Abstract

Explicit antigen–antibody binding has accelerated the development of immunosensors for the detection of various analytes in biomedical and environmental domains. Being a subclass of biosensors, immunosensors have been a significant area of research in attaining high sensitivity and an ultralow sensing limit to detect biological analytes present in trace levels. The highly porous structure, large surface area, and excellent biocompatibility of hydrogels enabling the retainability of the activity and innate framework of the attached biomolecules make them a suitable candidate for immunosensor fabrication. Hydrogels based on polycarboxylate, cellulose, polyaniline, polypyrrole, sodium alginate, chitosan, and agarose are exploited in conjunction with other nanomaterials such as AuNPs, GO, and MWCNTs to augment the electron transfer during the immunosensing mechanism. Surface plasmon resonance, electrochemiluminescence, colorimetric, and electrochemical assays are different strategies utilized for the signal transduction in hydrogel-based immunosensors during the formation of the antigen–antibody complex. These hydrogel-based immunosensors exhibit rapid response, excellent stability, reproducibility, high selectivity and high sensitivity, a broad range of detection, an ultralow limit of detection, and display results similar to those for the ELISA test. This review propounds different hydrogel-functionalized immunosensing platforms classified on the basis of their signal transduction for the detection of disparate cancer biomarkers (tumor necrosis factor, α-fetoprotein, prostate-specific antigen, carbohydrate antigen 24-2, carcinoembryonic antigen, neuron-specific enolase, and cytokeratin antigen 21-1), hormones (cortisol, cortisone, and human chorionic gonadotropin), human IgG, and ractopamine in animal feeds.
1. Introduction
In the past several years, exponential growth has been evidenced in the field of biosensors. A biosensor is an analytical device in a miniaturized form which comprises a bioreceptor and a transducer. The bioreceptor recognizes the target analyte, and the transducer converts the biochemical signal produced to a measurable signal wherein the signal is further processed to display the concentration.1,2
Immunosensors are a subclass of biosensors fabricated in order to recognize different analytes with regard to the explicit binding between the antigen and its respective antibody and have developed immense requirements in fields such as cancer diagnosis and food quality control. For the effectual construction of immunosensors with potential analytical performance, it is essential to prepare an immunosensing platform so as to ensure the immobilization of immunologically sensitive agents (antigen or antibody) and signal transduction.3 Enzyme-linked immunosorbent assay (ELISA), colorimetric, piezoelectric, radiometric, and electrochemiluminescent assays are the various immunoassays developed to date for the sensitive detection of different biomarkers.4 Biomolecules such as proteins, steroids, and several others are complex molecules that evince slightly similar chemical structures. To bypass false indications, the response of the sensor has to be analyzed gingerly. Whether the analysis is in vitro or in vivo, besides good sensitivity, the biosensor should also acquire good target specificity.5 Because the biomarkers in biological fluids are present in low concentrations, the primary objective of an immunosensor is to improve the sensitivity and to attain an ultralow limit of detection, whereby different approaches for signal amplification have been explored.6,7
Various immunosensing platforms have been recorded by utilizing nanomaterials (magnetic or metal nanoparticles, carbon nanotubes), polymers (molecularly imprinted polymers, self-assembled monomers), and gels (hydrogel, sol–gels).3 These materials should possess good biocompatibility to preserve the activity and innate framework of the attached biomolecule as well as good conductivity to improve the transport of electrons across the device surface and should exhibit good stability.3,8 The electrical properties, a large surface area, and the ability to inhibit the clustering of metal NPs during immobilization are all attributable to the utilization of carbon-based nanomaterials such as MWCNTs and graphene oxide (GO).9
A hydrogel is a three-dimensional porous material made up of interpenetrating polymeric networks (IPNs) that possess extensive biomedical applications, in particular, biosensing, drug delivery, and tissue engineering. The excellent hydrophilicity, large surface area (due to the interconnected porous structure), and excellent biocompatibility of hydrogels are due to their potential employment in biosensors. The nonrigid porous hydrated gel reduced the steric hindrance and augmented the immobilization of biomolecules and target binding compared to those of conventionally used surface-based assays. Different types of hydrogels such as conductive hydrogels and redox hydrogels, promoting rapid electron transfer, play a vital role in analyte detection and signal amplification, so they are exploited to modify the immunosensing platform.3,4,10−12
The present review reports state-of-the-art literature analysis and summarizes the employment of hydrogel-based immunosensing platforms of varied compositions utilizing various signal-transducer principles (SPR, electrochemiluminescence, colorimetrics, and electrochemical signals) for the recognition of a variety of analytes such as cancer biomarkers (cytokeratin antigen 21-1 (CYFRA21-1), α-fetoprotein, prostate-specific antigen (PSA), carbohydrate antigen 24-2 (CA242), neuron-specific enolase (NSE), carcinoembryonic antigen (CEA), tumor necrosis factor (TNF-α)), hormones (cortisol, cortisone, human chorionic gonadotropin (HCG)), human IgG, and ractopamine in animal feed. The fabrication, mechanism of detection, range of detection, and limit of detection of all of the hydrogel-based immunosensors have also been discussed.
2. Transduction Principle in Hydrogel-Based Immunosensors
The biochemical signal produced during the antigen–antibody interaction is converted to a measurable signal by the transducer. This conversion can occur via different transduction pathways based on the type of transducer. And the signals that are generated are directly proportional to the concentration of analyte detected. As shown in Figure 1, the signal-transduction methods in hydrogel-based immunosensors can be classified into four primary methods—surface plasmon resonance (SPR), electrochemical, electrochemiluminescence, and colorimetric wherein the electromagnetic field generated, change in electrical properties, electrogenerated chemiluminescence, and change in color are measured, respectively. Disparate hydrogels employed for immunoassays are discussed elaborately in this section and summarized in Table 1 along with their signal transduction technique, the linear range of detection, and the limit of detection.
Figure 1.
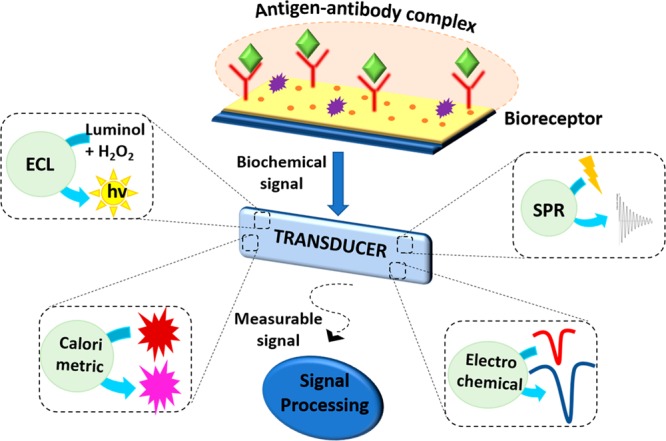
Schematic representation of different signal transduction techniques in a hydrogel-based immunosensor.
Table 1. Characteristics of a Hydrogel-Based Immunoassay.
| hydrogel composition | biomolecule detected | signal transduction method | detection range | limit of detection | ref |
|---|---|---|---|---|---|
| polycarboxylate hydrogel | cortisol and cortisone | surface plasmon resonance | cortisol: 5–154 μg/L; cortisone: 30–174 μg/L | cortisol: 2 μg/L; cortisone: 9 μg/L | (5) |
| microcrystalline cellulose hydrogel | α-fetoprotein | colorimetry | 0.1–10 000 ng/mL | 0.46 ng/mL | (22) |
| polyaniline hydrogel | human chorionic gonadotropin | electro chemiluminescence | 0.001–500 mIU/mL | 0.0003 mIU/mL | (24) |
| phenylalanine with ferrocene | human IgG | electrochemical | 0.1–100 pg/mL | 50 fg/mL | (7) |
| phenylalanine with ferrocene | tumor necrosis factor (TNF-α) | electrochemical | 1 pg/mL–10 ng/mL | 0.5 pg/mL | (6) |
| phenylalanine with ferrocene | prostate-specific antigen (PSA) | electrochemical | 1pg/mL–10 ng/mL | 0.5 pg/mL | (3) |
| polypyrrole hydrogel | carcinoembryonic antigen (CEA) | electrochemical | 1 fg/mL–200 ng/mL | 0.16 fg/mL | (11) |
| sodium alginate-Pb2+-graphene oxide (SA-Pb2+-GO) hydrogel | carbohydrate antigen 24-2 (CA242) | electrochemical | 0.005 U/mL–500 U/mL | 0.067 mU/mL | (4) |
| chitosan hybrid hydrogel | neuron-specific enolase (NSE) | electrochemical | 1pg/mL–100 ng/mL | 0.483 pg/mL | (9) |
| polyaniline hydrogel | prostate-specific antigen (PSA) | electrochemical | 10 fg/mL–100 ng/mL | 1.25 fg/mL | (14) |
| Fe3+ and 1,3,5-benzenetricarboxylic acid | neuron-specific enolase (NSE) | electrochemical | 1pg/mL–200 ng/mL | 0.26 pg/mL | (15) |
| aniline and vinyl-ferrocene based redox hydrogel | prostate-specific antigen (PSA) | electrochemical | 0.001–200 ng/mL | 0.54 pg/mL | (16) |
| pyrrole and thionine | neuron-specific enolase | electrochemical | 1pg/mL–100 ng/mL | 0.65 pg/mL | (17) |
| agarose hydrogel | ferritin | electrochemical | (5–50) × 10–5 g/L | 1.5 × 10–5 g/L | (18) |
| agarose gel | ractopamine | electrochemical | 1–1000 ng/mL | (8) | |
| polyaniline hydrogel | neuron-specific enolase (NSE) | electrochemical | 0.01–1000 ng/mL | 4.6 pg/mL | (25) |
| Fe3+-alginate hydrogel | neuron-specific enolase (NSE) | electrochemical | 0.001–100 ng/mL | 0.447 pg/mL | (19) |
| phytic acid and lead(II) | cytokeratin antigen 21-1 (CYFRA21-1) | electrochemical | 50 fg/mL–100 ng/mL | 38 fg/mL | (20) |
| sodium alginate | prostate-specific antigen (PSA) | electrochemical | 1 fg/mL–100 ng/mL | 0.09 fg/mL | (21) |
2.1. Surface Plasmon Resonance (SPR) Immunoassay
This optical surface-sensitive technique enables real-time monitoring of biological interactions that are in close proximity to a transducer without the need for labels. An SPR immunosensor comprises a light emitter, a detecting device, a transducer, a prism, a biomolecule, and a flow system. SPR corresponds to the generation of the electromagnetic field on the thin metal film when light of an appropriate angle and wavelength is incident on it. The generated field is strong at the surface and decreases with an increase in depth from the interface. The SPR principle of detection is depicted in Figure 2.13 SPR is a nonselective technique; it requires a well-planned strategy to obtain selectivity.
Figure 2.
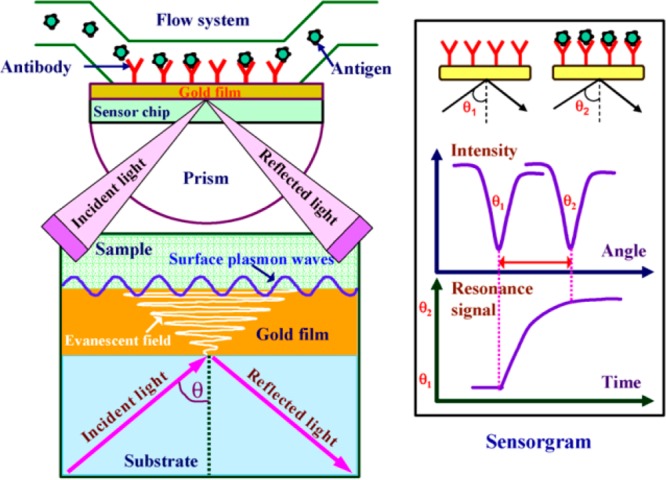
Diagrammatic representation of the surface plasmon resonance immunoassay. Reprinted with permission from ref (13). Copyright 2006 Elsevier.
In 2009, Fransconi et al.5 were the first to report an immunosensor based on the SPR principle of detecting corticosteroids in saliva and urine. The amine coupling enabled the covalent immobilization of antibodies, and the binding capacity of antibodies (anticortisol) on the SPR-based gold disk was increased by modifying it with polycarboxylate hydrogel. The SPR signal was observed when cortisol binds to the immobilized anticortisol, and the strong interaction made the resonant angle shift steadily. The highly sensitive and rapid immunoassay offers a response time of 15–20 min against the analysis of cortisol and cortisone. They exhibit detection ranges of 30 to 174 μg/L for cortisone and 5 to 154 μg/L for cortisol with detection limis of 9 and 2 μg/L, respectively. The sensitivity of the immunosensor is affected by interfering molecules after a certain concentration. This immunosensor is stable and reusable for 100 cycles.
2.2. Electrochemical Immunoassay
The performance of an electrochemical immunosensor is associated with the electrode chemical interface characteristics. Amperometric and impedimetric are two types of electrochemical immunosensors which work on the principle of change in current or impedance. The electrical conductivity, the specified surface area, and the structure and biocompatibility of the interfacial layer play vital roles in electrochemical signal transduction. Electrochemical methods have several advantages, including high sensitivity, simple and feasible instrumentation, miniaturization, and cost-effectiveness.
2.2.1. Sandwich-Type Electrochemical Immunoassay
Zhou et al. in 2013 developed a novel supramolecular hydrogel-based sandwich immunosensor to sense IgG in humans, and its function is illustrated in Figure 3.7 The hydrogel produced by modifying amino acid phenylalanine with ferrocene (Fc) was drop cast on the glassy carbon electrode along with chitosan to render the hydrogel stable on the device surface, and on this reformed surface, gold nanoparticles were immobilized to enable the absorption of antibody anti-IgG (Ab1). The immunosensor label (CNT-GOx-Ab2) was prepared by the functionalization of a carbon nanotube (CNT) with glucose oxidase (GOx) and anti-IgG (Ab2). The Fc moieties present in the hydrogel exhibit strong redox activity, but GOx catalyzes glucose to produce a byproduct, H2O2, such that it, in turn, oxidizes the Fc groups by diffusing into the chitosan/hydrogel layer, thereby causing a decrease in the redox peak of the electrode. The redox activity decrease is in proportion to the IgG concentration analyzed. The immunosensor displayed high selectivity and sensitivity, considerable reproducibility with a relative standard deviation of 2.7%, and a broad linear range (0.1 to 100 pg/mL) with a LOD of 50 fg/mL.
Figure 3.
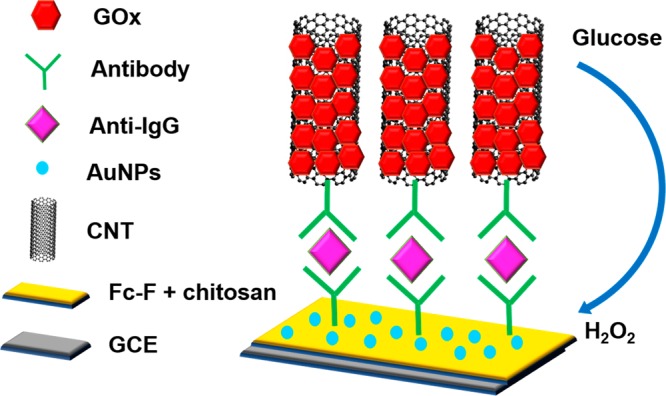
Diagrammatic representation of an immunosensor in the sandwich form to detect IgG. Reproduced with permission from ref (7). Copyright 2013, Elsevier.
In a sandwich format, a hydrogel-based immunosensor for the sensing of TNF-α was fabricated by Hou et al. in 2013.6 The hydrogel prepared by functionalizing amino acid and phenylalanine with ferrocene (Fc) couched a considerable number of Fc moieties and imparted the redox properties. The stability of the hydrogel immobilized on the electrode was rendered by mixing it with chitosan. The adsorption of anti-TNF-antibody (Ab1) on the hydrogel-modified electrode was facilitated by AuNPs, and this electrode acted as the platform for sensing. For the label, a polystyrene sphere (PS) was adopted as the material to support the freighting of alkaline phosphatase (ALP) and anti-TNF (Ab2) and thereby make the immunosensor highly sensitive. When TNF-α was captured by Ab1 and Ab2, ascorbic acid (AA) was produced as ALP catalyzed ascorbic acid 2-phosphate (AA-p) hydrolysis. The formed AA reduced the Fc moieties present in the hydrogel, resulting in a decline in current peak and hence detects the TNF concentration. This sandwich-type immunosensor has detectability from 1 pg/mL to 10 ng/mL and 0.5 pg/mL LOD, reproducibility with a relative standard deviation of 4.3%, and good storage stability. The results of clinical analyzed samples showed the proper consistency, with ELISA indicating reliability in the method.
In another work, Zang et al. developed a sandwich-based immunosensor with an AuNP-loaded polyaniline hydrogel as the substrate, and the labels were mesoporous silica nanoparticles (MSN) coated with polydopamine (PDA) which enveloped methylene blue (MB).14 Both the hydrogel and the label were antibody immobilized by AuNPs and blocked by BSA. These labels were considered “smart” because they were pH-triggered, and before the analysis, under acidic conditions, they were destroyed. Concurrently, the hydrogel adsorbed the liberated MB, and the distance reduction between the electrode and redox particles yielded signal amplification. The disengagement of the insulant layer (antigen–antibody complex) and the labels decreased the interfacial resistance and increased the current. To detect PSA, this immunosensor displayed excellent selectivity, reproducibility, and a broad range of detection (10 fg/mL to 100 ng/mL) with a low LOD (1.25 fg/mL). The optimized parameters were the temperature (40 °C), pH (7.5), and incubation time (50 min). The immunosensor exhibited good stability by retaining 89.1% of its current response and recovery in the range of 98.5 to 106.5%.
2.2.2. Label-Free Electrochemical Immunoassay
In 2014, Huang et al. developed an immunosensor to detect PSA utilizing a redox-active hydrogel.3 The ferrocene (Fc) incorporated amino acid, phenylalanine, and the hydrogel was embodied with nanofibers of diameter 50 to 100 nm and a length of 1 mm. The Fc groups in the hydrogel promoted electron flow, and the hydrogel-modified electrode exhibited strong redox peaks. The chitosan layer stabilized the hydrogel on the electrode, and the gold nanoparticles adsorbed on the chitosan enabled antibody (anti-PSA) immobilization. The quantitative detection of antigen (PSA) was proportional to the redox peak suppression during the antigen–antibody interaction. They displayed recovery in the range of 96.9 to 108%, and the relative standard deviation obtained was lower than 6%. This cost-effective immunosensor exhibits high sensitivity, good PSA selectivity, a broad range (1 pg/mL to 10 ng/mL), and a limit of detection of 0.5 pg/mL.
CEA was detected by a label-free amperometric immunosensor fabricated by Rong et al. in 201511 by employing a nanostructured conducting network of polypyrrole hydrogel and gold nanoparticles. The glassy carbon electrode (GCE) had been modified using the polypyrrole hydrogel, formed by the reaction between phytic acid and the pyrrole monomer. AuNPs integrated into the hydrogel via electrodeposition enhanced the electron conductivity of the electrode and served as an immobilization matrix for anti-CEA. The immunological reaction among anti-CEA and CEA resulted in the suppression of the current response. This immunosensor displayed excellent selectivity, reproducibility, stability, a broad detectability (1 fg/mL to 200 ng/mL), and an ultralow LOD of 0.16 fg/mL. The CEA concentration detection conducted on human serum samples by this immunosensor was compared with ELISA to obtain a relative error of −4.2 to 7.1%, indicating accuracy and considerable reliability.
Tang et al. in 2016 designed an immunosensor for the ultrasensitive detection of CA242 employing the sodium alginate-Pb2+-graphene oxide (SA-Pb2+-GO) redox hydrogel.4 The sensing platform was obtained by first coating the GCE with the SA-Pb2+-GO hydrogel and chitosan, to enrich it with Pb2+ further, and then immobilizing anti-CA242 utilizing glutaraldehyde. The conductivity of SA-Pb2+-GO/GCE was enhanced by the addition of Pb2+ and GO and resulted in signal amplification. The interaction of CA242 with anti-CA242 increased the resistance of the electrode and caused a significant drop in current. This immunosensor exhibits a broad linear range (0.005 to 500 U/mL) and an ultralow LOD (0.067 mU/mL). The proposed immunosensor indicated stability by retaining 90% of its current response even after storage at 4 °C for a month, and when tested against human serum samples and compared with ELISA, it showed a relative error lower than 10%.
In order to detect NSE, an immunosensor was proposed by Wang et al. in 2016 by employing a conductive hydrogel.15 Fe3+ and 1,3,5-benzenetricarboxylic acid were used as the metal ion and ligand, respectively, for the preparation of the hydrogel by a cross-linking coordination method. The electrodeposition of AuNPs on the hydrogel, drop coated on GCE, enhanced the immobilization of anti-NSE and electron transport. Blocking agent bovine serum albumin (BSA) was used, and the specific binding of NSE inhibited the transfer of electrons, ensuing a drop in the peak current. The immunoassay displayed good repeatability, excellent analytical performance, stability, NSE specificity, and a broad linear detection range (1 pg/mL to 200 ng/mL) with 0.26 pg/mL as the limit of detection. This immunosensor obtained accuracy and good reliability because the relative standard error of its practical test on human serum samples was less than 7% when compared with ELISA.
A catalytically conductive aniline- and vinyl-ferrocene (Fc)-based redox hydrogel was utilized by Li et al. in 2017 to fabricate a prostate-specific antigen (PSA)-detecting immunosensor.16 AuNPs facilitated the adsorption of antibody (anti-PSA) on the hydrogel-modified electrode and enhanced the conductivity. The catalytic activity of Fc enabled signal amplification by the oxidation of ascorbic acid to dehydroascorbic acid. The binding of PSA on anti-PSA inhibited electron transfer; thus, the decrease in the redox currents enabled the detection of PSA. The proposed immunosensor displayed a detection range from 0.001 to 200 ng/mL and 0.54 pg/mL LOD. As −7.3 to 6.0% was the relative standard deviation between the ELISA and the practical application of the immunosensor, it showed good reliability. The immunosensor displayed high storage stability by retaining 90.4% of its current response even after 4 weeks of storage at 4 °C.
In 2017, Wang et al. introduced a conductive hydrogel-based amperometric immunosensor for NSE detection.17 Thionine and pyrrole were the monomers, ammonium persulfate and HAuCl4 were co-oxidizing agents, and glucose was the doping agent used for the synthesis of the conductive hydrogel. This hydrogel (polypyrrole-polythionine hydrogel) functions as the substrate for sensing (by enhancing the transfer of electrons and signal amplification) and immobilizing the antibodies (anti-NSE). The electron transfer was further enhanced by the electrodeposition of AuNPs, and good analytical performance was obtained by the signal amplification via a cascading reaction. H2O2 produced during the catalytic reaction of glucose with GOx was further catalyzed by polythionine. Both reactions resulted in an enhancement in the redox signal, enabling its exploitation as a platform for sensitive detection. The anti-NSE/NSE complex was formed as the concentration of NSE increased, and current suppression was observed. pH 6.5 and 50 min were the optimized pH and incubation temperature. A good detection range (1 pg/mL to 100 ng/mL) with a low LOD (0.65 pg/mL) was displayed by the immunosensor. This immunosensor exhibited storage stability wherein the current response was decreased by only 10% and the relative standard deviation lower than 10% when compared to electrochemiluminescent immunoassay (ECLI) tests.
The AuPd nanoparticle-multiwalled carbon nanotube composite (AuPd-MWCNT), ferrocene carboxaldehyde (Fc-CHO), and a chitosan hydrogel were utilized by Yin et al. in 2017 to develop an NSE-detecting immunosensor.9 Chitosan provided numerous binding locations to the electroactive species, Fc-CHO, such that they display electrocatalytic activity toward H2O2 and generate a redox response via electron transfer. Signal amplification was further achieved by the synergistic enzyme-free catalytic activity of Au-MWCNT and Fc-CHO composites on H2O2. Glutaraldehyde enabled the immobilization of anti-NSE on the Au-MWCNT/CS-Fc hydrogel, and BSA was used as the blocking agent. The resistance increase during the antigen/antibody interaction exhibited a drop in the current peaks, evidencing the detection of NSE. This amperometric immunosensor provided a range of detection from 1 pg/mL to 100 ng/mL with a limit of detection of 0.483 pg/mL. The optimum incubation time was 50 min at pH 7.5, and the substrate with a volume ratio of Au-Pd-MWCNT to CS-Fc was considered optimum for producing a strong signal. The results after practical clinical tests displayed a relative standard error lower than 10% when compared with ELISA.
An immunosensor for the determination of serum ferritin was a novel proposal.18 In this immunosensor, ferritin antibody (FeAb) was immobilized on a GCE modified with the agarose hydrogel. The current response reduction percentage (CR%) was proportionate to the ferritin concentration and was maximized at 0.1 g/L, enabling a wide range of detectability ((5–50) × 10–5 g/L) with a LOD of 1.5 × 10–5 g/L, but this immunosensor has only 10 days of stability whereby a 15% decrease in peak current occurs. The optimum incubation time and temperature for the formation of the antigen–antibody complex were 30 min and 32 °C. The detected ferritin can be acceptable for low concentrations but not for high concentrations when compared to the clinical RIA method.
Yin et al. fabricated an immunosensor based on the decomposition of the Fe3+-alginate hydrogel by the Fenton reaction.19 To enhance the conductivity, MWCNT was utilized to modify the electrode, and the Fe3+-alginate hydrogel was developed in situ. The NSE sandwiched labeled immunoprobes of anti-NSE (Ab1) magnetic beads (MB) and Ab2-GOx-SiO2-GOx oxidized glucose to produce H2O2 and gluconic acid. When the modified electrode is submerged in the enzyme reaction mixture, the Fenton reaction occurs by the conversion of Fe3+ to Fe2+ in the presence of H2O2. The immunoreaction was performed within the MWCNT, and the Fe3+ consumption led to the decomposition of the Fe3+-alginate hydrogel, thereby decreasing the interface resistance. As the NSE concentration increased, the current signal also increased and thereby showcased an ultralow LOD of 0.447 pg/mL and a detection range of 0.001 to 100 ng/mL. The 0.5 wt % alginate volume, pH 5.0, and 40 min were the optimized parameters. Good clinical analysis can be obtained because its relative error is lower than 10%, thereby providing reproducibility and around 99% recovery.
In the agarose gel, Shen et al. incorporated ractopamine-bovine thyroglobulin antigen to fabricate an immunosensor to detect ractopamine in animal feeds.8 The GCE was coated with the hydrogel modified with antigens, and K3[Fe(CN)6]/K4[Fe(CN)6] was made use of as the redox probe to monitor the reaction. The specific binding of ractopamine-bovine thyroglobulin antigen with antiractopamine antibodies hindered the electron flow of the redox marker toward the GCE by insulating it. Hence, the immunosensor’s percentage of current response reduction measured was proportionate to the concentration of ractopamine in the range of 1–1000 ng/mL. The optimum incubation time, temperature, and pH were 30 min, 37 °C, and 7.4, respectively. And 40 μg mL–1 was the optimum concentration of the antibody chosen. Ractopamine was practically determined in animal feeds by this immunosensor, and in comparison with ELISA, the immunosensor results showed good consistency with a recovery of around 80–110%.
By the cross-linking coordination method, a conductive hydrogel was prepared from phytic acid (ligand) and lead (II-metal ion) that displayed strong electroconductivity at −0.5 V.20 To fabricate the immunosensor, Wang et al. utilized this hydrogel to drop coat on GCE such that it was used as a substrate for sensing cytokeratin antigen 21-1 (CYFRA21-1). The antibody (anti-CYFRA21-1) immobilization was attributable to AuNPs which were electrodeposited on the hydrogel and further enhanced the conductivity of the electrode. BSA was used as the blocking agent, and a drop in the current was witnessed during the antigen–antibody conjugation. The fabrication procedure of this immunosensor is illustrated in Figure 4. As the pH and incubation time affected the results, they were optimized to pH 6.5 and 50 min during the experiments. The designed immunosensor displayed a good linear range of detection and a low LOD of 50 fg/mL to 100 ng/mL and 38 fg/mL, respectively. From the test conducted on clinical samples, it could be deduced that they exhibited selectivity toward CYFRA21-1 and good consistence with ELISA wherein the relative standard error lower than 10% and the relative standard deviation of 6% indicated good reliability and repeatability. Less than 10% was the change in the electrical response observed when kept under 4 °C for a week, which indicated good storage stability.
Figure 4.
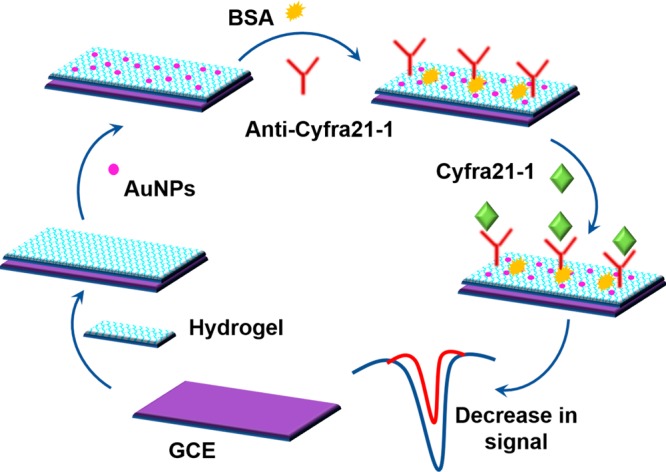
Electrochemical immunosensor fabrication is represented diagrammatically. Reproduced with permission from ref (20). Copyright 2017, Springer-Verlag Wien.
To overcome the challenges of signal amplification, Zhao et al. constructed a sandwich-type immunosensor based on Ca2+-elicited pH-response sodium alginate precipitation for PSA detection.21 On the GCE, anti-PSA (Ab1) was immobilized after the electrodeposition of AuNPs. The immunoprobes were labeled with gold nanoparticles-CaCO3 microspheres (AuNP-CaCO3), anti-PSA, and bovine serum albumin (BSA), and they imply a strong hindrance effect. The immunoprobes were conjugated with the sensing platform after Ab1 captured PSA molecules. Alginate gelation was triggered by the Ca2+ ions released under weakly acidic conditions, and the further elevation in the impedance signal is attributable to the negatively charged alginate surface which is pH-responsive. The cascading impedance response (ΔRct) was measured for the ultrasensitive detection of PSA. The potential of this immunosensor was realized when an acceptable relative error of 5.88–5% was observed when compared with the chemiluminescence immunoassay analyzer (CMIA). The immunosensor displayed good specificity, stability, and reproducibility, a rapid response time, and a linear detection range of 1 fg/mL to 100 ng/mL with a limit of detection of 0.09 fg/mL.
2.3. Colorimetric Immunoassay
In colorimetric immunoassays, alkaline phosphatase and horseradish oxidase were the probes commonly used, and the biological signals are indicated by color to provide direct and rapid readout.
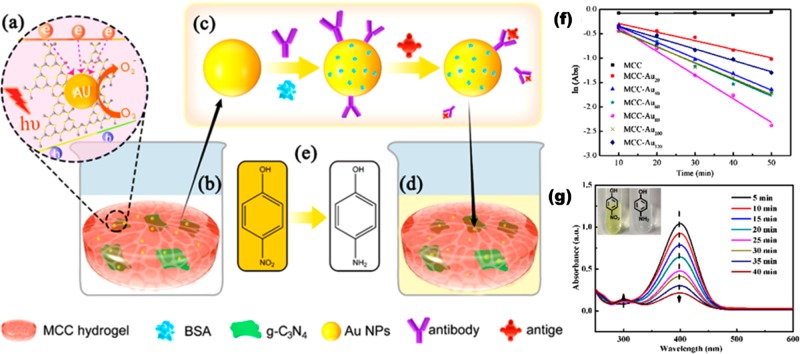
In 2019, for α-fetoprotein (AFP) detection a colorimetric immunosensor based on cellulose hydrogel was developed by Ma et al.22 In this, the microcrystalline cellulose (MCC) hydrogel employed provided the antibody binding site, and graphitelike carbon nitride (g-C3N4) nanosheets loaded with AuNPs were fixed in it to form a hybrid hydrogel (Au@g-C3N4/MCC). On antibody–antigen conjugation, the catalytically active AuNPs displayed a “switching on/off” mechanism and the superoxide ion radicals that were produced reduced the yellow 4-nitrophenol to a colorless 4-aminophenol substrate during the transfer of electrons from g-C3N4 to AuNPs. The catalytic activity of the hybrid hydrogel was “turned off” and “turned on” while AFP detached and conjugated with the anti-AFP, respectively. For the immunoassay, this color-changing mechanism was used for the quantitative analysis of AFP, and it is represented in Figure 5. The immunosensor used exhibits good stability (10% less than for the fresh composite) and repeatability and a selectivity toward AFP of within 0.1–10 000 ng/mL with a limit of detection of 0.46 ng/mL. The results of the immunosensor tested on real samples showed agreement with ELISA with a relative error of −4.3 to 2.8%.
Figure 5.
(a) Electron-transfer mechanism between g-C3N4 and AuNPs. (b) Catalysis of 4-nitrophenol by Au@g-C3N4/MCC leading to color fading. (c) Antibody/antigen interaction with Au@g-C3N4/MCC. (d) Partial color fading after Au@g-C3N4/MCC was incubated with the antigen. (e) Structure and color of 4-nitrophenol (yellow) and 4-AP (colorless). (f) Graph of the natural logarithmic value of 4-nitrophenol absorption [ln(Abs)] at 400 nm over time catalyzed by hydrogels with different MCC and AU compositions. (g) UV–vis absorption spectra of 4-nitrophenol reduced by NaBH4 for the Au@g-C3N4/MCC hydrogel. Reproduced with permission from ref (22). Copyright 2019 American Chemical Society.
2.4. Electrochemiluminescent (ECL) Immunoassay
Electrochemiluminescence or electrogenerated chemiluminescence is a type of luminescence produced during an electrochemical reaction when electrogenerated intermediates undergo excitation and relax to the lower state by emitting light.23
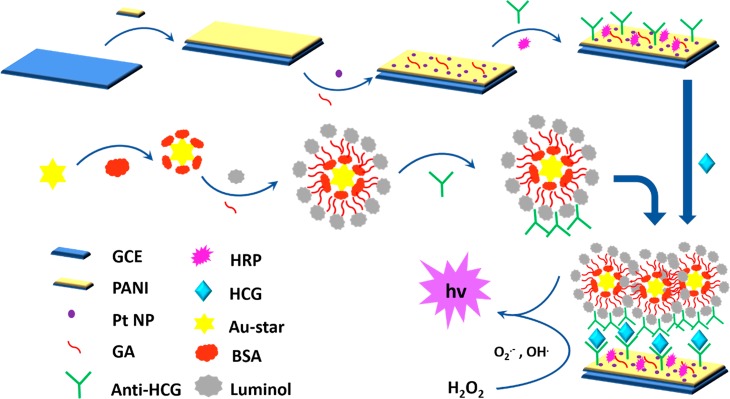
Zhang et al. in 2017 constructed an ultrasensitive immunosensor for the analysis of HCG based on the sandwich protocol.24 The GCE was coated with polyaniline hydrogel and loaded with Pt nanoparticles to enhance the electron transfer, and via glutaraldehyde, antibodies (Ab1) were captured on the substrate. In this, horseradish peroxidase (HRP) was employed as the H2O2-catalyzing enzyme and as the blocking agent for nonspecific binding sites. The luminescent probe was prepared by adding BSA to Au star and enabling the capture of luminol, a luminescent material by GA, which further promoted the immobilization of antibodies (Ab2) on its surface. Sandwiched immunoreactions exist between the synthesized luminol-Au star@BSA-Ab2 complex and the modified sensor during the conjugation of the HCG antigen. The electrochemical signal was amplified by the ability of nano-Pt and HRP to catalyze H2O2 to engender reactive O2.– and OH· which consequently act as a coreactant of luminol. The fabrication of both the label and the substrate is schematically represented in Figure 6. This immunosensor exhibited stability, selectivity, reproducibility, a detection range of 0.001 to 50 mIU/mL, and a limit of detection of 0.0003 mIU/mL. The ranges of relative deviations (0.19 to 3.31%) and recoveries (92.20 to 101.49%) indicate their reliability.
Figure 6.
Diagrammatic depiction of the fabrication and working of the electrochemiluminescent immunosensor. Reproduced with permission from ref (24). Copyright 2017, Elsevier.
3. Conclusion and Future Perspectives
Owing to its highly porous structure, large surface area, and excellent biocompatibility, hydrogels have been successfully employed in nanobiosensing. Hydrogels of varied compositions have been explored for the immobilization of biomolecules (antigen or antibody) to fabricate immunosensors with great analytical potential. SPR, electrochemiluminescence, colorimetrics, and electrochemical assays were the different strategies involved in the signal transduction in hydrogel-based immunosensors during the formation of the antigen–antibody complex. Electron transfer to the electrode during immunoreaction was augmented by the functionalization of these 3D gels by nanomaterials such as AuNPs, GO, and MWCNTs.
In this review, we have recapitulated disparate hydrogels such as conductive, redox, and smart hydrogels employed in immunosensors for the recognition of cancer biomarkers (TNF-α, AFP, PSA, CEA, CA242, NSE, CYFRA21-1), hormones (cortisol and cortisone, HCG), human IgG, and ractopamine in animal feed. Herein, the hydrogel-based immunosensing platforms are classified by virtue of their mode of signal transduction. The immunosensors discussed here exhibit rapid responses, good stability, reproducibility, and high sensitivity and selectivity and display results similar to those in the ELISA test.
The global market of biosensors has predicted growth of 16.9 billion USD in 2017 to 25.9 billion USD by 2022, which reveals a compound annual growth rate of around 9%. Concurrently, 27.2 billion USD represents the reported global market for hydrogels by 2022 with an estimated 6.3% CAGR. The future of hydrogels as an immunosensing platform relies on the progressive requirement for the recognition of several other analytes for food quality control, environmental monitoring, and diseases other than cancer in both humans and animals. The full potential of hydrogels has to be further explored because hydrogels other than conductive hydrogels and hydrogels with metal ion chelation act as sensing substrates for signal enhancement, and most of the hydrogels were employed just as an immobilization matrix. New methods have to be developed to monitor the hydrogel porosity wherein during immobilization the biomolecule entrapped within large pores tends to become inactive or cause bioreceptor leakage. Immunoassays with a shorter incubation time would be favorable because the lowest optimum incubation time for the formation of an antigen–antibody complex in hydrogel-based immunosensors reported so far is 50 min. More research could be done to prevent the destruction of hydrogels when detaching the bioreceptors in irreversible immobilizations. Piezoelectric, colorimetric, and electrochemiluminescent signal transduction principles with various natural and synthetic polymeric hydrogels need to be analyzed more. The potential of MXenes in immunosensors needs to be explored by functionalizing different hydrogels with MXene nanosheets. Thus, the hydrogel-based immunosensors may encounter a considerable market hike in the near future.
Acknowledgments
The authors acknowledge Dr. C. P. Ramanarayanan, Vice-Chancellor of Defence Institute of Advanced Technology (DU), Pune, India, for his persistent support and motivation. The authors also thank Mr. Prakash Gore, Mr. Raviprakash Magisetty, and Mr. Gaurav Sharma for useful technical discussions and constant encouragement. The authors are thankful to the Editor and all anonymous reviewers for improving the quality of the revised manuscript by their valuable comments and suggestions.
Biographies

Suchi Mercy George is currently pursuing her master’s degree in materials engineering at DIAT, Pune, India. She received her Bachelor of Technology in polymer engineering from Mahatma Gandhi University, Kottayam, India in 2017. Her research interests include biopolymers, hydrogels, immunosensors, biomaterials, polymer- and ceramic-based scaffolds for tissue engineering, and MXene/polymer nanocomposites for biomedical applications.
Saloni Tandon is currently pursuing her B. Tech. + M. Tech. dual degree in converging technologies (with a major in biotechnology) from the Centre of Converging Technologies, University of Rajasthan, Jaipur, India. Her research interests include, biofuels, tissue engineering, immunology, drug delivery, and agricultural nanobiotechnology.

Balasubramanian Kandasubramanian is a Dean of Student Affairs and a professor in the Department of Metallurgical & Materials Engineering, DIAT (DU), Ministry of Defence, India, and has been highly acclaimed for his contributions to polymer processing and fabrication for various applications including antibacterials, smart textiles, hydrophobic coatings, ablative materials, fire retardant fabrics, waste-water treatments, and polymer nanocomposites for Defence applications. He was actively involved in the development of various research laboratories including the Polymer Processing Laboratory, Nano Texturing Laboratory, Advanced Characterization Laboratoriess (field emission scanning electron microscope, high-resolution transmission electron microscope, and small-angle X-ray scattering laboratories), the Additive Manufacturing Laboratory, and so forth. The research activity of Prof. Balasubramanian’s research group can be classified into five categories: water management, smart textiles, biomimicking of polymers, structural composites, and advanced coatings for Defence applications. Prof. Balasubramanian has been recognized by The Royal Society of Chemistry (RSC) for being one of the most cited authors of the year (2015) for his erudite work on superhydrophobicity. He has recently received the prestigious Technology Innovation in Petrochemicals and Downstream Plastic Processing Industry Award from the Ministry of Chemicals and Fertilizers, Government of India, for his contribution to waste/water management and design of advanced coatings. In the last 5 years, he has developed various technologies that exist as patents, peer-reviewed journal articles, and international conferences. Apart from his scholarly achievements, Prof. Balasubramanian has been actively involved in various collaborative ventures with academia and industries around the world.
The authors declare no competing financial interest.
References
- Prajapati D. G.; Kandasubramanian B. Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors. Macromol. Chem. Phys. 2019, 1800561, 1–26. 10.1002/macp.201800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollap A.; Kochana J.. Electrochemical Immunosensors for Antibiotic Detection. Biosensors 2019, 9 ( (2), ). 61. 10.3390/bios9020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Ding Y.; Li T.; Yang M. Redox Hydrogel Based Immunosensing Platform for the Label-Free Detection of a Cancer Biomarker. Anal. Methods 2015, 7 (2), 411–415. 10.1039/C4AY02640C. [DOI] [Google Scholar]
- Tang Z.; Fu Y.; Ma Z. Multiple Signal Amplification Strategies for Ultrasensitive Label-Free Electrochemical Immunoassay for Carbohydrate Antigen 24–2 Based on Redox Hydrogel. Biosens. Bioelectron. 2017, 91, 299–305. 10.1016/j.bios.2016.12.049. [DOI] [PubMed] [Google Scholar]
- Frasconi M.; Mazzarino M.; Botrè F.; Mazzei F. Surface Plasmon Resonance Immunosensor for Cortisol and Cortisone Determination. Anal. Bioanal. Chem. 2009, 394 (8), 2151–2159. 10.1007/s00216-009-2914-6. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Li T.; Huang H.; Quan H.; Miao X.; Yang M. Electrochemical Immunosensor for the Detection of Tumor Necrosis Factor α Based on Hydrogel Prepared from Ferrocene Modified Amino Acid. Sens. Actuators, B 2013, 182, 605–609. 10.1016/j.snb.2013.03.067. [DOI] [Google Scholar]
- Zhou M.; Sun Z.; Shen C.; Li Z.; Zhang Y.; Yang M. Application of Hydrogel Prepared from Ferrocene Functionalized Amino Acid in the Design of Novel Electrochemical Immunosensing Platform. Biosens. Bioelectron. 2013, 49, 243–248. 10.1016/j.bios.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Shen L.; He P. An Electrochemical Immunosensor Based on Agarose Hydrogel Films for Rapid Determination of Ractopamine. Electrochem. Commun. 2007, 9 (4), 657–662. 10.1016/j.elecom.2006.10.049. [DOI] [Google Scholar]
- Yin S.; Zhao L.; Ma Z. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Neuron-Specific Enolase Based on Enzyme-Free Catalytic Amplification. Anal. Bioanal. Chem. 2018, 410 (4), 1279–1286. 10.1007/s00216-017-0767-y. [DOI] [PubMed] [Google Scholar]
- Souza S. F.; Kogikoski S.; Silva E. R.; Alves W. A. Nanostructured Antigen-Responsive Hydrogels Based on Peptides for Leishmaniasis Detection. J. Braz. Chem. Soc. 2017, 28 (9), 1619–1629. 10.21577/0103-5053.20160301. [DOI] [Google Scholar]
- Rong Q.; Han H.; Feng F.; Ma Z. Network Nanostructured Polypyrrole Hydrogel/Au Composites as Enhanced Electrochemical Biosensing Platform. Sci. Rep. 2015, 5 (March), 1–8. 10.1038/srep11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M. N.; Wei S. C.; Guo S.; Phan D. T.; Zhang Y.; Chen C. H. Smart Hydrogel Microfluidics for Single-Cell Multiplexed Secretomic Analysis with High Sensitivity. Small 2018, 14 (49), 1802918. 10.1002/smll.201802918. [DOI] [PubMed] [Google Scholar]
- Shankaran D. R.; Gobi K. V.; Miura N. Recent Advancements in Surface Plasmon Resonance Immunosensors for Detection of Small Molecules of Biomedical, Food and Environmental Interest. Sens. Actuators, B 2007, 121 (1), 158–177. 10.1016/j.snb.2006.09.014. [DOI] [Google Scholar]
- Zhang D.; Li W.; Ma Z. Improved Sandwich-Format Electrochemical Immunosensor Based on “Smart” SiO2@polydopamine Nanocarrier. Biosens. Bioelectron. 2018, 109 (March), 171–176. 10.1016/j.bios.2018.03.027. [DOI] [PubMed] [Google Scholar]
- Wang H.; Han H.; Ma Z. Conductive Hydrogel Composed of 1,3,5-Benzenetricarboxylic Acid and Fe3 + Used as Enhanced Electrochemical Immunosensing Substrate for Tumor Biomarker. Bioelectrochemistry 2017, 114, 48–53. 10.1016/j.bioelechem.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Li W.; Ma Z. Conductive Catalytic Redox Hydrogel Composed of Aniline and Vinyl-Ferrocene for Ultrasensitive Detection of Prostate Specific Antigen. Sens. Actuators, B 2017, 248, 545–550. 10.1016/j.snb.2017.04.021. [DOI] [Google Scholar]
- Wang H.; Ma Z. A Cascade Reaction Signal-Amplified Amperometric Immunosensor Platform for Ultrasensitive Detection of Tumour Marker. Sens. Actuators, B 2018, 254, 642–647. 10.1016/j.snb.2017.07.135. [DOI] [Google Scholar]
- Zhang X.; Wang S.; Hu M.; Xiao Y. An Immunosensor for Ferritin Based on Agarose Hydrogel. Biosens. Bioelectron. 2006, 21 (11), 2180–2183. 10.1016/j.bios.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Yin S.; Ma Z. Smart” Sensing Interface for the Improvement of Electrochemical Immunosensor Based on Enzyme-Fenton Reaction Triggered Destruction of Fe 3+ Cross-Linked Alginate Hydrogel. Sens. Actuators, B 2019, 281 (May 2018), 857–863. 10.1016/j.snb.2018.11.030. [DOI] [Google Scholar]
- Wang H.; Ma Z. Ultrasensitive Amperometric Detection of the Tumor Biomarker Cytokeratin Antigen Using a Hydrogel Composite Consisting of Phytic Acid, Pb(II) Ions and Gold Nanoparticles. Microchim. Acta 2017, 184 (4), 1045–1050. 10.1007/s00604-017-2101-y. [DOI] [Google Scholar]
- Zhao L.; Yin S.; Ma Z. Ca 2+ -Triggered Ph-Response Sodium Alginate Hydrogel Precipitation for Amplified Sandwich-Type Impedimetric Immunosensor of Tumor Marker. ACS Sensors 2019, 4 (2), 450–455. 10.1021/acssensors.8b01465. [DOI] [PubMed] [Google Scholar]
- Ma F.; Yuan C. W.; Liu J. N.; Cao J. H.; Wu D. Y. Colorimetric Immunosensor Based on Au@g-C3N4-Doped Spongelike 3D Network Cellulose Hydrogels for Detecting α-Fetoprotein. ACS Appl. Mater. Interfaces 2019, 11 (22), 19902–19912. 10.1021/acsami.9b06769. [DOI] [PubMed] [Google Scholar]
- Forster R.; Bertoncello P.; Keyes T. Electrogenerated Chemiluminescence. Annu Rev Anal Chem 2:359. Annu. Rev. Anal. Chem. 2009, 2, 359–385. 10.1146/annurev-anchem-060908-155305. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Guo W.; Ke H.; Zhang X.; Zhang H.; Huang C.; Yang D.; Jia N.; Cui D. Sandwich-Format ECL Immunosensor Based on Au Star@BSA-Luminol Nanocomposites for Determination of Human Chorionic Gonadotropin. Biosens. Bioelectron. 2018, 101, 219–226. 10.1016/j.bios.2017.10.040. [DOI] [PubMed] [Google Scholar]
- Li W.; Shu D.; Han H.; Ma Z. An Amperometric Immunoprobe Based on Multifunctional Nanogel for Sensitive Detection of Tumor Marker. Sens. Actuators, B 2018, 273 (March), 1451–1455. 10.1016/j.snb.2018.07.067. [DOI] [Google Scholar]