Abstract
Cabozantinib is approved for the treatment of renal cell carcinoma (RCC). However, prognostic factors are still lacking in this context. The aim of this study was to evaluate prognostic factors in RCC patients treated with second- or third-line cabozantinib. A multicenter retrospective real-world study was conducted, involving 32 worldwide centers. A total of 237 patients with histologically confirmed clear-cell and non-clear-cell RCC who received cabozantinib as second- or third-line therapy for metastatic disease were included. We analyzed overall survival (OS), progression-free survival (PFS) and time-to-strategy failure (TTSF) using Kaplan–Meier curves. Cox proportional models were used at univariate and multivariate analyses.The median PFS and OS of cabozantinib were 7.76 months (95% CI 6.51–10.88) and 11.57 months (95% CI 10.90–not reached (NR)) as second-line and 11.38 months (95% CI 5.79–NR) and NR (95% CI 11.51–NR) as third-line therapy. The median TTSF and OS were 11.57 and 15.52 months with the sequence of cabozantinib–nivolumab and 25.64 months and NR with nivolumab–cabozantinib, respectively. The difference between these two sequences was statistically significant only in good-risk patients. In the second-line setting, hemoglobin (Hb) levels (HR= 2.39; 95% CI 1.24–4.60, p = 0.009) and IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) group (HR = 1.72, 95% CI 1.04–2.87, p = 0.037) were associated with PFS while ECOG-PS (HR = 2.33; 95%CI, 1.16–4.69, p = 0.018) and Hb levels (HR = 3.12; 95%CI 1.18–8.26, p = 0.023) correlated with OS at multivariate analysis, while in the third-line setting, only Hb levels (HR = 2.72; 95%CI 1.04–7.09, p = 0.042) were associated with OS. Results are limited by the retrospective nature of the study.This real-world study provides evidence on the presence of prognostic factors in RCC patients receiving cabozantinib.
Keywords: cabozantinib, nivolumab, prognosis, real-world data, renal cell carcinoma, targeted therapy
1. Introduction
Renal cell carcinoma (RCC), the most common kidney cancer in adults, represents 5% of all cancers in men and 3% in women, with an estimated 65,340 new cases and 14,970 deaths in 2018 in the United States alone [1]. Agents able to target altered pathways promoting neoangiogenesis (e.g., sunitinib, pazopanib, sorafenib, axitinib and tivozanib [2,3,4,5,6,7,8,9]) have demonstrated activity in metastatic RCC (mRCC), as well as immunecheckpoint inhibitors used alone as nivolumab [10] or combined with other immunotherapy (nivolumab plus ipilimumab [11]) or targeted therapies (axitinib plus pembrolizumab or avelumab) [12,13].
Cabozantinib is an orally administered tyrosine kinase inhibitor acting mainly on VEGFR2, MET (mesenchymal epithelial transition receptor) and AXL (anexelekto pathway) [14]. In the randomized phase III METEOR trial comparing cabozantinib to everolimus in pretreated patients, cabozantinib improved overall survival (OS), progression-free survival (PFS) and objective response rate (ORR) [15]. Serious adverse events with cabozantinib occurred in 39% of patients, with the most common toxicities being hypertension, diarrhea and fatigue [16].
Most recently, a randomized phase II clinical trial (CABOSUN) randomized 157 patients with mRCC and intermediate or poorrisk of disease according to IMDC (International Metastatic Renal Cell Carcinoma Database Consortium) criteria (based on the presence of anemia, neutrophilia, thrombocytosis, Karnofskyperformance status <80, hypercalcemia and <1 year from diagnosis to metastatic disease) to receive cabozantinib or sunitinib as first-line therapy [17]. Compared to sunitinib, cabozantinib improved PFS and ORR in this subgroup of patients. Despite the fact that these findings were also confirmed on a subsequent analysis based on independent review [18], the results of this study are still controversial [19].
To date, cabozantinib is indicated for the treatment of patients with advanced RCC in treatment-naïve adults with intermediate poor-risk features (Food and Drug Administration, FDA), and by EMA (European Medical Agency) for adults progressed to prior vascular endothelial growth factor/receptor inhibitors. Here, we report results of a real-world analysis on cabozantinib in previously treated patients with mRCC andaimed to evaluate the presence of prognostic factors and the different therapeutic sequences in this setting.
2. Results
2.1. Overall Population
A total of 237 patients were included in this analysis; 174 (73.42%) were males and 63 (26.58%) females. The median age was 62.56y (range 24.55–85.76). The majority of patients had clear-cell RCC (182 patients, 76.79%), while in 55 patients (23.21%) non-clear-cell RCC (17 papillary type I, 14 papillary type II, 14 clear-cell RCC with sarcomatoid differentiation, 1 with rhabdoid differentiation, 1 chromophobe, 1 with XP11.3 translocation and 7 unclassified RCC tumors) was diagnosed. There were 120 patients (50.63%) who were metastatic at time of diagnosis. At first diagnosis, the Fuhrman or WHO/ISUP grade was G3 in 86 (36.29%) and G4 in 32 (13.50%). The number of metastatic sites was ≥2 in 160 cases (67.51%). The most frequent sites of metastasis were lung (154 patients, 64.98%), lymph nodes (133 patients, 56.12%) and bone (80 patients, 34.04%). According to IMDC criteria, 57 patients (24.05%) were at favorable-risk, 146 (61.60%) at intermediate-risk and 34 (14.35%) had poor-risk features. Patients’ characteristics are reported in Table 1. The distribution of IMDC criteria across the study population is showed in Table 2.
Table 1.
Patients’ characteristics. Immunotherapy combinations included axitinib plus pembrolizumab, axitinib plus avelumab and nivolumab plus ipilimumab.IMDC—International Metastatic Renal Cell Carcinoma Database Consortium.
| Clinicopathological Features | N. of Patients (%) |
|---|---|
| Age | |
| Median | 62.56y |
| Range | 24.55–85.76y |
| Gender | |
| Male | 174 (73.42) |
| Female | 63 (26.58) |
| T-Stage at Diagnosis | |
| T1 | 37 (15.61) |
| T2 | 35 (14.77) |
| T3 | 97 (40.93) |
| T4 | 26 (10.97) |
| Unknown | 42 (17.72) |
| Histology | |
| Clear-cell RCC | 182 (76.79) |
| Non-clear-cell RCC | 55 (23.21) |
| Fuhrman or WHO/ISUP Grade | |
| Grade 1 | 4 (1.69) |
| Grade 2 | 62 (26.16) |
| Grade 3 | 86 (36.39) |
| Grade 4 | 32 (13.50) |
| Unknown | 59 (22.36) |
| N. of Metastatic Sites at Recurrence | |
| 1 site | 77 (32.49) |
| ≥2 sites | 160 (67.51) |
| Site of Metastasis | |
| Lung | 154 (64.98) |
| Lymph nodes | 133 (56.12) |
| Bone | 80 (34.04) |
| Liver | 53 (22.36) |
| Brain | 20 (8.44) |
| IMDC Risk Group | |
| Good | 57 (24.05) |
| Intermediate | 146 (61.60) |
| Poor | 34 (14.35) |
| First-Line Therapy | |
| Sunitinib | 141 (59.49) |
| Pazopanib | 81 (34.18) |
| Immunotherapy combinations | 9 (3.80) |
| Other | 6 (2.53) |
| Second-Line Therapy | 237 (100) |
| Cabozantinib | 112 (47.26) |
| Nivolumab | 89 (37.55) |
| Axitinib | 19 (8.01) |
| Everolimus | 14 (5.91) |
| Other | 3 (1.27) |
| Third-Line Therapy | 178 (100) |
| Cabozantinib | 125 (70.22) |
| Nivolumab | 29 (16.29) |
| Other | 24 (13.49) |
Table 2.
Distribution of risk factors according to IMDC criteria in the study populations. LLN = lower limit of normal; ULN = upper limit of normal.
| IMDC Criteria | N of Patients (%) |
|---|---|
| <1 y from Diagnosis to Systemic Therapy | |
| Yes | 120 (50.63) |
| No | 117 (49.37) |
| Performance Status < 80% (Karnofsky) | |
| Yes | 19 (8.02) |
| No | 214 (91.98) |
| Hb Level < LLN | |
| Yes | 88 (37.13) |
| No | 149 (62.87) |
| Calcium Level > ULN | |
| Yes | 21 (8.86) |
| No | 216 (91.14) |
| Neutrophil > ULN | |
| Yes | 29 (12.24) |
| No | 208 (87.76) |
| Platelets > ULN | |
| Yes | 31 (13.08) |
| No | 206 (86.92) |
The median follow-up time from diagnosis was 182.79 months (95% CI 131.00 to not reached;NR) and median OS from the start of first-line therapy was 103.23 months (95% CI 63.40–NR). During the follow-up, 73 patients (30.80%) died. A further 112 patients (47.26%) were treated with cabozantinib as second-line therapy, while 125 (52.74%) received cabozantinib in the third-line setting. In 41 patients, second-line cabozantinib was ongoing at the time of data collection. Among the 71 patients who progressed on second-line cabozantinib, 53 (74.65%) received a third-line therapy, which was nivolumab in 29 patients (54.72%). Drug distribution and sequence is reported in Table 1.
2.2. Progression-Free Survival of Cabozantinib as Second-Line Therapy
The median PFS of cabozantinib as second-line therapy was 7.76 months (95% CI 6.51–10.88, Table 3).
Table 3.
Progression-free survival and overall survival obtained by cabozantinib in our study.
| Groups | Second-Line Cabozantinib | Third-Line Cabozantinib | ||
|---|---|---|---|---|
| All Patients | PFS [Median (95% CI)] |
OS [Median (95% CI)] |
PFS [Median (95% CI)] |
OS [Median (95% CI)] |
| 7.76 (6.51–10.88) | 11.57 (10.90–NR) | 11.38 (5.79–NR) | NR (11.5–NR) | |
| Second-line Cabozantinib (29 patients, 25.9%) |
Third-line Cabozantinib (28 patients, 22.4%) |
|||
| Favourable Group | PFS [Median (95% CI)] |
OS [Median (95% CI)] |
PFS [Median (95% CI)] |
OS [Median (95% CI)] |
| 11.28 (7.89–NR) | 12.53 (11.57–NR) | 11.38 (4.24–NR) | NR (7.40–NR) | |
| Second-line Cabozantinib (64 patients, 57.1%) |
Third-line Cabozantinib (78 patients, 68.4%) |
|||
|
Intermediate
Group |
PFS [Median (95% CI)] |
OS [Median (95% CI)] |
PFS [Median (95% CI)] |
OS [Median (95% CI)] |
| 7.59 (5.52–NR) | 10.95 (9.11–NR) | 7.63 (5.56–NR) | NR (11.51–NR) | |
| Second-line Cabozantinib (19 patients, 17.0%) |
Third-line Cabozantinib (19 patients, 9.2%) |
|||
| Poor-Risk Group | PFS [Median (95% CI)] |
OS [Median (95% CI)] |
PFS [Median (95% CI)] |
OS [Median (95% CI)] |
| 7.13 (2.66–NR) | 11.05 (7.46–NR) | 5.75 (3.19–NR) | NR (4.01–NR) | |
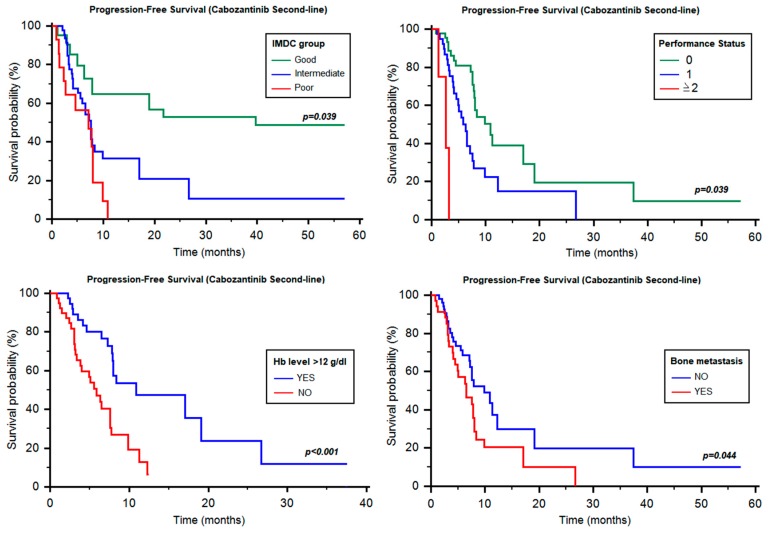
This figure significantly changed when patients were analyzed according to their IMDC status, yielding 11.28 months (95% CI 7.89–NR, Table 3, Figure 1) in good-risk, 7.59 months (95% CI 5.52–NR, Table 3, Figure 1) in intermediate-risk and 7.13 months (95% CI 2.66–NR, Table 3, Figure 1) in poor-risk patients (p = 0.039). Similarly, PFS was different according to ECOG-performance status (PS; 0 vs. 1 vs. ≥2; 10.88 months vs. 5.88 months vs. 2.66 months, p < 0.001, Figure 1) and hemoglobin (Hb) ≥12 g/dL vs. <12 g/dL (10.88 vs. 5.88 months, HR = 0.39, 95% CI 0.18–0.62, p < 0.001, Figure 1). Otherwise, no significant difference was found based on time from diagnosis to systemic therapy (≥1y vs. <1y, 11.28 vs. 7.13 months, HR = 0.62, 95% CI 0. 73–1.14, p = 0.130), neutrophilia (7.76 vs. 4.01 months, HR = 0.48, 95% CI 0.13–1.01, p = 0.051), thrombocytosis (7.89 vs. 6.51 months, HR = 0.50, 95% CI 0.15–1.02, p = 0.055) and hypercalcemia (7.82 vs. 3.06 months, HR = 0.50, 95% CI 0.12–1.22, p = 0.106).
Figure 1.
Progression-free survival of second-line cabozantinib according to different prognostic factors. Hb = hemoglobin; IMDC = International Metastatic Renal Cell Carcinoma Database Consortium.
Interestingly, no significant differences were also found between clear-cell and non-clear-cell histology (7.89 vs. 5.06 months, HR = 0.73, 95% CI 0.35–1.40, p = 0.310), age < 70y and ≥70y (7.89 vs. 7.13 months, HR = 0.74, 95% CI 0.37–1.41, p = 0.334), gender (p = 0.678), Fuhrman or WHO/ISUP grade (p = 0.756) or number of metastatic sites (1 site vs. ≥2 sites, 7.59 vs. 7.82 months, HR = 0.99, 95% CI 0.56–1.76, p = 0.987).
By stratifying patients based on the site of metastasis, a significant difference was found between patients with or without bone metastases (6.51 vs. 9.86 months, HR = 0.58, 95% CI 0.31–0.98, p = 0.044, Figure 1), whilst no differences were found between patients with lung (6.05 vs. 6.31 months, HR = 0.88, 95% CI 0.64–1.21, p = 0.446), liver (7.59 vs. 12.3 months, HR = 1.48, 95% CI 0.73–2.81, p = 0.297), lymph node (7.59 vs. 7.89 months, HR = 1.23, 95% CI 0.71–2.16, p = 0.447), or brain metastases (7.76 vs. 7.59 months, HR = 1.24, 95% CI 0.52–2.89, p = 0.638).
Furthermore, we analyzed the eventual prognostic role of the received first-line therapy, with any significant difference between sunitinib and pazopanib (7.89 vs. 7.82 months, HR = 1.25, 95% CI 0.70–2.38, p = 0.418).
Univariate analysis showed that ECOG-PS (HR = 2.47; 95% CI, 1.40–4.36, p = 0.002), Hb levels (HR = 2.90; 95% CI, 1.55–5.42, p < 0.001), IMDC group (HR = 1.77; 95% CI, 1.12–2.80, p = 0.015) and bone metastases (HR = 1.75; 95% CI, 1.10–3.02, p = 0.047) were significantly associated with the PFS of cabozantinib, given as second-line therapy. At multivariate analysis, only Hb levels (HR = 2.39; 95% CI, 1.24–4.60, p = 0.009) and IMDC group (HR = 1.72, 95% CI, 1.04–2.87, p = 0.037) maintained their prognostic significance in this setting.
2.3. Overall Survival of Cabozantinib as Second-Line Therapy
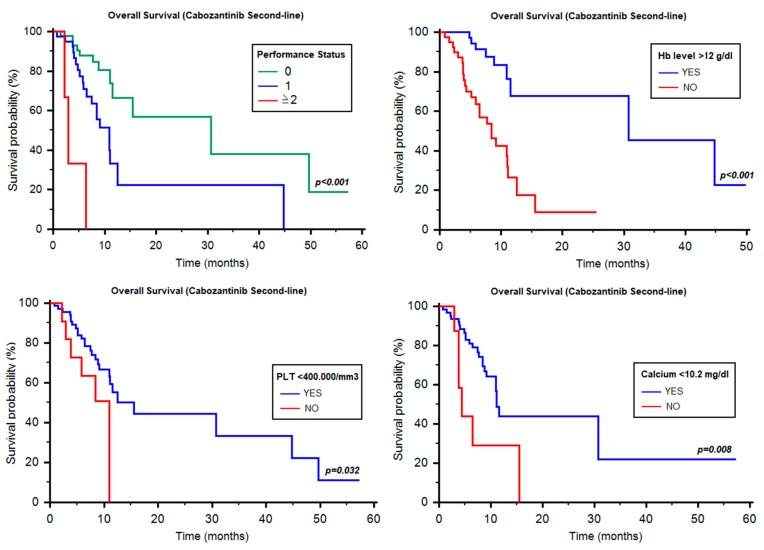
The median OS of cabozantinib as second-line therapy was 11.57 months (95% CI 10.90–NR, Table 3). Differently from PFS, IMDC classification was not associated with OS in the three prognostic groups (12.53 vs. 10.95 vs. 11.05 months, p = 0.349, Table 3). Conversely, the median OS was significantly different according to ECOG-PS (0 vs. 1 vs. ≥2; 30.71 months vs. 10.95 months vs. 2.96 months, p < 0.001, Figure 2), Hb ≥12 g/dL vs. <12 g/dL (30.71 vs. 8.42 months, HR = 0.24, 95% CI 0.10–0.44, p < 0.001, Figure 2), thrombocytosis (15.52 vs. 10.95 months, HR = 0.42, 95% CI 0.09–0.90, p = 0.032, Figure 2) and hypercalcemia (11.08 vs. 4.37 months, HR = 0.32, 95% CI 0.04–0.60, p = 0.008, Figure 2). Of note, no significant differences were found for neutrophilia (12.53 vs. 11.57 months, HR = 0.57, 95% CI 0.17–1.48, p = 0.211), time from diagnosis to systemic therapy (≥1y vs. <1y, 11.57 vs. 11.05 months, HR = 1.02, 95% CI 0.51–2.07, p = 0.949), clear-cell and non-clear-cell histology (11.57 months vs. notreached (NR), HR = 0.83, 95% CI 0.33–1.98, p = 0.648), age < 70y and ≥70y (11.57 vs. 11.08 months, HR = 0.93, 95% CI 0.42–2.04, p = 0.856), Fuhrman grade (p = 0.899), choice of first-line therapy (sunitinib vs.pazopanib: 15.52 vs. 11.08 months, HR = 1.44, 95% CI 0.71–3.26, p = 0.281), site of metastasis and number of metastatic sites (1 site vs. ≥2 sites, 15.52 vs. 11.05 months, HR = 0.82, 95% CI 0.41–1. 46, p = 0.573).
Figure 2.
Overall survival of second-line cabozantinib according to different prognostic factors. Hb = hemoglobin.
At univariate analysis, ECOG-PS (HR = 3.51; 95% CI, 1.86–6.63, p < 0.001), Hb levels (HR = 5.07; 95% CI, 2.18–11.76, p < 0.001), thrombocytosis (HR = 2.52; 95% CI, 1.05–6.01, p = 0.039) and hypercalcemia (11.08 vs. 4.37 months, HR = 3.24, 95% CI 0.31–8.03, p = 0.015) were significant predictors of OS, while at multivariate analysis, only ECOG-PS (HR = 2.33; 95% CI, 1.16–4.69, p = 0.018) and Hb levels (HR = 3.12; 95% CI, 1.18–8.26, p = 0.023) correlated with OS.
2.4. Progression-Free Survival of Cabozantinib as Third-Line Therapy
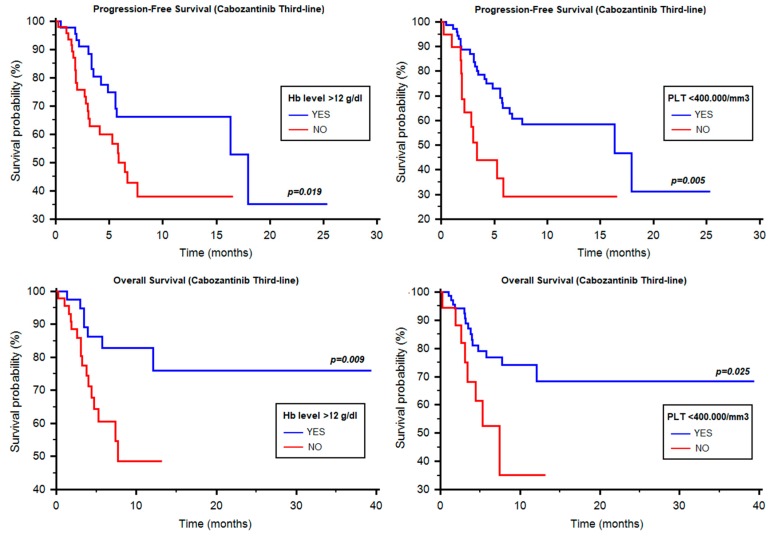
The median PFS of cabozantinib in the third-line setting was 11.38 months (95% CI 5.79–NR, Table 3). The median PFS was not statistically different among the three IMDC groups (11.38 vs. 7.63 vs. 5.75 months, p = 0.772, Table 3) or according to ECOG-PS (11.38 vs. 5.26 months, HR = 0.54, 95% CI 0.16–1.24, p = 0.120), time from diagnosis to systemic therapy (≥1y vs. <1y, NR vs. NR, HR = 0.57, 95% CI 0.27–1.35, p = 0.217), neutrophilia (7.63 months vs. NR, HR = 1.20, 95% CI 0.55–2.62, p = 0.657) and hypercalcemia (16.34 vs. 6.71 months, HR = 1.23, 95% CI 0.50–3.00, p = 0.652). Otherwise, PFS was statistically different according to Hb levels (17.95 vs. 6.44 months, HR = 0.47, 95% CI 0.24–0.88, p = 0.019, Figure 3) and thrombocytosis (16.34 vs. 3.35 months, HR = 0.39, 95% CI 0.12–0.68, p = 0.005, Figure 3).
Figure 3.
Progression-free survival and overall survival of third-line cabozantinib according to different prognostic factors. Hb = hemoglobin; PLT = platelets.
Of note, no significant differences were found by stratifying patients by clear-cell vs. non-clear-cell histology (6.71 vs. 11.38 months, HR = 1.26, 95% CI 0.61–2.59, p = 0.539), age < 70y and ≥70y (6.44 vs. 11.38 months, HR = 1.57, 95% CI 0.82–2.80, p = 0.183), Fuhrman grade (p = 0.474) or number or site of metastases.
Hb levels (HR = 2.19; 95% CI, 1.12–4.26, p = 0.022) and thrombocytosis (HR = 2.60; 95% CI, 1.30–5.19, p = 0.007), were significantly correlated with PFS at univariate but not at multivariate analysis.
2.5. Overall Survival of Cabozantinib as Third-Line Therapy
In the 125 patients treated with cabozantinib in third-line setting, the median OS was NR (95% CI 11.51–NR, Table 3). Hb ≥12 g/dL vs. <12 g/dL (NR vs. 7.73 months, HR = 0.33, 95% CI 0.14–0.76, p = 0.009, Figure 3), thrombocytosis (NR vs. 7.40 months, HR = 0.39, 95% CI 0.10–0.86, p = 0.025, Figure 3). Interestingly, no significant differences were found according to IMDC group (p = 0.739, Table 3), ECOG-PS (NR vs. 7.73 months, HR = 0.45, 95% CI 0.10–1.11, p = 0.073), neutrophilia (NR vs. NR months, HR = 0.73, 95% CI 0.26–1.97, p = 0.509), time from diagnosis to systemic therapy (NR vs. NR months, HR = 0.57, 95% CI 0.27–1.35, p = 0.217), hypercalcemia (NR vs. 7.40 months, HR = 0.73, 95% CI 0.21–2.34, p = 0.560), clear-cell vs. non-clear-cell histology (NR vs. NR, HR = 1.06, 95% CI 0.41–2.74, p = 0.906), age < 70y and ≥70y (12.10 months vs. NR, HR = 2.61, 95% CI 0.96–4.77, p = 0.063), Fuhrman or WHO/ISUP grade (p = 0.574) and neither specific sites nor number of metastatic sites (1 site vs. ≥2 sites, NR vs. NR, HR = 0.72, 95% CI 0.34–1.57, p = 0.422).
Fromunivariate analysis, Hb levels (HR = 3.11; 95% CI, 1.27–7.72, p = 0.014) and thrombocytosis (HR = 2.59; 95% CI, 1.10–6.13, p = 0.030) were found to be associated with OS, while for multivariate analysis, only Hb levels (HR = 2.72; 95% CI, 1.04–7.09, p = 0.042) were correlated with OS.
2.6. Time to Strategy Failure and Sequencing: Cabozantinib vs. Nivolumab
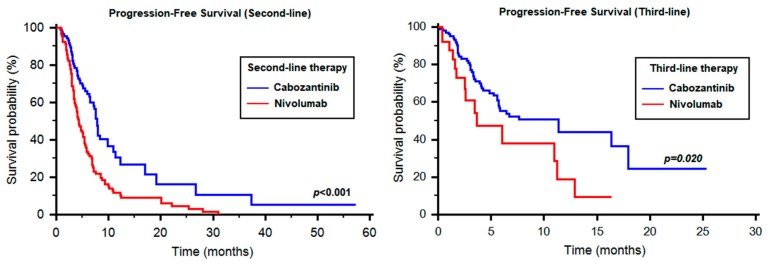
In the 89 patients treated with second-line nivolumab, we observed a median PFS of 4.31 months (95% CI 3.65–5.46), which was significantly different from cabozantinib in the same setting (HR 0.50, 95% CI 0.35–0.69, p < 0.001, Figure 4). Similarly, the 29 patients treated with third-line nivolumab had a median PFS of 3.68 months vs. 11.38 months registered by cabozantinib (HR 0.50, 95% CI 0.19–0.87, p = 0.020, Figure 4). The median TTSF from the start of second-line therapy was 18.61 months (95% CI 16.30–21.80).
Figure 4.
Comparison between cabozantinib and nivolumab in the second- and third-line setting.
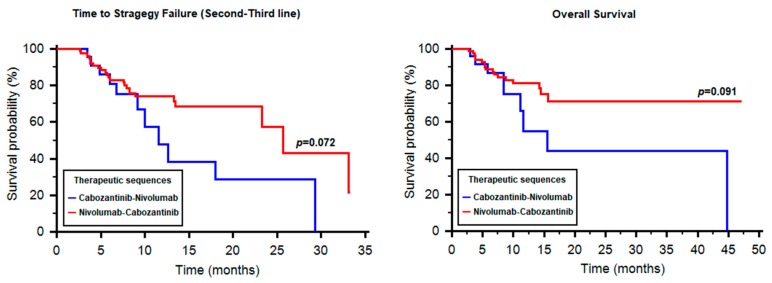
By stratifying patients according to the therapeutic sequence received, we reported 29 patients treated with cabozantinib followed by nivolumab and 89 patients who received nivolumab followed by cabozantinib. The median TTSF was 11.57 months with cabozantinib–nivolumab (95% CI 9.17–NR, Figure 5) and 25.64 months with nivolumab–cabozantinib (95% CI 23.24–NR, Figure 5). The difference was statistically significant only in the good-risk group (11.57 vs. 25.64 months, HR 8.99, 95% CI 3.91–671.63, p = 0.003).
Figure 5.
Time to strategy failure and overall survival in patients treated with the sequences cabozantinib–nivolumab and nivolumab–cabozantinib.
The median OS from the start of second-line therapy in the cabozantinib–nivolumab and nivolumab–cabozantinib groups were 15.52 months (95% CI 11.10–NR) and NR (95% CI NR–NR), respectively (HR 1.95, 95% CI 0.87–5.83, p = 0.091, Figure 5). Similarly to TTSF, the median OS was significantly longer with nivolumab–cabozantinib only in the good-risk subgroup (13.55 months vs. NR, p = 0.004).
3. Discussion
Cabozantinib represents an effective strategy in untreated and pretreated RCC patients. The lack of validated molecular or clinical predictive and prognostic factors aimed to optimize the efficacy and safety of this agent represents a major challenge for uro-oncologists. In our analysis, only Hb levels were significantly correlated with OS in the second- and third-line settings. The negative prognostic significance of anemia suggests that a prompt management of this condition could have a potential impact on the outcome of RCC patients. Moreover, these data support the necessity of investigating the prognostic significance of anemia in patients treated with nivolumab to understand if this condition may represent a key factor in the decision-making process between these two agents.In particular, reversible causes of anemia need to be addressed, but the data do not indicate attempting erythropoietin(EPO)-induced correction, particularly given the association of EPO with adverse outcomes in cancersin large studies [20].
Interestingly, no difference in terms of efficacy has been found between clear-cell and non-clear-cell histologies. This may be partially explained by the prevalence in the non-clear-cell group of papillary tumors (31/55), in which cabozantinib has demonstrated to be effective [21].
There is a clear statistical difference between the two sequences in favorable-risk RCCpatients (p = 0.003); and for all patients analyzed, there is a trend towards statistical significance with a clear separation of curves for TTSF and OS beyond 12 months. Even though cabozantinibhas better PFSin the second-line setting than nivolumab (Figure 4), there appears to be utility in trying the sequencenivolumab–cabozantinib, rather than cabozantinib–nivolumab, particularly in favorable-risk patients, which needs to be further investigated with a larger sample size. These data also call for studies investigating the biological rationale for differences in outcomes between the sequences. However, the small number of patients in each prognostic group and the retrospective nature of our study do not allow to definitively clarify this issue.
4. Patients and Methods
4.1. Study Population
The study population included adults (>18 years) with clear-cell or non-clear-cell mRCC, treated with cabozantinib as second- or third-line therapy. Patients were treated in 32 worldwide institutions between November 2004 and January 2019. Data were retrospectively collected from patients’ electronic medical records and paper charts. Patients were excluded from this study if they had missing data regarding thesite of metastasis and tumor response to therapy. The research was carried out in accordance with the approval by the ethical committee of the participating institutions. The study has been accepted by the “Comitato Etico Regionale delle Marche”, the accepting number is 2019-403.
4.2. Treatment Regimens and Statistical Analysis
Cabozantinib was administered orally, usually at a starting dose of 60 mg once daily. Treatment was administered until clinical or radiological disease progression, serious adverse events or death. Follow-up commonly consisted of periodic physical examination, laboratory analysis and imaging assessment by computed tomography (CT) or magnetic resonance imaging (MRI) not earlier than 4 weeks, and not later than 6 weeks, according to local regulations. Disease progression was defined by the Response Evaluation Criteria in Solid Tumors, RECIST v.1.1 [22]. Progression-free survival (PFS) was defined as the time from the start of therapy to progression or death from any cause, whichever occurred first. Patients with no tumor progression or death at time of data collection were censored at the last date of evaluation. Time to strategy failure (TTSF) was defined as the interval from the start of first-line therapy to progression on full therapy or death. PFS and OS were estimated using Kaplan–Meier method with Rothman’s 95% confidence intervals (CI) and compared across the groups using the log-rank test. Neutrophilia was defined as ≥7500 neutrophils/mm3; thrombocytosis was defined as ≥400,000 platelets/mm3, while corrected hypercalcemia as ≥10.2 mg/dL.
In order to investigate patients’ characteristics predictors of survival, Cox proportional-hazards models were used at univariate and multivariate analyses. All the significance levels were set at a 0.05 value and all p values were two-sided. The statistical analysis was performed by MedCalc version 11.4.4.0 (MedCalc Software, Broekstraat 52,9030 Mariakerke, Belgium).
5. Conclusions
Our results support the prognostic role of Hb levels in patients treated with cabozantinib and the importance of sequencing immunotherapy and targeted therapy. Further perspective studies should be provided in order to validate these prognostic factors and compare the sequential approaches available in this disease.
Author Contributions
Conceptualization, M.S. (Matteo Santoni) and R.M.; Data curation, V.P., J.G., E.P., S.S., P.S., E.V., S.M., M.R. and G.S.; Formal analysis, A.C. (Alessandro Conti) and F.P.; Funding acquisition, R.M. and N.B.; Investigation, M.S., D.Y.H., S.B. (Sergio Bracarda), G.P., M.M., C.P., M.R.M., G.C., S.J.C., U.D.G., U.B., C.M., F.C. (Fabio Calabrò), M.G.V., D.S., F.M. (Francesco Massari), L.G., G.F., R.R., S.B. (Sebastiano Buti), P.Z., O.C., F.M. (Franco Morelli), F.C. (Francesco Carrozza), A.M. (Angelo Martignetti), A.G., R.I., A.M. (Alessandra Mosca), F.A., N.V., L.I. and C.O.; Methodology, M.S. (Matteo Santoni); Project administration, N.B.; Software, F.P.; Supervision, A.L.-B., L.C., M.S. (Marina Scarpelli) and R.M.; Validation, D.Y.H., S.B. (Sergio Bracarda), G.P., M.M., C.P., M.R.M., C.G., S.J.C., U.D.G., U.B., C.M., F.C. (Fabio Calabrò), M.G.V., D.S., F.M. (Francesco Massari), L.G., G.F., R.R., S.B. (Sebastiano Buti), P.Z., O.C., F.M. (Franco Morelli), F.C. (Francesco Carrozza), A.M. (Angelo Martignetti), A.G., R.I., A.M. (Alessandra Mosca), F.A., N.V., L.I. and C.O.; Writing—original draft, M.S. (Matteo Santoni); Writing—review & editing, M.S. (Matteo Santoni) and A.C. (Alessia Cimadamore). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Motzer R.J., Hutson T.E., Cella D., Reeves J., Hawkins R., Guo J., Nathan P., Staehler M., de Souza P., Merchan J.R., et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 3.Motzer R.J., Escudier B., Tomczak P., Hutson T.E., Michaelson M.D., Negrier S., Oudard S., Gore M.E., Tarazi J., Hariharan S., et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomized phase 3 trial. Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 4.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M., Negrier S., Chevreau C., Solska E., Desai A.A., et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., Chevreau C., Filipek M., Melichar B., Bajetta E., et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: Arandomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 6.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O., Oudard S., Negrier S., Szczylik C., Kim S.T., et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Gore M.E., Szczylik C., Porta C., Bracarda S., Bjarnason G.A., Oudard S., Lee S.H., Haanen J., Castellano D., Vrdoljak E., et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br. J. Cancer. 2015;113:12–19. doi: 10.1038/bjc.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer R.J., Nosov D., Eisen T., Bondarenko I., Lesovoy V., Lipatov O., Tomczak P., Lyulko O., Alyasova A., Harza M., et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J. Clin. Oncol. 2013;31:3791–3799. doi: 10.1200/JCO.2012.47.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoni M., Massari F., Piva F., Carrozza F., Di Nunno V., Cimadamore A., Martignetti A., Montironi R., Battelli N. Tivozanib for the treatment of renal cell carcinoma. Expert Opin. Pharmacother. 2018;19:1021–1025. doi: 10.1080/14656566.2018.1480722. [DOI] [PubMed] [Google Scholar]
- 10.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer R.J., Tannir N.M., McDermott D.F., Arén Frontera O., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer R.J., Penkov K., Haanen J., Rini B., Albiges L., Campbell M.T., Venugopal B., Kollmannsberger C., Negrier S., Uemura M., et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Alekseev B., Soulières D., Melichar B., et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 14.Di Nunno V., Cubelli M., Massari F. The role of the MET/AXL pathway as a new target for multikinase inhibitors in renal cell carcinoma. Expert Rev. Precis. Med. Drug Dev. 2017;2:169–175. doi: 10.1080/23808993.2017.1347481. [DOI] [Google Scholar]
- 15.Choueiri T.K., Escudier B., Powles T., Mainwaring P.N., Rini B.I., Donskov F., Hammers H., Hutson T.E., Lee J.L., Peltola K., et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri T.K., Escudier B., Powles T., Tannir N.M., Mainwaring P.N., Rini B.I., Hammers H.J., Donskov F., Roth B.J., Peltola K., et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 17.Choueiri T.K., Halabi S., Sanford B.L., Hahn O., Michaelson M.D., Walsh M.K., Feldman D.R., Olencki T., Picus J., Small E.J., et al. Cabozantinib Versus Sunitinib as initial targeted therapy for patients with metastatic renal cell Carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017;35:591–597. doi: 10.1200/JCO.2016.70.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choueiri T.K., Hessel C., Halabi S., Sanford B., Michaelson M.D., Hahn O., Walsh M., Olencki T., Picus J., Small E.J., et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer. 2018;94:115–125. doi: 10.1016/j.ejca.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buti S., Bersanelli M. Is Cabozantinib Really Better Than Sunitinib As First-Line Treatment of Metastatic Renal Cell Carcinoma? J. Clin. Oncol. 2017;35:1858–1859. doi: 10.1200/JCO.2016.71.6506. [DOI] [PubMed] [Google Scholar]
- 20.Debeljak N., Solár P., Sytkowski A.J. Erythropoietin and Cancer: The Unintended Consequences of Anemia Correction. Front. Immunol. 2014;5:563. doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H., Nolley R., Chan A.M.W., Rankin E.B., Peehl D.M. Cabozantinib inhibits tumor growth and metastasis of a patient-derived xenograft model of papillary renal cell carcinoma with MET mutation. Cancer Biol. Ther. 2017;18:863–871. doi: 10.1080/15384047.2016.1219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]