studies used G‐CSF, with a total of 107 infants receiving treatment. All commented on the absence of adverse events attributable to G‐CSF therapy. Only one study (Bedford‐Russell 2001) quantified possible adverse events, including oedema, bradycardia, anaemia, thrombocytopenia, hyponatraemia and oxygen index. There were no differences between the groups, though the oxygen index worsened in more placebo group infants.
GM‐CSF: Five studies used GM‐CSF, with a total of 225 infants receiving GM‐CSF as sepsis treatment or prophylaxis. Again all authors commented that GM‐CSF was well tolerated with no adverse reactions. Only one study (Carr 1999) specifically analysed clinical outcomes which might be attributable to GM‐CSF and found no difference in fever, weight change, oxygen requirement during therapy, chronic lung disease or necrotising enterocolitis.
Heterogeneity: In part because of the small size of the studies available for inclusion, there was no evidence of significant heterogeneity between the studies.
Discussion
TREATMENT STUDIES There is no evidence from the seven published studies included in this meta‐analysis that the addition of G‐CSF or GM‐CSF to antibiotic therapy in preterm infants with suspected systemic infection reduces immediate all cause mortality. No significant survival advantage was seen at 14 days from the start of therapy [RR 0.71 (95% CI 0.38, 1.33); RD ‐0.05 (95% CI ‐0.14, 0.04)]. However, all seven of the treatment studies were small, the largest recruiting only 60 infants. As this review is limited to studies where there is a full peer reviewed publication, there is substantial potential for publication bias.
The subgroup analysis of 97 infants from three treatment studies (Bedford‐Russell 2001; Bilgin 2001; Schibler 1998) who, in addition to systemic infection, had clinically significant neutropenia (< 1.7 x109/l) at trial entry, shows a significant reduction in mortality to day 14 in those infants receiving G‐CSF or GM‐CSF [RR 0.34 (95% CI 0.12, 0.92); RD ‐0.18 (95% CI ‐0.33, ‐0.03); NNT 6 (95% CI 3 ‐ 33)]. That CSF therapy influences mortality directly related to the septic episode is suggested by noting that only 10% of deaths in these analyses occurred beyond 14 days and was equally distributed between treated and control infants. However the conclusion that CSF therapy reduces sepsis related mortality in infants who are neutropenic cannot be viewed as definitive. Sixty seven percent of subjects in the neutropenic subgroup were from a single study (Bilgin 2001) and in sensitivity analyses using a random effects model the reduction in mortality is no longer significant. Despite this caveat, the Bilgin study may have an important message when selecting infants for future CSF studies, in that all recruited infants were severely neutropenic (< 1.5 x109/l) and 75% were bacteraemic with a gram negative or group B Streptococcal infection.
A similar conclusion, that CSF therapy may improve survival in septic neonates who are also neutropenic, was reached by the one previous published meta‐analysis (Bernstein 2001). This analysis of five studies including 155 subjects did not include the quasi‐randomised Bilgin study but did include two small non‐randomised studies excluded from this review.
When the effect of G‐CSF on outcome is analysed having excluded the two trials in which neutropenia <1.7 x109/l was a pre‐specified recruitment criterion (Bilgin 2001; Schibler 1998), mortality is similar in both treated and control infants. The three treatment studies (Drossou‐Agakidou '98; Gillan 1994; Miura 2001) in which neutropenia, as defined in this review, was not a recruitment criterion, when combined with the non‐neutropenic subjects reported by Bedford Russell, had a RR 1.22 (95% CI 0.50, 2.99); RD 0.03 (95% CI ‐0.10, 0.15); [comparison 01.08]. The lack of effect of G‐CSF on sepsis related mortality in the absence of neutropenia is supported by the largest randomised controlled trial of CSF therapy undertaken in septic neonates (La Gamma 2000). This large multicentre study, which has only been reported in abstract form and so excluded from this meta‐analysis, randomised 137 infants with late onset nosocomial infection, as indicated by clinical and laboratory signs, to receive G‐CSF 10 mcg/kg/d for 5 days or placebo. There was no difference in mortality between the groups, which was 14% at 28 days from trial entry in each arm.
PROPHYLAXIS STUDIES Only three prophylaxis studies have been published. Although all three used GM‐CSF, they adopted very differing treatment regimens and do not use comparable criteria for identifying the primary outcome, episodes of systemic infection. The incidence of sepsis will differ depending on whether septic episodes are identified by both clinical and microbiological criteria together, or by either clinical criteria or microbiological criteria alone. This is because the majority of blood culture isolates from preterm newborns are coagulase negative staphylococci and it is estimated that approximately half of such isolates represent contamination rather than true systemic infection. In the protocol for this review we stated that studies using these two different levels of diagnostic criteria were not comparable and should be analysed separately. Thus, only the outcome of short term mortality could be combined for all three studies. GM‐CSF prophylaxis did not lead to a significant reduction in mortality. Prophylactic GM‐CSF may provide protection against infection when given to infants less that 32 weeks gestation who are neutropenic (< 1.7 x109/l) or at high risk of developing postnatal neutropenia. However this high risk group has only been recruited into one study (Carr 1999). The incidence of systemic infection in this subgroup was reduced from 53% to 31%, but the numbers were small (n = 31) and the difference not statistically significant [RR 0.59 (95% CI 0.25, 1.39); RD ‐0.22 (95% CI ‐0.56, 0.12)]. A similar effect was observed in a small non‐randomised prophylactic study using short term G‐CSF in neonates with established neutropenia (Kocherlakota 1998).
The authors are currently investigating the effect of prophylactic GM‐CSF in high neutropenia risk neonates in a large multicentre randomised controlled trial in the UK. This trial is recruiting infants who are both less that 32 weeks gestation and less than the tenth centile for birth weight (PROGRAMS).
EFFECTS ON LABORATORY PARAMETERS AND CLINICAL TOXICITY Both G‐CSF and GM‐CSF appear to be effective at correcting neutropenia, as they are in adult clinical practice. No toxicity associated with either G‐CSF or GM‐CSF was reported in any study included in this review, or in other published non‐randomised studies. However, some anxieties have been expressed about the theoretical possibility of late haematological toxicities. These include the development of leukaemia, cytopenias as a result of "lineage steal" effect, and the formation of antibodies to exogenously administered CSFs. In a recent review (Carr 2003) we concluded that cellular biology studies of the mode of CSF action, and clinical studies in children and adults, have not provided any evidence in support of these theoretical risks in neonates. Although rare outcomes are unlikely to be detected in RCTs, future studies should monitor for clinical outcomes which might be related to unexpected long term adverse effects.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to support the introduction of either G‐CSF or GM‐CSF into neonatal practice, either as treatment of established systemic infection to reduce resulting mortality, or as prophylaxis to prevent systemic infection in high risk neonates. No toxicity associated with the use of either G‐CSF or GM‐CSF was reported in any study included in this review, or in other published non‐randomised studies.
Implications for research.
The reduction in mortality observed when G‐CSF or GM‐CSF have been given to infants with suspected or microbiologically proven systemic infection associated with severe neutropenia needs to be investigated further in adequately powered, well designed RCTs. Data from existing studies suggest that future trials should focus on infants with systemic infection and a neutrophil count less than 1.7 x 109/l, and should be designed to recruit sufficient infants infected with organisms associated with a significant mortality risk. The data available do not support further study of CSFs in septic infants who are not neutropenic.
Neutropenia or high risk of neutropenia also appears to be of relevance to efficacy in prophylactic studies. Whether prophylactic GM‐CSF can reduce systemic infection or mortality in infants at high risk of postnatal neutropenia is currently being investigated in a UK study funded by Action Research, with a long term follow up study funded by the Wellcome Trust (PROGRAMS). This study will complete recruitment in 2004 and it is hoped that it will provide definitive guidance on the role of prophylactic CSFs in neonatal practice.
As infection in neonates not only causes short term mortality but may also be a major contributor to long term neurodisability, all future studies should include long term neurological and neurodevelopmental follow up.
What's new
| Date | Event | Description |
|---|---|---|
| 21 October 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 28 April 2003 | New citation required and conclusions have changed | Substantive amendment |
Data and analyses
Comparison 1. Treatment studies: CSF versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality to 14 days | 7 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.33] |
| 1.1 G‐CSF | 6 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.29] |
| 1.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.94] |
| 2 All cause mortality to 28 days | 5 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.98] |
| 2.1 G‐CSF | 4 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.29, 1.39] |
| 2.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.11] |
| 3 All cause mortality to discharge | 5 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.16] |
| 3.1 G‐CSF | 4 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.27, 2.21] |
| 3.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.11] |
| 4 Failure to correct neutropenia in those neutropenic at trial entry | 3 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.46] |
| 4.1 G‐CSF | 2 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.07, 2.46] |
| 4.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.44] |
| 5 All cause mortality to 14 days, in those neutropenic at trial entry | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.12, 0.92] |
| 5.1 G‐CSF | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.14, 2.43] |
| 5.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.94] |
| 6 All cause mortality to 28 days, in those neutropenic at trial entry | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.95] |
| 6.1 G‐CSF | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.83] |
| 6.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.11] |
| 7 All cause mortality to discharge, in those neutropenic at trial entry | 3 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.95] |
| 7.1 G‐CSF | 2 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.12, 1.83] |
| 7.2 GM‐CSF | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.11] |
| 8 All cause mortality to 14 days, in those without known neutropenia at trial entry | 4 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.50, 2.99] |
| 8.1 G‐CSF | 4 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.50, 2.99] |
| 8.2 GM‐CSF | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 1 All cause mortality to 14 days.
1.2. Analysis.
Comparison 1 Treatment studies: CSF versus control, Outcome 2 All cause mortality to 28 days.
1.3. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 3 All cause mortality to discharge.
1.4. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 4 Failure to correct neutropenia in those neutropenic at trial entry.
1.5. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 5 All cause mortality to 14 days, in those neutropenic at trial entry.
1.6. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 6 All cause mortality to 28 days, in those neutropenic at trial entry.
1.7. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 7 All cause mortality to discharge, in those neutropenic at trial entry.
1.8. Analysis.

Comparison 1 Treatment studies: CSF versus control, Outcome 8 All cause mortality to 14 days, in those without known neutropenia at trial entry.
Comparison 2. Prophylactic studies: CSF versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality to 10 days after intervention complete | 3 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.44] |
| 1.1 G‐CSF | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 GM‐CSF | 3 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.24, 1.44] |
| 2 Sepsis incidence to 10 days after intervention complete, in neutropenic / neutropenia high risk infants | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.39] |
| 3 Sepsis incidence to 10 days after intervention complete, stratified by sepsis identification criteria | 3 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.21] |
| 3.1 Sepsis identified by clinical plus microbiological criteria | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.36, 1.20] |
| 3.2 Sepis identified by either clinical or microbiological criteria | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.37] |
2.1. Analysis.

Comparison 2 Prophylactic studies: CSF versus control, Outcome 1 All cause mortality to 10 days after intervention complete.
2.2. Analysis.
Comparison 2 Prophylactic studies: CSF versus control, Outcome 2 Sepsis incidence to 10 days after intervention complete, in neutropenic / neutropenia high risk infants.
2.3. Analysis.

Comparison 2 Prophylactic studies: CSF versus control, Outcome 3 Sepsis incidence to 10 days after intervention complete, stratified by sepsis identification criteria.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2002.
| Methods | Multi‐centre randomised double‐blind placebo controlled trial | |
| Participants | 28 infants with early or late onset sepsis. Inclusion criteria were clinical signs of sepsis plus either neutropenia or proven bacteraemia. Sepsis defined as one of: Shock (requiring fluid boluses, vasopressors, or developing acidosis), Respiratory failure requiring mechanical ventilation; Necrotising enterocolitis (radiological or surgical diagnosis). Neutropenia defined as <1 x 10(9)/l Neutropenia secondary to maternal preeclampsia was not counted as a criteria for recruitment . GA < 32w, BW 445 ‐ 1960g, no postnatal age limit. | |
| Interventions | Randomisation was to G‐CSF, GM‐CSF or placebo. 10 infants received G‐CSF 10 mg/kg/day in 2 divided doses by iv inf. 10 infants received GM‐CSF 8 mg/kg/day in 2 divided doses by iv inf. 8 infants received placebo. Treatment continued for 6 days, or until ANC > 10 x 10(9)/l. G‐CSF (Filgrastim, Neupogen, Amgen, Thousand Oaks, CA) GM‐CSF (Sargramostim, Leukine, Immunex, Seattle, WA) Placebo was 2 ml saline. | |
| Outcomes | Mortality to discharge. Neutrophil counts on days 2,5,7 from study entry. * 3 infants died directly related to the septic episode: 2 G‐CSF; 1 GM‐CSF group. 1 additional infant who received G‐CSF died from a new infection 1 month later. | |
| Notes | 12 infants had early onset sepsis (G‐CSF 2; GM‐CSF 4; Placebo 6) 16 infants had late onset sepsis (G‐CSF 8; GM‐CSF 6; Placebo 2) Positive blood cultures at recruitment:11 Treated infants; of which Gram negs = 5. 5 Placebo infants; of which Gram negs = 1. (Gram negs were E.coli (4) or Klebsiella sp (1). There were no GBS or Staph aureus isolates) Outcome of infants with positive cultures not reported separately. Number of infants with neutropenia at recruitment not reported, nor outcomes of neutropenic subgroup. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bedford‐Russell 2001.
| Methods | Multi‐centre randomised double‐blind placebo controlled trial, stratified by centre and age at infection onset (< 72, > 72h) | |
| Participants | 28 infants with clinical signs of sepsis, and neutrophils < 5 x 10(9)/l Sepsis defined as >/= 2 objective clinical signs of sepsis requiring treatment with antibiotics. Microbiological confirmation not an entry criteria. GA > 25w, BW 500 ‐ 1500g, age < 29d. 4 centres in the UK. Dates not given. | |
| Interventions | 13 infants received 10 mcg/kg G‐CSF IV daily for a maximum of 14d or until discontinuation of antibiotics 15 infants received placebo. Both were clear colourless fluids (Filgrastim, Amgen, Thousand Oaks, CA, US) | |
| Outcomes | Mortality during the 42d from study entry. Survival to 12m post‐menstrual age. Treatment related adverse events (peripheral oedema, bradycardia, anaemia, thrombocytopenia and hyponatraemia) occurred in five placebo treated infants and two infants receiving G‐CSF. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bilgin 2001.
| Methods | Single centre, controlled trial, quasi‐randomised (alternate allocation) stratified by growth status (SGA, AGA, LGA) | |
| Participants | 60 infants with clinical signs of sepsis and neutropenia. Definition of sepsis for recruitment: New symptons/objective clinical signs of sepsis plus at least 1 positive blood culture, plus neutropenia. Definition of neutropenia for recruitment: Neutrophils < 1.5 x 10(9)/l GA <42w, BW 1100 ‐ 3500g, age < 29d. 25 treated and 24 controls had early onset sepsis. 1 centre, Turkey. Conducted January 1994 to March 1995. | |
| Interventions | 30 infants received 5 mcg/kg GM‐CSF SC daily for 7d 30 infants were controls (no placebo used). (Leucomax, Novartis, UK) | |
| Outcomes | Mortality to discharge. All surviving infants were discharged on or before day 21 from admission. All neonates tolerated GM‐CSF well with no adverse reactions. | |
| Notes | All infants included had positive blood cultures. Was recruitment and randomisation delayed until culture positive sepsis confirmed? Were infants with negative blood cultures excluded post randomisation? If so it is not stated and no information is provided on their outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Cairo 1995.
| Methods | Multi‐centre randomised placebo controlled trial, not stratified. | |
| Participants | 20 infants, BW 500 ‐ 1500g, GA < 34w, age < 72h. 3 centres in the US. December 1992 to Aug. 1994. | |
| Interventions | 5 infants received 5 mcg/kg GM‐CSF (Immunex, Seattle, US) IV daily for 7d, 5 infants received 5 mcg/kg GM‐CSF IV twice per d for 7d, 5 infants received 10 mcg/kg GM‐CSF IV daily for 7d, 5 infants received placebo daily for 7d. | |
| Outcomes | Positive blood cultures during the 10d observation period. Mortality to 30d. The GM‐CSF was well tolerated at all doses administered. One patient receiving 5 mcg/kg twice per day developed grade III disseminated intravascular coagulation secondary to necrotising enterocolitis on day 8. There were no grade III or IV pulmonary, hepatic, or haematologic toxicities. | |
| Notes | Group receiving two IV doses per day were not blind | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cairo 1999.
| Methods | Multi‐centre randomised double‐blind placebo controlled trial, stratified by centre and BW (501 ‐ 750, 751 ‐ 1000g). | |
| Participants | 264 infants without evidence of infection, BW 501 ‐ 1000g, all AGA (small for gestation infants excluded), age < 72h. Neutropenia not an entry criteria. 12 centres in the US. February 1995 to April 1997. | |
| Interventions | 134 infants received 8 mcg/kg GM‐CSF IV daily for 7d then every other day for an additional 21d, Treatment discontinued If WBC > 20 or if venous access no longer available 5% of treated infants needed a "dose reduction" (unspecified) GM‐CSF (Immunex, Seattle, US) 130 infants received placebo. | |
| Outcomes | Incidence of "confirmed nosocomial infections"* during treatment (ie to day 28 from trial entry). Mortality to 28d. Infants followed to day 58 but outcomes at that time not reported. * Infection incidence (as defined by authors) during treatment. No grade III/IV toxicity (National Cancer Institute common toxicity criteria) or adverse events were associated with GM‐CSF. No further details given | |
| Notes | Criteria for diagnosing "Confirmed nosocomial sepsis"*:
One or more of:
1. Positive blood culture: Single positive for pathogenic bacteria,
Positive on 2 occasions 4 days apart for commensal organisms.
2. Positive urine culture: Suprapubic or urethral catheter
3. Positive CSF
4. Positive culture from bone abscess or pleura
5. Radiological evidence of NEC Incidence distribution of these criteria or organisms not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Carr 1999.
| Methods | Multi‐centre, randomised controlled trial, stratified by growth status (AGA or SGA) | |
| Participants | 75 infants, BW 480 ‐ 1760g, GA < 32w, age < 72h. 2 centres in UK. Dates not given | |
| Interventions | 36 infants received 10 mcg/kg GM‐CSF (Leucomax, Novartis, UK) SC daily for 5d, 39 infants were controls (no placebo used) | |
| Outcomes | Symptomatic blood culture positive sepsis to 14d. Mortality to 28d. Mortality to 6m. During GM‐CSF administration there was no local toxicity at injection sites, no evidence of increased irritability, which might suggest bone pain, and no difference from controls in terms of pyrexia or weight change. There was no significant difference between the groups in oxygen requirement during treatment or during the subsequent week. Similar numbers of treated and control infants were oxygen‐dependent at 28 days postnatal age and 36 weeks post‐menstrual age. No gastrointestinal toxicity was observed, in particular necrotising enterocolitis was suspected or confirmed in 2 infants in each treatment group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Drossou‐Agakidou '98.
| Methods | Single centre randomised controlled trial, not stratified | |
| Participants | 35 infants with proven sepsis and neutrophils < 5 x 10(9)/l Definition of sepsis for recruitment: Presence of objective clinical signs indicative of sepsis plus at least 2 laboratory findings indicative of sepsis plus a positive blood culture. GA 24 ‐ 37w, BW 720 ‐ 2940g, age < 28d. 1 centre, Greece. Dates not given. | |
| Interventions | 19 infants received 10 mcg/kg G‐CSF SC daily for 3d, 16 infants were controls (no placebo used). (Granulokine, Roche) | |
| Outcomes | Mortality within 10d of sepsis onset. G‐CSF was well tolerated and no short term adverse effects were observed. | |
| Notes | All infants included had positive blood cultures. Was recruitment and randomisation delayed until culture positive sepsis confirmed? Were infants with negative blood cultures excluded post randomisation? If so it is not stated and no information is provided on their outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gillan 1994.
| Methods | Multi‐centre, randomised placebo controlled trial, stratified by GA (26 ‐ 30, 31 ‐ 35, 36 ‐ 40w) | |
| Participants | 42 infants with presumed sepsis. Definition of sepsis for recruitment: "blood cultures obtained and antibiotic therapy initiated" GA 26 ‐ 41w, BW > 800g, age < 3d, 2 centres in US. Conducted July 1991 to March 1993. | |
| Interventions | 9 infants received 1 mcg/kg/d G‐CSF (Amgen, Thousand Oaks, CA, US), 9 infants received 5 mcg/kg/d, 9 infants received 10 mcg/kg/d, 3 infants received 5 mcg/kg every 12h, 3 infants received 10 mcg/kg every 12h, all for 3d. 9 infants received placebo daily for 3d. | |
| Outcomes | Mortality during study. Mortality during follow‐up period (median follow‐up 15m). The use of G‐CSF seemed to be safe and well tolerated. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miura 2001.
| Methods | Single centre, randomised double‐masked placebo controlled trial, not stratified. | |
| Participants | 44 infants with clinical diagnosis of sepsis. Definition of sepsis for recruitment: >/= 3 objective clinical signs of sepsis plus >/= 1 laboratory criterion for sepsis plus blood culture taken and antibiotics commenced. Definition of neutropenia: neutrophils < 1.5 x 10(9)/l (not used as an entry criterion. GA < 37w, BW 500 ‐ 2000g, age <5d. 1 centre, Brazil. Conducted July 1996 to July 1997. | |
| Interventions | 22 infants received 10 mcg/kg G‐CSF IV daily for 3d, 22 infants received placebo (identical volume, visually indistinguishable). (Amgen, Thousand Oaks, CA, US) | |
| Outcomes | All cause mortality within 30d. Mortality to discharge. Nosocomial infections during the 14d after the final dose. We observed no adverse effects of G‐CSF. | |
| Notes | Trial was stopped early due to overwhelming evidence of non efficacy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schibler 1998.
| Methods | Multi‐centre randomised double‐blind placebo controlled trial, not stratified. | |
| Participants | 20 infants with early onset sepsis and neutropenia. Definition of sepsis for recruitment: Neutropenia plus elevated I/T ratio (>/= 0.25) plus ventilation. *Neutropenia based on Manroe criteria. This is variable and > 1.7 x 10(9)/l during first 3 days after birth. Therefore infants in this study may not be truly neutrophil depleted. 10 infants culture positive; 6 (3 from each group) blood culture positive. GA 24 ‐ 40w, BW 530 ‐ 3667g, age <3d. 2 centres, US. Conducted February 1993 to April 1995. | |
| Interventions | 10 infants received 10 mcg/kg G‐CSF) IV daily for 3d, 10 infants received placebo (identical volume). (Amgen, Thousand Oaks, CA, US | |
| Outcomes | Mortality to 7d. Mortality to discharge. No adverse effects of G‐CSF administration were noted. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of ongoing studies [ordered by study ID]
PROGRAMS.
| Trial name or title | PROGRAMS. Principal Investigators: Modi N, Carr R, Brocklehurst P. Trial statistician. Dore C. A multicentre, single blind, randomised controlled trial of prophylactic GM‐CSF to reduce sepsis in preterm neonates. |
| Methods | |
| Participants | Neonates <31 completed weeks GA and <10th centile BW. <72h postnatal age at trial entry. Trial size 320 subjects. |
| Interventions | Subjects randomised to GM‐CSF 10mcg/gk/d by sc inj for 5 days, or to no GM‐CSF |
| Outcomes | Primary outcome: Sepsis free survival at 14 days from trial entry. Secondary Outcomes: 1) survival without moderate or severe disability at 2 and 5 years corrected age. 2) microbiologically confirmed sepsis at 14 days and at 28 days from trial entry. 3)probable (culture negative) sepsis at 14 days and at 28 days from trial entry. Sepsis identification is based on predefined and objective clinical plus immunological plus microbiological criteria, assessed blind to treatment allocation. |
| Starting date | July 2000 |
| Contact information | Anne Smith, Trial Administrator, National Perinatal Epidemiology Unit, Oxford. email: anne.smith@perinatal‐epidemiology.ox.ac.uk |
| Notes | Anticipated to complete recruitment early 2004. |
Contributions of authors
All three reviewers contributed to data extraction. CD entered the data into RevMan, checked data accuracy and was responsible for statistical analysis. RC contacted authors for additional information. RC wrote the text of the review with contributions from NM and CD.
Declarations of interest
The authors were the investigators responsible for one prophylaxis study included in this review (Carr 1999).
The authors are currently investigating the effect of prophylactic GM‐CSF in neonates at high risk for neutropenia in a large multicentre randomised controlled trial in the UK (PROGRAMS).
Edited (no change to conclusions)
References
References to studies included in this review
Ahmad 2002 {published data only}
- Ahmad A, Laborada G, Bussel J, Nesin M. Comparison of recombinant G‐CSF, recombinant human GM‐CSF and placebo for treatment of septic preterm infants. Pediatric Infectious Disease Journal 2002;21:1061‐5. [DOI] [PubMed] [Google Scholar]
Bedford‐Russell 2001 {published and unpublished data}
- Bedford‐Russell AR, Emmerson AJB, Wilkinson N, Chant T, Sweet DG, Halliday HL, Holland B, Davies EG. A trial of recombinant human granulocyte colony stimulating factor for the treatment of very low birthweight infants with presumed sepsis and neutropenia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;84:F172‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilgin 2001 {published data only}
- Bilgin K, Yaramis A, Haspolat K, Tas A, Gunbey S, Derman O. A randomized trial of granulocyte‐macrophage colony‐stimulating factor in neonates with sepsis and neutropenia. Pediatrics 2001;107:36‐41. [DOI] [PubMed] [Google Scholar]
Cairo 1995 {published data only}
- Cairo MS, Christensen RD, Sender LS, Ellis R, Rosenthal J, Ven C, Worcester C, Agosti JM. Results of a phase I/II trial of recombinant human granulocyte‐macrophage colony‐stimulating factor in very low birthweight neonates: significant induction of circulatory neutrophils, monocytes, platelets, and bone marrow neutrophils. Blood 1995;86:2509‐15. [PubMed] [Google Scholar]
Cairo 1999 {published data only}
- Cairo MS, Agosti J, Ellis R, Laver JJ, Puppala B, DeLemos R, Givner L, Nesin M, Wheeler G, Seth T, Ven C, Fanaroff A. A randomised double‐blind placebo‐controlled trial of prophylactic recombinant human GM‐CSF to reduce nosocomial infection in very low birthweight neonates. Journal of Pediatrics 1999;134:64‐70. [DOI] [PubMed] [Google Scholar]
Carr 1999 {published and unpublished data}
- Carr R, Modi N, Doré CJ, El‐Rifai, Lindo D. A randomised controlled trial of prophylactic GM‐CSF in human newborns less than 32 weeks gestation. Pediatrics 1999;103:796‐802. [DOI] [PubMed] [Google Scholar]
Drossou‐Agakidou '98 {published data only}
- Drossou‐Agakidou V, Kanakoudi‐Tsakalidou F, Taparkou A, Tzimouli V, Tsandali H, Kremenopoulos G. Administration of recombinant human granulocyte‐colony stimulating factor to septic neonates induces neutrophilia and enhances the neutrophil respiratory burst and beta2 integrin expression Results of a randomized controlled trial. Neonatology 1998;157:583‐8. [DOI] [PubMed] [Google Scholar]
Gillan 1994 {published data only}
- Gillan ER, Christensen RD, Suen Y, et al. A randomized, placebo‐controlled trial of recombinant human granulocyte colony‐stimulating factor administration in newborn infants with presumed sepsis: significant induction of peripheral and bone marrow neutrophilia. Blood 1994;84:1427‐33. [PubMed] [Google Scholar]
- Rosenthal J, Healey T, Ellis R, Gillan E, Cairo M. A two year follow‐up of neonates with presumed sepsis treated with recombinant human G‐CSF during the first week of life. Journal of Pediatrics 1996;128:135‐7. [DOI] [PubMed] [Google Scholar]
Miura 2001 {published data only}
- Miura E, Procianoy RS, Bittar C, Miura CS, Miura MS, Mello C, Christensen RD. A randomized double‐masked, placebo controlled trial of recombinant granulocyte colony‐stimulating factor administration to preterm infants with the clinical diagnosis of early‐onset sepsis. Pediatrics 2001;107:30‐5. [DOI] [PubMed] [Google Scholar]
Schibler 1998 {published data only}
- Schibler KR, Osborne KA, Leung LY, Le TV, Baker SI, Thompson DD. A randomized placebo‐controlled trial of granulocyte colony‐stimulating factor administration to newborn infants with neutropenia and clinical signs of early‐onset sepsis. Pediatrics 1998;102:6‐13. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kucukoduk 2002 {published data only}
- Kucukoduk S, Sezer T, Yildiran A, Albayrak D. Randomised, double‐blind, placebo controlled trial of early administration of recombinant human G‐CSF to non‐neutropenic preterm newborns between 33 and 36 weeks, with presumed sepsis. Scandinavian Journal of Infectious Diseases 2002;34:893‐7. [DOI] [PubMed] [Google Scholar]
La Gamma 2000 {published data only}
- Gamma E, Welch W, Jeffs R. A multicentre, double‐blind, randomised, placebo‐controlled safety and efficacy study of filgrastim (rHu‐G‐CSF) in the treament of late‐onset neonatal sepsis. Pediatric Research 2000;47:409A. [Google Scholar]
References to ongoing studies
PROGRAMS {unpublished data only}
- Modi N, Carr R, Brocklehurst P. PROGRAMS.
Additional references
Bernstein 2001
- Bernstein HM, Pollock BH, Calhoun DA, Christensen RD. Administration of recombinant G‐CSF to neonates with septicaemia: a meta‐analysis. Pediatrics 2001;138:917‐20. [DOI] [PubMed] [Google Scholar]
Carr 1997
- Carr R, Modi N. Haemopoietic colony stimulating factors for preterm neonates. Archives of Disease in Childhood 1997;76:F128‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2000a
- Carr R. Neutrophil production and function in newborn infants. British Journal of Haematology 2000;110:18‐28. [DOI] [PubMed] [Google Scholar]
Carr 2000b
- Carr R, Huizinga TWJ. Low sFcRIII demonstrates reduced neutrophil reserves in preterm neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;83:F160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2003
- Carr R, Modi N. Haemopoietic growth factors for neonates: assessing risks and benefits. Acta Paediatrica 2003:In Press. [DOI] [PubMed] [Google Scholar]
Christensen 1989
- Christensen RD. Neutrophil kinetics in the fetus and neonate. Am J Pediatr Hematol Oncol 1989;11:215‐223. [PubMed] [Google Scholar]
Cooke 1997
- Cooke RW, Nycyk JA, Okuonghuae H, et al. Low dose vancomycin prophylaxis reduces coagulase‐negative staphylococcal bacteraemia in very low birthweight infants. J Hosp Infect 1997;37:297‐303. [DOI] [PubMed] [Google Scholar]
Damman 1998
- Damman O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol 1998;5:190‐201. [DOI] [PubMed] [Google Scholar]
Engle 1984
- Engle WD, Rosenfeld CR. Neutropenia in high risk neonates. J Pediatr 1984;105:982‐986. [DOI] [PubMed] [Google Scholar]
Gessler 1995
- Gessler P, Luders R, Konig S, et al. Neonatal neutropenia in low birthweight premature infants. Am J Perinatol 1995;12:34‐38. [DOI] [PubMed] [Google Scholar]
Hill 1987
- Hill HR. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res 1987;22:375‐382. [DOI] [PubMed] [Google Scholar]
Kocherlakota 1998
- Kocherlakota P, Gamma EF. Preliminary report: rhG‐CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia‐associated neutropenia. Pediatrics 1998;102:1107‐1111. [DOI] [PubMed] [Google Scholar]
Koening 1989
- Koenig JH, Christensen RD. Incidence, neutrophil kinetics and natural history of neonatal neutropenia associated with maternal hypertension. N Eng J Med 1989;321:557‐62. [DOI] [PubMed] [Google Scholar]
Lejeune 1999
- Lejeune M, Cantineiux B, Harag S, et al. Defective functional activity and accelerated apoptosis in neutrophils from children with cancer are differentially corrected by granulocyte and granulocyte‐macrophage colony stimulating factors in vitro. Br J Haematol 1999;106:756‐761. [DOI] [PubMed] [Google Scholar]
Manroe 1979
- Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. i. Reference values for neutrophilic cells. J Pediatr 1979;95:89‐98. [DOI] [PubMed] [Google Scholar]
Modi 2000
- Modi N, Carr R. Promising stratagems for reducing the burden of neonatal sepsis. Arch Dis Child Fetal & Neonatal edition 2000;83:F150‐F153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1991
- Roberts RL, Szelc CM, Scates SM, Boyd MT, Soderstrom KM, Davis MW, Glaspy JA. Neutropenia in an extremely premature infant treated with recombinant human granulocyte colony‐stimulating factor. Am J Dis Child 1991;145:808‐812. [PubMed] [Google Scholar]
Rodwell 1993
- Rodwell RL, Taylor KMCD, Tudehope DI, Gray PH. Hematologic scoring system in early diagnosis of sepsis in neutropenic newborns. Pediatr Inf Dis J 1993;12:372‐376. [DOI] [PubMed] [Google Scholar]
Stoll 1996
- Stoll BJ, Gordon T, Korones SB, et al. Late‐onset sepsis in very low birthweight neonates: A report from the National Institute of Child Health and Human Development neonatal research network. J Pediatr 1996;129:63‐71. [DOI] [PubMed] [Google Scholar]


